The Addition of Hermetia illucens to Feed: Influence on Nutritional Composition, Protein Digestion Characteristics, and Antioxidant Activity of Acheta domesticus
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Insects
2.3. Proximate Composition
2.4. Osborne Fractionation of Protein
2.5. Total Amino Acid and Nutritional Quality Indices of Amino Acids
2.6. Fatty Acid Composition
2.7. In Vitro Protein Digestion
2.8. Degree of Hydrolysis (DH%)
2.9. Antioxidant Activity In Vitro
2.9.1. DPPH Radical Scavenging Activity
2.9.2. ABTS Radical Scavenging Activity
2.9.3. Hydroxide Radical (OH−) Scavenging Activity
2.9.4. Potassium Hexacyanoferrate (III) Total Reducing Power Assay (TRP)
2.9.5. Ferric Reducing Antioxidant Power (FRAP)
2.9.6. Cupric Ion-Reducing Activity (CUPRAC)
2.10. Statistical Analysis
3. Results and Discussion
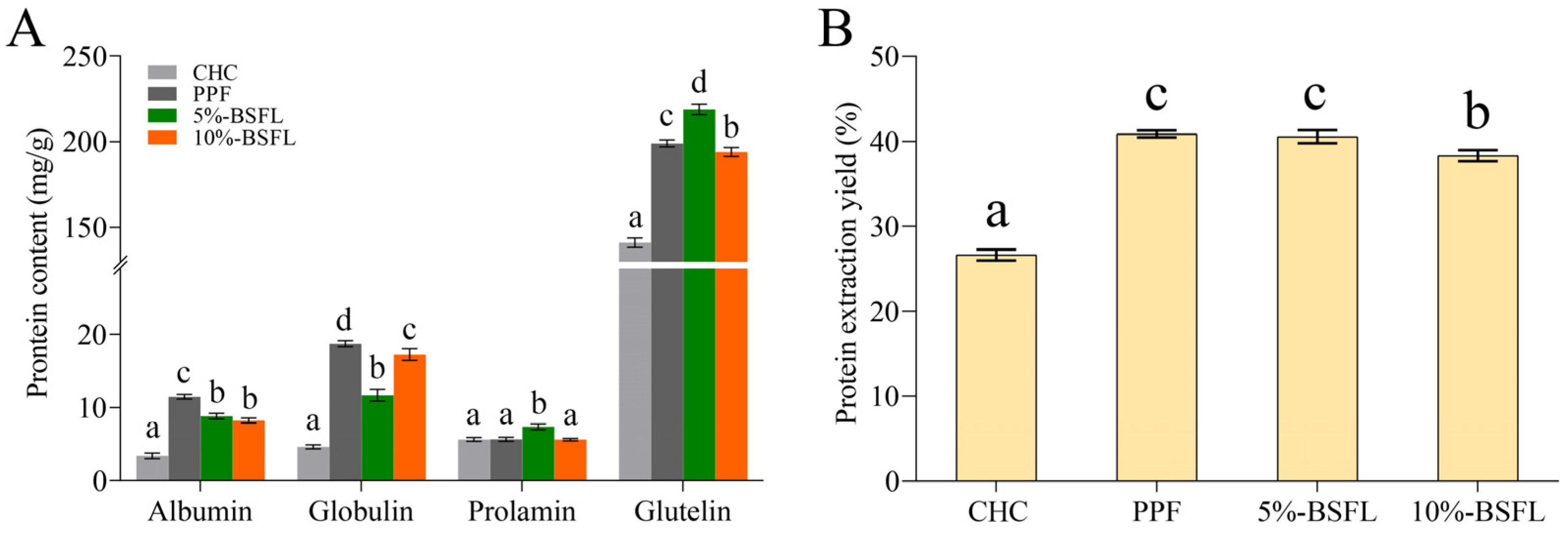
3.1. Yield and Proximate Analysis
3.2. Osborne Fractionation
3.3. Free Amino Acid (FAA) Profile
3.4. Fatty Acids Profile
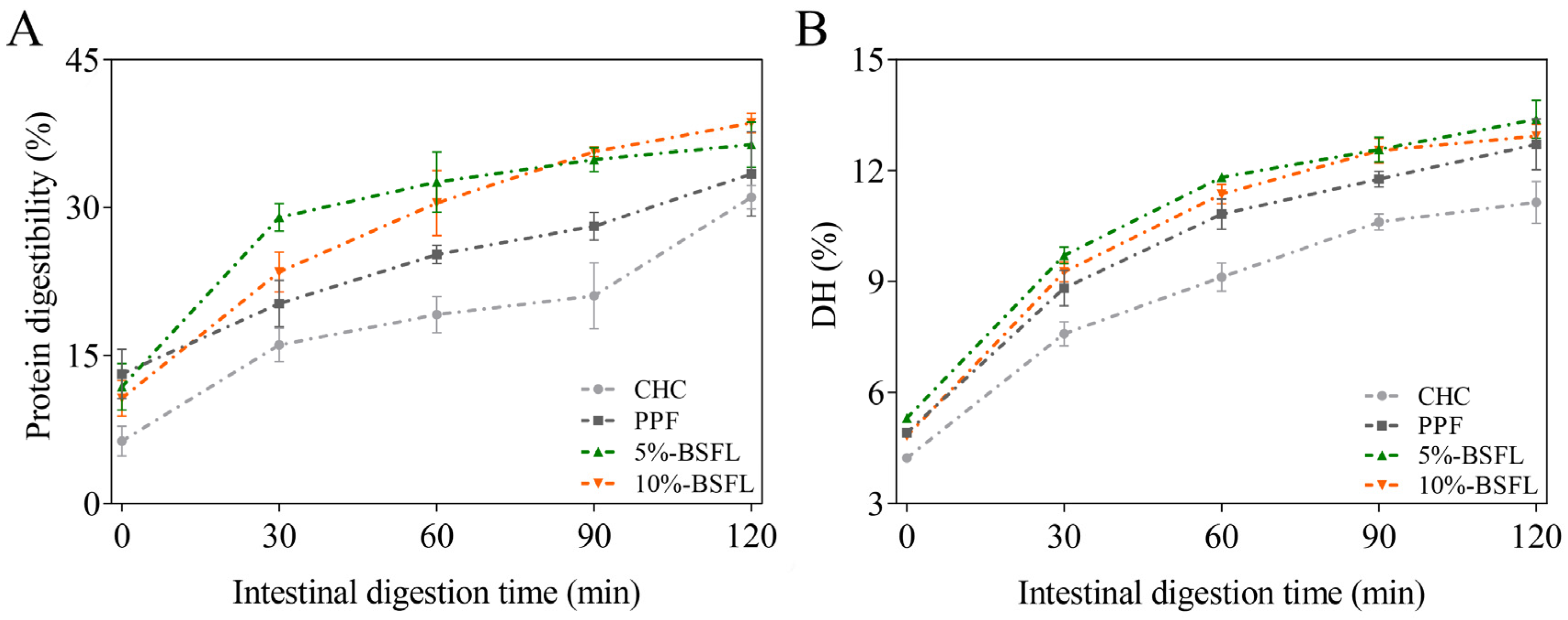
3.5. Protein Digestibility and DH (%) of Protein
3.6. Antioxidant Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EFSA. Risk profile related to production and consumption of insects as food and feed. EFSA J. 2015, 13, 4257. [Google Scholar] [CrossRef]
- Oloo, J.A.; Ayieko, M.; Nyongesah, J.M. Acheta domesticus (Cricket) feed resources among smallholder farmers in Lake Victoria region of Kenya. Food Sci. Nutr. 2020, 8, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Megido, R.C.; Alabi, T.; Nieus, C.; Blecker, C.; Danthine, S.; Bogaert, J.; Haubruge, É.; Francis, F. Optimisation of a cheap and residential small-scale production of edible crickets with local by-products as an alternative protein-rich human food source in Ratanakiri Province, Cambodia. J. Sci. Food Agric. 2016, 96, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Yang, H.M.; Sun, Y.; Niu, J.; Liu, S.Y. Purification and identification of antioxidant peptides from walnut (Juglans regia L.) protein hydrolysates. Peptides 2012, 38, 344–349. [Google Scholar] [CrossRef]
- Gasco, L.; Henry, M.; Piccolo, G.; Marono, S.; Gai, F.; Renna, M.; Lussiana, C.; Antonopoulou, E.; Mola, P.; Chatzifotis, S. Tenebrio molitor meal in diets for European sea bass (Dicentrarchus labrax L.) juveniles: Growth performance, whole body composition and in vivo apparent digestibility. Anim. Feed Sci. Technol. 2016, 220, 34–45. [Google Scholar] [CrossRef]
- Iaconisi, V.; Bonelli, A.; Pupino, R.; Gai, F.; Parisi, G. Mealworm as dietary protein source for rainbow trout: Body and fillet quality traits. Aquaculture 2018, 484, 197–204. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Henzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Gasco, L.; Gai, F.; Maricchiolo, G.; Genovese, L.; Ragonese, S.; Bottari, T.; Caruso, G. Fishmeal Alternative Protein Sources for Aquaculture Feeds. In Springer Briefs in Molecular Science; Springer International Publishing: Berlin, Germany, 2018; pp. 1–28. [Google Scholar] [CrossRef]
- Weththasinghe, P.; Hansen, J.O.; Mydland, L.T.; Overland, M. A systematic meta-analysis based review on black soldier fly (Hermetia illucens) as a novel protein source for salmonids. Rev. Aquac. 2022, 14, 938–956. [Google Scholar] [CrossRef]
- Bessa, L.W.; Pieterse, E.; Marais, J.; Hoffman, L.C. Why for feed and not for human consumption? The black soldier fly larvae. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2747–2763. [Google Scholar] [CrossRef]
- Onuh, J.O.; Aluko, R.E. Metabolomics as a tool to study the mechanism of action of bioactive protein hydrolysates and peptides: A review of current literature. Trends Food Sci. Tech. 2019, 91, 625–633. [Google Scholar] [CrossRef]
- Zielinska, E.; Baraniak, B.; Karas, M. Antioxidant and Anti-Inflammatory Activities of Hydrolysates and Peptide Fractions Obtained by Enzymatic Hydrolysis of Selected Heat-Treated Edible Insects. Nutrients 2017, 9, 970. [Google Scholar] [CrossRef] [PubMed]
- Hall, F.; Johnson, P.E.; Liceaga, A. Effect of enzymatic hydrolysis on bioactive properties and allergenicity of cricket (Gryllodes sigillatus) protein. Food Chem. 2018, 262, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Pastell, H.; Mellberg, S.; Ritvanen, T.; Raatikainen, M.; Mykkänen, S.; Niemi, J.; Latomäki, I.; Wirtanen, G. How Does Locally Produced Feed Affect the Chemical Composition of Reared House Crickets (Acheta domesticus)? ACS Food Sci. Technol. 2021, 1, 625–635. [Google Scholar] [CrossRef]
- AOAO. Official Methods of Analysis of AOAC International; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Zhang, Z.Q.; Chen, S.C.; Wang, Q.L.; Liu, C.Q.; Xiao, J.H.; Huang, D.W. Effects of traditional grinding and superfine grinding technologies on the properties and volatile components of larvae powder. LWT Food Sci. Technol. 2023, 173, 114307. [Google Scholar] [CrossRef]
- Huang, X.; Liang, K.H.; Liu, Q.; Qiu, J.; Wang, J.; Zhu, H. Superfine grinding affects physicochemical, thermal and structural properties of leaf powders. Ind. Crops Prod. 2020, 151, 112472. [Google Scholar] [CrossRef]
- Adebiyi, A.P.; Adebiyi, A.O.; Hasegawa, Y.; Ogawa, T.; Muramoto, K. Isolation and characterization of protein fractions from deoiled rice bran. Eur. Food Res. Technol. 2009, 228, 391–401. [Google Scholar] [CrossRef]
- Oser, B.L. An integrated essential amino acid index for predicting the Biological value of proteins. In Protein and Amino Acid Nutrition; Albanese, A.A., Ed.; Academic Press: New York, NY, USA, 1959; pp. 295–311. [Google Scholar]
- FAO/WHO/UNU. Protein and Amino Acid Requirements in Human Nutrition, Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Li, Y.X.; Zou, J.; Zhu, H.H.; He, J.Q.; Setter, T.L.; Wang, Y.H.; Meng, Y.L.; Chen, B.L.; Zhao, W.Q.; Wang, S.S.; et al. Drought deteriorated the nutritional quality of cottonseed by altering fatty acids and amino acids compositions in cultivars with contrasting drought sensitivity. Environ. Exp. Bot. 2022, 194, 104747. [Google Scholar] [CrossRef]
- Poelaert, C.; Francis, F.; Alabi, T.; Megido, R.C.; Crahay, B.; Bindelle, J.; Beckers, Y. Protein value of two insects, subjected to various heat treatments, using growing rats and the protein digestibility-corrected amino acid score. J. Insects Food Feed 2018, 4, 77–87. [Google Scholar] [CrossRef]
- Andreadis, S.S.; Panteli, N.; Mastoraki, M.; Rizou, E.; Stefanou, V.; Tzentilasvili, S.; Sarrou, E.; Chatzifotis, S.; Krigas, N.; Antonopoulou, E. Towards Functional Insect Feeds: Agri-Food By-Products Enriched with Post-Distillation Residues of Medicinal Aromatic Plants in Tenebrio molitor (Coleoptera: Tenebrionidae) Breeding. Antioxidants 2022, 11, 68. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Ketnawa, S.; Wickramathilaka, M.; Liceaga, A.M. Changes on antioxidant activity of microwave-treated protein hydrolysates after simulated gastrointestinal digestion: Purification and identification. Food Chem. 2018, 254, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, H.R.; Wang, L.J.; Qiu, J.; Li, Z.G.; Wang, L.L. Milling of wheat bran: Influence on digestibility, hydrolysis and nutritional properties of bran protein during digestion. Food Chem. 2023, 404, 134559. [Google Scholar] [CrossRef] [PubMed]
- Mulet-Cabero, A.I.; Rigby, N.M.; Brodkorb, A.; Mackie, A.R. Dairy food structures influence the rates of nutrient digestion through different in vitro gastric behaviour. Food Hydrocoll. 2017, 67, 63–73. [Google Scholar] [CrossRef]
- Zahir, M.; Fogliano, V.; Capuano, E. Food matrix and processing modulate in vitro protein digestibility in soybeans. Food Funct. 2018, 9, 6327–6337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Q.; Chen, S.C.; Wei, X.F.; Geng, J.; Sui, Z.X.; Wang, Q.L.; Liu, C.Q.; Xiao, J.H.; Huang, D.W. Characterization of bioactives and in vitro biological activity from Protaetia brevitarsis larval extracts obtained by different pretreatment extractions. Food Chem. 2023, 405, 134891. [Google Scholar] [CrossRef]
- Ribeiro, D.A.; Camilo, C.J.; Nonato, C.D.A.; Rodrigues, F.F.G.; Menezes, I.R.A.; Ribeiro, J.; Xiao, J.B.; Souza, M.M.D.; da Costa, J.G.M. Influence of seasonal variation on phenolic content and in vitro antioxidant activity of Secondatia floribunda A. DC. (Apocynaceae). Food Chem. 2020, 315, 126277. [Google Scholar] [CrossRef]
- Yildirim-Aksoy, M.; Eljack, R.; Schrimsher, C.; Beck, B.H. Use of dietary frass from black soldier fly larvae, Hermetia illucens, in hybrid tilapia (Nile x Mozambique, Oreocromis niloticus x O. mozambique) diets improves growth and resistance to bacterial diseases. Aquac. Rep. 2020, 17, 100373. [Google Scholar] [CrossRef]
- Ismail, B.P.; Senaratne-Lenagala, L.; Stube, A.; Brackenridge, A. Protein demand: Review of plant and animal proteins used in alternative protein product development and production. Anim. Front. 2020, 10, 53–63. [Google Scholar] [CrossRef]
- Magara, H.J.O.; Tanga, C.M.; Ayieko, M.A.; Hugel, S.; Mohamed, S.A.; Khamis, F.M.; Salifu, D.; Niassy, S.; Sevgan, S.; Fiaboe, K.K.M.; et al. Performance of Newly Described Native Edible Cricket Scapsipedus icipe (Orthoptera: Gryllidae) on Various Diets of Relevance for Farming. J. Econ. Entomol. 2019, 112, 653–664. [Google Scholar] [CrossRef]
- Osimani, A.; Garofalo, C.; Milanovic, V.; Taccari, M.; Cardinali, F.; Aquilanti, L.; Pasquini, M.; Mozzon, M.; Raffaelli, N.; Ruschioni, S.; et al. Insight into the proximate composition and microbial diversity of edible insects marketed in the European Union. Eur. Food Res. Technol. 2017, 243, 1157–1171. [Google Scholar] [CrossRef]
- Kulma, M.; Kourimská, L.; Plachy, V.; Bozik, M.; Adámková, A.; Vrabec, V. Effect of sex on the nutritional value of house cricket, Acheta domestica L. Food Chem. 2019, 272, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Cantero-Bahillo, E.; Del Hierro, J.N.; Hernandez, D.M.; Fernandez-Felipe, M.T.; Fornari, T.; Martin, D. Supercritical-CO2 for defatting and production of bioactive extracts from black soldier fly (Hermetia illucens) larvae. J. Insects Food Feed 2022, 8, 1441–1453. [Google Scholar] [CrossRef]
- Pilco-Romero, G.; Chisaguano-Tonato, A.M.; Herrera-Fontana, M.E.; Chimbo-Gándarac, L.F.; Sharifi-Rad, M.; Giampieri, F.; Battinof, M.; Vernaza, M.G.; Alvarez-Suárez, J.M. House cricket (Acheta domesticus): A review based on its nutritional composition, quality, and potential uses in the food industry. Trends Food Sci. Tech. 2023, 142, 104226. [Google Scholar] [CrossRef]
- Stone, A.K.; Tanaka, T.; Nickerson, M.T. Protein quality and physicochemical properties of commercial cricket and mealworm powders. J. Food Sci. Tech. Mys. 2019, 56, 3355–3363. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; Finke, M.D. Nutritional value of insects and ways to manipulate their composition. J. Insects Food Feed 2021, 7, 639–659. [Google Scholar] [CrossRef]
- Ferrari, L.; Panaite, S.A.; Bertazzo, A.; Visioli, F. Animal- and Plant-Based Protein Sources: A Scoping Review of Human Health Outcomes and Environmental Impact. Nutrients 2022, 14, 5115. [Google Scholar] [CrossRef]
- Prabakaran, M.; Lee, K.J.; An, Y.; Kwon, C.; Kim, S.; Yang, Y.; Ahmad, A.; Kim, S.H.; Chung, I.M. Changes in Soybean (Glycine max L.) Flour Fatty-Acid Content Based on Storage Temperature and Duration. Molecules 2018, 23, 2713. [Google Scholar] [CrossRef]
- Toishimanov, M.; Suleimenova, Z.; Myrzabayeva, N.; Dossimova, Z.; Shokan, A.; Kenenbayev, S.; Yessenbayeva, G.; Serikbayeva, A.; Clark, S. Effects of Organic Fertilizers on the Quality, Yield, and Fatty Acids of Maize and Soybean in Southeast Kazakhstan. Sustainability 2024, 16, 162. [Google Scholar] [CrossRef]
- Tsuzuki, W.; Suzuki, Y.; Yamada, S.; Kano, S.; Ohnishi, H.; Fujimoto, T.; Horigane, A. Effect of oxygen absorber on accumulation of free fatty acids in brown rice and whole grain wheat during storage. LWT Food Sci. Technol. 2014, 58, 222–229. [Google Scholar] [CrossRef]
- Skvorová, P.; Kulma, M.; Bozik, M.; Kurecka, M.; Plachy, V.; Slavíková, D.; Sebelová, K.; Kourimská, L. Evaluation of rapeseed cake as a protein substitute in the feed of edible crickets: A case study using Gryllus assimilis. Food Chem. 2024, 441, 138254. [Google Scholar] [CrossRef]
- Li, S.L.; Ji, H.; Zhang, B.X.; Tian, J.J.; Zhou, J.S.; Yu, H.B. Influence of black soldier fly (Hermetia illucens)) larvae oil on growth performance, body composition, tissue fatty acid composition and lipid deposition in juvenile Jian carp (Cyprinus carpio var. Jian). Aquaculture 2016, 465, 43–52. [Google Scholar] [CrossRef]
- Xu, X.X.; Ji, B.B.; Lu, R.H.; Ji, H. Black Soldier Fly Oil in Different Lipid Diets Could Regulate Tissue Lipid Metabolism and Fatty Acid Composition of Juvenile Mirror Carp. Aquac. Nutr. 2024, 2024, 8718694. [Google Scholar] [CrossRef] [PubMed]
- Leyton, J.; Drury, P.J.; Crawford, M.A. Differential oxidation of saturated and unsaturated fatty acids in vivo in the rat. Br. J. Nutr. 1987, 57, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Nordlund, E.; Katina, K.; Aura, A.M.; Poutanen, K. Changes in bran structure by bioprocessing with enzymes and yeast modifies the in vitro digestibility and fermentability of bran protein and dietary fibre complex. J. Cereal Sci. 2013, 58, 200–208. [Google Scholar] [CrossRef]
- Gong, X.; Hui, X.D.; Wu, G.; Morton, J.D.; Brennan, M.A.; Brennan, C.S. In vitro digestion characteristics of cereal protein concentrates as assessed using a pepsin-pancreatin digestion model. Food Res. Int. 2022, 152, 110715. [Google Scholar] [CrossRef]
- Albin, D.M.; Wubben, J.E.; Gabert, V.M. The influence of hydrochloric acid concentration and measurement method on the determination of amino acid levels in soya bean products. Anim. Feed Sci. Technol. 2000, 87, 173–186. [Google Scholar] [CrossRef]
- Hall, F.; Reddivari, L.; Liceaga, A.M. Identification and Characterization of Edible Cricket Peptides on Hypertensive and Glycemic In Vitro Inhibition and Their Anti-Inflammatory Activity on RAW 264.7 Macrophage Cells. Nutrients 2020, 12, 3588. [Google Scholar] [CrossRef]
- Tu, M.L.; Cheng, S.Z.; Lu, W.H.; Du, M. Advancement and prospects of bioinformatics analysis for studying bioactive peptides from food-derived protein: Sequence, structure, and functions. TrAC Trends Anal. Chem. 2018, 105, 7–17. [Google Scholar] [CrossRef]
- Nwachukwu, I.D.; Aluko, R.E. Structural and functional properties of food protein-derived antioxidant peptides. J. Food Biochem. 2019, 43, e12761. [Google Scholar] [CrossRef]
- Liu, C.L.; Guo, Y.; Zhao, F.R.; Qin, H.X.; Lu, H.Y.; Fang, L.; Wang, J.; Min, W.H. Potential mechanisms mediating the protective effects of a peptide from walnut (Juglans mandshurica Maxim.) against hydrogen peroxide induced neurotoxicity in PC12 cells. Food Funct. 2019, 10, 3491–3501. [Google Scholar] [CrossRef]
- Xu, N.J.; Chen, G.Q.; Liu, H. Antioxidative Categorization of Twenty Amino Acids Based on Experimental Evaluation. Molecules 2017, 22, 2066. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.H.; Chen, Y.N.; Yu, W.P.; Lin, M.J.; Yang, G.K.; Qin, C.B.; Meng, X.L.; Zhang, Y.M.; Ji, H.; Nie, G.X. Defatted black soldier fly (Hermetia illucens) larvae meal can replace soybean meal in juvenile grass carp (Ctenopharyngodon idellus) diets. Aquac. Rep. 2020, 18, 100520. [Google Scholar] [CrossRef]
- Ardra, M.; Pradhan, C.; Das, S.; Pillai, D. The effect of fishmeal replacement with organic acid fermented black soldier fly (Hermetia illucens) larvae meal on growth, nutrient utilization, metabolic enzyme activity, antioxidant status and immunity in Pangasius (Pangasianodon hypophthalmus). Aquaculture 2024, 591, 741114. [Google Scholar] [CrossRef]
- Wong, F.C.; Xiao, J.B.; Wang, S.Y.; Ee, K.Y.; Chai, T.T. Advances on the antioxidant peptides from edible plant sources. Trends Food Sci. Technol. 2020, 99, 44–57. [Google Scholar] [CrossRef]

| Corn (g/100 g) | Soybean (g/100 g) | Whole Wheat (g/100 g) | Yeast (g/100 g) | H. illucens (g/100 g) | Protein (g/100 g) | Fat (g/100) | Energy (J/100 g) | |
|---|---|---|---|---|---|---|---|---|
| PPF | 25 | 45 | 25 | 5 | 0 | 23.4 | 10.10 | 1449.20 |
| 5% BSFL | 30 | 38 | 22 | 5 | 5 | 23.36 | 10.11 | 1457.28 |
| 10% BSFL | 34 | 31 | 20 | 5 | 10 | 23.41 | 10.12 | 1462.52 |
| CHC | PPF | 5% BSFL | 10% BSFL | p-Value | |
|---|---|---|---|---|---|
| Yield (g) | — | 129.37 ± 12.12 a | 169.76 ± 3.42 c | 146.81 ± 6.42 b | <0.001 |
| Moisture (%) | 10.68 ± 0.69 d | 6.39 ± 0.31 c | 5.49 ± 0.11 a | 5.59 ± 0.10 b | <0.001 |
| Fat (%) | 24.19 ± 0.17 c | 22.90 ± 0.23 b | 20.38 ± 0.13 a | 20.56 ± 0.74 a | <0.001 |
| Protein (%) | 58.12 ± 0.32 ab | 57.47 ± 0.53 a | 60.83 ± 0.74 c | 58.75 ± 0.11 b | <0.001 |
| Ash (%) | 4.83 ± 0.01 b | 4.50 ± 0.01 a | 4.52 ± 0.04 a | 4.50 ± 0.03 a | <0.001 |
| Chitin (%) | 8.32 ± 0.33 | 7.62 ± 0.11 | 7.80 ± 0.60 | 7.86 ± 0.09 | 0.167 |
| TPC (mg/g) | 11.93 ± 0.17 ab | 11.43 ± 0.05 a | 12.10 ± 0.44 b | 12.93 ± 0.48 c | 0.004 |
| CHC | PPF | 5% BSFL | 10% BSFL | p-Value | |
|---|---|---|---|---|---|
| Threonine | 36.96 ± 0.75 a | 37.56 ± 1.34 a | 37.96 ± 0.00 a | 39.66 ± 0.78 b | 0.025 |
| Valine | 45.65 ± 0.00 a | 47.85 ± 2.79 ab | 51.48 ± 0.76 b | 51.38 ± 1.35 b | 0.05 |
| Methionine | 12.61 ± 1.51 | 12.97 ± 0.78 | 14.40 ± 0.00 | 13.07 ± 3.40 | 0.687 |
| Isoleucine | 31.74 ± 0.75 a | 34.43 ± 1.55 b | 35.34 ± 1.31 bc | 37.41 ± 0.78 c | 0.002 |
| Leucine | 67.39 ± 0.75 a | 70.21 ± 3.38 ab | 66.75 ± 1.31 a | 73.02 ± 1.35 b | 0.016 |
| Phenylalanine | 26.96 ± 0.75 a | 28.62 ± 2.05 ab | 29.23 ± 0.76 ab | 31.10 ± 1.35 b | 0.032 |
| Histidine | 26.09 ± 0.00 | 25.94 ± 1.69 | 25.96 ± 0.38 | 27.04 ± 1.17 | 0.543 |
| Lysine | 47.39 ± 0.75 | 47.40 ± 3.10 | 48.43 ± 1.31 | 49.13 ± 0.78 | 0.583 |
| EAA | 294.79 ± 1.30 a | 304.98 ± 14.79 ab | 309.55 ± 5.59 ab | 321.82 ± 3.38 b | 0.022 |
| Arginine | 67.83 ± 4.52 b | 64.84 ± 0.78 b | 52.79 ± 1.51 a | 55.44 ± 4.88 a | 0.002 |
| Proline | 56.52 ± 0.75 a | 58.13 ± 2.05 ab | 62.83 ± 3.46 b | 62.65 ± 2.82 b | 0.031 |
| Aspartic | 80.00 ± 1.51 b | 80.94 ± 4.31 b | 71.99 ± 1.31 a | 81.58 ± 4.35 b | 0.021 |
| Serine | 48.26 ± 0.00 b | 52.77 ± 2.05 c | 41.45 ± 0.76 a | 54.09 ± 1.35 c | <0.001 |
| Glutamic | 108.70 ± 2.72 | 110.9 ± 6.20 | 106.89 ± 2.00 | 115.39 ± 2.07 | 0.096 |
| Glycine | 56.52 ± 1.51 a | 65.29 ± 0.78 b | 64.14 ± 0.00 b | 63.10 ± 1.56 b | <0.001 |
| Alanine | 91.31 ± 1.30 a | 114.93 ± 1.55 b | 113.00 ± 3.78 b | 110.43 ± 2.82 b | <0.001 |
| Cystine | 5.65 ± 0.75 | 5.37 ± 1.34 | 5.24 ± 0.00 | 6.31 ± 0.78 | 0.472 |
| Tyrosine | 44.78 ± 0.75 a | 45.17 ± 2.79 a | 56.72 ± 0.76 b | 47.33 ± 1.35 a | <0.001 |
| NEAA | 491.75 ± 4.70 a | 533.5 ± 14.47 b | 522.25 ± 4.53 b | 540.87 ± 15.36 b | 0.003 |
| TAA | 854.36 ± 1.3 a | 903.32 ± 28.95 b | 884.59 ± 9.49 ab | 918.12 ± 22.80 b | 0.017 |
| EAAI | 56.82 ± 0.76 a | 58.65 ± 2.63 ab | 59.96 ± 0.99 ab | 61.38 ± 1.04 b | 0.035 |
| SRC | 0.27 ± 0.01 | 0.26 ± 0.03 | 0.27 ± 0.00 | 0.25 ± 0.03 | 0.667 |
| PDCAAS | 56.74 ± 4.05 | 56.97 ± 6.37 | 61.01 ± 0.00 | 60.22 ± 8.75 | 0.735 |
| CHC | PPF | 5% BSFL | 10% BSFL | p-Value | |
|---|---|---|---|---|---|
| Lauric acid (C12:0) | 0.34 ± 0.00 b | ND | ND | 0.19 ± 0.00 a | <0.001 |
| Myristic acid (C14:0) | 0.65 ± 0.01 d | 0.33 ± 0.01 a | 0.39 ± 0.00 b | 0.57 ± 0.01 c | <0.001 |
| Pentadecanoic acid (C15:0) | 0.13 ± 0.01 | ND | ND | ND | - |
| Palmitic acid (C16:0) | 29.39 ± 0.02 c | 14.64 ± 0.09 a | 14.98 ± 0.22 b | 14.92 ± 0.09 b | <0.001 |
| Palmitoleic acid (C16:1n7) | 0.63 ± 0.01 c | 0.47 ± 0.02 a | 0.58 ± 0.04 b | 0.86 ± 0.01 d | <0.001 |
| Heptadecanoic acid (C17:0) | 0.21 ± 0.00 b | 0.19 ± 0.02 ab | 0.17 ± 0.02 ab | 0.16 ± 0.02 a | 0.042 |
| Stearic acid (C18:0) | 12.11 ± 0.01 c | 7.52 ± 0.14 b | 7.49 ± 0.11 ab | 7.28 ± 0.14 a | <0.001 |
| Oleic trans acid (C18:1n9t) | 0.13 ± 0.02 a | 1.48 ± 0.09 d | 0.88 ± 0.06 c | 0.75 ± 0.04 b | <0.001 |
| Oleic acid (C18:1n9c) | 24.42 ± 0.03 c | 17.78 ± 0.33 a | 17.88 ± 0.25 a | 19.97 ± 0.16 b | <0.001 |
| Linoleic trans acid (C18:2n6t) | ND | ND | 0.20 ± 0.02 b | 0.17 ± 0.01 a | 0.063 |
| Linoleic acid (C18:2n6c) | 29.76 ± 0.03 a | 50.82 ± 0.25 b | 51.38 ± 0.11 b | 50.97 ± 0.56 b | <0.001 |
| γ-Linolenic acid (C18:3n6) | 0.24 ± 0.01 | 0.25 ± 0.03 | 0.20 ± 0.01 | ND | 0.442 |
| α-Linolenic acid (C18:3n3) | 1.10 ± 0.01 a | 4.27 ± 0.06 c | 3.4 ± 0.11 b | 3.38 ± 0.03 b | <0.001 |
| Arachidic acid (C20:1) | 0.18 ± 0.01 a | ND | ND | 1.18 ± 0.05 b | <0.001 |
| Dohomo–γ–Linolenic Acid(C20:3n6) | ND | 1.10 ± 0.03 b | 1.25 ± 0.04 b | 0.81 ± 0.12 a | 0.001 |
| Eicosapentaenoic Acid (C20:5n3) | 0.73 ± 0.04 a | 1.14 ± 0.09 b | ND | ND | 0.002 |
| SFA | 42.82 ± 0.02 c | 22.68 ± 0.11 a | 23.04 ± 0.28 b | 22.93 ± 0.13 b | <0.001 |
| MUFA | 25.36 ± 0.02 d | 19.73 ± 0.27 a | 20.52 ± 0.19 b | 21.58 ± 0.18 c | <0.001 |
| PUFA | 31.82 ± 0.01 a | 57.58 ± 0.21 d | 56.44 ± 0.16 c | 55.53 ± 0.42 b | <0.001 |
| n-3 | 1.10 ± 0.01 a | 4.27 ± 0.06 c | 3.40 ± 0.11 b | 3.38 ± 0.03 b | <0.001 |
| n-6 | 30.00 ± 0.04 a | 52.17 ± 0.24 b | 53.04 ± 0.13 c | 52.16 ± 0.39 b | <0.001 |
| n-3/n-6 | 0.04 ± 0.00 a | 0.08 ± 0.00 c | 0.06 ± 0.00 b | 0.06 ± 0.00 b | <0.001 |
| AI | 0.54 ± 0.00 c | 0.20 ± 0.00 a | 0.20 ± 0.00 ab | 0.20 ± 0.00 b | <0.001 |
| TI | 1.35 ± 0.00 c | 0.45 ± 0.00 a | 0.48 ± 0.01 b | 0.47 ± 0.00 b | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Yu, T.; Yuan, B.; Xiao, J.; Huang, D. The Addition of Hermetia illucens to Feed: Influence on Nutritional Composition, Protein Digestion Characteristics, and Antioxidant Activity of Acheta domesticus. Foods 2025, 14, 1140. https://doi.org/10.3390/foods14071140
Huang J, Yu T, Yuan B, Xiao J, Huang D. The Addition of Hermetia illucens to Feed: Influence on Nutritional Composition, Protein Digestion Characteristics, and Antioxidant Activity of Acheta domesticus. Foods. 2025; 14(7):1140. https://doi.org/10.3390/foods14071140
Chicago/Turabian StyleHuang, Junkui, Tinghao Yu, Binqiao Yuan, Jinhua Xiao, and Dawei Huang. 2025. "The Addition of Hermetia illucens to Feed: Influence on Nutritional Composition, Protein Digestion Characteristics, and Antioxidant Activity of Acheta domesticus" Foods 14, no. 7: 1140. https://doi.org/10.3390/foods14071140
APA StyleHuang, J., Yu, T., Yuan, B., Xiao, J., & Huang, D. (2025). The Addition of Hermetia illucens to Feed: Influence on Nutritional Composition, Protein Digestion Characteristics, and Antioxidant Activity of Acheta domesticus. Foods, 14(7), 1140. https://doi.org/10.3390/foods14071140






