Bioactive Polyphenolic Compounds from Propolis of Tetragonula carbonaria in the Gibberagee Region, New South Wales, Australia
Abstract
1. Introduction
2. Materials and Methods
2.1. Solvents and Reagents
2.2. Sample Collection and Extraction
2.3. Isolation and Purification
2.4. Evaluation of Antioxidant Activity Using DPPH Free Radical Scavenging Assay
2.5. NMR Analysis
2.6. LC-QTOF MS Analysis
2.7. Molecular Networking Analysis
2.8. In Silico Screening of Compounds Against α-Glucosidase
3. Results and Discussion
3.1. Antioxidant Activity of the Gibberagee Propolis and the Pure Polyphenols
3.2. Mining Polyphenolic Compounds Through Molecular Networking Analysis
3.3. In Silico Assay
3.4. Potential Bioactivity of Compounds Identified from the Gibberagee Propolis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Hatamleh, M.A.I.; Boer, J.C.; Wilson, K.L.; Plebanski, M.; Mohamud, R.; Mustafa, M.Z. Antioxidant-Based Medicinal Properties of Stingless Bee Products: Recent Progress and Future Directions. Biomolecules 2020, 10, 923. [Google Scholar] [CrossRef] [PubMed]
- Ghisalberti, E.L. Propolis: A review. Bee World 1979, 60, 59–84. [Google Scholar] [CrossRef]
- Leonhardt, S.D.; Blüthgen, N. A Sticky Affair: Resin Collection by Bornean Stingless Bees. Biotropica 2009, 41, 730–736. [Google Scholar] [CrossRef]
- Bankova, V.; Popova, M.; Trusheva, B. The phytochemistry of the honeybee. Phytochemistry 2018, 155, 1–11. [Google Scholar] [CrossRef]
- Popova, M.; Trusheva, B.; Bankova, V. Propolis of stingless bees: A phytochemist’s guide through the jungle of tropical biodiversity. Phytomedicine 2021, 86, 153098. [Google Scholar] [CrossRef] [PubMed]
- Zulhendri, F.; Perera, C.O.; Chandrasekaran, K.; Ghosh, A.; Tandean, S.; Abdulah, R.; Herman, H.; Lesmana, R. Propolis of stingless bees for the development of novel functional food and nutraceutical ingredients: A systematic scoping review of the experimental evidence. J. Funct. Foods 2022, 88, 104902. [Google Scholar] [CrossRef]
- Tran, T.D.; Ogbourne, S.M.; Brooks, P.R.; Sánchez-Cruz, N.; Medina-Franco, J.L.; Quinn, R.J. Lessons from Exploring Chemical Space and Chemical Diversity of Propolis Components. Int. J. Mol. Sci. 2020, 21, 4988. [Google Scholar] [CrossRef]
- Reynolds, O.L.; Robinson, M. Australian Native Bee Strategic RD&E Plan (2022–2027); AgriFutures Australia Publication: Wagga Wagga, NSW, Australia, 2022; p. 40. [Google Scholar]
- Halcroft, M.; Spooner-Hart, R.; Dollin, L.A. Australian Stingless Bees. In Pot-Honey: A Legacy of Stingless Bees; Vit, P., Pedro, S.R.M., Roubik, D., Eds.; Springer: New York, NY, USA, 2013; pp. 35–72. [Google Scholar] [CrossRef]
- Massaro, F.C.; Brooks, P.R.; Wallace, H.M.; Russell, F.D. Cerumen of Australian stingless bees (Tetragonula carbonaria): Gas chromatography-mass spectrometry fingerprints and potential anti-inflammatory properties. Naturwissenschaften 2011, 98, 329–337. [Google Scholar] [CrossRef]
- Massaro, F.C.; Brooks, P.R.; Wallace, H.M.; Nsengiyumva, V.; Narokai, L.; Russell, F.D. Effect of Australian Propolis from Stingless Bees (Tetragonula carbonaria) on Pre-Contracted Human and Porcine Isolated Arteries. PLoS ONE 2013, 8, e81297. [Google Scholar] [CrossRef]
- Massaro, C.F.; Katouli, M.; Grkovic, T.; Vu, H.; Quinn, R.J.; Heard, T.A.; Carvalho, C.; Manley-Harris, M.; Wallace, H.M.; Brooks, P. Anti-staphylococcal activity of C-methyl flavanones from propolis of Australian stingless bees (Tetragonula carbonaria) and fruit resins of Corymbia torelliana (Myrtaceae). Fitoterapia 2014, 95, 247–257. [Google Scholar] [CrossRef]
- Nishimura, E.; Murakami, S.; Suzuki, K.; Amano, K.; Tanaka, R.; Shinada, T. Structure Determination of Monomeric Phloroglucinol Derivatives with a Cinnamoyl Group Isolated from Propolis of the Stingless Bee, Tetragonula carbonaria. Asian J. Org. Chem. 2016, 5, 855–859. [Google Scholar] [CrossRef]
- Hamilton, K.D.; Czajkowski, D.; Kong, N.J.; Tran, T.D.; Gustafson, K.R.; Pauly, G.; Boyle, G.M.; Simmons, J.L.; Steadman, R.; Moseley, R.; et al. Anti-Fibrotic Potential of Tomentosenol A, a Constituent of Cerumen from the Australian Native Stingless Bee, Tetragonula carbonaria. Antioxidants 2022, 11, 1604. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.P. Oxidative Stress in Health and Disease. Biomedicines 2023, 11, 2925. [Google Scholar] [CrossRef]
- Bondonno, N.P.; Dalgaard, F.; Murray, K.; Davey, R.J.; Bondonno, C.P.; Cassidy, A.; Lewis, J.R.; Kyrø, C.; Gislason, G.; Scalbert, A.; et al. Higher Habitual Flavonoid Intakes Are Associated with a Lower Incidence of Diabetes. J. Nutr. 2021, 151, 3533–3542. [Google Scholar] [CrossRef]
- Tomic, D.; Chen, L.; Moran, L.L.; Magliano, D.J.; Shaw, J.E. Causes of death among Australians with type 1 or type 2 diabetes, 2002–2019. Diabet. Med. 2024, 41, e15206. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Sui, L.; Yang, F.; Ren, X.; Xing, Y.; Xiu, Z. Reducing the intestinal side effects of acarbose by baicalein through the regulation of gut microbiota: An in vitro study. Food Chem. 2022, 394, 133561. [Google Scholar] [CrossRef]
- Vinayagam, R.; Jayachandran, M.; Xu, B. Antidiabetic Effects of Simple Phenolic Acids: A Comprehensive Review. Phytother. Res. 2016, 30, 184–199. [Google Scholar] [CrossRef]
- Pieczykolan, A.; Pietrzak, W.; Gawlik-Dziki, U.; Nowak, R. Antioxidant, Anti-Inflammatory, and Anti-Diabetic Activity of Phenolic Acids Fractions Obtained from Aerva lanata (L.) Juss. Molecules 2021, 26, 3486. [Google Scholar] [CrossRef]
- Tran, C.T.N.; Brooks, P.R.; Bryen, T.J.; Williams, S.; Berry, J.; Tavian, F.; McKee, B.; Tran, T.D. Quality assessment and chemical diversity of Australian propolis from Apis mellifera bees. Sci. Rep. 2022, 12, 13574. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- Uddin, S.; Brooks, P.R.; Tran, T.D. Chemical Characterization, α-Glucosidase, α-Amylase and Lipase Inhibitory Properties of the Australian Honey Bee Propolis. Foods 2022, 11, 1964. [Google Scholar] [CrossRef] [PubMed]
- The Galaxy, C. The Galaxy platform for accessible, reproducible, and collaborative data analyses: 2024 update. Nucleic Acids Res. 2024, 52, W83–W94. [Google Scholar] [CrossRef] [PubMed]
- Tukiran, B.; Mahmudah, F.; Hidayati, N.; Shimizu, K. A phenolic acid and its antioxidant activity from stem bark of chloroform fraction of Syzygium littorale (Blume) amshoff (Myrtaceae). Molekul 2016, 11, 180–189. [Google Scholar] [CrossRef]
- Davis, A.L.; Cai, Y.; Davies, A.P.; Lewis, J.R. 1H and 13C NMR Assignments of Some Green Tea Polyphenols. Magn. Reson. Chem. 1996, 34, 887–890. [Google Scholar] [CrossRef]
- Park, S.Y.; Bae, Y.S. Antioxidative Activity of Prunus sargentii Outer Bark Extractives. J. Korean Wood Sci. Technol. 2012, 40, 141–146. [Google Scholar] [CrossRef]
- He, D.; Gu, D.; Huang, Y.; Ayupbek, A.; Yang, Y.; Aisa, H.A.; Ito, Y. Separation and Purification of Phenolic Acids and Myricetin from Black Currant by High-Speed Countercurrent Chromatography. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 3077–3088. [Google Scholar] [CrossRef]
- Chu, L.L.; Pandey, R.P.; Jung, N.; Jung, H.J.; Kim, E.-H.; Sohng, J.K. Hydroxylation of diverse flavonoids by CYP450 BM3 variants: Biosynthesis of eriodictyol from naringenin in whole cells and its biological activities. Microb. Cell Factories 2016, 15, 135. [Google Scholar] [CrossRef]
- Granados-Pineda, J.; Uribe-Uribe, N.; García-López, P.; Ramos-Godinez, M.D.; Rivero-Cruz, J.F.; Pérez-Rojas, J.M. Effect of Pinocembrin Isolated from Mexican Brown Propolis on Diabetic Nephropathy. Molecules 2018, 23, 852. [Google Scholar] [CrossRef]
- Malterud, K.E. C-methylated dihydrochalcones from Myrica gale fruit exudate. Acta Pharm. Nord. 1992, 4, 65–68. [Google Scholar]
- Wollenweber, E.; Wehde, R.; Dörr, M.; Lang, G.; Stevens, J.F. C-Methyl-flavonoids from the leaf waxes of some Myrtaceae. Phytochemistry 2000, 55, 965–970. [Google Scholar] [CrossRef]
- Freitas, M.O.; Ponte, F.A.F.; Lima, M.A.S.; Silveira, E.R. Flavonoids and triterpenes from the nest of the stingless bee Trigona spinipes. J. Braz. Chem. Soc. 2008, 19, 532–535. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Department of Agriculture, Water and the Environment. Conservation Advice for Melichrus sp. Gibberagee (Narrow-Leaf Melichrus). Department of Agriculture, Water and the Environment, The Australian Government Canberra, 2021. Available online: https://www.environment.gov.au/biodiversity/threatened/species/pubs/86881-conservation-advice-23112021.pdf (accessed on 24 February 2025).
- Samson, S.L.; Garber, A.J. Prevention of type 2 Diabetes Mellitus: Potential of pharmacological agents. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 357–371. [Google Scholar] [CrossRef]
- Gao, H.; Nishioka, T.; Kawabata, J.; Kasai, T. Structure-activity relationships for alpha-glucosidase inhibition of baicalein, 5,6,7-trihydroxyflavone: The effect of A-ring substitution. Biosci. Biotechnol. Biochem. 2004, 68, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Proença, C.; Freitas, M.; Ribeiro, D.; Oliveira, E.F.T.; Sousa, J.L.C.; Tomé, S.M.; Ramos, M.J.; Silva, A.M.S.; Fernandes, P.A.; Fernandes, E. α-Glucosidase inhibition by flavonoids: An in vitro and in silico structure–activity relationship study. J. Enzym. Inhib. Med. Chem. 2017, 32, 1216–1228. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Yang, H.; Dong, Y.; Du, H.; Shi, H.; Peng, Y.; Li, X. Antioxidant Compounds from Propolis Collected in Anhui, China. Molecules 2011, 16, 3444–3455. [Google Scholar] [CrossRef]
- Jasemi, S.V.; Khazaei, H.; Morovati, M.R.; Joshi, T.; Aneva, I.Y.; Farzaei, M.H.; Echeverría, J. Phytochemicals as treatment for allergic asthma: Therapeutic effects and mechanisms of action. Phytomedicine 2024, 122, 155149. [Google Scholar] [CrossRef]
- Jasemi, S.V.; Khazaei, H.; Momtaz, S.; Farzaei, M.H.; Echeverría, J. Natural products in the treatment of pulmonary emphysema: Therapeutic effects and mechanisms of action. Phytomedicine 2022, 99, 153988. [Google Scholar] [CrossRef]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran. J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar]
- Hadidi, M.; Liñán-Atero, R.; Tarahi, M.; Christodoulou, M.C.; Aghababaei, F. The Potential Health Benefits of Gallic Acid: Therapeutic and Food Applications. Antioxidants 2024, 13, 1001. [Google Scholar] [CrossRef]
- Obafemi, T.O.; Jaiyesimi, K.F.; Olomola, A.A.; Olasehinde, O.R.; Olaoye, O.A.; Adewumi, F.D.; Afolabi, B.A.; Adewale, O.B.; Akintayo, C.O.; Ojo, O.A. Combined effect of metformin and gallic acid on inflammation, antioxidant status, endoplasmic reticulum (ER) stress and glucose metabolism in fructose-fed streptozotocin-induced diabetic rats. Toxicol. Rep. 2021, 8, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef]
- Ito, H.; Shoji, Y.; Matsumoto, K.-i.; Fukuhara, K.; Nakanishi, I. Anti-cancer Effect of a Planar Catechin Analog through the Decrease in Mitochondrial Membrane Potential. ACS Med. Chem. Lett. 2023, 14, 1478–1481. [Google Scholar] [CrossRef] [PubMed]
- Stefaniu, A.; Pirvu, L.C. In Silico Study Approach on a Series of 50 Polyphenolic Compounds in Plants; A Comparison on the Bioavailability and Bioactivity Data. Molecules 2022, 27, 1413. [Google Scholar] [CrossRef]
- Fan, F.Y.; Sang, L.X.; Jiang, M. Catechins and Their Therapeutic Benefits to Inflammatory Bowel Disease. Molecules 2017, 22, 484. [Google Scholar] [CrossRef]
- Gopal, J.; Muthu, M.; Paul, D.; Kim, D.-H.; Chun, S. Bactericidal activity of green tea extracts: The importance of catechin containing nano particles. Sci. Rep. 2016, 6, 19710. [Google Scholar] [CrossRef] [PubMed]
- Ayuda-Durán, B.; Garzón-García, L.; González-Manzano, S.; Santos-Buelga, C.; González-Paramás, A.M. Insights into the Neuroprotective Potential of Epicatechin: Effects against Aβ-Induced Toxicity in Caenorhabditis elegans. Antioxidants 2024, 13, 79. [Google Scholar] [CrossRef]
- Taheri, Y.; Suleria, H.A.R.; Martins, N.; Sytar, O.; Beyatli, A.; Yeskaliyeva, B.; Seitimova, G.; Salehi, B.; Semwal, P.; Painuli, S.; et al. Myricetin bioactive effects: Moving from preclinical evidence to potential clinical applications. BMC Complement. Med. Ther. 2020, 20, 241. [Google Scholar] [CrossRef]
- Islam, A.; Islam, M.S.; Rahman, M.K.; Uddin, M.N.; Akanda, M.R. The pharmacological and biological roles of eriodictyol. Arch. Pharmacal Res. 2020, 43, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Elbatreek, M.H.; Mahdi, I.; Ouchari, W.; Mahmoud, M.F.; Sobeh, M. Current advances on the therapeutic potential of pinocembrin: An updated review. Biomed. Pharmacother. 2023, 157, 114032. [Google Scholar] [CrossRef] [PubMed]
- Vechi, G.; da Silva, R.d.C.M.V.d.A.F.; de Souza, P.; da Silva, L.M.; de Andrade, S.F.; Cechinel Filho, V. Cryptostrobin and catechin isolated from Eugenia mattosii D. Legrand leaves induce endothelium-dependent and independent relaxation in spontaneously hypertensive rat aorta. Pharmacol. Rep. 2019, 71, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, N.; Nobushi, Y.; Wada, T.; Sonoda, K.; Okazaki, Y.; Tsutsumi, S.; Park, Y.K.; Kurokawa, M.; Shimba, S.; Yasukawa, K. Inhibitory effects of compounds isolated from the dried branches and leaves of murta (Myrceugenia euosma) on lipid accumulation in 3T3-L1 cells. J. Nat. Med. 2016, 70, 502–509. [Google Scholar] [CrossRef]
- Gafner, S.; Wolfender, J.L.; Mavi, S.; Hostettmann, K. Antifungal and antibacterial chalcones from Myrica serrata. Planta Medica 1996, 62, 67. [Google Scholar] [CrossRef]
- Abdel Bar, F.M. Genus Melaleuca—A Review on the Phytochemistry and Pharmacological Activities of the Non-Volatile Components. Rec. Nat. Prod. 2021, 15, 219–242. [Google Scholar] [CrossRef]
- Cuong, N.M.; Khanh, P.N.; Nhung, L.T.H.; Ha, N.X.; Huong, T.T.; Bauerova, K.; Kim, Y.H.; Tung, D.D.; Thuy, T.T.; Anh, N.T.H. Acetylcholinesterase inhibitory activities of some flavonoids from the root bark of Pinus krempfii Lecomte: In vitro and in silico study. J. Biomol. Struct. Dyn. 2024, 42, 4888–4901. [Google Scholar] [CrossRef]
- Tarawneh, A.H.; Ibrahim, M.A.; Radwan, M.M.; Ma, G.; Cutler, H.G.; Cutler, S.J. Therapeutic Efficacy of Phenolic Compounds from Micnia prasina. in Acute Lymphoblastic Leukemia. Planta Medica 2013, 79, P55. [Google Scholar] [CrossRef]
- Stompor, M. A Review on Sources and Pharmacological Aspects of Sakuranetin. Nutrients 2020, 12, 513. [Google Scholar] [CrossRef]
- Uçar, K.; Göktaş, Z. Biological activities of naringenin: A narrative review based on in vitro and in vivo studies. Nutr. Res. 2023, 119, 43–55. [Google Scholar] [CrossRef]
- Norkaew, C.; Subkorn, P.; Chatupheeraphat, C.; Roytrakul, S.; Tanyong, D. Pinostrobin, a fingerroot compound, regulates miR-181b-5p and induces acute leukemic cell apoptosis. Sci. Rep. 2023, 13, 8084. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-L.; Jayeoye, T.J.; Ashaolu, T.J.; Olatunji, O.J. Pinostrobin, a dietary bioflavonoid exerts antioxidant, anti-inflammatory, and anti-apoptotic protective effects against methotrexate-induced ovarian toxicity in rats. Tissue Cell 2023, 85, 102254. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, I.; Al-Abbasi, F.A.; Afzal, M.; Shahid Nadeem, M.; Altayb, H.N. Sterubin protects against chemically-induced Alzheimer’s disease by reducing biomarkers of inflammation- IL-6/ IL-β/ TNF-α and oxidative stress- SOD/MDA in rats. Saudi J. Biol. Sci. 2023, 30, 103560. [Google Scholar] [CrossRef] [PubMed]
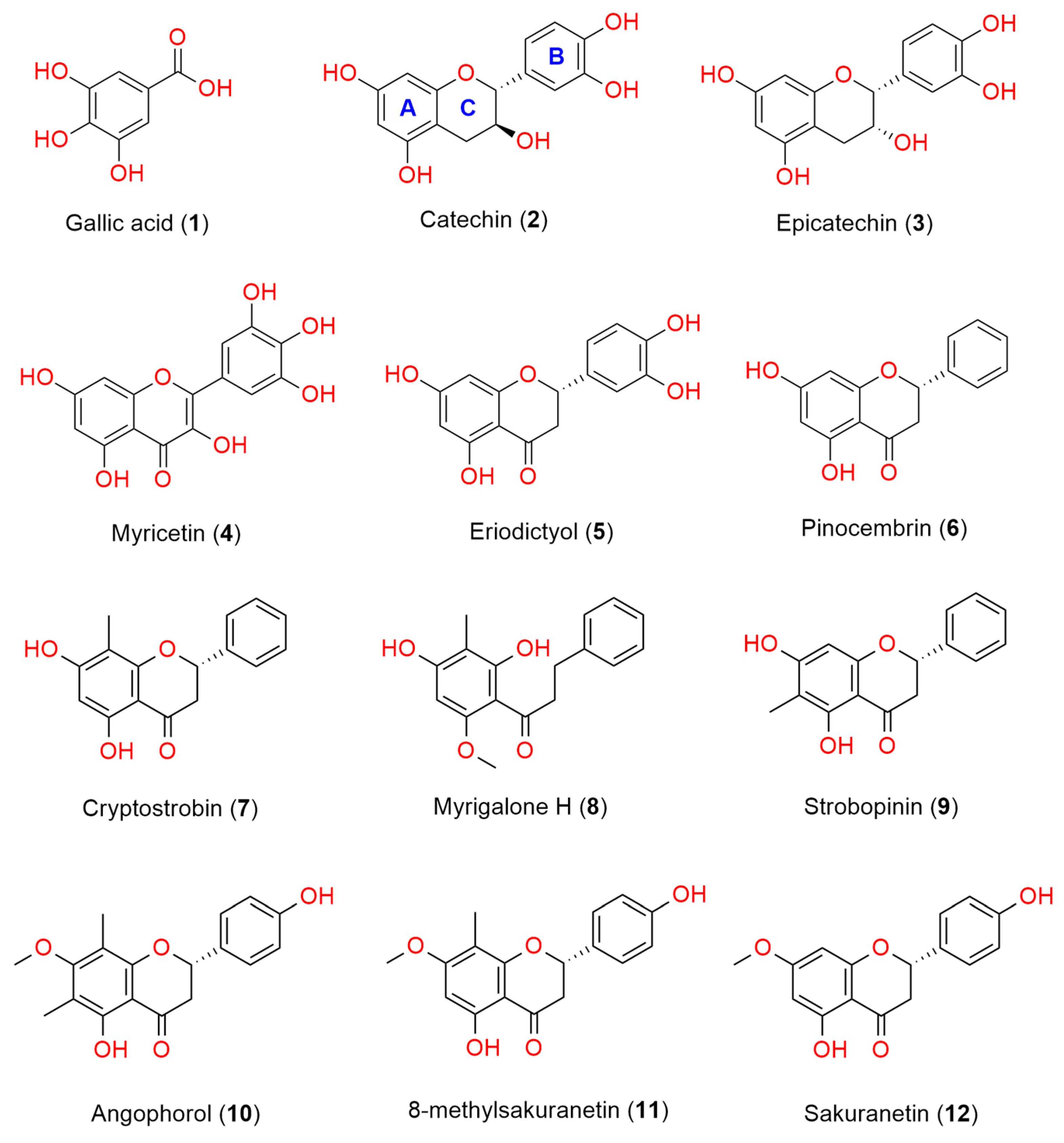
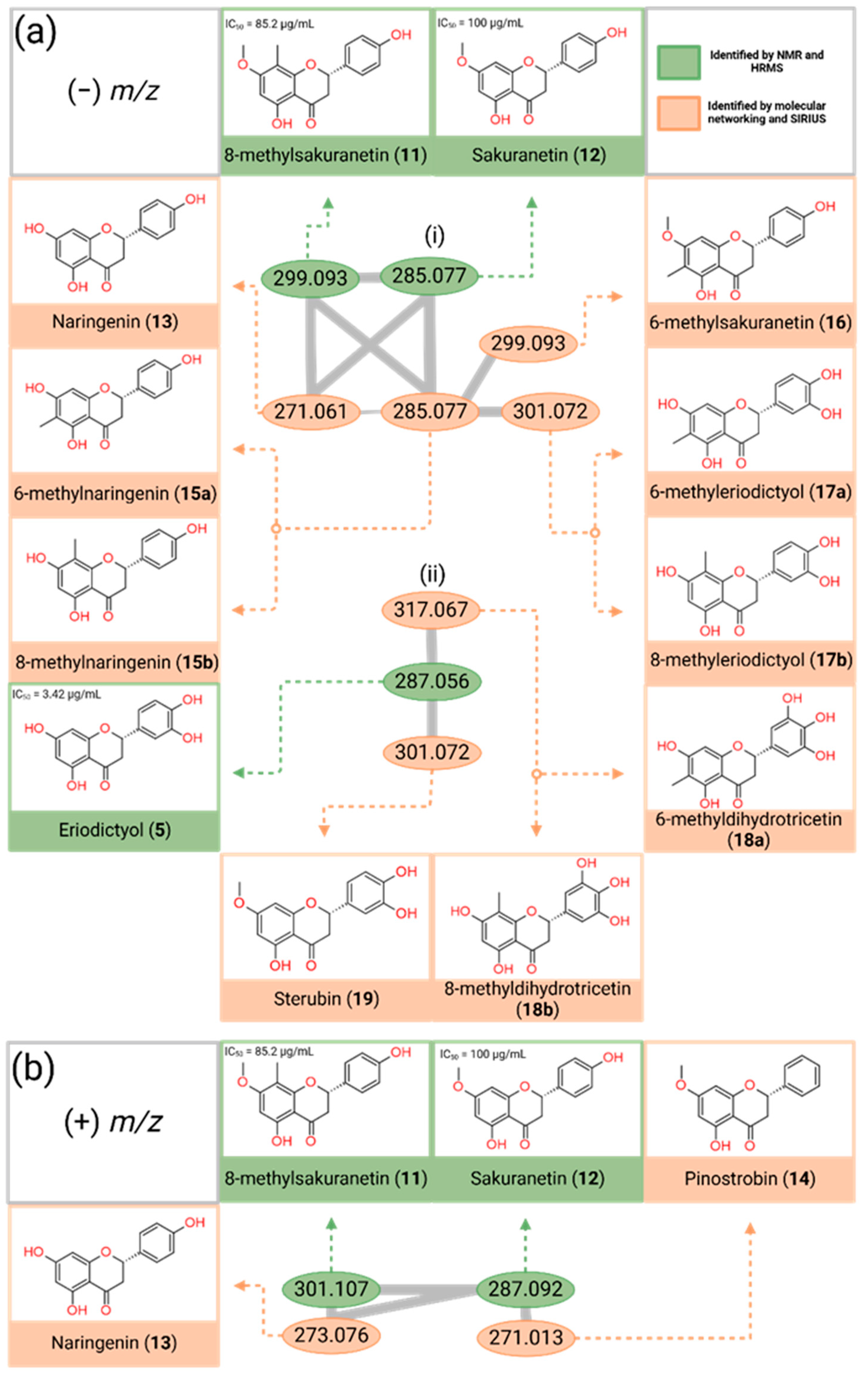
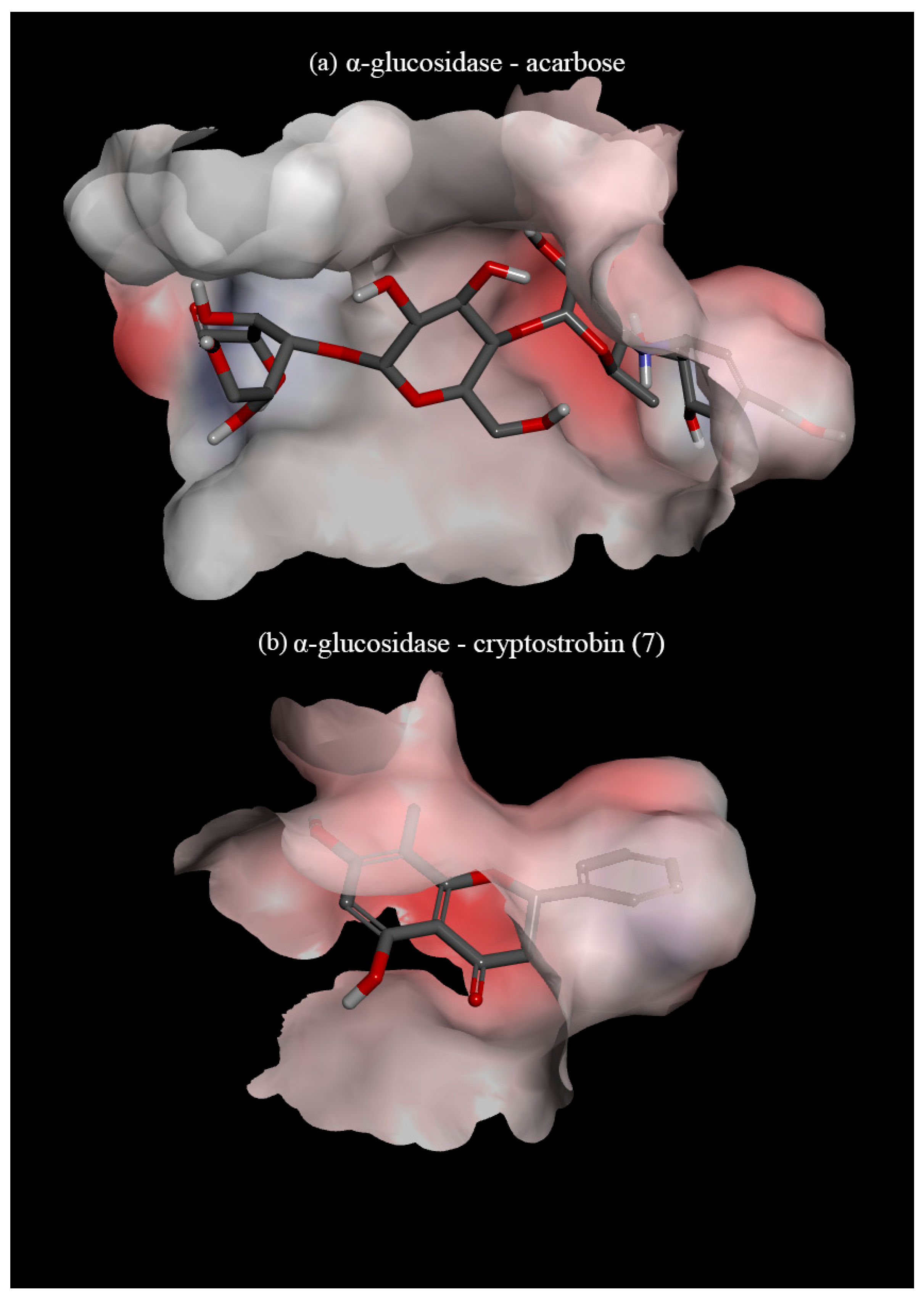
| Extract/Compounds | % Inhibition at 100 µg/mL | IC50 (µg/mL) | IC50 (µM) |
|---|---|---|---|
| Propolis extract | 80 ± 2 | 24.5 ± 0.3 | - |
| Gallic acid (1) | 100 ± 1 | 0.9 ± 0.1 | 5.3 ± 0.6 |
| Catechin (2) | 100 ± 0 | 2.5 ± 0.2 | 8.6 ± 0.7 |
| Epicatechin (3) | 99 ± 1 | 2.3 ± 0.1 | 7.9 ± 0.3 |
| Myricetin (4) | 100 ± 1 | 1.8 ± 0.1 | 5.7 ± 0.3 |
| Eriodictyol (5) | 100 ± 0 | 3.4 ± 0.2 | 11.8 ± 0.7 |
| Pinocembrin (6) | 38 ± 3 | N.D a | N.D a |
| Cryptostrobin (7) | 41 ± 5 | N.D a | N.D a |
| Myrigalone H (8) | 80 ± 2 | 20.0 ± 0.5 | 69.9 ± 1.7 |
| Strobopinin (9) | 40 ± 1 | N.D a | N.D a |
| Angophorol (10) | 63 ± 4 | 58.4 ± 0.8 | 186 ± 2.5 |
| 8-Methylsakuranetin (11) | 56 ± 4 | 85.2 ± 1.1 | 284 ± 3.7 |
| Sakuranetin (12) | 50 ± 1 | 100 ± 0.1 | 350 ± 0.3 |
| Ascorbic acid (Vitamin C) | 100 ± 0 | 5.0 ± 0.1 | 28.4 ± 0.6 |
| Compound | Known Bioactivities |
|---|---|
| Gallic Acid (1) | Antiallergic [41], Anti-angiogenic [42], Anticancer [43], Antidiabetic [44,45], Anti-inflammatory [46], Antimicrobial [43,44], Antioxidant [44], Antiviral [44], Neuroprotective [44] |
| Catechin (2) | Cardiovascular Protective [47], Anticancer [48], Antidiabetic [49], Anti-inflammatory [50], Antimicrobial [51], Antioxidant [47], Neuroprotective [47] |
| Epicatechin (3) | Anticancer [47], Antidiabetic [49,52], Antioxidant [47], Cardiovascular protective [47], Neuroprotective [52] |
| Myricetin (4) | Anticancer [53], Antidiabetic [53], Antihypertensive [53], Antimicrobial [53], Antioxidant [53], Immunomodulatory [53], Neuroprotective [53] |
| Eriodictyol (5) | Anticancer [54], Antidiabetic [54], Anti-inflammatory [54], Antioxidant [54], Cardioprotective [54], Hepatoprotective [54], Neuroprotective [54] |
| Pinocembrin (6) | Anticancer [55], Antifibrotic [55], Anti-inflammatory [55], Antimicrobial [55], Antioxidant [55], Cardiovascular protective [55], Neuroprotective [55] |
| Cryptostrobin (7) | Antibacterial [12,56], Antidiabetic [57], Antihypertensive [56] |
| Myrigalone H (8) | Antibacterial [58] |
| Strobopinin (9) | Anti-inflammatory [59], Antimicrobial [59], Antioxidant [59], Antiparasitic [59], Neuroprotective [60] |
| Angophorol (10) | Anticancer [61] |
| Sakuranetin (12) | Antiallergic [62], Anticancer [62], Anti-inflammatory [62], Antimicrobial [62], Antimutagenic [62], Antioxidant [62], Antiparasitic [62], Antiviral [62] |
| Naringenin (13) | Anticancer [63], Antidiabetic [63], Antimicrobial [63], Antidiabetic [63], Antioxidant [63], Cardiovascular protective [63], Gastroprotective [63], Immunomodulatory [63], Neuroprotective [63] |
| Pinostrobin (14) | Antibacterial [64], Anticancer [64,65], Antidiabetic [65], Anti-inflammatory [65], Antioxidant [64], Antiviral [64] |
| Sterubin (19) | Anti-inflammatory [66], Antioxidant [66], Neuroprotective [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebner, D.W.; Woods, D.C.; Tran, T.D. Bioactive Polyphenolic Compounds from Propolis of Tetragonula carbonaria in the Gibberagee Region, New South Wales, Australia. Foods 2025, 14, 965. https://doi.org/10.3390/foods14060965
Ebner DW, Woods DC, Tran TD. Bioactive Polyphenolic Compounds from Propolis of Tetragonula carbonaria in the Gibberagee Region, New South Wales, Australia. Foods. 2025; 14(6):965. https://doi.org/10.3390/foods14060965
Chicago/Turabian StyleEbner, Dylan W., Damon C. Woods, and Trong D. Tran. 2025. "Bioactive Polyphenolic Compounds from Propolis of Tetragonula carbonaria in the Gibberagee Region, New South Wales, Australia" Foods 14, no. 6: 965. https://doi.org/10.3390/foods14060965
APA StyleEbner, D. W., Woods, D. C., & Tran, T. D. (2025). Bioactive Polyphenolic Compounds from Propolis of Tetragonula carbonaria in the Gibberagee Region, New South Wales, Australia. Foods, 14(6), 965. https://doi.org/10.3390/foods14060965







