Impact of Pulsed Electric Fields Combined with Dissolved Oxygen and Ferrous Ions on the Aroma and Components of Strong-Flavor Baijiu
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. PEF Treatment
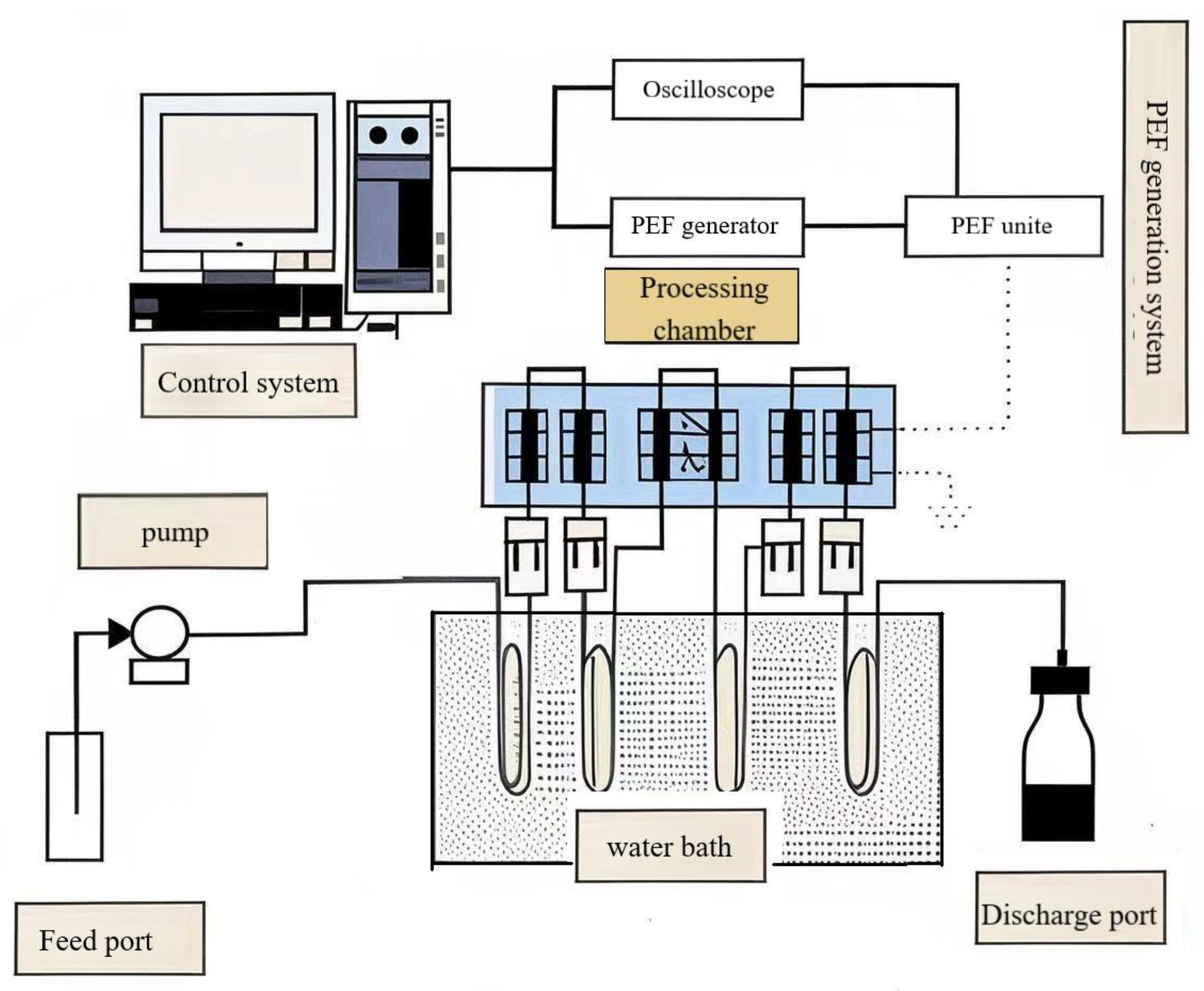
2.2.1. PEF System
2.2.2. Parameter Optimization
2.2.3. Experiment Design of Baijiu Sample Pretreated
2.3. Sensory Analysis
2.3.1. Panelists
2.3.2. Sample Evaluation
2.4. Analysis of Volatile Compounds
2.5. Alcoholic Strength, Electrical Potential, pH, DO, and Conductivity Analysis
2.6. ICP-MS Analysis
2.7. Electron Paramagnetic Resonance (EPR) Spin Trapping
2.8. Statistical Analysis
3. Results
3.1. Effects of PEF Treatment on Physicochemical Parameters of the Strong-Flavor Baijiu
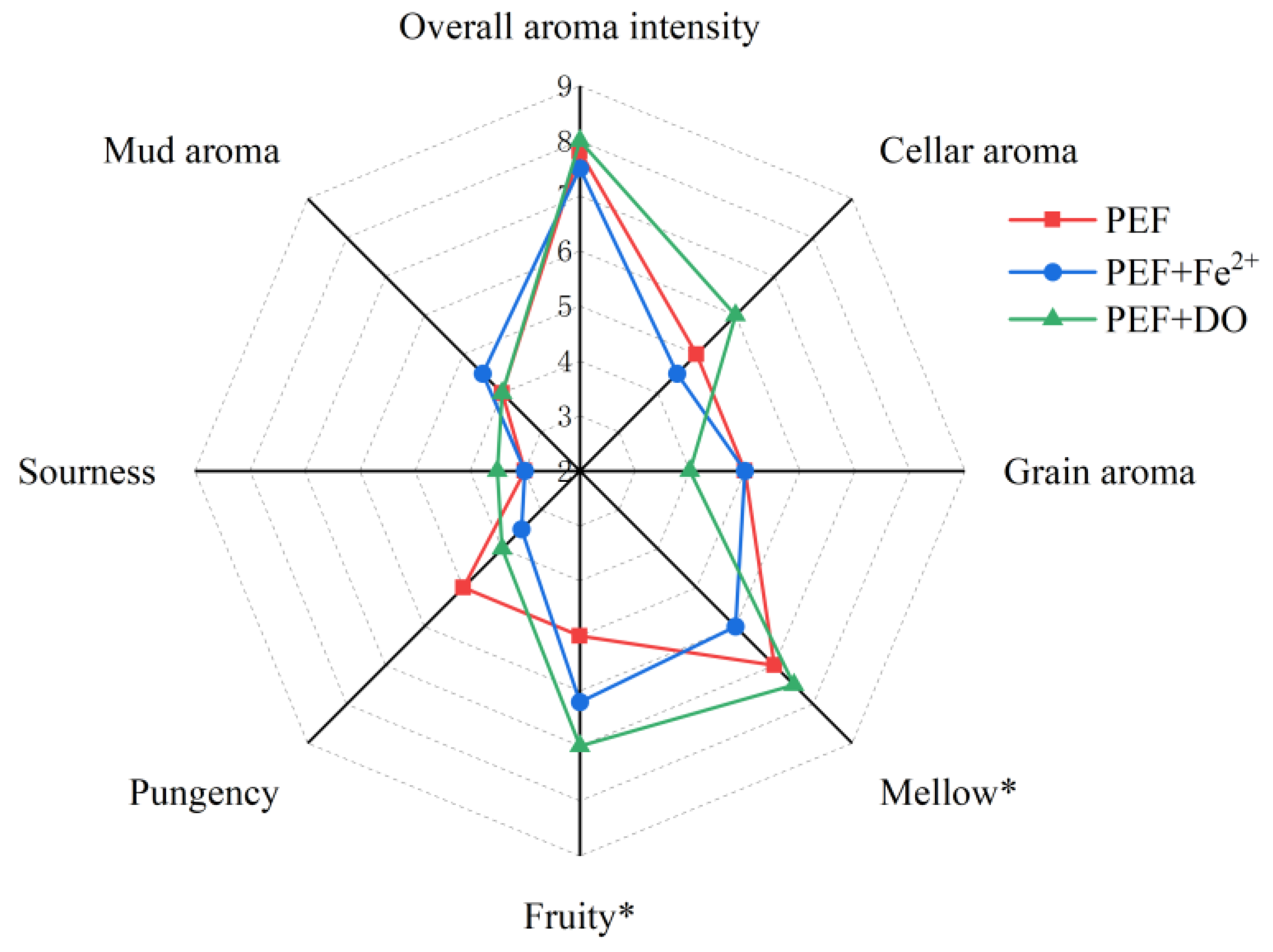
3.2. Effect of PEF Treatment on Baijiu Flavor Perception
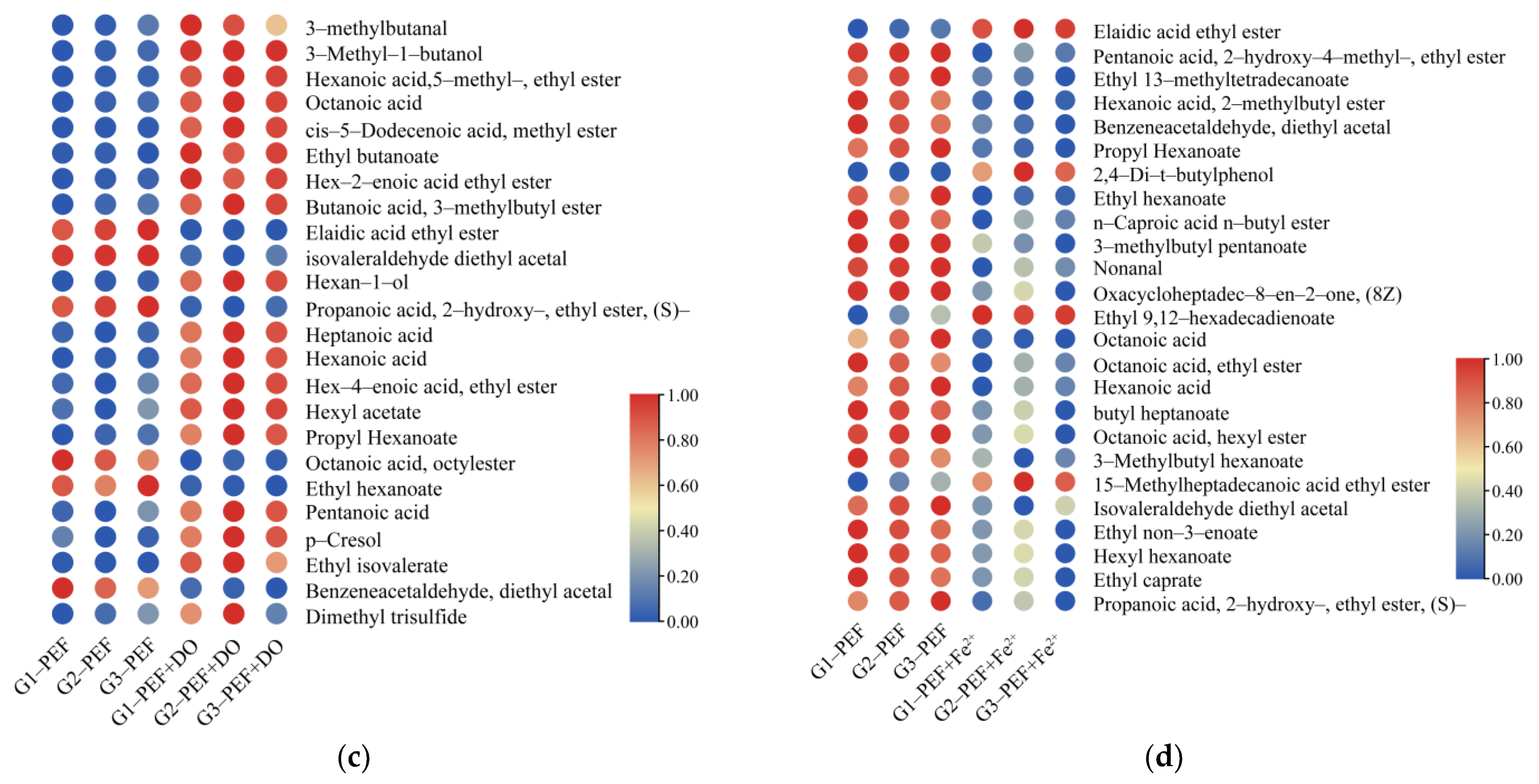
3.3. Effect of Pulse Parameters on the Flavor Compounds of Strong-Flavor Baijiu
3.3.1. Effect of Electric Field Strength and Pulse Frequency on Flavor of Strong-Flavor Baijiu
3.3.2. Correlation Analysis of Electric Field Strength, Pulse Frequency, and Differential Compound
3.4. Effect of Oxidant Combined with PEF on the Strong-Flavor Baijiu
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
| Compound Name | Blank (mg/L) | S1 (mg/L) | S2 (F2) (mg/L) | S3 (mg/L) | F1 (mg/L) | F3 (mg/L) |
|---|---|---|---|---|---|---|
| 3-methylbutyl pentanoate | 1.688 | 2.007 | 2.239 | 2.124 | 2.474 | 2.316 |
| Ethyl heptanoate | 348.671 | 234.362 | 263.123 | 242.753 | 297.115 | 264.634 |
| Pentyl Hexanoate | 40.843 | 24.228 | 24.708 | 23.626 | 35.625 | 29.245 |
| Propyl n-Octanoate | 4.186 | 2.377 | 2.477 | 2.725 | 3.085 | 2.983 |
| Hexyl hexanoate | 193.759 | 118.620 | 133.816 | 130.156 | 170.265 | 155.251 |
| Ethyl myristate | 23.994 | 14.210 | 15.430 | 13.204 | 19.178 | 17.115 |
| Ethyl valerate | 69.105 | 124.348 | 106.662 | 127.159 | 109.528 | 115.971 |
| Ethyl formate | 0.372 | 0.471 | 0.507 | 0.209 | 0.172 | 0.619 |
| Ethyl acetate | 59.371 | 112.534 | 112.723 | 95.301 | 90.590 | 92.946 |
| Ethyl propionate | 1.162 | 1.162 | 1.162 | 1.162 | - | - |
| Ethyl isobutyrate | 0.423 | 1.629 | 1.263 | 1.996 | 2.609 | 2.399 |
| Ethyl butyrate | 57.685 | 103.360 | 95.648 | 105.311 | 94.441 | 106.623 |
| Ethyl 2-methylbutyrate | 0.809 | 0.997 | 0.201 | 1.611 | 1.369 | 0.621 |
| Ethyl isovalerate | 1.148 | 4.184 | 4.307 | 3.259 | 2.138 | 1.352 |
| Isoamyl acetate | 3.519 | 5.322 | 5.084 | 6.516 | 5.488 | 6.410 |
| Ethyl pyruvate | 2.555 | 2.468 | 2.201 | 4.034 | 2.571 | 2.518 |
| Ethyl hexanoate | 1170.524 | 1206.133 | 1251.237 | 1217.046 | 1264.031 | 1261.510 |
| Isoamyl butyrate | 4.105 | 3.635 | 3.371 | 3.579 | 3.424 | 3.828 |
| Hexyl acetate | 7.506 | 6.819 | 7.589 | 8.093 | 7.306 | 7.197 |
| Ethyl cyclopropanepropionate | 0.830 | 0.287 | 0.419 | 0.626 | 0.135 | - |
| Ethyl 5-methylhexanoate | 4.005 | 1.536 | 5.301 | 5.090 | 4.654 | 5.446 |
| Ethyl 3-hexenoate | 0.479 | 1.128 | 0.991 | - | 1.055 | 0.987 |
| Butyl valerate | 3.118 | 1.652 | 1.795 | 3.381 | 0.108 | 0.360 |
| Propyl hexanoate | 35.818 | 27.427 | 30.692 | 27.106 | 26.679 | 34.411 |
| Ethyl hexadienoate | 1.498 | 0.666 | 0.554 | 1.117 | 1.663 | 1.070 |
| Isobutyl hexanoate | 9.020 | 1.268 | - | - | 8.555 | - |
| Ethyl lactate | 9.536 | 14.838 | 13.251 | 10.544 | 11.834 | 12.452 |
| Heptyl acetate | 0.333 | 0.189 | - | - | - | - |
| Butyl hexanoate | 86.300 | 69.305 | 32.877 | 59.224 | 67.344 | 72.534 |
| Ethyl octanoate | 539.066 | 383.160 | 431.965 | 445.513 | 492.647 | 545.636 |
| Isoamyl hexanoate | 60.389 | 51.464 | 52.187 | 49.559 | 57.250 | 64.045 |
| Ethyl 7-octenoate | 0.353 | 0.252 | 0.294 | 0.091 | 0.280 | 0.323 |
| Ethyl nonanoate | 28.989 | 19.477 | 25.425 | 21.802 | 25.755 | 27.996 |
| Ethyl 2-hydroxy-4-methylpentanoate | 2.439 | 3.925 | 3.430 | 3.353 | 3.288 | 3.646 |
| Isobutyl heptanoate | 0.465 | 0.770 | 0.473 | 0.279 | 0.912 | 0.365 |
| Isobutyl octanoate | 1.066 | 0.947 | 0.953 | 1.117 | 1.164 | 1.225 |
| Ethyl 3-nonenoate | 0.677 | 1.223 | 1.396 | 1.647 | 1.466 | 1.691 |
| Octyl butyrate | 0.373 | 0.357 | 0.373 | 0.367 | 0.458 | 0.457 |
| Ethyl decanoate | 56.115 | 33.791 | 42.831 | 45.291 | 47.867 | 44.538 |
| Isoamyl octanoate | 5.173 | 3.660 | 4.377 | 4.622 | 4.324 | 5.168 |
| Diethyl succinate | 1.593 | 2.684 | 2.316 | 2.126 | 2.337 | 2.677 |
| Ethyl 4-decenoate | 0.287 | 0.097 | 0.095 | 0.119 | - | - |
| Butyl heptanoate | 15.740 | 4.652 | 5.758 | 5.205 | 5.481 | 5.481 |
| Ethyl undecanoate | 2.149 | 0.785 | 1.142 | 1.349 | 1.046 | 1.373 |
| Ethyl 2-decenoate | 0.496 | 0.353 | 0.370 | 0.413 | 0.385 | 0.458 |
| Ethyl phenylacetate | 6.967 | 9.960 | 10.421 | 10.783 | 11.475 | 12.405 |
| Hexyl octanoate | 18.397 | 16.506 | 18.701 | 14.839 | 19.805 | 21.311 |
| Ethyl laurate | 24.536 | 3.556 | 12.572 | 21.615 | 17.119 | 6.766 |
| Ethyl 3-phenylpropionate | 7.525 | 9.245 | 11.140 | 10.896 | 11.533 | 11.648 |
| Ethyl tridecanoate | 1.067 | 0.737 | 0.739 | 0.723 | 0.869 | 0.915 |
| Ethyl 4-phenylbutyrate | - | 0.727 | 0.739 | 0.757 | 0.845 | 0.829 |
| Octyl octanoate | 0.654 | 0.497 | 0.502 | 0.477 | 0.576 | 0.667 |
| Ethyl pentadecanoate | 3.340 | 2.067 | 2.177 | 2.298 | 1.987 | 2.599 |
| Methyl palmitate | 0.079 | 0.246 | 0.232 | 0.281 | 0.270 | 0.315 |
| Ethyl palmitate | 156.714 | 98.682 | 99.126 | 114.452 | 106.419 | 115.440 |
| Ethyl 9-hexadecenoate | 9.645 | 5.471 | 6.320 | 6.188 | 5.866 | 6.968 |
| Ethyl 15-methylheptadecanoate | 0.629 | 0.286 | 0.296 | 0.223 | 0.244 | 0.096 |
| Ethyl oleate | 28.083 | 13.952 | 15.010 | 14.786 | 13.586 | 14.314 |
| Ethyl linoleate | 31.557 | 15.490 | 16.625 | 15.487 | 14.238 | 15.593 |
| Dibutyl phthalate | 0.224 | 0.873 | 1.130 | 1.568 | 1.061 | 1.455 |
| Propyl octanoate | 4.186 | 2.377 | 2.477 | 2.725 | 2.650 | 3.236 |
| Ethyl undecanoate | 2.149 | 0.785 | 1.142 | 1.349 | 1.046 | 1.373 |
| Ethyl 2-decenoate | 0.496 | 0.353 | 0.370 | 0.413 | 0.385 | 0.458 |
| Ethyl phenylacetate | 6.967 | 9.960 | 10.421 | 10.783 | 11.475 | 12.405 |
| Hexyl octanoate | 18.397 | 16.506 | 18.701 | 14.839 | 19.805 | 21.311 |
| Ethyl palmitate | 156.714 | 98.682 | 99.126 | 114.452 | 106.419 | 115.440 |
| Ethyl 9-hexadecenoate | 9.645 | 5.471 | 6.320 | 6.188 | 5.866 | 6.968 |
| Ethyl oleate | 28.083 | 13.952 | 15.010 | 14.786 | 13.586 | 14.314 |
| Ethyl linoleate | 31.557 | 15.490 | 16.625 | 15.487 | 14.238 | 15.593 |
| Butyl valerate | 3.118 | 1.652 | 1.795 | 3.381 | 0.108 | 0.360 |
| Acetaldehyde | 0.327 | 0.477 | 0.600 | 0.714 | 0.469 | 0.675 |
| Isovaleraldehyde | 3.576 | 5.371 | 5.545 | 6.509 | 5.626 | 6.706 |
| Isobutyraldehyde | 0.225 | 0.446 | 0.447 | 0.402 | 0.394 | 0.470 |
| Nonanal | 1.301 | 0.466 | 0.266 | 1.075 | 1.075 | 1.075 |
| Butyraldehyde | - | 0.157 | 0.324 | 0.363 | - | - |
| 2-Nonanone | 1.324 | 1.233 | 1.224 | 1.559 | 1.544 | 1.108 |
| 2-Undecanone | 0.828 | 0.512 | 0.587 | 0.649 | 0.682 | 0.723 |
| 2-Pentadecanone | 0.720 | 0.511 | 0.511 | 0.477 | 0.574 | 0.717 |
| Fitone | 0.508 | 0.314 | 0.277 | 0.449 | 0.221 | 0.384 |
| 2-Nonen-4-one | 0.185 | - | 0.158 | - | - | - |
| Benzeneacetaldehyde, diethyl acetal | 0.634 | 1.556 | 1.597 | 1.342 | 1.446 | 1.925 |
| Decane, 1,1-diethoxy- | 0.005 | 0.639 | 0.728 | 0.648 | 0.777 | 0.837 |
| 1,1-diethoxynonane | 1.933 | 2.781 | 3.118 | 2.759 | 2.782 | 3.626 |
| Nonanoic acid | 7.760 | 15.258 | 21.191 | 11.021 | 12.849 | 25.987 |
| Acetic acid | 1.933 | 2.781 | 3.118 | 2.759 | 3.158 | 3.980 |
| Isobutyric acid | 4.761 | 3.808 | 3.681 | 4.290 | 4.222 | 4.638 |
| Butyric acid | 1.048 | 4.010 | 2.852 | - | 0.650 | 3.162 |
| Valeric acid | 1.858 | 2.831 | 2.417 | 2.380 | 2.419 | 2.566 |
| Hexanoic acid | 72.065 | 141.949 | 111.350 | 103.087 | 102.015 | 120.088 |
| Heptanoic acid | 5.540 | 8.184 | 7.818 | 5.644 | 6.423 | 9.758 |
| Octanoic acid | 7.760 | 15.258 | 21.191 | 12.086 | 14.749 | 27.998 |
| Nonanoic acid | - | 0.145 | - | 0.176 | 0.239 | 0.059 |
| 3-Hydroxylauric acid | 0.036 | 0.072 | 0.189 | 0.134 | - | - |
| α-Linolenic acid | 0.479 | 0.442 | 0.124 | 0.478 | 0.444 | 0.445 |
| 2-Dodecenoic acid | 0.474 | 1.148 | 1.262 | 1.555 | 1.323 | 1.497 |
| 5-Dodecenoic acid | - | 0.524 | 0.534 | 1.504 | 1.504 | 1.504 |
| 9-Hexadecenoic acid | - | 0.070 | 0.232 | 0.263 | 0.074 | 0.110 |
| 13-Octadecenoic acid | - | 0.090 | 0.174 | 0.158 | 0.238 | 0.363 |
| 17-Octadecynoic acid | - | 0.393 | - | - | - | - |
| Eicosenoic acid | 0.605 | 0.432 | 0.333 | 0.364 | 0.263 | 1.144 |
| Hexanoic anhydride | 5.516 | 6.436 | 7.460 | 6.499 | 8.178 | 7.740 |
| 14-Pentadecanoic acid | - | 0.188 | 0.448 | 0.397 | 0.452 | 0.166 |
| Ethanol | 103.724 | 160.902 | 130.184 | 234.262 | 232.228 | 155.995 |
| Hexanol | 19.775 | 36.768 | 36.902 | 36.275 | 34.207 | 43.713 |
| Isobutanol | 1.103 | 2.400 | 1.050 | 1.197 | 1.390 | 1.699 |
| 2-Propyl-1-pentanol | - | 0.298 | 0.328 | 0.211 | 0.526 | 0.238 |
| 3-Methyl-1-heptanol | - | 1.372 | 3.318 | 2.759 | 1.376 | - |
| 2-Ethylhexanol | - | - | 0.171 | 0.589 | 0.302 | 0.574 |
| Isoamyl alcohol | - | 10.298 | 21.137 | 6.880 | - | - |
| 2-Heptanol | - | 0.278 | - | - | 0.316 | - |
| 2-[(Z)-9-Octadecenyloxy]ethanol | 0.324 | 0.089 | 0.075 | 0.058 | - | 0.200 |
| 2-Methyl-1-propanol | - | 0.204 | 0.218 | - | - | - |
| Methanol | 0.320 | 0.129 | 0.317 | 0.683 | 0.126 | 0.696 |
| Propanol | - | 0.108 | 0.355 | 0.209 | 0.314 | 0.154 |
| Butanol | 0.167 | 1.887 | 0.428 | 0.988 | 0.380 | 1.527 |
| Pentanol | 0.431 | 0.431 | 0.431 | 0.431 | 0.431 | 0.431 |
| sec-Butanol | - | 0.555 | 0.636 | - | 0.454 | 0.645 |
| 2-Heptanol | - | 0.278 | - | - | 0.316 | - |
| p-Cresol | 0.280 | 0.290 | - | 0.210 | 0.089 | 0.322 |
| 2,4-Di-t-butylphenol | 6.512 | 5.731 | 16.466 | 5.701 | 19.059 | 19.406 |
| 2-(12-Pentadecynyloxy)tetrahydro-2H-pyran | - | 0.242 | 0.783 | 0.740 | 0.661 | 0.958 |
| 2-Methylphenol | - | - | - | - | 4.428 | 10.754 |
| 2,6-Di-tert-butyl-p-cresol | - | 0.122 | 0.311 | 0.094 | 0.097 | 0.491 |
| Guaiacol | - | 0.264 | - | 0.591 | 0.212 | 0.667 |
| Name | Pearson’s r | p Value |
|---|---|---|
| Octanoic acid | 0.2288 | 0.012 |
| Hexanoic acid | 0.1040 | 0.018 |
| 1,1-diethoxynonane | 0.2706 | 0.007 |
| 2-(12-Pentadecynyloxy) tetrahydro-2H-pyran | 0.1336 | 0.018 |
| Ethyl caprate | 0.1758 | 0.011 |
| Hexyl hexanoate | 0.3733 | 0.001 |
| Decane, 1,1-diethoxy- | 0.2290 | 0.010 |
| Hexan-1-ol | 0.1239 | 0.038 |
| Hexanoic acid, anhydride | 0.2752 | 0.003 |
| Hexanoic acid,5-methyl-, ethyl ester | 0.1240 | 0.009 |
| 1,1-diethoxynonane | 0.2484 | 0.010 |
| Ethyl caprate | 0.1332 | 0.012 |
| Hexyl hexanoate | 0.3357 | 0.003 |
| Decane, 1,1-diethoxy- | 0.2026 | 0.015 |
| Butyl valerate | 0.0846 | 0.048 |
| Hexanoic acid, anhydride | 0.2961 | 0.002 |
References
- Qiao, L.; Wang, J.; Wang, R. A review on flavor of Baijiu and other world-renowned distilled liquors. Food Chem. X 2023, 20, 100870. [Google Scholar] [CrossRef]
- Jia, W.; Ma, R.; Hu, L.B.; Mo, H. Synergy of physicochemical reactions occurred during aging for harmonizing and improving flavor. Food Chem. X 2022, 17, 100554. [Google Scholar] [CrossRef] [PubMed]
- Shui, Z.; Zhao, J.; Li, Y. Fast identification of Baijius based on organic acid response colorimetric sensor array. J. Food Compos. Anal. 2025, 137, 106862. [Google Scholar] [CrossRef]
- Sun, Q.; Yang, R.; Wu, L. High-voltage pulsed electric field has sterilization and aging effects on fermented orange vinegar. Sci. Technol. Food Ind. 2015, 12, 133–137. [Google Scholar]
- Jia, W.; Fan, Z.; Du, A. Untargeted foodomics reveals molecular mechanism of magnetic field effect on Feng-flavor Baijiu ageing. Food Res. Int. 2021, 149, 110681. [Google Scholar] [CrossRef]
- Rosellini, T.K.; Aline, A.; Alessandro, N. Current Technologies to Accelerate the Aging Process of Alcoholic Beverages: A Review. Beverages 2022, 8, 65. [Google Scholar] [CrossRef]
- Parisa, M.G.; Shima, J.; Jia, G. Ozone in wineries and wine processing: A review of the benefits, application, and perspectives. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3129–3152. [Google Scholar]
- Andreou, V.; Giannoglou, M.; Xanthou, M.Z.; Metafa, M.; Katsaros, G. Aging acceleration of balsamic vinegar applying micro-oxygenation technique. Food Chem. 2023, 419, 136077. [Google Scholar] [CrossRef]
- Puértolas, E.; López, N.; Condón, S. Potential applications of PEF to improve red wine quality. Trends Food Sci. Technol. 2010, 21, 247–255. [Google Scholar] [CrossRef]
- Bai, C.X.; Yang, Y. Pulsed electric fields as an alternative to thermal processing for preservation of nutritive and physicochemical properties of beverages: A review. J. Food Process Eng. 2018, 41, 12638. [Google Scholar]
- Zi, L.; Pang, Z.; Wen, M. Pulsed electric field processing of green tea-infused chardonnay wine: Effects on physicochemical properties, antioxidant activities, phenolic and volatile compounds. Food Biosci. 2023, 54, 102884. [Google Scholar]
- Toulaki, A.K.; Bozinou, E.; Athanasiadis, V. Accelerating Xinomavro Red Wine Flavor Aging Using a Pulsed Electric Field and Various Wood Chips. Appl. Sci. 2023, 13, 12844. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, W.; Yan, W. Effect of pulsed electric field pretreatment on oil content of potato chips. LWT 2021, 135, 110198. [Google Scholar] [CrossRef]
- Lin, Z.; Zeng, X.; Yu, S. Enhancement of Ethanol–Acetic Acid Esterification Under Room Temperature and Non-catalytic Condition via Pulsed Electric Field Application. Food Bioprocess Technol. 2011, 5, 2637–2645. [Google Scholar]
- Yu, Q.; Zeng, X. Effect of PEF treatments on enhancing the chelation reaction between glycine and copper sulfate. Food Ferment. Ind. 2013, 10, 69–72. [Google Scholar]
- Del, A.S.; Pando, M.; Nevares, V. Investigation and correction of the interference of ethanol, sugar and phenols on dissolved oxygen measurement in wine. Anal. Chim. Acta 2014, 809, 162–173. [Google Scholar]
- Zhang, Q.; Whang, Z.; Xiong, A. Elucidating oxidation-based flavour formation mechanism in the aging process of Chinese distilled spirits by electrochemistry and UPLC-Q-Orbitrap-MS/MS. Food Chem. 2021, 355, 129596. [Google Scholar]
- Elias, R.J.; Mogens, L.A.; Leif, H.S. Identification of Free Radical Intermediates in Oxidized Wine Using Electron Paramagnetic Resonance Spin Trapping. J. Agric. Food Chem. 2009, 57, 4359–4365. [Google Scholar] [CrossRef]
- Qing, Z.; Yuan, S.; Xue, F. Free radical generation induced by ultrasound in red wine and model wine: An EPR spin-trapping study. Ultrason. Sonochem. 2015, 27, 96–101. [Google Scholar]
- Xie, F.; Mao, H.; Lin, C.; Feng, Y.; Stoddart, J.F.; Young, R.M.; Wasielewski, M.R. Quantum Sensing of Electric Fields Using Spin-Correlated Radical Ion Pairs. J. Am. Chem. Soc. 2023, 145, 14922–14931. [Google Scholar]
- Deng, Y.; Xiong, A.; Zhao, K. Mechanisms of the regulation of ester balance between oxidation and esterification in aged Baijiu. Sci. Rep. 2020, 10, 17169. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.S.; Martínez, G.A.; Bueno, H.M. Kinetics of oxygen consumption, a key factor in the changes of young wines composition. LWT 2023, 182, 114786. [Google Scholar]
- Zhao, W.; Yang, R.; Gu, Y.; Tang, Y.; Li, C. Assessment of pulsed electric fields induced cellular damage in Saccharomyces cerevisiae: Change in performance of mitochondria and cellular enzymes. LWT—Food Sci. Technol. 2014, 58, 55–62. [Google Scholar] [CrossRef]
- He, Y.; Tang, K.; Yu, X. Identification of Compounds Contributing to Trigeminal Pungency of Baijiu by Sensory Evaluation, Quantitative Measurements, Correlation Analysis, and Sensory Verification Testing. J. Agric. food Chem. 2022, 70, 598–606. [Google Scholar] [CrossRef] [PubMed]
- ISO 8586:2012; Sensory analysis-General guidelines for the selection, training and monitoring of selected assessors and expert sensory assessors. International Organization for Standardization: Geneva, Switzerland, 2012.
- Sun, X.; Qian, Q.; Xiong, Y. Characterization of the key aroma compounds in aged Chinese Xiaoqu Baijiu by means of the sensomics approach. Food Chem. 2022, 384, 132452. [Google Scholar]
- Liu, Q.; Zhang, X.; Zheng, L. Machine learning based age-authentication assisted by chemo-kinetics: Case study of strong-flavor Chinese Baijiu. Food Res. Int. 2023, 167, 112594. [Google Scholar]
- GB 10345–2022; Baijiu Analyticl Methods. China Standard Press: Beijing, China, 2018.
- Zhong, P.; Lin, Z.; Liu, Z.; Kong, L. Source, species and determination method of reactive oxygen species in water environment. Ecol. Sci. 2005, 4, 364–367. [Google Scholar]
- Wang, M.S.; Wang, S.N. Study on the Variable-Frequency Electric Field Assisted Aging Mechanism of Liquor. Nucl. Ind. Inst. Chem. Eng. 2021, 7, 22. [Google Scholar]
- Everendilek, G.A. Pulsed Electric Field Processing of Red Wine: Effect on Wine Quality and Microbial Inactivation. Bverages 2022, 8, 78. [Google Scholar]
- Wei, Z.; Yang, X.; Ru, D. An electric-field instrument for accelerated aging to improve flavor of Chinese Baijiu. LWT-Food Sci. Technol. 2023, 174, 114446. [Google Scholar]
- Qing, R.; Liu, X.L. Influence on the volatilization of ethyl esters: Nonnegligible role of long-chain fatty acids on Baijiu flavor via intermolecular interaction. Food Chem. 2024, 436, 137731. [Google Scholar]
- Feng, Y.; Yang, T.; Zhang, Y.; Zhang, A.; Gai, L.; Niu, D. Potential applications of pulsed electric field in the fermented wine industry. Front. Nutr. 2022, 9, 1048632. [Google Scholar] [CrossRef]
- Zhang, Q.A.; Zheng, H.; Lin, J. The state-of-the-art research of the application of ultrasound to winemaking: A critical review. Ultrason. Sonochem. 2023, 95, 106384. [Google Scholar] [CrossRef]
- Martin, J.F.G.; Sun, D.W. Ultrasound and electric fields as novel techniques for assisting the wine ageing process: The state of the art research. Trends in Food Sci. Technol. 2013, 33, 40–53. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, X.; Song, X. Evolution of the key odorants and aroma profiles in traditional Laowuzeng baijiu during its one-year ageing. Food Chem. 2020, 310, 125898. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Chen, X.; Wang, X. Esters and higher alcohols regulation to enhance wine fruity aroma based on oxidation-reduction potential. LWT 2024, 200, 116165. [Google Scholar] [CrossRef]
- Comuzzo, P.; Marconi, M.; Zanella, G. Pulsed electric field processing of white grapes (cv. Garganega): Effects on wine composition and volatile compounds. Food Chem. 2018, 264, 16–23. [Google Scholar] [CrossRef]
- He, J.; Chen, Q.; Jia, X. The effects of gamma irradiation and natural aging on the composition of Nongxiangxing baijiu. J. Food Process. Preserv. 2022, 46, 1. [Google Scholar] [CrossRef]
- Cheng, Z.; Xiao, L.; Arshad, R.N. Pulsed electric field as a promising technology for solid foods processing: A review. Food Chem. 2023, 403, 134367. [Google Scholar]
- Zheng, Q.; Hu, Y.; Xiong, A.; Su, Y.; Wang, Z.; Zhao, K.; Yu, Y. Elucidating metal ion-regulated flavour formation mechanism in the aging process of Chinese distilled spirits (Baijiu) by electrochemistry, ICP-MS/OES, and UPLC-Q-Orbitrap-MS/MS. Food Funct. 2021, 12, 8899–8906. [Google Scholar] [CrossRef]
- Jang, M.L.; Hu, X.J.; Lei, Y. Research progress of liquor aging. Chin. Brew. 2022, 41, 13–17. [Google Scholar]
- Wei, L.; Hu, J.; Pan, C. Effects of different storage containers on the flavor characteristics of Jiangxiangxing baijiu. Food Res. Int. 2023, 172, 113196. [Google Scholar] [CrossRef]
- Sha, S.; Chen, S.; Qian, M. Characterization of the typical potent odorants in Chinese roasted sesame-like flavor type liquor by headspace solid phase microextraction-aroma extract dilution analysis, with special emphasis on sulfur-containing odorants. J. Agric. Food Chem. 2017, 65, 123–131. [Google Scholar]
- Yan, Y.; Chen, S.; Nie, Y. Characterization of volatile sulfur compounds in soy sauce aroma type Baijiu and changes during fermentation by GC × GC-TOFMS, organoleptic impact evaluation, and multivariate data analysis. Food Res. Int. 2020, 130, 109043. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.J.; Zeng, Y.H.; Liu, W.H.; Wang, S.T. Effects of metals released in strong-flavor baijiu on the evolution of aroma compounds during storage. Food Sci. Nutr. 2020, 8, 1904–1913. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Millero, F.J. The solubility of iron hydroxide in sodium chloride solutions. Geochim. Cosmochim. Acta 1999, 63, 3487–3497. [Google Scholar] [CrossRef]
- Thomas, B.N. Chemistry of Iron in Natural Water. United States Government Printing Office. 1963. Available online: https://pubs.usgs.gov/wsp/1459a/report.pdf (accessed on 19 March 2025).
- Modesti, M.; Macaluso, M.; Taglieri, I. Ozone and Bioactive Compounds in Grapes and Wine. Foods 2021, 10, 2934. [Google Scholar] [CrossRef]






| Factors | CK | S1 | S2 (F2) | S3 | F1 | F3 |
|---|---|---|---|---|---|---|
| Electric field strength (kV/cm) | - | 15 | 25 | 35 | 25 | 25 |
| Pulse frequency (Hz) | - | 350 | 350 | 350 | 200 | 500 |
| Alcohol Content (%vol) | 67.20 ± 0.70 b | 69.47 ± 0.55 b | 65.10 ± 1.10 a | 67.73 ± 0.95 ab | 66.08 ± 1.00 a | 66.03 ± 0.15 a |
| Conductivity (μS/cm) | 11.80 ± 0.07 a | 13.57 ± 0.80 b | 15.10 ± 0.96 b | 13.97 ± 0.12 b | 17.47 ± 0.74 c | 13.90 ± 0.12 b |
| pH | 3.98 ± 0.20 a | 3.92 ± 0.05 a | 3.94 ± 0.07 a | 3.94 ± 0.02 a | 4.02 ± 0.08 a | 3.94 ± 0.02 a |
| Dissolve oxygen (mg/L) | 7.34 ± 0.14 a | 7.34 ± 0.14 a | 7.58 ± 0.05 b | 7.50 ± 0.09 b | 7.42 ± 0.08 ab | 7.50 ± 0.09 b |
| Electrical potential (mV) | 175.00 ± 3.34 a | 175.83 ± 4.34 a | 172.40 ± 2.63 a | 177.27 ± 3.68 a | 177.27 ± 1.20 a | 177.27 ± 3.68 a |
| Categories (mg/L) | Esters | Alcohols | Acids | Aromatics | Aldehydes and Ketones |
|---|---|---|---|---|---|
| CK | 2707.3 ± 238.92 a | 124.92 ± 14.8 a | 96.11 ± 3.69 a | 17.7 ± 9.47 a | 10.65 ± 1.26 a |
| S1 | 3167.31 ± 363.68 a | 200.39 ± 11.57 b | 181.32 ± 27.57 d | 21.76 ± 0.35 b | 12.86 ± 0.19 a |
| S2 (F2) | 2901.81 ± 208.24 a | 271.73 ± 44.61 c | 132.36 ± 16.36 b | 24.37 ± 9.9 a | 15.99 ± 0.52 b |
| S3 | 2883.73 ± 629.44 a | 168.45 ± 6.75 b | 155.75 ± 1.81 c | 26.7 ± 0.68 c | 13.52 ± 1.71 a |
| F1 | 3028.13 ± 152.9 a | 267.93 ± 23.4 c | 137.14 ± 10.3 b | 26.02 ± 1.67 c | 13.57 ± 1.75 a |
| F3 | 3897.49 ± 281.74 b | 201.8 ± 22.02 b | 181.9 ± 19.2 d | 34.09 ± 0.89 d | 16.66 ± 1.54 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.; Zhou, Z.; Huang, M.; Ji, Z.; Qin, H.; Mao, J. Impact of Pulsed Electric Fields Combined with Dissolved Oxygen and Ferrous Ions on the Aroma and Components of Strong-Flavor Baijiu. Foods 2025, 14, 1097. https://doi.org/10.3390/foods14071097
Lu J, Zhou Z, Huang M, Ji Z, Qin H, Mao J. Impact of Pulsed Electric Fields Combined with Dissolved Oxygen and Ferrous Ions on the Aroma and Components of Strong-Flavor Baijiu. Foods. 2025; 14(7):1097. https://doi.org/10.3390/foods14071097
Chicago/Turabian StyleLu, Jin, Zhilei Zhou, Mengyang Huang, Zhongwei Ji, Hui Qin, and Jian Mao. 2025. "Impact of Pulsed Electric Fields Combined with Dissolved Oxygen and Ferrous Ions on the Aroma and Components of Strong-Flavor Baijiu" Foods 14, no. 7: 1097. https://doi.org/10.3390/foods14071097
APA StyleLu, J., Zhou, Z., Huang, M., Ji, Z., Qin, H., & Mao, J. (2025). Impact of Pulsed Electric Fields Combined with Dissolved Oxygen and Ferrous Ions on the Aroma and Components of Strong-Flavor Baijiu. Foods, 14(7), 1097. https://doi.org/10.3390/foods14071097







