Pulse Proteins: Processing, Nutrition, and Functionality in Foods
Abstract
:1. Introduction
2. Worldwide Pulse Production
3. Composition of Pulses
3.1. Protein
3.2. Carbohydrates
3.3. Bioactive Compounds
3.4. Off-Flavour Compounds
3.5. Antinutritional Factors
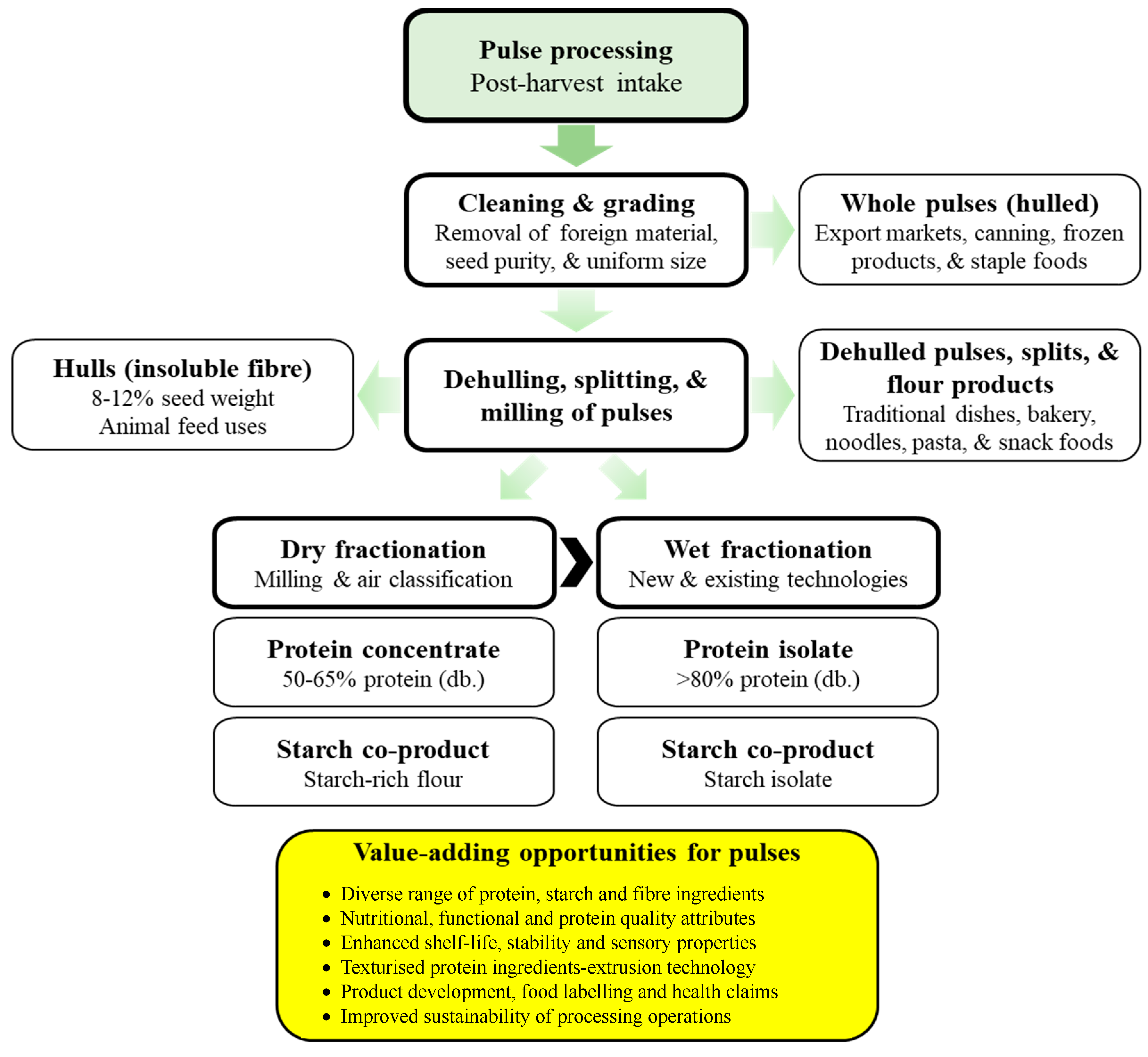
4. Pulse Protein Extraction
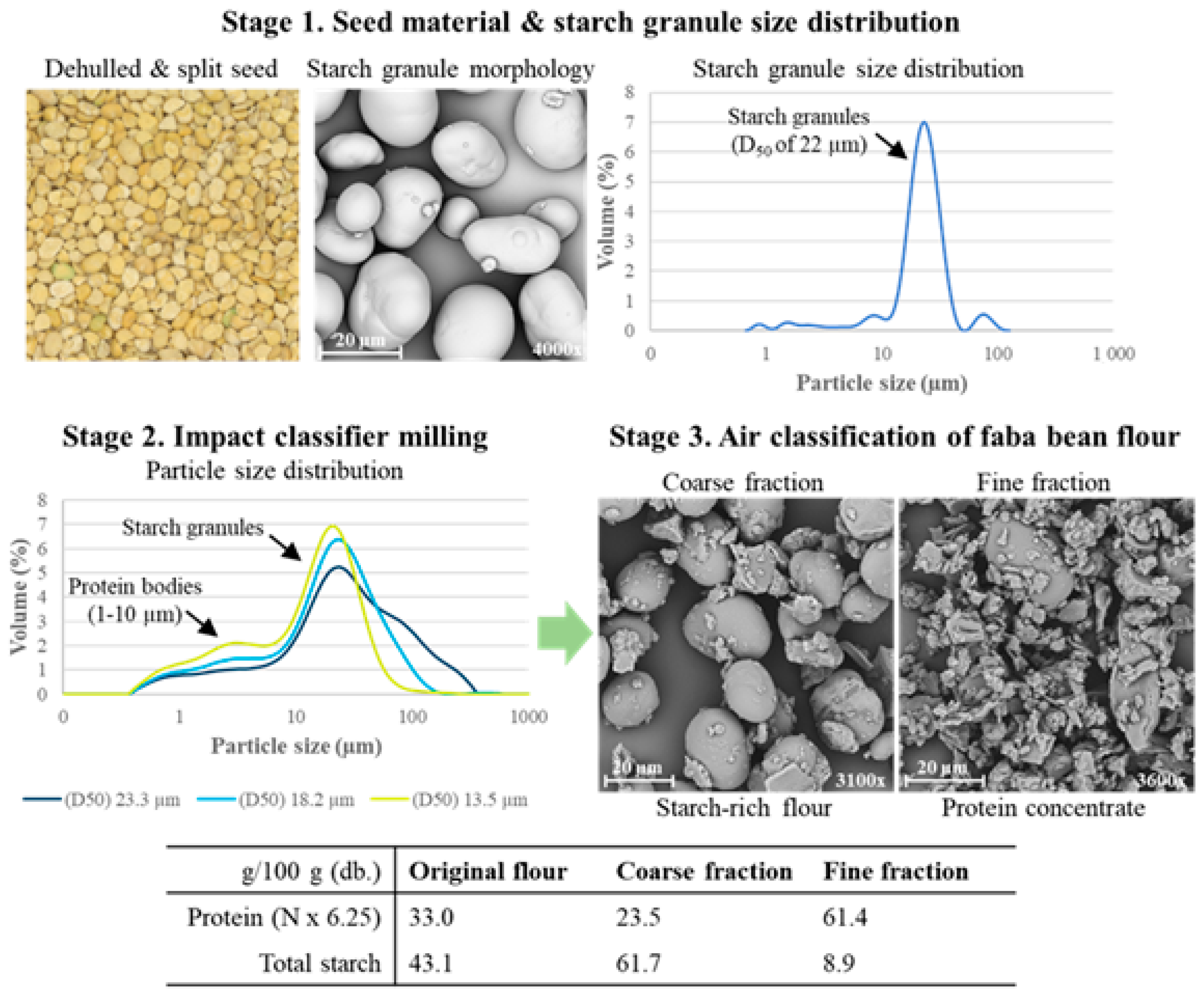
4.1. Dehulling, Splitting, and Milling of Pulses
4.2. Dry Fractionation
4.3. Tribo-Electric Separation
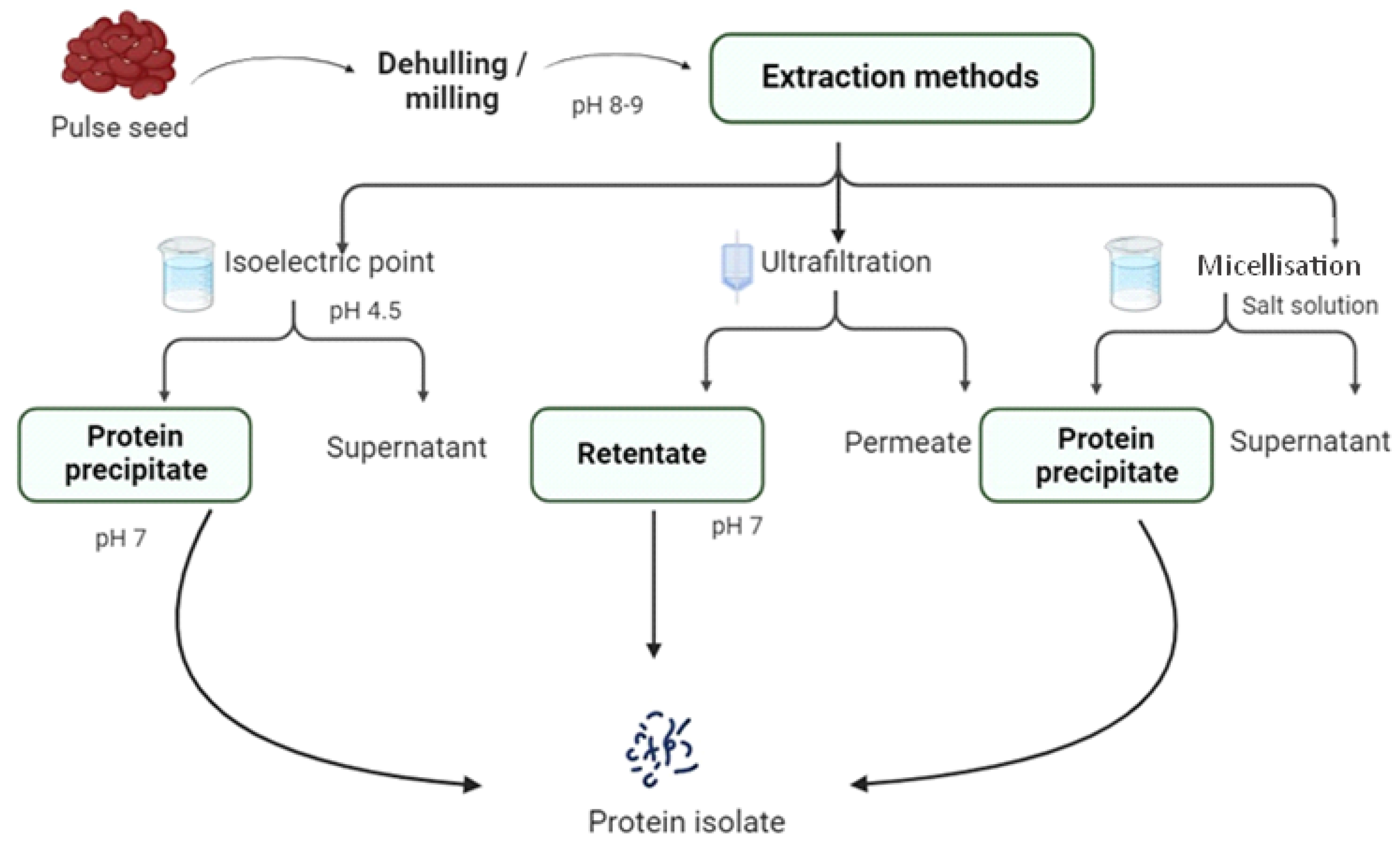
4.4. Wet Fractionation
4.5. Isoelectric Point
4.6. Ultrafiltration
4.7. Micellisation (Salt Extraction)
4.8. Modification and Improvement of Pulse Proteins
5. Functional Properties of Pulse Proteins
5.1. Solubility
5.2. Water- and Oil-Holding Capacity
5.3. Emulsifying Properties
5.4. Foaming Properties
5.5. Gelation
6. Uses of Pulse Proteins in Foods
6.1. Bakery Products and Pasta
6.2. Dairy Alternatives
6.3. Meat Analogues
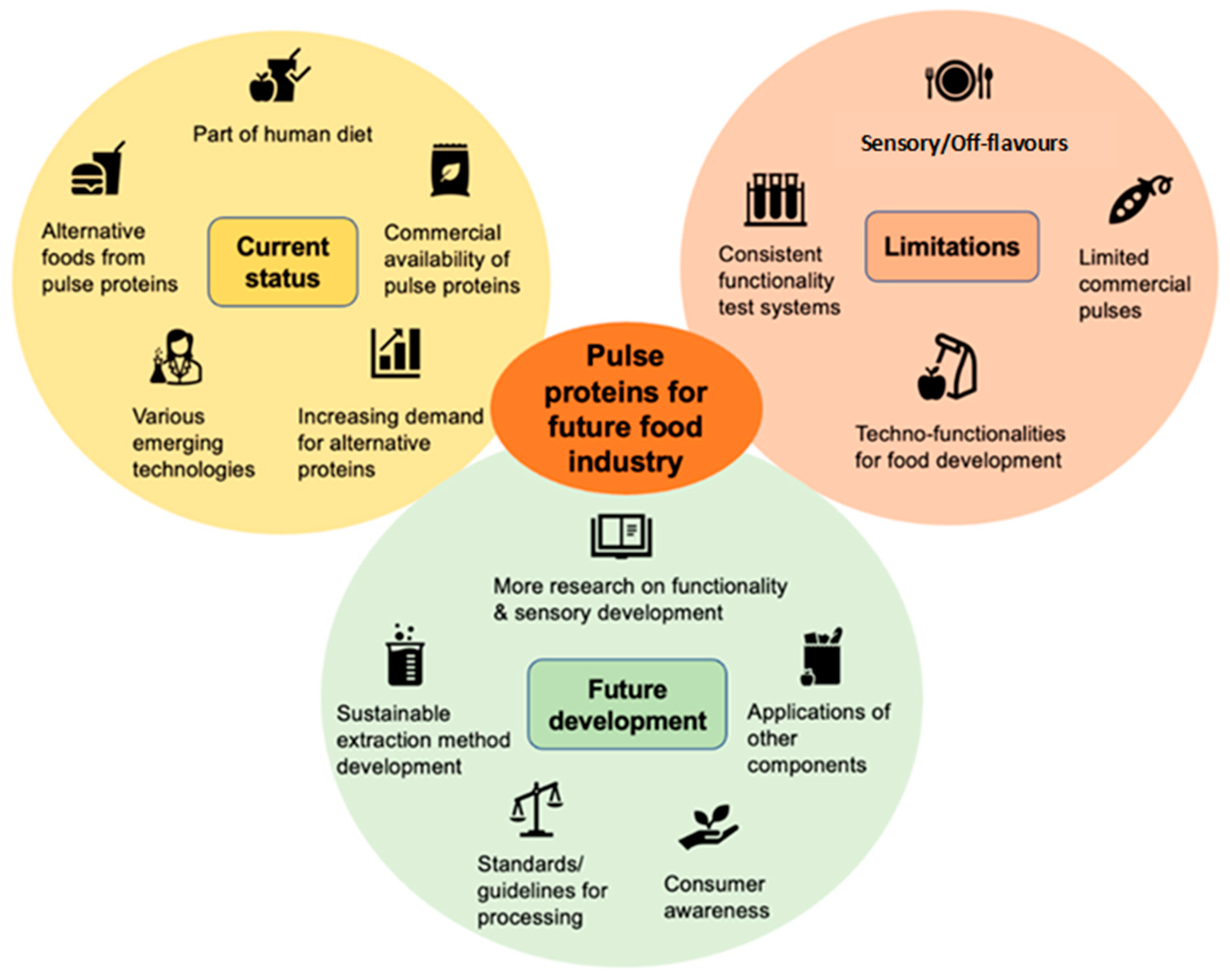
7. Future Trends
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAs | Amino acids |
| C | Concentrate |
| CLSM | Confocal laser scanning microscopy |
| DF | Dry fractionation |
| I | Isolate |
| IEP | Isoelectric precipitation |
| LGC | Least gelling concentration |
| OHC | Oil-holding capacity |
| RFOs | Raffinose family of oligosaccharides |
| TVP | Texturised vegetable proteins |
| UF | Ultrafiltration |
| WF | Wet fractionation |
| WHC | Water-holding capacity |
References
- Schutyser, M.A.; Pelgrom, P.J.; Van der Goot, A.J.; Boom, R.M. Dry fractionation for sustainable production of functional legume protein concentrates. Trends Food Sci. Technol. 2015, 45, 327–335. [Google Scholar]
- Akharume, F.U.; Aluko, R.E.; Adedeji, A.A. Modification of plant proteins for improved functionality: A review. Comp. Rev. Food Sci. Food Saf. 2021, 20, 198–224. [Google Scholar] [CrossRef]
- Vogelsang-O’Dwyer, M.; Sahin, A.W.; Arendt, E.K.; Zannini, E. Enzymatic hydrolysis of pulse proteins as a tool to improve techno-functional properties. Foods 2022, 20, 198–224. [Google Scholar]
- Derbyshire, E.J. Flexitarian Diets and Health: A Review of the Evidence-Based Literature. Front. Nutr. 2017, 3, 55. [Google Scholar]
- FAO. 2019. Available online: https://www.fao.org/fao-who-codexalimentarius/en/ (accessed on 13 October 2019).
- Kumar, Y.; Basu, S.; Goswami, D.; Devi, M.; Shivhare, U.S.; Vishwakarma, K. Anti-nutritional compounds in pulses: Implications and alleviation methods. Legume Sci. 2022, 4, e111. [Google Scholar]
- Bouchard, J.; Malalgoda, M.; Storsley, J.; Malunga, L.; Netticadan, T.; Thandapilly, S.J. Health benefits of cereal grain- and pulse-derived proteins. Molecules 2022, 27, 3746. [Google Scholar] [CrossRef]
- Aiking, H.; De Boer, J. The next protein transitions. Trends Food Sci. Technol. 2020, 105, 515–522. [Google Scholar] [CrossRef]
- Asif, M.; Rooney, L.W.; Ali, R.; Riaz, M.N. Application and opportunities of pulses in food system: A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 1168–1179. [Google Scholar]
- Boye, J.; Zare, F.; Pletch, A. Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar]
- Jones, J.M.; García, C.G.; Braun, H.J. Perspective: Whole and refined grains and health-evidence supporting make half your grains whole. Adv. Nutr. 2020, 11, 492–506. [Google Scholar]
- Ramdath, D.; Renwick, S.; Duncan, A.M. The role of pulses in the dietary management of diabetes. Can. J. Diabetes 2016, 40, 355–363. [Google Scholar] [CrossRef]
- Padhi, E.M.T.; Ramdath, D.D. A review of the relationship between pulse consumption and reduction of cardiovascular disease risk factors. J. Funct. Foods 2017, 38, 635–643. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: Systematic review and dose-response meta-analysis of prospective studies. Br. Med. J. 2016, 353, i2716. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, J.; Du, L.; Li, K.; Zhou, Y. Association of whole grain, refined grain, and cereal consumption with gastric cancer risk: A meta-analysis of observational studies. Food Sci. Nutr. 2019, 7, 256–265. [Google Scholar] [CrossRef]
- Rao, S.; Chinkwo, K.A.; Santhakumar, A.B.; Blanchard, C.L. Inhibitory effects of pulse bioactive compounds on cancer development pathways. Diseases 2018, 6, 72. [Google Scholar] [CrossRef]
- Rawal, V.; Charrondiere, R.; Xipsiti, M.; Grande, F. Pulses: Nutritional Benefits and Consumption Patterns. In The Global Economy of Pulses; Rawal, V., Navarro, D.K., Eds.; FAO: Rome, Italy, 2019; pp. 9–19. [Google Scholar]
- Devkota, L.; Kyriakopoulou, K.; Bergia, R.; Dhital, S. Structural and thermal characterization of protein isolates from Australian lupin varieties as affected by processing conditions. Foods 2023, 12, 908. [Google Scholar] [CrossRef]
- OECD/FAO. OECD-FAO Agricultural Outlook 2022–2031; OECD Publishing: Paris, France, 2023. [Google Scholar]
- He, Y.; Shim, Y.Y.; Mustafa, R.; Meda, V.; Reaney, M.J.T. Chickpea cultivar selection to produce aquafaba with superior emulsion properties. Foods 2019, 8, 685. [Google Scholar] [CrossRef]
- Yegrem, L.; Mengestu, D.; Legesse, O.; Abebe, W.; Girma, N. Nutritional compositions and functional properties of New Ethiopian chickpea varieties: Effects of variety, grown environment and season. Int. J. Food. Prop. 2022, 25, 1485–1497. [Google Scholar] [CrossRef]
- Wood, J.A.; Knights, E.J.; Campbell, G.M.; Choct, M. Differences between easy- and difficult-to-mill chickpea (Cicer arietinum L.) genotypes. Part I: Broad chemical composition. J. Sci. Food Agric. 2014, 94, 1437–1445. [Google Scholar]
- Vishwakarma, R.K.; Shivhare, U.S.; Gupta, R.K.; Yadav, D.N.; Jaiswal, A.; Prasad, P. Status of pulse milling processes and technologies: A review. Crit. Rev. Food Sci. Nutr. 2017, 58, 1615–1628. [Google Scholar] [CrossRef]
- Sumo, C.; De Angelis, D.; Ricciardi, L.; Caponio, F.; Lotti, C.; Pavan, S.; Pasqualone, A. Nutritional, physico-chemical and functional characterization of a global chickpea collection. J. Food Compost. Anal. 2019, 84, 103306. [Google Scholar]
- Guldiken, B.; Konieczny, D.; Franczyk, A.; Satiro, V.; Pickard, M.; Wang, N.; House, J.; Nickerson, M.T. Impacts of infrared heating and tempering on the chemical composition, morphological, functional properties of navy bean and chickpea flours. Eur. Food. Res. Technol. 2022, 2483, 767–781. [Google Scholar] [CrossRef]
- Chatur, P. Identifying the Nutritional Properties of Australian Kabuli Chickpeas to Inform Future Novel Food Product Development. Ph.D. Thesis, Curtin University, Bentley, WA, Australia, 2021. [Google Scholar]
- Gharibzahedi, S.M.T.; Mousavi, S.M.; Jafari, S.M.; Faraji, K. Proximate composition, mineral content, and fatty acids profile of two varieties of lentil seeds cultivated in Iran. Chem. Nat. Compd. 2012, 47, 976–978. [Google Scholar]
- Ramdath, D.; Lu, Z.; Maharaj, P.; Winberg, J.; Brummer, Y.; Hawke, A. Proximate analysis and nutritional evaluation of twenty Canadian lentils by principal component and cluster analyses. Foods 2020, 9, 175. [Google Scholar] [CrossRef]
- Lee, H.C.; Htoon, A.K.; Paterson, J.L. Alkaline extraction of starch from Australian lentil cultivars Matilda and Digger optimised for starch yield and starch and protein quality. Food Chem. 2007, 102, 551–559. [Google Scholar] [CrossRef]
- Goswami, K.; Shukla, P. Evaluation of improved varieties of field pea (Pisum sativum) for nutritional and functional quality. Int. J. Chem. Stud. 2019, 7, 2260–2266. [Google Scholar]
- Bashir, S.; Shah, S.Z.A.; Naz, R.M.M.; Hamid, A.; Anjum, S.; Zahid, N.; Maqbool, M.; Khan, M.; Yaqoob, A.; Afzal, A. Physicochemical evaluation of field pea (Pisum sativum L.) landraces under rainfed conditions of AJ&K-Pakistan. Pure App. Biol. 2019, 8, 1033–1042. [Google Scholar]
- Ashenafi, N.; Mezgebe, A.; Leka, L. Optimization of amount of spices, roasting temperature and time for field pea (Pisum sativum) Shiro flour using response surface methodology. Appl. Food Res. 2023, 3, 100257. [Google Scholar]
- Black, R.G.; Brouwer, J.B.; Meares, C.; Iyer, L. Variation in physico-chemical properties of field peas (Pisum sativum). Food Res. Int. 1998, 31, 81–86. [Google Scholar]
- Millar, K.A.; Gallagher, E.; Burke, R.; McCarthy, S.; Barry-Ryan, C. Proximate composition and anti-nutritional factors of fava-bean (Vicia faba), green-pea and yellow-pea (Pisum sativum) flour. J. Food Compos. Anal. 2019, 82, 103233. [Google Scholar]
- Salazar, D.; Rodas, M.; Arancibia, M. Production of tortillas from nixtamalized corn flour enriched with Andean crops flours: Faba-bean (Vicia faba) and white-bean (Phaseolus vulgaris). Emir. J. Food. Agric. 2020, 32, 731–738. [Google Scholar]
- Skylas, D.J.; Johnson, B.J.; Kalitsis, J.; Richard, S.; Whiteway, C.; Wesley, I.; Naiker, M.; Quail, K.J. Optimised dry processing of protein concentrates from Australian pulses: A comparative study of faba bean, yellow pea and red lentil seed material. Legume Sci. 2023, 5, e161. [Google Scholar]
- Blessing, I.A.; Gregory, L.O. Effect of processing on the proximate composition of the dehulled and undehulled mung bean [Vigna radiata (L.) Wilczek] flours. Pak. J. Nutr. 2010, 9, 1006–1016. [Google Scholar]
- Wenhao, L.; Chang, S.; Shuqin, Y.; Qun, S. Characteristics of sixteen mung bean cultivars and their protein isolates. Int. J. Food. Sci. Technol. 2010, 45, 1205–1211. [Google Scholar]
- Nagrale, S.C.; Patil, A.N.; Tayade, N.; Jadhav, P.V.; Wakode, Y.S. Proximate composition and estimation of mineral content from different mung bean (Vigna radiata (L). Wilczek) genotypes. J. Pharmacogn. Phytochem. 2018, 7, 434–3436. [Google Scholar]
- Skylas, D.J.; Molloy, M.P.; Willows, R.D.; Salman, H.; Blanchard, C.L.; Quail, K.J. Effect of processing on Mung bean (Vigna radiata) flour nutritional properties and protein composition. J. Agri. Sci. 2018, 10, 16–28. [Google Scholar]
- Mubarak, A.E. Chemical, nutritional and sensory properties of bread supplemented with lupin seed (Lupinus albus) products. Food/Nahrung 2001, 45, 241–245. [Google Scholar]
- Tizazu, H.; Emire, S.A. Chemical composition, physicochemical and functional properties of lupin (Lupinus albus) seeds grown in Ethiopia. Afr. J. Food Agric. Nutr. Dev. 2010, 10, 3029–3046. [Google Scholar]
- Chamone, M.E.R.; Ascheri, J.L.R.; Vargas-Solórzano, J.W.; Stephan, M.P.; Carvalho, C.W.P. Chemical characterization of white lupin (Lupinus albus) flour treated by extrusion cooking and aqueous debittering processes. Plant Foods Hum. Nutr. 2023, 78, 292–298. [Google Scholar]
- Villarino, C.B.J.; Jayasena, V.; Coorey, R.; Chakrabarti-Bell, S.; Johnson, S.K. The effects of Australian sweet lupin (ASL) variety on physical properties of flours and breads. LWT-Food Sci. Technol. 2015, 60, 435–443. [Google Scholar]
- Hall, A.E.; Moraru, C.I. Structure and function of pea, lentil and faba bean proteins treated by high pressure processing and heat treatment. LWT-Food Sci. Technol. 2021, 152, 112349. [Google Scholar] [CrossRef]
- Mazumder, K.; Biswas, B.; Kerr, P.G.; Blanchard, C.; Nabila, A.; Golder, M.; Gulzarul Aziz, M.; Farahnaky, A. Comparative assessment of nutritional, thermal, rheological and functional properties of nine Australian lupin cultivars. Sci. Rep. 2021, 11, 21515. [Google Scholar] [CrossRef] [PubMed]
- Skylas, D.J.; Paull, J.G.; Hughes, D.G.D.; Gogel, B.; Long, H.; Williams, B.; Mundree, S.; Blanchard, C.L.; Quail, K.J. Nutritional and anti-nutritional seed-quality traits of faba bean (Vicia faba) grown in South Australia. Crop Pasture Sci. 2019, 70, 463–472. [Google Scholar] [CrossRef]
- Hood-Niefer, S.D.; Warkentin, T.D.; Chibbar, R.N.; Vandenberg, A.; Tyler, T.R. Effect of genotype and environment on the concentrations of starch and protein in, and the physicochemical properties of starch from, field pea and faba bean. J. Sci. Food Agric. 2012, 92, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Skylas, D.J.; Blanchard, C.L.; Quail, K.J. Variation in nutritional composition of Australian mung bean varieties. J. Agric. Sci. 2017, 9, 45–53. [Google Scholar]
- Khattab, R.Y.; Arntfield, S.D.; Nyachoti, C.M. Nutritional quality of legume seeds as affected by some physical treatments, part 1: Protein quality evaluation. LWT-Food Sci. Technol. 2009, 42, 1107–1111. [Google Scholar] [CrossRef]
- Chukwuejim, S.; Utioh, A.; Choi, T.D.; Aluko, R.E. Lupin seed proteins: A comprehensive review of composition, extraction technologies, food functionality, and health benefits. Food Rev. Int. 2023, 40, 691–714. [Google Scholar] [CrossRef]
- Chéreau, D.; Videcoq, P.; Ruffieux, C.; Pichon, L.; Motte, J.C.; Belaid, S.; Ventureira, J.; Lopez, M. Combination of existing and alternative technologies to promote oilseeds and pulses proteins in food applications. OCL 2016, 23, D406. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Chen, Y.; Kaur, A.; Yu, L. Pulse proteins: Secondary structure, functionality and applications. J. Food Sci. Technol. 2019, 56, 2787–2798. [Google Scholar] [CrossRef]
- Ghumman, A.; Kaur, A.; Singh, N. Functionality and digestibility of albumins and globulins from lentil and horse gram and their effect on starch rheology. Food Hydrocoll. 2016, 61, 843–850. [Google Scholar] [CrossRef]
- Tripathi, A.; Iswarya, V.; Rawson, A.; Singh, N.; Dave Oomah, B.; Patras, A. Chemistry of pulses—Macronutrients. In Pulse Foods; Academic Press: Cambridge, MA, USA, 2021; pp. 31–59. [Google Scholar]
- Singh, N. Functional and physicochemical properties of pulse starch. In Pulse Foods; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 87–112. [Google Scholar]
- Venkidasamy, B.; Selvaraj, D.; Nile, A.S.; Ramalingam, S.; Kai, G.; Nile, S.H. Indian pulses: A review on nutritional, functional and biochemical properties with future perspectives. Trends Food Sci. Technol. 2019, 88, 228–242. [Google Scholar]
- Hall, C.; Hillen, C.; Robinson, J.G. Composition, nutritional value, and health benefits of pulses. Cereal Chem. 2017, 94, 11–31. [Google Scholar]
- Hou, D.; Feng, Q.; Tang, J.; Shen, Q.; Zhou, S. An update on nutritional profile, phytochemical compounds, health benefits, and potential applications in the food industry of pulses seed coats: A comprehensive review. Crit. Rev. Food Sci. Nutri 2023, 63, 1960–1982. [Google Scholar]
- Parveen, S.; Jamil, A.; Pasha, I.; Ahmad, F. Pulses: A Potential Source of Valuable Protein for Human Diet; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Tiwari, U.; Cummins, E. Legume fiber characterization, functionality, and process effects. In Pulse Foods; Academic Press: Cambridge, MA, USA, 2021; pp. 147–175. [Google Scholar]
- Khazaei, H.; Purves, R.W.; Hughes, J.; Link, W.; O’Sullivan, D.M.; Schulman, A.H.; Björnsdotter, E.; Geu-Flores, F.; Nadzieja, M.; Andersen, S.U.; et al. Eliminating vicine and convicine, the main anti-nutritional factors restricting faba bean usage. Trends Food Sci. Technol. 2019, 9, 1549–1556. [Google Scholar]
- Xiang, J.; Zhang, M.; Apea-Bah, F.B.; Beta, T. Hydroxycinnamic acid amide (HCAA) derivatives, flavonoid C-glycosides, phenolic acids and antioxidant properties of foxtail millet. Food Chem. 2019, 295, 214–223. [Google Scholar]
- Siah, S.; Konczak, I.; Wood, J.A.; Agboola, S.; Blanchard, C. Effects of Roasting on Phenolic Composition and In vitro Antioxidant Capacity of Australian Grown Faba Beans (Vicia faba L.). Plant Foods Hum. Nutr. 2014, 69, 85–91. [Google Scholar] [PubMed]
- Siah, S.D.; Wood, J.A.; Agboola, S.; Konczak, I.; Blanchard, C.L. Effects of soaking, boiling and autoclaving on the phenolic contents and antioxidant activities of faba beans (Vicia faba L.) differing in seed coat colours. Food Chem. 2013, 142, 461–468. [Google Scholar]
- Johnson, J.B.; Collins, T.; Skylas, D.; Quail, K.; Blanchard, C.; Naiker, M. Profiling the varietal antioxidative contents and macrochemical composition in Australian faba beans (Vicia faba L.). Legume Sci. 2020, 2, e28. [Google Scholar]
- Johnson, J.B.; Skylas, D.J.; Mani, J.S.; Xiang, J.; Walsh, K.B.; Naiker, M. Phenolic Profiles of Ten Australian Faba Bean Varieties. Molecules 2021, 26, 4642. [Google Scholar] [CrossRef]
- Conti, M.V.; Guzzetti, L.; Panzeri, D.; De Giuseppe, R.; Coccetti, P.; Labra, M.; Cena, H. Bioactive compounds in legumes: Implications for sustainable nutrition and health in the elderly population. Trends Food Sci. Technol. 2021, 117, 139–147. [Google Scholar]
- Kouris-Blazos, A.; Belski, R. Health benefits of legumes and pulses with a focus on Australian sweet lupins. Asia Pacific J. Clin. Nutr. 2016, 25, 1–17. [Google Scholar]
- Roland, W.S.; Pouvreau, L.; Curran, J.; Van de Velde, F.; de Kok, P.M. Flavour aspects of pulse ingredients. Cereal Chem. 2017, 94, 58–65. [Google Scholar] [CrossRef]
- Malcolmson, L.; Han, J. Pulse processing and utilization of pulse ingredients in foods. In Health Benefits of Pulses; Springer International Publishing AG: Berlin/Heidelberg, Germany, 2019; pp. 129–149. [Google Scholar]
- Heng, L.; Vincken, J.P.; Van Koningsveld, G.A.; Legger, L.; Roozen, J.P.; Gruppen, H.; Van Boekel, M.A.J.S.; Voragen, A.G.J. Bitterness of saponins and their contents in peas. J. Sci. Food Agric. 2006, 86, 1225–1231. [Google Scholar] [CrossRef]
- Schindler, S.; Wittig, M.; Zelena, K.; Krings, U.; Bez, J.; Eisner, P.; Berger, R.G. Lactic fermentation to improve the aroma of protein extracts of sweet lupin (Lupinus angustifolius). Food Chem. 2011, 128, 330–337. [Google Scholar] [CrossRef]
- Troszyńska, A.; Estrella, I.; Lamparski, G.; Hernández, T.; Amarowicz, R.; Pegg, R.B. Relationship between the sensory quality of lentil (Lens culinaris) sprouts and their phenolic constituents. Food Res. Int. 2011, 44, 3195–3201. [Google Scholar]
- Simons, R. Prenylated Isoflavonoids from Soya and Licorice: Analysis, Induction and In Vitro Estrogenicity. Ph.D. Thesis, Wageningen University, Wageningen, The Netherland, 2011. [Google Scholar]
- Roland, W.S.U.; Vincken, J.P.; Gouka, R.J.; Van Buren, L.; Gruppen, H.; Smit, G. Soy isoflavones and other isoflavonoids activate the human bitter taste receptors hTAS2R14 and hTAS2R39. J. Agric. Food Chem. 2011, 59, 11764–11771. [Google Scholar]
- Oomah, B.D.; Razafindrainibe, M.; Drover, J.C.G. Headspace volatile components of Canadian grown low-tannin faba bean (Vicia faba L.) genotypes. J. Sci. Food Agric. 2014, 94, 473–481. [Google Scholar] [CrossRef]
- Shi, L.; Arntfield, S.D.; Nickerson, M. Changes in Levels of Phytic Acid, Lectins and Oxalates during Soaking and Cooking of Canadian pulses. Food Res. Int. 2018, 2107, 660–668. [Google Scholar]
- Aspri, M.; Mustač, N.Č.; Tsaltas, D. Non-cereal and legume-based sourdough metabolites. In Sourdough Innovations; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2023; pp. 63–86. [Google Scholar]
- Wang, J.; Zhao, J.; De Wit, M.; Boom, R.M.; Schutyser, M. Lupine protein enrichment by milling and electrostatic separation. IFSET 2016, 33, 596–602. [Google Scholar]
- Amarakoon, D.; McPhee, K.; Thavarajah, P. Iron-, zinc-, and magnesium-rich field peas (Pisum sativum L.) with naturally low phytic acid: A potential food-based solution to global micronutrient malnutrition. J. Food Compos. Anal. 2012, 27, 8–13. [Google Scholar]
- Bautista-Expósito, S.; Vandenberg, A.; Peñas, E.; Frias, J.; Martínez-Villaluenga, C. Lentil and fava bean with contrasting germination kinetics: A focus on digestion of proteins and bioactivity of resistant peptides. Front. Plant Sci. 2021, 12, 754287. [Google Scholar]
- Jiang, Z.Q.; Pulkkinen, M.; Wang, Y.J.; Lampi, A.M.; Stoddard, F.L.; Salovaara, H.; Piironen, V.; Sontag-Strohm, T. Faba bean flavour and technological property improvement by thermal pre-treatments. LWT—Food Sci. Technol. 2016, 68, 295–305. [Google Scholar] [CrossRef]
- Nadhifa, D.G.; Mahendradatta, M.; Bastian, F. Effect of spontaneous fermentation and germination on antinutrients, total ash, and iron contents of several types of legumes. Acta Aliment. 2025, 54, 109–119. [Google Scholar]
- Nsabimana, S.; Ismail, T.; Lazarte, C.E. Enhancing iron and zinc bioavailability in maize (Zea mays) through phytate reduction: The impact of fermentation alone and in combination with soaking and germination. Front. Nutr. 2024, 11, 1478155. [Google Scholar]
- Elliott, H.; Wood, P.; Green, B.; Nugent, A. Can sprouting reduce phytate and improve the nutritional composition and nutrient bio accessibility in cereals and legumes? Nutr. Bull. 2022, 47, 138–156. [Google Scholar] [CrossRef]
- Godrich, J.; Rose, P.; Muleya, M.; Gould, J. The effect of popping, soaking, boiling and roasting processes on antinutritional factors in chickpeas and red kidney beans. Int. J. Food Sci. Technol. 2023, 58, 279–289. [Google Scholar] [CrossRef]
- De Angelis, D.; Pasqualone, A.; Allegretta, I.; Porfido, C.; Terzano, R.; Squeo, G.; Summo, C. Antinutritional factors, mineral composition and functional properties of dry fractionated flours as influenced by the type of pulse. Heliyon 2021, 7, e06177. [Google Scholar]
- Elango, D.; Rajendran, K.; Van der Laan, L.; Sebastiar, S.; Raigne, J.; Thaiparambil, N.A.; Haddad, N.; Raja, B.; Wang, W.; Ferela, A.; et al. Raffinose family oligosaccharides: Friend or foe for human and plant health? Front. Plant Sci. 2022, 13, 829118. [Google Scholar] [CrossRef]
- Li, L.; Yuan, T.Z.; Setia, R.; Raja, R.B.; Zhang, B.; Ai, Y. Characteristics of pea, lentil and faba bean starches isolated from air-classified flours in comparison with commercial starches. Food Chem. 2019, 276, 599–607. [Google Scholar]
- Rosa-Sibakov, N.; Karsma, M.R.; Laitila, A.; Nordlund, E. Phytic acid reduction by bioprocessing as a tool to improve the in vitro digestibility of faba bean protein. J. Agric. Food Chem. 2018, 6640, 10394–10399. [Google Scholar]
- Queirós, R.P.; Saraiva, J.A.; Silva, J.A.L. Tailoring structure and technological properties of plant proteins using high hydrostatic pressure. Crit. Rev. Food Sci. Nutr. 2018, 58, 1538–1556. [Google Scholar]
- Samaei, S.P.; Ghorbani, M.; Tagliazucchi, D.; Martini, S.; Gotti, R.; Themelis, T.; Tesini, F.; Gianotti, A.; Gallina Toschi, T.; Babini, E. Functional, Nutritional, Antioxidant, Sensory Properties and Comparative Peptidomic Profile of Faba Bean (Vicia faba L.) Seed Protein Hydrolysates and Fortified Apple Juice. Food Chem. 2020, 330, 127120. [Google Scholar]
- Champ, M.M.J. Non-nutrient bioactive substances of pulses. Br. J. Nutr. 2020, 88, 307–319. [Google Scholar]
- Alonso, R.; Aguirre, A.; Marzo, F. Effects of extrusion and traditional processing methods on antinutrients and in vitro digestibility of protein and starch in faba and kidney beans. Food Chem. 2000, 68, 159–165. [Google Scholar]
- Osman, A.M.A.; Hassan, A.B.; Osman, G.A.; Mohammed, N.; Rushdi, M.A.; Diab, E.E.; Babiker, E.E. Effects of gamma irradiation and/or cooking on nutritional quality of faba bean (Vicia faba L.) cultivars seeds. J. Food Sci. Technol. 2014, 51, 1554–1560. [Google Scholar] [PubMed]
- Moses, T.; Papadopoulou, K.K.; Osbourn, A. Metabolic and functional diversity of saponins, biosynthetic intermediates and semi-synthetic derivatives. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 439–462. [Google Scholar]
- Luo, J.; Cai, W.; Wu, T.; Xu, B. Phytochemical distribution in hull and cotyledon of adzuki bean (Vigna angularis L.) and mung bean (Vigna radiate L.), and their contribution to antioxidant, anti-inflammatory and anti-diabetic activities. Food Chem. 2016, 201, 350–360. [Google Scholar] [PubMed]
- Saldanha do Carmo, C.; Silventoinen, P.; Nordgård, C.T.; Poudroux, C.; Dessev, T.; Zobel, H.; Holtekjølen, A.K.; Draget, K.I.; Holopainen-Mantila, U.; Knutsen, S.H.; et al. Is dehulling of peas and faba beans necessary prior to dry fractionation for the production of protein- and starch-rich fractions? Impact on physical properties, chemical composition and techno-functional properties. J. Food Eng. 2020, 278, 109937. [Google Scholar]
- Patterson, C.A.; Curran, J.; Der, T. Effect of processing on antinutrient compounds in pulses. Cereal Chem. 2017, 94, 2–10. [Google Scholar]
- Wood, J.A.; Knights, E.J.; Campbell, G.M.; Harden, S.; Choct, M. Enzyme pre-milling treatments improved milling performance of chickpeas by targeting mechanisms of seed coat and cotyledon adhesion with various effects on dhal quality. J. Sci. Food Agric. 2022, 102, 62–72. [Google Scholar]
- Rivera, J.; Siliveru, K.; Li, L. A comprehensive review on pulse protein fractionation and extraction: Processes, functionality, and food applications. Crit. Rev. Food Sci. Nutr. 2022, 64, 4179–4201. [Google Scholar] [CrossRef] [PubMed]
- Supun, F. Production of protein-rich pulse ingredients through dry fractionation: A review. LWT-Food Sci. Technol. 2021, 141, 110961. [Google Scholar]
- Fernando, S.; Manthey, F.A. Milling method affects the physical properties of black bean flour. Cereal Chem. 2021, 98, 749–758. [Google Scholar]
- Pulivarthi, M.K.; Buenavista, R.M.; Bangar, S.P.; Li, Y.; Pordesimo, L.O.; Bean, R.; Siliveru, K. Dry fractionation process operations in the production of protein concentrates: A review. Compr. Rev. Food Sci. Food Saf. 2023, 22, 4670–4697. [Google Scholar]
- Eze, C.R.; Kwofie, E.M.; Adewale, P.; Lam, E.; Ngadi, M. Advances in legume protein extraction technologies: A review. Innov. Food Sci. Emerg. Technol. 2022, 10, 103199. [Google Scholar]
- Zhu, H.G.; Wang, Y.Q.; Cheng, Z.G.; Li, Z.; Tong, T. Optimization of the powder state to enhance the enrichment of functional mung bean protein concentrates obtained by dry separation. Powder Technol. 2020, 373, 681–688. [Google Scholar]
- Tabtabaei, S.; Vitelli, M.; Rajabzadeh, A.R.; Legge, R.L. Analysis of protein enrichment during single- and multi-stage tribo-electrostatic bioseparation processes for dry fractionation of legume flour. Sep. Purif. Technol. 2017, 176, 48–58. [Google Scholar]
- Pelgrom, P.J.; Boom, R.; Schutyser, M.A. Method development to increase protein enrichment during dry fractionation of starch-rich legumes. Food Bioproc. Technol. 2015, 8, 1495–1502. [Google Scholar]
- Ghribi, A.M.; Gafsi, I.M.; Blecker, C.; Danthine, S.; Attia, H.; Besbes, S. Effect of drying methods on physico-chemical and functional properties of chickpea protein concentrates. J. Food Eng. 2015, 165, 179–188. [Google Scholar]
- Johansson, M.; Johansson, D.; Ström, A.; Rydén, J.; Nilsson, K.; Karlsson, J.; Moriana, R.; Langton, M. Effect of starch and fibre on faba bean protein gel characteristics. Food Hydrocoll. 2022, 131, 107741. [Google Scholar]
- Gunes, Z.S.; Can Karaca, A. Examining the amino acid composition, secondary structure, and physicochemical and functional properties of proteins isolated from local lentil landraces of Anatolia. Cereal Chem. 2022, 99, 78–89. [Google Scholar] [CrossRef]
- Guldiken, B.; Stobbs, J.; Nickerson, M. Heat induced gelation of pulse protein networks. Food Chem. 2021, 350, 129158. [Google Scholar] [CrossRef] [PubMed]
- Kornet, R.; Veenemans, J.; Venema, P.; Van der Goot, A.J.; Meinders, M.; Sagis, L.; Van der Linden, E. Less is more: Limited fractionation yields stronger gels for pea proteins. Food Hydrocoll. 2021, 112, 106285. [Google Scholar] [CrossRef]
- Eckert, E.; Wismer, W.; Waduthanthri, K.; Babii, O.; Yang, J.; Chen, L. Application of barley- and lentil-protein concentrates in the production of protein-enriched doughnuts. J. Am. Oil Chem. Soc. 2018, 95, 1027–1040. [Google Scholar] [CrossRef]
- Jarpa-Parra, M.; Wong, L.; Wismer, W.; Temelli, F.; Han, J.; Huang, W.; Ecjhart, E.; Tian, Z.; Shi, K.; Chen, L. Quality characteristics of angel food cake and muffin using lentil protein as egg/milk replacer. Int. J. Food Sci. Technol. 2017, 52, 1604–1613. [Google Scholar] [CrossRef]
- Higa, F.A.; Boyd, L.; Sopiwnyk, E.; Nickerson, M.T. Effect of particle size, flour:water ratio and type of pulse on the physicochemical and functional properties of wet protein extraction. Cereal Chem. 2022, 99, 1049–1062. [Google Scholar] [CrossRef]
- Burger, T.; Singh, I.; Mayfield, C.; Baumert, J.L.; Zhang, Y. The impact of spray drying conditions on the physicochemical and emulsification properties of pea protein isolate. LWT—Food Sci. Technol. 2022, 153, 112495. [Google Scholar] [CrossRef]
- Shrestha, S.; Van’t Hag, L.; Haritos, V.; Dhital, S. Comparative study on molecular and higher-order structures of legume seed protein isolates: Lentil, mung bean and yellow pea. Food Chem. 2023, 411, 135464. [Google Scholar] [CrossRef]
- Chew, P.G.; Casey, A.J.; Johnson, S.K. Protein quality and physico-functionality of Australian sweet lupin (Lupinus angustifolius cv. Gungurru) protein concentrates prepared by isoelectric precipitation or ultrafiltration. Food Chem. 2003, 83, 575–583. [Google Scholar]
- Shrestha, S.; Hag Van’t, L.V.; Haritos, S.; Dhital, S. Lupin proteins: Structure, isolation and application. Trends Food Sci. Technol. 2021, 116, 928–939. [Google Scholar] [CrossRef]
- Massmann, C.M.; Berhow, M.; Gibbons, W.R.; Karki, B. The effects of fungal bioprocessing on air-classified pea protein concentrates. LWT-Food Sci. Technol. 2022, 154, 112686. [Google Scholar]
- Karve, N. Impact of Germination on Yellow Pea Flour Functionality; Charles Stuart University: Bathurst, Australia, 2018; Available online: https://researchoutput.csu.edu.au/ws/portalfiles/portal/44493837/Neeta_Karve_Final_PhD_thesis_100520_.pdf (accessed on 11 October 2018).
- Devi, C.B.; Kushwaha, A.; Kumar, A. Sprouting characteristics and associated changes in nutritional composition of cowpea (Vigna unguiculata). J. Food Sci. Technol. 2010, 52, 6821–6827. [Google Scholar] [CrossRef]
- Bellaio, S.; Kappeler, S.; Zamprogna Rosenfeld, E. Partially germinated ingredients for naturally healthy and tasty products. Cereal Foods World 2013, 58, 55–59. [Google Scholar] [CrossRef]
- Vogelsang-O’Dwyer, M.; Petersen, I.L.; Joehnke, M.S.; Sørensen, J.C.; Bez, J.; Detzel, A.; Busch, M.; Krueger, M.; O’Mahony, A.; Arendt, E.K.; et al. Comparison of faba bean protein ingredients produced using dry fractionation and isoelectric precipitation: Techno-functional, nutritional, and environmental performance. Foods 2020, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Stone, A.K.; Karalash, A.; Tyler, R.T.; Warkentin, T.D.; Nickerson, M.T. Functional attributes of pea protein isolates prepared using different extraction methods and cultivars. Food Res. Int. 2015, 76, 31–38. [Google Scholar]
- Samard, S.; Ryu, G.H. Physicochemical and functional characteristics of plant protein-based meat analogs. J. Food Process. Preserv. 2019, 43, e14123. [Google Scholar]
- Shrestha, S.; van ’t Hag, L.; Haritos, V.; Dhital, S. Rheological and textural properties of heat-induced gels from pulse protein isolates: Lentil, mung bean and yellow pea. Food Hydrocoll. 2023, 143, 1089. [Google Scholar] [CrossRef]
- Singh, N.; Nakaura, Y.; Inouchi, N.; Nishinari, K. Structure and viscoelastic properties of starches separated from different legumes. Starch 2008, 60, 349–357. [Google Scholar]
- Vioque, J.; Alaiz, M.; Girón-Calle, J. Nutritional and functional properties of Vicia faba protein isolates and related fractions. Food Chem. 2012, 132, 67–72. [Google Scholar] [CrossRef]
- Schlangen, M.; Taghian, S.; Schutyser, M.; Van der Goot, A. Dry fractionation to produce functional fractions from mung bean, yellow pea and cowpea flour. Innov. Food Sci. Emerg. Technol. 2022, 78, 103018. [Google Scholar]
- Shevkani, K.; Singh, N.; Kaur, A.; Rana, J. Structural and functional characterization of kidney bean and field pea protein isolates: A comparative study. Food Hydrocol. 2015, 43, 679–689. [Google Scholar] [CrossRef]
- Paredes-Lopez, O.; Ordorica-Falomir, C.; Olivares-Vazquez, M.R. Chickpea protein isolates: Physicochemical, functional and nutritional characterization. J. Food Sci. 1991, 56, 726–729. [Google Scholar] [CrossRef]
- Fernández-Quintela, A.; Macarulla, M.T.; del Barrio, A.S.; Martínez, J.A. Composition and functional properties of protein isolates obtained from commercial legumes grown in northern Spain. Plant Foods Hum. Nutr. 1997, 51, 331–341. [Google Scholar] [PubMed]
- Zhang, T.; Jiang, B.; Wang, Z. Gelation properties of chickpea protein isolates. Food Hydrocoll. 2007, 21, 280–286. [Google Scholar]
- Badjona, A.; Bradshaw, R.; Millman, C.; Howarth, M.; Dubey, B. Faba bean processing: Thermal and non-thermal processing on chemical, antinutritional factors, and pharmacological properties. Molecules 2023, 28, 5431. [Google Scholar] [CrossRef]
- Shanthakumar, P.; Klepacka, J.; Bains, A.; Chawla, P.; Dhull, S.B.; Najda, J. The current situation of pea protein and its application in the food industry. Molecules 2022, 27, 5354. [Google Scholar] [CrossRef]
- Jeske, S.; Bez, J.; Arendt, E.; Zannini, E. Formation, stability, and sensory characteristics of a lentil-based milk substitute as affected by homogenisation and pasteurisation. Eur. Food Res. Technol. 2019, 245, 1519–1531. [Google Scholar]
- Tiwari, A.K.; Shivhare, A.K. Pulses in India: Retrospect and Prospects; Directory, Government of India, Ministry of Agriculture & Farmers Welfare (DAC&FW); Directorate of Pulses Development, VindhyachalBhavan: Bhopal, India, 2016. [Google Scholar]
- Sim, S.; Srv, A.; Chiang, J.; Henry, C. Plant proteins for future foods: A roadmap. Foods 2021, 10, 967. [Google Scholar] [CrossRef]
- Loveday, S. Plant protein ingredients with food functionality potential. Nutr. Bull. 2020, 45, 321–327. [Google Scholar]
- Sreerama, Y.N.; Sashikala, V.B.; Pratape, V.M.; Singh, V. Nutrients and antinutrients in cowpea and horse gram flours in comparison to chickpea flour: Evaluation of their flour functionality. Food Chem. 2012, 131, 462–468. [Google Scholar] [CrossRef]
- Shen, Y.; Hong, S.; Singh, G.; Koppel, K.; Li, L. Improving functional properties of pea protein through “green” modifications using enzymes and polysaccharides. Food Chem. 2022, 385, 132687. [Google Scholar] [PubMed]
- McClements, D.; Grossmann, L. The science of plant-based foods: Constructing next-generation meat, fish, milk, and egg analogs. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4049–4100. [Google Scholar] [CrossRef]
- Gumus, E.; Decker, E.; McClements, D. Formation and stability of ω-3 oil emulsion-based delivery systems using plant proteins as emulsifiers: Lentil, pea, and faba bean proteins. Food Biophy. 2017, 12, 186–197. [Google Scholar]
- Ladjal-Ettoumi, Y.; Boudries, H.; Chibane, M.; Romero, A. Pea, chickpea and lentil protein isolates: Physicochemical characterization and emulsifying properties. Food Biophy. 2016, 11, 43–51. [Google Scholar] [CrossRef]
- Campbell, G.M. A history of aerated foods. In Bubbles in Food 2; Elsevier: Amsterdam, The Netherlands, 2008; pp. 1–10. [Google Scholar]
- Deotale, S.; Dutta, S.; Moses, J.A.; Balasubramaniam, V.M.; Anandharamakrishnan, C. Foaming characteristics of beverages and its relevance to food processing. Food Eng. Rev. 2020, 12, 229–250. [Google Scholar]
- Amagliani, L.; Silva, J.V.; Saffon, M.; Dombrowski, J. On the foaming properties of plant proteins: Current status and future opportunities. Trends Food Sci.Technol. 2021, 118, 261–272. [Google Scholar]
- Dachmann, E.; Nobis, V.; Kulozik, U.; Dombrowski, J. Surface and foaming properties of potato proteins: Impact of protein concentration, pH value and ionic strength. Food Hydrocoll. 2020, 107, 105981. [Google Scholar]
- Lee, J.; Duggan, E. Whey protein microgels for stabilisation of foams. Int. Dairy J. 2022, 132, 105399. [Google Scholar]
- Tan, T.; Nawaz, M.; Buckow, R. Functional and food application of plant proteins–a review. Food Rev. Int. 2021, 39, 2428–2456. [Google Scholar] [CrossRef]
- Toews, R.; Wang, N. Physicochemical and functional properties of protein concentrate from pulses. Food Res. Int. 2013, 52, 445–451. [Google Scholar]
- Kaur, M.; Singh, N. Characterization of protein isolates from different Indian chickpea (Cicer arietinum L.) cultivars. Food Chem. 2007, 102, 366–374. [Google Scholar] [CrossRef]
- Ma, K.K.; Grossmann, L.; Nolden, A.A.; McClements, D.J.; Kinchla, A.J. Functional and physical properties of commercial pulse proteins compared to soy derived protein. J. Future Foods 2022, 6, 100155. [Google Scholar] [CrossRef]
- Grossmann, L.; Weiss, J. Alternative protein sources as techno functional food ingredients. Annu. Rev. Food Sci. Technol. 2021, 12, 93–117. [Google Scholar] [CrossRef] [PubMed]
- Obatolu, V.A.; Fasoyiro, S.B.; Ogunsunmi, L. Processing and functional properties of yam beans (Sphenostylis stenocarpa). J. Food Process. Preserv. 2007, 31, 240–249. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics (ABS). Australian Health Survey: Consumption of Food Groups from the Australian Dietary Guidelines; Australian Bureau of Statistics: Canberra, Australia, 2016. [Google Scholar]
- Figueira, N.; Curtain, F.; Beck, E.; Grafenauer, S. Consumer understanding and culinary use of legumes in Australia. Nutrients 2019, 11, 1575. [Google Scholar] [CrossRef]
- GLNC. State of the Industry: An Overview of Plant-Based Products. 2022. Available online: http://www.glnc.org.au (accessed on 2 June 2023).
- Gangola, M.P.; Ramadoss, B.R.; Jaiswal, S.; Fabek, H.; Tulbek, M.; Anderson, G.H.; Chibbar, R.N. Nutritional composition and in vitro starch digestibility of crackers supplemented with faba bean whole flour, starch concentrate, protein concentrate and protein isolate. Foods 2022, 11, 645. [Google Scholar] [CrossRef] [PubMed]
- Guajardo-Flores, D.; Pérez-Carrillo, E.; Romo-López, I.; Ramírez-Valdez, L.E.; Moreno-García, B.E.; Gutiérrez-Uribe, J.A. Effect of dehulling and germination on physicochemical and pasting properties of black beans (Phaseolus vulgaris L.). Cereal Chem. 2017, 94, 98–103. [Google Scholar] [CrossRef]
- Pal, R.S.; Bhartiya, A.; Yadav, P.; Kant, L.; Mishra, K.K.; Aditya, J.P.; Pattanayak, A. Effect of dehulling, germination and cooking on nutrients, anti-nutrients, fatty acid composition and antioxidant properties in lentil (Lens culinaris). J. Food Sci. Technol. 2017, 54, 909–920. [Google Scholar] [CrossRef]
- Romano, A.; Giosafatto, C.V.L.; Masi, P.; Mariniello, L. Impact of dehulling on the physico-chemical properties and in vitro protein digestion of common beans (Phaseolus vulgaris L.). Food Funct. 2015, 6, 1345–1351. [Google Scholar] [CrossRef]
- Grasso, N.; Bot, F.; Roos, Y.H.; Crowley, S.V.; Arendt, E.K.; O’Mahony, J.A. Plant-based alternatives to cheese formulated using blends of zein and chickpea protein ingredients. Foods 2023, 12, 1492. [Google Scholar] [CrossRef]
- Duarte, R.V.; Pinto, C.A.; Gomes, A.M.; Delgadillo, I.; Saraiva, J.A. A microbiological perspective of raw milk preserved at room temperature using hyperbaric storage compared to refrigerated storage. Innov. Food Sci. Emerg. Technol. 2022, 78, 103019. [Google Scholar]
- Hickisch, A.; Beer, R.; Vogel, R.F.; Toelstede, S. Influence of lupin-based milk alternative heat treatment and exopolysaccharide-producing lactic acid bacteria on the physical characteristics of lupin-based yogurt alternatives. Food Res. Int. 2016, 84, 180–188. [Google Scholar]
- Kantanen, K.; Oksanen, A.; Edelmann, M.; Suhonen, H.; Sontag-Strohm, T.; Piironen, V.; Ramos Diaz, J.M.; Jouppila, K. Physical properties of extrudates with fibrous structures made of faba bean protein ingredients using high moisture extrusion. Foods 2022, 11, 1280. [Google Scholar] [CrossRef] [PubMed]
- Osen, R.; Toelstede, S.; Wild, F.; Eisner, P.; Schweiggert-Weisz, U. High moisture extrusion cooking of pea protein isolates: Raw material characteristics, extruder responses, and texture properties. J. Food Eng. 2014, 127, 67–74. [Google Scholar]
- Goldstein, N.; Reifen, R. The potential of legume-derived proteins in the food industry. Grain Oil Sci. Technol. 2022, 5, 167–178. [Google Scholar]
- Day, L.; Cakebread, J.; Loveday, S. Food proteins from animals and plants: Differences in the nutritional and functional properties. Trends Food Sci. Technol. 2022, 119, 428–442. [Google Scholar]
- Mariotti, M.; Lucisano, M.; Ambrogina Pagani, M.; Ng, P.K. The role of corn starch, amaranth flour, pea isolate, and Psyllium flour on the rheological properties and the ultrastructure of gluten-free doughs. Food Res. Int. 2009, 42, 963–975. [Google Scholar]
- Bojnanska, T.; Musilova, J.; Vollmannova, A. Effects of adding legume flours on the rheological and breadmaking properties of dough. Foods 2021, 10, 1087. [Google Scholar] [CrossRef]
- Xing, Q.; Kyriakopoulou, K.; Zhang, L.; Boom, R.M.; Schutyser, M.A.I. Protein fortification of wheat bread using dry fractionated chickpea protein-enriched fraction or its sourdough. LWT—Food Sci Technol. 2021, 142, 110931. [Google Scholar]
- Shukla, P.; Sudhakar, A.; Sharma, A. Study of machine parameters in twin-screw extruder for pulses based extrudate. CIGR 2021, 23, 274–281. [Google Scholar]
- Messia, M.C.; Cuomo, F.; Falasca, L.; Trivisonno, M.C.; De Arcangelis, E.; Marconi, E. Nutritional and technological quality of high protein pasta. Foods 2021, 10, 589. [Google Scholar] [CrossRef]
- Sahagún, M.; Gómez, M. Influence of protein source on characteristics and quality of gluten-free cookies. J. Food Sci. Technol. 2018, 55, 4131–4138. [Google Scholar] [CrossRef] [PubMed]
- Mota, J.; Lima, A.B.; Ferreira, R.; Raymundo, A. Lupin Seed Protein Extract Can Efficiently Enrich the Physical Properties of Cookies Prepared with Alternative Flours. Foods 2020, 9, 1064. [Google Scholar] [CrossRef]
- Boukid, E.; Zannini, E.; Carini, E.; Vittadini, E. Pulses for bread fortification: A necessity or a choice. Trends Food Sci. Technol. 2019, 88, 416–428. [Google Scholar] [CrossRef]
- Doğan, S.N.; Yılmaz, İ. Physicochemical and sensory properties of vegetarian pasta produced with pea (Pisum sativum) protein powder. Harran Tarım Gıda Bilim. Derg. 2024, 28, 267–279. [Google Scholar] [CrossRef]
- Cardello, A.V.; Llobell, F.; Giacalone, D.; Roigard, C.M.; Jaeger, S.R. Plant-based alternatives vs dairy milk: Consumer segments and their sensory, emotional, cognitive and situational use responses to tasted products. Food Qual. Prefer. 2022, 100, 104599. [Google Scholar] [CrossRef]
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-based milk alternatives an emerging segment of functional beverages: A review. J. Food Sci. Technol. 2016, 53, 3408–3423. [Google Scholar] [CrossRef]
- Makinen, O.E.; Wanhalinna, V.; Zannini, E.; Arendt, E.K. Foods for special dietary needs: Non-dairy plant-based milk substitutes and fermented dairy-type products. Crit. Rev. Food Sci. Nutr. 2016, 56, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.; Pierrepont, C.; Duarte, C.M.; Filipe, A.; Medronho, B.; Sousa, I. Legume beverages from chickpea and lupin, as new milk alternatives. Foods 2020, 9, 1458. [Google Scholar] [CrossRef]
- Boeck, T.; Sahin, A.W.; Zannini, E.; Arendt, E.K. Nutritional properties and health aspects of pulses and their use in plant-based yogurt alternatives. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3858–3880. [Google Scholar] [CrossRef]
- Klost, M.; Giménez-Ribes, G.; Drusch, S. Enzymatic hydrolysis of pea protein: Interactions and protein fractions involved in fermentation induced gels and their influence on rheological properties. Food Hydrocoll. 2020, 105, 105793. [Google Scholar]
- Ashraf, Z.U.; Gani, A.; Shah, A.; Gani, A. Techno-Functional Characterization of Pulse Proteins (Broad Bean, Mung Bean, and Lentil Bean) as Sustainable Plant-Based Meat and Dairy Alternatives. ACS Food Sci. Technol. 2024, 4, 1341–1351. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, X.; Blank, I.; Liu, Y. Strategies to improve meat-like properties of meat analogs meeting consumers expectations. Biomaterials 2022, 287, 121648. [Google Scholar]
- Dekkers, B.; Boom, R.; Van der Goot, V. Structuring processes for meat analogues. Trends Food Sci. Technol. 2018, 81, 25–36. [Google Scholar]
- Zhang, J.; Chen, Q.; Kaplan, D.L.; Wang, Q. High-moisture extruded protein fiber formation toward plant-based meat substitutes applications: Science, technology, and prospect. Trends Food Sci. Technol. 2022, 128, 202–216. [Google Scholar]
- Wittek, P.; Walther, G.; Karbstein, H.P.; Emin, M.A. Comparison of the rheological properties of plant proteins from various sources for extrusion applications. Foods 2021, 10, 1700. [Google Scholar] [CrossRef]
- Zahari, I.; Östbring, K.; Purhagen, J.; Rayner, M. Plant-based meat analogues from alternative protein: A systematic literature review. Foods 2022, 11, 2870. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Ruffo de Oliveira, V. Overview of the incorporation of legumes into new food options: An approach on versatility, nutritional, technological, and sensory quality. Foods 2023, 12, 2586. [Google Scholar] [CrossRef]
- Zhu, H.G.; Tang, H.; Cheng, Y.; Li, Z.; Tong, L. Novel electromagnetic separation technology for the production of pea protein concentrate. Innov. Food Sci. Emerg. Technol. 2021, 70, 102668. [Google Scholar]
- Penchalaraju, M.; Narayandas, A.; Mithun, K.; Reddy, M.; Rajesh, K.; Tejaswini, V.; Archana, K. Textural and sensorial properties of meatball analogues produced from alkaline extracted Indian pulse protein concentrates and other plant-based composites. J. Food Sci. Technol. 2024, 9, 1–9. [Google Scholar] [CrossRef]
- Rajpurohit, B.; Li, Y. Overview on pulse proteins for future foods: Ingredient development and novel applications. J. Future Foods 2023, 3, 340–356. [Google Scholar] [CrossRef]
- Ratnayake, W.S.; Naguleswaran, S. Utilising side streams of pulse protein processing: A review. Legume Sci. 2021, 4, 1–15. [Google Scholar]
- Tassoni, A.; Tedeschi, T.; Zurlini, C.; Cigognini, I.M.; Petrusan, J.I.; Rodríguez, O.; Neri, S.; Celli, A.; Sisti, L.; Cinelli, P.; et al. State-of-the-art production chains for peas, beans and chickpeas-valorization of agro-industrial residues and applications of derived extracts. Molecules 2020, 25, 1383. [Google Scholar] [CrossRef] [PubMed]

| Pulse Production (mmt) | 2018 | 2019 | 2020 | 2021 | 2022 |
|---|---|---|---|---|---|
| Beans, dry | 27.5 | 25.6 | 27.4 | 27.7 | 28.5 |
| Chickpeas, dry | 16.9 | 14.2 | 15.1 | 15.9 | 16.5 |
| Peas, dry | 13.4 | 14.0 | 14.7 | 12.4 | 13.0 |
| Cowpeas, dry | 8.4 | 8.5 | 9.0 | 9.0 | 9.2 |
| Broad beans and horse beans, dry | 5.6 | 5.4 | 5.7 | 6.0 | 6.2 |
| Lentils, dry | 6.6 | 5.8 | 6.5 | 5.6 | 6.0 |
| Pigeon peas, dry | 5.4 | 4.4 | 5.0 | 5.5 | 5.8 |
| Other pulses (*n.e.c.) | 4.8 | 4.3 | 4.6 | 4.7 | 5.0 |
| Lupins | 1.2 | 1.3 | 1.0 | 1.4 | 1.5 |
| Vetches | 0.6 | 0.7 | 0.7 | 0.6 | 0.7 |
| Bambara beans, dry | 0.2 | 0.2 | 0.3 | 0.2 | 0.3 |
| Common Name | Latin Name | Country | Cultivar | Protein (%) | Fat (%) | Carbohydrate (%) | Minerals (%) | Fibre (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Chickpea | Cicer arietinum | India | Chana | 18.5 | 4.3 | 55.0 | 3.5 | 4.0 | [20] |
| Canada | CDC leader | 20.9 | 6.5 | 65.4 | 3.0 | 4.3 | [21] | ||
| Ethiopia | Natoli | 17.7 | 5.2 | 62.4 | 3.7 | 6.7 | [22] | ||
| Australia | Desi | 15.7 | 4.4 | - | 3.7 | - | [23] | ||
| Kabuli chickpea | Cicer arietinum | India | Kabuli chana | 20.5 | 5.7 | 55.0 | 3.8 | 4.0 | [22] |
| Italy | MG_1 | 17.4 | 3.7 | 54.3 | 3.3 | 5.5 | [24] | ||
| Canada | CDC Orion | 20.0 | 6.6 | 43.2 | 2.9 | - | [25] | ||
| Australia | Kimberley Large | 18.2 | 5.9 | 52.6 | 2.9 | 16.5 | [26] | ||
| Lentil | Lens culinaris | India | Masoor | 25.0 | 1.0 | 59.0 | 2.0 | 1.0 | [23] |
| Iran | Red | 25.9 | 2.7 | 59.0 | 3.6 | 2.7 | [27] | ||
| Canada | Roxy | 30.4 | 1.5 | 65.0 | 3.1 | - | [28] | ||
| Australia | Matilda | 32.6 | 2.8 | - | - | - | [29] | ||
| Field pea | Pisum sativum | India | Pant pea 25 | 20.3 | 2.0 | 58.5 | 2,9 | 3.4 | [30] |
| Pakistan | Paniola | 20.0 | 1.7 | 89.7 | - | 0.8 | [31] | ||
| Ethiopia | Bilalo | 23.9 | 2.7 | 59.2 | 3.4 | 3.6 | [32] | ||
| Australia | Commercial | 20.7 | 0.3 | 4.7 | 0.5 | 25.1 | [33] | ||
| Faba bean | Vicia faba | India | Bell bean | 26.1 | 1.5 | 58.3 | - | 2.5 | [23] |
| Ireland | Victor | 28.0 | 1.6 | 40.9 | 3.4 | 13.8 | [34] | ||
| Ecuador | Commercial | 22.5 | 0.6 | 63.7 | 1.3 | 2.1 | [35] | ||
| Australia | Commercial | 33.0 | 1.5 | - | 2.4 | - | [36] | ||
| Mung bean | Vigna radiata | Nigeria | Wilczek | 24.1 | 1.9 | 55.7 | 3.0 | 5.0 | [37] |
| China | Bailv 522 | 26.2 | 1.3 | 56.8 | 3.8 | 3.6 | [38] | ||
| India | AKM-8802 | 21.9 | - | 41.9 | 3.6 | - | [39] | ||
| Australia | Dahl | 27.6 | 1.9 | 46.5 | 3.5 | 10.6 | [40] | ||
| Lupin | Lupinus albus | Ethiopia | Commercial | 34.9 | 1.3 | 50.5 | 3.3 | 9.9 | [41] |
| Ethiopia | Commercial | 28.6 | 9.4 | - | 3.0 | 9.7 | [42] | ||
| Brazil | White | 34.1 | 9.9 | 14.9 | 2.6 | 38.4 | [43] | ||
| Australia | Jenabillup | 38.6 | 7.1 | 6.7 | 3.5 | 36.1 | [44] |
| Method | Advantages | Disadvantages |
|---|---|---|
| Wet fractionation |
|
|
| Dry fractionation |
|
|
| Functionality | Pulse Protein | Fractionation | Results | Reference |
|---|---|---|---|---|
| Solubility | Faba bean (I) | WF and DF | 32%; 85%, resp. | [126] |
| Pea and faba bean (C) | DF | 19–25%; 14–29%, resp. | [99] | |
| Pea (I) | WF | 53.9–70.3% | [127] | |
| Soy (I) | WF | 21% | [128] | |
| Water-holding capacity | Lentil, mung bean, and yellow pea (I) | WF | 2.9 g/g; 1.5 g/g; 2.6 g/g, resp. | [129] |
| Chickpea (I) | WF | 2.6 g/g | [130] | |
| Chickpea (I) | WF | 2.3–3.5 g/g | [131] | |
| Mung bean, yellow pea, and cowpea (C) | DF | 2.1 g/g; 1.5 g/g; 2.1 g/g resp. | [132] | |
| Pea and faba bean (C) | DF | 0.93–0.97 g/g; 0.58–0.63 g/g | [99] | |
| Pea (I) | WF | 1.88–2.37 g/g | [127] | |
| Soy and mung bean (I) | WF | 3.5 g/g; 2.9 g/g, resp. | [128] | |
| Oil-holding | Lentil, mung bean, and yellow pea (I) | WF | 1.4–1.6 g/g, 1.6 g/g; 1.5 g/g, resp. | [129] |
| capacity | Faba, kidney bean, cowpea and field pea (I) | WF | 2.3 g/g; 4.7–6.9 g/g, 1.4–2.0 g/g; 5.5–7.2 g/g, resp. | [133] |
| Pea and faba bean (C) | DF | 1.1 g/g; 1.1 g/g, resp. | [99] | |
| Pea (I) | WF | 1.1–1.4 g/g | [127] | |
| Soy and mung bean (I) | WF | 1.0 g/g; 1.1 g/g, resp. | [128] | |
| Foaming properties | Chickpea (I) | WF | 59.2–66.6% | [134] |
| Faba bean (C) and faba bean (I) | DF | 30–60%; 82–95%, resp. | [126] | |
| Pea and faba bean (I) | AC | 54.8–55.5%, 26.5–41.9%, resp. | [99] | |
| Gelation | Faba bean (I) | WF | 140 g/kg (* LGC) | [135] |
| Chickpea (I) | WF | 140 g/L (* LGC) | [136] | |
| Kidney bean and field pea (I) | WF | 87.4 °C; 84 °C, resp. | [133] | |
| Faba bean (C) | DF | 65 °C | [137] |
| Food Product | Pulse Protein Type | Outcomes | Reference |
|---|---|---|---|
| Bakery products | |||
| Crackers | Faba bean (C) (I) | Improved nutritional and functional properties (lower starch and fat content) | [162] |
| Donuts | Lentils (C) | Increased cooking characteristics, delayed the loss of moisture, and decreased the rate of hardening | [115] |
| Cookies | Pea (I) | Increased water absorption capacity and rheological properties, but it does not improve spread ratio or hardness | [99] |
| Muffins | Lentils (C) | Improved overall score | [116] |
| Wheat bread | Pea (I) | Improved rheological, structural, and nutritional parameters | [163] |
| Pasta | |||
| Gluten-free pasta | Pea and faba bean (I) | An overall change in colour, hardness, water uptake, and cooking losses | [164] |
| High protein pasta | Pea (I) | Improved chemical score, amino acid digestibility, colour, and cooking quality | [165] |
| Alternative dairy products | |||
| Cheese | Chickpea (I) | Stretchability, meltability, texture, rheological, thermal behaviour, and microstructure were improved | [166] |
| Milk | Sweet lupin and chickpea (C) | Improved protein content | [167] |
| Yoghurt | Lupin (I) | Higher viscosity and with more firmness | [168] |
| Meat analogues | |||
| Pea (C) (I) | Lower hardness, high oil absorption, neutral sensory profiles | [53] | |
| Faba bean(C) (I) | High-moisture and fibrous structures | [169] | |
| Faba bean (C) (I) | Fibrous structures | [170] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messina, V.; Skylas, D.J.; Roberts, T.H.; Valtchev, P.; Whiteway, C.; Li, Z.; Hopf, A.; Dehghani, F.; Quail, K.J.; Kaiser, B.N. Pulse Proteins: Processing, Nutrition, and Functionality in Foods. Foods 2025, 14, 1151. https://doi.org/10.3390/foods14071151
Messina V, Skylas DJ, Roberts TH, Valtchev P, Whiteway C, Li Z, Hopf A, Dehghani F, Quail KJ, Kaiser BN. Pulse Proteins: Processing, Nutrition, and Functionality in Foods. Foods. 2025; 14(7):1151. https://doi.org/10.3390/foods14071151
Chicago/Turabian StyleMessina, Valeria, Daniel J. Skylas, Thomas H. Roberts, Peter Valtchev, Chris Whiteway, Ziqi Li, Andreas Hopf, Fariba Dehghani, Ken J. Quail, and Brent N. Kaiser. 2025. "Pulse Proteins: Processing, Nutrition, and Functionality in Foods" Foods 14, no. 7: 1151. https://doi.org/10.3390/foods14071151
APA StyleMessina, V., Skylas, D. J., Roberts, T. H., Valtchev, P., Whiteway, C., Li, Z., Hopf, A., Dehghani, F., Quail, K. J., & Kaiser, B. N. (2025). Pulse Proteins: Processing, Nutrition, and Functionality in Foods. Foods, 14(7), 1151. https://doi.org/10.3390/foods14071151






