Recent Advances in Food Safety: Nanostructure-Sensitized Surface-Enhanced Raman Sensing
Abstract
:1. Introduction
2. Analytical Mechanism of SERS
2.1. Basic Theory of SERS
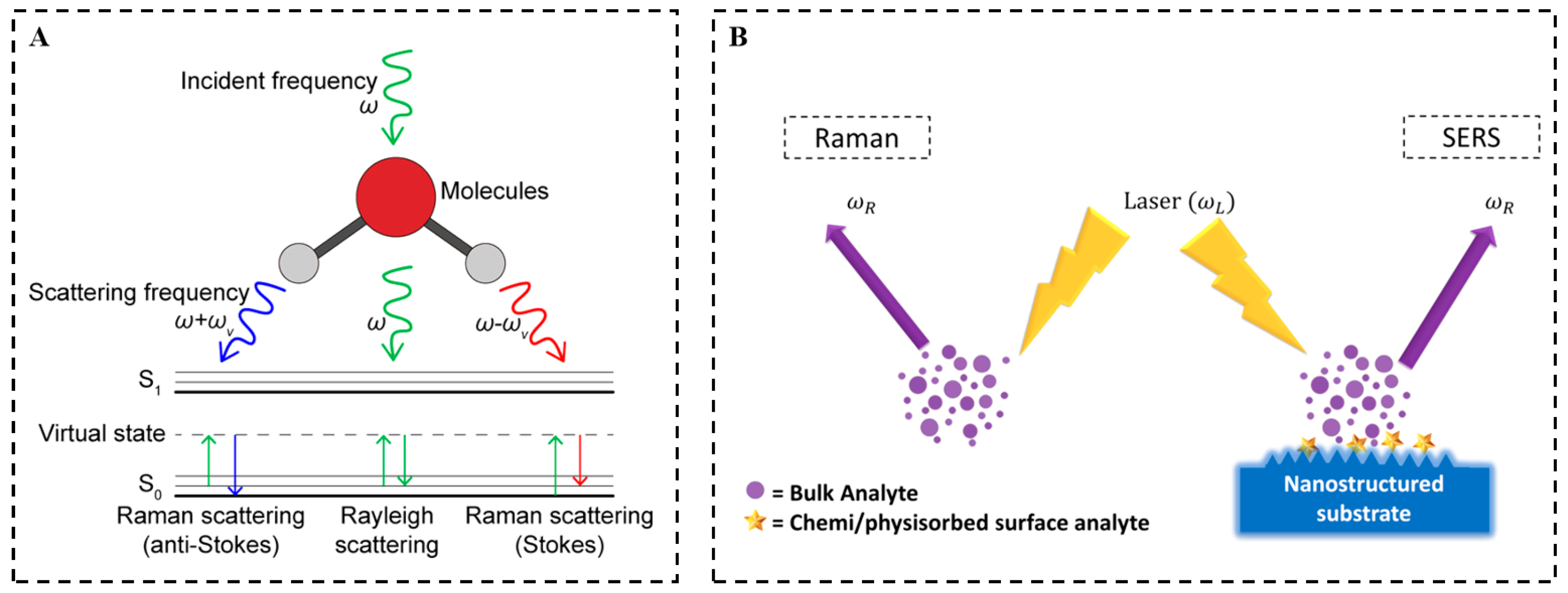
2.2. Raman Signal Acquisition
2.3. SERS Superiority
2.4. Spectral Statistics Characteristics
3. Sensitizing Effect of Nanostructures in the SERS Assay
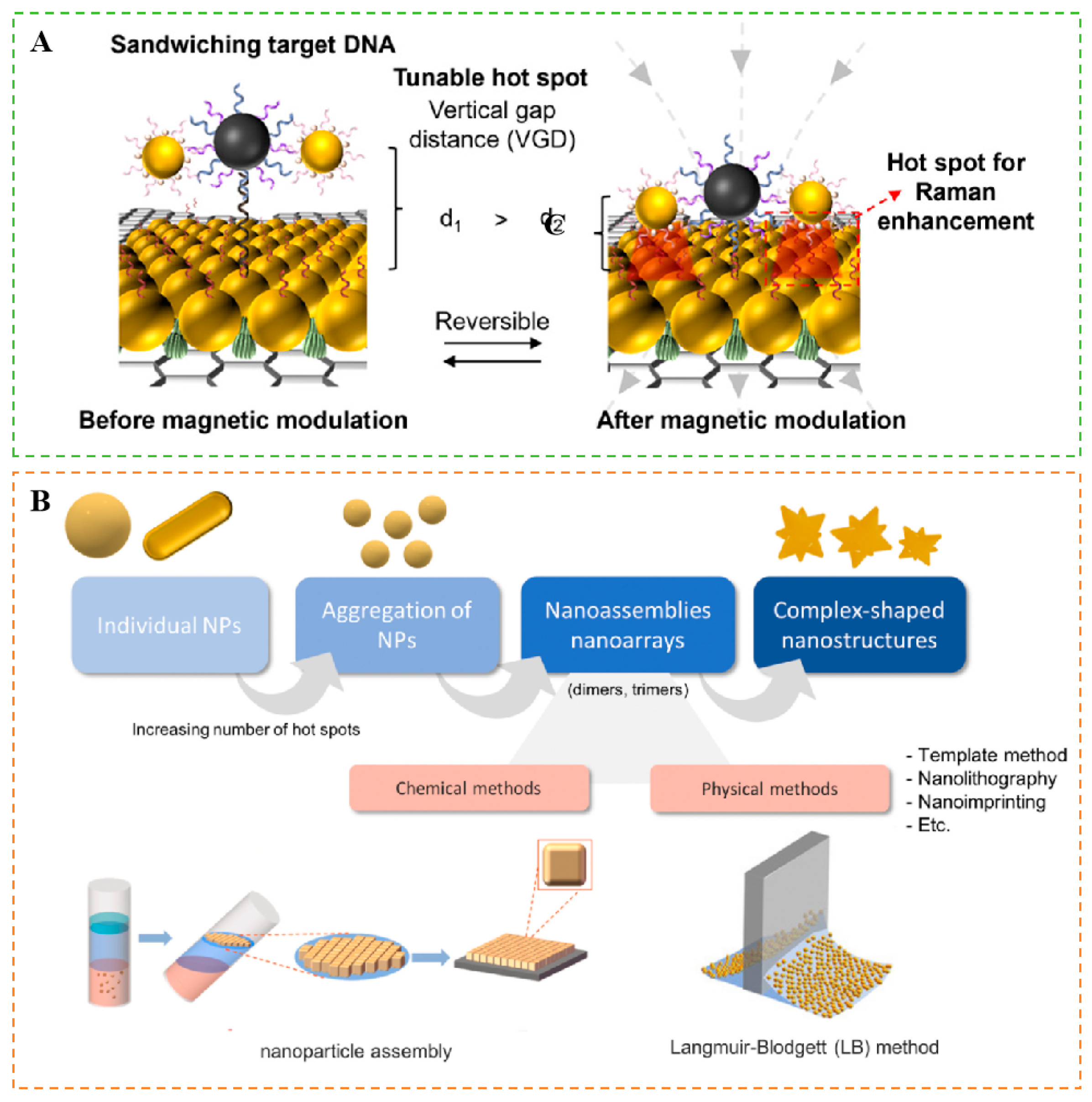
3.1. Classification and Preparation of Nanostructures in SERS
3.1.1. Classification of Nanostructures for Enhancing SERS Signals
3.1.2. Preparation of Nanostructure-Sensitized SERS Substrates
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
3.2. Enhancement Behavior of Nanostructure-Sensitized SERS
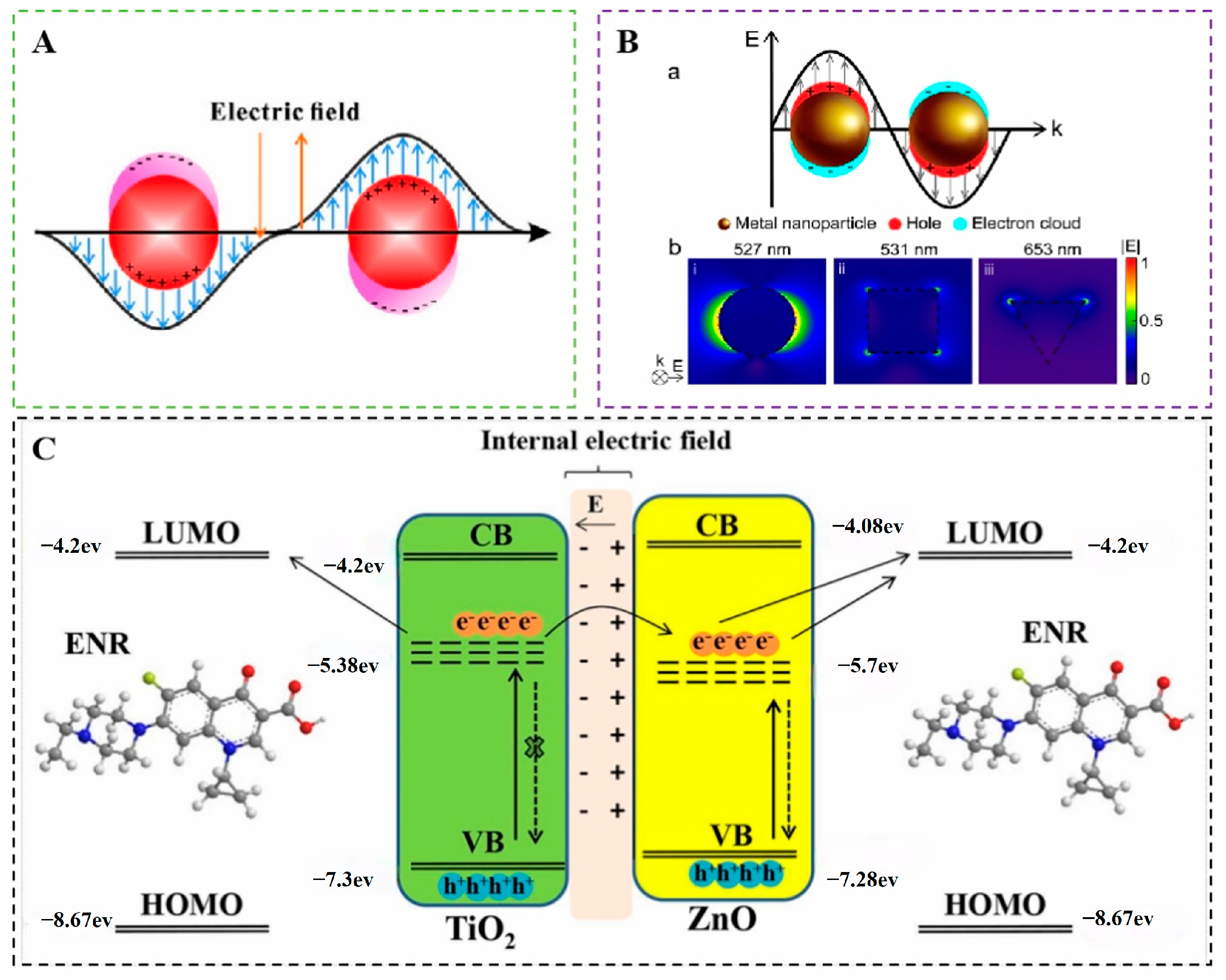
3.2.1. Electromagnetic Enhancement Mechanism
3.2.2. Chemical Enhancement Mechanism
3.2.3. “Hot Spot” Formation
3.2.4. Effect of Nanostructure Morphology and Composition on Signal Enhancement
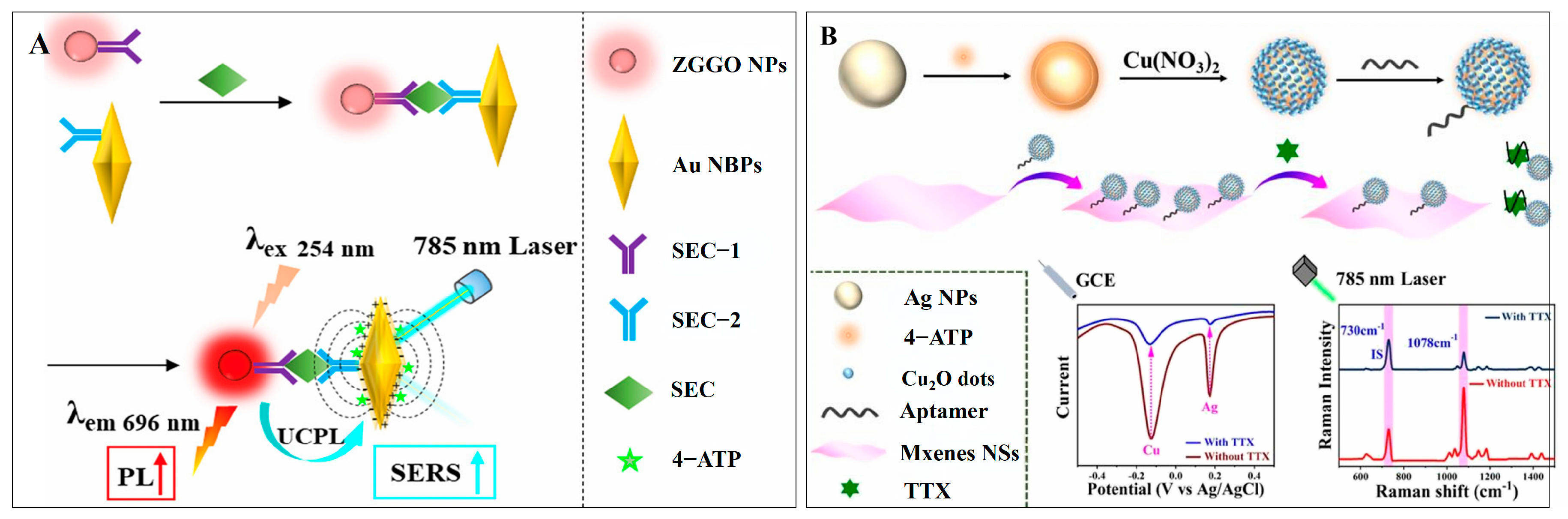
3.2.5. Multi-Modal Analysis of Signal Enhancement
4. Nanostructure-Sensitized SERS for Assessing Harmful Substances in Food
4.1. Nanostructure-Sensitized SERS to Assess Microbial Contamination in Food
4.1.1. Sensing Foodborne Pathogens
4.1.2. Sensing Fungi, Molds, and Their Toxins
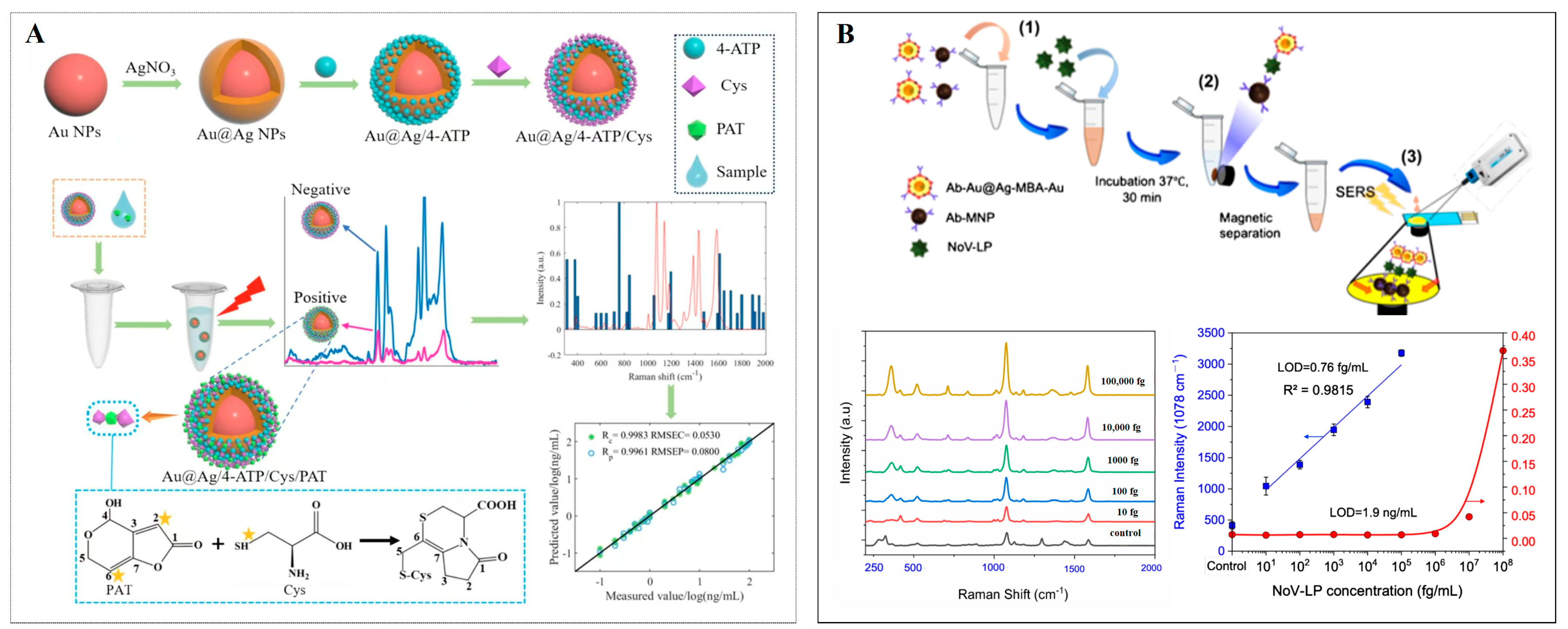
4.1.3. Sensing of Viruses
4.2. Nanostructure-Sensitized SERS Assessing Chemical Contamination in Food
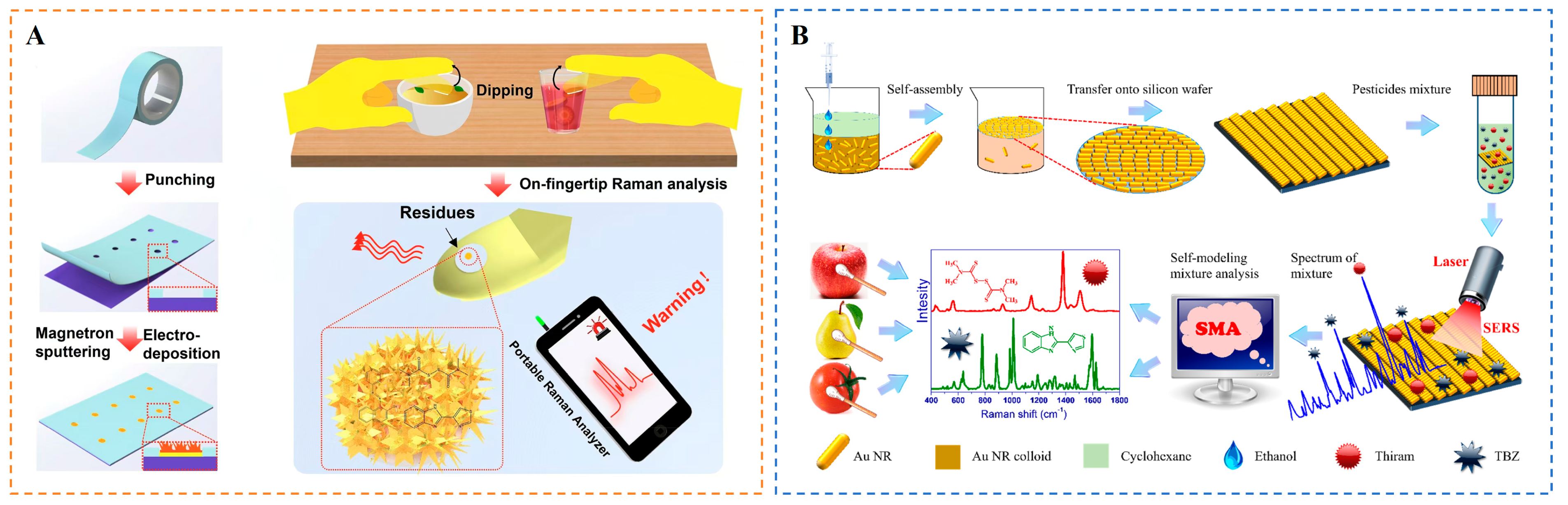
4.2.1. Sensing of Pesticide Residues
4.2.2. Sensing Veterinary Drug Residues
4.2.3. Sensing of Heavy Metals
4.2.4. Sensing Food Additives and Illicit Adulterants
4.3. Nanostructure-Sensitized SERS to Assess Physical Contamination in Food
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, W.; Wang, Q.; Chang, K.; Zhao, Y. Surface-enhanced Raman spectroscopy substrates for monitoring antibiotics in dairy products: Mechanisms, advances, and prospects. Compr. Rev. Food Sci. Food Saf. 2024, 23, e70024. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, Y.; Han, Y.; Liu, X.; Han, B.; Mao, H.; Chen, Q. A dual-mode optical sensor for sensitive detection of saxitoxin in shellfish based on three-in-one functional nanozymes. J. Food Compos. Anal. 2024, 130, 106190. [Google Scholar] [CrossRef]
- Wu, G.; Qiu, H.; Liu, X.; Luo, P.; Wu, Y.; Shen, Y. Nanomaterials-based fluorescent assays for pathogenic bacteria in food-related matrices. Trends Food Sci. Technol. 2023, 142, 104214. [Google Scholar] [CrossRef]
- Liu, S.; Lou, Y.; Li, Y.; Zhao, Y.; Laaksonen, O.; Li, P.; Zhang, J.; Battino, M.; Yang, B.; Gu, Q. Aroma characteristics of volatile compounds brought by variations in microbes in winemaking. Food Chem. 2023, 420, 136075. [Google Scholar] [CrossRef]
- Song, S.-H.; Gao, Z.-F.; Guo, X.; Chen, G.-H. Aptamer-Based Detection Methodology Studies in Food Safety. Food Anal. Methods 2019, 12, 966–990. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, Y.; Zhao, L.; Li, M.; Liang, D.; Li, M.; Zhao, G.; Ma, Y.; Tu, Q. Characteristic substance analysis and rapid detection of bacteria spores in cooked meat products by surface enhanced Raman scattering based on Ag@AuNP array substrate. Anal. Chim. Acta 2024, 1308, 342616. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Wang, M.; Barimah, A.O.; Chen, Q.; Li, H.; Shi, J.; El-Seedi, H.R.; Zou, X. Label-free surface enhanced Raman scattering spectroscopy for discrimination and detection of dominant apple spoilage fungus. Int. J. Food Microbiol. 2021, 338, 108990. [Google Scholar] [CrossRef]
- Atta, O.M.; Manan, S.; Shahzad, A.; Ul-Islam, M.; Ullah, M.W.; Yang, G. Biobased materials for active food packaging: A review. Food Hydrocoll. 2022, 125, 107419. [Google Scholar] [CrossRef]
- Nasiru, M.M.; Frimpong, E.B.; Muhammad, U.; Qian, J.; Mustapha, A.T.; Yan, W.; Zhuang, H.; Zhang, J. Dielectric barrier discharge cold atmospheric plasma: Influence of processing parameters on microbial inactivation in meat and meat products. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2626–2659. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, W.; Shi, J.; Li, Z.; Huang, X.; Zou, X.; Tan, W.; Zhang, X.; Hu, X.; Wang, X.; et al. Impedimetric aptasensor based on highly porous gold for sensitive detection of acetamiprid in fruits and vegetables. Food Chem. 2020, 322, 126762. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, P.; Yosri, N.; Chen, Q.; Elseedi, H.R.; Zou, X.; Yang, H. Detection of Heavy Metals in Food and Agricultural Products by Surface-enhanced Raman Spectroscopy. Food Rev. Int. 2021, 39, 1440–1461. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, P.; Yin, L.; Zuo, M.; Chen, Q.; El-Seedi, H.R.; Zou, X. Determination of lead in food by surface-enhanced Raman spectroscopy with aptamer regulating gold nanoparticles reduction. Food Control 2022, 132, 108498. [Google Scholar] [CrossRef]
- Rong, Y.; Ali, S.; Ouyang, Q.; Wang, L.; Li, H.; Chen, Q. Development of a bimodal sensor based on upconversion nanoparticles and surface-enhanced Raman for the sensitive determination of dibutyl phthalate in food. J. Food Compos. Anal. 2021, 100, 103929. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.; Huang, X.; Hu, X.; Huang, X.; Yin, L.; Huang, Q.; Wen, Y.; Li, B.; Shi, J.; et al. Switchable aptamer-fueled colorimetric sensing toward agricultural fipronil exposure sensitized with affiliative metal-organic framework. Food Chem. 2023, 407, 135115. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Ahmad, W.; Hassan, M.M.; Wu, J.; Ouyang, Q.; Chen, Q. An upconversion biosensor based on inner filter effect for dual-role recognition of sulfadimethoxine in aquatic samples. Food Chem. 2024, 437, 137832. [Google Scholar] [CrossRef]
- Yue, Z.; Liu, X.; Mei, T.; Zhang, Y.; Pi, F.; Dai, H.; Zhou, Y.; Wang, J. Reducing microplastics in tea infusions released from filter bags by pre-washing method: Quantitative evidences based on Raman imaging and Py-GC/MS. Food Chem. 2024, 445, 138740. [Google Scholar] [CrossRef]
- Yu, F.; Qu, C.; Ding, Z.; Wang, X.; Zheng, L.; Su, M.; Liu, H. Liquid Interfacial Coassembly of Plasmonic Arrays and Trace Hydrophobic Nanoplastics in Edible Oils for Robust Identification and Classification by Surface-Enhanced Raman Spectroscopy. J. Agric. Food Chem. 2023, 71, 14342–14350. [Google Scholar] [CrossRef]
- Shi, Y.; Yi, L.; Du, G.; Hu, X.; Huang, Y. Visual characterization of microplastics in corn flour by near field molecular spectral imaging and data mining. Sci. Total Environ. 2023, 862, 160714. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Zhou, W. A Review of Recent Advances for the Detection of Biological, Chemical, and Physical Hazards in Foodstuffs Using Spectral Imaging Techniques. Foods 2023, 12, 2266. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, M.; Chen, Q.; Ouyang, Q. Multifunctional Metal–Organic Frameworks Driven Three-Dimensional Folded Paper-Based Microfluidic Analysis Device for Chlorpyrifos Detection. J. Agric. Food Chem. 2024, 72, 14375–14385. [Google Scholar] [CrossRef]
- Zou, Y.; Shi, Y.; Wang, T.; Ji, S.; Zhang, X.; Shen, T.; Huang, X.; Xiao, J.; Farag, M.A.; Shi, J.; et al. Quantum dots as advanced nanomaterials for food quality and safety applications: A comprehensive review and future perspectives. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13339. [Google Scholar] [CrossRef] [PubMed]
- Mehedi Hassan, M.; He, P.; Xu, Y.; Zareef, M.; Li, H.; Chen, Q. Rapid detection and prediction of chloramphenicol in food employing label-free HAu/Ag NFs-SERS sensor coupled multivariate calibration. Food Chem. 2022, 374, 131765. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Lu, J.; Wu, Y.; Meng, M.; Yu, C.; Sun, C.; Chen, M.; Da, Z.; Yan, Y. Antifouling molecularly imprinted membranes for pretreatment of milk samples: Selective separation and detection of lincomycin. Food Chem. 2020, 333, 127477. [Google Scholar] [CrossRef]
- Mei, J.; Zhao, F.; Xu, R.; Huang, Y. A review on the application of spectroscopy to the condiments detection: From safety to authenticity. Crit. Rev. Food Sci. Nutr. 2021, 62, 6374–6389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hassan, M.M.; Rong, Y.; Liu, R.; Li, H.; Ouyang, Q.; Chen, Q. A solid-phase capture probe based on upconvertion nanoparticles and inner filter effect for the determination of ampicillin in food. Food Chem. 2022, 386, 132739. [Google Scholar] [CrossRef]
- Pan, C.; Zhu, B.; Yu, C. A Dual Immunological Raman-Enabled Crosschecking Test (DIRECT) for Detection of Bacteria in Low Moisture Food. Biosensors 2020, 10, 200. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, L.; Liang, Z.; Pang, Y.; Wang, X.; Zhou, L. Recent Advances in Nanomaterials-Based Sensing Platforms for the Determination of Multiple Bacterial Species: A Minireview. Anal. Lett. 2023, 57, 920–939. [Google Scholar] [CrossRef]
- Wu, S.; Hulme, J.P. Recent Advances in the Detection of Antibiotic and Multi-Drug Resistant Salmonella: An Update. Int. J. Mol. Sci. 2021, 22, 3499. [Google Scholar] [CrossRef]
- Wu, S.; Duan, N.; He, C.; Yu, Q.; Dai, S.; Wang, Z. Surface-enhanced Raman spectroscopic–based aptasensor for Shigella sonnei using a dual-functional metal complex-ligated gold nanoparticles dimer. Colloids Surf. B Biointerfaces 2020, 190, 110940. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Z.; Wang, B.; Liao, J.; Zhang, M. Non-thermal Microbial Inactivation of Honey Raspberry Wine Through the Application of High-Voltage Electrospray Technology. Food Bioprocess Technol. 2022, 15, 177–189. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, L.; Pu, H.; Sun, D.-W. Magnetic surface-enhanced Raman scattering (MagSERS) biosensors for microbial food safety: Fundamentals and applications. Trends Food Sci. Technol. 2021, 113, 366–381. [Google Scholar] [CrossRef]
- Lu, R.; Liu, Z.; Shao, Y.; Su, J.; Li, X.; Sun, F.; Zhang, Y.; Li, S.; Zhang, Y.; Cui, J.; et al. Nitric Oxide Enhances Rice Resistance to Rice Black-Streaked Dwarf Virus Infection. Rice 2020, 13, 24. [Google Scholar] [CrossRef]
- Xie, Y.; Du, X.; Li, D.; Wang, X.; Xu, C.; Zhang, C.; Sun, A.; Schmidt, S.; Liu, X. Seasonal occurrence and abundance of norovirus in pre- and postharvest lettuce samples in Nanjing, China. LWT 2021, 152, 112226. [Google Scholar] [CrossRef]
- Park, E.Y.; Maehata, S.; Khoris, I.M.; Achadu, O.J. Signal-amplified surface-enhanced Raman scattering using core/shell satellite nanoparticles for norovirus detection. Microchim. Acta 2024, 191, 560. [Google Scholar] [CrossRef]
- Song, M.; Khan, I.M.; Wang, Z. Research Progress of Optical Aptasensors Based on AuNPs in Food Safety. Food Anal. Methods 2021, 14, 2136–2151. [Google Scholar] [CrossRef]
- Zhang, H.; Serwah Boateng, N.A.; Ngolong Ngea, G.L.; Shi, Y.; Lin, H.; Yang, Q.; Wang, K.; Zhang, X.; Zhao, L.; Droby, S. Unravelling the fruit microbiome: The key for developing effective biological control strategies for postharvest diseases. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4906–4930. [Google Scholar] [CrossRef]
- Hassan, M.M.; Yi, X.; Zareef, M.; Li, H.; Chen, Q. Recent advancements of optical, electrochemical, and photoelectrochemical transducer-based microfluidic devices for pesticide and mycotoxins in food and water. Trends Food Sci. Technol. 2023, 142, 104230. [Google Scholar] [CrossRef]
- Guo, Z.; Guo, C.; Sun, L.; Zuo, M.; Chen, Q.; El-Seedi, H.R.; Zou, X. Identification of the apple spoilage causative fungi and prediction of the spoilage degree using electronic nose. J. Food Process Eng. 2021, 44, e13816. [Google Scholar] [CrossRef]
- Shi, X.; He, C.; Jiang, L.; Liang, H.; Zhang, X.; Yuan, R.; Yang, X. Mo-doped Co LDHs as Raman enhanced substrate for detection of roxarsine. Anal. Chim. Acta 2024, 1318, 342947. [Google Scholar] [CrossRef]
- Liang, M.; Zhang, G.; Song, J.; Tan, M.; Su, W. Paper-Based Microfluidic Chips for Food Hazard Factor Detection: Fabrication, Modification, and Application. Foods 2023, 12, 4107. [Google Scholar] [CrossRef]
- Li, C.; Song, M.; Wu, S.; Wang, Z.; Duan, N. Selection of aptamer targeting levamisole and development of a colorimetric and SERS dual-mode aptasensor based on AuNPs/Cu-TCPP(Fe) nanosheets. Talanta 2023, 251, 123739. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Xu, Y.; Peng, F.; Ma, W.; Zhao, Y. Plasmonic metal NP-bismuth composite film with amplified SERS activity for multiple detection of pesticides and veterinary drugs. Chem. Eng. J. 2023, 474, 145933. [Google Scholar] [CrossRef]
- Li, H.; Mehedi Hassan, M.; Wang, J.; Wei, W.; Zou, M.; Ouyang, Q.; Chen, Q. Investigation of nonlinear relationship of surface enhanced Raman scattering signal for robust prediction of thiabendazole in apple. Food Chem. 2021, 339, 127843. [Google Scholar] [CrossRef]
- Jiao, T.; Mehedi Hassan, M.; Zhu, J.; Ali, S.; Ahmad, W.; Wang, J.; Lv, C.; Chen, Q.; Li, H. Quantification of deltamethrin residues in wheat by Ag@ZnO NFs-based surface-enhanced Raman spectroscopy coupling chemometric models. Food Chem. 2021, 337, 127652. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, M.; Xu, K.; Song, W.; Chen, Q.; Wen, H. Safeguarding food safety: Nanomaterials-based fluorescent sensors for pesticide tracing. Food Chem. 2025, 463, 141288. [Google Scholar] [CrossRef]
- Li, M.; Hong, X.; Qiu, X.; Yang, C.; Mao, Y.; Li, Y.; Liu, Z.; Du, D. Ultrasensitive monitoring strategy of PCR-like levels for zearalenone contamination based DNA barcode. J. Sci. Food Agric. 2021, 101, 4490–4497. [Google Scholar] [CrossRef]
- Wang, J.; Ahmad, W.; Mehedi Hassan, M.; Zareef, M.; Viswadevarayalu, A.; Arslan, M.; Li, H.; Chen, Q. Landing microextraction sediment phase onto surface enhanced Raman scattering to enhance sensitivity and selectivity for chromium speciation in food and environmental samples. Food Chem. 2020, 323, 126812. [Google Scholar] [CrossRef]
- Hassan, M.M.; Ahmad, W.; Zareef, M.; Rong, Y.; Xu, Y.; Jiao, T.; He, P.; Li, H.; Chen, Q. Rapid detection of mercury in food via rhodamine 6G signal using surface-enhanced Raman scattering coupled multivariate calibration. Food Chem. 2021, 358, 129844. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Dai, X.; Yang, R.; Liu, Z.; Chen, H.; Zhang, Y.; Zhang, X. Fenton-like catalytic MOFs driving electrochemical aptasensing toward tracking lead pollution in pomegranate fruit. Food Control 2025, 169, 111006. [Google Scholar] [CrossRef]
- Mukherjee, A.G.; Renu, K.; Gopalakrishnan, A.V.; Veeraraghavan, V.P.; Vinayagam, S.; Paz-Montelongo, S.; Dey, A.; Vellingiri, B.; George, A.; Madhyastha, H.; et al. Heavy Metal and Metalloid Contamination in Food and Emerging Technologies for Its Detection. Sustainability 2023, 15, 1195. [Google Scholar] [CrossRef]
- Wang, X.; Hu, X.; Zhai, X.; Huang, X.; Li, Z.; Zou, X.; Shi, J. A simple and sensitive electrochemical sensing based on amine-functionalized metal–organic framework and polypyrrole composite for detection of lead ions in meat samples. J. Food Meas. Charact. 2024, 18, 5813–5825. [Google Scholar] [CrossRef]
- Huang, X.; Huang, C.; Zhou, L.; Hou, G.; Sun, J.; Zhang, X.; Zou, X. Allosteric switch for electrochemical aptasensor toward heavy metals pollution of Lentinus edodes sensitized with porphyrinic metal-organic frameworks. Anal. Chim. Acta 2023, 1278, 341752. [Google Scholar] [CrossRef]
- Dominguez, A.N.; Jimenez, L.E.; Álvarez, R.M.S. Rapid detection of pyraclostrobin fungicide residues in lemon with surface-enhanced Raman spectroscopy. J. Food Meas. Charact. 2023, 17, 6350–6362. [Google Scholar] [CrossRef]
- Balram, D.; Lian, K.-Y.; Sebastian, N.; Rasana, N. Ultrasensitive detection of cytotoxic food preservative tert-butylhydroquinone using 3D cupric oxide nanoflowers embedded functionalized carbon nanotubes. J. Hazard. Mater. 2021, 406, 124792. [Google Scholar] [CrossRef]
- Sun, F.; Li, P.; Wu, G.; He, F.; Liu, S.; Shen, Y.; Wu, Y.; Li, L. Carbon nanomaterials-based smart dual-mode sensors for colorimetric and fluorescence detection of foodborne hazards. Trends Food Sci. Technol. 2024, 152, 104681. [Google Scholar] [CrossRef]
- Hassan, M.M.; Xu, Y.; Zareef, M.; Li, H.; Rong, Y.; Chen, Q. Recent advances of nanomaterial-based optical sensor for the detection of benzimidazole fungicides in food: A review. Crit. Rev. Food Sci. Nutr. 2021, 63, 2851–2872. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, M.; Wang, Q.; Wang, J.; Zhu, Y.; Bu, L.; Zhang, H.; Li, P.; Xu, W. Necklace-like Te-Au reticula platform with three dimensional hotspots Surface-Enhanced Raman Scattering (SERS) sensor for food hazards analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 311, 124037. [Google Scholar] [CrossRef]
- Zhao, Y.; Jing, X.; Zheng, F.; Liu, Y.; Fan, Y. Surface-Enhanced Raman Scattering-Active Plasmonic Metal Nanoparticle-Persistent Luminescence Material Composite Films for Multiple Illegal Dye Detection. Anal. Chem. 2021, 93, 8945–8953. [Google Scholar] [CrossRef]
- Okeke, E.S.; Ezeorba, T.P.C.; Okoye, C.O.; Chen, Y.; Mao, G.; Feng, W.; Wu, X. Analytical detection methods for azo dyes: A focus on comparative limitations and prospects of bio-sensing and electrochemical nano-detection. J. Food Compos. Anal. 2022, 114, 104778. [Google Scholar] [CrossRef]
- Rong, N.; He, S.; Li, B.; Lin, X.; Liu, X.; Yu, Y.; Feng, Y. Coupled magnetic nanoparticle-mediated isolation and single-cell image recognition to detect Bacillus’ cell size in soil. Eur. J. Soil Sci. 2022, 73, e13236. [Google Scholar] [CrossRef]
- Dai, J.; Bai, M.; Li, C.; Cui, H.; Lin, L. Advances in the mechanism of different antibacterial strategies based on ultrasound technique for controlling bacterial contamination in food industry. Trends Food Sci. Technol. 2020, 105, 211–222. [Google Scholar] [CrossRef]
- Song, S.; Liu, Z.; Huang, M.; Zhu, Q.; Qin, J.; Kim, M.S. Detection of fish bones in fillets by Raman hyperspectral imaging technology. J. Food Eng. 2020, 272, 109808. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, N.; Han, C.; Chen, Z.; Zhai, X.; Li, Z.; Zheng, K.; Zhu, J.; Wang, X.; Zou, X.; et al. Competitive immunosensor for sensitive and optical anti-interference detection of imidacloprid by surface-enhanced Raman scattering. Food Chem. 2021, 358, 129898. [Google Scholar] [CrossRef] [PubMed]
- Thapliyal, D.; Karale, M.; Diwan, V.; Kumra, S.; Arya, R.K.; Verros, G.D. Current Status of Sustainable Food Packaging Regulations: Global Perspective. Sustainability 2024, 16, 5554. [Google Scholar] [CrossRef]
- Abdul Halim, N.R.; Hashim, H.; Mutalib, S.A.; Ghani, M.A. Food safety regulations implementation and their impact on food security level in Malaysia: A review. Int. Food Res. J. 2024, 31, 20–31. [Google Scholar] [CrossRef]
- Cioca, A.-A.; Tušar, L.; Langerholc, T. Food Risk Analysis: Towards a Better Understanding of “Hazard” and “Risk” in EU Food Legislation. Foods 2023, 12, 2857. [Google Scholar] [CrossRef]
- Díaz-Galiano, F.J.; Murcia-Morales, M.; Gómez-Ramos, M.J.; Gómez-Ramos, M.d.M.; Fernández-Alba, A.R. Economic poisons: A review of food contact materials and their analysis using mass spectrometry. TrAC Trends Anal. Chem. 2024, 172, 117550. [Google Scholar] [CrossRef]
- Gu, Y.-X.; Yan, T.-C.; Yue, Z.-X.; Liu, F.-M.; Cao, J.; Ye, L.-H. Recent developments and applications in the microextraction and separation technology of harmful substances in a complex matrix. Microchem. J. 2022, 176, 107241. [Google Scholar] [CrossRef]
- Tsochatzis, E.D.; Alberto Lopes, J.; Hoekstra, E.; Emons, H. Development and validation of a multi-analyte GC-MS method for the determination of 84 substances from plastic food contact materials. Anal. Bioanal. Chem. 2020, 412, 5419–5434. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, J.; Wang, X.; Li, C.; Peng, T.; Miao, Q.; Du, X.; Lu, X. A facile molecularly imprinted column coupled to GC-MS/MS for sensitive and selective determination of polycyclic aromatic hydrocarbons and study on their migration in takeaway meal boxes. Talanta 2022, 243, 123385. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Q.; Belwal, T.; Lin, X.; Luo, Z. Insights into chemometric algorithms for quality attributes and hazards detection in foodstuffs using Raman/surface enhanced Raman spectroscopy. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2476–2507. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; He, Y.; Xu, W.; Chen, Q. Quantitative Detection of Acid Value During Edible Oil Storage by Raman Spectroscopy: Comparison of the Optimization Effects of BOSS and VCPA Algorithms on the Characteristic Raman Spectra of Edible Oils. Food Anal. Methods 2021, 14, 1826–1835. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, Y.; Shi, J.; Zhang, W.; Zhang, X.; Huang, X.; Zou, X.; Li, Z.; Wei, R. Facile synthesis of Au@Ag core–shell nanorod with bimetallic synergistic effect for SERS detection of thiabendazole in fruit juice. Food Chem. 2022, 370, 131276. [Google Scholar] [CrossRef] [PubMed]
- Fakhlaei, R.; Babadi, A.A.; Sun, C.; Ariffin, N.M.; Khatib, A.; Selamat, J.; Xiaobo, Z. Application, challenges and future prospects of recent nondestructive techniques based on the electromagnetic spectrum in food quality and safety. Food Chem. 2024, 441, 138402. [Google Scholar] [CrossRef]
- Hassane Hamadou, A.; Zhang, J.; Li, H.; Chen, C.; Xu, B. Modulating the glycemic response of starch-based foods using organic nanomaterials: Strategies and opportunities. Crit. Rev. Food Sci. Nutr. 2022, 63, 11942–11966. [Google Scholar] [CrossRef]
- Li, H.; Sheng, W.; Haruna, S.A.; Hassan, M.M.; Chen, Q. Recent advances in rare earth ion-doped upconversion nanomaterials: From design to their applications in food safety analysis. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3732–3764. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Huang, X.; Hu, X.; Li, Y.; Zhou, Y.; Wang, X.; Zhang, R.; Wei, X.; Zhai, X.; et al. H-Bond Modulation Mechanism for Moisture-driven Bacteriostat Evolved from Phytochemical Formulation. Adv. Funct. Mater. 2023, 34, 2312053. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Li, X.; Xiao, W.; Zou, X.; Huang, Q.; Zhou, L. Competitive electrochemical sensing for cancer cell evaluation based on thionine-interlinked signal probes. Analyst 2023, 148, 912–918. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.; Wang, H.; Huang, X.; Shi, Y.; Zou, Y.; Hu, X.; Li, Z.; Shi, J.; Zou, X. Energy difference-driven ROS reduction for electrochemical tracking crop growth sensitized with electron-migration nanostructures. Anal. Chim. Acta 2024, 1304, 342515. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Tian, J.; Li, Y.; Liu, Q.; Chen, X.; Feng, F.; Yu, X.; Yang, C. Multiomics analysis reveals that peach gum colouring reflects plant defense responses against pathogenic fungi. Food Chem. 2022, 383, 132424. [Google Scholar] [CrossRef]
- Qiu, J.; Gu, H.; Wang, S.; Ji, F.; He, C.; Jiang, C.; Shi, J.; Liu, X.; Shen, G.; Lee, Y.-W.; et al. A diverse Fusarium community is responsible for contamination of rice with a variety of Fusarium toxins. Food Res. Int. 2024, 195, 114987. [Google Scholar] [CrossRef]
- Hassan, M.M.; Zareef, M.; Xu, Y.; Li, H.; Chen, Q. SERS based sensor for mycotoxins detection: Challenges and improvements. Food Chem. 2021, 344, 128652. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tan, R.; Zeng, M.; Yuan, X.; Zhuang, K.; Feng, C.; He, Y.; Luo, X. SERS detection of triazole pesticide residues on vegetables and fruits using Au decahedral nanoparticles. Food Chem. 2024, 439, 138110. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Huang, C.; Wu, X.; Liu, X.-F.; You, E.-M.; Liu, S.-H.; Wang, A.; Jin, S.; Zhang, F.-L. 3D hot spot construction on the hydrophobic interface with SERS tags for quantitative detection of pesticide residues on food surface. Food Chem. 2025, 463, 141391. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, X.; Wang, Z.; Zhang, Y.; Huang, X.; Li, Z.; Daglia, M.; Xiao, J.; Shi, J.; Zou, X. Bioinspired nanozyme enabling glucometer readout for portable monitoring of pesticide under resource-scarce environments. Chem. Eng. J. 2022, 429, 132243. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Huang, X.; Huang, Q.; Wen, Y.; Li, B.; Holmes, M.; Shi, J.; Zou, X. Uniform stain pattern of robust MOF-mediated probe for flexible paper-based colorimetric sensing toward environmental pesticide exposure. Chem. Eng. J. 2023, 451, 138928. [Google Scholar] [CrossRef]
- Shenashen, M.A.; Emran, M.Y.; El Sabagh, A.; Selim, M.M.; Elmarakbi, A.; El-Safty, S.A. Progress in sensory devices of pesticides, pathogens, coronavirus, and chemical additives and hazards in food assessment: Food safety concerns. Prog. Mater. Sci. 2022, 124, 100866. [Google Scholar] [CrossRef]
- Ali, A.; Nettey-Oppong, E.E.; Effah, E.; Yu, C.Y.; Muhammad, R.; Soomro, T.A.; Byun, K.M.; Choi, S.H. Miniaturized Raman Instruments for SERS-Based Point-of-Care Testing on Respiratory Viruses. Biosensors 2022, 12, 590. [Google Scholar] [CrossRef]
- Beeram, R.; Vepa, K.R.; Soma, V.R. Recent Trends in SERS-Based Plasmonic Sensors for Disease Diagnostics, Biomolecules Detection, and Machine Learning Techniques. Biosensors 2023, 13, 328. [Google Scholar] [CrossRef]
- Chen, J.; Lin, H.; Guo, M.; Cao, L.; Sui, J.; Wang, K. Improving the detection accuracy of the dual SERS aptasensor system with uncontrollable SERS “hot spot” using machine learning tools. Anal. Chim. Acta 2024, 1307. [Google Scholar] [CrossRef]
- Lin, L.; Bi, X.; Gu, Y.; Wang, F.; Ye, J. Surface-enhanced Raman scattering nanotags for bioimaging. J. Appl. Phys. 2021, 129, 191101. [Google Scholar] [CrossRef]
- Petersen, M.; Yu, Z.; Lu, X. Application of Raman Spectroscopic Methods in Food Safety: A Review. Biosensors 2021, 11, 187. [Google Scholar] [CrossRef]
- Cai, L.; Fang, G.; Tang, J.; Cheng, Q.; Han, X. Label-Free Surface-Enhanced Raman Spectroscopic Analysis of Proteins: Advances and Applications. Int. J. Mol. Sci. 2022, 23, 3868. [Google Scholar] [CrossRef]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A Review on Surface-Enhanced Raman Scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Pisano, F.; Masmudi-Martín, M.; Andriani, M.S.; Cid, E.; Kazemzadeh, M.; Pisanello, M.; Balena, A.; Collard, L.; Parras, T.J.; Bianco, M.; et al. Vibrational fiber photometry: Label-free and reporter-free minimally invasive Raman spectroscopy deep in the mouse brain. Nat. Methods 2024, 22, 371–379. [Google Scholar] [CrossRef]
- Bonifacio, A.; Cervo, S.; Sergo, V. Label-free surface-enhanced Raman spectroscopy of biofluids: Fundamental aspects and diagnostic applications. Anal. Bioanal. Chem. 2015, 407, 8265–8277. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Czajkowsky, D.M.; Shao, Z.; Ye, J. Digital colloid-enhanced Raman spectroscopy by single-molecule counting. Nature 2024, 628, 771–775. [Google Scholar] [CrossRef]
- Ogundare, S.A.; van Zyl, W.E. A review of cellulose-based substrates for SERS: Fundamentals, design principles, applications. Cellulose 2019, 26, 6489–6528. [Google Scholar] [CrossRef]
- Harshita; Wu, H.F.; Kailasa, S.K. Recent advances in nanomaterials-based optical sensors for detection of various biomarkers (inorganic species, organic and biomolecules). Luminescence 2022, 38, 954–998. [Google Scholar] [CrossRef]
- Zhao, Y. On the Measurements of the Surface-Enhanced Raman Scattering Spectrum: Effective Enhancement Factor, Optical Configuration, Spectral Distortion, and Baseline Variation. Nanomaterials 2023, 13, 2998. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Auguié, B. Enhancement Factors: A Central Concept during 50 Years of Surface-Enhanced Raman Spectroscopy. ACS Nano 2024, 18, 9773–9783. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Mo, T.; Liu, Q. Surface-enhanced Raman scattering-based strategies for tumor markers detection: A review. Talanta 2024, 280, 126717. [Google Scholar] [CrossRef] [PubMed]
- Freund, R.; Canossa, S.; Cohen, S.M.; Yan, W.; Deng, H.; Guillerm, V.; Eddaoudi, M.; Madden, D.G.; Fairen-Jimenez, D.; Lyu, H.; et al. 25 Years of Reticular Chemistry. Angew. Chem. Int. Ed. 2021, 60, 23946–23974. [Google Scholar] [CrossRef]
- Litti, L.; Trivini, S.; Ferraro, D.; Reguera, J. 3D Printed Microfluidic Device for Magnetic Trapping and SERS Quantitative Evaluation of Environmental and Biomedical Analytes. ACS Appl. Mater. Interfaces 2021, 13, 34752–34761. [Google Scholar] [CrossRef]
- Karthick Kannan, P.; Shankar, P.; Blackman, C.; Chung, C.H. Recent Advances in 2D Inorganic Nanomaterials for SERS Sensing. Adv. Mater. 2019, 31, 1803432. [Google Scholar] [CrossRef]
- Sakthivel, R.; Keerthi, M.; Chung, R.-J.; He, J.-H. Heterostructures of 2D materials and their applications in biosensing. Prog. Mater. Sci. 2023, 132, 101024. [Google Scholar] [CrossRef]
- Naguib, M.; Barsoum, M.W.; Gogotsi, Y. Ten Years of Progress in the Synthesis and Development of MXenes. Adv. Mater. 2021, 33, 2103393. [Google Scholar] [CrossRef]
- Nithya, R.; Thirunavukkarasu, A.; Sathya, A.B.; Sivashankar, R. Magnetic materials and magnetic separation of dyes from aqueous solutions: A review. Environ. Chem. Lett. 2021, 19, 1275–1294. [Google Scholar] [CrossRef]
- Jabłońska, A.; Jaworska, A.; Kasztelan, M.; Berbeć, S.; Pałys, B. Graphene and Graphene Oxide Applications for SERS Sensing and Imaging. Curr. Med. Chem. 2019, 26, 6878–6895. [Google Scholar] [CrossRef]
- Butmee, P.; Samphao, A.; Tumcharern, G. Reduced graphene oxide on silver nanoparticle layers-decorated titanium dioxide nanotube arrays as SERS-based sensor for glyphosate direct detection in environmental water and soil. J. Hazard. Mater. 2022, 437, 129344. [Google Scholar] [CrossRef]
- Nasiri, H.; Abbasian, K. High-sensitive surface plasmon resonance sensor for melamine detection in dairy products based on graphene oxide /chitosan nanocomposite. Food Control 2024, 166, 110761. [Google Scholar] [CrossRef]
- Scaramuzza, S.; Polizzi, S.; Amendola, V. Magnetic tuning of SERS hot spots in polymer-coated magnetic–plasmonic iron–silver nanoparticles. Nanoscale Adv. 2019, 1, 2681–2689. [Google Scholar] [CrossRef] [PubMed]
- Parnsubsakul, A.; Ngoensawat, U.; Wutikhun, T.; Sukmanee, T.; Sapcharoenkun, C.; Pienpinijtham, P.; Ekgasit, S. Silver nanoparticle/bacterial nanocellulose paper composites for paste-and-read SERS detection of pesticides on fruit surfaces. Carbohydr. Polym. 2020, 235, 115956. [Google Scholar] [CrossRef]
- Kumar, A.; Santhanam, V. Paper swab based SERS detection of non-permitted colourants from dals and vegetables using a portable spectrometer. Anal. Chim. Acta 2019, 1090, 106–113. [Google Scholar] [CrossRef]
- He, Z.-H.; Zhu, W.-W.; Jiang, Y.-L.; Zhao, S.-S.; Yan, J.; Tan, X.-C. Green synthesis of paper-based SERS substrate for the quantitative detection of thiabendazole by wipe sampling. Microchem. J. 2024, 197, 109729. [Google Scholar] [CrossRef]
- Guan, T.; Yang, H.; Ou, M.; Zhang, J. Storage period affecting dynamic succession of microbiota and quality changes of strong-flavor Baijiu Daqu. LWT 2021, 139, 110544. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, A.; Lv, Z.; Gao, Z. Nondestructive measurement of kiwifruit firmness, soluble solid content (SSC), titratable acidity (TA), and sensory quality by vibration spectrum. Food Sci. Nutr. 2020, 8, 1058–1066. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Zhu, F.; Song, Y.; Yu, K.; Zhao, Y. Rapid qualitative detection of titanium dioxide adulteration in persimmon icing using portable Raman spectrometer and Machine learning. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 290, 122221. [Google Scholar] [CrossRef]
- Wu, X.; Liang, X.; Wang, Y.; Wu, B.; Sun, J. Non-Destructive Techniques for the Analysis and Evaluation of Meat Quality and Safety: A Review. Foods 2022, 11, 3713. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, X.; Xu, Y.; Wang, X.; Guo, Z.; Huang, X.; Li, Z.; Shi, J.; Zou, X. Single-step electrochemical sensing of ppt-level lead in leaf vegetables based on peroxidase-mimicking metal-organic framework. Biosens. Bioelectron. 2020, 168, 112544. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, Y.; Zhu, M.; Xu, Y.; Guo, Z.; Shi, J.; Han, E.; Zou, X.; Wang, D. Electrochemical DNA sensor for inorganic mercury(II) ion at attomolar level in dairy product using Cu(II)-anchored metal-organic framework as mimetic catalyst. Chem. Eng. J. 2020, 383, 123182. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, M.; Jiang, Y.; Wang, X.; Guo, Z.; Shi, J.; Zou, X.; Han, E. Simple electrochemical sensing for mercury ions in dairy product using optimal Cu2+-based metal-organic frameworks as signal reporting. J. Hazard. Mater. 2020, 400, 123222. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, J.; Hwang, S.; Choi, H.; Kim, J.; Lim, S.-H. UiO-66–coated hybrid micro-gas chromatography column chips: Evaluation of gas preconcentration and separation performances as a function of particle size, pore size, time, concentration, and flow rate. Chem. Eng. J. 2024, 500, 157075. [Google Scholar] [CrossRef]
- Tian, Z.; Xu, D.; Yang, S.; Wang, B.; Zhang, Z. Highly ordered nanocavity as photonic-plasmonic-polaritonic resonator for single molecule miRNA SERS detection. Biosens. Bioelectron. 2024, 254, 116231. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Zhang, Z. Photonic–plasmonic resonator for SERS biodetection. Analyst 2024, 149, 3123–3130. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.; Wang, J.; Huang, X.; El-Mesery, H.S.; Shi, Y.; Zou, Y.; Li, Z.; Li, Y.; Shi, J.; et al. Simple-easy electrochemical sensing mode assisted with integrative carbon-based gel electrolyte for in-situ monitoring of plant hormone indole acetic acid. Food Chem. 2025, 467, 142342. [Google Scholar] [CrossRef]
- Ying, Y.; Tang, Z.; Liu, Y. Material design, development, and trend for surface-enhanced Raman scattering substrates. Nanoscale 2023, 15, 10860–10881. [Google Scholar] [CrossRef]
- Chang, K.; Zhao, Y.; Wang, M.; Xu, Z.; Zhu, L.; Xu, L.; Wang, Q. Advances in metal-organic framework-plasmonic metal composites based SERS platforms: Engineering strategies in chemical sensing, practical applications and future perspectives in food safety. Chem. Eng. J. 2023, 459, 141539. [Google Scholar] [CrossRef]
- Guo, Z.; Zheng, Y.; Yin, L.; Xue, S.; Ma, L.; Zhou, R.; El-Seedi, H.R.; Zhang, Y.; Yosri, N.; Jayan, H.; et al. Flexible Au@AgNRs/MAA/PDMS-based SERS sensor coupled with intelligent algorithms for in-situ detection of thiram on apple. Sens. Actuators B Chem. 2024, 404, 135303. [Google Scholar] [CrossRef]
- Hu, B.; Sun, D.-W.; Pu, H.; Wei, Q. Rapid nondestructive detection of mixed pesticides residues on fruit surface using SERS combined with self-modeling mixture analysis method. Talanta 2020, 217, 120998. [Google Scholar] [CrossRef]
- Wang, K.; Sun, D.-W.; Pu, H.; Wei, Q. Two-dimensional Au@Ag nanodot array for sensing dual-fungicides in fruit juices with surface-enhanced Raman spectroscopy technique. Food Chem. 2020, 310, 125923. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Xu, Q.; Yin, L.; Zou, C.; Wu, W.; Wang, C.; Zhou, R.; Guo, Z.; Cai, J. Surface-enhanced Raman scattering sensor based on cysteine-mediated nucleophilic addition reaction for detection of patulin. Microchem. J. 2024, 204, 111021. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, N.; Yan, J.; Cui, K.; Chu, Q.; Chen, X.; Luo, X.; Deng, X. A dual-signaling surface-enhanced Raman spectroscopy ratiometric strategy for ultrasensitive Hg2+ detection based on Au@Ag/COF composites. Food Chem. 2024, 456, 139998. [Google Scholar] [CrossRef]
- Ding, Q.; Wang, J.; Chen, X.; Liu, H.; Li, Q.; Wang, Y.; Yang, S. Quantitative and Sensitive SERS Platform with Analyte Enrichment and Filtration Function. Nano Lett. 2020, 20, 7304–7312. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, Y.; Wei, J.; Wang, Z.; Ma, X. A facile dual-mode SERS/fluorescence aptasensor for AFB1 detection based on gold nanoparticles and magnetic nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 315, 124268. [Google Scholar] [CrossRef]
- Sakir, M.; Salem, S.; Sanduvac, S.T.; Sahmetlioglu, E.; Sarp, G.; Onses, M.S.; Yilmaz, E. Photocatalytic green fabrication of Au nanoparticles on ZnO nanorods modified membrane as flexible and photocatalytic active reusable SERS substrates. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124088. [Google Scholar] [CrossRef]
- Chen, J.; Cao, X.; Liu, W.; Liu, J.; Qi, L.; Wei, M.; Zou, X. Functionalized MXene (Ti3C2TX) Loaded with Ag Nanoparticles as a Raman Scattering Substrate for Rapid Furfural Detection in Baijiu. Foods 2024, 13, 3064. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, D.-W.; Pu, H.; Wei, Q.; Lin, X. Ti3C2Tx MXenes loaded with Au nanoparticle dimers as a surface-enhanced Raman scattering aptasensor for AFB1 detection. Food Chem. 2022, 372, 131293. [Google Scholar] [CrossRef]
- Hu, X.; Yang, B.; Wen, X.; Su, J.; Jia, B.; Fu, F.; Zhang, Y.; Yu, Q.; Liu, X. One-Pot Synthesis of a Three-Dimensional Au-Decorated Cellulose Nanocomposite as a Surface-Enhanced Raman Scattering Sensor for Selective Detection and in Situ Monitoring. ACS Sustain. Chem. Eng. 2021, 9, 3324–3336. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, X.; Zhang, W.; Song, L.; Wei, H.; Xiu, H.; Zhang, M.; Shang, M.; Wang, C. A SERS-based point-of-care testing approach for efficient determination of diquat and paraquat residues using a flexible silver flower-coated melamine sponge. Food Chem. 2024, 454, 139831. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.; Jeon, Y.; Kwon, G.; Kim, U.-J.; Oh, C.-S.; Kim, J.; You, J. Plasmonic nanoparticle-analyte nanoarchitectronics combined with efficient analyte deposition method on regenerated cellulose-based SERS platform. Cellulose 2021, 28, 11493–11502. [Google Scholar] [CrossRef]
- Song, S.W.; Kim, D.; Kim, J.; You, J.; Kim, H.M. Flexible nanocellulose-based SERS substrates for fast analysis of hazardous materials by spiral scanning. J. Hazard. Mater. 2021, 414, 125160. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, J.; Henzie, J.; Ko, Y.; Lim, H.; Kwon, G.; Na, J.; Kim, H.-J.; Yamauchi, Y.; You, J. Mesoporous Au films assembled on flexible cellulose nanopaper as high-performance SERS substrates. Chem. Eng. J. 2021, 419, 129445. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Xue, Z.; Peng, B.; Kou, X.; Gao, Z. Research progress of dual-mode sensing technology strategy based on SERS and its application in the detection of harmful substances in foods. Trends Food Sci. Technol. 2024, 148, 104487. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, L.; Jing, X.; Miao, H.; Zhao, Y. SERS-Active Composites with Au–Ag Janus Nanoparticles/Perovskite in Immunoassays for Staphylococcus aureus Enterotoxins. ACS Appl. Mater. Interfaces 2022, 14, 3293–3301. [Google Scholar] [CrossRef]
- Tang, X.; Hao, Q.; Hou, X.; Lan, L.; Li, M.; Yao, L.; Zhao, X.; Ni, Z.; Fan, X.; Qiu, T. Exploring and Engineering 2D Transition Metal Dichalcogenides toward Ultimate SERS Performance. Adv. Mater. 2024, 36, 2312348. [Google Scholar] [CrossRef]
- Ma, H.; Pan, S.-Q.; Wang, W.-L.; Yue, X.; Xi, X.-H.; Yan, S.; Wu, D.-Y.; Wang, X.; Liu, G.; Ren, B. Surface-Enhanced Raman Spectroscopy: Current Understanding, Challenges, and Opportunities. ACS Nano 2024, 18, 14000–14019. [Google Scholar] [CrossRef]
- Hang, Y.; Wang, A.; Wu, N. Plasmonic silver and gold nanoparticles: Shape- and structure-modulated plasmonic functionality for point-of-caring sensing, bio-imaging and medical therapy. Chem. Soc. Rev. 2024, 53, 2932–2971. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zheng, X.; Wang, H.; Yan, M.; Chen, Z.; Yang, Q.; Shao, Y. Research advances of SERS analysis method based on silent region molecules for food safety detection. Microchim. Acta 2023, 190, 387. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, J.; Zhu, X.; Liu, Z.; Huang, J.; Jiang, X.; Fu, F.; Lin, Z.; Dong, Y. Hybridizing Silver Nanoparticles in Hydrogel for High-Performance Flexible SERS Chips. ACS Appl. Mater. Interfaces 2022, 14, 26216–26224. [Google Scholar] [CrossRef]
- Pino-Sandoval, D.; Villanueva-Rodríguez, M.; Cantú-Cárdenas, M.E.; Hernández-Ramírez, A. Performance of Ag-Cu/TiO2 photocatalyst prepared by sol-gel method on the inactivation of Escherichia coli and Salmonella typhimurium. J. Environ. Chem. Eng. 2020, 8, 104539. [Google Scholar] [CrossRef]
- Dong, H.; Bai, W.; Zheng, S.; Wang, Q.; Zhang, L.; Hu, Q.; Liu, Y.; Wang, C.; Wang, S. Fabrication of Raman reporter molecule–embedded magnetic SERS tag for ultrasensitive immunochromatographic monitoring of Cd ions and clenbuterol in complex samples. Colloids Surf. A Physicochem. Eng. Asp. 2024, 702, 135159. [Google Scholar] [CrossRef]
- Jiang, L.; Wei, W.; Liu, S.; Haruna, S.A.; Zareef, M.; Ahmad, W.; Hassan, M.M.; Li, H.; Chen, Q. A tailorable and recyclable TiO2 NFSF/Ti@Ag NPs SERS substrate fabricated by a facile method and its applications in prohibited fish drugs detection. J. Food Meas. Charact. 2022, 16, 2890–2898. [Google Scholar] [CrossRef]
- Liu, R.; Wang, J.; Cui, L.; Tan, R.; Li, S.; Qin, N.; Yu, B. Reusable Ag SERS substrates fabricated by tip-based mechanical lithography. Opt. Mater. 2024, 156, 115977. [Google Scholar] [CrossRef]
- Peng, J.; Liu, P.; Chen, Y.; Guo, Z.-H.; Liu, Y.; Yue, K. Templated synthesis of patterned gold nanoparticle assemblies for highly sensitive and reliable SERS substrates. Nano Res. 2022, 16, 5056–5064. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, S. Highly sensitive, reproducible, and stable core–shell MoN SERS substrate synthesized via sacrificial template method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 327, 125322. [Google Scholar] [CrossRef]
- Yang, M.-C.; Chien, T.-Y.; Cheng, Y.-W.; Hsieh, C.-K.; Syu, W.-L.; Wang, K.-S.; Chen, Y.-C.; Chen, J.-S.; Chen, C.-C.; Liu, T.-Y. Reproducible SERS substrates manipulated by interparticle spacing and particle diameter of gold nano-island array using in-situ thermal evaporation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 303, 123190. [Google Scholar] [CrossRef]
- Zhou, Z.; Xie, A.; Tan, Y.; Zhang, J.; Xue, C. Vacuum-assisted thermal evaporation deposition for the preparation of AgNPs/NF 3D SERS substrates and their applications. New J. Chem. 2023, 47, 21225–21231. [Google Scholar] [CrossRef]
- Siddiqui, S.; Niazi, J.H.; Qureshi, A. Mn3O4–Au nanozymes as peroxidase mimic and the surface-enhanced Raman scattering nanosensor for the detection of hydrogen peroxide. Mater. Today Chem. 2021, 22, 100560. [Google Scholar] [CrossRef]
- Zhang, N.; Cui, L.; Yu, X.; Yu, Q.; Zhao, J. Fabrication of blue silver substrate with 10 nm grains by an electrochemical deposition and application in SERS. J. Electroanal. Chem. 2023, 946, 117700. [Google Scholar] [CrossRef]
- Peng, R.; Zhang, T.; Wang, S.; Liu, Z.; Pan, P.; Xu, X.; Song, Y.; Liu, X.; Yan, S.; Wang, J. Self-Assembly of Strain-Adaptable Surface-Enhanced Raman Scattering Substrate on Polydimethylsiloxane Nanowrinkles. Anal. Chem. 2024, 96, 10620–10629. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, Y.; Wang, W.; Yu, D.; Yang, L.; Jiang, X.; Song, W.; Zhao, B. A new semiconductor heterojunction SERS substrate for ultra-sensitive detection of antibiotic residues in egg. Food Chem. 2024, 431, 137163. [Google Scholar] [CrossRef]
- Li, B.; Liu, S.; Huang, L.; Jin, M.; Wang, J. Nanohybrid SERS substrates intended for food supply chain safety. Coord. Chem. Rev. 2023, 494, 215349. [Google Scholar] [CrossRef]
- Yin, B.; Ho, W.K.H.; Zhang, Q.; Li, C.; Huang, Y.; Yan, J.; Yang, H.; Hao, J.; Wong, S.H.D.; Yang, M. Magnetic-Responsive Surface-Enhanced Raman Scattering Platform with Tunable Hot Spot for Ultrasensitive Virus Nucleic Acid Detection. ACS Appl. Mater. Interfaces 2022, 14, 4714–4724. [Google Scholar] [CrossRef]
- Lee, H.K.; Lee, Y.H.; Koh, C.S.L.; Phan-Quang, G.C.; Han, X.; Lay, C.L.; Sim, H.Y.F.; Kao, Y.-C.; An, Q.; Ling, X.Y. Designing surface-enhanced Raman scattering (SERS) platforms beyond hotspot engineering: Emerging opportunities in analyte manipulations and hybrid materials. Chem. Soc. Rev. 2019, 48, 731–756. [Google Scholar] [CrossRef]
- López-Lorente, Á.I. Recent developments on gold nanostructures for surface enhanced Raman spectroscopy: Particle shape, substrates and analytical applications. A review. Anal. Chim. Acta 2021, 1168, 338474. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Chen, Y.; Wei, Y.; Li, G.; Tang, X.; Chen, D.; Zhu, X.; Yao, L.; Zhao, X.; Li, M.; et al. Mechanism Switch in Surface-Enhanced Raman Scattering: The Role of Nanoparticle Dimensions. J. Phys. Chem. Lett. 2024, 15, 7183–7190. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhu, K.; Wang, Y.; Qian, Z.; Zhang, Y.; Yang, Z.; Wang, Z.; Wu, L.; Zong, S.; Cui, Y. Ti3C2Tx MXene-Loaded 3D Substrate toward On-Chip Multi-Gas Sensing with Surface-Enhanced Raman Spectroscopy (SERS) Barcode Readout. ACS Nano 2021, 15, 12996–13006. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; Wang, C.; Tian, X.; Chang, X.; Ren, Y.; Yu, S. Applications of surface functionalized Fe3O4 NPs-based detection methods in food safety. Food Chem. 2021, 342, 128343. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, L.; Miao, H.; Jing, X. “Add on” Dual-Modal Optical Immunoassay by Plasmonic Metal NP-Semiconductor Composites. Anal. Chem. 2021, 93, 3250–3257. [Google Scholar] [CrossRef]
- Yao, J.; Jin, Z.; Zhao, Y. Electroactive and SERS-Active Ag@Cu2O NP-Programed Aptasensor for Dual-Mode Detection of Tetrodotoxin. ACS Appl. Mater. Interfaces 2023, 15, 10240–10249. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; He, B.; Liu, Y.; Wang, L.; Liang, Y.; Wang, J.; Jin, H.; Wei, M.; Ren, W.; Suo, Z.; et al. A dual-signal mode electrochemical aptasensor based on tetrahedral DNA nanostructures for sensitive detection of citrinin in food using PtPdCo mesoporous nanozymes. Food Chem. 2024, 460, 140739. [Google Scholar] [CrossRef]
- Xu, X.; Lu, S.; Zhang, Z. Hydrogel/MOF Dual-Modified Photoelectrochemical Biosensor for Antibiofouling and Biocompatible Dopamine Detection. Langmuir 2024, 40, 10718–10725. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; He, B.; Zhao, R.; Ren, W.; Suo, Z.; Xu, Y.; Xie, D.; Zhao, W.; Wei, M.; Jin, H. Electrochemical aptasensor based on CRISPR/Cas12a-mediated and DNAzyme-assisted cascade dual-enzyme transformation strategy for zearalenone detection. Chem. Eng. J. 2024, 493, 152431. [Google Scholar] [CrossRef]
- Li, J.; Zhou, X.; Zhang, C.; Zhao, Y.; Zhu, Y.; Zhang, J.; Bai, J.; Xiao, X. The Effects of Carbendazim on Acute Toxicity, Development, and Reproduction in Caenorhabditis elegans. J. Food Qual. 2020, 2020, 8853537. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, C.; Guo, Z.; Yang, T.; Zhang, X.; Huang, X.; Shi, J.; Gao, S.; Zou, X. Ultrasensitive Analysis of Escherichia coli O157:H7 Based on Immunomagnetic Separation and Labeled Surface-Enhanced Raman Scattering with Minimized False Positive Identifications. J. Agric. Food Chem. 2024, 72, 22349–22359. [Google Scholar] [CrossRef]
- Suzuki, Y.; Shimizu, H.; Tamai, S.; Hoshiko, Y.; Maeda, T.; Nukazawa, K.; Iguchi, A.; Masago, Y.; Ishii, S. Simultaneous detection of various pathogenic Escherichia coli in water by sequencing multiplex PCR amplicons. Environ. Monit. Assess. 2023, 195, 264. [Google Scholar] [CrossRef]
- Gao, M.; Tan, F.; Shen, Y.; Peng, Y. Rapid detection method of bacterial pathogens in surface waters and a new risk indicator for water pathogenic pollution. Sci. Rep. 2024, 14, 1614. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, Y.; Wei, J.; Deng, Y.; Deng, R.; Yang, H. Endoprotein-activating DNAzyme assay for nucleic acid extraction- and amplification-free detection of viable pathogenic bacteria. Biosens. Bioelectron. 2024, 266, 116715. [Google Scholar] [CrossRef]
- Deng, R.; Bai, J.; Yang, H.; Ren, Y.; He, Q.; Lu, Y. Nanotechnology-leveraged nucleic acid amplification for foodborne pathogen detection. Coord. Chem. Rev. 2024, 506, 215745. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, S.; Zhang, D.; Zhou, T.; Huang, J.; Gao, M.; Jiang, Y.; Liu, Y.; Yang, J. Ultrasensitive dual-enhanced sandwich strategy for simultaneous detection of Escherichia coli and Staphylococcus aureus based on optimized aptamers-functionalized magnetic capture probes and graphene oxide-Au nanostars SERS tags. J. Colloid Interface Sci. 2023, 634, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Sun, D.-W.; Wu, Z.; Pu, H.; Wei, Q. Reproducible, shelf-stable, and bioaffinity SERS nanotags inspired by multivariate polyphenolic chemistry for bacterial identification. Anal. Chim. Acta 2021, 1167, 338570. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; He, Y.; Song, Z.; Khan, I.M.; Wang, Z.; Jiang, C.; Ma, X. Satellite nanostructures composed of CdTe quantum dots and DTNB-labeled AuNPs used for SERS-fluorescence dual-signal detection of AFB1. Food Control 2024, 156, 110112. [Google Scholar] [CrossRef]
- Xie, G.; Liu, L.; Gong, Y.; Zhang, G.; Huang, J.; Xu, H.; Wang, J. Development of tri-mode lateral flow immunoassay based on tailored porous gold nanoflower for sensitive detection of aflatoxin B1. Food Biosci. 2024, 61, 104700. [Google Scholar] [CrossRef]
- Peng, R.; Qi, W.; Deng, T.; Si, Y.; Li, J. Development of surface-enhanced Raman scattering-sensing Method by combining novel Ag@Au core/shell nanoparticle-based SERS probe with hybridization chain reaction for high-sensitive detection of hepatitis C virus nucleic acid. Anal. Bioanal. Chem. 2024, 416, 2515–2525. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, X.; Liu, L.; Jiang, S.; Xia, L.; Li, D.; Wu, G.; Sun, Z.; Zhang, Z.; Li, Y. Low-Temperature Substrate: Detection of Viruses on Cold Chain Food Packaging Based on Surface-Enhanced Raman Spectroscopy. ACS Mater. Lett. 2024, 6, 4649–4657. [Google Scholar] [CrossRef]
- Sharma, S.; Sahu, B.K.; Cao, L.; Bindra, P.; Kaur, K.; Chandel, M.; Koratkar, N.; Huang, Q.; Shanmugam, V. Porous nanomaterials: Main vein of agricultural nanotechnology. Prog. Mater. Sci. 2021, 121, 100812. [Google Scholar] [CrossRef]
- Chen, T.; Liang, W.; Zhang, X.; Wang, Y.; Lu, X.; Zhang, Y.; Zhang, Z.; You, L.; Liu, X.; Zhao, C.; et al. Screening and identification of unknown chemical contaminants in food based on liquid chromatography–high-resolution mass spectrometry and machine learning. Anal. Chim. Acta 2024, 1287, 342116. [Google Scholar] [CrossRef]
- Sun, Q.; Dong, Y.; Wen, X.; Zhang, X.; Hou, S.; Zhao, W.; Yin, D. A review on recent advances in mass spectrometry analysis of harmful contaminants in food. Front. Nutr. 2023, 10, 1244459. [Google Scholar] [CrossRef]
- Su, X.; Chen, Z.; Wang, H.; Yuan, L.; Zheng, K.; Zhang, W.; Zou, X. Ratiometric immunosensor with DNA tetrahedron nanostructure as high-performance carrier of reference signal and its applications in selective phoxim determination for vegetables. Food Chem. 2022, 383, 132445. [Google Scholar] [CrossRef]
- Ding, Y.; Sun, Y.; Liu, C.; Jiang, Q.Y.; Chen, F.; Cao, Y. SERS-Based Biosensors Combined with Machine Learning for Medical Application**. ChemistryOpen 2023, 12, e202200192. [Google Scholar] [CrossRef]
- Dong, Y.; Hu, J.; Jin, J.; Zhou, H.; Jin, S.; Yang, D. Advances in machine learning-assisted SERS sensing towards food safety and biomedical analysis. TrAC Trends Anal. Chem. 2024, 180, 117974. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, M.; Chen, Y.; Tang, F.; Dai, J.; Jin, Y.; Wang, C.; Xue, F. Ultrasensitive and selective detection of sulfamethazine in milk via a Janus-labeled Au nanoparticle-based surface-enhanced Raman scattering-immunochromatographic assay. Talanta 2024, 267, 125208. [Google Scholar] [CrossRef]
- He, X.; Yang, S.; Xu, T.; Song, Y.; Zhang, X. Microdroplet-captured tapes for rapid sampling and SERS detection of food contaminants. Biosens. Bioelectron. 2020, 152, 112013. [Google Scholar] [CrossRef]
- Hu, W.; Xia, L.; Hu, Y.; Li, G. Fe3O4-WO3−X@AuNPs for magnetic separation, enrichment and surface-enhanced Raman scattering analysis all-in-one of albendazole and streptomycin in meat samples. Sens. Actuators B Chem. 2024, 402, 135131. [Google Scholar] [CrossRef]
- Tu, J.; Wu, T.; Yu, Q.; Li, J.; Zheng, S.; Qi, K.; Sun, G.; Xiao, R.; Wang, C. Introduction of multilayered magnetic core–dual shell SERS tags into lateral flow immunoassay: A highly stable and sensitive method for the simultaneous detection of multiple veterinary drugs in complex samples. J. Hazard. Mater. 2023, 448, 130912. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Hassan, M.M.; Ali, S.; Li, H.; Sheng, R.; Chen, Q. Evolving trends in SERS-based techniques for food quality and safety: A review. Trends Food Sci. Technol. 2021, 112, 225–240. [Google Scholar] [CrossRef]
- Yue, P.; Zhang, M.; Zhao, T.; Liu, P.; Peng, F.; Yang, L. Eco-friendly epoxidized Eucommia ulmoides gum based composite coating with enhanced super-hydrophobicity and corrosion resistance properties. Ind. Crop. Prod. 2024, 214, 118523. [Google Scholar] [CrossRef]
- Xia, X.; Zhou, C.; Zhu, Y.; Dong, Y.; He, Q.; Khan, M.R.; Chi, Y.; Busquets, R.; Deng, R.; Ren, Y. Tb 3+-nucleic acid probe-based label-free and rapid detection of mercury pollution in food. Food Sci. Hum. Wellness 2024, 13, 993–998. [Google Scholar] [CrossRef]
- Li, M.; Liu, H.; He, B.; Xie, L.; Cao, X.; Jin, H.; Wei, M.; Ren, W.; Suo, Z.; Xu, Y. Ultrasensitive label-free electrochemical aptasensor for Pb2+ detection exploiting Exo III amplification and AgPt/GO nanocomposite-enhanced transduction. Talanta 2024, 276, 126260. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Zhang, W.; Li, Y.; Zhang, X.; Huang, X.; Shi, Y.; Zou, Y.; Li, Z.; Shi, J.; et al. Highly catalytic Ce-based MOF for powering electrochemical aptasensing toward evaluating dissolution rate of microelement copper from tea-leaves. J. Food Compos. Anal. 2025, 140, 107266. [Google Scholar] [CrossRef]
- Chen, Q.; Tang, J.; Huang, Z.; Li, H.; Chen, Z.; Xiang, L. SERS scaffold based on silver nanoparticles with multi-ingredient heavy metal ligands for the determination of Mn(II). Colloid Polym. Sci. 2023, 301, 949–956. [Google Scholar] [CrossRef]
- Chen, P.; Yin, L.; El-Seedi, H.R.; Zou, X.; Guo, Z. Green reduction of silver nanoparticles for cadmium detection in food using surface-enhanced Raman spectroscopy coupled multivariate calibration. Food Chem. 2022, 394, 133481. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, H.; Zou, X.; Meng, G.; Wu, N. Raman spectroscopy for food quality assurance and safety monitoring: A review. Curr. Opin. Food Sci. 2022, 47, 100910. [Google Scholar] [CrossRef]
- Santhoshkumar, S.; Murugan, E. Rationally designed SERS AgNPs/GO/g-CN nanohybrids to detect methylene blue and Hg2+ ions in aqueous solution. Appl. Surf. Sci. 2021, 553, 149544. [Google Scholar] [CrossRef]
- Lu, Z.; Wei, W.; Yang, J.; Xu, Q.; Hu, X.-Y. Improved SERS performance of a silver triangular nanoparticle/TiO2 nanoarray heterostructure and its application for food additive detection. New J. Chem. 2022, 46, 7070–7077. [Google Scholar] [CrossRef]
- Ramachandran, K.; Hamdi, A.; Columbus, S.; Meselmene, N.A.; Dogheche, E.; Daoudi, K.; Gaidi, M. Synergism induced sensitive SERS sensing to detect 2,6-Di-t-butyl-p-hydroxytoluene (BHT) with silver nanotriangles sensitized ZnO nanorod arrays for food security applications. Surf. Interfaces 2022, 35, 102407. [Google Scholar] [CrossRef]
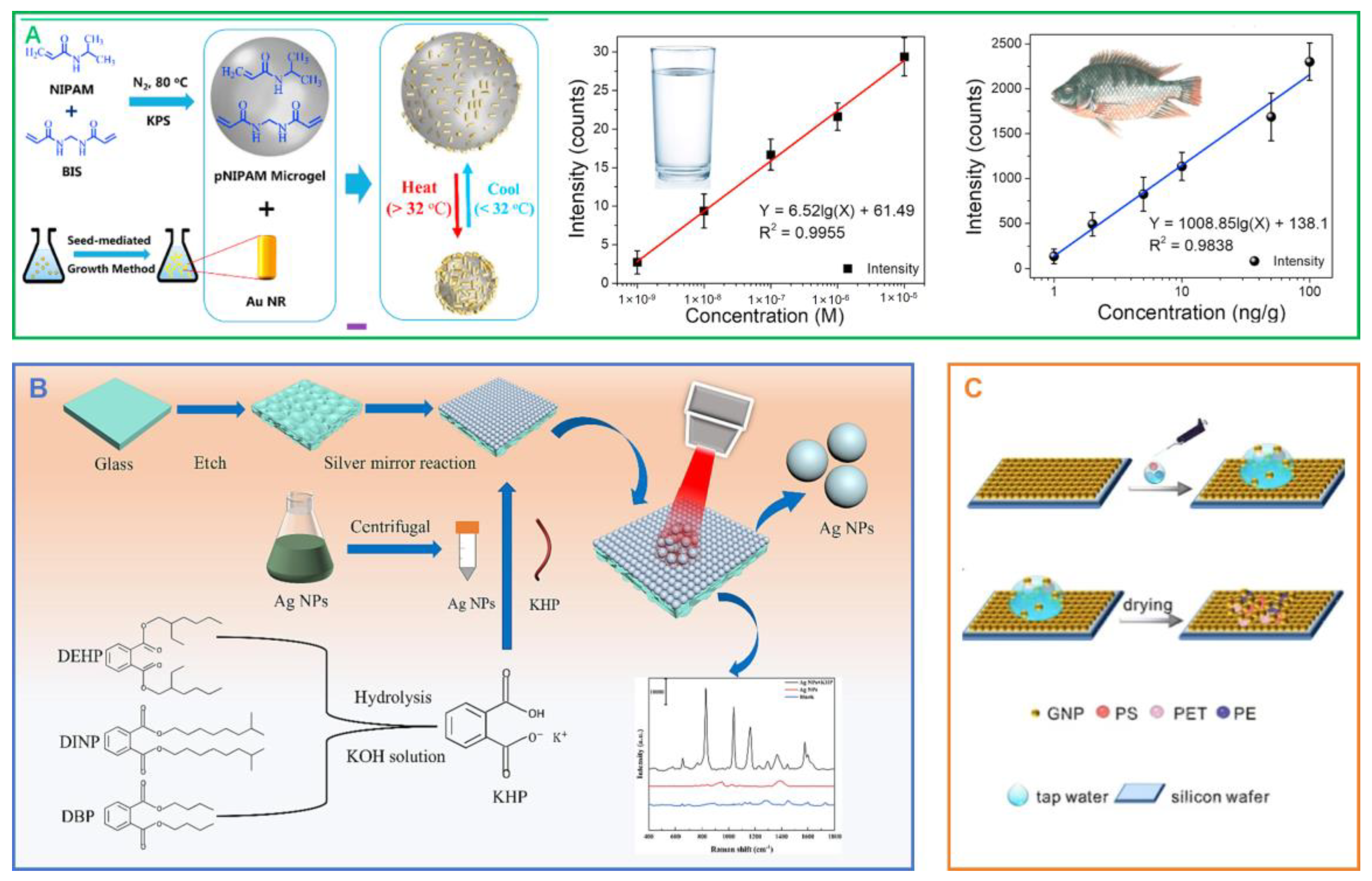
- Hu, B.; Sun, D.-W.; Pu, H.; Wei, Q. A dynamically optical and highly stable pNIPAM @ Au NRs nanohybrid substrate for sensitive SERS detection of malachite green in fish fillet. Talanta 2020, 218, 121188. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Huang, J.; Liu, Y.; Wu, Y.; You, R.; Zhang, J.-H.; Lu, Y.; Shen, H. Preparation of SERS substrate with 2D silver plate and nano silver sol for plasticizer detection in edible oil. Food Chem. 2023, 409, 135363. [Google Scholar] [CrossRef]
- Michaels, B.S.; Ayers, T.; Brooks-McLaughlin, J.; McLaughlin, R.J.; Sandoval-Warren, K.; Schlenker, C.; Ronaldson, L.; Ardagh, S. Potential for Glove Risk Amplification via Direct Physical, Chemical, and Microbiological Contamination. J. Food Prot. 2024, 87, 100283. [Google Scholar] [CrossRef]
- Pakdel, M.; Olsen, A.; Bar, E.M.S. A Review of Food Contaminants and Their Pathways Within Food Processing Facilities Using Open Food Processing Equipment. J. Food Prot. 2023, 86, 100184. [Google Scholar] [CrossRef] [PubMed]
- Thakali, A.; MacRae, J.D.; Isenhour, C.; Blackmer, T. Composition and contamination of source separated food waste from different sources and regulatory environments. J. Environ. Manag. 2022, 314, 115043. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Yang, J.-Y.; Wang, Y.-T.; Zhang, H.-C.; Chen, M.-L.; Yang, T.; Wang, J.-H. M13 phage-based nanoprobe for SERS detection and inactivation of Staphylococcus aureus. Talanta 2021, 221, 121668. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Wang, Z.; Peng, L.; Xu, Y.; Jiao, T.; Ouyang, Q.; Chen, Q. Sensitive SERS detection of S. aureus via HCR-mediated G-quadruplex DNAzyme assembly. Sens. Actuators B Chem. 2025, 425, 136977. [Google Scholar] [CrossRef]
- Kumari, A.; Das, S.; Thapa, P.; Meenakshi; Kumar, A.; Nagpal, P.; Dubey, S.K.; Perumal, V.; Mehta, D.S. Surface plasmon enhanced auto-fluorescence and Raman spectroscopy for low-level detection of biological pathogens. Methods Appl. Fluoresc. 2025, 13, 015004. [Google Scholar] [CrossRef]
- Yuwen, L.; Ni, J.; Liang, J.; Liu, X.; Chen, Z.; Li, X.; Lv, H.; Zhang, J.; Song, C. Portable SERS biosensor based on aptamer-assisted catalytic hairpin assembly signal amplification for ultrasensitive detection of Staphylococcus aureus. Talanta 2024, 278, 126565. [Google Scholar] [CrossRef]
- Ma, X.; Lin, X.; Xu, X.; Wang, Z. Fabrication of gold/silver nanodimer SERS probes for the simultaneous detection of Salmonella typhimurium and Staphylococcus aureus. Microchim. Acta 2021, 188, 202. [Google Scholar] [CrossRef]
- Zhao, Y.; Guan, M.; Mi, F.; Zhang, Y.; Geng, P.; Zhang, S.; Song, H.; Chen, G. A SERS/colorimetric biosensor based on AuNSs@Ag core–shell Prussian blue nanozyme for non-interference and rapid detection of Staphylococcus aureus in milk. Microchim. Acta 2025, 192, 83. [Google Scholar] [CrossRef]
- Huang, X.; Yang, Y.; Zhou, H.; Hu, L.; Yang, A.; Jin, H.; Zheng, B.; Pi, J.; Xu, J.; Sun, P.; et al. Coupling of an Au@AgPt nanozyme array with an micrococcal nuclease-specific responsiveness strategy for colorimetric/SERS sensing of Staphylococcus aureus in patients with sepsis. J. Pharm. Anal. 2025, 15, 101085. [Google Scholar] [CrossRef]
- Zhu, A.; Ahmad, W.; Xu, Y.; Wei, W.; Jiao, T.; Ouyang, Q.; Chen, Q. Trace detection of S. aureus cells in food samples via RCA-assisted SERS signal amplification with core-shell nanoprobe. Talanta 2025, 286, 127458. [Google Scholar] [CrossRef]
- Muthukumar, D.; Shtenberg, G. SERS-based immunosensor for E. coli contaminants detection in milk using silver-coated nanoporous silicon substrates. Talanta 2023, 254, 124132. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, D.; Shtenberg, G. Quantitative detection of Staphylococcus aureus using aptamer-based bioassay coupled with porous Si SERS platform. J. Sci. Adv. Mater. Devices 2024, 9, 100690. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, D.; Zhou, T.; Huang, J.; Wang, Y.; Li, B.; Chen, L.; Yang, J.; Liu, Y. Aptamer-conjugated magnetic Fe3O4@Au core-shell multifunctional nanoprobe: A three-in-one aptasensor for selective capture, sensitive SERS detection and efficient near-infrared light triggered photothermal therapy of Staphylococcus aureus. Sens. Actuators B Chem. 2022, 350, 130879. [Google Scholar] [CrossRef]
- Qu, X.; Zhou, P.; Zhao, W.; Shi, B.; Zheng, Y.; Jiang, L. Efficient capture and ultra-sensitive detection of drug-resistant bacteria ESBL-E. coli based on self-assembled Au NPs and MXene-Au SERS platform. Microchem. J. 2024, 199, 110069. [Google Scholar] [CrossRef]
- Pang, Y.; Wan, N.; Shi, L.; Wang, C.; Sun, Z.; Xiao, R.; Wang, S. Dual-recognition surface-enhanced Raman scattering (SERS)biosensor for pathogenic bacteria detection by using vancomycin-SERS tags and aptamer-Fe3O4@Au. Anal. Chim. Acta 2019, 1077, 288–296. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Sun, D.-W.; Pu, H.; Huang, L. Bridging Fe3O4@Au nanoflowers and Au@Ag nanospheres with aptamer for ultrasensitive SERS detection of aflatoxin B1. Food Chem. 2020, 324, 126832. [Google Scholar] [CrossRef]
- Lin, S.; Zheng, Y.; Xing, Y.; Dou, K.; Wang, R.; Cui, H.; Wang, R.; Yu, F. Highly sensitive SERS nanoplatform based on aptamer and vancomycin for detection of S. aureus and its clinical application. Talanta 2024, 280, 126691. [Google Scholar] [CrossRef]
- Dai, J.; Li, J.; Jiao, Y.; Yang, X.; Yang, D.; Zhong, Z.; Li, H.; Yang, Y. Colorimetric-SERS dual-mode aptasensor for Staphylococcus aureus based on MnO2@AuNPs oxidase-like activity. Food Chem. 2024, 456, 139955. [Google Scholar] [CrossRef]
- Dai, H.; Zhang, Y.; Zhao, W.; Guo, R.; Qian, S.; Xu, Y.; Li, Y.; Liu, Y.; Liu, H. A multifunctional biosensor for selective identification, sensitive detection and efficient photothermal sterilization of Salmonella typhimurium and Staphylococcus aureus. Anal. Chim. Acta 2025, 1338, 343589. [Google Scholar] [CrossRef]
- Sridhar, K.; Inbaraj, B.S.; Chen, B.-H. An improved surface enhanced Raman spectroscopic method using a paper-based grape skin-gold nanoparticles/graphene oxide substrate for detection of rhodamine 6G in water and food. Chemosphere 2022, 301, 134702. [Google Scholar] [CrossRef]
- Kasztelan, M.; Słoniewska, A.; Gorzkowski, M.; Lewera, A.; Pałys, B.; Zoladek, S. Ammonia modified graphene oxide—Gold nanoparticles composite as a substrate for surface enhanced Raman spectroscopy. Appl. Surf. Sci. 2021, 554, 149060. [Google Scholar]
- Zhang, C.; Zhao, B.; Hao, R.; Wang, Z.; Hao, Y.; Zhao, B.; Liu, Y. Graphene oxide-highly anisotropic noble metal hybrid systems for intensified surface enhanced Raman scattering and direct capture and sensitive discrimination in PCBs monitoring. J. Hazard. Mater. 2020, 385, 121510. [Google Scholar] [PubMed]
- Xie, H.; Li, P.; Shao, J.; Huang, H.; Chen, Y.; Jiang, Z.; Chu, P.K.; Yu, X.-F. Electrostatic Self-Assembly of Ti3C2Tx MXene and Gold Nanorods as an Efficient Surface-Enhanced Raman Scattering Platform for Reliable and High-Sensitivity Determination of Organic Pollutants. ACS Sens. 2019, 4, 2303–2310. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Tang, M.; Qin, L.; Kang, S.-Z.; Li, X. Aluminum sheet induced flower-like carbon nitride anchored with silver nanowires for highly efficient SERS detection of trace malachite green. Environ. Res. 2022, 204, 112289. [Google Scholar] [PubMed]
- Murugan, E.; Santhoshkumar, S.; Govindaraju, S.; Palanichamy, M. Silver nanoparticles decorated g-C3N4: An efficient SERS substrate for monitoring catalytic reduction and selective Hg2+ ions detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 246, 119036. [Google Scholar]
- Liu, H.; Guo, Y.; Wang, Y.; Zhang, H.; Ma, X.; Wen, S.; Jin, J.; Song, W.; Zhao, B.; Ozaki, Y. A nanozyme-based enhanced system for total removal of organic mercury and SERS sensing. J. Hazard. Mater. 2021, 405, 124642. [Google Scholar] [CrossRef]
- He, J.; Song, G.; Wang, X.; Zhou, L.; Li, J. Multifunctional magnetic Fe3O4/GO/Ag composite microspheres for SERS detection and catalytic degradation of methylene blue and ciprofloxacin. J. Alloys Compd. 2022, 893, 162226. [Google Scholar] [CrossRef]
- Fu, Z.; Shen, Z.; Fan, Q.; Hao, S.; Wang, Y.; Liu, X.; Tong, X.; Kong, X.; Yang, Z. Preparation of multi-functional magnetic–plasmonic nanocomposite for adsorption and detection of thiram using SERS. J. Hazard. Mater. 2020, 392, 122356. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, M.; Yin, Z.; Ding, C.; Chen, P.; Gan, W.; Yu, H.; Sun, Z. Construction of black phosphorus nanosheets and Ag nanoparticles co-sensitized TiO2 nanorod arrays as high-performance SERS substrate and photocatalyst. Appl. Surf. Sci. 2022, 592, 153265. [Google Scholar]
- Ma, J.; Xu, L.; Zhang, Y.; Dong, L.; Gu, C.; Wei, G.; Jiang, T. Multifunctional SERS chip mediated by black phosphorus@gold-silver nanocomposites inserted in bilayer membrane for in-situ detection and degradation of hazardous materials. J. Colloid Interface Sci. 2022, 626, 787–802. [Google Scholar]
- Li, H.; Huang, X.; Hassan, M.; Zuo, M.; Wu, X.; Chen, Y.; Chen, Q. Dual-channel biosensor for Hg2+ sensing in food using Au@Ag/graphene-upconversion nanohybrids as metal-enhanced fluorescence and SERS indicators. Microchem. J. 2020, 154, 104563. [Google Scholar] [CrossRef]
- Lv, L.; He, L.; Jiang, S.; Chen, J.; Zhou, C.; Qu, J.; Lu, Y.; Hong, P.; Sun, S.; Li, C. In situ surface-enhanced Raman spectroscopy for detecting microplastics and nanoplastics in aquatic environments. Sci. Total Environ. 2020, 728, 138449. [Google Scholar] [CrossRef] [PubMed]
- Mei, R.; Wang, Y.; Yu, Q.; Yin, Y.; Zhao, R.; Chen, L. Gold Nanorod Array-Bridged Internal-Standard SERS Tags: From Ultrasensitivity to Multifunctionality. ACS Appl. Mater. Interfaces 2019, 12, 2059–2066. [Google Scholar] [CrossRef] [PubMed]

| Classification | Examples | Specific Materials | Characteristics | References |
|---|---|---|---|---|
| Metal Nanomaterials |  | Au, Ag, metal alloy | Widely used in SERS substrates due to their excellent LSPR property. | [7,130] |
| Core–Shell Structure |  | Au@Ag, Ag@Au | Optimizing SERS signals by adjusting the properties of the outer metal layer. | [13,131,132] |
| Porous Materials |  | Porous carbon (PC), porous silicon (PS), MOFs | High specific surface area and good molecular sieving effects, effectively adsorbing target molecules and enhancing SERS signals. | [39,133,134] |
| Semiconductor Nanostructures |  | Titanium dioxide (TiO2), zinc oxide (ZnO) | Weak SERS activity on their own, but can enhance signal strength when combined with metal nanoparticles. | [13,135,136] |
| Carbon-Based Nanostructures |  | Graphene and its derivatives (e.g., reduced graphene oxide) | Excellent conductivity and large specific surface area, effectively enhancing SERS signals. | [137,138] |
| Polymer-Based Nanostructures |  | Functionalized polymers (e.g., PMMA, PDMS) | Combining with metal nanoparticles to form a composition for enhancing SERS signals. | [139,140] |
| Biomass-Based Nanostructures |  | Natural polymers (e.g., chitosan, gelatin, cellulose nanofibers) | Biocompatibility and tunability allow them to combine with metal nanoparticles for enhancing signals. | [141,142,143] |
| Composite Nanostructures |  | Combination of different nanostructures (e.g., metals with semiconductors, metals with porous materials) | Achieving synergistic effects to further enhance SERS signals. | [13,133,134,144,145] |
| Advantage | Description |
|---|---|
| High Sensitivity Detection | SERS technology uses the surface plasmon resonance of metal nanoparticles to significantly enhance the Raman signals of adsorbed molecules, enabling the highly sensitive detection of trace hazardous substances in food. |
| Rapid Response | SERS technology can provide rapid assay results, which is crucial for an immediate response and the management of food safety incidents. |
| No Need for Labeling and Pretreatment | SERS detection does not require complex sample pretreatment or labeling; it can directly test food samples, simplifying the operational process. |
| High Selectivity | SERS technology exhibits high selectivity, enabling the rapid quantitative or qualitative detection of hazardous substances in complex food matrices. |
| Multiplex Detection Capability | SERS technology is capable of detecting multiple hazardous substances simultaneously, enhancing the efficiency and scope of detection. |
| Real-Time Monitoring Capability | Combined with portable devices, SERS technology can perform the real-time monitoring of hazardous substances during food processing and storage. |
| Data Traceability | The Raman spectra provided by SERS have unique fingerprint characteristics, aiding in tracing contamination sources and food safety traceability. |
| Strong Environmental Adaptability | Nanostructures can be used under various environmental conditions, enhancing the application potential of SERS technology in diverse food testing scenarios. |
| Cost-Effectiveness | Although the initial investment may be high, SERS technology reduces the costs associated with repeated testing and erroneous results, making it cost-effective in the long run. |
| Biocompatibility | Selecting appropriate nanostructures ensures that SERS detection is safe for both the food and operators, avoiding secondary contamination. |
| Types of Food Contamination | Types of Nanostructures | SERS Substrate | EF | Analytes | LODs | Ref. |
|---|---|---|---|---|---|---|
| Microbial Contamination | Metal | Au NR | E. coli | 102 CFU/g | [26] | |
| AuNPs | S. Aureus | 10 CFU/mL | [213] | |||
| Au NPs | 1.8 × 105 | S. Aureus | 1.5 CFU/mL | [214] | ||
| Ag-NDs | 3.9 × 106 | S. Aureus | 102 CFU/mL | [215] | ||
| HPGN | AFB1 | 0.05 ng/mL | [184] | |||
| Ag@ICNPs | 108 | Monkeypox Virus | 6.25 × 103 copies/mL | [186] | ||
| Au NPs-DNA (H2)-DTNB Ag NRs-DNA (H1) | S. Aureus | 5 CFU/mL | [216] | |||
| Au-Ag nanodimer | S. Aureus | 96 CFU/mL | [217] | |||
| Core–Shell | Ag@AuNPs | HCV | 0.47 fM | [185] | ||
| Au@Ag/4-ATP/Cys | PAT | 0.27 ng/mL | [132] | |||
| Au@Ag-MBA-AuNPs | 108 | NOV | 0.76 fg/mL | [34] | ||
| AuNSs@PB@Ag-Apt | S. Aureus | 1 CFU/mL | [218] | |||
| Au@AgPt | S. Aureus | 6 CFU/mL | [219] | |||
| Au@NTP@SiO₂-ssDNA | 9.6 × 105 | S. Aureus | 2 CFU/mL | [220] | ||
| Porous Materials | Ag-pSi | E. coli | 3 CFU/mL | [221] | ||
| Ag-pSi-DTNB | S. Aureus | 2 CFU/mL | [222] | |||
| Biomass-Based | AgNPs@4-MB@MPNs | 106 | E. coli O157:H7, S. Aureus | 102 CFU/mL | [182] | |
| Semiconductor | QD-SNPs DTNB-AuNPs | AFB1 | 0.100 pg/mL | [183] | ||
| Au@Ag@MBA@SiO2 | S. Aureus | 2 CFU/mL | [223] | |||
| Carbon-Based | Ti3C2Tx@Au NPs@4-MBN | E. coli | 10 CFU/mL | [224] | ||
| Composite | Fe3O4@SiO2-Au NPs | E. coli and S. Aureus | 10 CFU/mL | [181] | ||
| Fe3O4@Au | S. Aureus | 25 CFU/mL | [225] | |||
| Fe3O4@Au NFs | AFB1 | 0.40 pg/mL | [226] | |||
| Fe3O4-Apt MNPs Van-Au NPs | S. Aureus | 3.27 CFU/mL | [227] | |||
| MnO₂@AuNPs | S. Aureus | 1.561 CFU/mL | [228] | |||
| ZnO/Ag–Au@Ag | 4.67 × 105 | S. Aureus | 10 CFU/mL | [229] | ||
| Chemical Contamination | Carbon-Based | GE-AuNPs/GO | 1.92 × 109 | R6G | 7.33 × 10−11 M | [230] |
| AuNPs/NH3-GO | 7.0 × 103 | R6G | 10−7 M | [231] | ||
| AgNFs-GO-AuNSts | 2.59 × 107 | R6G | 1.0 × 10−13 M | [232] | ||
| MXene/AuNRs | 4.5 × 106 | Thiram | 1 × 10−10 M | [233] | ||
| Al/f-C3N4/AgNWs | 3.37 × 105 | MG | 8.38 × 10−12 M | [234] | ||
| Ag NPs@g-C3N4 | 1.4 × 108 | Hg2+ | 0.02005 ppm | [235] | ||
| Composite | Spiky Au@Ag NPs-Bi₂WO₆ films | Spiky Au@Ag NPs-Bi₂WO₆ films (1.8 times), CuB₂O₄ (1.3 times), BiOI (1.1 times) | CAP, paraquat | 8.46 ng/L (CAP), 75.71 ng (paraquat) | [42] | |
| mAbAuNpDTNB nanoparticles | SM2 | 0.1 pg/mL | [193] | |||
| Au-NiFe LDH/rGO | Hg2+ | 1 × 10−8 M | [236] | |||
| Fe3O4-WO3-X@AuNPs | 6.1 × 105 (ABZ), 7.8 × 105 (SPT) | ABZ, SPT | 12.5 μg/L (ABZ), 11.6 μg/L (SPT) | [195] | ||
| Fe3O4/GO/Ag | 2.26 × 107 | MB | 10−9 M | [237] | ||
| Fe@RAu | 5.25 × 106 | Cd2+ | 1.88 pg/mL | [152] | ||
| Fe@Ag | Thiram | 0.01 ppm | [238] | |||
| BP/Ag/TNR | 3.81 × 105 | R6G | 1.0 × 10−14 M | [239] | ||
| BP@Au-Ag | 2.5 × 107 | Thiram | 2.6 × 10−6 mg/mL | [240] | ||
| pNIPAM@Au NRs | MG | 3.95 × 10−10 M (aqueous solution), 0.73 ng/g (1.58 × 10−9 m, fillet tissue) | [207] | |||
| Metal | Au DNs | 4.2 × 106 | Triazolone | 0.00011 μg/mL | [83] | |
| Au nanodendrite tape substrate | 108 | Thiram, Thiabendazole | 1 × 10−5 M | [194] | ||
| Au NRs array | 1.64 × 106 | TBZ, Thiram | TBZ (0.79 ng/cm2 on apple, 0.76 ng/cm2 on tomato and 0.80 ng/cm2 on pear), Thiram (0.041 ng/cm2 for apples, 0.029 ng/cm2 for tomatoes, and 0.047 ng/cm2 for pears) | [130] | ||
| 3D Au@Ag NPs | 3.4 × 106 | Thiram | 10−6 M | [241] | ||
| MNA-MA-AgNPs | Mn2+ | 4.0 × 10⁻6 mol/L (220 μg/L) | [202] | |||
| 2D silver plate | PAE | 10−9 mol/L | [208] | |||
| Core–Shell | MDAu@Ag-based SERS-LFA | Kana, RAC, CLE, CAP | 0.52 pg/mL (Kana), 2.5 pg/mL (RAC), 0.87 pg/mL (CLE), 6.2 pg/mL (CAP) | [196] | ||
| Au@Ag-GU | Hg2+ | 0.33 ppb | [242] | |||
| Porous Materials | Au@Ag/COF | Hg2+ | 5.0 × 10−16 M | [133] | ||
| Semiconductor | AgNTs/TNA | R6G, CV, MG, Thiram | 10−10 M(R6G), 10−9 M(CV), 10−8 M(MG), and 10−7 M(Thiram) | [206] | ||
| ZNR/AgT | Z3/AgT (5.4 × 10⁷, comparison with a glass substrate containing only silver nanotriangles) | BHT | 0.1 ng/mL | [209] | ||
| TiO2 NTs/AgNPs-rGO | 7.1 × 108 | MB | 1.0 × 10−14 M | [110] | ||
| Polymer-Based | PS@Au@1,4-BDT@Au | 108 | MB, T1, MV, MG | 10−12 M (MB), 2.5 × 10−8 M (T1), 10−8 M (MV), 10−11 M (MG) | [84] | |
| Physical Contamination | Metal | GNPs | Nanoplastic components (e.g., PS, PE, PP) | 1 μg/mL | [17] | |
| silver colloid | 7 × 102 (100 nm PS ball), 1.1 × 104 (500 nm PS ball) | PS, PE, PP | 40 μg/mL (100 nm plastic particles), 0.08 mg/mL (500 nm plastic particles) | [243] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Yang, R.; Chen, H.; Zhang, X. Recent Advances in Food Safety: Nanostructure-Sensitized Surface-Enhanced Raman Sensing. Foods 2025, 14, 1115. https://doi.org/10.3390/foods14071115
Liu Z, Yang R, Chen H, Zhang X. Recent Advances in Food Safety: Nanostructure-Sensitized Surface-Enhanced Raman Sensing. Foods. 2025; 14(7):1115. https://doi.org/10.3390/foods14071115
Chicago/Turabian StyleLiu, Zeyan, Renqing Yang, Haili Chen, and Xinai Zhang. 2025. "Recent Advances in Food Safety: Nanostructure-Sensitized Surface-Enhanced Raman Sensing" Foods 14, no. 7: 1115. https://doi.org/10.3390/foods14071115
APA StyleLiu, Z., Yang, R., Chen, H., & Zhang, X. (2025). Recent Advances in Food Safety: Nanostructure-Sensitized Surface-Enhanced Raman Sensing. Foods, 14(7), 1115. https://doi.org/10.3390/foods14071115






