Chemical and Functional Characteristics of Strawberry Tree (Arbutus unedo L.) Honey from Western Greece
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Strawberry Tree Honey Samples
2.2. Analyses of Physicochemical Characteristics
2.3. Evaluation of Biological Properties In Vitro
2.4. Carbohydrate and Phenolic Compounds Profile Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characteristics
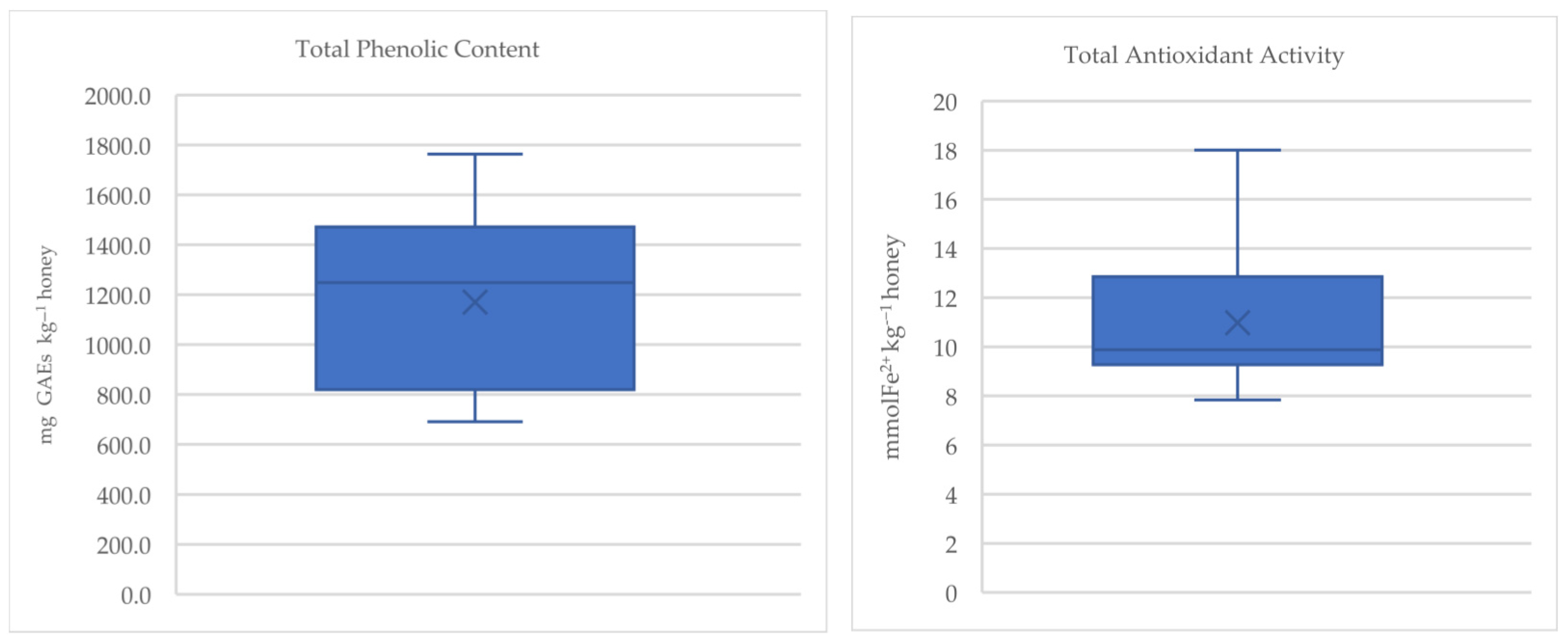
3.2. Antioxidant Properties
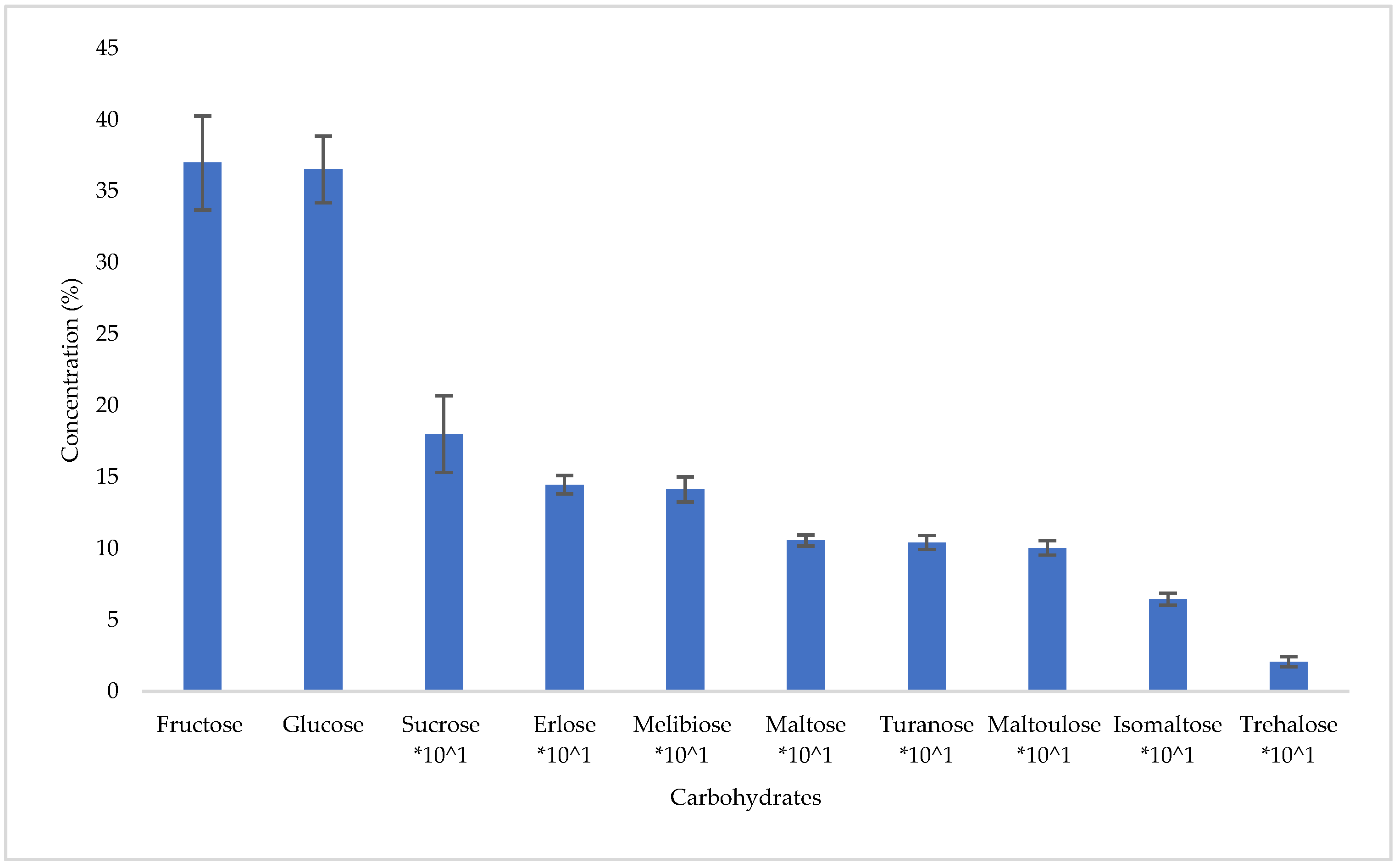
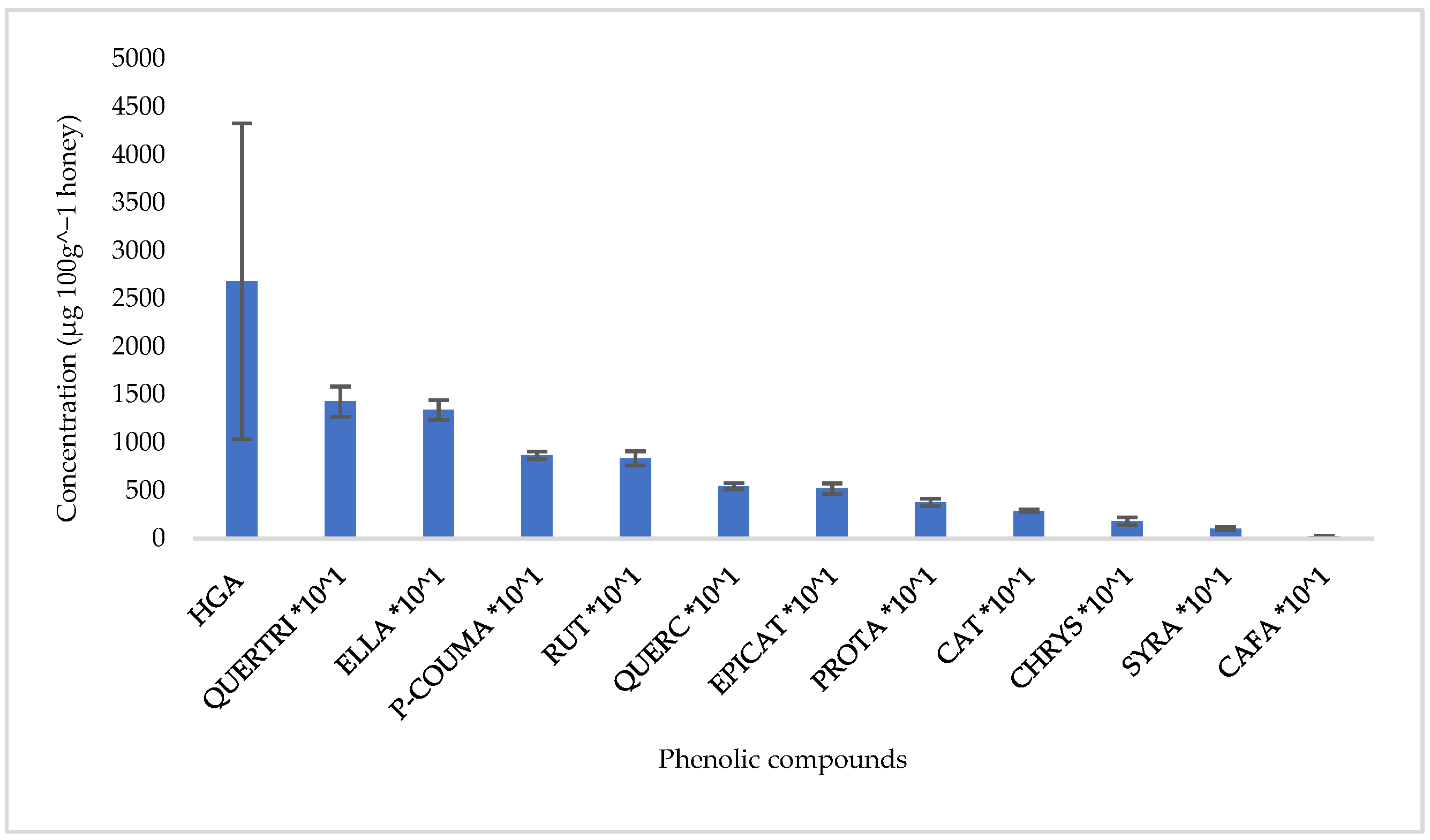
3.3. Carbohydrate and Phenolic Compounds Profile
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tuberoso, C.I.G.; Bifulco, E.; Caboni, P.; Cottiglia, F.; Cabras, P.; Floris, I. Floral Markers of strawberry tree (Arbutus unedo L.) honey. J. Agric. Food Chem. 2010, 58, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Deiana, V.; Tuberoso, C.; Satta, A.; Pinna, C.; Camarda, I.; Spano, N.; Ciulu, M.; Floris, I. Relationship between markers of botanical origin in nectar and honey of the strawberry tree (Arbutus unedo) throughout flowering periods in different years and in different geographical areas. J. Apic. Res. 2016, 54, 342–349. [Google Scholar] [CrossRef]
- Bianchi, F.; Careri, M.; Musci, M. Volatile norisoprenoids as markers of botanical origin of Sardinian strawberry-tree (Arbutus unedo L.) honey: Characterisation of aroma compounds by dynamic headspace extraction and gas chromatography–mass spectrometry. Food Chem. 2005, 89, 527–532. [Google Scholar] [CrossRef]
- Afrin, S.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Bompadre, S.; Quiles, J.L.; Sanna, G.; Spano, N.; Giampieri, F.; Battino, M. Strawberry-Tree honey induces growth inhibition of human colon cancer cells and increases ROS generation: A Comparison with Manuka Honey. Int. J. Mol. Sci. 2017, 18, 613. [Google Scholar] [CrossRef]
- Floris, I.; Pusceddu, M.; Satta, A. The Sardinian Bitter Honey: From Ancient Healing Use to Recent Findings. Antioxidants 2021, 10, 506. [Google Scholar] [CrossRef]
- Preti, R.; Tarola, A.M. Influence of geographic origin on the profile and level of phenolic compounds in Italian strawberry tree (Arbutus unedo L.) honey. J. Food Nut. Res. 2022, 61, 352–360. [Google Scholar]
- Persano Oddo, L.; Piazza, M.G.; Sabatini, A.G.; Accorti, M. Characterization of unifloral honeys. Apidologie 1995, 26, 453–465. [Google Scholar] [CrossRef]
- Rosa, A.; Tuberoso, C.I.G.; Atzeri, A.; Melis, M.P.; Bifulco, E.; Dessì, M.A. Antioxidant profile of strawberry tree honey and its marker homogentisic acid in several models of oxidative stress. Food Chem. 2011, 129, 1045–1053. [Google Scholar] [CrossRef]
- Lovaković, B.T.; Lazarus, M.; Brčić Karačonji, I.; Juricab, K.; Živković Semren, T.; Lušić, D.; Brajenović, N.; Pelaić, Z.; Pizent, A. Multi-elemental composition and antioxidant properties of strawberry tree (Arbutus unedo L.) honey from the coastal region of Croatia: Risk-benefit analysis. J. Trace Elem. Med. Biol. 2018, 45, 85–92. [Google Scholar] [CrossRef]
- Osés, S.M.; Nieto, S.; Rodrigo, S.; Pérez, S.; Rojo, S.; Sancho, M.T.; Fernández-Muiño, M.Á. Authentication of strawberry tree (Arbutus unedo L.) honeys from southern Europe based on compositional parameters and biological activities. Food Biosci. 2020, 38, 100768. [Google Scholar] [CrossRef]
- Jurič, A.; Brčić Karačonji, I.; Gašić, U.; Milojković Opsenica, D.; Prđun, S.; Bubalo, D.; Lušić, D.; Vahčić, N.; Kopjar, N. Protective Effects of Arbutus unedo L. Honey in the Alleviation of Irinotecan-Induced Cytogenetic Damage in Human Lymphocytes—An In Vitro Study. Int. J. Mol. Sci. 2023, 24, 1903. [Google Scholar] [CrossRef] [PubMed]
- Cabras, P.; Angioni, A.; Tuberoso, C.; Floris, I.; Reniero, F.; Guillou, C.; Ghelli, S. Homogentisic acid: A phenolic acid as a marker of strawberry-tree (Arbutus unedo) honey. J. Agric. Food Chem. 1999, 47, 4064–4067. [Google Scholar] [CrossRef]
- Montoro, P.; D’Urso, G.; Kowalczyk, A.; Tuberoso, C.I.G. LC-ESI/LTQ-Orbitrap-MS Based Metabolomics in evaluation of bitter taste of Arbutus unedo honey. Molecules 2021, 26, 2765. [Google Scholar] [CrossRef]
- Von Der Ohe, W.; Persano Oddo, L.; Piana, M.L.; Morlot, M.; Martin, P. Harmonized methods of Melissopalynology. Apidologie 2004, 35, 18–25. [Google Scholar] [CrossRef]
- Piana, M.L.; Persano Oddo, L.; Bentabol, A.; Bruneau, E.; Bogdanov, S.; Guyot-Declerck, C. Sensory analysis applied to honey: State of the art. Apidologie 2004, 35, 26–37. [Google Scholar] [CrossRef]
- Bogdanov, S.; Ruoff, K.; Oddo, L.P. Physico-chemical methods for the characterization of unifloral honey: A review. Apidologie 2004, 35, 275–282. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef]
- Bogdanov, S. Nature and Origin of the Antibacterial Substances in Honey. LWT-Food Sci. Technol. 1997, 30, 748–753. [Google Scholar] [CrossRef]
- Can, Z.; Yildiz, O.; Sahin, H.; Akyuz Turumtay, E.; Silici, S.; Kolayli, S. An investigation of Turkish honeys: Their physico-chemical properties, antioxidant capacities and phenolic profiles. Food Chem. 2015, 180, 133–141. [Google Scholar] [CrossRef]
- Akyuz, E.; Şahin, H.; Islamoglu, F.; Kolayli, S.; Sandra, P. Evaluation of Phenolic Compounds in Tilia rubra Subsp. Caucasica by HPLC-UV and HPLC-UV-MS/MS. Int. J. Food Prop. 2014, 17, 331–343. [Google Scholar] [CrossRef]
- European Economic Community EEC Council directive of 20 December 2001 relating to honey. OJEC 2002, 110, 47–50.
- Thrasyvoulou, A.; Tananaki, C.; Goras, G.; Karazafiris, E.; Dimou, M.; Liolios, V.; Kanelis, D.; Gounari, S. Legislation of honey criteria and standards. J. Apic. Res. 2018, 57, 88–96. [Google Scholar] [CrossRef]
- Flanjak, I.; Kenjerić, D.; Bubalo, D.; Primorac, L. Characterisation of selected Croatian honey types based on the combination of antioxidant capacity, quality parameters, and chemometrics. Eur. Food Res. Technol. 2016, 242, 467–475. [Google Scholar] [CrossRef]
- Rodopoulou, M.-A.; Tananaki, C.; Kanelis, D.; Liolios, V.; Dimou, M.; Thrasyvoulou, A. A chemometric approach for the differentiation of 15 monofloral honeys based on physicochemical parameters. J. Sci. Food Agric. 2021, 102, 139–146. [Google Scholar] [CrossRef]
- Castiglioni, S.; Stefano, M.; Astolfi, P.; Carloni, P. Chemometric approach to the analysis of antioxidant properties and colour of typical Italian monofloral honeys. Int. J. Food Sci. Technol. 2017, 52, 1138–1146. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Boban, M.; Bifulco, E.; Budimir, D.; Pirisi, F.M. Antioxidant capacity and vasodilatory properties of Mediterranean food: The case of Cannonau wine, myrtle berries liqueur and strawberry-tree honey. Food Chem. 2013, 140, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Jurič, A.; Brčić Karačonji, I.; Kopjar, N. Homogentisic acid, a main phenolic constituent of strawberry tree honey, protects human peripheral blood lymphocytes against irinotecan-induced cytogenetic damage in vitro. Chem. Biol. Int. 2021, 349, 109672. [Google Scholar] [CrossRef]
- Jurič, A.; Brčić Karačonji, I.; Žunec, S.; Katić, A.; Gašić, U.; Milojković Opsenica, D.; Kopjar, N. Protective role of strawberry tree (Arbutus unedo L.) honey against cyto/genotoxic effects induced by ultraviolet B radiation in vitro. J. Apic. Res. 2022, 63, 513–522. [Google Scholar] [CrossRef]
- Escriche, I.; Kadar, M.; Juan-Borrás, M.; Domenech, E. Suitability of antioxidant capacity, flavonoids and phenolic acids for floral authentication of honey. Impact of industrial thermal treatment. Food Chem. 2014, 142, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chen, F.; Zhou, B. Antioxidative, anti-inflammatory and anti-apoptotic effects of ellagic acid in liver and brain of rats treated by D-galactose. Sci. Rep. 2018, 8, 1465. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, G.; Sun, C.; Peng, F.; Yu, L.; Chen, Y.; Tan, Y.; Cao, X.; Tang, Y.; Xie, X.; et al. Chemistry, pharmacokinetics, pharmacological activities, and toxicity of Quercitrin. Phytother. Res. 2022, 36, 1545–1575. [Google Scholar] [CrossRef] [PubMed]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef]
- Negahdari, R.; Bohlouli, S.; Sharifi, S.; Maleki Dizaj, S.; Rahbar Saadat, Y.; Khezri, K.; Jafari, S.; Ahmadian, E.; Gorbani Jahandizi, N.; Raeesi, S. Therapeutic benefits of rutin and its nanoformulations. Phytother. Res. 2021, 35, 1719–1738. [Google Scholar] [CrossRef]
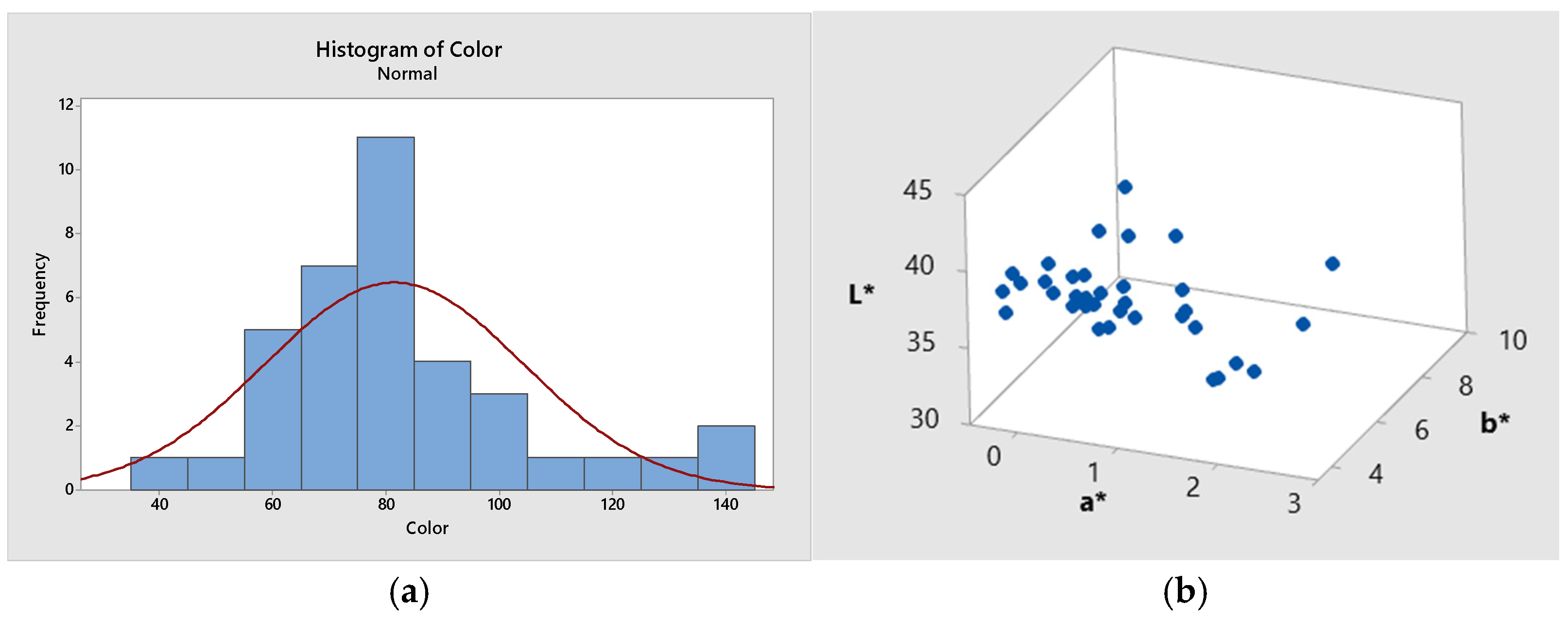
| Physicochemical Parameters | Moisture (%) | Electrical Conductivity (mS cm−1) | HMF (mg kg−1) | Diastase Activity (DN) | pH | Acidity (meq kg−1) |
|---|---|---|---|---|---|---|
| Mean value ± Standard deviation | 19.2 ± 1.9 | 0.784 ± 0.132 | 3.1 ± 3.3 | 9.6 ± 3.8 | 4.3 ± 0.16 | 34.1 ± 7.6 |
| Min-Max | 16.4–24.0 | 0.454–1.120 | 0.0–12.0 | 3.2–18.0 | 3.72–4.41 | 17.5–49.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tananaki, C.; Kanelis, D.; Liolios, V.; Rodopoulou, M.A.; Papadopoulou, F. Chemical and Functional Characteristics of Strawberry Tree (Arbutus unedo L.) Honey from Western Greece. Foods 2025, 14, 1473. https://doi.org/10.3390/foods14091473
Tananaki C, Kanelis D, Liolios V, Rodopoulou MA, Papadopoulou F. Chemical and Functional Characteristics of Strawberry Tree (Arbutus unedo L.) Honey from Western Greece. Foods. 2025; 14(9):1473. https://doi.org/10.3390/foods14091473
Chicago/Turabian StyleTananaki, Chrysoula, Dimitrios Kanelis, Vasilios Liolios, Maria Anna Rodopoulou, and Fotini Papadopoulou. 2025. "Chemical and Functional Characteristics of Strawberry Tree (Arbutus unedo L.) Honey from Western Greece" Foods 14, no. 9: 1473. https://doi.org/10.3390/foods14091473
APA StyleTananaki, C., Kanelis, D., Liolios, V., Rodopoulou, M. A., & Papadopoulou, F. (2025). Chemical and Functional Characteristics of Strawberry Tree (Arbutus unedo L.) Honey from Western Greece. Foods, 14(9), 1473. https://doi.org/10.3390/foods14091473






