Applications of Microextraction Technology for the Analysis of Alcoholic Beverages Quality: Current Perspectives and Future Directions
Abstract
1. Introduction
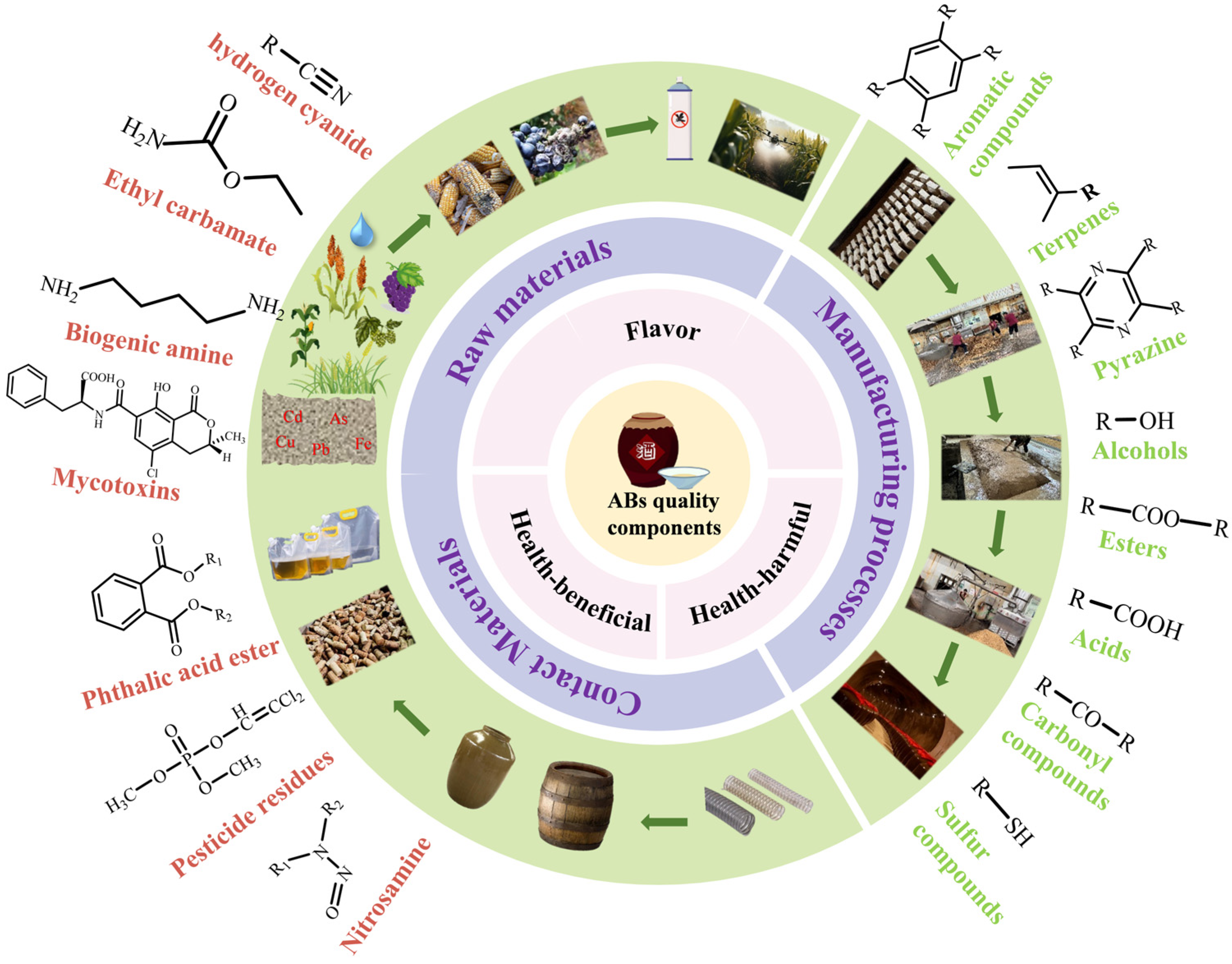
2. Substances Related to AB Quality and Their Key Influencing Factors
2.1. Substances That Affect AB Quality
2.1.1. Flavor Substances
2.1.2. Functional Substances
2.1.3. Harmful Substances
2.2. Key Factors Affecting the Formation of Substances Related to AB Quality
2.2.1. Raw Materials and Contact Materials
2.2.2. Manufacturing Processes
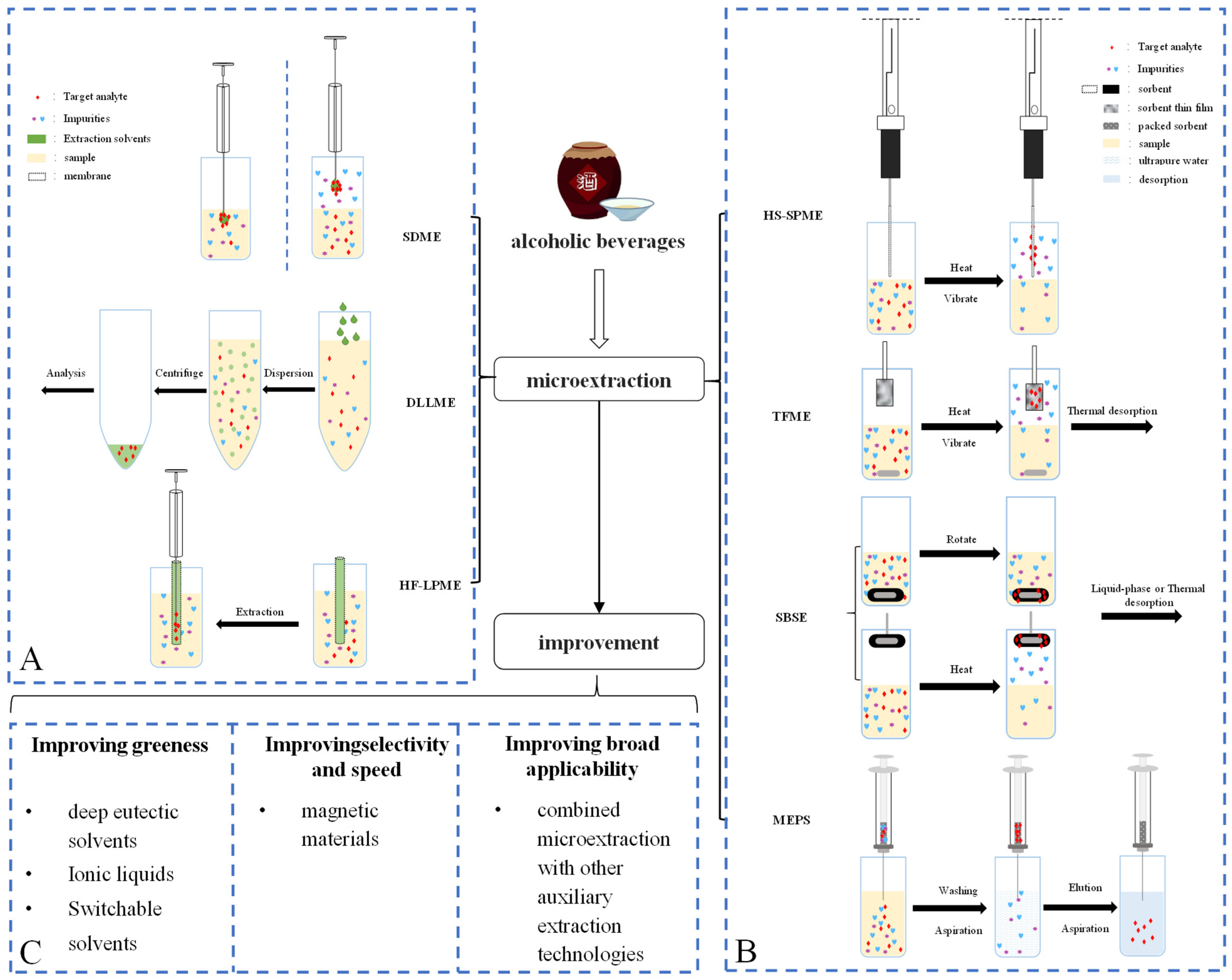
3. Microextraction in AB Quality Analysis
3.1. The Application of LPME in AB Quality Analysis
3.1.1. Single-Drop Microextraction
3.1.2. Hollow-Fiber Liquid-Phase Microextraction
3.1.3. Dispersive Liquid-Liquid Microextraction
3.2. The Application of SPME in AB Quality Analysis
3.2.1. Solid-Phase Microextraction
3.2.2. Stir Bar Sorptive Extraction
3.2.3. Thin-Film Microextraction
3.2.4. Quick, Easy, Cheap, Effective, Rugged, Safe Method
3.2.5. Microextraction by Packed Sorbent
3.3. The Application of Other Microextraction Techniques in AB Quality Analysis
4. Improvements Based on Commonly Used Microextraction Techniques
4.1. Improving the Greenness of Microextraction Using Green Solvents
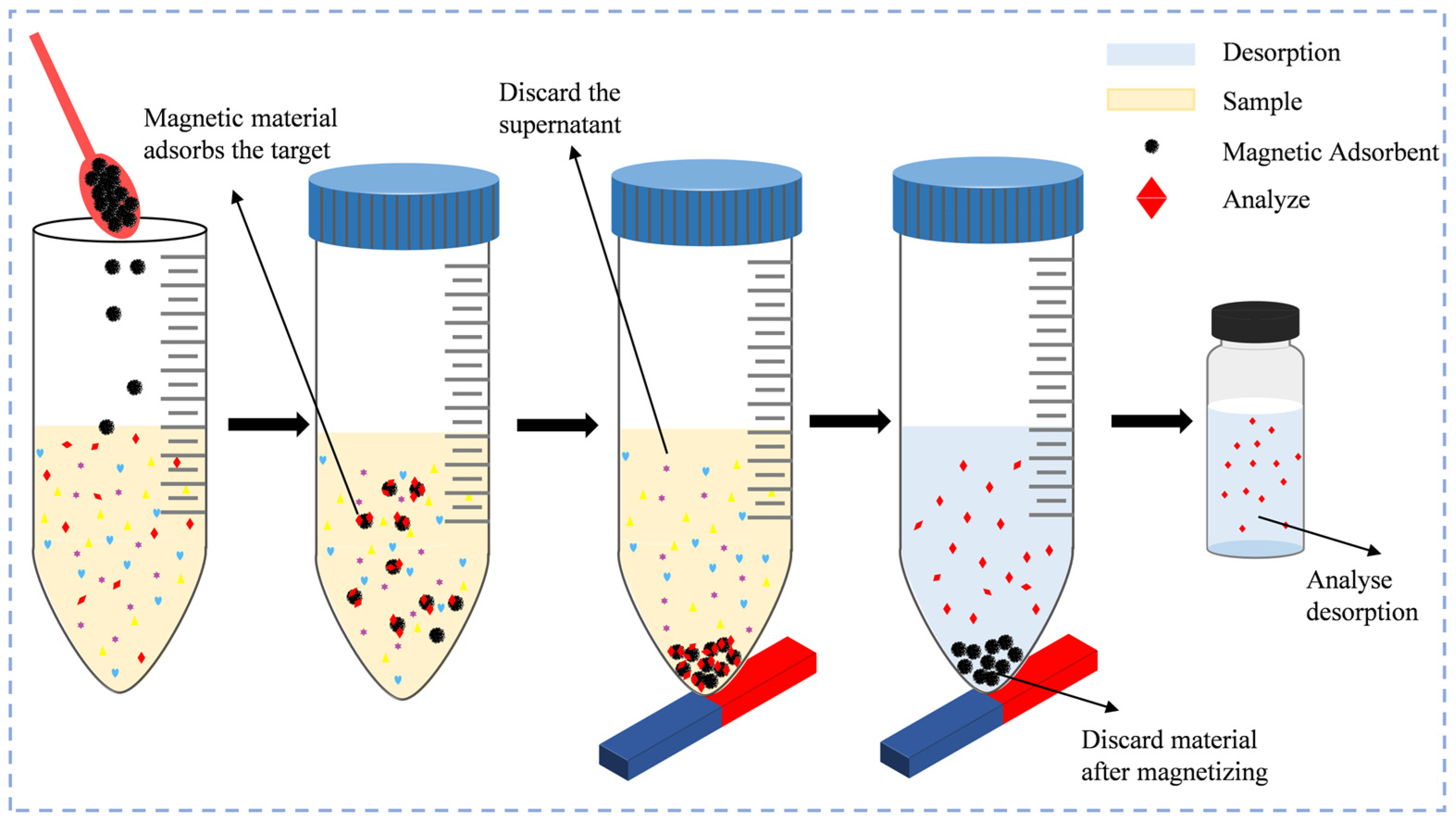
4.2. Improving the Selectivity and Speed of Microextraction Using Magnetic Nanomaterials
4.3. Improving Applicability Using Multiple Combined Technologies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABs | Alcoholic beverages |
| USD | United States dollar |
| OAV | Odor activity value |
| LC-MS | Liquid chromatograph mass spectrometer |
| GC-MS | Gas chromatograph mass spectrometer |
| NMR | Nuclear magnetic resonance |
| FT-IR | Fourier transform infrared spectrometer |
| EU | Europäische Union |
| LODs | Limits of detection |
| LOQs | Limits of quantitation |
| RSD | Relative standard deviation |
| VOCs | volatile organic compounds |
| LC-UV | Liquid chromatograph ultraviolet and visible spectrum |
| LC-FID | Liquid chromatograph flame ionization detector |
| GC-μECD | Gas chromatograph micro electron capture detector |
| UHPLC-Orbitrap-MS | Ultra performance liquid chromatography orbitrap mass spectrometer |
| GC-NPD | Gas chromatograph nitrogen-phosphorus detector |
References
- Lin, M.; Yang, B.; Dai, M.; Xu, Y.; Li, X.; Sun, B. East meets west in alcoholic beverages: Flavor comparison, microbial metabolism and health effects. Food Biosci. 2023, 56, 103385. [Google Scholar] [CrossRef]
- Kaczyński, P.; Iwaniuk, P.; Hrynko, I.; Łuniewski, S.; Łozowicka, B. The effect of the multi-stage process of wheat beer brewing on the behavior of pesticides according to their physicochemical properties. Food Control 2024, 160, 110356. [Google Scholar] [CrossRef]
- Statista. Revenue of the Alcoholic Drinks Market Worldwide from 2018 to 2028 (in Billion U.S. Dollars). 2024. Available online: https://www.statista.com/forecasts/696641/market-value-alcoholic-beverages-worldwide (accessed on 10 October 2024).
- Lachenmeier, D.; Rehm, J. Is There a Relationship Between Alcohol Quality and Health? Alcohol Alcohol. 2012, 48, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Qiu, J.; Du, Z.; He, M.; Xin, X.; Wang, W. Research progress and control methods of methanol in fermented fruit wine. China Brew. 2024, 43, 33–40. [Google Scholar] [CrossRef]
- Xu, P.; Wu, Y.; Chen, H.; Liu, Y.; Yang, N.; Mao, Y.; Tian, L.; He, Z.; Qiu, X.; Guan, T. Promoting microbial community succession and improving the content of esters and aromatic compounds in strong-flavor Daqu via bioaugmentation inoculation. Food Biosci. 2023, 56, 103299. [Google Scholar] [CrossRef]
- Zhao, G.; Dang, K.; Gong, X.; Wang, H.; Zhang, S.; Ma, Y.; Guo, L.; Feng, B. Analysis on the physicochemical properties and Baijiu-making characteristics in grain of japonica sorghum and glutinous sorghum. China Brew. 2021, 40, 77–82. [Google Scholar] [CrossRef]
- Ntuli, R.G.; Saltman, Y.; Ponangi, R.; Jeffery, D.W.; Bindon, K.; Wilkinson, K.L. Impact of fermentation temperature and grape solids content on the chemical composition and sensory profiles of Cabernet Sauvignon wines made from flash détente treated must fermented off-skins. Food Chem. 2022, 369, 130861. [Google Scholar] [CrossRef]
- Arslan, M.; Tahir, H.E.; Zareef, M.; Shi, J.; Rakha, A.; Bilal, M.; Huang, X.; Li, Z.; Zou, X. Recent trends in quality control, discrimination and authentication of alcoholic beverages using nondestructive instrumental techniques. Trends Food Sci. Technol. 2021, 107, 80–113. [Google Scholar] [CrossRef]
- Piergiovanni, M.; Gosetti, F.; Rocio-Bautista, P.; Termopoli, V. Aroma determination in alcoholic beverages: Green MS-based sample preparation approaches. Mass Spectrom. Rev. 2022, 43, e21802. [Google Scholar] [CrossRef]
- Tabago, M.; Calingacion, M.N.; Garcia, J. Recent advances in NMR-based metabolomics of alcoholic beverages. Food Chem. 2021, 2, 100009. [Google Scholar] [CrossRef]
- Jia, W.; Fan, Z.; Du, A.; Li, Y.; Zhang, R.; Shi, Q.; Shi, L.; Chu, X. Recent advances in Baijiu analysis by chromatography based technology—A review. Food Chem. 2020, 324, 126899. [Google Scholar] [CrossRef] [PubMed]
- Płotka-Wasylka, J.; Owczarek, K.; Namieśnik, J. Modern solutions in the field of microextraction using liquid as a medium of extraction. TrAC Trends Anal. Chem. 2016, 85, 46–64. [Google Scholar] [CrossRef]
- Tintrop, L.K.; Salemi, A.; Jochmann, M.A.; Engewald, W.R.; Schmidt, T.C. Improving greenness and sustainability of standard analytical methods by microextraction techniques: A critical review. Anal. Chim. Acta 2023, 1271, 341468. [Google Scholar] [CrossRef] [PubMed]
- Jagirani, M.S.; Soylak, M. Review: Microextraction Technique Based New Trends in Food Analysis. Crit. Rev. Anal. Chem. 2022, 52, 968–999. [Google Scholar] [CrossRef]
- Dong, W.; Dai, X.; Jia, Y.; Ye, S.; Shen, C.; Liu, M.; Lin, F.; Sun, X.; Xiong, Y.; Deng, B. Association between Baijiu chemistry and taste change: Constituents, sensory properties, and analytical approaches. Food Chem. 2024, 437, 137826. [Google Scholar] [CrossRef]
- GB/T 10781.1-2021; Quailty Requirements for Baijiu-Part 1: Nongxiangxing Baijiu. State Administration For Market Regulation: Beijing, China, 2021.
- Shi, T.; Li, P.; Xiao, D. Effect of disrupting ILV2 gene on growth and diacetyl metabolism of brewer’s yeast. Microbiol. China 2016, 43, 1732–1738. [Google Scholar] [CrossRef]
- Qiao, L.; Wang, J.; Wang, R.; Zhang, N.; Zheng, F. A review on flavor of Baijiu and other world-renowned distilled liquors. Food Chem. X 2023, 20, 100870. [Google Scholar] [CrossRef]
- GB/T 4927-2008; Beer. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2008.
- Hu, D.; Chen, Y.; Li, H.; Wu, H.; Lin, Y. Removal of turbidity in low-alcohol Chinese baijiu by coalescence separation. Results Eng. 2024, 22, 102056. [Google Scholar] [CrossRef]
- Ferraz da Costa, D.C.; Pereira Rangel, L.; Quarti, J.; Santos, R.A.; Silva, J.L.; Fialho, E. Bioactive Compounds and Metabolites from Grapes and Red Wine in Breast Cancer Chemoprevention and Therapy. Molecules 2020, 25, 3531. [Google Scholar] [CrossRef]
- Kang, Q.; Sun, J.; Wang, B.; Sun, B. Wine, beer and Chinese Baijiu in relation to cardiovascular health: The impact of moderate drinking. Food Sci. Hum. Wellness 2023, 12, 1–13. [Google Scholar] [CrossRef]
- Baselga-Escudero, L.; Blade, C.; Ribas-Latre, A.; Casanova, E.; Salvado, M.J.; Arola, L.; Arola-Arnal, A. Grape seed proanthocyanidins repress the hepatic lipid regulators miR-33 and miR-122 in rats. Mol. Nutr. Food Res. 2012, 56, 1636–1646. [Google Scholar] [CrossRef] [PubMed]
- Terzo, M.; Iantomasi, M.; Tsiani, E. Effects of Resveratrol on Adipocytes: Evidence from In Vitro and In Vivo Studies. Molecules 2024, 29, 5359. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Liao, W.; Xia, H.; Wang, S.; Sun, G. The Effect of Resveratrol on Blood Lipid Profile: A Dose-Response Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 3755. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gomez, A.; Caballero, I.; Blanco, C.A. Phenols and Melanoidins as Natural Antioxidants in Beer. Structure, Reactivity and Antioxidant Activity. Biomolecules 2020, 10, 400. [Google Scholar] [CrossRef]
- Salanță, L.C.; Coldea, T.E.; Ignat, M.V.; Pop, C.R.; Tofană, M.; Mudura, E.; Borșa, A.; Pasqualone, A.; Anjos, O.; Zhao, H. Functionality of Special Beer Processes and Potential Health Benefits. Processes 2020, 8, 1613. [Google Scholar] [CrossRef]
- Fernandez-Sola, J. Cardiovascular risks and benefits of moderate and heavy alcohol consumption. Nat. Rev. Cardiol. 2015, 12, 576–587. [Google Scholar] [CrossRef]
- Sun, B.; Huang, M.; Wang, J. Research Progress on Flavor Chemistry and Healthy Function of Baijiu. J. Chin. Inst. Food Sci. Technol. 2021, 21, 1–13. [Google Scholar] [CrossRef]
- He, N.X.; Bayen, S. An overview of chemical contaminants and other undesirable chemicals in alcoholic beverages and strategies for analysis. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3916–3950. [Google Scholar] [CrossRef]
- Yang, Q.; Wei, S.; Liu, N.; Gu, Z. The Dissipation of Cyazofamid and Its Main Metabolite CCIM during Wine-Making Process. Molecules 2020, 25, 777. [Google Scholar] [CrossRef]
- Pazzirota, T.; Martin, L.; Mezcua, M.; Ferrer, C.; Fernandez-Alba, A.R. Processing factor for a selected group of pesticides in a wine-making process: Distribution of pesticides during grape processing. Food Addit. Contam. Part A 2013, 30, 1752–1760. [Google Scholar] [CrossRef]
- Dal Bosco, C.; Mariani, F.; Gentili, A. Hydrophobic Eutectic Solvent-Based Dispersive Liquid-Liquid Microextraction Applied to the Analysis of Pesticides in Wine. Molecules 2022, 27, 908. [Google Scholar] [CrossRef] [PubMed]
- Abt, E.; Incorvati, V.; Robin, L.P.; Redan, B.W. Occurrence of Ethyl Carbamate in Foods and Beverages: Review of the Formation Mechanisms, Advances in Analytical Methods, and Mitigation Strategies. J. Food Prot. 2021, 84, 2195–2212. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Ji, L.; Han, X.; Wu, T.; Han, B.; Li, C.; Zhan, J.; Huang, W.; You, Y. Research progress on the application of different controlling strategies to minimizing ethyl carbamate in grape wine. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1495–1516. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yoo, M.; Shin, D. The identification and quantification of biogenic amines in Korean turbid rice wine, Makgeolli by HPLC with mass spectrometry detection. LWT—Food Sci. Technol. 2015, 62, 350–356. [Google Scholar] [CrossRef]
- Ji, Y.; Zhao, M.; Li, A.; Zhao, L. Hydrophobic deep eutectic solvent-based ultrasonic-assisted dispersive liquid-liquid microextraction for preconcentration and determination of trace cadmium and arsenic in wine samples. Microchem. J. 2021, 164, 105974. [Google Scholar] [CrossRef]
- Algazzali, V.; Shellhammer, T. Bitterness Intensity of Oxidized Hop Acids: Humulinones and Hulupones. J. Am. Soc. Brew. Chem. 2016, 74, 36–43. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, X.; Jiang, X.; Wang, L.; Su, Z.; Li, W.; Jiang, W.; Tian, F.; Han, X.; Hu, Y. Process optimization for production of multi-grain light aroma type Baijiu. Food Ferment. Ind. 2020, 47, 140–145. [Google Scholar] [CrossRef]
- Noguerol-Pato, R.; Fernandez-Cruz, T.; Sieiro-Sampedro, T.; Gonzalez-Barreiro, C.; Cancho-Grande, B.; Cilla-Garcia, D.A.; Garcia-Pastor, M.; Martinez-Soria, M.T.; Sanz-Asensio, J.; Simal-Gandara, J. Dissipation of Fungicide Residues during Winemaking and Their Effects on Fermentation and the Volatile Composition of Wines. J. Agric. Food Chem. 2016, 64, 1344–1354. [Google Scholar] [CrossRef]
- Pflaum, T.; Hausler, T.; Baumung, C.; Ackermann, S.; Kuballa, T.; Rehm, J.; Lachenmeier, D.W. Carcinogenic compounds in alcoholic beverages: An update. Arch. Toxicol. 2016, 90, 2349–2367. [Google Scholar] [CrossRef]
- Lee, Y.; Baek, J.; Kwon, Y. Assessing dietary bisphenol A exposure among Koreans: Comprehensive database construction and analysis using the Korea National Health and Nutrition Examination Survey. Food Addit. Contam. Part A 2024, 41, 1018–1055. [Google Scholar] [CrossRef]
- Dong, W.; Guo, R.; Sun, X.; Li, H.; Zhao, M.; Zheng, F.; Sun, J.; Huang, M.; Wu, J. Assessment of phthalate ester residues and distribution patterns in Baijiu raw materials and Baijiu. Food Chem. 2019, 283, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhao, J.; Liu, X.; Zhang, C.; Zhao, Z.; Li, X.; Sun, B. Flavor mystery of Chinese traditional fermented baijiu: The great contribution of ester compounds. Food Chem. 2022, 369, 130920. [Google Scholar] [CrossRef] [PubMed]
- Kruis, A.J.; Bohnenkamp, A.C.; Patinios, C.; van Nuland, Y.M.; Levisson, M.; Mars, A.E.; van den Berg, C.; Kengen, S.W.M.; Weusthuis, R.A. Microbial production of short and medium chain esters: Enzymes, pathways, and applications. Biotechnol. Adv. 2019, 37, 107407. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.; Liu, X.; Li, X.; Zhang, C.; Li, W.; Sun, X.; Wang, W.; Sun, B. Discovery and development of a novel short-chain fatty acid ester synthetic biocatalyst under aqueous phase from Monascus purpureus isolated from Baijiu. Food Chem. 2021, 338, 128025. [Google Scholar] [CrossRef]
- Wu, S.; Yang, Y.; Huo, X.; Liu, C.; Shen, G.; Zhao, Z.; Li, J. Effect of Two Distillation Processes on the Characteristic Aroma of Whisky. Food Sci. 2022, 44, 341–350. [Google Scholar] [CrossRef]
- Deng, Y.; Xiong, A.; Zhao, K.; Hu, Y.; Kuang, B.; Xiong, X.; Yang, Z.; Yu, Y.; Zheng, Q. Mechanisms of the regulation of ester balance between oxidation and esterification in aged Baijiu. Sci. Rep. 2020, 10, 17169. [Google Scholar] [CrossRef]
- Alexander, J.; Audunsson, G.A.; Benford, D.; Cockburn, A.; Cravedi, J.-P.; Dogliotti, E.; Di Domenico, A.; Fernandez-Cruz, M.L.; Fuerst, P.; Fink-Gremmels, J.; et al. Ethyl carbamate and hydrocyanic acid in food and beverages—Scientific Opinion of the Panel on Contaminants. EFSA J. 2007, 551, 1–44. [Google Scholar] [CrossRef]
- Wang, P.; Sun, J.; Li, X.; Wu, D.; Li, T.; Lu, J.; Chen, J.; Xie, G. Contribution of citrulline to the formation of ethyl carbamate during Chinese rice wine production. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2014, 31, 587–592. [Google Scholar] [CrossRef]
- Cao, S.; Wu, Q.; Xu, Y. Effects of Different Sorghum Cultivars on the Formation of Ethyl Carbamate in the Solid-state Fermentation Process of Chinese Liquor. J. Food Sci. Biotechnol. 2015, 35, 677–683. [Google Scholar] [CrossRef]
- Cui, K.; Wu, Q.; Xu, Y. Biodegradation of Ethyl Carbamate and Urea with Lysinibacillus sphaericus MT33 in Chinese Liquor Fermentation. J. Agric. Food Chem. 2018, 66, 1583–1590. [Google Scholar] [CrossRef]
- Lago, L.O.; Welke, J.E. Carbonyl compounds in wine: Factors related to presence and toxic effects. Ciência Rural 2019, 49, e20190349. [Google Scholar] [CrossRef]
- Dong, W.; Guo, R.; Liu, M.; Shen, C.; Sun, X.; Zhao, M.; Sun, J.; Li, H.; Zheng, F.; Huang, M.; et al. Characterization of key odorants causing the roasted and mud-like aromas in strong-aroma types of base Baijiu. Food Res. Int. 2019, 125, 108546. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Yang, S.; Zhang, G.; Xu, L.; Li, H.; Sun, J.; Huang, M.; Zheng, F.; Sun, B. Exploration of key aroma active compounds in strong flavor Baijiu during the distillation by modern instrument detection technology combined with multivariate statistical analysis methods. J. Food Compos. Anal. 2022, 110, 104577. [Google Scholar] [CrossRef]
- Fabjanowicz, M.; Różańska, A.; Kalinowska, K.; Płotka-Wasylka, J. Miniaturized, green salting-out liquid–liquid microextraction coupled with GC–MS used to evaluate biogenic amines in wine samples. Microchem. J. 2022, 180, 107616. [Google Scholar] [CrossRef]
- Jin, F.; Yin, X.; Wan, Y.; Zhang, J.; Wang, J.; Fu, X.; Fu, T.; Liu, B.; Chen, Y.; Tian, B.; et al. Ultrasonic–microwave synergistic supramolecular solvent liquid–liquid microextraction of trace biogenic amines in fish and beer based on solidification of floating organic droplet. Food Chem. 2023, 429, 136965. [Google Scholar] [CrossRef]
- Pereira, C.; Cunha, S.C.; Fernandes, J.O. Commercial beers: A source of phthalates and di-ethylhexyl adipate. Food Chem. X 2023, 19, 100768. [Google Scholar] [CrossRef]
- Gure, A.; Lara, F.J.; García-Campaña, A.M.; Megersa, N. Vortex-assisted ionic liquid dispersive liquid–liquid microextraction for the determination of sulfonylurea herbicides in wine samples by capillary high-performance liquid chromatography. Food Chem. 2015, 170, 348–353. [Google Scholar] [CrossRef]
- Lai, X.; Ruan, C.; Liu, R.; Liu, C. Application of ionic liquid-based dispersive liquid–liquid microextraction for the analysis of ochratoxin A in rice wines. Food Chem. 2014, 161, 317–322. [Google Scholar] [CrossRef]
- Cao, D.; Xu, X.; Xue, S.; Feng, X.; Zhang, L. An in situ derivatization combined with magnetic ionic liquid-based fast dispersive liquid-liquid microextraction for determination of biogenic amines in food samples. Talanta 2019, 199, 212–219. [Google Scholar] [CrossRef]
- Jing, X.; Huang, X.; Wang, H.; Xue, H.; Wu, B.; Wang, X.; Jia, L. Popping candy-assisted dispersive liquid–liquid microextraction for enantioselective determination of prothioconazole and its chiral metabolite in water, beer, Baijiu, and vinegar samples by HPLC. Food Chem. 2021, 348, 129147. [Google Scholar] [CrossRef]
- Carneiro, A.F.; Carneiro, C.N.; Gomez, F.J.V.; Spisso, A.; Silva, M.F.; Minho, L.A.C.; Dos Santos, W.N.L.; Dias, F.D.S. Doehlert matrix for the optimization of ultrasound dispersive liquid–liquid microextraction of melatonin in Argentine and Brazilian wine samples. Microchem. J. 2020, 159, 105313. [Google Scholar] [CrossRef]
- Timofeeva, I.; Kanashina, D.; Moskvin, L.; Bulatov, A. An evaporation-assisted dispersive liquid–liquid microextraction technique as a simple tool for high performance liquid chromatography tandem–mass spectrometry determination of insecticides in wine. J. Chromatogr. A 2017, 1512, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.-P.; Tseng, W.-C.; Kong, P.-H.; Huang, C.-K.; Chen, J.-H.; Chen, P.-S.; Huang, S.-D. Up-and-down-shaker-assisted dispersive liquid–liquid microextraction coupled with gas chromatography–mass spectrometry for the determination of fungicides in wine. Food Chem. 2015, 185, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Stój, A.; Płotka-Wasylka, J.; Simeonov, V.; Kapłan, M. The content of biogenic amines in Rondo and Zweigelt wines and correlations between selected wine parameters. Food Chem. 2022, 371, 131172. [Google Scholar] [CrossRef]
- Bergler, G.; Nolleau, V.; Picou, C.; Perez, M.; Ortiz-Julien, A.; Brulfert, M.; Camarasa, C.; Bloem, A. Dispersive Liquid–Liquid Microextraction for the Quantitation of Terpenes in Wine. J. Agric. Food Chem. 2020, 68, 13302–13309. [Google Scholar] [CrossRef]
- Martínez, D.; Grindlay, G.; Gras, L.; Mora, J. Determination of cadmium and lead in wine samples by means of dispersive liquid–liquid microextraction coupled to electrothermal atomic absorption spectrometry. J. Food Compos. Anal. 2018, 67, 178–183. [Google Scholar] [CrossRef]
- Zhou, Z.; Ni, W.; Ji, Z.; Liu, S.; Han, X.; Li, X.; Mao, J. Development of a Rapid Method for Determination of Main Higher Alcohols in Fermented Alcoholic Beverages Based on Dispersive Liquid-Liquid Microextraction and Gas Chromatography-Mass Spectrometry. Food Anal. Methods 2020, 13, 591–600. [Google Scholar] [CrossRef]
- Silveira, J.R.K.; Brudi, L.C.; Waechter, S.R.; Mello, P.A.; Costa, A.B.; Duarte, F.A. Copper determination in beer by flame atomic absorption spectrometry after extraction and preconcentration by dispersive liquid–liquid microextraction. Microchem. J. 2023, 184, 108181. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Simeonov, V.; Namieśnik, J. An in situ derivatization—Dispersive liquid–Liquid microextraction combined with gas-chromatography—Mass spectrometry for determining biogenic amines in home-made fermented alcoholic drinks. J. Chromatogr. A 2016, 1453, 10–18. [Google Scholar] [CrossRef]
- Sousa, F.M.; Ferreira, R.J.R.; Sá, S.V.M.; Cunha, S.C.S.; Oliveira Fernandes, J. Novel analytical approach to assess the profile of volatile phenols in Portuguese red wines. Aust. J. Grape Wine Res. 2020, 26, 90–100. [Google Scholar] [CrossRef]
- Seeger, T.S.; Rosa, F.C.; Bizzi, C.A.; Dressler, V.L.; Flores, E.M.M.; Duarte, F.A. Feasibility of dispersive liquid–liquid microextraction for extraction and preconcentration of Cu and Fe in red and white wine and determination by flame atomic absorption spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2015, 105, 136–140. [Google Scholar] [CrossRef]
- Zhang, K.; Guo, R.; Wang, Y.; Wang, J.; Nie, Q.; Zhu, G. Terpenes based hydrophobic deep eutectic solvents for dispersive liquid-liquid microextraction of aliphatic aldehydes in drinking water and alcoholic beverages. Chemosphere 2024, 354, 141706. [Google Scholar] [CrossRef] [PubMed]
- Carbonell-Rozas, L.; Canales, R.; Lara, F.J.; García-Campaña, A.M.; Silva, M.F. A natural deep eutectic solvent as a novel dispersive solvent in dispersive liquid-liquid microextraction based on solidification of floating organic droplet for the determination of pesticide residues. Anal. Bioanal. Chem. 2021, 413, 6413–6424. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jia, S.; Yoon, S.J.; Lee, S.J.; Kwon, S.W.; Lee, J. Ion-pair dispersive liquid–liquid microextraction solidification of floating organic droplets method for the rapid and sensitive detection of phenolic acids in wine samples using liquid chromatography combined with a core–shell particle column. J. Food Compos. Anal. 2016, 45, 73–79. [Google Scholar] [CrossRef]
- Bernardi, G.; Kemmerich, M.; Machado, F.F.; Prestes, O.D.; Adaime, M.B.; Zanella, R. Multiresidue Determination of Fungicides in Wine by Solvent Demulsification-Dispersive Liquid-Liquid Microextraction and Ultra-High Performance Liquid Chromatography–Tandem Mass Spectrometry. Food Anal. Methods 2022, 15, 2026–2035. [Google Scholar] [CrossRef]
- Ma, Z.; Zhao, T.; Cui, S.; Zhao, X.; Fan, Y.; Song, J. Determination of ethyl carbamate in wine by matrix modification-assisted headspace single-drop microextraction and gas chromatography–mass spectrometry technique. Food Chem. 2022, 373, 131573. [Google Scholar] [CrossRef]
- Dos Anjos, J.P.; De Andrade, J.B. Simultaneous determination of pesticide multiresidues in white wine and rosé wine by SDME/GC-MS. Microchem. J. 2015, 120, 69–76. [Google Scholar] [CrossRef]
- Qin, B.; Wang, X.; Tang, L.; Wang, S.; Shi, Y.; Zhao, L.; Jiang, H. Comparative study of headspace and headspace single drop microextraction combined with GC for the determination of methanol in wine. J. Chromatogr. A 2022, 1673, 463079. [Google Scholar] [CrossRef]
- Jia, L.; Huang, X.; Zhao, W.; Wang, H.; Jing, X. An effervescence tablet-assisted microextraction based on the solidification of deep eutectic solvents for the determination of strobilurin fungicides in water, juice, wine, and vinegar samples by HPLC. Food Chem. 2020, 317, 126424. [Google Scholar] [CrossRef]
- Nascimento, M.M.; Dos Anjos, J.P.; Nascimento, M.L.; Assis Felix, C.S.; Da Rocha, G.O.; De Andrade, J.B. Development of a green liquid-phase microextraction procedure using a customized device for the comprehensive determination of legacy and current pesticides in distinct types of wine samples. Talanta 2024, 266, 124914. [Google Scholar] [CrossRef]
- Altunay, N.; Elik, A.; Unal, Y.; Kaya, S. Optimization of an ultrasound-assisted alcohol-based deep eutectic solvent dispersive liquid-phase microextraction for separation and preconcentration of quercetin in wine and food samples with response surface methodology. J. Sep. Sci. 2021, 44, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Jeannot, M.A.; Cantwell, F.F. Solvent Microextraction into a Single Drop. Anal. Chem. 1996, 68, 2236–2240. [Google Scholar] [CrossRef] [PubMed]
- Martendal, E.; Budziak, D.; Carasek, E. Application of fractional factorial experimental and Box-Behnken designs for optimization of single-drop microextraction of 2,4,6-trichloroanisole and 2,4,6-tribromoanisole from wine samples. J. Chromatogr. A 2007, 1148, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Zaruba, S.; Vishnikin, A.B.; Škrlíková, J.; Andruch, V. Using an Optical Probe as the Microdrop Holder in Headspace Single Drop Microextraction: Determination of Sulfite in Food Samples. Anal. Chem. 2016, 88, 10296–10300. [Google Scholar] [CrossRef]
- Pedersen-Bjergaard, S.; Rasmussen, K.E. Liquid−Liquid−Liquid Microextraction for Sample Preparation of Biological Fluids Prior to Capillary Electrophoresis. Anal. Chem. 1999, 71, 2650–2656. [Google Scholar] [CrossRef]
- Carasek, E.; Merib, J. Membrane-based microextraction techniques in analytical chemistry: A review. Anal. Chim. Acta 2015, 880, 8–25. [Google Scholar] [CrossRef]
- Ouyang, G.; Pawliszyn, J. Kinetic Calibration for Automated Hollow Fiber-Protected Liquid-Phase Microextraction. Anal. Chem. 2006, 78, 5783–5788. [Google Scholar] [CrossRef]
- Bolaños, P.P.; Romero-González, R.; Frenich, A.G.; Vidal, J.L.M. Application of hollow fibre liquid phase microextraction for the multiresidue determination of pesticides in alcoholic beverages by ultra-high pressure liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. A 2008, 1208, 16–24. [Google Scholar] [CrossRef]
- Romero-Gonzalez, R.; Frenich, A.G.; Vidal, J.L.; Aguilera-Luiz, M.M. Determination of ochratoxin A and T-2 toxin in alcoholic beverages by hollow fiber liquid phase microextraction and ultra high-pressure liquid chromatography coupled to tandem mass spectrometry. Talanta 2010, 82, 171–176. [Google Scholar] [CrossRef]
- Rezaee, M.; Assadi, Y.; Milani Hosseini, M.R.; Aghaee, E.; Ahmadi, F.; Berijani, S. Determination of organic compounds in water using dispersive liquid-liquid microextraction. J. Chromatogr. A 2006, 1116, 1–9. [Google Scholar] [CrossRef]
- Fariña, L.; Boido, E.; Carrau, F.; Dellacassa, E. Determination of volatile phenols in red wines by dispersive liquid–liquid microextraction and gas chromatography–mass spectrometry detection. J. Chromatogr. A 2007, 1157, 46–50. [Google Scholar] [CrossRef]
- Pizarro, C.; Sáenz-González, C.; Pérez-del-Notario, N.; González-Sáiz, J.M. Development of a dispersive liquid–liquid microextraction method for the simultaneous determination of the main compounds causing cork taint and Brett character in wines using gas chromatography–tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 1576–1584. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Z.; Liu, M.; Ao, L.; Sun, B.; Sun, J.; Zheng, F.; Huang, M.; Li, H. Determination of Tetramethylpyrazine-4-Methyl Guaiacol and 4-Ethyl Guaiacol in 67 Chinese Baijiu Samples by Vortex Assisted Liquid-Liquid Microextration Combined with Gas Chromatography-Mass Spectrometry. Food Sci. 2017, 38, 73–79. [Google Scholar] [CrossRef]
- De Souza, F.L.A.; De Souza Ramos, T.J.; Montenegro, M.C.B.S.M.; Pinto, L.; Cassol, T.M.; Paim, A.P.S. Chemometric cleanup to eliminate ionic liquid interferences and enable its application on in-situ IL-DLLME using HPLC-DAD detection. J. Mol. Liq. 2021, 330, 115627. [Google Scholar] [CrossRef]
- Rodríguez-Cabo, T.; Rodríguez, I.; Ramil, M.; Cela, R. Dispersive liquid–liquid microextraction using non-chlorinated, lighter than water solvents for gas chromatography–mass spectrometry determination of fungicides in wine. J. Chromatogr. A 2011, 1218, 6603–6611. [Google Scholar] [CrossRef]
- Zhou, W.; Wieczorek, M.N.; Pawliszyn, J. High throughput and automated solid-phase microextraction and determination by liquid chromatography-mass spectrometry for the analysis of mycotoxins in beer. Food Chem. 2023, 426, 136557. [Google Scholar] [CrossRef]
- Bates, T.L.; Sacks, G.L. Rapid headspace solid-phase microextraction sheets with direct analysis in real time mass spectrometry (SPMESH-DART-MS) of derivatized volatile phenols in grape juices and wines. Anal. Chim. Acta 2023, 1275, 341577. [Google Scholar] [CrossRef]
- Milheiro, J.; Filipe-Ribeiro, L.; Cosme, F.; Nunes, F.M. Development and validation of a multiple headspace solid-phase microextraction method for accurate and precise analysis of the aroma of Tawny and White Port wines. Food Chem. 2023, 421, 136154. [Google Scholar] [CrossRef]
- Wu, J.; Huang, X. Electric field-reinforced solid phase microextraction based on anion-exchange monolith for efficient entrapment of anions in aqueous and wine samples. J. Chromatogr. A 2022, 1676, 463291. [Google Scholar] [CrossRef]
- Resende Dos Santos, R.; Orlando, R.M.; De Lourdes Cardeal, Z.; Menezes, H.C. Assessment of polycyclic aromatic hydrocarbons and derivatives in beer using a new cold fiber-solid phase microextraction system. Food Control 2021, 126, 108104. [Google Scholar] [CrossRef]
- Lenti, L.; Scortichini, S.; Pacetti, D.; Cespi, M.; Fiorini, D. Polydimethylsiloxane/divinylbenzene overcoated fiber and its application to extract and analyse wine volatile compounds by solid-phase microextraction and gas chromatography coupled to mass spectrometry: Direct immersion, headspace or both? Food Res. Int. 2021, 148, 110632. [Google Scholar] [CrossRef]
- Wieczorek, M.N.; Zhou, W.; Pawliszyn, J. Sequential thin film-solid phase microextraction as a new strategy for addressing displacement and saturation effects in food analysis. Food Chem. 2022, 389, 133038. [Google Scholar] [CrossRef]
- Tesoro, C.; Acquavia, M.A.; Giussani, B.; Bianco, G.; Pascale, R.; Lelario, F.; Ciriello, R.; Capece, A.; Pietrafesa, R.; Siesto, G.; et al. An Interplay between a Face-Centred Composite Experimental Design and Solid-Phase Microextraction for Wine Aroma GC/MS Analysis. Appl. Sci. 2023, 13, 4609. [Google Scholar] [CrossRef]
- Qin, D.; Duan, J.; Li, H.; Zheng, F.; Cheng, H.; Ye, X.; Sun, B. Characterization and comparison of the aroma-active compounds on different grades of sesame-flavor Baijiu by headspace solid-phase microextraction and gas chromatography-olfactometry-mass spectrometry. Food Sci. Hum. Wellness 2023, 12, 79–88. [Google Scholar] [CrossRef]
- Jasmins, G.; Perestrelo, R.; Coïsson, J.D.; Sousa, P.; Teixeira, J.A.; Bordiga, M.; Câmara, J.S. Tracing the Volatilomic Fingerprint of the Most Popular Italian Fortified Wines. Foods 2023, 12, 2058. [Google Scholar] [CrossRef]
- Amareh, N.; Yamini, Y.; Saeidi, M.; Ghaemmaghami, M. Zeolitic imidazole framework-67 coated stainless steel fiber for solid-phase microextraction of some alcohols in alcoholic beverage samples. Chem. Pap. 2023, 77, 4923–4934. [Google Scholar] [CrossRef]
- Peña-Alvarez, A.; Capella, S.; Juárez, R.; Labastida, C. Determination of terpenes in tequila by solid phase microextraction-gas chromatography–mass spectrometry. J. Chromatogr. A 2006, 1134, 291–297. [Google Scholar] [CrossRef]
- He, X.; Yangming, H.; Górska-Horczyczak, E.; Wierzbicka, A.; Jeleń, H.H. Rapid analysis of Baijiu volatile compounds fingerprint for their aroma and regional origin authenticity assessment. Food Chem. 2021, 337, 128002. [Google Scholar] [CrossRef]
- Liu, Y.; Qian, X.; Xing, J.; Li, N.; Li, J.; Su, Q.; Chen, Y.; Zhang, B.; Zhu, B. Accurate Determination of 12 Lactones and 11 Volatile Phenols in Nongrape Wines through Headspace-Solid-Phase Microextraction (HS-SPME) Combined with High-Resolution Gas Chromatography-Orbitrap Mass Spectrometry (GC-Orbitrap-MS). J. Agric. Food Chem. 2022, 70, 1971–1983. [Google Scholar] [CrossRef]
- Zhang, X.-J.; Meng, L.-J.; Lu, Z.-M.; Chai, L.-J.; Wang, S.-T.; Shi, J.-S.; Shen, C.-H.; Xu, Z.-H. Identification of age-markers based on profiling of Baijiu volatiles over a two-year maturation period: Case study of Lu-flavor Baijiu. LWT 2021, 141, 110913. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, A.; Fan, C.; Li, X.; Cui, Z.; Zhang, Z. Determination of Multihalo- Phenols and Anisoles in Wine by Gas Chromatography Tandem Mass Spectrometry Through Online Derivatization and Head Space Solid Phase Microextraction. Food Anal. Methods 2022, 15, 3435–3443. [Google Scholar] [CrossRef]
- Sudol, P.E.; Galletta, M.; Tranchida, P.Q.; Zoccali, M.; Mondello, L.; Synovec, R.E. Untargeted profiling and differentiation of geographical variants of wine samples using headspace solid-phase microextraction flow-modulated comprehensive two-dimensional gas chromatography with the support of tile-based Fisher ratio analysis. J. Chromatogr. A 2022, 1662, 462735. [Google Scholar] [CrossRef]
- Fitzgerald, N.; Edwards, J.C. Investigation of Solid Phase Microextraction Gas Chromatography–Mass Spectrometry, Fourier Transform Infrared Spectroscopy and 1H qNMR Spectroscopy as Potential Methods for the Authentication of Baijiu Spirits. Beverages 2023, 9, 25. [Google Scholar] [CrossRef]
- Vyviurska, O.; Thai, H.A.; Garančovská, D.; Gomes, A.A.; Špánik, I. Enhanced multi-stir bar sorptive extraction for wine analysis: Alteration in headspace mode. Food Res. Int. 2022, 158, 111510. [Google Scholar] [CrossRef]
- Morales, M.L.; Fierro-Risco, J.; Ríos-Reina, R.; Ubeda, C.; Paneque, P. Influence of Saccharomyces cerevisiae and Lachancea thermotolerans co-inoculation on volatile profile in fermentations of a must with a high sugar content. Food Chem. 2019, 276, 427–435. [Google Scholar] [CrossRef]
- Arbulu, M.; Sampedro, M.C.; Sanchez-Ortega, A.; Gómez-Caballero, A.; Unceta, N.; Goicolea, M.A.; Barrio, R.J. Characterisation of the flavour profile from Graciano Vitis vinifera wine variety by a novel dual stir bar sorptive extraction methodology coupled to thermal desorption and gas chromatography–mass spectrometry. Anal. Chim. Acta 2013, 777, 41–48. [Google Scholar] [CrossRef]
- Ubeda, C.; Callejón, R.M.; Troncoso, A.M.; Peña-Neira, A.; Morales, M.L. Volatile profile characterisation of Chilean sparkling wines produced by traditional and Charmat methods via sequential stir bar sorptive extraction. Food Chem. 2016, 207, 261–271. [Google Scholar] [CrossRef]
- Picard, M.; Franc, C.; De Revel, G.; Marchand, S. Dual solid-phase and stir bar sorptive extraction combined with gas chromatography-mass spectrometry analysis provides a suitable tool for assaying limonene-derived mint aroma compounds in red wine. Anal. Chim. Acta 2018, 1001, 168–178. [Google Scholar] [CrossRef]
- Xiao, Z.; Dai, X.; Zhu, J.; Yu, H. Classification of Chinese Rice Wine According to Geographic Origin and Wine Age Based on Chemometric Methods and SBSE-TD-GC-MS Analysis of Volatile Compounds. Food Sci. Technol. Res. 2015, 21, 371–380. [Google Scholar] [CrossRef]
- Niu, Y.; Yu, D.; Xiao, Z.; Zhu, J.; Song, S.; Zhu, G. Use of Stir Bar Sorptive Extraction and Thermal Desorption for Gas Chromatography-Mass Spectrometry Characterization of Selected Volatile Compounds in Chinese Liquors. Food Anal. Methods 2015, 8, 1771–1784. [Google Scholar] [CrossRef]
- Hjelmeland, A.K.; Wylie, P.L.; Ebeler, S.E. A comparison of sorptive extraction techniques coupled to a new quantitative, sensitive, high throughput GC–MS/MS method for methoxypyrazine analysis in wine. Talanta 2016, 148, 336–345. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Zhang, B.; Shen, C.; Xu, Y.; Tang, K. Identification, quantitation and sensorial contribution of lactones in brandies between China and France. Food Chem. 2021, 357, 129761. [Google Scholar] [CrossRef]
- Ma, L.; Meng, Q.; Chen, F.; Gao, W. SAFE and SBSE combined with GC-MS and GC-O for characterization of flavor compounds in Zhizhonghe Wujiapi medicinal liquor. J. Food Sci. 2022, 87, 939–956. [Google Scholar] [CrossRef]
- Niu, Y.; Zhao, W.; Xiao, Z.; Zhu, J.; Xiong, W.; Chen, F. Characterization of aroma compounds and effects of amino acids on the release of esters in Laimao baijiu. J. Sci. Food Agric. 2023, 103, 1784–1799. [Google Scholar] [CrossRef]
- Ahammed Shabeer, T.; Somkuwar, R.; Sharma, A.K.; Deshmukh, U.; Hingmire, S. Multi-residue method validation, processing factor and monitoring of thirteen targeted fungicide residues in the process of wine making. J. Food Compos. Anal. 2023, 115, 104912. [Google Scholar] [CrossRef]
- Kosma, C.I.; Koloka, O.L.; Albanis, T.A.; Konstantinou, I.K. Accurate mass screening of pesticide residues in wine by modified QuEChERS and LC-hybrid LTQ/Orbitrap-MS. Food Chem. 2021, 360, 130008. [Google Scholar] [CrossRef]
- Bernardi, G.; Kemmerich, M.; Adaime, M.B.; Prestes, O.D.; Zanella, R. Miniaturized QuEChERS method for determination of 97 pesticide residues in wine by ultra-high performance liquid chromatography coupled with tandem mass spectrometry. Anal. Methods 2020, 12, 2682–2692. [Google Scholar] [CrossRef]
- Navickiene, S.; Santos, L.F.S.; Dos Reis Silva, A. Use of Magnesium Silicate as a New Type of Adsorbent for Dispersive Solid-Phase Extraction Cleanup of the Quick, Cheap, Effective, Rugged, and Safe Method for Pesticides During Analysis of Lager Beer by Gas Chromatography-Tandem Mass Spectrometry. J. AOAC Int. 2019, 102, 619–624. [Google Scholar] [CrossRef]
- Słowik-Borowiec, M.; Szpyrka, E. Multiresidue Analysis of Pesticides in Wine and Grape Using Gas Chromatography with Microelectron Capture and Nitrogen–Phosphorus Detection. Food Anal. Methods 2018, 11, 3516–3530. [Google Scholar] [CrossRef]
- Freitas, J.; Perestrelo, R.; Cassaca, R.; Castillo, M.; Santos, M.; Pereira, J.; Camara, J.S. A fast and environment-friendly MEPSPEP/UHPLC-PDA methodology to assess 3-hydroxy-4,5-dimethyl-2(5H)-furanone in fortified wines. Food Chem. 2017, 214, 686–693. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, C.L.; Camara, J.S. Quantification of furanic derivatives in fortified wines by a highly sensitive and ultrafast analytical strategy based on digitally controlled microextraction by packed sorbent combined with ultrahigh pressure liquid chromatography. J. Chromatogr. A 2015, 1381, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Leca, J.M.; Pereira, V.; Pereira, A.C.; Marques, J.C. Rapid and sensitive methodology for determination of ethyl carbamate in fortified wines using microextraction by packed sorbent and gas chromatography with mass spectrometric detection. Anal. Chim. Acta 2014, 811, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Arthur, C.L.; Pawliszyn, J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Ng, L.-K.; Hupé, M.; Harnois, J.; Moccia, D. Characterisation of Commercial Vodkas by Solid-Phase Microextraction and Gas Chromatography/Mass Spectrometry Analysis. J. Sci. Food Agric. 1996, 70, 380–388. [Google Scholar] [CrossRef]
- Metafa, M.; Economou, A. Chemometrical development and comprehensive validation of a solid phase microextraction/gas chromatography–mass spectrometry methodology for the determination of important free and bound primary aromatics in Greek wines. J. Chromatogr. A 2013, 1305, 244–258. [Google Scholar] [CrossRef]
- Jeleń, H.; Majcher, M.; Gracka, A. Application of Solid Phase Microextraction in Food Analysis—Flavor and Off-Flavor Sampling. In Solid Phase Microextraction: Recent Developments and Applications; Ouyang, G., Jiang, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 223–246. [Google Scholar]
- Yang, X.; Peppard, T. Solid-Phase Microextraction for Flavor Analysis. J. Agric. Food Chem. 1994, 42, 1925–1930. [Google Scholar] [CrossRef]
- Jeleń, H.H.; Wieczorek, M.N. Commentary: “Quantitative” vs quantitative Headspace Solid-Phase Microextraction (HS-SPME) in food volatile and flavor compounds analysis. J. Food Compos. Anal. 2023, 115, 104955. [Google Scholar] [CrossRef]
- Aresta, A.; Cotugno, P.; Massari, F.; Zambonin, C. Determination of Trans-resveratrol in Wines, Spirits, and Grape Juices Using Solid-Phase Micro Extraction Coupled to Liquid Chromatography with UV Diode-Array Detection. Food Anal. Methods 2018, 11, 426–431. [Google Scholar] [CrossRef]
- Feng, M.; Li, C.; Wang, C.; Zhu, G.; Lu, J.; Chen, Y.; Xiao, D.; Guo, X. Determination of terpenoids in Baijiu using solid-phase extraction combined with headspace solid-phase microextraction. Int. J. Food Prop. 2022, 25, 2445–2456. [Google Scholar] [CrossRef]
- Baltussen, E.; Sandra, P.; David, F.; Cramers, C. Stir bar sorptive extraction (SBSE), a novel extraction technique for aqueous samples: Theory and principles. J. Microcolumn Sep. 1999, 11, 737–747. [Google Scholar] [CrossRef]
- Marín-San Román, S.; Rubio-Bretón, P.; Pérez-Álvarez, E.P.; Garde-Cerdán, T. Advancement in analytical techniques for the extraction of grape and wine volatile compounds. Food Res. Int. 2020, 137, 109712. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, M.; Liu, Z.; Chen, S.; Xu, Y. Three Extraction Methods in Combination with GC×GC-TOFMS for the Detailed Investigation of Volatiles in Chinese Herbaceous Aroma-Type Baijiu. Molecules 2020, 25, 4429. [Google Scholar] [CrossRef] [PubMed]
- Weldegergis, B.T.; Crouch, A.M. Analysis of Volatiles in Pinotage Wines by Stir Bar Sorptive Extraction and Chemometric Profiling. J. Agric. Food Chem. 2008, 56, 10225–10236. [Google Scholar] [CrossRef] [PubMed]
- Perestrelo, R.; Nogueira, J.M.F.; Câmara, J.S. Potentialities of two solventless extraction approaches—Stir bar sorptive extraction and headspace solid-phase microextraction for determination of higher alcohol acetates, isoamyl esters and ethyl esters in wines. Talanta 2009, 80, 622–630. [Google Scholar] [CrossRef]
- Bruheim, I.; Liu, X.; Pawliszyn, J. Thin-Film Microextraction. Anal. Chem. 2003, 75, 1002–1010. [Google Scholar] [CrossRef]
- Jiang, R.; Pawliszyn, J. Thin-film microextraction offers another geometry for solid-phase microextraction. TrAC Trends Anal. Chem. 2012, 39, 245–253. [Google Scholar] [CrossRef]
- Deng, Z.; Chen, X.; Wang, Y.; Fang, E.; Zhang, Z.; Chen, X. Headspace Thin-Film Microextraction Coupled with Surface-Enhanced Raman Scattering as a Facile Method for Reproducible and Specific Detection of Sulfur Dioxide in Wine. Anal. Chem. 2015, 87, 633–640. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and Easy Multiresidue Method Employing Acetonitrile Extraction/Partitioning and “Dispersive Solid-Phase Extraction” for the Determination of Pesticide Residues in Produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef]
- Peng, P.L.; Lim, L.H. Polycyclic Aromatic Hydrocarbons (PAHs) Sample Preparation and Analysis in Beverages: A Review. Food Anal. Methods 2022, 15, 1042–1061. [Google Scholar] [CrossRef]
- Tuzimski, T.; Rejczak, T.; Pieniążek, D.; Buszewicz, G.; Teresiński, G. Comparison of SPE/d-SPE and QuEChERS-Based Extraction Procedures in Terms of Fungicide Residue Analysis in Wine Samples by HPLC–DAD and LC-QqQ-MS. J. AOAC Int. 2016, 99, 1436–1443. [Google Scholar] [CrossRef]
- Patil, S.H.; Banerjee, K.; Dasgupta, S.; Oulkar, D.P.; Patil, S.B.; Jadhav, M.R.; Savant, R.H.; Adsule, P.G.; Deshmukh, M.B. Multiresidue analysis of 83 pesticides and 12 dioxin-like polychlorinated biphenyls in wine by gas chromatography-time-of-flight mass spectrometry. J. Chromatogr. A 2009, 1216, 2307–2319. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rodriguez, R.M.; Cancho-Grande, B.; Simal-Gandara, J. Multiresidue determination of 11 new fungicides in grapes and wines by liquid-liquid extraction/clean-up and programmable temperature vaporization injection with analyte protectants/gas chromatography/ion trap mass spectrometry. J. Chromatogr. A 2009, 1216, 6033–6042. [Google Scholar] [CrossRef] [PubMed]
- Walorczyk, S.; Drożdżyński, D.; Gnusowski, B. Multiresidue determination of 160 pesticides in wines employing mixed-mode dispersive-solid phase extraction and gas chromatography–tandem mass spectrometry. Talanta 2011, 85, 1856–1870. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yu, C.; Meng, L.; Ji, W.; Liu, S.; Pan, C.; Lan, T.; Wang, L.; Qu, B. Research progress of applications for nano-materials in improved QuEChERS method. Crit. Rev. Food Sci. Nutr. 2023, 64, 10517–10536. [Google Scholar] [CrossRef]
- Abdel-Rehim, M. New trend in sample preparation: On-line microextraction in packed syringe for liquid and gas chromatography applications: I. Determination of local anaesthetics in human plasma samples using gas chromatography–mass spectrometry. J. Chromatogr. B 2004, 801, 317–321. [Google Scholar] [CrossRef]
- Silva, C.L.; Goncalves, J.L.; Camara, J.S. A sensitive microextraction by packed sorbent-based methodology combined with ultra-high pressure liquid chromatography as a powerful technique for analysis of biologically active flavonols in wines. Anal. Chim. Acta 2012, 739, 89–98. [Google Scholar] [CrossRef]
- Haider, W.; Barillier, D.; Hayat, A.; Gaillard, J.-L.; Ledauphin, J. Rapid quantification and comparison of major volatile compounds of ciders from France (Normandy and Brittany) using microextraction by packed sorbent (MEPS). Anal. Methods 2014, 6, 1364–1376. [Google Scholar] [CrossRef]
- Pacheco, J.G.; Valente, I.M.; Goncalves, L.M.; Rodrigues, J.A.; Barros, A.A. Gas-diffusion microextraction. J. Sep. Sci. 2010, 33, 3207–3212. [Google Scholar] [CrossRef]
- Kishikawa, N.; El-Maghrabey, M.H.; Kuroda, N. Chromatographic methods and sample pretreatment techniques for aldehydes determination in biological, food, and environmental samples. J. Pharm. Biomed. Anal. 2019, 175, 112782. [Google Scholar] [CrossRef]
- Albarri, R.; Vardara, H.F.; Al, S.; Önal, A. Chromatographic Methods and Sample Pretreatment Techniques for Aldehydes, Biogenic Amine, and Carboxylic Acids in Food Samples. Crit. Rev. Anal. Chem. 2024, 48, 1–22. [Google Scholar] [CrossRef]
- Ferreira, I.M.; Carvalho, D.O.; Da Silva, M.G.; Guido, L.F. Gas-Diffusion Microextraction (GDME) Combined with Derivatization for Assessing Beer Staling Aldehydes: Validation and Application. Foods 2021, 10, 1704. [Google Scholar] [CrossRef] [PubMed]
- de Lima, L.F.; Brandao, P.F.; Donegatti, T.A.; Ramos, R.M.; Goncalves, L.M.; Cardoso, A.A.; Pereira, E.A.; Rodrigues, J.A. 4-hydrazinobenzoic acid as a derivatizing agent for aldehyde analysis by HPLC-UV and CE-DAD. Talanta 2018, 187, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Chisvert, A.; Cárdenas, S.; Lucena, R. Dispersive micro-solid phase extraction. TrAC Trends Anal. Chem. 2019, 112, 226–233. [Google Scholar] [CrossRef]
- Safaríková, M.; Safarík, I. Magnetic solid-phase extraction. J. Magn. Magn. Mater. 1999, 194, 108–112. [Google Scholar] [CrossRef]
- Tian, M.-M.; Chen, D.-X.; Sun, Y.-L.; Yang, Y.-W.; Jia, Q. Pillararene-functionalized Fe3O4 nanoparticles as magnetic solid-phase extraction adsorbent for pesticide residue analysis in beverage samples. RSC Adv. 2013, 3, 22111–22119. [Google Scholar] [CrossRef]
- Xu, G.; Hou, L.; Liu, C.; Wang, X.; Liu, L.; Li, N.; Lin, J.-M.; Zhao, R.-S. Fabrication of a Magnetic Fluorinated Covalent Organic Framework for the Selective Capture of Benzoylurea Insecticide Residue in Beverages. ACS Appl. Mater. Interfaces 2021, 13, 51535–51545. [Google Scholar] [CrossRef]
- Ao, L.; Lian, X.; Lin, W.; Guo, R.; Xu, Y.; Dong, W.; Liu, M.; Shen, C.; Sun, X.; Sun, B.; et al. Insights into a new alternative method with graphene oxide/polyacrylamide/Fe3O4 nanocomposite for the extraction of six odor-active esters from Strong-aroma types of Baijiu. Food Chem. X 2022, 15, 100379. [Google Scholar] [CrossRef]
- Ye, S.; Shang, X.; Ao, L.; Sun, B.; Chen, X.; Shen, C.H.; Liu, M.; Lin, F.; Dong, W.; Sun, X.; et al. Decoding Long-Chain Fatty Acid Ethyl Esters during the Distillation of Strong Aroma-Type Baijiu and Exploring the Adsorption Mechanism with Magnetic Nanoparticles. J. Agric. Food Chem. 2024, 72, 21752–21762. [Google Scholar] [CrossRef]
- Gong, L.; Li, A.; Sun, J.; Li, H.; Sun, X.; Huang, M.; Zheng, F.; Sun, B. Analysis on volatile flavor compounds of fermented grains by SAFE and HS-SPME coupled with GC-MS. Food Ferment. Ind. 2016, 42, 169–177. [Google Scholar]
- Fontana, A.; Rodríguez, I.; Cela, R. Accurate determination of 3-alkyl-2-methoxypyrazines in wines by gas chromatography quadrupole time-of-flight tandem mass spectrometry following solid-phase extraction and dispersive liquid–liquid microextraction. J. Chromatogr. A 2017, 1515, 30–36. [Google Scholar] [CrossRef]
- Gil, M.; Cabellos, J.M.; Arroyo, T.; Prodanov, M. Characterization of the volatile fraction of young wines from the Denomination of Origin “Vinos de Madrid” (Spain). Anal. Chim. Acta 2006, 563, 145–153. [Google Scholar] [CrossRef]
- Hong, J.; Huang, H.; Zhao, D.; Sun, J.; Huang, M.; Sun, X.; Sun, B. Investigation on the key factors associated with flavor quality in northern strong aroma type of Baijiu by flavor matrix. Food Chem. 2023, 426, 136576. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Wang, J.; Zhang, C.; Zhao, Z.; Tian, W.; Wu, Y.; Chen, H.; Zhao, D.; Sun, J. Unraveling variation on the profile aroma compounds of strong aroma type of Baijiu in different regions by molecular matrix analysis and olfactory analysis. RSC Adv. 2021, 11, 33511–33521. [Google Scholar] [CrossRef]
- Chen, S.; Xu, Y.; Qian, M.C. Comparison of the aromatic profile of traditional and modern types of Huang Jiu (Chinese rice wine) by aroma extract dilution analysis and chemical analysis. Flavour Fragr. J. 2018, 33, 263–271. [Google Scholar] [CrossRef]
- Gao, W.; Fan, W.; Xu, Y. Characterization of the key odorants in light aroma type chinese liquor by gas chromatography-olfactometry, quantitative measurements, aroma recombination, and omission studies. J. Agric. Food Chem. 2014, 62, 5796–5804. [Google Scholar] [CrossRef]
- Guan, Q.; Meng, L.; Mei, Z.; Liu, Q.; Chai, L.; Zhong, X.; Zheng, L.; Liu, G.; Wang, S.; Shen, C. Volatile Compound Abundance Correlations Provide a New Insight into Odor Balances in Sauce-Aroma Baijiu. Foods 2022, 11, 3916. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Gao, X.; Shi, X.; Chen, S.; Xu, Y.; Tang, K. Comparison of the Aroma-Active Compounds and Sensory Characteristics of Different Grades of Light-Flavor Baijiu. Foods 2023, 12, 1238. [Google Scholar] [CrossRef]
- Zhao, P.; Qian, Y.; He, F.; Li, H.; Qian, M. Comparative Characterization of Aroma Compounds in Merlot Wine by LiChrolut-EN-Based Aroma Extract Dilution Analysis and Odor Activity Value. Chemosens. Percept. 2017, 10, 149–160. [Google Scholar] [CrossRef]
- Jiang, B.; Xi, Z.; Luo, M.; Zhang, Z. Comparison on aroma compounds in Cabernet Sauvignon and Merlot wines from four wine grape-growing regions in China. Food Res. Int. 2013, 51, 482–489. [Google Scholar] [CrossRef]
- Li, H.; Kong, W.; Zhou, X.; Zhang, W. Analysis and evaluation of aroma components in wine brewed with new variety of Cabernet Gernischt. China Brew. 2020, 39, 164–170. [Google Scholar] [CrossRef]
- Langos, D.; Granvogl, M.; Schieberle, P. Characterization of the key aroma compounds in two bavarian wheat beers by means of the sensomics approach. J. Agric. Food Chem. 2013, 61, 11303–11311. [Google Scholar] [CrossRef] [PubMed]
- Methner, Y.; Dancker, P.; Maier, R.; Latorre, M.; Hutzler, M.; Zarnkow, M.; Steinhaus, M.; Libkind, D.; Frank, S.; Jacob, F. Influence of Varying Fermentation Parameters of the Yeast Strain Cyberlindnera saturnus on the Concentrations of Selected Flavor Components in Non-Alcoholic Beer Focusing on (E)-beta-Damascenone. Foods 2022, 11, 1038. [Google Scholar] [CrossRef]
- Nicolotti, L.; Mall, V.; Schieberle, P. Characterization of Key Aroma Compounds in a Commercial Rum and an Australian Red Wine by Means of a New Sensomics-Based Expert System (SEBES)-An Approach To Use Artificial Intelligence in Determining Food Odor Codes. J. Agric. Food Chem. 2019, 67, 4011–4022. [Google Scholar] [CrossRef] [PubMed]
- Fechir, M.; Reglitz, K.; Mall, V.; Voigt, J.; Steinhaus, M. Molecular Insights into the Contribution of Specialty Barley Malts to the Aroma of Bottom-Fermented Lager Beers. J. Agric. Food Chem. 2021, 69, 8190–8199. [Google Scholar] [CrossRef]
- Kishimoto, T.; Noba, S.; Yako, N.; Kobayashi, M.; Watanabe, T. Simulation of Pilsner-type beer aroma using 76 odor-active compounds. J. Biosci. Bioeng. 2018, 126, 330–338. [Google Scholar] [CrossRef]
- Long, Y.; Tang, J.; Wang, X.; Shi, W.; Wu, D. Research progress on health factors and their enrichment pathways in Baijiu. China Brew. 2021, 41, 23–28. [Google Scholar] [CrossRef]
- Wu, T.; Zhu, S.; Sun, X.; Zhao, W.; Cui, G. Analysis of Health Factors of Meilanchun Sesame-flavor Liquor. Liquor-Mak. Sci. Technol. 2013, 8, 125–130. [Google Scholar]
- Huo, J.; Huang, M.; Sun, B.; Zheng, F.; Sun, J.; Sun, X.; Li, H. Research Progress in Functional Factors in Baijiu. Liquor-Mak. Sci. Technol. 2017, 9, 17–23. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, Y.; Zhou, Z.; Tang, Q. Study on Healthy & Functional Compositions in Jian’nanchun Liquor. Liquor-Mak. Sci. Technol. 2008, 5, 41–44. [Google Scholar]
- Gao, C. Study on Flavor Compounds and Biological Activity of Sesame Flavor Liquor. Master’s Thesis, Hebei University Of Technology, Wuhan, China, 2017. [Google Scholar]
- Fan, W.; Xu, Y. Review of Important Functional Compounds Terpenes in Baijiu. Liquor. Mak. 2013, 40, 11–16. [Google Scholar] [CrossRef]
- Cheng, F. Study on the Mechanism of Baijiu-Induced Liver Injuryand Regulation of Intestinal Flora Based on Omic Approaches. Ph.D. Thesis, Jiangnan University, Wuxi, China, 2019. [Google Scholar]
- Yan, H.; Zhao, Y.; Huan, D.; Zong, W.; Song, F. Research Progress in Analysis and Detection of Trace Harmful Components in Baijiu. Liquor-Mak. Sci. Technol. 2022, 10, 94–106. [Google Scholar]
- Piornos, J.A.; Balagiannis, D.P.; Methven, L.; Koussissi, E.; Brouwer, E.; Parker, J.K. Elucidating the Odor-Active Aroma Compounds in Alcohol-Free Beer and Their Contribution to the Worty Flavor. J. Agric. Food Chem. 2020, 68, 10088–10096. [Google Scholar] [CrossRef] [PubMed]
- Takoi, K.; Koie, K.; Itoga, Y.; Katayama, Y.; Shimase, M.; Nakayama, Y.; Watari, J. Biotransformation of hop-derived monoterpene alcohols by lager yeast and their contribution to the flavor of hopped beer. J. Agric. Food Chem. 2010, 58, 5050–5058. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cheng, P.; You, X.; Fan, Q.; Zhu, A.; Zhang, J. Research Progress in Functional Active Ingredients in Jiangxiang Baijiu. Liquor-Mak. Sci. Technol. 2023, 09, 109–113. [Google Scholar] [CrossRef]
- Hong, J.; Zhao, D.; Sun, B. Research Progress on the Profile of Trace Components in Baijiu. Food Rev. Int. 2023, 39, 1666–1693. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, Y.; Zhou, Z.; Tang, Q. Research of Functional Ingredients in Notable Chinese Liquor-JIANNANCHUN Liquor. Sichuan Food Ferment. 2008, 44, 24–27. [Google Scholar]
- Fu, H.; Chen, X.; Zhao, Y.; Chen, M.; Zhou, D.; Jia, W. Research progress on the component analysis in Baijiu. Food Ferment. Ind. 2021, 47, 320–327. [Google Scholar] [CrossRef]
- Du, P.; Jiao, G.; Zhang, Z.; Wang, J.; Li, P.; Dong, J.; Wang, R. Relationship between Representative Trace Components and Health Functions of Chinese Baijiu: A Review. Fermentation 2023, 9, 658. [Google Scholar] [CrossRef]
- Zhao, D.; Shi, D.; Sun, J.; Li, H.; Zhao, M.; Sun, B. Quantification and cytoprotection by vanillin, 4-methylguaiacol and 4-ethylguaiacol against AAPH-induced abnormal oxidative stress in HepG2 cells. RSC Adv. 2018, 8, 35474–35484. [Google Scholar] [CrossRef]
- Zhao, D.; Sun, J.; Sun, B.; Zhao, M.; Zheng, F.; Huang, M.; Sun, X.; Li, H. Intracellular antioxidant effect of vanillin, 4-methylguaiacol and 4-ethylguaiacol: Three components in Chinese Baijiu. RSC Adv. 2017, 7, 46395–46405. [Google Scholar] [CrossRef]
- Fujitaka, K.; Otani, H.; Jo, F.; Jo, H.; Nomura, E.; Iwasaki, M.; Nishikawa, M.; Iwasaka, T.; Das, D.K. Modified resveratrol Longevinex improves endothelial function in adults with metabolic syndrome receiving standard treatment. Nutr. Res. 2011, 31, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham Ul, H.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Sharma, K.; Kumar, V.; Kaur, J.; Tanwar, B.; Goyal, A.; Sharma, R.; Gat, Y.; Kumar, A. Health effects, sources, utilization and safety of tannins: A critical review. Toxin Rev. 2019, 40, 432–444. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, Z.; Tian, D.; Pan, L.; Liu, W. Research Progress on Healthy Functional Components of Nongxiangxing Baijiu. China Food Saf. Mag. 2022, 15, 110–113. [Google Scholar] [CrossRef]
- Zhu, Z.; Rao, J.; Zhang, J.; Li, D.; Wang, J.; Cai, F.; Zhang, R.; Xu, J. Research progress of health factors in Huangjiu. China Brew. 2021, 40, 26–31. [Google Scholar]
- Shi, X. Study on the Analysis and Detection of different Quality Baijiu and the Method of removing Harmful Substances. Master’s Thesis, Xiangtan University, Xiangtan, China, 2022. [Google Scholar]
- Vahdat-Lasemi, F.; Aghaee-Bakhtiari, S.H.; Tasbandi, A.; Jaafari, M.R.; Sahebkar, A. Targeting interleukin-beta by plant-derived natural products: Implications for the treatment of atherosclerotic cardiovascular disease. Phytother. Res. 2021, 35, 5596–5622. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, X.; Chen, C.; Mao, W. Systematic Investigation of Quercetin for Treating Cardiovascular Disease Based on Network Pharmacology. Comb. Chem. High Throughput Screen. 2019, 22, 411–420. [Google Scholar] [CrossRef]
- Jiang, C.; Sun, T.; Xiang, D.; Wei, S.; Li, W. Anticancer Activity and Mechanism of Xanthohumol: A Prenylated Flavonoid From Hops (Humulus lupulus L.). Front. Pharmacol. 2018, 9, 530. [Google Scholar] [CrossRef]
- Samuels, J.S.; Shashidharamurthy, R.; Rayalam, S. Novel anti-obesity effects of beer hops compound xanthohumol: Role of AMPK signaling pathway. Nutr. Metab. 2018, 15, 42. [Google Scholar] [CrossRef]
- Liou, S.; Nguyen, T.; Hsu, J.; Sulistyowati, E.; Huang, S.; Wu, B.; Lin, M.; Yeh, J. The Preventive Effects of Xanthohumol on Vascular Calcification Induced by Vitamin D3 Plus Nicotine. Antioxidants 2020, 9, 956. [Google Scholar] [CrossRef]
- Sun, X.; Shen, D.; Shi, T.; Cui, G. Research on decomposition of triglycerides activities and α-glycosidase inhibitory of sulfide and pyrazine compositions in sesame-flavor liquor. Liquor. Mak. 2014, 41, 56–59. [Google Scholar]
- Gao, C.; Tian, T.; Xin, Y. The activities of the extracts of zhimaxiang Baijiu (sesame-flavor liquor) and the 4 kinds of characteristic compounds. Liquor-Mak. Sci. Technol. 2015, 250, 61–64. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, F.; Guo, X.; Zhao, T.; Luo, Z.; Wu, D. Research progress on formation mechanism of health factor 2,3,5,6-tetramethylpyrazine in Baijiu. China Brew. 2023, 43, 27–33. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, T.; Huang, M.; Wei, J.; Wu, J.; Huo, J. Intracellular Antioxidant Activity of Two Terpenoids in Baijiu. Food Sci. Nutr. 2020, 41, 66–73. [Google Scholar] [CrossRef]
- Henriques, J.F.; Serra, D.; Dinis, T.C.P.; Almeida, L.M. The Anti-Neuroinflammatory Role of Anthocyanins and Their Metabolites for the Prevention and Treatment of Brain Disorders. Int. J. Mol. Sci. 2020, 21, 8653. [Google Scholar] [CrossRef]
- Huang, W.; Liu, Y.; Wang, J.; Wang, X.; Li, C. Anti-Inflammatory Effect of the Blueberry Anthocyanins Malvidin-3-Glucoside and Malvidin-3-Galactoside in Endothelial Cells. Molecules 2014, 19, 12827–12841. [Google Scholar] [CrossRef]
- Pu, J.; Wang, M.; Chen, J.; Wang, M. Simultaneous detection of amide herbicides and their intermediates in beerby solid-phase extraction coupled with gas chromatography-massspectrometry. J. Food Saf. Qual. 2018, 9, 1369–1376. [Google Scholar] [CrossRef]
- Lv, H.; Geng, D.; Xie, H.; Guo, C.; Cai, Y.; Yang, G. Optimization of detection method and migration amount for phthalate acid esters in beer. China Brew. 2021, 40, 157–162. [Google Scholar] [CrossRef]
| Type of LPME | Sample | Quality Factor | Analyte | Extractant | Ext. Solvent Volume | Sample Volume a | Extract Time b | Auxiliary Equipment | Detection System | LOD | LOQ | Linear Range | RSD/% | Recovery /% | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VS-LLME | Baijiu | harmful components | 14 Phthalate esters | tetrachloroethylene | 500 μL | ++ | + | Emulsifier (Tween 20), vortex | GC-MS | 0.05–10.0 μg/kg | 0.125–20.0 μg/kg | - | 0.1–6.2 | 83.4–122.3 | [44] |
| Baijiu | Aroma | roasted and mud-like aroma | CCl4, ether, CH2Cl2 | 250 μL | ++ | + | Emulsifier (Tween 20, Cetyl-trimethylammonium bromide), vortex | GC-MS | 0.1–88.9 μg/L | - | 0.005–450 μg/L | - | 72–120.9 | [55] | |
| VA-LLME | Baijiu | aroma | volatile compounds | dichloromethane | 1 mL | ++ | + | vortex | GC-MS, GC-O-MS | - | - | - | - | - | [56] |
| SA-LLME | wine | harmful components | biogenic amines | ethyl acetate | 50 μL | + | ++ | salting-out | GC-MS | 1.5–8.1 μg/L | 5.0–27 μg/L | - | 2.3–10.4 | 84–106 | [57] |
| LLME | beer | harmful components | biogenic amines | supramolecular solvent (mixture of 1-Dodecanol and tetrahydrofuran | 600 μL | +++ | ++ | ultrasonic–microwave synergistic | HPLC | 0.004–0.06 μg/L | 0.013–0.2 μg/L | 0.1–2.0 × 105 μg/L | 1.2–2.1 | 96.28–102.56 | [58] |
| VA-DLLME | beer | harmful components | phthalates and adipates. | n-hexane | 200 μL | +++ | + | Ethano, vortex | GC-MS/MS | 0.3–1.5 μg/L | 1–5 μg/L | - | 1.72–20.4 | - | [59] |
| Wine | harmful components | four sulfonylurea herbicides | The ionic liquid (1-hexyl-3-methylimidazolium hexafluorophosphate) | 80 mg | ++ | + | Methanol, vortex | capillary liquid chromatography (CLC) | 3.2 × 10−6–6.6 × 10−6 | 10–22 μg/kg | 11–450 μg/kg | 0.6–6.9 | 80–106 | [60] | |
| huangjiu | harmful components | ochratoxin A | The ionic liquid (1-hexyl-3-methylimidazolium hexafluorophosphate ([HMIM] [PF6])) | 100 μL | ++ | ++ | Ethanol, vortex | HPLC | 0.04 μg/L | 0.1 μg/L | - | 2.7–10.4 | 76–82.1 | [61] | |
| wine | harmful components | 6 biogenic amines | trihexyltetradecylphosphonium tetrachlorocobalt (II) [P6,6,6,14+]2[CoCl42−] | 20 mg | ++ | +++ | methanol, vortex | HPLC-UV | 1.3–3.9 μg/L | 4.1–9.9 μg/L | 10–1000 μg/L | <4.9 | 93.2–103.1 | [62] | |
| PCA-DLLME | Baijiu | harmful components | Triazole fungicides | green medium-chain fatty acid (decanoic acid) | 175 μL | ++ | + | Popping candy | HPLC-DAD | 8.1–11.2 μg/L | 27.1–37.3 μg/L | 27.1–1000 μg/L | 1.1–7.1 | 80.8–102.5 | [63] |
| UA-DLLME | wine | harmful components | Cd, As | deep eutectic solvent (DL-lactic acid/trioctylmethylammonium chloride-based) | 400 μL | ++ | +++ | Methanol, vortex, sonicate | FAAS | 0.08, 0.3 μg/L | 0.25, 1.0 μg/L | 0.5–8, 2–50 μg/L | 2.9–4.5 | 90.6–103.6 | [38] |
| wine | illegal additives | melatonin | dichloromethane | 250 μL | ++ | +++ | ultrasound | HPLC-DAD | 0.23 μg/L | 0.7 μg/L | 0.70 μg/L–15 mg/L | 0.4–1.1 | 92–103 | [64] | |
| EVA-DLLME | wine | harmful components | organophosphate insecticides (malathion, diazinon, phosalone) | hexanol and dichloromethane mixture | 400 μL | +++ | + | dichloromethane evaporate | HPLC-MS/MS | 3 × 10−10–3 × 10−7 g/L | - | 10−9–10−2 g/L | - | 92–103 | [65] |
| UD-SA-DLLME | wine | harmful components | fungicides | 1-octanol | 11 μL | ++ | ++ | shaker | GC-MS | 0.007–0.025 μg/L | 0.024–0.082 μg/L | 0.05–100 μg/L | <12 | 83–108 | [66] |
| DLLME | wine | harmful components | 19 pesticides | L-menthol and butylated hydroxytoluene | 150 μL | +++ | ++ | endogenous ethanol | HPLC-MS | 7 × 10−10~1.6 × 10−6 g/L | 0.0024–5.0 μg/L | - | 3–14 | 56–100 | [34] |
| wine | harmful components | biogenic amines (BAs) | chloroform | 400 μL | ++ | ++ | methanol | GC-MS | 1.4–4.2 μg/L | 4.6–12.6 μg/L | - | 4–12 | 76–105 | [67] | |
| White wine | aroma | terpenes | dichloromethane | 500 μL | ++ | + | acetone | GC-MS | 5.6–11.3 g/L | 18.7–37.6 g/L | 10–200 μg/L | 3.3–19.4 | 97.9–105.3 | [68] | |
| wine | harmful components | Cd/Pb | 1-Butyl-3-methyl-imidazolium hexafluorophosphate | 150 mg | ++ | ++ | methanol | ETAAS | 0.01/0.08 μg/L | - | - | - | 96–100 | [69] | |
| huangjiu | aroma | Higher alcohols | dichloromethane | 600 μL | ++ | + | acetonitrile | GC-MS | 0.14–1.04 mg/L | 0.47–3.45 mg/L | 1.39–309.8 mg/L | 1.4–9.3 | 80–124 | [70] | |
| beer | harmful components | Cu | CCl4 | 100 μL | ++ | No used | FAAS | 3.2 μg/L | 9.1 μg/L | - | 3–16 | 92–116 | [71] | ||
| wine | harmful components | biogenic amines | chloroform | 400 μL | ++ | + | methanol | GC-MS | 1.1–4.1 μg/L | 3.3–12.3 μg/L | - | 2–13 | 77–105 | [72] | |
| wine | Aroma | vinylphenols and ethylphenols | trichloroethylene | 60 μL | ++ | + | acetone | GC-MS | 1.5–3.9 μg/mL | 1–5 μg/mL | - | - | 70–113 | [73] | |
| Wine | harmful components | Cu, Fe | 1,2-dichlorobenzene | 40 μL | +++ | - | methanol | FAAS | 2.4, 6.3 μg/L | 7.2, 19 μg/L | - | - | 89–113 | [74] | |
| Beer, alcoholic beverage | harmful components | Aliphatic aldehydes | DES (hexafluoroisopropanol and menthol/thymol) | 100 μL | ++ | + | acetonitrile | HPLC-UV | 0.1–0.5 μg/L | 0.2–1.0 μg/L | - | 1.1–5.3 | 77.3–119 | [75] | |
| DLLME-SFO | Wine | harmful components | pesticide residues (fipronil, fipronil-sulfide, fipronil-sulfone, and boscalid) | 1-dodecanol | 100 μL | ++ | + | natural deep eutectic solvents (NADESs): glucose, anhydrous, citric acid anhydrous, lactic acid | HPLC-UV | 0.8–1.3 μg/L | 2.7–4.4 μg/L | 2.7–200 μg/L | 1.0–12.4 | >80 | [76] |
| wine | Appearance factor, Taste, Functional components | phenolic acids (gallic acid and protocatechuic acid) | 1-dodecanol | 50 μL | ++ | ++++ | tetrahexylammonium bromide (ion-pairing technique) | LC using a coreshell particle column | 0.005–0.1 g/L | 0.01–0.30 g/L | 0.01–15.00 g/L | 0.18–9.33 | 77.2–117 | [77] | |
| SD-DLLME | wine | harmful components | 30 fungicides | 1-octanol | 100 μL | +++ | + | Acetonitrile, vortex | HPLC-MS/MS | 0.03–0.06 μg/L | 0.1–0.2 μg/L | - | 4–22 | 70–117 | [78] |
| SDME | wine | harmful components | ethyl carbamate | butyl acetate | 2 μL | + | ++ | microsyringe | GC-MS | 1.5 ng/mL | 5 ng/mL | 2–1000 ng/mL | <5 | 94.9–99.9 | [79] |
| SDME | wine | harmful components | eighteen pesticide residues | toluene | 10 μL | +++ | ++++ | microsyringe | GC-MS | 0.1–4.62 μg/L | 1.78–18.6 μg/L | 0.25–25 μg/L | - | 5–120 | [80] |
| HS-SDME | wine | harmful components | Methanol | N, N-dimethylformamide | 2 μL | +++ | ++ | stirring magnet | GC-FID | 0.001 mg/L | 0.003 mg/L | 0.05–2.0 mg/L | 1.9–4.7 | 83.99–117.24 | [81] |
| ETA-ME | water, juice, wine, and vinegar samples | harmful components | strobilurin fungicides | Deep eutectic solvents (thymol with octanoic acid) | 120 μL | ++ | - | effervescence tablet (sodium bicarbonate and citric acid) | HPLC | 0.15–0.38 μg/L | 0.49–1.25 μg/L | - | 1.0–8.6 | 77.4–106.9 | [82] |
| LPME | Wine | harmful components | 47 multiclass pesticides | toluene | 70 μL | +++ | ++++ | vortex | GC-MS | 2.29–533 ng/L | 7.63–1776 ng/L | - | <10.2 | 81.7–119 | [83] |
| UA-LPME | Wine and food sample | FunctionComponents, pigment | quercetin | DES (tetrabutyl ammonium chloride and ethyl glycol) | 450 μL | +++ | + | Tetrahydrofuran, ultrasound bath | spectrophotometry | 6.1 μg/L | 20 μg/L | 20–850 μg/L | 1.9 | 98.5 | [84] |
| Type of SPME | Material | Sample | Quality Factor | Analyte | Sample Volume a | Extract Time b | Detection System | LOD | LOQ | Linear Range | RSD/% | Recovery/% | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High-throughput and automated SPME | HLB (co-polymer of hydrophilic N-vinylpyrrolidone and lipophilic divinylbenzene), (particle size: 5 μm) at length of 1 mm and thickness of 10 μm on both sides of the blade | Beer | Harmful components | Mycotoxins | ++ | +++ | LC-MS | 0.02–3 ng/mL | 0.05–10 ng/mL | 0.1–200 ng/mL | <13 | 79–121 | [99] |
| HS-SPMESH | PDMS sheet (12.5 cm × 0.5 mm × 8.5 cm) | Wine | Aroma | Volatile phenols | +++ | ++ | DART-MS | <1 μg/L | 6 μg/L | 6–250 μg/L | 5–6 | 86–102 | [100] |
| MHS-SPME | DVB/CAR/PDMS, 50/30 μm | Wine | Aroma | 23 aroma compounds | ++ | ++++ | GC-MS | <1 μg/L | <1 μg/L | 0.001–50 mg/L | <5 | >95 | [101] |
| ER-SPME | Anion-exchange monolith (AEM) (the pore sizes are around 450 nm) | Various aqueous and wine samples | Harmful component, flavor | F−, Cl−, NO2−, NO3−, Br−, BrO3− | ++++ | +++ | IC/CD | 0.015–1.5 μg/L | 0.051–4.95 μg/L | 0.1–500 μg/L | 1.4–9.1 | 83.2–115 | [102] |
| CF-SPME | PDMS/DVB, 65 μm | Beer | Harmful components | polycyclic aromatic hydrocarbons (PAHs), and their nitrated (nitro-PAHs) and oxygenated (oxy-PAHs) derivatives | + | +++ | GC-MS | 0.003–0.128 μg/L | 0.011–0.427 μg/L | - | 3.0–18.7 | 80.1–100.3 | [103] |
| DI-HS-SPME | PDMS/DVB 75 μm, including 65 μm coating + 10 μm overcoating, length: 1 cm | wine | Wine quality | Volatile compounds | ++ | ++ | GC-MS | - | - | - | - | - | [104] |
| TF-SPME | PDMS was used to deplete non-polar and other compounds HLB/PDMS for the direct microextraction of the remaining compounds | Beer | - | polar and low volatility compounds | - | ++ | GC-MS | - | - | - | - | - | [105] |
| HS-SPME | PDMS, 100 μm | wine | aroma | volatile organic compounds | ++ | ++ | GC-MS | - | - | - | - | - | [106] |
| divinybenzene/carboxen/polydimethy-lsiloxane (DVB/CAR/PDMS), 50/30 μm | Baijiu | aroma | aroma-active compounds | +++ | +++ | GC-O-MS | - | - | - | - | - | [107] | |
| DVB/CAR/PDMS, 50/30 μm | wine | aroma | volatile organic compounds | ++ | +++ | GC-MS | - | - | - | - | - | [108] | |
| zeolitic imidazole framework-67 (thickness of the coating, which is around 15 μm) | Beer, vodka | harmful components, aroma | Some alcohols | +++ | +++ | GC-MS, GC-FID | 0.17 μg/L | - | 0.5–100.0 μg/L | 6.8–9.6 | 67.5–108 | [109] | |
| PDMS/DVB, 65 μm | tequila | aroma | terpenes | + | ++ | GC-MS | 2.0–8.1 ng/mL | 6.3–10.33 ng/mL | 50–1000 ng/mL | <10 | - | [110] | |
| DVB/CAR/PDMS | Baijiu | Authenticity (aroma types and geographical origin) | volatile compounds | + | + | GC-MS/MS | - | - | - | - | - | [111] | |
| DVB/CAR/PDMS, 50/30 μm | nongrape wine | aroma | lactones and volatile phenols | ++ | ++ | GC-Orbitrap-MS | 0.003–37.44 μg/L, 0.02–104.28 μg/L | 0.01–124.8 μg/L, 0.05–347.6 μg/L | - | 8.2–18.56 | 80–119 | [112] | |
| DVB/CAR/PDMS, 50/30 μm | Baijiu | Authenticity (age-markers) | Volatile compounds | ++ | +++ | GC-MS | - | - | - | - | - | [113] | |
| DVB/CAR/PDMS, 50/30 μm | Wine | harmful components, taste | 9 Multihalo- Phenols and Anisoles | ++ | +++ | GC-MS/MS | 3–30 ng/L | 10–100 ng/L | 10–10,000 ng/L | 2.8–19.4 | 75.2–119.8 | [114] | |
| DVB/CAR/PDMS, 50/30 μm | Wine | Authenticity (geographical origin) | volatile fraction | ++ | ++ | FM GC×GC-TOFMS | - | - | - | - | - | [115] | |
| SPME | DVB/PDMS, 65 μm | Baijiu | Authenticity | volatile components | - | + | GC-MS | - | - | - | - | - | [116] |
| multi-SBSE | PDMS and polyethyleneglycol-modified silicone (EG-Silicone), 1 cm × 1 mm | botrytized wines | Authenticity (geographic origin) | volatile organic compounds | ++ | ++++ | GC-GC | - | - | - | - | - | [117] |
| dual sequential-SBSE | PDMS | Wine | Flavor | volatile composition | +++ | +++ | GC-MS | - | - | - | - | - | [118] |
| PDMS (10 mm × 0.5 mm) | wine | Flavor | volatile and semivolatile compounds. | +++ | ++++ | GC-MS | - | - | - | - | - | [119] | |
| PDMS (10 mm × 0.5 mm) | wine | aroma | volatile compositions | +++ | +++ | GC-MS | - | - | - | - | - | [120] | |
| SPE+SBSE | PDMS (20 mm × 1 mm; length film thickness) | wine | aroma | limonene-derived monoterpenes | ++++ | +++ | GC-MS | 2–45 ng/L | 8–150 ng/L | - | 3.9–18.1 | 83–120 | [121] |
| SBSE with thermal desorption | PDMS (10 mm × 0.5 mm) | huangjiu | Authenticity (Geographic Origin and Age | volatile compounds | +++ | ++ | GC-MS | - | - | - | - | - | [122] |
| PDMS (10 mm × 0.5 mm × 24 μL) | Baijiu | aroma | Volatile Compounds | +++ | ++++ | GC-MS | 0.007–17.89 μg/L | 0.02–69.6 μg/L | - | 0.2–7.0 | 76.3–105.6 | [123] | |
| SBSE | PDMS, 10 mm | wine | aroma | Methoxypyrazines | ++ | ++ | GC-MS/MS | 0.25 ng/L | 0.5 ng/L | - | 0.44–19 | 92–108 | [124] |
| ethylene glycol-silicone (EG) | Brandy | Taste, aroma | Lactones | +++ | +++ | GC×GC-TOFMS | - | - | - | - | - | [125] | |
| PDMS, 10 mm/0.5 mm | medicinal liquor | aroma | Volatile compounds | ++++ | +++ | GC-MS | - | - | - | - | - | [126] | |
| PDMS, 10 × 0.5 mm (length × film thickness) | Baijiu | Aroma, Functional components | active-aroma compounds and amino acids | ++++ | +++ | GC-O, GC-FID, GC-MS | - | - | - | - | - | [127] | |
| QuEChERS | Extract: ethyl acetate Cleanup: Primary secondary amine (PSA), 40 μm | wine | harmful components | 13 fungicide residues | +++ | ++ | LC-MS/MS | 0.0003 mg/kg | 0.001–0.003 mg/kg | 1–50 ng/mL | 3.45–6.14 | 80.56–97.85 | [128] |
| Extract: acetonitrile Cleanup: PSA, 40 μm | wine | harmful components | pesticide residues | +++ | ++ | UHPLC-Orbitrap-MS | 0.7–21.5 μg/kg | 2.5–72 μg/kg | - | <11 | 70–120 | [129] | |
| Extract: 10 mL acetonitrile containing 1% (v/v) acetic acid Cleanup: PSA (125 mg) and C18 (250 mg) | wine | harmful components | 97 pesticides | ++ | + | UHPLC-MS/MS | 3.0-6.0 μg/L | 10-20 μg/L | - | <20 | 70–120 | [130] | |
| Extract: acetonitrile containing 1% (v/v) acetic acid Cleanup: 50 mg C18 | Beer | harmful components | Pesticides | +++ | ++ | GC-MS/MS | 0.0001–0.0007 μg/mL | 0.001–0.006 μg/mL | 0.001–2.5 μg/mL. | 0.3–10.5 | 70–123 | [131] | |
| Extract: 10 mL acetonitrile Cleanup: 150 mg PSA | wine | harmful components | over 131 pesticides | +++ | + | GC-μECD, GC-NPD | - | 0.009–0.023 mg/kg | 0.009 and 0.023 mg/kg | ≤20 | 72–113 | [132] | |
| MEPS | Extract:PEP (Polar Enhanced Polymer) Elution:100 μL of 50% MeOH. | wine | aroma | Sotolon | + | - | UHPLC-PDA | 0.45–2.51 μg/L | 1.49–8.36 μg/L | 10–800 mg/L | <5.6 | >81 | [133] |
| Extract: C8 Elution: 200 μL MeOH: H2O (95:5, v/v) | wine | harmful components | furanic derivatives | + | - | UHPLC-PDA | 4.5–129.3 ng/L | 14.9–431.0 ng/L | - | <5 | 74–97 | [134] | |
| Extract: C8 Elution: 100 μL dichloromethane | wine | harmful components | ethyl carbamate | + | - | GC-MS | 1.5 μg/L | 4.5 μg/L | 5–400 μg/L | <7 | 97–106 | [135] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Y.; Deng, Q.; Zhang, Y.; Sun, B.; Li, W.; Dong, W.; Sun, X. Applications of Microextraction Technology for the Analysis of Alcoholic Beverages Quality: Current Perspectives and Future Directions. Foods 2025, 14, 1152. https://doi.org/10.3390/foods14071152
Qiu Y, Deng Q, Zhang Y, Sun B, Li W, Dong W, Sun X. Applications of Microextraction Technology for the Analysis of Alcoholic Beverages Quality: Current Perspectives and Future Directions. Foods. 2025; 14(7):1152. https://doi.org/10.3390/foods14071152
Chicago/Turabian StyleQiu, Yue, Qi Deng, Yongqing Zhang, Baoguo Sun, Wenxian Li, Wei Dong, and Xiaotao Sun. 2025. "Applications of Microextraction Technology for the Analysis of Alcoholic Beverages Quality: Current Perspectives and Future Directions" Foods 14, no. 7: 1152. https://doi.org/10.3390/foods14071152
APA StyleQiu, Y., Deng, Q., Zhang, Y., Sun, B., Li, W., Dong, W., & Sun, X. (2025). Applications of Microextraction Technology for the Analysis of Alcoholic Beverages Quality: Current Perspectives and Future Directions. Foods, 14(7), 1152. https://doi.org/10.3390/foods14071152





