Exploration of Pea Protein Isolate–Sodium Alginate Complexes as a Novel Strategy to Substitute Sugar in Plant Cream: Synergistic Interactions Between the Two at the Interface
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PPI/SA Complexes
2.3. Characterization of PPI/SA Complexes
2.3.1. Fluorescence Spectroscopy
2.3.2. Zeta Potential
2.3.3. Measurement of Flexibility
2.3.4. Determination of SH Content
2.3.5. Circular Dichroism (CD) Spectra
2.3.6. Isothermal Titration Calorimetry (ITC)
2.3.7. Interfacial Tension
2.3.8. Interfacial Protein Concentrations
2.4. Preparation and Characterization of Whipped Creams
2.4.1. Preparation of Whipped Creams
2.4.2. Determination of Hardness of the Whipped Creams
2.4.3. Determination of Overrun of the Whipped Creams
2.4.4. Measurement of Stirring Time and Beating Rate
2.4.5. Separation Index of Cream Slurry
2.4.6. Observation of Stability of Mounting and Stability of Mounting Cutting Surface
2.4.7. Apparent Viscosity of the Whipped Creams
2.4.8. Frequency Sweeps of the Whipped Creams
2.4.9. Confocal Laser Scanning Microscopy (CLSM)
2.5. Statistical Analysis
3. Results
3.1. Fluorescence Spectroscopy
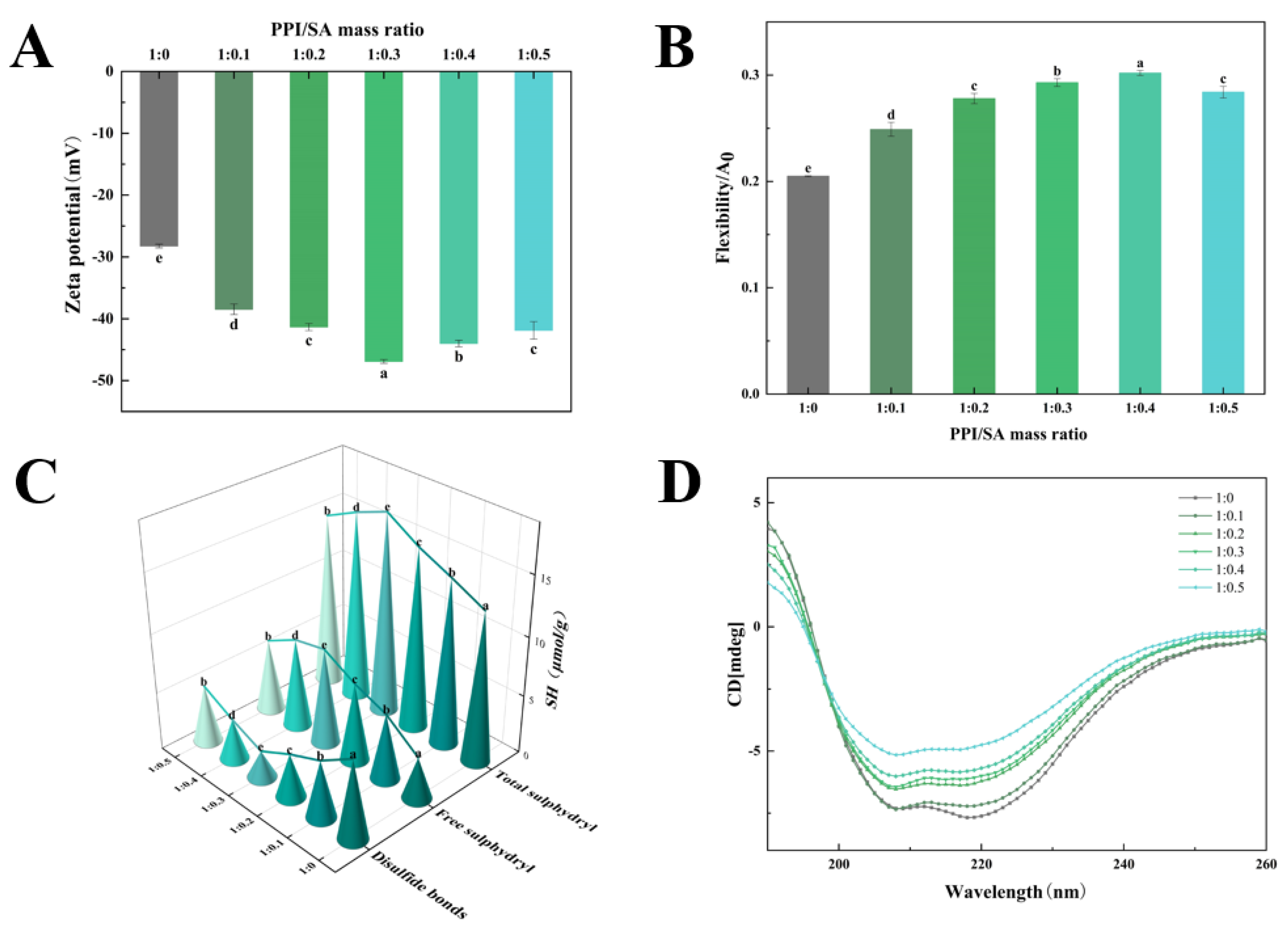
3.2. Zeta Potential
3.3. Protein Flexibility
3.4. Free Sulfhydryl Content
3.5. Secondary Structure
3.6. ITC
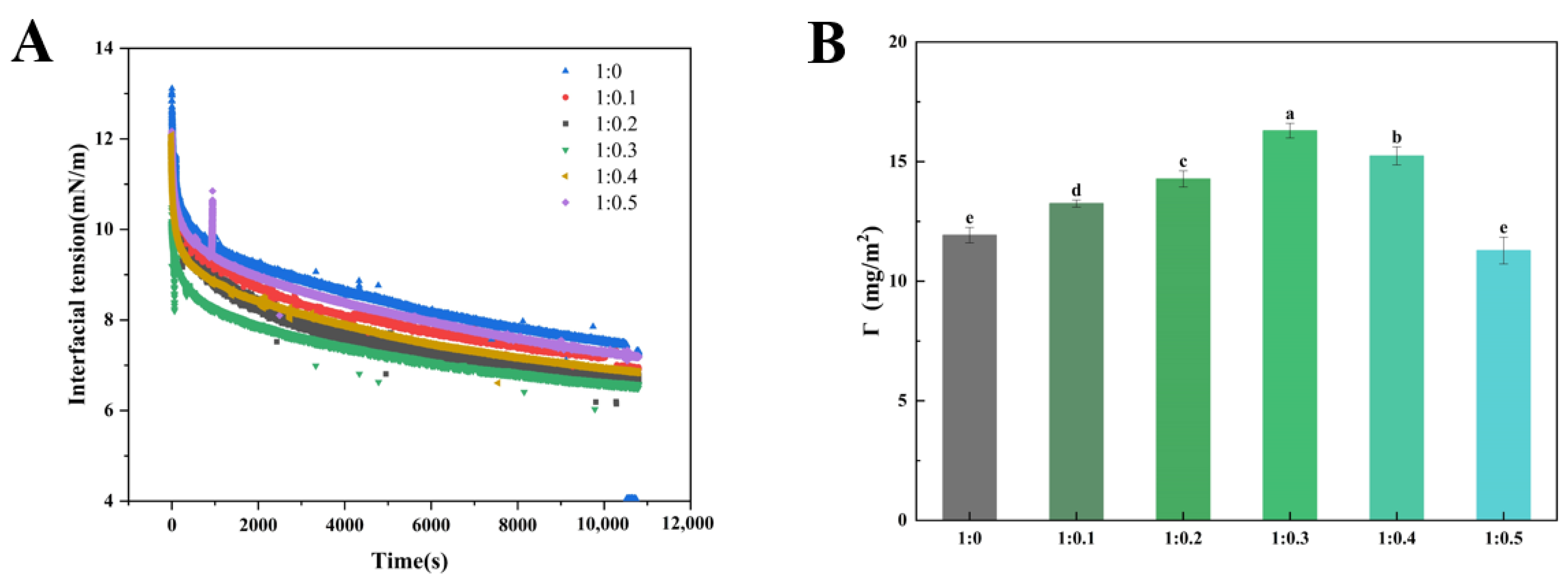
3.7. Adsorption Kinetics of Protein/Emulsifier Mixture
3.8. Interfacial Protein Concentration
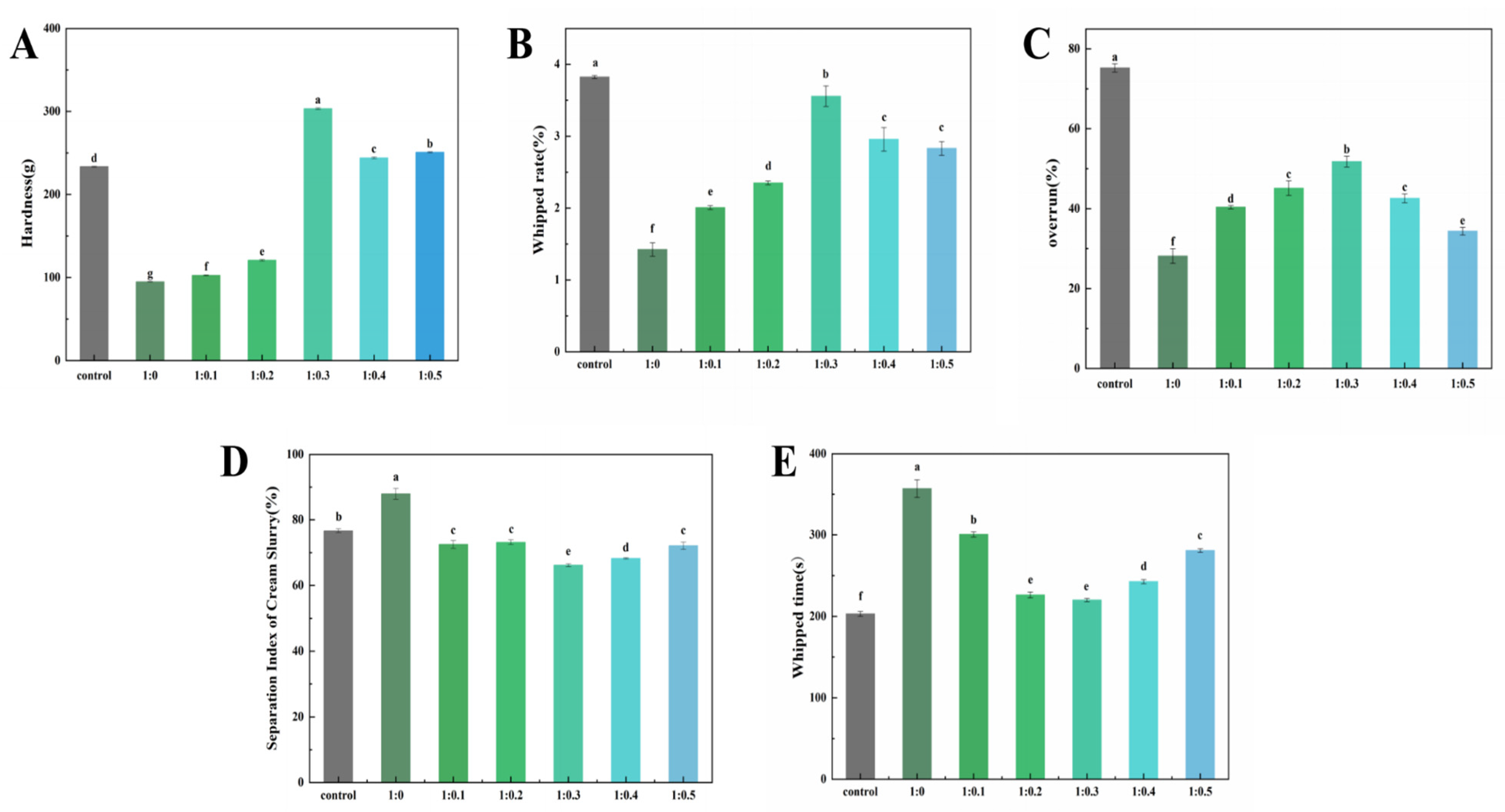
3.9. Texture Analysis of Cream
3.10. Analysis of Cream Whipped Rate
3.11. Analysis of Cream Overrun
3.12. Analysis of Separation Index of Cream Slurry
3.13. Analysis of Cream Whipped Time
3.14. Observation and Analysis of the Appearance and Section of Mounted Flowers
3.15. Analysis of Cream Whipped Time
3.16. Analysis of Frequency Scanning of Cream
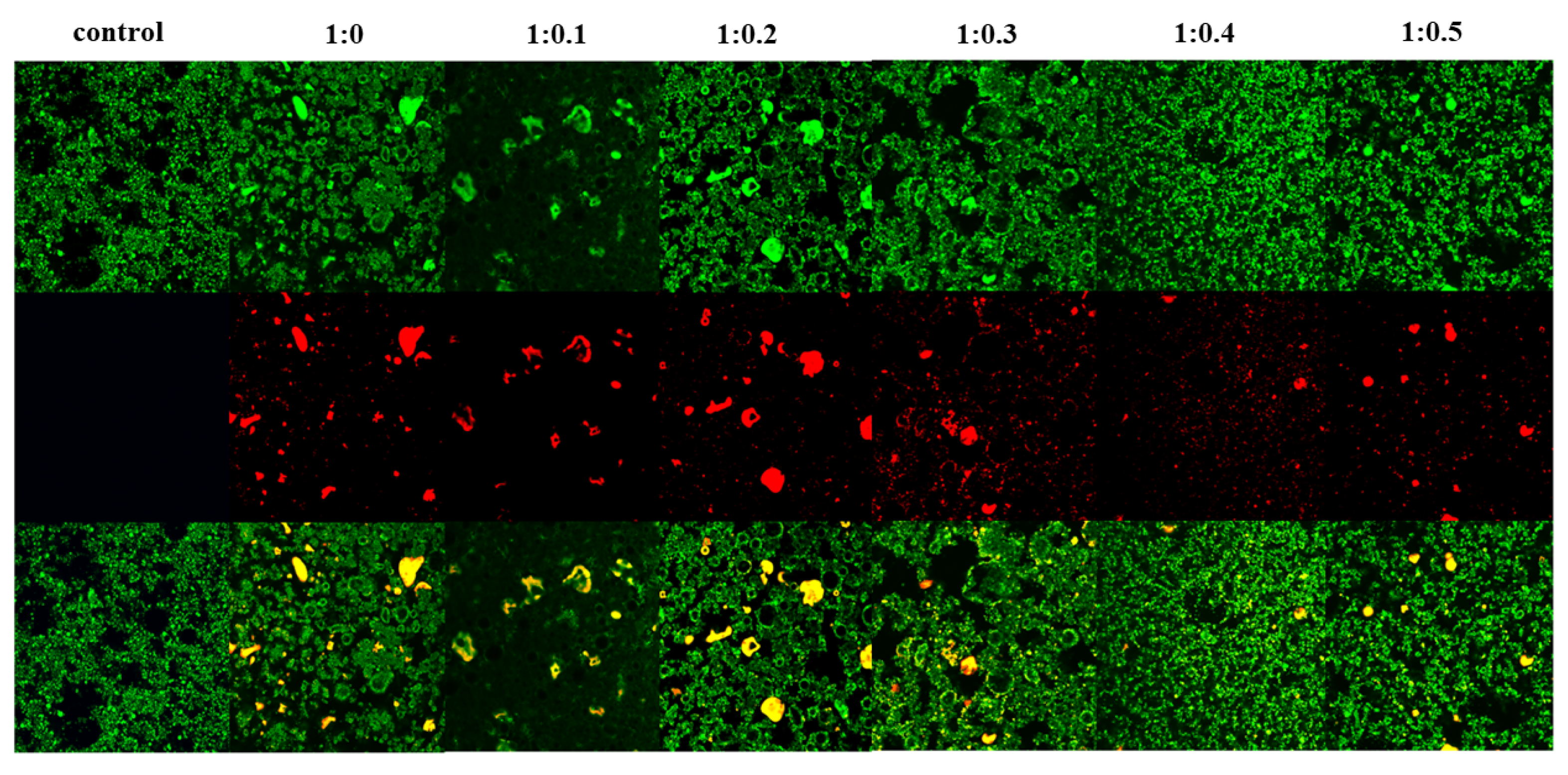
3.17. CLSM
3.18. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeng, Y.; Zeng, D.; Liu, T.; Cai, Y.; Li, Y.; Zhao, M.; Zhao, Q. Effects of glucose and corn syrup on the physical characteristics and whipping properties of vegetable-fat based whipped creams. Foods 2022, 11, 1195. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; Lucan, S.C.; O’Keefe, J.H. The evidence for saturated fat and for sugar related to coronary heart disease. Prog. Cardiovasc. Dis. 2016, 58, 464–472. [Google Scholar] [CrossRef] [PubMed]
- López-Martínez, M.I.; Moreno-Fernández, S.; Miguel, M. Development of functional ice cream with egg white hydrolysates. Int. J. Gastron. Food Sci. 2021, 25, 100334. [Google Scholar] [CrossRef]
- Aaltonen, T.; Kytö, E.; Ylisjunttila-Huusko, S.; Outinen, M. Effect of the milk-based ash-protein ratio on the quality and acceptance of chocolate with a reduced sugar content. Int. Dairy J. 2020, 105, 104663. [Google Scholar] [CrossRef]
- Nooshkam, M.; Varidi, M.; Alkobeisi, F. Licorice extract/whey protein isolate/sodium alginate ternary complex-based bioactive food foams as a novel strategy to substitute fat and sugar in ice cream. Food Hydrocoll. 2023, 135, 108206. [Google Scholar] [CrossRef]
- da Silva Faresin, L.; Devos, R.J.B.; Reinehr, C.O.; Colla, L.M. Development of ice cream with reduction of sugar and fat by the addition of inulin, Spirulina platensis or phycocyanin. Int. J. Gastron. Food Sci. 2022, 27, 100445. [Google Scholar] [CrossRef]
- Yan, X.; Yan, J.; Shi, X.; Song, Y.; McClements, D.J.; Ma, C.; Liu, X.; Chen, S.; Xu, D.; Liu, F. High internal phase double emulsions stabilized by modified pea protein-alginate complexes: Application for co-encapsulation of riboflavin and β-carotene. Int. J. Biol. Macromol. 2024, 270, 132313. [Google Scholar] [CrossRef]
- Zhu, Q.; Qiu, Y.; Zhang, L.; Lu, W.; Pan, Y.; Liu, X.; Li, Z.; Yang, H. Encapsulation of lycopene in Pickering emulsion stabilized by complexes of whey protein isolate fibrils and sodium alginate: Physicochemical property, structural characterization and in vitro digestion property. Food Res. Int. 2024, 191, 114675. [Google Scholar] [CrossRef]
- Gao, Z.; Huang, Y.; Hu, B.; Zhang, K.; Xu, X.; Fang, Y.; Nishinari, K.; Phillips, G.O.; Yang, J. Interfacial and emulsifying properties of the electrostatic complex of β-lactoglobulin fibril and gum Arabic (Acacia Seyal). Colloids Surf. A Physicochem. Eng. Asp. 2019, 562, 1–7. [Google Scholar] [CrossRef]
- Dong, Y.; Wei, Z.; Xue, C. Effect of interaction between ovotransferrin fibrils and pectin on properties of oleogel-based Pickering emulsions. Food Hydrocoll. 2023, 140, 108620. [Google Scholar] [CrossRef]
- Sun, C.; Liu, R.; Wu, T.; Liang, B.; Shi, C.; Cong, X.; Hou, T.; Zhang, M. Combined superfine grinding and heat-shearing treatment for the Microparticulation of whey proteins. Food Bioprocess Technol. 2015, 9, 378–386. [Google Scholar] [CrossRef]
- Rezvani, F.; Abbasi, H.; Nourani, M. Effects of protein-polysaccharide interactions on the physical and textural characteristics of low-fat whipped cream. J. Food Process. Preserv. 2020, 44, 14743. [Google Scholar] [CrossRef]
- Ghribi, A.M.; Zouari, M.; Attia, H.; Besbes, S. Study of protein/κ-carrageenan mixture’s effect on low-fat whipping cream formulation. LWT Food Sci. Tehcnol. 2021, 147, 111647. [Google Scholar] [CrossRef]
- Wang, S.; Yang, J.; Shao, G.; Qu, D.; Zhao, H.; Yang, L.; Zhu, L.; He, Y.; Liu, H.; Zhu, D. Soy protein isolated-soy hull polysaccharides stabilized O/W emulsion: Effect of polysaccharides concentration on the storage stability and interfacial rheological properties. Food Hydrocoll. 2020, 101, 105490. [Google Scholar] [CrossRef]
- Hu, H.; Cheung, I.W.; Pan, S.; Li-Chan, E.C. Effect of high intensity ultrasound on physicochemical and functional properties of aggregated soybean β-conglycinin and glycinin. Food Hydrocoll. 2015, 45, 102–110. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Geng, Q.; He, X.; Tang, Y.; Deng, L.; Li, T.; Liu, C.; Dai, T. Non-covalent interaction of the plant protein composites-proanthocyanidins and the utilization in oil in water emulsions. LWT 2024, 198, 116062. [Google Scholar] [CrossRef]
- Zeng, D.; Cai, Y.; Liu, T.; Huang, L.; Wang, J.; Zhao, M.; Zhu, S.; Zhao, Q. Effect of hydrophobic sucrose esters with different fat acid composition and esterification degree on whipped cream properties. Food Hydrocoll. 2024, 146, 109183. [Google Scholar] [CrossRef]
- Yan, G.; Li, Y.; Wang, H.; Cui, S.; Li, Y.; Zhang, L.; Yan, J. Multiscale approach to the characterization of the interfacial properties of micellar casein and whey protein blends and their effects on recombined dairy creams. Food Res. Int. 2024, 188, 114453. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, Z.; Zhang, H.; Wang, J.; Ren, S. Protein particles ameliorate the mechanical properties of highly polyunsaturated oil-based whipped cream: A possible mode of action. Food Hydrocoll. 2020, 99, 105350. [Google Scholar] [CrossRef]
- Van Aken, G.A. Aeration of emulsions by whipping. Colloids Surf. A Physicochem. Eng. Asp. 2001, 190, 333–354. [Google Scholar] [CrossRef]
- Agyare, K.K.; Xiong, Y.L.; Addo, K. Influence of salt and pH on the solubility and structural characteristics of transglutaminase-treated wheat gluten hydrolysate. Food Chem. 2008, 107, 1131–1137. [Google Scholar] [CrossRef]
- Wan, Y.; Liu, J.; Guo, S. Effects of succinylation on the structure and thermal aggregation of soy protein isolate. Food Chem. 2018, 245, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, J.; Sun, F.; Guo, Y.; Wang, D.; Cheng, T.; Xu, M.; Wang, Z.; Guo, Z. Spectroscopy combined with spatiotemporal multiscale strategy to study the adsorption mechanism of soybean protein isolate with meat flavor compounds (furan): Differences in position and quantity of the methyl. Food Chem. 2024, 451, 139415. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cheng, Y.; Zhang, Z.; Wang, Y.; Mintah, B.K.; Dabbour, M.; Jiang, H.; He, R.; Ma, H. Modification of rapeseed protein by ultrasound-assisted pH shift treatment: Ultrasonic mode and frequency screening, changes in protein solubility and structural characteristics. Ultrason. Sonochemistry 2020, 69, 105240. [Google Scholar] [CrossRef]
- Yuan, M.; Li, B.; Sun, F.; Cheng, T.; Liu, J.; Wang, D.; Guo, Z.; Wang, Z. Characterization of HIPEs stabilized by soy protein isolate with excellent rheological properties for 3D printing: Bridging the correlation among structure, function and application. Food Hydrocoll. 2024, 153, 110053. [Google Scholar] [CrossRef]
- Li, R.; Cui, Q.; Wang, G.; Liu, J.; Chen, S.; Wang, X.; Wang, X.; Jiang, L. Relationship between surface functional properties and flexibility of soy protein isolate-glucose conjugates. Food Hydrocoll. 2019, 95, 349–357. [Google Scholar] [CrossRef]
- Basak, S.; Singhal, R.S. Succinylation of food proteins-a concise review. LWT 2022, 154, 112866. [Google Scholar] [CrossRef]
- Ai, M.; Xiao, N.; Jiang, A. Molecular structural modification of duck egg white protein conjugates with monosaccharides for improving emulsifying capacity. Food Hydrocoll. 2021, 111, 106271. [Google Scholar] [CrossRef]
- Liu, M.; Shan, S.; Gao, X.; Shi, Y.; Lu, W. The effect of sweet tea polysaccharide on the physicochemical and structural properties of whey protein isolate gels. Int. J. Biol. Macromol. 2023, 240, 124344. [Google Scholar] [CrossRef]
- Jiang, L.; Ren, Y.; Xiao, Y.; Liu, S.; Zhang, J.; Yu, Q.; Chen, Y.; Xie, J. Effects of Mesona chinensis polysaccharide on the thermostability, gelling properties, and molecular forces of whey protein isolate gels. Carbohydr. Polym. 2020, 242, 116424. [Google Scholar] [CrossRef]
- Yi, J.; Chen, X.; Wen, Z.; Fan, Y. Improving the functionality of pea protein with laccase-catalyzed crosslinking mediated by chlorogenic acid. Food Chem. 2024, 433, 137344. [Google Scholar] [CrossRef] [PubMed]
- Zhi, Z.; Yan, L.; Li, H.; Dewettinck, K.; Van der Meeren, P.; Liu, R.; Van Bockstaele, F. A combined approach for modifying pea protein isolate to greatly improve its solubility and emulsifying stability. Food Chem. 2022, 380, 131832. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Shen, Y.; Zhao, R.; Deng, J.; Wang, C. Interactions with soy lecithin regulate the emulsification capacity of pea protein: Effects of soy lecithin concentration. Food Hydrocoll. 2024, 155, 110168. [Google Scholar] [CrossRef]
- Huo, C.; Liu, G.; Xu, M.; Li, X.; Zong, W.; Liu, R. Characterizing the binding interactions of sodium benzoate with lysozyme at the molecular level using multi-spectroscopy, ITC and modeling methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 263, 120213. [Google Scholar] [CrossRef]
- Archut, A.; Rolin, C.; Drusch, S.; Kastner, H. Interaction of sugar beet pectin and pea protein: Impact of neutral sugar side chains and acetyl groups. Food Hydrocoll. 2023, 138, 108454. [Google Scholar] [CrossRef]
- Jabarkhyl, S.; Barigou, M.; Badve, M.; Zhu, S. Rheological properties of wet foams generated from viscous pseudoplastic fluids. Innov. Food Sci. Emerg. Technol. 2020, 64, 102304. [Google Scholar] [CrossRef]
- Zaragoza, A.; Teruel, J.A.; Aranda, F.J.; Marqués, A.; Espuny, M.J.; Manresa, Á.; Ortiz, A. Interaction of a Rhodococcus sp. trehalose lipid biosurfactant with model proteins: Thermodynamic and structural changes. Langmuir 2012, 28, 1381–1390. [Google Scholar] [CrossRef]
- Cai, Z.; Wei, Y.; Shi, A.; Zhong, J.; Rao, P.; Wang, Q.; Zhang, H. Correlation between interfacial layer properties and physical stability of food emulsions: Current trends, challenges, strategies, and further perspectives. Adv. Colloid Interface Sci. 2023, 313, 102863. [Google Scholar] [CrossRef]
- Tao, Y.; Cai, J.; Wang, P.; Chen, J.; Zhou, L.; Yang, Z.; Xu, X. Exploring the relationship between the interfacial properties and emulsion properties of ultrasound-assisted cross-linked myofibrillar protein. Food Hydrocoll. 2024, 146, 109287. [Google Scholar] [CrossRef]
- Lin, J.; Yang, J.; Kong, J.; Shen, M.; Yu, Q.; Chen, Y.; Xie, J. Effect of cellulose nanofibrils on stability and digestive properties of legume protein-based emulsions. Food Hydrocoll. 2024, 151, 109779. [Google Scholar] [CrossRef]
- Lin, L.; Xiong, Y.L. Competitive adsorption and dilatational rheology of pork myofibrillar and sarcoplasmic proteins at the O/W emulsion interface. Food Hydrocoll. 2021, 118, 106816. [Google Scholar] [CrossRef]
- Sun, F.; Pan, C.; Liu, Y.; Yang, N. Carboxymethyl tamarind seed polysaccharide/chitosan complexes through electrostatic interaction stabilize high internal phase emulsions: Roles of the mass ratio and oil-water interfacial activity. LWT 2024, 195, 115833. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, B.; Xie, F.; Hu, M.; Sun, Y.; Han, L.; Li, L.; Zhang, S.; Li, Y. Emulsion stability and dilatational rheological properties of soy/whey protein isolate complexes at the oil-water interface: Influence of pH. Food Hydrocoll. 2021, 113, 106391. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Zhao, H.; He, Z.; Zeng, M.; Qin, F.; Chen, J. Improvement of emulsifying properties of soy protein through selective hydrolysis: Interfacial shear rheology of adsorption layer. Food Hydrocoll. 2016, 60, 453–460. [Google Scholar] [CrossRef]
- Cheng, J.; Cui, J.; Ma, Y.; Yan, T.; Wang, L.; Li, H.; Li, X. Effects of soy-to-milk protein ratio and sucrose fatty acid ester addition on the stability of ice cream emulsions. Food Hydrocoll. 2016, 60, 425–436. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Wang, R.; Wang, Y.; Li, Y.; Zhang, L. Effects of triglycerol monostearate on physical properties of recombined dairy cream. Int. Dairy J. 2020, 103, 104622. [Google Scholar] [CrossRef]
- Funami, T.; Nakauma, M. Instrumental food texture evaluation in relation to human perception. Food Hydrocoll. 2022, 124, 107253. [Google Scholar] [CrossRef]
- Han, Y.; Zhu, L.; Zhang, H.; Wu, G. Fabrication and characterization of non-fat whipped cream analogue: Effects of type and concentration of polysaccharide. Int. J. Biol. Macromol. 2024, 276, 133819. [Google Scholar] [CrossRef]
- Liu, X.; Sala, G.; Scholten, E. Impact of soy protein dispersibility on the structural and sensory properties of fat-free ice cream. Food Hydrocoll. 2024, 147, 109340. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, G.; Chen, W.; Qie, X.; Fu, L.; Li, X.; He, Z.; Zeng, M.; Goff, H.D.; Chen, J. Effects of soy proteins and hydrolysates on fat globule coalescence and whipping properties of recombined low-fat whipped cream. Food Biophys. 2022, 17, 324–334. [Google Scholar] [CrossRef]
- Long, Z.; Zhao, M.; Sun-Waterhouse, D.; Lin, Q.; Zhao, Q. Effects of sterilization conditions and milk protein composition on the rheological and whipping properties of whipping cream. Food Hydrocoll. 2016, 52, 11–18. [Google Scholar] [CrossRef]
- Feizi, R.; Goh, K.K.; Mutukumira, A.N. Effect of chia seed mucilage as stabiliser in ice cream. Int. Dairy J. 2021, 120, 105087. [Google Scholar] [CrossRef]
- Warren, M.M.; Hartel, R.W. Effects of emulsifier, overrun and dasher speed on ice cream microstructure and melting properties. J. Food Sci. 2018, 83, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Narala, V.R.; Orlovs, I.; Jugbarde, M.A.; Masin, M. Inulin as a fat replacer in pea protein vegan ice cream and its influence on textural properties and sensory attributes. Appl. Food Res. 2022, 2, 100066. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, T.; Sun, M.; Li, Y.; Zhang, G.; Hu, Z.; Wang, D.; Guo, Z.; Wang, Z. Application of soy protein isolate-naringenin complexes as fat replacers in low-fat cream: Based on protein conformational changes, aggregation states and interfacial adsorption behavior. Int. J. Biol. Macromol. 2024, 274, 133315. [Google Scholar] [CrossRef]
- Ihara, K.; Hirota, M.; Akitsu, T.; Urakawa, K.; Abe, T.; Sumi, M.; Okawa, T.; Fujii, T. Effects of emulsifying components in the continuous phase of cream on the stability of fat globules and the physical properties of whipped cream. J. Dairy Sci. 2015, 98, 2875–2883. [Google Scholar] [CrossRef]
- Xie, P.; Huang, M.; Liu, J.; Elbarbary, A.; Jin, Q.; Wang, X.; Jin, J. Effects of spans on whipping capabilities of aerated emulsions: Reinforcement of fat crystal-membrane interactions. J. Food Eng. 2024, 372, 112008. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, R.; Meng, Z. Whipped cream stabilized by faba bean protein isolate microgel particles substituted for sodium caseinate: Whipping performance and foam stabilization analysis. Food Hydrocoll. 2024, 157, 110388. [Google Scholar] [CrossRef]
- Sajedi, M.; Nasirpour, A.; Keramat, J.; Desobry, S. Effect of modified whey protein concentrate on physical properties and stability of whipped cream. Food Hydrocoll. 2014, 36, 93–101. [Google Scholar] [CrossRef]
- Wang, Y.; Hartel, R.W.; Zhang, L. The stability of aerated emulsions: Effects of emulsifier synergy on partial coalescence and crystallization of milk fat. J. Food Eng. 2021, 291, 110257. [Google Scholar] [CrossRef]
- Camacho, M.; Martı́nez-Navarrete, N.; Chiralt, A. Influence of locust bean gum/λ-carrageenan mixtures on whipping and mechanical properties and stability of dairy creams. Food Res. Int. 1998, 31, 653–658. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, Z.; Zhao, M.; Zhang, H.; Wang, J.; Sun, B. Synergistic influence of protein particles and low-molecular-weight emulsifiers on the stability of a milk fat-based whippable oil-in-water emulsion. Food Hydrocoll. 2022, 127, 107520. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Z.; Liu, C.; Ma, T. Effect of pH-shifting and sonication-assisted treatment on properties and stability of vegetable oil-based whipped cream stabilized by kidney bean protein aggregates. Food Hydrocoll. 2023, 141, 108736. [Google Scholar] [CrossRef]
- Grossi, M.; Rao, J.; Chen, B. Tuning the properties of plant-based whipped cream through zein nanoparticles-sodium stearate complex. Food Hydrocoll. 2024, 155, 110219. [Google Scholar] [CrossRef]
- Cui, H.; Tang, C.; Wu, S.; McClements, D.J.; Liu, S.; Li, B.; Li, Y. Fabrication of chitosan-cinnamaldehyde-glycerol monolaurate bigels with dual gelling effects and application as cream analogs. Food Chem. 2022, 384, 132589. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Q.; Rao, Z.; Lei, X.; Zhao, J.; Lei, L.; Ming, J. Formulation and characterization of bigels utilizing whey protein and polysaccharides: Potential applications as cream analogues. Food Hydrocoll. 2024, 152, 109884. [Google Scholar] [CrossRef]
- Hei, X.; Liu, Z.; Li, S.; Wu, C.; Jiao, B.; Hu, H.; Ma, X.; Zhu, J.; Adhikari, B.; Wang, Q. Freeze-thaw stability of Pickering emulsion stabilized by modified soy protein particles and its application in plant-based ice cream. Int. J. Biol. Macromol. 2024, 257, 128183. [Google Scholar] [CrossRef]
- Kurt, A.; Cengiz, A.; Kahyaoglu, T. The effect of gum tragacanth on the rheological properties of salep based ice cream mix. Carbohydr. Polym. 2016, 143, 116–123. [Google Scholar] [CrossRef]
- Chang, Y.; Hartel, R. Stability of air cells in ice cream during hardening and storage. J. Food Eng. 2002, 55, 59–70. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Zheng, Y.; Hong, Q.; Wang, Q.; Xu, X. Synergistic effects of microcrystalline cellulose and xanthan gum on the stability of milk fat-based UHT whipping cream. LWT 2023, 184, 114966. [Google Scholar] [CrossRef]
- Han, Y.; Zhu, L.; Zhang, H.; Liu, T.; Wu, G. Understanding the foam stability mechanisms of complex formed by soy protein isolate and different charged polysaccharides: Air/water interfacial behavior and rheological characteristics. Int. J. Biol. Macromol. 2024, 268, 131583. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, Z.; Zhang, C.; Zheng, B.; Tian, Y. Effects of microwave-vacuum pre-treatment with different power levels on the structural and emulsifying properties of lotus seed protein isolates. Food Chem. 2020, 311, 125932. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Chi, Y.; Chi, Y. High-temperature glycosylation of saccharides to modify molecular conformation of egg white protein and its effect on the stability of high internal phase emulsions. Food Res. Int. 2024, 176, 113825. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhu, L.; Zhang, H.; Liu, T.; Wu, G. Characteristic of the interaction mechanism between soy protein isolate and functional polysaccharide with different charge characteristics and exploration of the foaming properties. Food Hydrocoll. 2024, 150, 109615. [Google Scholar] [CrossRef]
- Martínez, K.D.; Farías, M.E.; Pilosof, A.M. Effects of soy protein hydrolysis and polysaccharides addition on foaming properties studied by cluster analysis. Food Hydrocoll. 2011, 25, 1667–1676. [Google Scholar] [CrossRef]




| PPI/SA Mass Ratios | 5% PPI/ml | 2% SA/mL | PBS/mL |
|---|---|---|---|
| 1:0 | 20 | 0 | 80 |
| 1:0.1 | 20 | 5 | 75 |
| 1:0.2 | 20 | 10 | 70 |
| 1:0.3 | 20 | 15 | 65 |
| 1:0.4 | 20 | 20 | 60 |
| 1:0.5 | 20 | 25 | 55 |
| Component | Content |
|---|---|
| D-sorbitol | 0.95% |
| White sugar | 7.51% |
| Sucrose syrup | 15.02% |
| Xanthan gum | 0.81% |
| Hydrogenated palm oil | 25% |
| Sodium stearoyl lactylate | 1.08% |
| PPI/SA complexes | Add according to experimental requirements |
| Water | Supplement to 100% |
| PPI/SA Mass Ratios | α-Helix (%) | β-Sheet (%) | β-Turn (%) | Random Coil (%) |
|---|---|---|---|---|
| 1:0 | 24.83 ± 0.06 a | 13.87 ± 0.15 e | 14.64 ± 0.05 c | 44.05 ± 0.15 c |
| 1:0.1 | 23.67 ± 0.07 b | 15.61 ± 1.07 d | 15.42 ± 0.23 bc | 43.92 ± 1.76 bc |
| 1:0.2 | 21.03 ± 0.53 c | 17.94 ± 0.27 c | 17.09 ± 0.81 ab | 40.10 ± 0.35 b |
| 1:0.3 | 20.36 ± 0.71 c | 18.93 ± 0.80 bc | 18.29 ± 1.76 a | 40.97 ± 0.95 b |
| 1:0.4 | 20.79 ± 1.14 c | 19.92 ± 0.34 b | 16.66 ± 0.44 a | 39.60 ± 0.37 a |
| 1:0.5 | 17.72 ± 0.21 d | 25.02 ± 0.56 a | 17.91 ± 0.89 a | 38.19 ± 0.53 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Yang, X.; Diao, J.; Wang, Y.; Wang, C. Exploration of Pea Protein Isolate–Sodium Alginate Complexes as a Novel Strategy to Substitute Sugar in Plant Cream: Synergistic Interactions Between the Two at the Interface. Foods 2025, 14, 991. https://doi.org/10.3390/foods14060991
Sun J, Yang X, Diao J, Wang Y, Wang C. Exploration of Pea Protein Isolate–Sodium Alginate Complexes as a Novel Strategy to Substitute Sugar in Plant Cream: Synergistic Interactions Between the Two at the Interface. Foods. 2025; 14(6):991. https://doi.org/10.3390/foods14060991
Chicago/Turabian StyleSun, Jingru, Xiyuan Yang, Jingjing Diao, Yichang Wang, and Changyuan Wang. 2025. "Exploration of Pea Protein Isolate–Sodium Alginate Complexes as a Novel Strategy to Substitute Sugar in Plant Cream: Synergistic Interactions Between the Two at the Interface" Foods 14, no. 6: 991. https://doi.org/10.3390/foods14060991
APA StyleSun, J., Yang, X., Diao, J., Wang, Y., & Wang, C. (2025). Exploration of Pea Protein Isolate–Sodium Alginate Complexes as a Novel Strategy to Substitute Sugar in Plant Cream: Synergistic Interactions Between the Two at the Interface. Foods, 14(6), 991. https://doi.org/10.3390/foods14060991






