Advancements in Inactivation of Soybean Trypsin Inhibitors
Abstract
1. Introduction
2. Soybean Proteins and Their Digestion
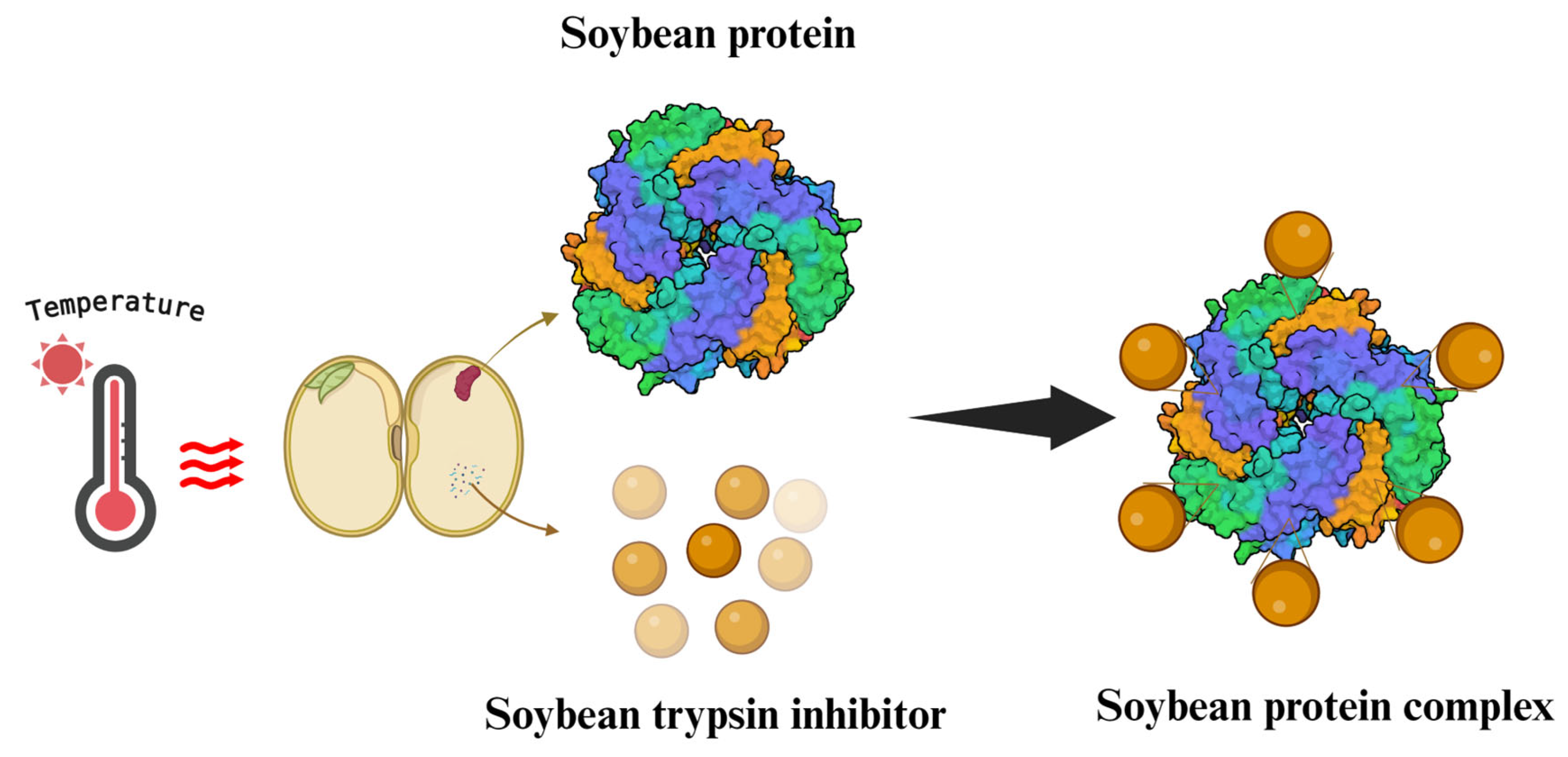
2.1. Soybean Proteins
2.2. The Digestion of Soybean Proteins
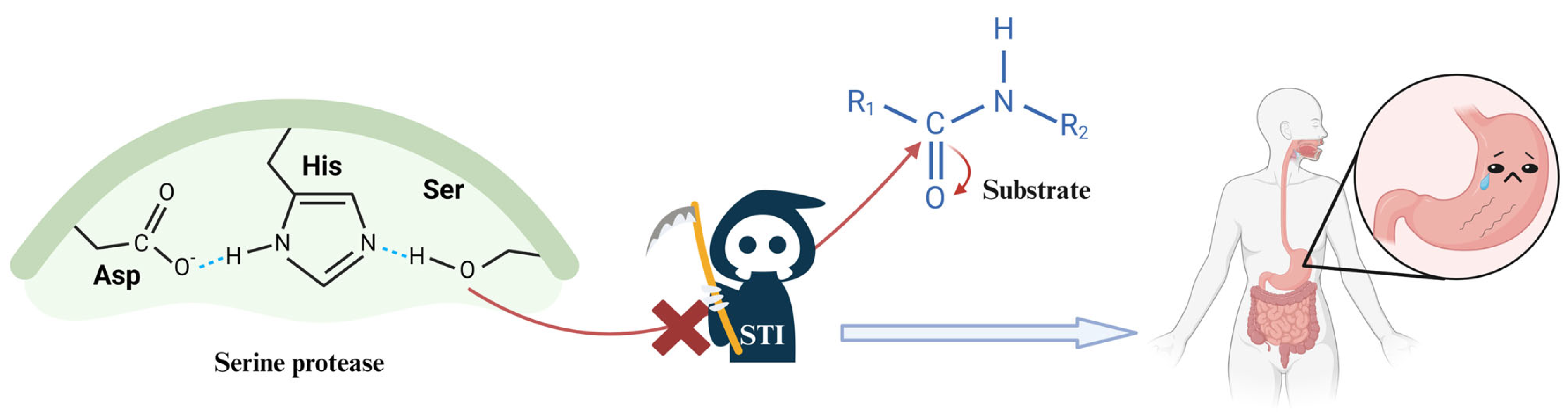
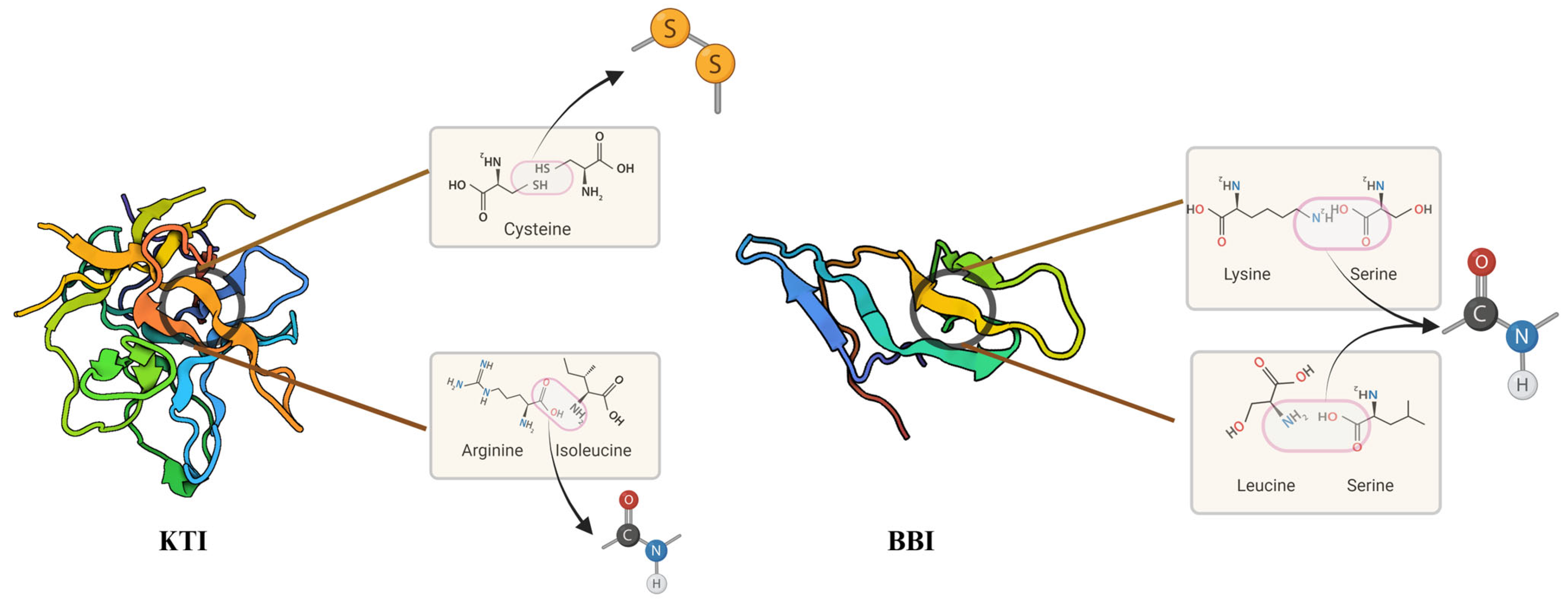
2.3. The Changes and Effects of STIs in Gastrointestinal Tract
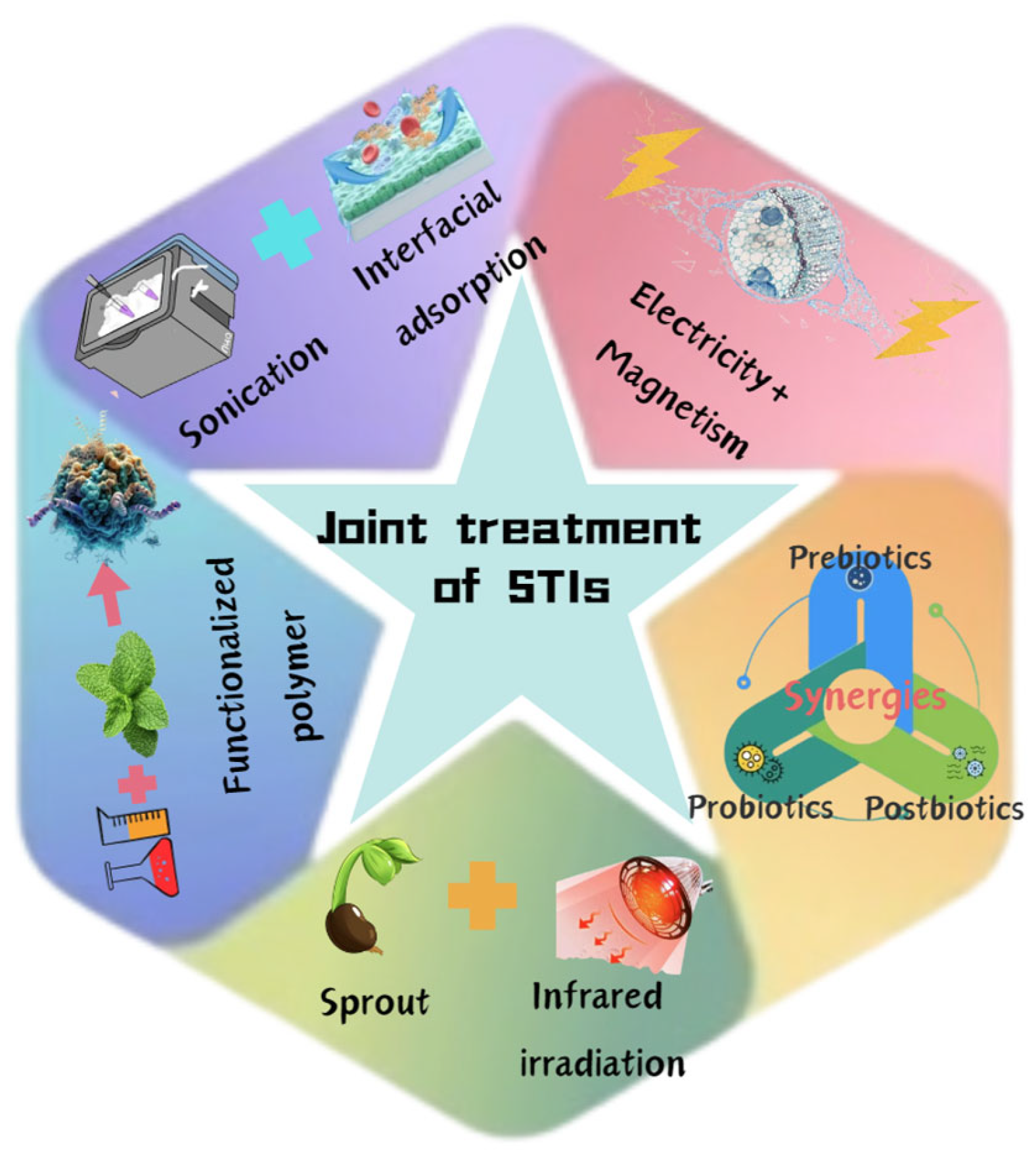
3. Inactivation Technologies for STIs
3.1. Conventional Treatments
3.1.1. Thermal Treatments
3.1.2. Microwave Processing
3.1.3. Polyphenols
3.2. Maillard Reaction
3.3. Novel Treatments
3.3.1. Radio Frequency (RF)
3.3.2. Electric Fields
3.3.3. Infrared Heating (IRH)
3.3.4. Pressure Treatments
3.4. Combinating Treatments
3.4.1. Temperature–Pressure Treatment
3.4.2. Extrusion Processing
3.5. Other Treatments
3.5.1. Ultrasonication
3.5.2. Irradiation
3.5.3. Dielectric-Barrier Discharge (DBD) Plasma
3.6. Chemical Treatments
3.6.1. Acid, Alkali, Salt
3.6.2. Reducing Agents
3.7. Biological Treatments
4. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Padalkar, G.; Mandlik, R.; Sudhakaran, S.; Vats, S.; Kumawat, S.; Kumar, V.; Kumar, V.; Rani, A.; Ratnaparkhe, M.B.; Jadhav, P.; et al. Necessity and challenges for exploration of nutritional potential of staple-food grade soybean. J. Food Compos. Anal. 2023, 117, 105093. [Google Scholar] [CrossRef]
- Anggraeni, A.A.; Triwitono, P.; Lestari, L.A.; Harmayani, E. Evaluation of glucomannan as a fat replacer in the dough and cookies made from fermented cassava flour and soy protein concentrate. Food Chem. 2024, 434, 137452. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sala, G.; Scholten, E. Impact of soy protein dispersibility on the structural and sensory properties of fat-free ice cream. Food Hydrocoll. 2024, 147, 109340. [Google Scholar] [CrossRef]
- Zeng, X.; Li, Y.; Li, P.; Zhao, J.; Li, X.; Wang, X.; Liu, B.; Ni, L.; Li, H.; Xi, Y. Encapsulation of roast beef flavor by soy protein isolate/chitosan complex pickering emulsions to improve its releasing properties during the processing of plant-based meat analogues. Food Chem. 2024, 450, 139313. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kumar, R.; Sabapathy, S.; Bawa, A. Functional and edible uses of soy protein products. Compr. Rev. Food Sci. Food Saf. 2008, 7, 14–28. [Google Scholar] [CrossRef]
- Gong, H.; Fu, H.; Zhang, J.; Zhang, Q.; Wang, Y.; Wang, D.; Cai, L.; Chen, J.; Yu, H.; Lyu, B. Preparation of soybean protein-based nanoparticles and its application as encapsulation carriers of bioactive substances. LWT 2024, 191, 115680. [Google Scholar] [CrossRef]
- Chen, J.; Mu, T.; Goffin, D.; Blecker, C.; Richard, G.; Richel, A.; Haubruge, E. Application of soy protein isolate and hydrocolloids based mixtures as promising food material in 3d food printing. J. Food Eng. 2019, 261, 76–86. [Google Scholar] [CrossRef]
- Zhang, J.; Long, Y.; Zhang, Y.; Lu, L.; Liu, C.; Xu, W.; Hu, Z.; Hu, C. Study on the effect of heat treatment process on the quality characteristics of soybean-citri reticulatae pericarpium (crp) based yogurt and related mechanisms. Food Hydrocoll. 2025, 164, 111179. [Google Scholar] [CrossRef]
- Fu, J.; Zhan, Z.; Duan, Q.; Yang, Y.; Xie, H.; Dong, X.; Zhang, H.; Yu, L. Application of soybean protein isolates-polysaccharides hybrid emulsion gels as alternative fats in fabricating plant-based meats with two-phase. LWT 2025, 218, 117524. [Google Scholar] [CrossRef]
- Tkaczewska, J.; Jamróz, E.; Zając, M.; Guzik, P.; Gedif, H.D.; Turek, K.; Kopeć, M. Antioxidant edible double-layered film based on waste from soybean production as a vegan active packaging for perishable food products. Food Chem. 2023, 400, 134009. [Google Scholar] [CrossRef]
- Liu, K. Soybeans: Chemistry, Technology, and Utilization; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Hu, M.; Gao, Y.; Wen, W.; Zhang, P.; Zhang, F.; Fan, B.; Wang, F.; Li, S. The aggregation behavior between soybean whey protein and polysaccharides of diverse structures and their implications in soybean isoflavone delivery. Food Chem. 2024, 439, 138061. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.; Makkar, H.P.; Becker, K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 2001, 199, 197–227. [Google Scholar] [CrossRef]
- Li, S.; Sauer, W.C.; Huang, S.; Hardin, R.T. Response of pancreatic secretions to feeding diets with low and high levels of soybean trypsin inhibitors in growing pigs. J. Sci. Food Agric. 1998, 76, 347–356. [Google Scholar] [CrossRef]
- Häußler, D.; Scheidt, T.; Stirnberg, M.; Steinmetzer, T.; Gütschow, M. A bisbenzamidine phosphonate as a janus-faced inhibitor for trypsin-like serine proteases. ChemMedChem 2015, 10, 1641–1646. [Google Scholar] [CrossRef]
- Li, C.; Li, W.; Zhang, Y.; Simpson, B.K. Comparison of physicochemical properties of recombinant buckwheat trypsin inhibitor (rbti) and soybean trypsin inhibitor (sbti). Protein Expr. Purif. 2020, 171, 105614. [Google Scholar] [CrossRef]
- Vagadia, B.H.; Vanga, S.K.; Raghavan, V. Inactivation methods of soybean trypsin inhibitor—A review. Trends Food Sci. Technol. 2017, 64, 115–125. [Google Scholar] [CrossRef]
- Song, H.; Suh, S. Kunitz-type soybean trypsin inhibitor revisited: Refined structure of its complex with porcine trypsin reveals an insight into the interaction between a homologous inhibitor from Erythrina caffra and tissue-type plasminogen activator. J. Mol. Biol. 1998, 275, 347–363. [Google Scholar] [CrossRef]
- Koide, T.; Ikenaka, T. Studies on Soybean Trypsin Inhibitors. 3. Amino-Acid Sequence of the Carboxyl-Terminal Region and the Complete Amino-Acid Sequence of Soybean Trypsin Inhibitor (Kunitz). Eur. J. Biochem. 2010, 32, 417–431. [Google Scholar] [CrossRef]
- Losso, J.N. The biochemical and functional food properties of the bowman-birk inhibitor. Crit. Rev. Food Sci. Nutr. 2008, 48, 94–118. [Google Scholar] [CrossRef]
- Birk, Y. The Bowman-Birk inhibitor. Trypsin-and chymotrypsin-inhibitor from soybeans. Int. J. Pept. Protein Res. 1985, 25, 113–131. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Zhang, C.; Kong, X.; Hua, Y. Heat-induced inactivation mechanisms of Kunitz trypsin inhibitor and Bowman-Birk inhibitor in soymilk processing. Food Chem. 2014, 154, 108–116. [Google Scholar] [CrossRef]
- He, H.; Li, X.; Kong, X.; Hua, Y.; Chen, Y. Heat-induced inactivation mechanism of soybean Bowman-Birk inhibitors. Food Chem. 2017, 232, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Luo, L.; Wu, Y.; Yang, X.; Dong, J.; Luo, F.; Zou, Y.; Shen, Y.; Lin, Q. Inactivation of soybean Bowman–Birk inhibitor by stevioside: Interaction studies and application to soymilk. J. Agric. Food Chem. 2019, 67, 2255–2264. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Veloza, A.; Wang, Z.; Zhong, Q.; D’Souza, D.; Krishnan, H.B.; Dia, V.P. Lunasin protease inhibitor concentrate decreases pro-inflammatory cytokines and improves histopathological markers in dextran sodium sulfate-induced ulcerative colitis. Food Sci. Hum. Wellness 2022, 11, 1508–1514. [Google Scholar] [CrossRef]
- Li, Q.; Huang, L.; Luo, Z.; Tamer, T.M. Stability of trypsin inhibitor isolated from potato fruit juice against ph and heating treatment and in vitro gastrointestinal digestion. Food Chem. 2020, 328, 127152. [Google Scholar] [CrossRef]
- Cruz-Huerta, E.; Fernández-Tomé, S.; Arques, M.C.; Amigo, L.; Recio, I.; Clemente, A.; Hernández-Ledesma, B. The protective role of the bowman-birk protease inhibitor in soybean lunasin digestion: The effect of released peptides on colon cancer growth. Food Funct. 2015, 6, 2626–2635. [Google Scholar] [CrossRef]
- Fang, E.F.; Wong, J.H.; Ng, T.B. Thermostable kunitz trypsin inhibitor with cytokine inducing, antitumor and hiv-1 reverse transcriptase inhibitory activities from korean large black soybeans. J. Biosci. Bioeng. 2010, 109, 211–217. [Google Scholar] [CrossRef]
- de Siqueira Patriota, L.L.; Ramos, D.d.B.M.; e Silva, M.G.; dos Santos, A.C.L.A.; Silva, Y.A.; de Oliveira Marinho, A.; Coelho, L.C.B.B.; Paiva, P.M.G.; Pontual, E.V.; Mendes, R.L. The trypsin inhibitor from moringa oleifera flowers (mofti) inhibits acute inflammation in mice by reducing cytokine and nitric oxide levels. S. Afr. J. Bot. 2021, 143, 474–481. [Google Scholar] [CrossRef]
- Kennedy, A.R. The bowman-birk inhibitor from soybeans as an anticarcinogenic agent. Am. J. Clin. Nutr. 1998, 68, 1406S–1412S. [Google Scholar] [CrossRef]
- Kuenz, S.; Thurner, S.; Hoffmann, D.; Kraft, K.; Wiltafsky-Martin, M.; Damme, K.; Windisch, W.; Brugger, D. Effects of gradual differences in trypsin inhibitor activity on the estimation of digestible amino acids in soybean expellers for broiler chickens. Poult. Sci. 2022, 101, 101740. [Google Scholar] [CrossRef]
- Aderibigbe, A.; Cowieson, A.; Ajuwon, K.; Adeola, O. Contribution of purified soybean trypsin inhibitor and exogenous protease to endogenous amino acid losses and mineral digestibility. Poult. Sci. 2021, 100, 101486. [Google Scholar] [CrossRef] [PubMed]
- Embaby, H.E.-S. Effect of heat treatments on certain antinutrients and in vitro protein digestibility of peanut and sesame seeds. Food Sci. Technol. Res. 2010, 17, 31–38. [Google Scholar] [CrossRef]
- Cabrera-Orozco, A.; Jiménez-Martínez, C.; Dávila-Ortiz, G. Soybean: Non-nutritional factors and their biological functionality. Soybean-Bio-Act. Compd. 2013, 2013, 387–410. [Google Scholar]
- Fasina, Y.; Garlich, J.; Classen, H.; Ferket, P.; Havenstein, G.; Grimes, J.; Qureshi, M.; Christensent, V. Response of turkey poults to soybean lectin levels typically encountered in commercial diets. 1. Effect on growth and nutrient digestibility. Poult. Sci. 2004, 83, 1559–1571. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, S.K. Trypsin inhibitor activity, phenolic content and antioxidant capacity of soymilk as affected by grinding temperatures, heating methods and soybean varieties. LWT 2022, 153, 112424. [Google Scholar] [CrossRef]
- Andrade, J.; Mandarino, J.; Kurozawa, L.; Ida, E. The effect of thermal treatment of whole soybean flour on the conversion of isoflavones and inactivation of trypsin inhibitors. Food Chem. 2016, 194, 1095–1101. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, Z.; Zhao, Y. Impact of radio frequency, microwaving, and high hydrostatic pressure at elevated temperature on the nutritional and antinutritional components in black soybeans. J. Food Sci. 2015, 80, C2732–C2739. [Google Scholar] [CrossRef]
- Agrahar-Murugkar, D.; Jha, K. Effect of drying on nutritional and functional quality and electrophoretic pattern of soyflour from sprouted soybean (Glycine max). J. Food Sci. Technol. 2010, 47, 482–487. [Google Scholar] [CrossRef]
- Cheng, S.; Langrish, T.A. Fluidized bed drying of chickpeas: Developing a new drying schedule to reduce protein denaturation and remove trypsin inhibitors. J. Food Eng. 2023, 351, 111515. [Google Scholar] [CrossRef]
- Wang, L.; Li, D.; Tatsumi, E.; Liu, Z.; Chen, X.; Li, L. Application of two-stage ohmic heating to tofu processing. Chem. Eng. Process. Process. Intensif. 2007, 46, 486–490. [Google Scholar] [CrossRef]
- Rajan, A.; Velusamy, M.; Baskaran, K.; Rangarajan, J.; Natarajan, V.; Radhakrishnan, M. High pressure processing of whole soymilk: Effect on allergenicity, anti-nutritional factor, lipoxygenase activity and E-nose-aroma characteristics. Food Chem. Adv. 2023, 3, 100427. [Google Scholar] [CrossRef]
- Vanga, S.K.; Singh, A.; Raghavan, V. Changes in soybean trypsin inhibitor by varying pressure and temperature of processing: A molecular modeling study. Innov. Food Sci. Emerg. Technol. 2018, 49, 31–40. [Google Scholar] [CrossRef]
- Haddad, J.; Allaf, K. A study of the impact of instantaneous controlled pressure drop on the trypsin inhibitors of soybean. J. Food Eng. 2007, 79, 353–357. [Google Scholar] [CrossRef]
- Tewari, K.; Kumari, S.; Vinutha, T.; Singh, B.; Dahuja, A. Gamma irradiation induces reduction in the off-flavour generation in soybean through enhancement of its antioxidant potential. J. Radioanal. Nucl. Chem. 2015, 303, 2041–2051. [Google Scholar] [CrossRef]
- Rathod, R.P.; Annapure, U.S. Effect of extrusion process on antinutritional factors and protein and starch digestibility of lentil splits. LWT—Food Sci. Technol. 2016, 66, 114–123. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, Y.; Huang, K.; Li, J.; Zhong, C.; He, X. Inactivation of soybean trypsin inhibitor by dielectric-barrier discharge plasma and its safety evaluation and application. Foods 2022, 11, 4017. [Google Scholar] [CrossRef]
- Dabade, A.; Kahar, S.; Acharjee, A.; Bhushette, P.; Annapure, U. Effect of atmospheric pressure non-thermal pin to plate cold plasma on structural and functional properties of soy protein isolate. J. Agric. Food Res. 2023, 12, 100538. [Google Scholar] [CrossRef]
- Che Man, Y.; Wei, L.; Nelson, A.; Yamashita, N. Effects of soaking soybeans in dilute acids on biologically active components. J. Am. Oil Chem. Soc. 1991, 68, 471–473. [Google Scholar] [CrossRef]
- Wedemeyer, W.J.; Welker, E.; Narayan, M.; Scheraga, H.A. Disulfide bonds and protein folding. Biochemistry 2000, 39, 4207–4216. [Google Scholar] [CrossRef]
- Baker, E.; Mustakas, G. Heat inactivation of trypsin inhibitor, lipoxygenase and urease in soybeans: Effect of acid and base additives. J. Am. Oil Chem. Soc. 1973, 50, 137–141. [Google Scholar] [CrossRef]
- Avilés-Gaxiola, S.; Chuck-Hernández, C.; del Refugio Rocha-Pizaña, M.; García-Lara, S.; López-Castillo, L.M.; Serna-Saldívar, S.O. Effect of thermal processing and reducing agents on trypsin inhibitor activity and functional properties of soybean and chickpea protein concentrates. LWT 2018, 98, 629–634. [Google Scholar] [CrossRef]
- Rehder, A.; Sørensen, J.C.; Markedal, K.E.; Sørensen, H.; Sørensen, S.; Petersen, I.L. Targeted inactivation of soybean proteinase inhibitors using zinc. Food Chem. 2021, 349, 129049. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Li, Y.; Liu, X. Purification of soybean Kunitz trypsin inhibitor and the mechanism of its passivation by lysine and disulfide bond modifications. Food Biosci. 2023, 55, 103042. [Google Scholar] [CrossRef]
- Ge, G.; Guo, W.; Zheng, J.; Zhao, M.; Sun, W. Effect of interaction between tea polyphenols with soymilk protein on inactivation of soybean trypsin inhibitor. Food Hydrocoll. 2021, 111, 106177. [Google Scholar] [CrossRef]
- Rackis, J.J. Biological and physiological factors in soybeans. J. Am. Oil Chem. Soc. 1974, 51 Pt 2, 161A–174A. [Google Scholar] [CrossRef]
- Johnson, R.A.; Jakobs, K.H.; Schultz, G. Extraction of adenylate cyclase-activating factor of bovine sperm and its identification as trypsin-like protease. J. Biol. Chem. 1985, 260, 114–121. [Google Scholar] [CrossRef]
- Sharma, S.; Sahni, P. Dynamics of germination behaviour, protein secondary structure, technofunctional properties, antinutrients, antioxidant capacity and mineral elements in germinated dhaincha. Food Technol. Biotechnol. 2021, 59, 238–250. [Google Scholar] [CrossRef]
- Adeyemo, S.M.; Onilude, A.A. Enzymatic reduction of anti-nutritional factors in fermenting soybeans by Lactobacillus plantarum isolates from fermenting cereals. Niger. Food J. 2013, 31, 84–90. [Google Scholar] [CrossRef]
- Salim, R.; Nehvi, I.B.; Mir, R.A.; Tyagi, A.; Ali, S.; Bhat, O.M. A review on anti-nutritional factors: Unraveling the natural gateways to human health. Front. Nutr. 2023, 10, 1215873. [Google Scholar] [CrossRef]
- Herreman, L.; Nommensen, P.; Pennings, B.; Laus, M.C. Comprehensive overview of the quality of plant-And animal-sourced proteins based on the digestible indispensable amino acid score. Food Sci. Nutr. 2020, 8, 5379–5391. [Google Scholar] [CrossRef]
- Orborne, T.B. The Vegetable Proteins. Nature 1924, 114, 822. [Google Scholar]
- Tandang-Silvas, M.R.G.; Tecson-Mendoza, E.M.; Mikami, B.; Utsumi, S.; Maruyama, N. Molecular design of seed storage proteins for enhanced food physicochemical properties. Annu. Rev. Food Sci. Technol. 2011, 2, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, N.; Mikami, B.; Utsumi, S. The development of transgenic crops to improve human health by advanced utilization of seed storage proteins. Biosci. Biotechnol. Biochem. 2011, 75, 823–828. [Google Scholar] [CrossRef]
- Mori, T.; Maruyama, N.; Nishizawa, K.; Higasa, T.; Yagasaki, K.; Ishimoto, M.; Utsumi, S. The composition of newly synthesized proteins in the endoplasmic reticulum determines the transport pathways of soybean seed storage proteins. Plant J. 2004, 40, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Zhang, T.; Jiang, L. Soy protein: Molecular structure revisited and recent advances in processing technologies. Annu. Rev. Food Sci. Technol. 2021, 12, 119–147. [Google Scholar] [CrossRef]
- Taliercio, E.; Loveless, T.; Turano, M.J. Identification of epitopes of the A1aBx and A5A4B3 subunits of glycinin antigenic in three animal species. Food Agric. Immunol. 2015, 26, 271–281. [Google Scholar] [CrossRef]
- Adachi, M.; Takenaka, Y.; Gidamis, A.B.; Mikami, B.; Utsumi, S. Crystal structure of soybean proglycinin A1aB1b homotrimer. J. Mol. Biol. 2001, 305, 291–305. [Google Scholar] [CrossRef]
- Hughes, G.J.; Ryan, D.J.; Mukherjea, R.; Schasteen, C.S. Protein digestibility-corrected amino acid scores (PDCAAS) for soy protein isolates and concentrate: Criteria for evaluation. J. Agric. Food Chem. 2011, 59, 12707–12712. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Greff, B.; Varga, L. The structure–function relationships and techno-functions of β-conglycinin. Food Chem. 2024, 462, 140950. [Google Scholar] [CrossRef]
- Zahir, M.; Fogliano, V.; Capuano, E. Food matrix and processing modulate in vitro protein digestibility in soybeans. Food Funct. 2018, 9, 6326–6336. [Google Scholar] [CrossRef]
- Han, K.; Feng, G.; Li, T.; Deng, Z.; Zhang, Z.; Wang, J.; Yang, X. Digestion Resistance of Soybean 7S Protein and Its Implications for Reinforcing the Gastric Mucus Barrier. J. Agric. Food Chem. 2022, 70, 8776–8787. [Google Scholar] [CrossRef] [PubMed]
- Rio, A.R.D.; Boom, R.M.; Janssen, A.E.M. Effect of Fractionation and Processing Conditions on the Digestibility of Plant Proteins as Food Ingredients. Foods 2022, 11, 870. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Jiao, M.; Zhao, M.; Sun, W. In vitro gastrointestinal digest of catechin-modified β-conglycinin oxidized by lipoxygenase-catalyzed linoleic acid peroxidation. Food Chem. 2019, 280, 154–163. [Google Scholar] [CrossRef]
- De Muelenaere, H. Studies on the digestion of soybeans. J. Nutr. 1964, 82, 197–205. [Google Scholar] [CrossRef]
- Krogdahl, A.; Holm, H. Soybean proteinase inhibitors and human proteolytic enzymes: Selective inactivation of inhibitors by treatment with human gastric juice. J. Nutr. 1981, 111, 2045–2051. [Google Scholar] [CrossRef]
- Laskowski, M., Jr.; Kato, I. Protein inhibitors of proteinases. Annu. Rev. Biochem. 1980, 49, 593–626. [Google Scholar] [CrossRef]
- Huber, R.; Kukla, D.; Bode, W.; Schwager, P.; Bartels, K.; Deisenhofer, J.; Steigemann, W. Structure of the complex formed by bovine trypsin and bovine pancreatic trypsin inhibitor: II. Crystallographic refinement at 1.9 Å resolution. J. Mol. Biol. 1974, 89, 73–101. [Google Scholar] [CrossRef]
- Polticelli, F.; Ascenzi, P.; Bolognesi, M.; Honig, B. Structural determinants of trypsin affinity and specificity for cationic inhibitors. Protein Sci. 1999, 8, 2621–2629. [Google Scholar] [CrossRef]
- Janin, J.; Chothia, C. Stability and specificity of protein-protein interactions: The case of the trypsin-trypsin inhibitor complexes. J. Mol. Biol. 1976, 100, 197–211. [Google Scholar] [CrossRef]
- Vincent, J.P.; Lazdunski, M. Trypsin-pancreatic trypsin inhibitor association. Dynamics of the interaction and role of disulfide bridges. Biochemistry 1972, 11, 2967–2977. [Google Scholar] [CrossRef]
- Fersht, A.R. Catalysis, binding and enzyme-substrate complementarity. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1974, 187, 397–407. [Google Scholar]
- Tsunogae, Y.; Tanaka, I.; Yamane, T.; Kikkawa, J.; Ashida, T.; Ishikawa, C.; Watanabe, K.; Nakamura, S.; Takahashi, K. Structure of the trypsin-binding domain of Bowman-Birk type protease inhibitor and its interaction with trypsin. J. Biochem. 1986, 100, 1637. [Google Scholar] [CrossRef] [PubMed]
- Tang, C. Effect of Temperature on Molecular Conformation and Immunoreactivity of Soybean Lectin. Ph.D. Thesis, The Florida State University, Tallahassee, FL, USA, 2021. [Google Scholar]
- Machado, F.; Queiróz, J.; Oliveira, M.; Piovesan, N.; Peluzio, M.; Costa, N.; Moreira, M. Effects of heating on protein quality of soybean flour devoid of kunitz inhibitor and lectin. Food Chem. 2008, 107, 649–655. [Google Scholar] [CrossRef]
- Finley, J.W.; Wheeler, E.L.; Witt, S.C. Oxidation of glutathione by hydrogen peroxide and other oxidizing agents. J. Agric. Food Chem. 1981, 29, 404–407. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, Y.; Zhang, C.; Kong, X.; Hua, Y. The heat-induced protein aggregate correlated with trypsin inhibitor inactivation in soymilk processing. J. Agric. Food Chem. 2012, 60, 8012–8019. [Google Scholar] [CrossRef]
- Volkin, D.B.; Klibanov, A.M. Thermal destruction processes in proteins involving cystine residues. J. Biol. Chem. 1987, 262, 2945–2950. [Google Scholar] [CrossRef]
- Guzik, P.; Kulawik, P.; Zając, M.; Migdał, W. Microwave applications in the food industry: An overview of recent developments. Crit. Rev. Food Sci. Nutr. 2022, 62, 7989–8008. [Google Scholar] [CrossRef]
- Kala, B.; Mohan, V. Effect of microwave treatment on the antinutritional factors of two accessions of velvet bean, Mucuna pruriens (L.) DC. var. utilis (Wall. ex Wight) Bak. ex Burck. Int. Food Res. J. 2012, 19, 961–969. [Google Scholar]
- Kubo, M.T.; dos Reis, B.H.; Sato, L.N.; Gut, J.A. Microwave and conventional thermal processing of soymilk: Inactivation kinetics of lipoxygenase and trypsin inhibitors activity. LWT 2021, 145, 111275. [Google Scholar] [CrossRef]
- Vanga, S.K.; Wang, J.; Jayaram, S.; Raghavan, V. Effects of pulsed electric fields and ultrasound processing on proteins and enzymes: A review. Processes 2021, 9, 722. [Google Scholar] [CrossRef]
- Kubo, M.T.; Siguemoto, É.S.; Funcia, E.S.; Augusto, P.E.; Curet, S.; Boillereaux, L.; Sastry, S.K.; Gut, J.A. Non-thermal effects of microwave and ohmic processing on microbial and enzyme inactivation: A critical review. Curr. Opin. Food Sci. 2020, 35, 36–48. [Google Scholar] [CrossRef]
- El Mecherfi, K.E.; Curet, S.; Lupi, R.; Larré, C.; Rouaud, O.; Choiset, Y.; Rabesona, H.; Haertlé, T. Combined microwave processing and enzymatic proteolysis of bovine whey proteins: The impact on bovine β-lactoglobulin allergenicity. J. Food Sci. Technol. 2019, 56, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Sun, Y.; Huangfu, B.; He, X.; Huang, K. Ultra-high-pressure passivation of soybean agglutinin and safety evaluations. Food Chem. X 2023, 18, 100726. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Chang, S.K.; Liu, Z.; Xu, B. Elimination of trypsin inhibitor activity and beany flavor in soy milk by consecutive blanching and ultrahigh-temperature (UHT) processing. J. Agric. Food Chem. 2008, 56, 7957–7963. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Y.; Xue, Z.; Gao, X.; Jia, Y.; Wang, Y.; Lu, Y.; Zhang, J.; Zhang, M.; Chen, H. Insight into the inactivation mechanism of soybean Bowman-Birk trypsin inhibitor (BBTI) induced by epigallocatechin gallate and epigallocatechin: Fluorescence, thermodynamics and docking studies. Food Chem. 2020, 303, 125380. [Google Scholar] [CrossRef]
- Oste, R.E.; Brandon, D.L.; Bates, A.H.; Friedman, M. Effect of maillard browning reactions of the kunitz soybean trypsin inhibitor on its interaction with monoclonal antibodies. J. Agric. Food Chem. 1990, 38, 258–261. [Google Scholar] [CrossRef]
- Hodge, J.E. Dehydrated foods, chemistry of browning reactions in model systems. J. Agric. Food Chem. 1953, 1, 928–943. [Google Scholar] [CrossRef]
- Mao, Y.; Wang, S. Simultaneous hot-air assisted radio frequency drying and disinfestation for in-shell walnuts using a two-stage strategy. LWT 2021, 151, 112134. [Google Scholar] [CrossRef]
- Jiao, S.; Johnson, J.; Tang, J.; Wang, S. Industrial-scale radio frequency treatments for insect control in lentils. J. Stored Prod. Res. 2012, 48, 143–148. [Google Scholar] [CrossRef]
- Takács, K.; Szabó, E.E.; Nagy, A.; Cserhalmi, Z.; Falusi, J.; Gelencsér, É. The effect of radiofrequency heat treatment on trypsin inhibitor activity and in vitro digestibility of soybean varieties (Glycine max.(L.) Merr.). J. Food Sci. Technol. 2022, 59, 4436–4445. [Google Scholar] [CrossRef]
- Ye, X.; Jiang, S.; Niu, W.; Bai, R.; Yang, C.; Wang, S.; Li, Z.; Zhang, L.; Han, H.; Xi, J. Glycosylated gelatin prepared based on electron beam irradiation and its physicochemical properties. Int. J. Biol. Macromol. 2024, 279, 135369. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.; Zeng, X.; Ren, E.; Xu, F.; Li, J.; Wang, M.; Wang, R. Review of the application of pulsed electric fields (PEF) technology for food processing in China. Food Res. Int. 2020, 137, 109715. [Google Scholar] [CrossRef] [PubMed]
- Vagadia, B.H.; Vanga, S.K.; Singh, A.; Raghavan, V. Effects of thermal and electric fields on soybean trypsin inhibitor protein: A molecular modelling study. Innov. Food Sci. Emerg. Technol. 2016, 35, 9–20. [Google Scholar] [CrossRef]
- Guo, W.; Llave, Y.; Jin, Y.; Fukuoka, M.; Sakai, N. Mathematical modeling of ohmic heating of two-component foods with non-uniform electric properties at high frequencies. Innov. Food Sci. Emerg. Technol. 2017, 39, 63–78. [Google Scholar] [CrossRef]
- Dos Santos, I.F.; Pimentel, T.C.; da Cruz, A.G.; Stringheta, P.C.; Martins, E.; Campelo, P.H. Ohmic Heating in Food Processing: An Overview of Plant-Based Protein Modification. Processes 2024, 12, 1800. [Google Scholar] [CrossRef]
- Lu, L.; Zhao, L.; Zhang, C.; Kong, X.; Hua, Y.; Chen, Y. Comparative effects of ohmic, induction cooker, and electric stove heating on soymilk trypsin inhibitor inactivation. J. Food Sci. 2015, 80, C495–C503. [Google Scholar] [CrossRef]
- Anbarasan, R.; Jaganmohan, R.; Anandakumar, S.; Mahendran, R. Pulsed electric field treatment of soymilk: Impact on Kunitz trypsin inhibitor allergenicity, antinutritional factor, and aroma characteristics. J. Food Sci. 2023, 88, 5093–5107. [Google Scholar] [CrossRef]
- Yalcin, S.; Basman, A. Effects of infrared treatment on urease, trypsin inhibitor and lipoxygenase activities of soybean samples. Food Chem. 2015, 169, 203–210. [Google Scholar] [CrossRef]
- Maetens, E.; Hettiarachchy, N.; Dewettinck, K.; Horax, R.; Moens, K. Reductions of anti-nutritional factors of germinated soybeans by ultraviolet and infrared treatments for snack chips preparation. LWT 2018, 90, 513–518. [Google Scholar] [CrossRef]
- Sakare, P.; Giri, S.K.; Kate, A. Optimization of sprouting and infrared radiation combination treatment for production of ready-to-eat sprouted soybean: Ready-to-eat sprouted soybean process optimization. J. Sci. Ind. Res. (JSIR) 2023, 82, 691–699. [Google Scholar]
- Avelar, Z.; Vicente, A.A.; Saraiva, J.A.; Rodrigues, R.M.M. The role of emergent processing technologies in tailoring plant protein functionality: New insights. Trends Food Sci. Technol. 2021, 113, 219–231. [Google Scholar] [CrossRef]
- Van Der Ven, C.; Matser, A.M.; Van den Berg, R.W. Inactivation of soybean trypsin inhibitors and lipoxygenase by high-pressure processing. J. Agric. Food Chem. 2005, 53, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.E.; Moraru, C.I. Effect of High Pressure Processing and heat treatment on in vitro digestibility and trypsin inhibitor activity in lentil and faba bean protein concentrates. LWT 2021, 152, 112342. [Google Scholar] [CrossRef]
- Masson, L.M.; Rosenthal, A.; Calado, V.M.; Deliza, R.; Tashima, L. Effect of ultra-high pressure homogenization on viscosity and shear stress of fermented dairy beverage. LWT—Food Sci. Technol. 2011, 44, 495–501. [Google Scholar] [CrossRef]
- Poliseli-Scopel, F.H.; Hernández-Herrero, M.; Guamis, B.; Ferragut, V. Comparison of ultra high pressure homogenization and conventional thermal treatments on the microbiological, physical and chemical quality of soymilk. LWT—Food Sci. Technol. 2012, 46, 42–48. [Google Scholar] [CrossRef]
- Suslick, K.S.; Mdleleni, M.M.; Ries, J.T. Chemistry induced by hydrodynamic cavitation. J. Am. Chem. Soc. 1997, 119, 9303–9304. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, X.; Liu, S.; Ren, F.; Wang, J. Effects of high hydrostatic pressure on nutritional composition and cooking quality of whole grains and legumes. Innov. Food Sci. Emerg. Technol. 2023, 83, 103239. [Google Scholar] [CrossRef]
- Linsberger-Martin, G.; Weiglhofer, K.; Phuong, T.P.T.; Berghofer, E. High hydrostatic pressure influences antinutritional factors and in vitro protein digestibility of split peas and whole white beans. LWT—Food Sci. Technol. 2013, 51, 331–336. [Google Scholar] [CrossRef]
- Garcia-Mora, P.; Peñas, E.; Frías, J.; Gómez, R.; Martinez-Villaluenga, C. High-pressure improves enzymatic proteolysis and the release of peptides with angiotensin I converting enzyme inhibitory and antioxidant activities from lentil proteins. Food Chem. 2015, 171, 224–232. [Google Scholar] [CrossRef]
- Zhu, D.; Guan, D.; Fan, B.; Sun, Y.; Wang, F. Untargeted mass spectrometry-based metabolomics approach unveils molecular changes in heat-damaged and normal soybean. LWT 2022, 171, 114136. [Google Scholar] [CrossRef]
- Manassero, C.A.; Vaudagna, S.R.; Sancho, A.M.; Añón, M.C.; Speroni, F. Combined high hydrostatic pressure and thermal treatments fully inactivate trypsin inhibitors and lipoxygenase and improve protein solubility and physical stability of calcium-added soymilk. Innov. Food Sci. Emerg. Technol. 2016, 35, 86–95. [Google Scholar] [CrossRef]
- Rane, R.; Marar, T.; Sonawane, S.K.; Dabade, A. A review on Instant Controlled Pressure Drop Technology (DIC) associated with Drying technology and effect on quality characteristics. Food Chem. Adv. 2022, 1, 100114. [Google Scholar] [CrossRef]
- Pedrosa, M.M.; Cuadrado, C.; Burbano, C.; Allaf, K.; Haddad, J.; Gelencsér, E.; Takács, K.; Guillamón, E.; Muzquiz, M. Effect of instant controlled pressure drop on the oligosaccharides, inositol phosphates, trypsin inhibitors and lectins contents of different legumes. Food Chem. 2012, 131, 862–868. [Google Scholar] [CrossRef]
- Téllez-Pérez, C.; Alonzo-Macías, M.; Mounir, S.; Besombes, C.; Allaf, T.; Amami, E.; Allaf, K. Instant Controlled Pressure-Drop DIC as a Strategic Technology for Different Types of Natural Functional Foods. Funct. Foods 2019, 25, 81740. [Google Scholar]
- Haddad, J.; Greiner, R.; Allaf, K. Effect of instantaneous controlled pressure drop on the phytate content of lupin. LWT—Food Sci. Technol. 2007, 40, 448–453. [Google Scholar] [CrossRef]
- Berk, Z. Food extrusion. In Engineering Foods for Bioactives Stability and Delivery; Springer: Berlin/Heidelberg, Germany, 2017; pp. 309–339. [Google Scholar]
- Nikmaram, N.; Leong, S.Y.; Koubaa, M.; Zhu, Z.; Barba, F.J.; Greiner, R.; Oey, I.; Roohinejad, S. Effect of extrusion on the anti-nutritional factors of food products: An overview. Food Control 2017, 79, 62–73. [Google Scholar] [CrossRef]
- Singh, S.; Gamlath, S.; Wakeling, L. Nutritional aspects of food extrusion: A review. Int. J. Food Sci. Technol. 2007, 42, 916–929. [Google Scholar] [CrossRef]
- Shiferaw Terefe, N.; Buckow, R.; Versteeg, C. Quality-related enzymes in plant-based products: Effects of novel food-processing technologies part 3: Ultrasonic processing. Crit. Rev. Food Sci. Nutr. 2015, 55, 147–158. [Google Scholar] [CrossRef]
- Huang, H.; Kwok, K.C.; Liang, H.H. Inhibitory activity and conformation changes of soybean trypsin inhibitors induced by ultrasound. Ultrason. Sonochem. 2008, 15, 724–730. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rathod, V.K. Ultrasound assisted intensification of enzyme activity and its properties: A mini-review. World J. Microbiol. Biotechnol. 2017, 33, 170. [Google Scholar] [CrossRef]
- Wu, Y.; Li, W.; Martin, G.J.; Ashokkumar, M. Mechanism of low-frequency and high-frequency ultrasound-induced inactivation of soy trypsin inhibitors. Food Chem. 2021, 360, 130057. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, A.; Jayachandran, L.E.; Rao, P.S. Sequential Microwave–Ultrasound assisted extraction of soymilk and optimization of extraction process. LWT 2021, 151, 112220. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, W.; Shen, J.; Xu, X.; Zhou, G. Influence of gamma irradiation on porcine serum albumin structural properties and allergenicity. J. AOAC Int. 2018, 101, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.D.E.D.H. The nutritive value for chicks of full-fat soybeans irradiated at up to 60 kGy. Anim. Feed Sci. Technol. 1998, 73, 319–328. [Google Scholar] [CrossRef]
- Taghinejad-Roudbaneh, M.; Ebrahimi, S.; Azizi, S.; Shawrang, P. Effects of electron beam irradiation on chemical composition, antinutritional factors, ruminal degradation and in vitro protein digestibility of canola meal. Radiat. Phys. Chem. 2010, 79, 1264–1269. [Google Scholar] [CrossRef]
- Ebrahimi-Mahmoudabad, S.; Taghinejad-Roudbaneh, M. Investigation of electron beam irradiation effects on anti-nutritional factors, chemical composition and digestion kinetics of whole cottonseed, soybean and canola seeds. Radiat. Phys. Chem. 2011, 80, 1441–1447. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Niu, D.; Huang, Z.; Gai, L.; Hang, F. Effects of moderate dielectric barrier discharge (DBD) plasma treatment on the structure, antigenicity, and digestibility of casein. Food Hydrocoll. 2024, 153, 109973. [Google Scholar] [CrossRef]
- Dong, S.; Gao, A.; Zhao, Y.; Li, Y.; Chen, Y. Characterization of physicochemical and structural properties of atmospheric cold plasma (ACP) modified zein. Food Bioprod. Process. 2017, 106, 65–74. [Google Scholar] [CrossRef]
- Li, Q.; Shen, F.; He, X.; Xing, C.; Yan, W.; Fang, Y.; Hu, Q. Modification of soy protein isolate using dielectric barrier discharge cold plasma assisted by modified atmosphere packaging. Food Chem. 2023, 401, 134158. [Google Scholar] [CrossRef]
- Liu, Z.-W.; Niu, D.; Zhou, Y.-X.; Cheng, J.-H.; Bekhit, A.E.-D.; Aadil, R.M. Oxidation induced by dielectric-barrier discharge (dbd) plasma treatment reduces soybean agglutinin activity. Food Chem. 2021, 340, 128198. [Google Scholar] [CrossRef]
- Boutureira, O.; Bernardes, G.J. Advances in chemical protein modification. Chem. Rev. 2015, 115, 2174–2195. [Google Scholar] [CrossRef]
- Wu, Y.; Sessa, D.J. Conformation of bowman-birk inhibitor. J. Agric. Food Chem. 1994, 42, 2136–2138. [Google Scholar] [CrossRef]
- Wolf, R.B.; Cavins, J.F.; Kleiman, R.; Black, L.T. Effect of temperature on soybean seed constituents: Oil, protein, moisture, fatty acids, amino acids and sugars. J. Am. Oil Chem. Soc. 1982, 59, 230–232. [Google Scholar] [CrossRef]
- Nasri, M. Protein hydrolysates and biopeptides: Production, biological activities, and applications in foods and health benefits. A review. Adv. Food Nutr. Res. 2017, 81, 109–159. [Google Scholar]
- Ma, Y. Deactivation of Soybean Agglutinin by Enzyme Hydrolysis and Identification of Active Peptides from Soy Proteins. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2010. [Google Scholar]
- Sugawara, M.; Ito, D.; Yamamoto, K.; Akita, M.; Oguri, S.; Momonoki, Y.S. Kunitz soybean trypsin inhibitor is modified at its C-terminus by novel soybean thiol protease (protease T1). Plant Prod. Sci. 2007, 10, 314–321. [Google Scholar] [CrossRef]
- EI-Gawad, A.I.; Hefny, A.A.; EI-Sayed, E.M.; Saleh, F.A. Preparation Technique of Soymilk-Based Yoghurt and it’s Relation to Soybean Varieties and Anti-Nutritional Factors. J. Nutr. Food Sci. 2015, 5, 100041. [Google Scholar]
- Qi, N.; Zhan, X.; Milmine, J.; Chang, K.; Li, J. A novel thermophilic strain of Bacillus subtilis with antimicrobial activity and its potential application in solid-state fermentation of soybean meal. Microbiol. Spectr. 2024, 12, e02784-23. [Google Scholar] [CrossRef]
- Silva Júnior, S.; Tavano, O.; Demonte, A.; Rossi, E.; Pinto, R. Nutritional evaluation of soy yoghurt in comparison to soymilk and commercial milk yoghurt. Effect of fermentation on soy protein. Acta Aliment. 2012, 41, 443–450. [Google Scholar] [CrossRef]

| Treatment | Conditions | Advantage | Disadvantage | Studies |
|---|---|---|---|---|
| Cooking on the stove | 20 min |
|
| [36] |
| Microwave | 2450 MHZ, 4 min |
|
| [37,38] |
| Pressure and cooking | 120 °C, 10 min | —— |
| [37] |
| Regular hot air drying | 100 °C, 2 h | —— | —— | [39] |
| Fluidized bed drying | Initial inlet air temperature of 80 °C, 30 min after processing temperature 60 °C instead to continue processing |
|
| [40] |
| Ohmic heating | 220 V, 50 Hz |
| —— | [41] |
| High-pressure processing | 600 MPa, 25 min |
|
| [42] |
| High pressure and heating | Various combinations of temperature (300 K, 345 K, and 373 K) and pressure (1 bar, 3 kbar, and 6 kbar) |
| —— | [43] |
| 1 min, 6 min |
|
| [44] |
| Ultrasonic treatment | 25 kHz, 400 W, 16 min |
|
| [43] |
| Irradiation | 10 kGy or less irradiation |
|
| [45] |
| Extrusion processing | 160 °C of mold temperature and screw speed of 200 rpm |
|
| [46] |
| 23 V, 15 min or 33.8 KV, 5 min |
| —— | [47,48] |
| Treatment | Conditions | Advantage | Disadvantage | Studies |
|---|---|---|---|---|
| Acids, alkalis, and salt | HCl, 23 °C or 40 °C, 8 h |
| Nutritional Loss. Deterioration of sensory quality. Environmental Concerns. | [49] |
| Nacl, heating | Simplicity and ease of using. Low Cost. | Limiting inactivation effect, Impacting on sensory quality. | [21] | |
| NaoH |
| Nutritional Loss. Deterioration of sensory quality. Environmental Concerns. | [50] | |
| NaHCO3 | Mild Processing Conditions | Incompleting inactivation | [51] | |
| Disulfide bond modification | Sodium metabisulfite or L-cysteine, 25 °C, 2 h |
| —— | [52] |
| Zn |
|
| [53] | |
| Modification of amino acid residues | Maleicanhydride, pH = 3.5–9.5, 30 min | —— | Chemical residues are harmful to health. | [54] |
| CH3OH | —— |
| [16] | |
| Polyphenols | TPs |
|
| [55] |
| Stevioside |
|
| [24] |
| Treatment | Conditions | Advantage | Disadvantage | Studies |
|---|---|---|---|---|
| Enzymolysis | Alcalase, pH = 8, 60 °C, 4 h |
|
| [56] |
| High-pressure homogenization-assisted enzymatic digestion | —— |
|
| [57] |
| Germination | 3 d 32 °C, 90% relative humidity |
|
| [58] |
| Fermenting | Acidophilus, Bacillus subtilis, Lactobacillus bulgaricus, 5 d |
| —— | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Z.; Zhu, Y.; Xiang, H.; Wang, Z.; Jiang, Z.; Zhao, X.; Sun, X.; Guo, Z. Advancements in Inactivation of Soybean Trypsin Inhibitors. Foods 2025, 14, 975. https://doi.org/10.3390/foods14060975
Luo Z, Zhu Y, Xiang H, Wang Z, Jiang Z, Zhao X, Sun X, Guo Z. Advancements in Inactivation of Soybean Trypsin Inhibitors. Foods. 2025; 14(6):975. https://doi.org/10.3390/foods14060975
Chicago/Turabian StyleLuo, Zhanjun, Yujia Zhu, Huiyu Xiang, Ziqian Wang, Zhimo Jiang, Xinglong Zhao, Xiaomeng Sun, and Zengwang Guo. 2025. "Advancements in Inactivation of Soybean Trypsin Inhibitors" Foods 14, no. 6: 975. https://doi.org/10.3390/foods14060975
APA StyleLuo, Z., Zhu, Y., Xiang, H., Wang, Z., Jiang, Z., Zhao, X., Sun, X., & Guo, Z. (2025). Advancements in Inactivation of Soybean Trypsin Inhibitors. Foods, 14(6), 975. https://doi.org/10.3390/foods14060975










