Effect of Boiling Time on the Color, Water, Protein Secondary Structure, and Volatile Compounds of Beef
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Raw Material and Sample Preparation
2.3. Color Analysis
2.4. Water Analysis
2.5. Analysis of Volatile Compounds
2.5.1. Electronic Nose Analysis
2.5.2. GC–MS Analysis
2.5.3. Quantitation and OAVs Analysis of Volatile Compounds
2.6. Analysis of Protein Secondary Structure
2.7. Statistical Analysis
3. Results
3.1. Changes in the Water Content of Boiled Beef
3.2. Changes in the Surface Color Value of Beef
3.3. Volatile Compounds
3.3.1. E-Nose Analysis
3.3.2. Volatile Compounds Identified by GC–MS
3.4. Change in Protein Secondary Structure in Boiled Beef
4. Discussion
4.1. The Changes in Water Content
4.2. The Changes in Color
4.3. Key Aroma Compounds in Boiled Beef
4.4. Protein Secondary Structure in Boiled Beef
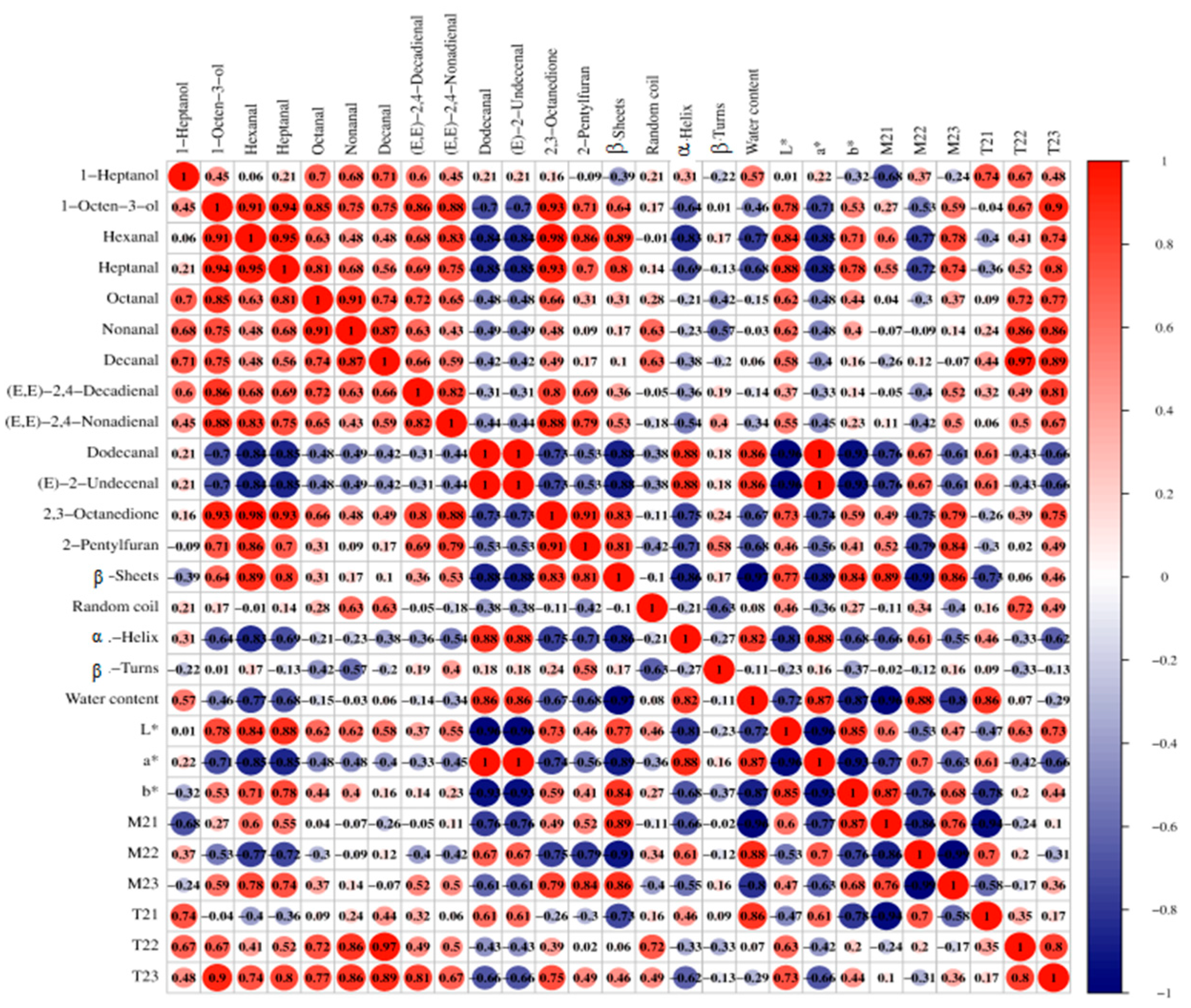
4.5. Relationship Between Color, Water, Protein Secondary Structure, and Key Aroma Compounds
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ren, Y.; Sun, D.W. Monitoring of moisture contents and rehydration rates of microwave vacuum and hot air dehydrated beef slices and splits using hyperspectral imaging. Food Chem. 2022, 382, 132346. [Google Scholar] [CrossRef]
- Kang, N.; Panzone, L.; Kuznesof, S. The role of cooking in consumers’ quality formation: An exploratory study of beef steaks. Meat Sci. 2022, 186, 108730. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Z.; Suleman, R.; Shen, Q.; Zhang, D. Effect of protein thermal stability and protein secondary structure on the roasted mutton texture and colour from different cuts. Meat Sci. 2019, 156, 52–58. [Google Scholar] [CrossRef]
- Shahrai, N.N.; Babji, S.; Maskat, M.Y.; Razali, A.F.; Yusop, S.M. Effects of marbling on physical and sensory characteristics of ribeye steaks from four different cattle breeds. Anim. Biosci. 2021, 34, 904–913. [Google Scholar] [CrossRef]
- Han, D.; Zhang, C.H.; Fauconnier, M.L.; Mi, S. Characterization and differentiation of boiled pork from Tibetan, Sanmenxia and Duroc×(Landrac× Yorkshire) pigs by volatiles profiling and chemometrics analysis. Food Res. Int. 2020, 130, 108910. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhu, Y.; Peng, J.; Zhang, Y.; Zhang, W.; Liu, Y. Characterization of stewed beef by sensory evaluation and multiple intelligent sensory technologies combined with chemometrics methods. Food Chem. 2023, 408, 135193. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.; Wu, W.; Soladoye, O.P.; Aluko, R.E.; Bak, K.H.; Fu, Y.; Zhang, Y. Maillard reaction of food-derived peptides as a potential route to generate meat flavor compounds: A review. Food Res. Int. 2022, 151, 110823. [Google Scholar] [CrossRef]
- Sohai, A.; Al-Dalali, S.; Wang, J.N.; Xie, J.C.; Shakoor, A.; Patil, P. Aroma compounds identified in cooked meat: A review. Food Res. Int. 2022, 157, 111385. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Cheng, Y.; Liu, Y. Volatile components of deep-fried soybean oil as indicator indices of lipid oxidation and quality degradation. Eur. Food Res. Technol. 2020, 246, 1183–1192. [Google Scholar] [CrossRef]
- Du, W.; Zhao, M.; Zhen, D.; Tan, J.; Wang, T.; Xie, J. Key aroma compounds in Chinese fried food of youtiao. Flavour. Frag. J. 2019, 35, 88–98. [Google Scholar] [CrossRef]
- Al-Dalali, S.; Li, C.; Xu, B. Insight into the effect of frozen storage on the changes in volatile aldehydes and alcohols of marinated roasted beef meat: Potential mechanisms of their formation. Food Chem. 2022, 385, 132629. [Google Scholar] [CrossRef]
- Vhangani, L.N.; Van, W.J. Heated plant extracts as natural inhibitors of enzymatic browning: A case of the Maillard reaction. J. Food Biochem. 2021, 45, e13611. [Google Scholar] [CrossRef]
- Wang, Z.; Cai, R.; Yang, X.; Gao, Z.; Yuan, Y.; Yue, T. Changes in aroma components and potential Maillard reaction products during the stir-frying of pork slices. Food Control 2021, 123, 107855. [Google Scholar] [CrossRef]
- Du, W.; Wang, Y.; Yan, Q.; Bai, S.; Huang, Y.; Li, L.; Wang, F. The number and position of unsaturated bonds in aliphatic aldehydes affect the cysteine-glucose Maillard reaction: Formation mechanism and comparison of volatile compounds. Food Res. Int. 2023, 173, 113337. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Tian, W.; Sun, L.; Liu, Y.; Li, M.; Zhao, G. Characterization of protein changes and development of flavor components induced by thermal modulation during the cooking of chicken meat. J. Food Process Pres. 2019, 43, e13949. [Google Scholar] [CrossRef]
- Qian, S.; Li, X.; Wang, H.; Wei, X.; Mehmood, W.; Zhang, C. Contribution of calpain to protein degradation, variation in myowater properties and the waterholding capacity of pork during postmortem ageing. Food Chem. 2020, 324, 126892. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Cui, J.; Gao, S.; Bai, S.; You, L.; Wang, S. Dynamic changes in the water and volatile compounds of chicken breast during the frying process. Food Res. Int. 2024, 175, 113715. [Google Scholar] [CrossRef]
- Zhang, J.; Kang, D.; Zhang, W.; Lorenzo, J.M. Recent advantage of interactions of protein-flavor in foods: Perspective of theoretical models, protein properties and extrinsic factors. Trends Food Sci. Tech. 2021, 111, 405–425. [Google Scholar] [CrossRef]
- Gianelli, M.P.; Flores, M.; Toldra, F. Interactions of soluble peptides and proteins from skeletal muscle on the release of volatile compounds. J. Agri. Food Chem. 2003, 51, 6828–6834. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, W.W.; Feng, X.C.; Yu, J.H.; Han, M.Y.; Deng, S.L.; Xu, X.L. Thermal degradation of gelatin enhances its ability to bind aroma compounds: Investigation of underlying mechanisms. Food Hydrocolloid 2018, 83, 497–510. [Google Scholar] [CrossRef]
- Gu, S.; Dai, W.; Chong, Y.; Lyu, F.; Zhou, X.; Ding, Y. The binding of key fishy off-flavor compounds to silver carp proteins: A thermodynamic analysis. RSC Adv. 2020, 10, 11292–11299. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.J.; Wang, R.Q. Changes in secondary structure of myofibrillar protein and its relationship with water dynamic changes during storage of battered and deep-fried pork slices. Food Sci. Biotechnol. 2018, 27, 1667–1673. [Google Scholar] [CrossRef]
- Nawaz, A.; Li, E.; Khalifa, I.; Walayat, N.; Liu, J.; Irshad, S.; Lorenzo, J.M. Effect of different processing methods on quality, structure, oxidative properties and water distribution properties of fish meat-based snacks. Foods 2021, 10, 2467. [Google Scholar] [CrossRef]
- Pérez-Juan, M.; Kondjoyan, A.; Picouet, P.; Realini, C.E. Effect of marination and microwave heating on the quality of Semimembranosus and Semitendinosus muscles from Friesian mature cows. Meat Sci. 2012, 92, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xue, Y.; Li, C.; Xie, Z.; Zhao, Z. Comparative study on the flavor and sensory characteristics of two animal oils in hotpot seasoning. China Condiment 2020, 45, 16–30. [Google Scholar]
- Wang, Y.R.; Luo, R.M.; Wang, S.L. Water distribution and key aroma compounds in the process of beef roasting. Front. Nutr. 2022, 9, 978622. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Li, K.; Luo, R.; Wang, S.; Chen, F.; Sun, Y. Dynamic changes in the water distribution and key aroma compounds of roasted chicken during roasting. Food Res. Int. 2023, 172, 113146. [Google Scholar] [CrossRef]
- Zhao, J.; Cui, J.; Chen, R.; Tang, Z.; Tan, Z.; Jiang, L.; Liu, F. Real-time in-situ quantification of protein secondary structures in aqueous solution based on ATR-FTIR subtraction spectrum. Biochem. Eng. J. 2021, 176, 108225. [Google Scholar] [CrossRef]
- Xu, S.T.; Seid, R.F. Juiciness of meat, meat products, and meat analogues: Definition, evaluation methods, and influencing factors. Food Rev. Int. 2024, 40, 2344–2377. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, X.J.; Liu, X.Z.; Xing, J.F.; Guo, C.; Du, Y.H.; Wang, W.H. Focusing on intramuscular connective tissue: Effect of cooking time and temperature on physical, textual, and structural properties of yak meat. Meat Sci. 2022, 184, 108690. [Google Scholar] [CrossRef]
- Shao, J.H.; Deng, Y.M.; Jia, N.; Li, R.R.; Cao, J.X.; Liu, D.Y. Low-field NMR determination of water distribution in meat batters with NaCl and polyphosphate addition. Food Chem. 2016, 204, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Muhoza, B.; Wang, X.W.; Feng, T.T.; Xia, S.Q.; Zhang, X.M. Comparison between microwave and traditional water bath cooking on saltiness perception, water distribution and microstructure of grass crap meat. Food Res. Int. 2019, 125, 108521. [Google Scholar] [CrossRef]
- Yu, X.; Li, L.; Xue, J.; Wang, J.; Song, G.; Zhang, Y.; Shen, Q. Effect of air-frying conditions on the quality attributes and lipidomic characteristics of surimi during processing. Innov. Food Sci. Emerg. 2020, 60, 102305. [Google Scholar] [CrossRef]
- Bıyıklı, M.; Akoğlu, A.; Kurhan, Ş.; Akoğlu, İ.T. Effect of different Sous Vide cooking temperature-time combinations on the physicochemical, microbiological, and sensory properties of turkey cutlet. Int. J. Gastron. Food Sci. 2020, 20, 100204. [Google Scholar] [CrossRef]
- Christensen, L.B.; Ertbjerg, P.; Aaslyng, M.D.; Christensen, M. Effect of prolonged heat treatment from 48 ◦C to 63 ◦C on toughness, cooking loss and color of pork. Meat Sci. 2011, 88, 280–285. [Google Scholar] [CrossRef]
- Roldán, M.; Antequera, T.; Martín, A.; Mayoral, A.I.; Ruiz, J. Effect of different temperature-time combinations on physicochemical, microbiological, textural and structural features of sous-vide cooked lamb loins. Meat Sci. 2013, 93, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hui, T.; Fang, F.; Ma, Q.; Li, S.; Zhang, D.; Wang, Z. Characterization and discrimination of key aroma compounds in pre-and postrigor roasted mutton by GC-O-MS, GC E-Nose and aroma recombination experiments. Foods 2021, 10, 2387. [Google Scholar] [CrossRef]
- Liu, H.; Hui, T.; Fang, F.; Li, S.; Wang, Z.; Zhang, D. The formation of key aroma compounds in roasted mutton during the traditional charcoal process. Meat Sci. 2022, 184, 108689. [Google Scholar] [CrossRef]
- Wang, Y.R.; Wang, S.L.; Luo, R.M. Evaluation of key aroma compounds and protein secondary structure in the roasted Tan mutton during the traditional charcoal process. Front. Nutr. 2022, 9, 1003126. [Google Scholar] [CrossRef]
- Ji, C.; Wu, B.; Gao, S.; Wang, Y. Impact of roasting time on the color, protein, water distribution and key volatile compounds of pork. J. Food Compos. Anal. 2024, 136, 106787. [Google Scholar] [CrossRef]
- Wang, Y.; Bu, X.; Yang, D.; Deng, D.; Lei, Z.; Guo, Z.; Yu, Q. Effect of cooking method and doneness degree on volatile compounds and taste substance of Pingliang Red Beef. Foods 2023, 12, 446. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; He, Z.; Yang, L.; Li, H. Generation of key aroma compounds in fat and lean portions of non-smoked bacon induced via lipid pyrolysis reaction. Food Chem. 2024, 437, 137684. [Google Scholar] [CrossRef] [PubMed]
- Schmidberger, P.C.; Schieberle, P. Changes in the key aroma compounds of raw shiitake mushrooms (Lentinula edodes) induced by pan-frying as well as by rehydration of dry mushrooms. J. Agric. Food Chem. 2020, 68, 4493–4506. [Google Scholar] [CrossRef]
- Bento-Silva, A.; Duarte, N.; Santos, M.; Costa, C.P.; Vaz Patto, M.C. Rocha SM and Bronze MR, Comprehensive two-dimensional gas chromatography as a powerful strategy for the exploration of Broas volatile composition. Molecules 2022, 27, 2728. [Google Scholar] [CrossRef]
- Cheng, L.; Li, X.; Tian, Y.; Wang, Q.; Li, X.; An, F.; Huang, Q. Mechanisms of cooking methods on flavor formation of Tibetan pork. Food Chem. X 2023, 19, 100873. [Google Scholar] [CrossRef] [PubMed]
- Mattice, K.D.; Marangoni, A.G. Comparing methods to produce fibrous material from zein. Food Res. Int. 2020, 128, 108804. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Q.; Liu, L.; Zhang, Y.; He, N.; Wang, Q. High-moisture extrusion process of transglutaminase-modified peanut protein: Effect of transglutaminase on the mechanics of the process forming a fibrous structure. Food Hydrocolloid 2021, 112, 106346. [Google Scholar] [CrossRef]
- Xu, X.L.; Han, M.Y.; Fei, Y.; Zhou, G.H. Raman spectroscopic study of heat-induced gelation of porkmyofibrillar proteins and its relationship with textural characteristic. Meat Sci. 2011, 87, 159–164. [Google Scholar] [CrossRef]
- Zhou, C.Y.; Cao, J.X.; Zhuang, X.B.; Bai, Y.; Li, C.B.; Xu, X.L.; Zhou, G.H. Evaluation of the secondary structure anddigestibility of myofibrillar proteins in cooked ham. CyTA-J. Food 2019, 17, 78–86. [Google Scholar] [CrossRef]
- Guo, X.Y.; Peng, Z.Q.; Zhang, Y.W.; Liu, B.; Cui, Y.Q. The solubility and conformational characteristics of porcine myosin as affected by the presence of L-lysine and L-histidine. Food Chem. 2015, 170, 212–217. [Google Scholar] [CrossRef]
- Liu, F.; Dong, X.; Shen, S.; Shi, Y.; Ou, Y.; Cai, W.; Zhu, B. Changes in the digestion properties and protein conformation of sturgeon myofibrillar protein treated by low temperature vacuum heating during in vitro digestion. Food Funct. 2021, 12, 6981–6991. [Google Scholar] [CrossRef] [PubMed]
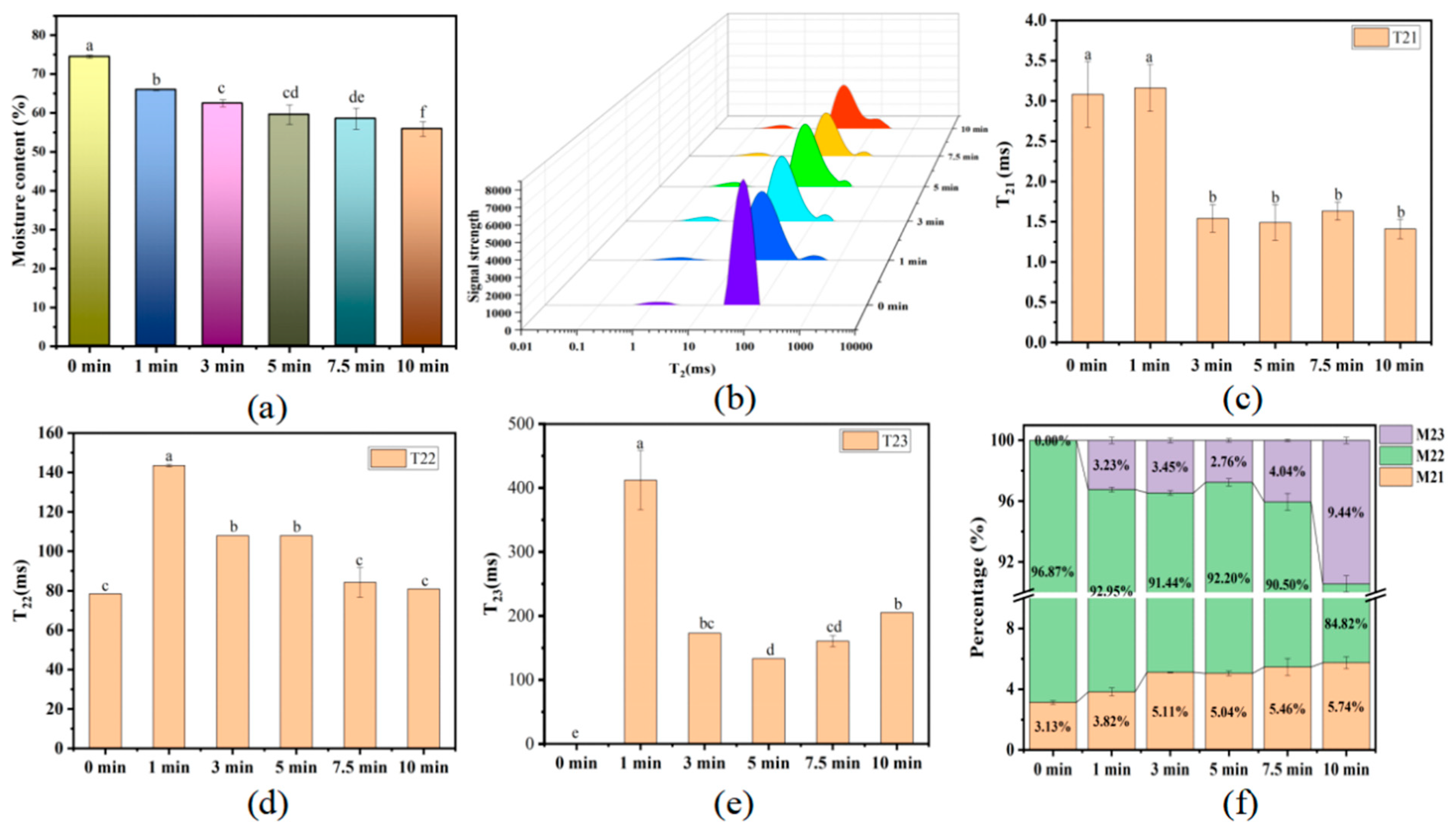



| Boling Time (min) | 0 | 1 | 3 | 5 | 7.5 | 10 |
|---|---|---|---|---|---|---|
| L* | 37.89 ± 1.66 d | 57.90 ± 2.08 a | 56.10 ± 1.09 ab | 57.48 ± 1.33 a | 52.46 ± 2.49 c | 54.09 ± 1.49 bc |
| a* | 27.44 ± 2.51 a | 11.40 ± 0.46 b | 11.55 ± 0.35 b | 11.45 ± 0.15 b | 11.29 ± 0.69 b | 10.95 ± 0.23 b |
| b* | 6.33 ± 1.66 d | 9.35 ± 0.31 c | 11.27 ± 0.57 bc | 9.96 ± 0.30 bc | 10.15 ± 0.51 abc | 10.77 ± 0.65 ab |
| w | 31.80 | 55.39 | 53.23 | 54.85 | 50.09 | 51.59 |
| ΔE | 0 | 25.82 | 24.67 | 25.55 | 22.08 | 23.54 |
| BI | 46.32 | 14.05 | 15.06 | 14.33 | 15.60 | 14.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, L.; Zhang, Y.; Ma, Y.; Wang, Y.; Wei, Z. Effect of Boiling Time on the Color, Water, Protein Secondary Structure, and Volatile Compounds of Beef. Foods 2025, 14, 1372. https://doi.org/10.3390/foods14081372
You L, Zhang Y, Ma Y, Wang Y, Wei Z. Effect of Boiling Time on the Color, Water, Protein Secondary Structure, and Volatile Compounds of Beef. Foods. 2025; 14(8):1372. https://doi.org/10.3390/foods14081372
Chicago/Turabian StyleYou, Liqin, Yanfeng Zhang, Yingjuan Ma, Yongrui Wang, and Zhaojun Wei. 2025. "Effect of Boiling Time on the Color, Water, Protein Secondary Structure, and Volatile Compounds of Beef" Foods 14, no. 8: 1372. https://doi.org/10.3390/foods14081372
APA StyleYou, L., Zhang, Y., Ma, Y., Wang, Y., & Wei, Z. (2025). Effect of Boiling Time on the Color, Water, Protein Secondary Structure, and Volatile Compounds of Beef. Foods, 14(8), 1372. https://doi.org/10.3390/foods14081372







