Current Progress and Future Trends of Genomics-Based Techniques for Food Adulteration Identification
Abstract
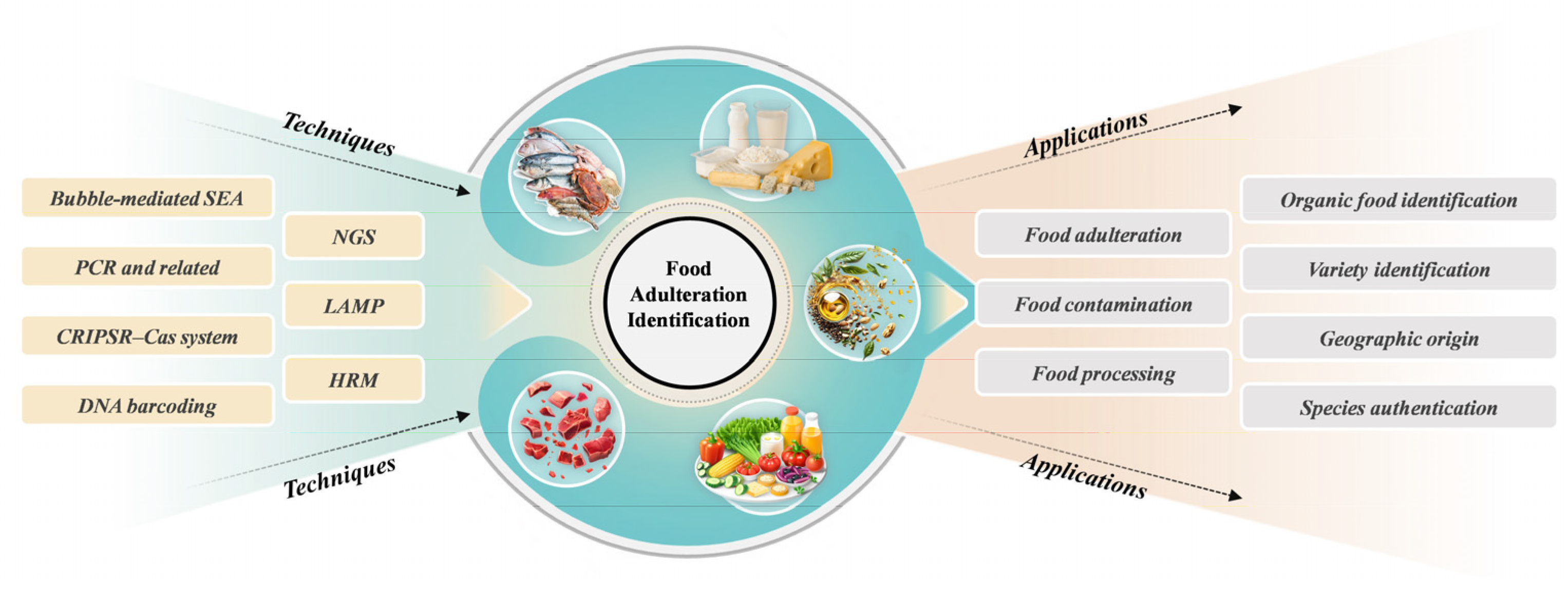
1. Introduction
2. Genomics Approaches
2.1. Traditional PCR Technology and Its Extensions
2.1.1. PCR-RFLP
2.1.2. Multiplex PCR
2.1.3. qRT-PCR
2.1.4. ddPCR
2.2. Next-Generation Sequencing (NGS)
2.3. DNA Barcoding

2.4. HRM
2.5. Loop-Mediated Isothermal Amplification (LAMP)
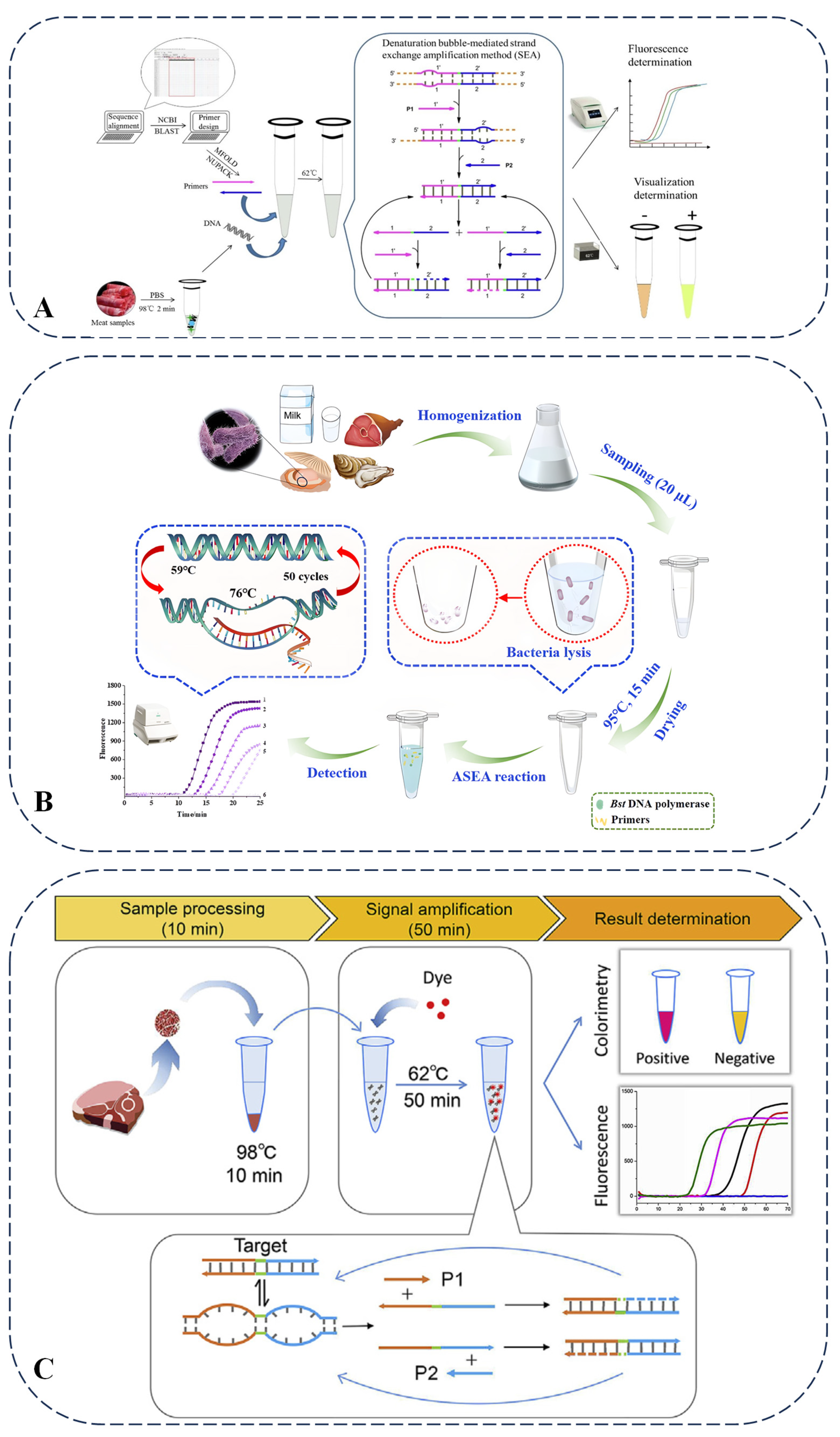
2.6. Bubble-Mediated SEA (Strand Exchange Amplification)
2.7. CRISP–CAS System
2.8. Other Techniques

3. Identification of Food Product Authentication
3.1. Meat Products
3.2. Aquatic Food Products
3.3. Milk and Dairy Products
3.4. Oils Products
3.5. Other Food Products
4. Challenges and Prospectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Elliott, C.T.; Petchkongkaew, A.; Wu, D. The classification, detection and ‘SMART’ control of the nine sins of tea fraud. Trends Food Sci. Technol. 2024, 149, 104565. [Google Scholar]
- Joenperä, J.; Vainio, A.; Lundén, J. External and internal food fraud prevention in Finnish food businesses. Food Control 2024, 164, 110496. [Google Scholar]
- Sharma, R.; Nath, P.C.; Lodh, B.K.; Mukherjee, J.; Mahata, N.; Gopikrishna, K.; Tiwari, O.N.; Bhunia, B. Rapid and sensitive approaches for detecting food fraud: A review on prospects and challenges. Food Chem. 2024, 454, 139817. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Zhou, C.; Zhu, Y.; Dong, Y.; He, Q.; Khan, M.R.; Chi, Y.; Busquets, R.; Deng, R.; Ren, Y. Tb3+-nucleic acid probe-based label-free and rapid detection of mercury pollution in food. Food Sci. Hum. Wellness 2024, 13, 993–998. [Google Scholar] [CrossRef]
- Chukwugozie, D.C.; Njoagwuani, E.I.; David, K.; Okonji, B.A.; Milovanova, N.; Akinsemolu, A.A.; Mazi, I.M.; Onyeaka, H.; Winnall, L.; Ghosh, S. Combatting food fraud IN SUB-SAHARAN Africa: Strategies for Strengthened safety and security. Trends Food Sci. Technol. 2024, 150, 104575. [Google Scholar]
- Bhat, M.A.; Rather, M.Y.; Singh, P.; Hassan, S.; Hussain, N. Advances in smart food authentication for enhanced safety and quality. Trends Food Sci. Technol. 2025, 155, 104800. [Google Scholar] [CrossRef]
- Yu, Y.; Chai, Y.; Yan, Y.; Li, Z.; Huang, Y.; Chen, L.; Dong, H. Near-infrared spectroscopy combined with support vector machine for the identification of Tartary buckwheat (Fagopyrum tataricum (L.) Gaertn) adulteration using wavelength selection algorithms. Food Chem. 2025, 463, 141548. [Google Scholar]
- Tang, X.; Lu, M.; Wang, J.; Man, S.; Peng, W.; Ma, L. Recent Advances of DNA-Templated Metal Nanoclusters for Food Safety Detection: From Synthesis, Applications, Challenges, and Beyond. J. Agric. Food Chem. 2024, 72, 5542–5554. [Google Scholar] [CrossRef]
- Tao, W.; Zhang, H.; Jiang, X.; Chen, N. Resveratrol combats chronic diseases through enhancing mitochondrial quality. Food Sci. Hum. Wellness 2024, 13, 597–610. [Google Scholar]
- Goyal, R.; Singha, P.; Singh, S.K. Spectroscopic food adulteration detection using machine learning: Current challenges and future prospects. Trends Food Sci. Technol. 2024, 146, 104377. [Google Scholar]
- González-Domínguez, R.; Sayago, A.; Morales, M.T.; Fernández-Recamales, Á. Assessment of virgin olive oil adulteration by a rapid luminescent method. Foods 2019, 8, 287. [Google Scholar] [CrossRef]
- Rahman, M.M.; Wang, S.; Xu, J.; Zhang, X.; Zhang, X.; Chingin, K. Rapid analysis of untreated food samples by gel loading tip spray ionization mass spectrometry. Anal. Bioanal. Chem. 2024, 416, 4435–4445. [Google Scholar] [CrossRef]
- Mazumder, A.; Ghosh, S.K. Rapid seafood fraud detection powered by multiple technologies: Food authenticity using DNA-QR codes. J. Food Compos. Anal. 2024, 131, 106204. [Google Scholar] [CrossRef]
- Okoye, C.O.; Jiang, H.; Nazar, M.; Tan, X.; Jiang, J. Redefining modern food analysis: Significance of omics analytical techniques integration, chemometrics and bioinformatics. TrAC Trends Anal. Chem. 2024, 175, 117706. [Google Scholar] [CrossRef]
- Ye, H.; Xu, H.; Xu, J.; Liang, J.; Huang, T.; Wang, X. A novel rapid detection method for chicken adulteration based on recombinant polymerase amplification and multicomponent nuclease (MNAzyme). Microchem. J. 2024, 204, 111148. [Google Scholar] [CrossRef]
- Deng, R.; Xu, L.; Zhang, Y.; Zhang, X.; Yuan, Z.; Chen, J.; Xia, X. CRISPR-based nucleic acid assays for food authentication. Trends Food Sci. Technol. 2024, 145, 104351. [Google Scholar] [CrossRef]
- Shawky, E.; Nahar, L.; Nassief, S.M.; Sarker, S.D.; Ibrahim, R.S. Dairy products authentication with biomarkers: A comprehensive critical review. Trends Food Sci. Technol. 2024, 147, 104445. [Google Scholar] [CrossRef]
- Ji, C.; He, Y.H.; Xing, Y.Y.; Liu, W.; Xie, Y.X.; Ba, H.R.; Yang, M.; He, X.H.; Zheng, W.J.; Lu, X.A. Development of a PCR-based lateral flow immunoassay for the identification of rainbow trout ingredient in foods. Food Control 2023, 154, 110034. [Google Scholar] [CrossRef]
- Kim, K.H.; Kang, T.S. Development of advanced PCR-based methods for accurate identification and authentication of commercial shrimp products. Food Control 2024, 159, 110288. [Google Scholar] [CrossRef]
- Dąbrowska, A. The use of PCR method for adulteration detection of goat dairy products manufactured by smallholders. Small Rumin. Res. 2024, 240, 107372. [Google Scholar] [CrossRef]
- Pohl, G.; Ie, M.S. Principle and applications of digital PCR. Expert Rev. Mol. Diagn. 2004, 4, 41–47. [Google Scholar] [CrossRef]
- Kang, T.S. Basic principles for developing real-time PCR methods used in food analysis: A review. Trends Food Sci. Technol. 2019, 91, 574–585. [Google Scholar] [CrossRef]
- Sint, D.; Raso, L.; Traugott, M. Advances in multiplex PCR: Balancing primer efficiencies and improving detection success. Methods Ecol. Evol. 2012, 3, 898–905. [Google Scholar] [PubMed]
- Li, B.Y.; Yu, M.X.; Xu, W.P.; Chen, L.; Han, J. Comparison of PCR techniques in adulteration identification of dairy products. Agriculture 2023, 13, 1450. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, S.J.; Park, H. Trend in serological and molecular diagnostic methods for Toxoplasma gondii infection. Eur. J. Med. Res. 2024, 29, 520. [Google Scholar]
- Roig, A.P.; Carmona-Salido, H.; Sanjuán, E.; Fouz, B.; Amaro, C. A multiplex PCR for the detection of Vibrio vulnificus hazardous to human and/or animal health from seafood. Int. J. Food Microbiol. 2022, 377, 109778. [Google Scholar]
- Wibowo, T.; Cahyadi, M.; Pramono, A.; Volkandari, S.D. Evaluation of commercial meat product food label conformity using multiplex PCR assay. Food Control 2023, 149, 109712. [Google Scholar]
- Feng, J.C.; Lan, H.Z.; Pan, D.D. Triplex-colored nucleic acid lateral flow strip and multiplex polymerase chain reaction coupled method for quantitative identification of beef, pork and chicken. J. Food Compos. Anal. 2023, 123, 105493. [Google Scholar]
- Xu, Q.; Li, J.; Zhang, Z.; Yang, Q.; Zhang, W.; Yao, J.; Zhang, Y.; Zhang, Y.; Guo, Z.; Li, C.; et al. Precise determination of reaction conditions for accurate quantification in digital PCR by real-time fluorescence monitoring within microwells. Biosens. Bioelectron. 2024, 244, 115798. [Google Scholar] [CrossRef]
- Bharati, R.; Sen, M.K.; Kumar, R.; Gupta, A.; Ziarovská, J.; Fernández-Cusimamani, E.; Leuner, O. Systematic identification of suitable reference genes for quantitative real-time PCR analysis in Melissa officinalis L. Plants 2023, 12, 470. [Google Scholar] [CrossRef]
- Ji, L.; Du, X.; Zhang, H.; Ji, F.; Nie, Z.; Xia, Y. Establishment of a multiplex qPCR method for sensitive detection of proteolytic psychrotrophic bacteria in raw milk. Food Biosci. 2024, 62, 105339. [Google Scholar]
- Suh, S.-M.; Kim, E.; Kim, M.-J.; Yang, S.-M.; Kim, H.-Y. Development of real-time PCR method for rapid and accurate detection of Centipedes (Scolopendra mutilans) in food. Food Sci. Biotechnol. 2023, 32, 979–985. [Google Scholar]
- He, Y.X.; Yan, W.; Dong, L.M.; Ma, Y.; Li, C.C.; Xie, Y.B.; Liu, N.; Xing, Z.J.; Xia, W.; Long, L.K.; et al. An effective droplet digital PCR method for identifying and quantifying meat adulteration in raw and processed food of beef (Bos taurus) and lamb (Ovis aries). Front. Sustain. Food Syst. 2023, 7, 1180301. [Google Scholar]
- Huang, J.; Zhai, L.G.; Wang, J.Y.; Sun, X.T.; Wang, B.S.; Wei, Z.H. An evaluation of the sensitivity and applicability of a droplet digital polymerase chain reaction assay to simultaneously detect pseudomonas aeruginosa and pseudomonas fragi in foods. Foods 2024, 13, 1453. [Google Scholar] [CrossRef]
- Nuraeni, U.; Malau, J.; Astuti, R.T.; Dewantoro, A.; Apriori, D.; Lusiana, E.D.; Prasetya, B. Droplet digital PCR versus real-time PCR for in-house validation of porcine detection and quantification protocol: An artificial recombinant plasmid approach. PLoS ONE 2023, 18, e0287712. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, K.; Sarup, P.; Oertelt, L.; Jahoor, A.; Orabi, J. Assessing myBaits target capture sequencing methodology using short-read sequencing for variant detection in oat genomics and breeding. Genes 2024, 15, 700. [Google Scholar] [CrossRef]
- Diaz, M.; Kellingray, L.; Akinyemi, N.; Adefiranye, O.O.; Olaonipekun, A.B.; Bayili, G.R.; Ibezim, J.; du Plessis, A.S.; Houngbédji, M.; Kamya, D.; et al. Comparison of the microbial composition of African fermented foods using amplicon sequencing. Sci. Rep. 2019, 9, 13863. [Google Scholar]
- Baert, L.; McClure, P.; Winkler, A.; Karn, J.; Bouwknegt, M.; Klijn, A. Guidance document on the use of whole genome sequencing (WGS) for source tracking from a food industry perspective. Food Control 2021, 130, 108148. [Google Scholar] [CrossRef]
- Jovel, J.; Patterson, J.; Wang, W.; Hotte, N.; O'Keefe, S.; Mitchel, T.; Perry, T.; Kao, D.; Mason, A.L.; Madsen, K.L.; et al. Characterization of the gut microbiome using 16S or shotgun metagenomics. Front. Microbiol. 2016, 7, 459. [Google Scholar]
- Haynes, E.; Jimenez, E.; Pardo, M.A.; Helyar, S.J. The future of NGS (Next Generation Sequencing) analysis in testing food authenticity. Food Control 2019, 101, 134–143. [Google Scholar]
- Rodriguez, R.; Krishnan, Y. The chemistry of next-generation sequencing. Nat. Biotechnol. 2023, 41, 1709–1715. [Google Scholar]
- Choate, L.A.; Koleilat, A.; Harris, K.; Vidal-Folch, N.; Guenzel, A.; Newman, J.; Peterson, B.J.; Peterson, S.E.; Rice, C.S.; Train, L.J.; et al. Confirmation of insertion, Deletion, and Deletion-Insertion variants detected by next-generation sequencing. Clin. Chem. 2023, 69, 1155–1162. [Google Scholar]
- Flügge, F.; Kerkow, T.; Kowalski, P.; Bornhöft, J.; Seemann, E.; Creydt, M.; Schütze, B.; Günther, U.L. Qualitative and quantitative food authentication of oregano using NGS and NMR with chemometrics. Food Control 2023, 145, 109497. [Google Scholar]
- He, M.Y.; Xing, S.G.; Yao, G.H.; Yao, M.Y.; Wen, X.F.; Ling, Y.; Guo, W. Application of next generation semiconductor based sequencing for species identification and meat derived products authentication. Food Control 2024, 165, 110639. [Google Scholar] [CrossRef]
- Prosser, S.W.J.; Hebert, P.D.N. Rapid identification of the botanical and entomological sources of honey using DNA metabarcoding. Food Chem. 2017, 214, 183–191. [Google Scholar] [CrossRef]
- Utzeri, V.J.; Ribani, A.; Schiavo, G.; Bertolini, F.; Bovo, S.; Fontanesi, L. Application of next generation semiconductor based sequencing to detect the botanical composition of monofloral, polyfloral and honeydew honey. Food Control 2018, 86, 342–349. [Google Scholar]
- Chen, Y.; Huang, X.; Zuo, D.; Li, Y.; Wang, Y.; Wang, Q.; Tian, X.; Ma, Y.; Wang, W. Exploring the influence of different processing conditions on DNA quality of collagen peptides and the feasibility of its raw material traceability. Food Chem. 2025, 463, 141556. [Google Scholar] [CrossRef]
- Qayoom, U.; Pawar, R.; Mohite, S.; Sawant, M.; Vivek, N.; Pawar, S.; Goswami, M.; Lakra, W. DNA barcoding of some commonly exploited fishes from the northern Western Ghats, India. Indian J. Anim. Sci. 2018, 88, 245–250. [Google Scholar] [CrossRef]
- Hebert, P.; Cywinska, A.; Ball, S.L.; deWaard, J. Biological identification through DNA barcodes. Proc. R. Soc. Lond. B 2003, 270, 313–321. [Google Scholar] [CrossRef]
- Giagkazoglou, Z.; Loukovitis, D.; Gubili, C.; Chatziplis, D.; Symeonidis, A.; Imsiridou, A. Untangling the cephalopod market: Authentication of seafood products in Greece with DNA-barcoding. Food Control 2024, 163, 110523. [Google Scholar] [CrossRef]
- Currò, S.; Babbucci, M.; Carletti, P.; Fasolato, L.; Novelli, E.; Balzan, S. The globalized fish Industry: Employing DNA-barcoding and NIRS technology to combat counterfeiting and safeguard traditional agro-food products. Food Control 2024, 158, 110246. [Google Scholar]
- Kim, D.Y.; Miranda-Romo, D.; Ten Cate, A.R.; Hellberg, R.S. Use of a novel combination of multiplex PCR and DNA barcoding in assessing authenticity of ginseng products. Food Control 2025, 168, 110893. [Google Scholar]
- Zhao, G.; Li, L.Y.; Shen, X.; Zhong, R.M.; Zhong, Q.P.; Lei, H.T. DNA barcoding unveils novel discoveries in authenticating high-value snow lotus seed food products. Foods 2024, 13, 2580. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Ferreiro, M.; Otero, A.; Morán, P. It’s what’s inside that counts: DNA-barcoding of porcini (Boletus sp., Basidiomycota) commercial products reveals product mislabelling. Food Control 2023, 144, 109346. [Google Scholar]
- Zeng, X.Y.; Yee, J.C.; Yang, H.P. Rapid and accurate identification of Mercenaria mercenaria and mercenaria campechiensis using high-resolution melting analysis. J. Shellfish Res. 2024, 43, 111–118. [Google Scholar] [CrossRef]
- Barrias, S.; Ibáñez, J.; Martins-Lopes, P. Wine authenticity throughout the wine-chain: Exploring the potential of HRM-SNP assays in varietal discrimination and quantification in wine blends. Food Control 2025, 167, 110814. [Google Scholar] [CrossRef]
- Moine, A.; Boccacci, P.; De Paolis, C.; Rolle, L.; Gambino, G. TaqMan® and HRM approaches for SNP genotyping in genetic traceability of musts and wines. Curr. Res. Food Sci. 2024, 8, 100707. [Google Scholar]
- Borkowska, M.; Kułakowski, M.; Myszka, K. High-Resolution melting analysis potential for Saccharomyces cerevisiae var. boulardii authentication in probiotic-enriched food matrices. BioTech 2024, 13, 48. [Google Scholar] [CrossRef]
- Barrias, S.; Ibáñez, J.; Martins-Lopes, P. High resolution melting analysis of microsatellite markers applied to grapevine varietal fingerprinting throughout the wine production chain. Food Control 2024, 160, 110368. [Google Scholar]
- Holz, N.; Wax, N.; Oest, M.; Fischer, M. REASSURED test system for food control-preparation of LAMP reaction mixtures for in-field identification of plant and animal species. Appl. Sci. 2024, 14, 10946. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, W.; Su, H.; Xie, W.; Jia, L. A LAMP-based colorimetric and fluorescence dual-channel assay for on-site identification of adulterated meat by a portable device. Food Control 2025, 169, 110984. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, J.; Xiao, B.; Sun, X.; Huang, F.; Chen, A. Disposable and instrument-free nucleic acid lateral flow cassette for rapid and on-site identification of adulterated goat milk. Talanta 2024, 267, 125205. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cui, J.; Chen, H.; Li, H.; Xie, Y.; Song, W.; Chen, R. Rapid colorimetric and fluorescence identification of Pinelliae Rhizoma and adulterate Rhizoma Typhonii Flagelliformis using direct-LAMP assay. Food Chem. 2024, 437, 137840. [Google Scholar] [CrossRef]
- Moorthy, G.; Benjakul, S.; Sukkapat, P.; Senathipathi, D.N.; Saetang, J. Development and validation of colorimetric LAMP assay for species authentication of economically important Mytildae mussels. Food Control 2024, 165, 110697. [Google Scholar] [CrossRef]
- Schäfer, L.; Völker, E.; Eule, M.; Ahrens, B.; Beyer, K.; Holzhauser, T. Development and validation of a loop-mediated isothermal amplification (LAMP) method for the rapid, sensitive, and specific detection of hazelnut as an allergen in food matrix. J. Agric. Food Chem. 2024, 72, 24093–24100. [Google Scholar] [CrossRef]
- Yang, N.; Zhang, H.; Han, X.; Liu, Z.; Lu, Y. Advancements and applications of loop-mediated isothermal amplification technology: A comprehensive overview. Front. Microbiol. 2024, 15, 1406632. [Google Scholar] [CrossRef]
- Yan, C.; Wang, X.; Zhao, X.; Wei, M.; Shi, C.; Ma, C. Development of a direct and visual isothermal method for meat adulteration detection in low resource settings. Food Chem. 2020, 319, 126542. [Google Scholar] [CrossRef]
- Chen, P.; Zhou, M.; Chen, X.; Xiong, S.; Su, Y.; Zhou, H.; Peng, J.; Xiong, Y. Quantum dot bead-based competitive immunochromatographic assay for enterotoxin aureus A detection in pasteurized milk. J. Dairy Sci. 2022, 105, 4938–4945. [Google Scholar] [CrossRef]
- Nodoushan, S.M.; Nasirizadeh, N.; Sedighian, H.; Kachuei, R.; Azimzadeh-Taft, M.; Fooladi, A.A.I. Detection of Staphylococcal Enterotoxin A (SEA) using a sensitive nanomaterial-based electrochemical aptasensor. Diam. Relat. Mater. 2022, 127, 109042. [Google Scholar] [CrossRef]
- Liu, R.; Wang, X.; Wang, X.; Shi, Y.; Shi, C.; Wang, W.; Ma, C. A simple isothermal nucleic acid amplification method for the effective on-site identification for adulteration of pork source in mutton. Food Control 2019, 98, 297–302. [Google Scholar] [CrossRef]
- Zhang, X.J.; Wang, X.J.; Shi, B.H.; Li, J.F.; Fan, Y.F.; Li, Y.; Shi, C.; Ma, C.P. A biphasic accelerated strand exchange amplification strategy for culture-independent and rapid detection of Salmonella enterica in food samples. Anal. Methods 2024, 16, 4083–4092. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, R.; Yang, X.; Jia, J.; Zhang, Q.; Ye, S.; Man, S.; Ma, L. Machine learning-assisted, dual-channel CRISPR/Cas12a biosensor-in-microdroplet for amplification-free nucleic acid detection for food authenticity testing. ACS Nano 2024, 18, 33505–33519. [Google Scholar] [PubMed]
- Chen, J.; Liu, J.; Wu, D.; Pan, R.; Chen, J.; Wu, Y.; Huang, M.; Li, G. CRISPR/Cas Precisely Regulated DNA-Templated Silver Nanocluster Fluorescence Sensor for Meat Adulteration Detection. J. Agric. Food Chem. 2022, 70, 14296–14303. [Google Scholar] [PubMed]
- Ge, H.; Wang, X.; Xu, J.; Lin, H.; Zhou, H.; Hao, T.; Wu, Y.; Guo, Z. A CRISPR/Cas12a-mediated dual-mode electrochemical biosensor for polymerase chain reaction-free detection of genetically modified soybean. Anal. Chem. 2021, 93, 14885–14891. [Google Scholar] [PubMed]
- Cui, Y.; Qu, X. CRISPR-Cas systems of lactic acid bacteria and applications in food science. Biotechnol. Adv. 2024, 71, 108323. [Google Scholar]
- Wang, Y.; Fu, L.; Tao, D.; Han, X.; Xu, B.; Deng, M.; Li, S.; Zhao, C.; Li, X.; Zhao, S.; et al. Development of a naked eye CRISPR-Cas12a and -Cas13a multiplex point-of-care detection of genetically modified swine. ACS Synth. Biol. 2023, 12, 2051–2060. [Google Scholar] [CrossRef]
- Shum, P.; Cusa, M.; Prasetyo, A.; Mariani, S. Nanopore sequencing facilitates screening of diversity and provenance of seafood and marine wildlife. Food Control 2024, 161, 110382. [Google Scholar]
- Detcharoen, M.; Khrueakaew, P.; Sukkapat, P.; Benjakul, S.; Saetang, J. Metabarcoding for authentication of fish species in surimi-based products by Nanopore sequencing. Food Biosci. 2024, 61, 104628. [Google Scholar] [CrossRef]
- Caliskan-Aydogan, O.; Alocilja, E.C. Parallel biosensor platform for the detection of carbapenemase-producing E. coli in spiked food and water samples. Food Control 2024, 163, 110485. [Google Scholar]
- Kim, E.; Yang, S.-M.; Kim, J.-S.; Kim, H.-Y. Rapid and portable microfluidic chip-based qPCR for quality assurance of commercial seaweeds (lavers) in the supply chain. Food Control 2024, 165, 110621. [Google Scholar]
- Ren, J.J.; Jiang, Z.K.; Li, W.J.; Kang, X.S.; Bai, S.L.; Yang, L.J.; Li, S.P.; Zhang, D.L. Characterization of glutenin genes in bread wheat by Third-Generation RNA Sequencing and the development of a Glu-1Dx5 marker specific for the extra cysteine residue. J. Agric. Food Chem. 2022, 70, 7211–7219. [Google Scholar] [CrossRef]
- Adenuga, B.M.; Biltes, R.; Villa, C.; Costa, J.; Spychaj, A.; Montowska, M.; Mafra, I. Unravelling red deer (Cervus elaphus) meat adulteration in gourmet foods by quantitative real-time PCR. Food Control 2025, 168, 110872. [Google Scholar] [CrossRef]
- Cai, C.; Hou, X.; Huang, B.; He, J.; Wu, X.; He, Q. LAMP assay coupled CRISPR/LbCas12a system in a single-tube method for visual detection of meat adulteration. Food Control 2025, 167, 110809. [Google Scholar] [CrossRef]
- Kurniawati, D.; Sismindari, S.; Rumiyati, R.; Wibowo, F.; Pebriyanti, N.; Irnawati, I.; Rohman, A. Specific real-time PCR assay targeting D-loop gene and short amplicon sequencing for identification of monkey meat in beef meatballs. Indones. J. Chem. 2024, 24, 315–324. [Google Scholar]
- Zhao, G.; Shen, X.; Li, Y.; Zhong, R.M.; Raza, S.H.A.; Zhong, Q.P.; Lei, H.T. Duplex recombinase polymerase amplification combined with CRISPR/Cas12a-Cas12a assay for on-site identification of yak meat adulteration. J. Food Compos. Anal. 2024, 134, 106455. [Google Scholar] [CrossRef]
- Saravia-Sánchez, R.F.; Molina-Quirós, J.L.; Chaves-Campos, J.; Elizondo-Sancho, M.; Martínez-Fernández, D.; Marrari, M.; Hernández-Muñoz, S. Molecular identification of billfish (Osteichthyes, families Xiphiidae and Istiophoridae) products in Costa Rica as a tool to reduce mislabeling and fraudulent sales of fish. Food Control 2025, 168, 110961. [Google Scholar]
- Giantsis, I.A.; Tokamani, M.; Triantaphyllidis, G.; Tzatzani, S.; Chatzinikolaou, E.; Toros, A.; Bouchorikou, A.; Chatzoglou, E.; Miliou, H.; Sarantopoulou, J.; et al. Development of multiplex PCR and Melt-Curve analysis for the molecular identification of four species of the mullidae family, available in the market. Genes 2023, 14, 960. [Google Scholar] [CrossRef]
- Zhao, J.L.; Timira, V.; Ahmed, I.; Chen, Y.; Wang, H.; Zhang, Z.Y.; Lin, H.; Li, Z.X. Crustacean shellfish allergens: Influence of food processing and their detection strategies. Crit. Rev. Food Sci. Nutr. 2024, 64, 3794–3822. [Google Scholar]
- Zhou, J.; Wang, Y.; Zhou, C.; Zheng, L.; Fu, L. A ratiometric fluorescent aptasensor based on EXPAR to detect shellfish tropomyosin in food system. Food Control 2023, 144, 109380. [Google Scholar]
- Sarkar, D.; Naha, S. Advancement in species specific adulteration identification in camel milk. J. Food Compos. Anal. 2024, 130, 106168. [Google Scholar]
- Baptista, M.; Cunha, J.T.; Domingues, L. DNA-based approaches for dairy products authentication: A review and perspectives. Trends Food Sci. Technol. 2021, 109, 386–397. [Google Scholar]
- Ma, X.Y.; Xia, H.L.; Pan, Y.Q.; Huang, Y.F.; Xu, T.; Guan, F. Double-Tube multiplex TaqMan Real-Time PCR for the detection of eight animal-derived dairy ingredients. J. Agric. Food Chem. 2024, 72, 11640–11651. [Google Scholar]
- Kanwal, N.; Musharraf, S.G. Analytical approaches for the determination of adulterated animal fats and vegetable oils in food and non-food samples. Food Chem. 2024, 460, 140786. [Google Scholar] [PubMed]
- Christopoulou, N.M.; Mamoulaki, V.; Mitsiakou, A.; Samolada, E.; Kalogianni, D.P.; Christopoulos, T.K. Screening method for the visual discrimination of olive oil from other vegetable oils by a multispecies DNA sensor. Anal. Chem. 2024, 96, 1803–1811. [Google Scholar]
- Christopoulou, N.M.; Figgou, E.; Kalaitzis, P.; Kalogianni, D.P.; Christopoulos, T.K. Multiallelic DNA sensors for molecular traceability of olive oil varietal origin. Sens. Actuators B-Chem. 2024, 406, 135423. [Google Scholar]
- Costa, J.; Silva, I.; Villa, C.; Mafra, I. A novel single-tube nested real-time PCR method to quantify pistachio nut as an allergenic food: Influence of food matrix. J. Food Compos. Anal. 2023, 115, 105042. [Google Scholar]
- Wei, Y.M.; Liu, Y.; Li, L.; Xiang, S.N.; Zhang, H.Y.; Shang, Y. Identification of s9ap used as an endogenous reference gene in qualitative and real-time quantitative PCR detection of Pleurotus eryngii. Mol. Biol. Rep. 2023, 50, 621–629. [Google Scholar] [PubMed]
- Wang, G.; Ren, Y.; Su, Y.Y.; Zhang, H.; Li, J.F.; Zhao, H.X.; Zhang, H.X.; Han, J.P. Identification of toxic Gelsemium elegans in processed food and honey based on real-time PCR analysis. Food Res. Int. 2024, 182, 114188. [Google Scholar]
- Baviera, J.M.B.; Bolognesi, C.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lambre, C.; Lampi, E.; Mengelers, M.; et al. Taxonomic identity of the Bacillus licheniformis strains used to produce food enzymes evaluated in published EFSA opinions. EFSA J. 2024, 22, e8770. [Google Scholar]
- Arellano, K.; Lim, J.; Bucheli, J.E.V.; Park, H.; Todorov, S.D.; Holzapfel, W.H. Identification of safe putative probiotics from various food products. Folia Microbiol. 2024, 69, 1053–1068. [Google Scholar] [CrossRef]
- Hillinger, S.; Saeckler, J.; Domig, K.J.; Dobrovolny, S.; Hochegger, R. Development of a DNA metabarcoding method for the identification of insects in food. Foods 2023, 12, 1086. [Google Scholar] [CrossRef]
- Hu, T.; Chitnis, N.; Monos, D.; Dinh, A. Next-generation sequencing technologies: An overview. Hum. Immunol. 2021, 82, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Kallastu, A.; Malv, E.; Aro, V.; Meikas, A.; Vendelin, M.; Kattel, A.; Nahku, R.; Kazantseva, J. Absolute quantification of viable bacteria abundances in food by next-generation sequencing: Quantitative NGS of viable microbes. Curr. Res. Food Sci. 2023, 6, 100443. [Google Scholar] [CrossRef]
- Sadurski, J.; Polak-Berecka, M.; Staniszewski, A.; Waśko, A. Step-by-Step metagenomics for food microbiome analysis: A detailed review. Foods 2024, 13, 2216. [Google Scholar] [CrossRef] [PubMed]
- Marien, A.; Dubois, B.; Anselmo, A.; Veys, P.; Berben, G.; Kohl, C.; Maljean, J.; Guillet, S.; Morin, J.-F.; Debode, F. Detection of Bombyx mori as a protein source in feedingstuffs by real-time PCR with a single-copy gene target. Agriculture 2024, 14, 1996. [Google Scholar] [CrossRef]
- Albini, S.; Palmieri, L.; Dubois, A.; Bourg, N.; Lostal, W.; Richard, I. Assessment of therapeutic potential of a dual AAV approach for duchenne muscular dystrophy. Int. J. Mol. Sci. 2023, 24, 11421. [Google Scholar] [CrossRef]
- Acharya, K.; Blackburn, A.; Mohammed, J.; Haile, A.T.; Hiruy, A.M.; Werner, D. Metagenomic water quality monitoring with a portable laboratory. Water Res. 2020, 184, 116112. [Google Scholar] [PubMed]
- Ikeogu, U.N.; Akdemir, D.; Wolfe, M.D.; Okeke, U.G.; Chinedozi, A.; Jannink, J.-L.; Egesi, C.N. Genetic correlation, genome-wide association and genomic prediction of portable NIRS predicted carotenoids in cassava roots. Front. Plant Sci. 2019, 10, 1570. [Google Scholar]
- Park, S.; Lee, D.; Kim, Y.; Lim, S.; Chae, H.; Kim, S. BioVLAB-Cancer-Pharmacogenomics: Tumor heterogeneity and pharmacogenomics analysis of multi-omics data from tumor on the cloud. Bioinformatics 2021, 38, 275–277. [Google Scholar] [CrossRef]
- Talukder, A.; Barham, C.; Li, X.; Hu, H. Interpretation of deep learning in genomics and epigenomics. Brief. Bioinform. 2020, 22, bbaa177. [Google Scholar]
- Ramos, P.D.; Almeida, M.S.; Olsson, I.A.S. Safe and purposeful genome editing under harmonized regulation for responsible use: Views of research experts. New Genet. Soc. 2023, 42, e2237177. [Google Scholar]
- Marklewitz, M.; Jaguparov, A.; Wilhelm, A.; Akande, O.W.; Musul, B.; Poates, A.L.; Afrough, B.; Norberg, A.; Hull, N.C.; Ehsani, S.; et al. Genomics costing tool: Considerations for improving cost-efficiencies through cross scenario comparison. Front. Public Health 2025, 12, 1498094. [Google Scholar] [CrossRef] [PubMed]
- Bombard, Y.; Ginsburg, G.S.; Sturm, A.C.; Zhou, A.Y.; Lemke, A.A. Digital health-enabled genomics: Opportunities and challenges. Am. J. Hum. Genet. 2022, 109, 1190–1198. [Google Scholar] [CrossRef]
- Perga, S.; Biolatti, C.; Martini, I.; Rossi, F.; Benso, A.; Acutis, P.L.; Bagnato, A.; Cognata, D.; Caroggio, P.; Peletto, S.; et al. Application of microsatellites to trace the dairy products back to the farm of origin. Foods 2023, 12, 4131. [Google Scholar] [CrossRef] [PubMed]
- Bonomi, L.; Huang, Y.; Ohno-Machado, L. Privacy challenges and research opportunities for genomic data sharing. Nat. Genet. 2020, 52, 646–654. [Google Scholar] [CrossRef]
- Andersen, S.C.; Fachmann, M.S.R.; Kiil, K.; Møller Nielsen, E.; Hoorfar, J. Gene-Based pathogen detection: Can we use qPCR to predict the outcome of diagnostic metagenomics? Genes 2017, 8, 332. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Chen, H.; Zhang, C.; Zhuang, Y.; Sharif, Y.; Cai, T.; Yang, Q.; Soni, P.; Pandey, M.K.; Varshney, R.K.; et al. Designing future peanut: The power of genomics-assisted breeding. Theor. Appl. Genet. 2024, 137, 66. [Google Scholar] [CrossRef]
- Yu, Y.; Chai, Y.; Li, Z.; Li, Z.; Ren, Z.; Dong, H.; Chen, L. Quantitative predictions of protein and total flavonoids content in Tartary and common buckwheat using near-infrared spectroscopy and chemometrics. Food Chem. 2025, 462, 141033. [Google Scholar] [CrossRef]
- Song, W.; Wang, H.; Yun, Y.-H. Smartphone video imaging: A versatile, low-cost technology for food authentication. Food Chem. 2025, 462, 140911. [Google Scholar] [CrossRef]
- Cavdaroglu, C.; Altug, N.; Serpen, A.; Öztop, M.H.; Ozen, B. Comparative performance of artificial neural networks and support vector Machines in detecting adulteration of apple juice concentrate using spectroscopy and time domain NMR. Food Res. Int. 2025, 201, 115616. [Google Scholar] [CrossRef]
- Sohn, S.-I.; Pandian, S.; Oh, Y.-J.; Zaukuu, J.-L.Z.; Na, C.-S.; Lee, Y.-H.; Shin, E.-K.; Kang, H.-J.; Ryu, T.-H.; Cho, W.-S.; et al. Vis-NIR spectroscopy and machine learning methods for the discrimination of transgenic Brassica napus L. and their hybrids with B. juncea. Processes 2022, 10, 240. [Google Scholar]
- Zhang, J.; Feng, X.; Wu, Q.; Yang, G.; Tao, M.; Yang, Y.; He, Y. Rice bacterial blight resistant cultivar selection based on visible/near-infrared spectrum and deep learning. Plant Methods 2022, 18, 49. [Google Scholar] [CrossRef]
- Sohn, S.-I.; Pandian, S.; Oh, Y.-J.; Zaukuu, J.-L.Z.; Kang, H.-J.; Ryu, T.-H.; Cho, W.-S.; Cho, Y.-S.; Shin, E.-K.; Cho, B.-K. An overview of near infrared spectroscopy and its applications in the detection of genetically modified organisms. Int. J. Mol. Sci. 2021, 22, 9940. [Google Scholar] [CrossRef]
- Song, Q.; Bai, C.; Dong, Y.; Chen, M.; Wang, S.; Hu, J.; Qiao, X.; Chen, J.; Li, S.; Liu, X.; et al. Highly selective Zn2+ near-infrared fluorescent probe and its application in biological imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 322, 124828. [Google Scholar] [CrossRef]
- Bose, D.; Padmavati, M. Honey Authentication: A review of the issues and challenges associated with honey adulteration. Food Biosci. 2024, 61, 105004. [Google Scholar]
- Ji, C.; Petchkongkaew, A.; van Ruth, S.; Wu, D.; Elliott, C. The crucial importance of soy sauce authenticity: Global trade, adulteration risks, and analytical challenges. Trends Food Sci. Technol. 2024, 152, 104666. [Google Scholar]
- Yang, H.; Wang, Y.; Zhao, J.; Li, P.; Li, Z.; Li, L.; Fan, B.; Wang, F. Data-driven pipeline modeling for predicting unknown protein adulteration in dairy products. Food Chem. 2025, 471, 142736. [Google Scholar] [CrossRef]
- Boodoo, C.; Dester, E.; David, J.; Patel, V.; KC, R.; Alocilja, E.C. Multi-Probe nano-genomic biosensor to detect S. aureus from magnetically-extracted food samples. Biosensors 2023, 13, 608. [Google Scholar] [CrossRef] [PubMed]
- Velmovitsky, P.E.; Bublitz, F.M.; Fadrique, L.X.; Morita, P.P. Blockchain applications in health care and public health: Increased transparency. JMIR Med. Inform. 2021, 9, e20713. [Google Scholar]
- Lanubile, A.; Stagnati, L.; Marocco, A.; Busconi, M. DNA-based techniques to check quality and authenticity of food, feed and medicinal products of plant origin: A review. Trends Food Sci. Technol. 2024, 149, 104568. [Google Scholar] [CrossRef]
- Kępińska-Pacelik, J.; Biel, W.; Natonek-Wiśniewska, M.; Krzyścin, P. Assessment of adulteration in the composition of dog food based on DNA identification by real-time PCR. Anim. Feed. Sci. Technol. 2023, 298, 115609. [Google Scholar]

| PCR Types | Time | Cost | Accuracy | Complexity | Applications | Ref. |
|---|---|---|---|---|---|---|
| Conventional PCR | Relatively fast, typically completed within a few hours | Relatively low, suitable for large-scale experiments | Lower, relies on gel electrophoresis analysis | Simple, relatively easy operation | Genotyping, cloning verification, etc. | [20] |
| PCR-RFLP | Slower due to additional enzymatic digestion steps | Moderate, requires purchasing restriction enzymes | Moderate, limited by presence of restriction sites | Moderate, involves additional enzymatic digestion steps | Genotyping, genetic disease detection, etc. | [21] |
| qRT-PCR | Faster with real-time monitoring | Higher, needs specialized equipment and reagents | High, precise quantification through fluorescence signals | Moderate to high, requires sophisticated instruments and data analysis | Gene expression analysis, viral load determination, etc. | [22] |
| Multiplex PCR | Can be time-consuming due to optimization of multiple primer pairs | Higher, especially when using multiple fluorescent probes | Moderate to high, depends on primer design and reaction conditions | High, complex primer design and optimization required | Pathogen detection, multi-gene expression analysis, etc. | [23] |
| ddPCR | Longer analysis process because of need to generate and analyze numerous droplets | High, requires special equipment and reagents | High, capable of absolute quantification | High, technically demanding | Absolute quantification, rare mutation detection, etc. | [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Yang, W.; Cai, H.; Cao, G.; Li, Z. Current Progress and Future Trends of Genomics-Based Techniques for Food Adulteration Identification. Foods 2025, 14, 1116. https://doi.org/10.3390/foods14071116
Zhao J, Yang W, Cai H, Cao G, Li Z. Current Progress and Future Trends of Genomics-Based Techniques for Food Adulteration Identification. Foods. 2025; 14(7):1116. https://doi.org/10.3390/foods14071116
Chicago/Turabian StyleZhao, Jing, Wei Yang, Hongli Cai, Guangtian Cao, and Zhanming Li. 2025. "Current Progress and Future Trends of Genomics-Based Techniques for Food Adulteration Identification" Foods 14, no. 7: 1116. https://doi.org/10.3390/foods14071116
APA StyleZhao, J., Yang, W., Cai, H., Cao, G., & Li, Z. (2025). Current Progress and Future Trends of Genomics-Based Techniques for Food Adulteration Identification. Foods, 14(7), 1116. https://doi.org/10.3390/foods14071116







