Chemistry of Mezcal: Volatile Profile of Artisanal Mezcal Made from Wild Agaves of Oaxaca
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Mezcal Samples
2.3. Physical Properties of the Samples
2.3.1. Viscosity
2.3.2. Density and Alcohol Content by Volume
2.3.3. Total Soluble Solids
2.4. Determination of Volatile Organic Compounds by HS-SPME and GC-MS
2.5. Quantification of Main Volatile Organic Compounds
2.6. Statistical Analysis
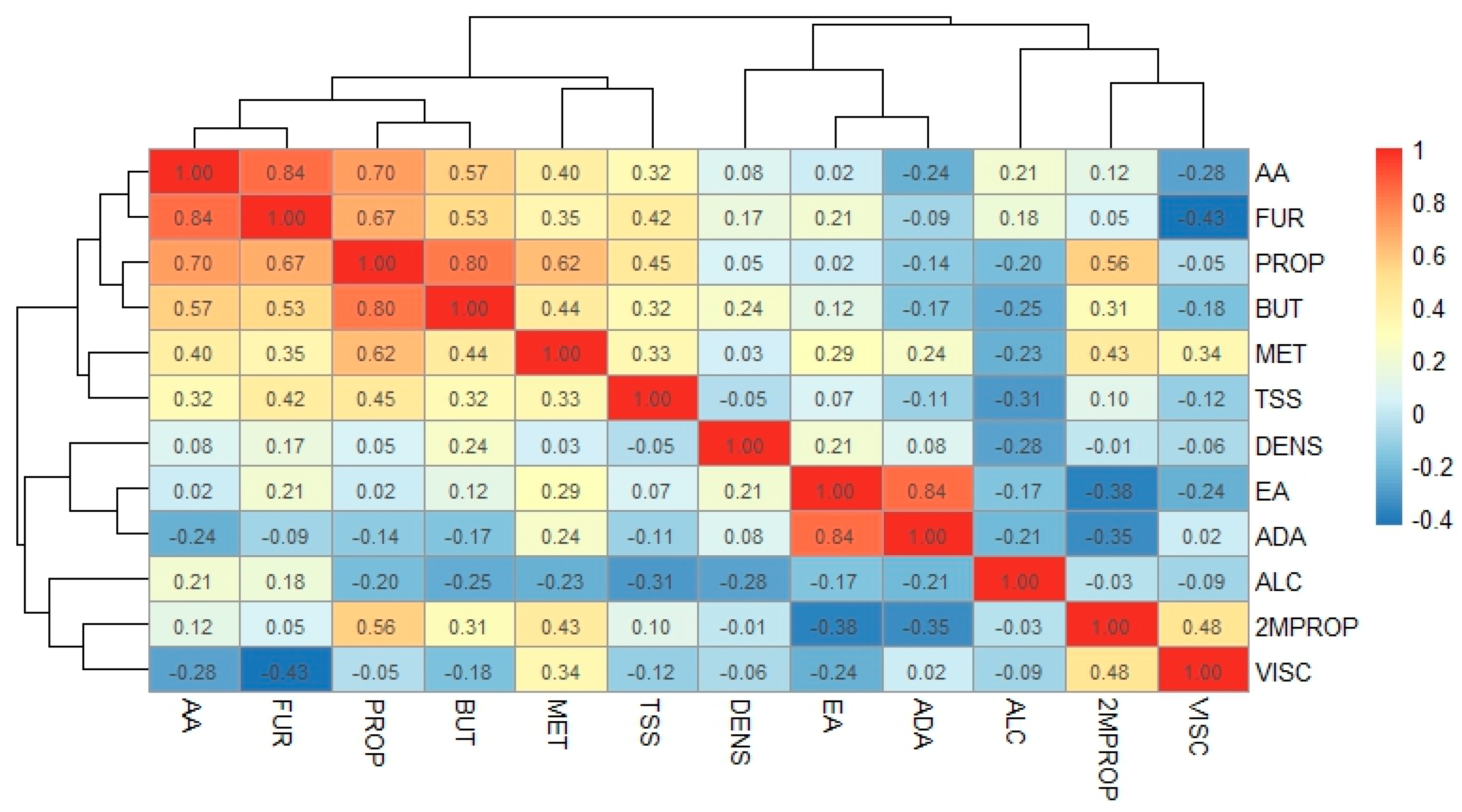
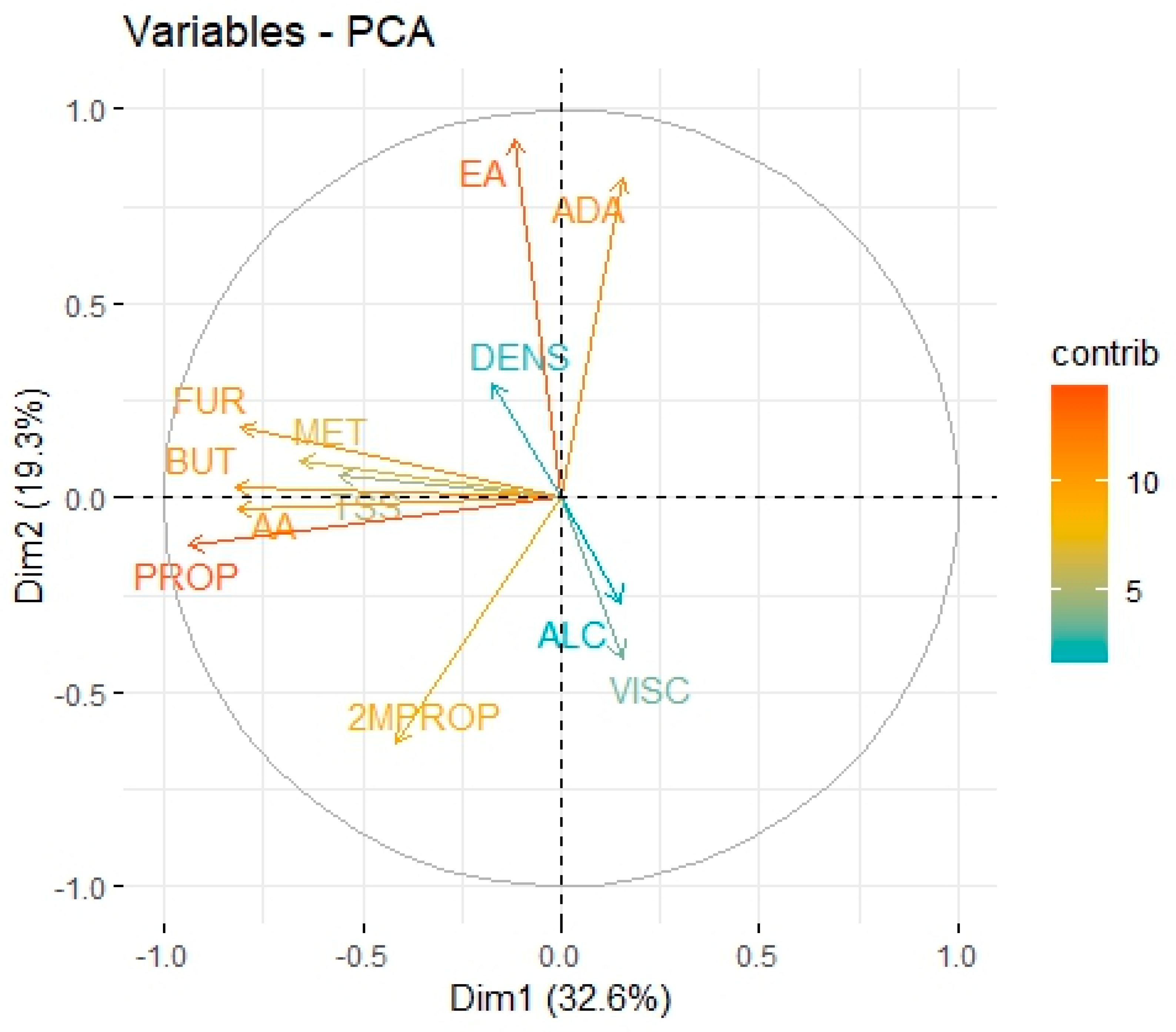
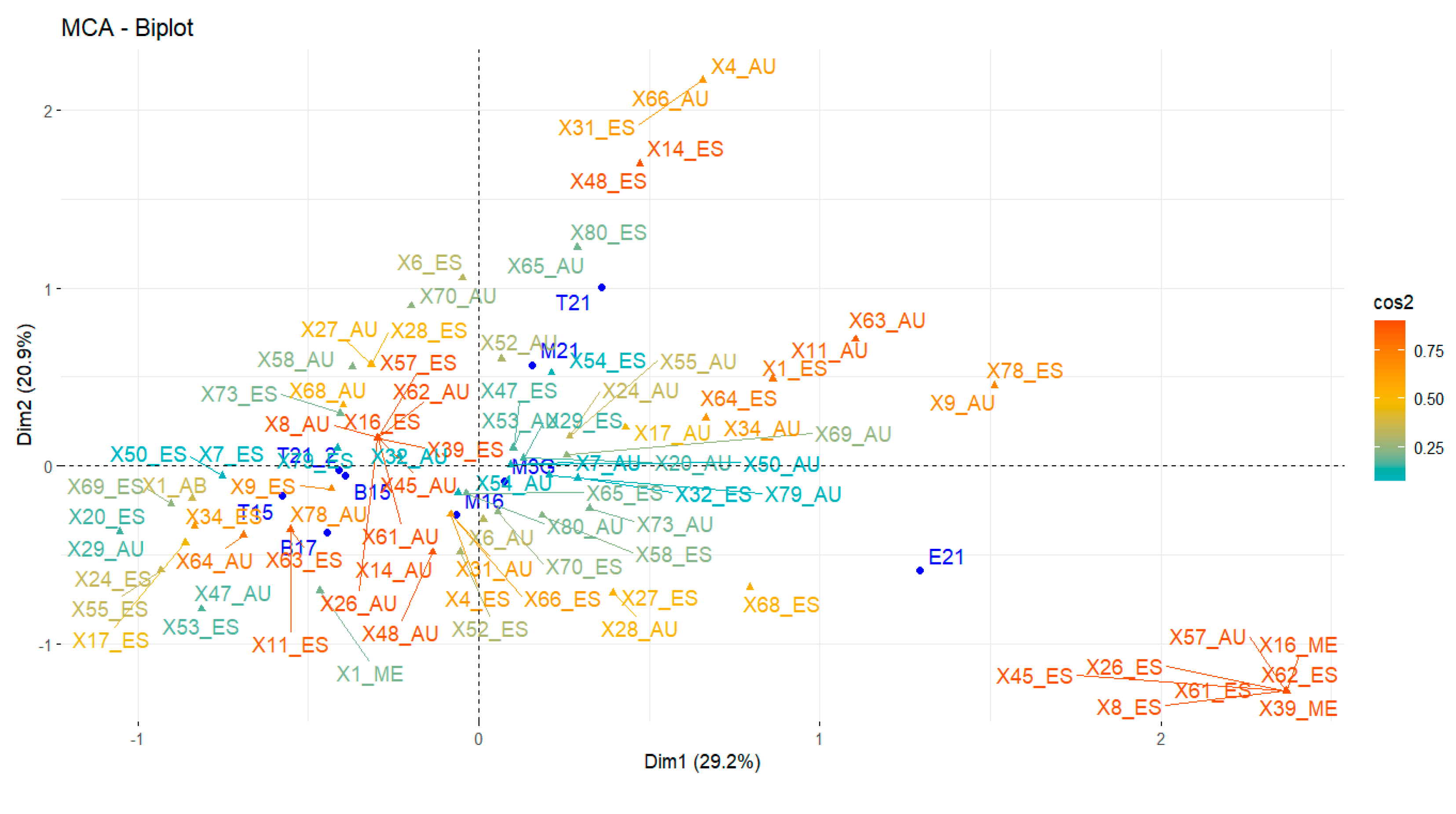
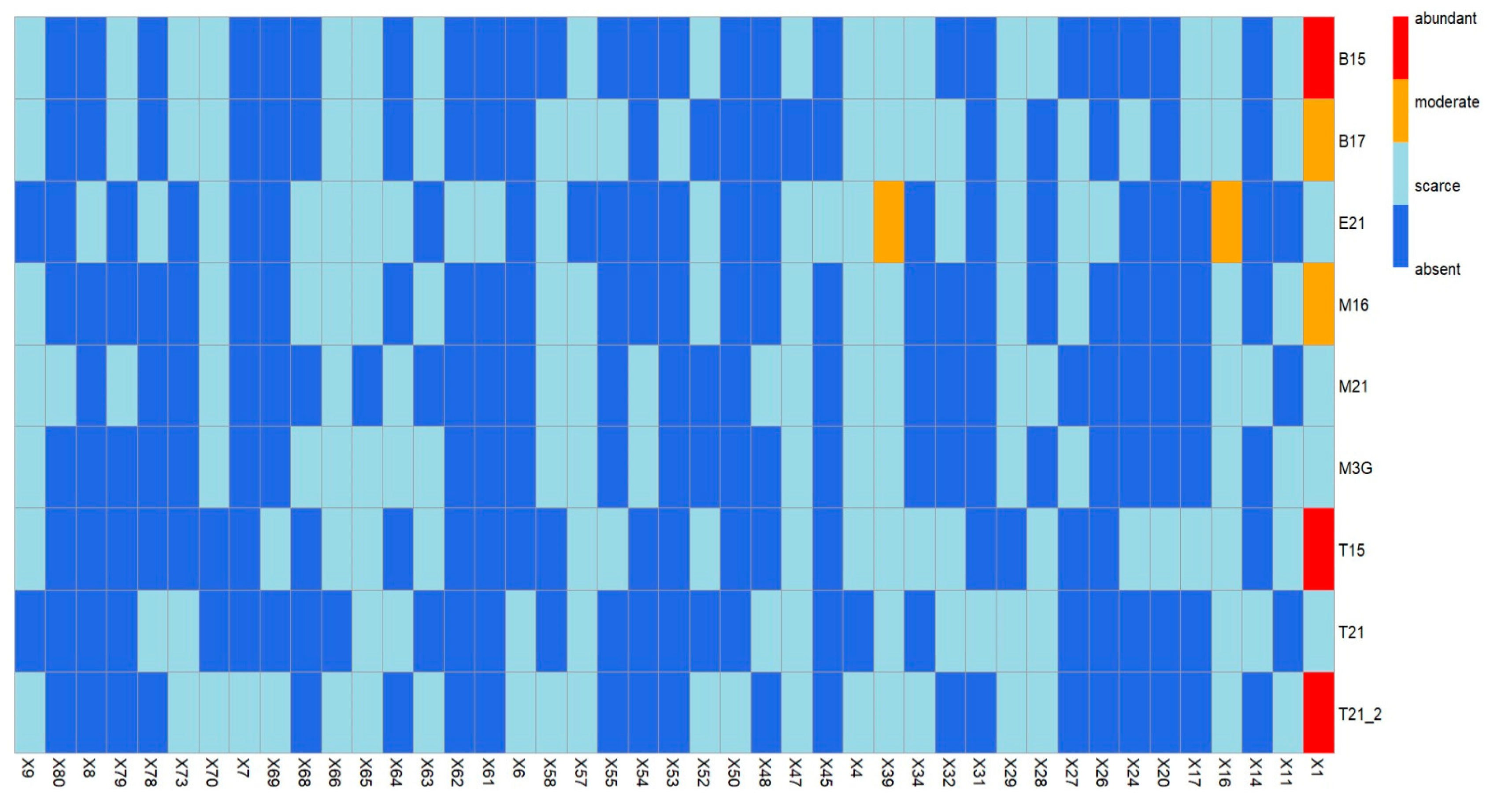
3. Results and Discussion
Volatile Compounds and Physical Properties of Mezcals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Consejo Mexicano Regulador de la Calidad del Mezcal. Informe Estadístico 2024; COMERCAM: Oaxaca, Mexico, 2024; Available online: https://comercam-dom.org.mx/wp-content/uploads/2024/04/PUBLICO_INFORME_2024.pdf (accessed on 1 January 2025).
- Arellano-Plaza, M.; Paez-Lerma, J.B.; Soto-Cruz, N.O.; Kirchmayr, M.R.; Gschaedler Mathis, A. Mezcal production in Mexico: Between tradition and commercial exploitation. Front. Sustain. Food Syst. 2022, 6, 832532. [Google Scholar] [CrossRef]
- López, M.G. Tequila Aroma. In Flavor Chemistry of Ethnic Foods; Shahidi, F., Ho, C., Eds.; Kluwer Academic: New York, NY, USA; Plenum Publishers: New York, NY, USA, 1999; pp. 211–217. [Google Scholar]
- Cole, V.C.; Noble, A.C. Flavor chemistry. In Fermented Beverage Production; Lea, A.G.H., Piggott, J.R., Eds.; Kluwer Academic: New York, NY, USA; Plenum Publishers: New York, NY, USA, 2003; pp. 393–396. [Google Scholar]
- Vera-Guzmán, A.M.; López, M.G.; Beristain, C.I. Composición volátil de mezcales elaborados con Agave angustifolia y Agave potatorum. Rev. Fitotec. Mex. 2009, 32, 297–302. Available online: https://revistafitotecniamexicana.org/documentos/32-4/3a.pdf (accessed on 1 January 2025).
- Sánchez-Álvarez, A.; Luna-Moreno, D.; Silva-Hernández, O.; Rodríguez-Delgado, M.M. Application of SPR method as an approach to gas phase sensing of volatile compound profile in mezcal spirits conferred by agave species. Chemosensors 2023, 11, 70. [Google Scholar] [CrossRef]
- Vázquez-Pérez, N. Variación Morfológica y Genética de Agave Karwinskii (Agavaceae) en Los Estados de Oaxaca y Puebla. Master’s Thesis, Universidad Nacional Autónoma de México, Mexico City, Mexico, 2015. [Google Scholar]
- Vera-Guzmán, A.M.; Guzmán-Gerónimo, R.I.; López, M.G.; Chávez-Servia, J.L. Volatile compound profiles in mezcal spirits as influenced by agave species and production processes. Beverages 2018, 4, 9. [Google Scholar] [CrossRef]
- Pérez Hernández, E.; Chávez Parga, M.C.; González Hernández, J.C. Review of agave and mezcal. Rev. Colomb. Biotecnol. 2016, 18, 148–164. [Google Scholar] [CrossRef]
- López, M.G.; Guevara, Y. Authenticity of three mexican alcoholic beverages by SPME-GCMS. In Proceedings of the Annual Meeting of Institute of Food Technologists, New Orleans, LA, USA; 2001; pp. 10–13. [Google Scholar]
- De León-Rodríguez, A.; González-Hernández, L.; Barba de la Rosa, A.P.; Escalante-Minakata, P.; López, M.G. Characterization of volatile compounds of mezcal, an ethnic alcoholic beverage obtained from Agave salmiana. J. Agric. Food Chem. 2006, 54, 1337–1341. [Google Scholar] [CrossRef]
- Barbosa-García, O.; Ramos-Ortiz Maldonado, J.L.; Pichardo-Molina, J.L.; Meneses-Nava, M.A.; Landgrave, J.E.A. UV–vis absorption spectroscopy and multivariate analysis as a method to discriminate tequila. Spectroschim. Acta Part A Mol. Biomol Spectrosc. 2007, 66, 129–134. [Google Scholar] [CrossRef]
- Fraustro-Reyes, C.; Medina-Gutierrez, C.; Sato-Berru, R.; Sahagún, L.R. Qualitative study of ethanol content in tequilas by Raman spectroscopy and principal component analysis. Spectroschim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 2657–2662. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Richling, E.; López, M.G.; Frank, W.; Scheier, P. Multivariate analysis of FTIR and ion chromatographic data for the quality control of tequila. J. Agric. Food Chem. 2005, 53, 2151–2157. [Google Scholar] [CrossRef]
- Bauer-Christoph, C.; Christoph, N.; Aguilar-Cisneros, B.O.; López, M.G.; Richling, E.; Rossmann, A. Authentication of tequila by gas chromatography and stable isotope ratio analyses. Eur. Food Res. Technol. 2003, 217, 438–443. [Google Scholar] [CrossRef]
- Peña-Alvarez, A.; Díaz, L.; Medina, A.; Labastida, C.; Capella, S.; Vera, L.E. Characterization of three agave species by gas chromatography and solid-phase microextraction-gas chromatography–mass spectrometry. J. Chromatogr. A 2004, 1027, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Ceballos-Magana, S.G.; Jurado, J.M.; Martin, M.J.; Pablos, F. Quantitation of twelve metals in tequila and Mezcal spirits as authenticity parameters. J. Agric. Food Chem. 2009, 57, 1372–1376. [Google Scholar] [CrossRef]
- Molina-Guerrero, J.A.; Botello-Álvarez, J.E.; Estrada-Baltazar, A.; Navarrete-Bolaños, J.L.; Jiménez-Islas, H.; Cárdenas-Manriquez, M.; Rico-Martínez, R. Compuestos volátiles en el mezcal. Rev. Mex. Ing. Química 2007, 6, 41–50. Available online: https://www.redalyc.org/articulo.oa?id=62060106 (accessed on 1 January 2025).
- Balcerek, M.; Pielech-Przybylska, K.; Dziekońska-Kubczak, U.; Patelski, P.; Strąk, E. Fermentation results and chemical composition of agricultural distillates obtained from rye and barley grains and the corresponding malts as a source of amylolytic enzymes and starch. Molecules 2016, 21, 1320. [Google Scholar] [CrossRef] [PubMed]
- Gschaedler, A.; Ramírez, J.; Díaz, D.; Herrera, E.; Arrizón, J.; Pinal, L.; Arellano, M. Fermentación. In Ciencia y Tecnología del Tequila Avances y Perspectivas; CIATEJ: Guadalajara, Mexico, 2004; pp. 61–120. [Google Scholar]
- Nykänen, L.; Suomalainen, H. Aroma of Beer, Wine and Distilled Alcoholic Beverages; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1983; Volume 3. [Google Scholar]
- Huang, X.; Cadwallader, K.R. A critical review of the flavor chemistry of tequila. In Chemistry of Alcoholic Beverages; Flynn, N., Ed.; ACS: Washington, DC, USA, 2023; Volume 1455, pp. 1–36. [Google Scholar] [CrossRef]
- Berry, D.R.; Watson, D.C. Production of organoleptic compounds. In Yeast Biotechnology; Berry, D.R., Russell, I., Stewart, G.G., Eds.; Springer: Dordrecht, The Netherlands, 1987; pp. 345–368. [Google Scholar]
- Pretorius, I.S. Tailoring wine yeast for the new millennium: Novel approaches to the ancient art of winemaking. Yeast 2000, 16, 675–729. [Google Scholar] [CrossRef]
- Arrizon, J.; Gschaedler, A. Effects of the addition of different nitrogen sources in the tequila fermentation process at high sugar concentration. J. Appl. Microbiol. 2007, 102, 1123–1131. [Google Scholar] [CrossRef]
- Devi, M.L.; Singh, N.B.; Sharma, K.C.; Rajashekar, Y.; Mishra, A.; Das, S. Volatile Compound Profile Analysis of Seasonal Flower, Fruit, Leaf, and Stem of Zanthoxylum armatum DC. from Manipur Using HS-SPME-GC-MS. Chemosensors 2023, 11, 273. [Google Scholar] [CrossRef]
- Guo, J.; Yu, Z.; Liu, M.; Guan, M.; Shi, A.; Hu, Y.; Li, S.; Yi, L.; Ren, D. Analysis of Volatile Profile and Aromatic Characteristics of Raw Pu-erh Tea during Storage Based on GC-MS and Odor Activity Value. Foods 2023, 12, 3568. [Google Scholar] [CrossRef]
- Kang, M.; Guo, Y.; Ren, Z.; Ma, W.; Luo, Y.; Zhao, K.; Wang, X. Volatile Fingerprint and Differences in Volatile Compounds of Different Foxtail Millet (Setaria italica Beauv.) Varieties. Foods 2023, 12, 4273. [Google Scholar] [CrossRef]
- Reyrolle, M.; Desauziers, V.; Pigot, T.; Gautier, L.; Le Bechec, M. Comparison of Untargeted and Markers Analysis of Volatile Organic Compounds with SIFT-MS and SPME-GC-MS to Assess Tea Traceability. Foods 2024, 13, 3996. [Google Scholar] [CrossRef]
- Espitia-López, J.; Luna, H.; Escalona-Buendía, H.B.; Verde-Calvo, J.R. Identification, quantification, and sensory profile of esters and alcohols of a Mexican red Merlot wine comparing barrel ageing with wood chips, using a multivariable analysis. J. Food Process. Preserv. 2017, 42, e13433. [Google Scholar] [CrossRef]
- Carreon-Alvarez, A.; Suárez-Gómez, A.; Zurita, F.; Gómez-Salazar, S.; Soltero, J.F.A.; Barcena-Soto, M.; Casillas, N.; Gutierrez, P.; Moreno-Medrano, E.D. Assessment of physicochemical properties of tequila brands: Authentication and quality. J. Chem. 2016, 2016, 6254942. [Google Scholar] [CrossRef]
- NOM-070-SCFI-2016; Bebidas Alcohólicas-Mezcal-Especificaciones. Secretaría de Economía (SE): Mexico City, Mexico, 2016. Available online: https://www.dof.gob.mx/normasOficiales/6437/seeco11_C/seeco11_C.html (accessed on 1 January 2025).
- NMX-V-013-NORMEX-2019; Bebidas Alcohólicas-Determinación Del Contenido Alcohólico (Por Ciento de Alcohol en Volumen a 20 °C) (% Alc.Vol.)-Métodos de Ensayo (Prueba) (Esta Norma Cancela la NMX-V-013-NORMEX-2013). Secretaría de Economía (SE): Mexico City, Mexico, 2020. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5594809&fecha=11/06/2020#gsc.tab=0 (accessed on 24 January 2025).
- Zhang, Y. Analysis of Some Alcohols, Aldehydes, and Esters in Distilled Spirits with the Agilent 8860 Gas Chromatograph; Agilent Technologies Application Note; 5994-0490EN; Agilent Technologies (Shanghai) Co. Ltd.: Shanghai, China, 2019; pp. 1–9. Available online: https://www.agilent.com/cs/library/applications/application-alcohols-aldehydes-esters-distilled-spirits-8860-gc-5994-0490en-agilent.pdf.pdf (accessed on 1 January 2025).
- Lucero, M.; Estell, R.; Tellez, M.; Fredrickson, E. A Retention Index Calculator Simplifies Identification of Plant Volatile Organic Compounds. Phytochem. Anal. 2009, 20, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Fernández, R.E.; Diaz, D.; Duarte, G.; Lappe-Oliveras, P.; Sánchez, S.; Macías-Rubalcava, M.L. Antifungal Volatile Organic Compounds from the Endophyte Nodulisporium sp. Strain GS4d2II1a: A Qualitative Change in the Intraspecific and Interspecific Interactions with Pythium aphanidermatum. Microb. Ecol. 2016, 71, 347–364. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 1 January 2025).
- RStudio Team. RStudio: Integrated Development for R. RStudio; PBC: Boston, MA, USA, 2023; Available online: http://www.rstudio.com/ (accessed on 1 January 2025).
- Acosta-García, E.D.; Páez-Lerma, J.B.; Martínez-Prado, M.A.; Soto-Cruz, N.O. Volatile compound analysis in mezcal based on multiple extraction/concentration methods, deconvolution software, and multivariate analysis. Food Control 2025, 168, 110852. [Google Scholar] [CrossRef]
- Kirchmayr, M.R.; Segura-García, L.E.; Lappe-Oliveras, P.; Moreno-Terrazas, R.; de la Rosa, M.; Gschaedler Mathis, A. Impact of environmental conditions and process modifications on microbial diversity, fermentation efficiency and chemical profile during the fermentation of mezcal in Oaxaca. LWT-Food Sci. Technol. 2017, 79, 160–169. [Google Scholar] [CrossRef]
- Arends, I.W.C.E.; Sheldon, R.A.; Bäckvall, J.E. (Eds.) Modern Oxidation Methods, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Boisvert, L.; Goldberg, K.I. Reactions of Late Transition Metal Complexes with Molecular Oxygen. Acc. Chem. Res. 2012, 45, 899–910. [Google Scholar] [CrossRef]
- Vera-Guzmán, A.M.; Guadalupe-López, M.; Chávez-Servia, J.J. Chemical composition and volatile compounds in the artisanal fermentation of mezcal in Oaxaca, Mexico. Afr. J. Biotechnol. 2012, 11, 14344–14353. [Google Scholar] [CrossRef]
- Espinoza-Martinez, V.A.; Alvarez-Gutierrez, P.E.; Palma-Cruz, F.d.J.; Enriquez-Valencia, R.; Ramirez-Lopez, M.P.; Lopez-Sanchez, C.; Vazquez-Lopez, H.G. Influence of the Biotechnological Process of Mezcal Fermentation on Yeast Diversity in Four palenques of Oaxaca, Mexico. Beverages 2023, 9, 99. [Google Scholar] [CrossRef]
- De León-Rodríguez, A.; Escalante-Minakata, P.; Jiménez-García, M.I.; Ordoñez-Acevedo, L.G.; Flores Flores, J.L.; Barba de la Rosa, A.P. Composition of Alcoholic Beverages from Agave. Food Technol. Biotechnol. 2008, 46, 448–455. [Google Scholar]
- González Seguí, H.O.; Hernández López, J.J.; Hendrik Giersiepen, J. Methanol: Tolerances and Requirements in Norm for Mezcal and Agave Drinks. Rivar 2020, 7, 1–21. [Google Scholar] [CrossRef]
- Muñoz-Muñoz, A.C.; Pichardo-Molina, J.L.; Ramos-Ortíz, G.; Barbosa-García, O.; Maldonado, J.L.; Meneses-Nava, M.A.; Ornelas-Soto, N.E.; Escobedo, A.; López-de-Alba, P.L. Identification and quantification of furanic compounds in tequila and mezcal using spectroscopy and chemometric methods. J. Braz. Chem. Soc. 2010, 21, 1003–1010. [Google Scholar] [CrossRef]
- Nolasco-Cancino, H.; Jarquín-Martínez, D.; Ruiz-Terán, F.; Santiago-Urbina, J.A. Behavior of the volatile compounds regulated by the Mexican Official Standard NOM-070-SCFI-2016 during the distillation of artisanal Mezcal. Biotecnia. Rev. Cienc. Biológicas Salud 2022, 24, 20–27. [Google Scholar] [CrossRef]
- Rodríguez-Félix, E.; Contreras-Ramos, S.M.; Davila-Vazquez, G.; Rodríguez-Campos, J.; Marino-Marmolejo, E.N. Identification and quantification of volatile compounds found in vinasses from two different processes of tequila production. Energies 2018, 11, 490. [Google Scholar] [CrossRef]
- Mancilla-Margalli, N.A.; López, M.G. Generation of Maillard Compounds from Inulin during the Thermal Processing of Agave tequilana Weber Var. azul. J. Agric. Food Chem. 2002, 50, 806–812. [Google Scholar] [CrossRef]
- International Organization of Legal Metrology (OIML). International Recommendation R 22: Alcoholometry and Alcohol Tables; OIML: Paris, France, 2022; Available online: https://www.oiml.org/en/publications (accessed on 1 January 2025).
| Name | Conferred Properties | References |
|---|---|---|
| Ethyl acetate | Ethyl acetate is produced by the yeast Saccharomyces cerevisiae through the enzyme acetyl alcohol transferase. It imparts fruity flavors and aromas to beverages. | [18,19,20] |
| Methanol | Agave contains pectin with methoxyl groups, which break down due to the high temperatures in ovens. Some yeasts have a pectin-methyl-esterase enzyme, which separates methoxyl groups from pectins and produces methanol during cooking. | [21] |
| Acetaldehyde diethyl acetal | Acetal is the primary compound produced during alcoholic fermentation. Its main biosynthesis occurs during the anabolic process through the enzyme pyruvate decarboxylase. Aldehydes are also produced during the maturation phase due to alcohol oxidation. Fruity aromatic note. | [18,21,22] |
| 1-Propanol | It can be produced from amino acids through catabolism. Aromatic notes: alcoholic, solvent-like, fruit, and sweet. The production of higher alcohols such as propanol, isobutanol, and amyl alcohols is associated with ethanol production. | [22,23] |
| 2-Methyl-1-propanol | Produced through amino acid catabolism, including aldehydes that are reduced by alcohol dehydrogenase into their respective alcohols, though at lower production rates than ethanol. Aromatic notes: sweet, chemical, bleach, chocolate, musty. | [24] |
| Acetic acid | Acetic acid originates from the Maillard reaction during cooking, as it has been identified in exudates of Agave tequilana Weber. Aromatic note: vinegar. | [18,25] |
| Furfural | Produced during thermal processes, occurring during both the cooking and distillation phases, due to the thermal degradation of sugars. Aromatic notes: floral, fruity. | [11,18] |
| 1-Butanol | It can result from a combination of chemical processes, including aldehyde reduction to alcohols and micro-oxidation, along with a possible relative concentration effect due to the selective evaporation of volatile compounds caused by storage in a semi-permeable container. Aromatic note: sweet, fusel. | [18] |
| Mezcal Sample | Year of Production | EA | MET | ADA | PROP | 2MPROP | BUT | AA | FUR | VISC (mPa∙s) | TSS (%) | ALC (% v/v) | DENS (g/cm3) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. potatorum | |||||||||||||
| Tobalá | 2015 | 503.54 ± 165.30 a | 257.81 ± 25.54 a | 41.35 ± 36.71 a | 32.1 ± 3.61 a | 89.3 ± 2.16 ab | 6.5 ± 1.86 a | 238.9 ± 26.30 abc | 4.9 ± 1.44 cd | 3.37 ± 0.02 ab | 15.93 ± 0.21 c | 44.83 ± 0.99 d | 0.93 ± 0.01 a |
| A. karwinskii Zucc. | |||||||||||||
| Bicuishe | 2015 | 263.16 ± 60.37 ab | 317.55 ± 183.12 a | 26.50 ± 11.95 a | 16.21 ± 9.92 a | 88.16 ± 33.39 ab | 1.99 ± 1.20 ab | 125.01 ± 26.64 bc | 2.40 ± 1.84 cd | 3.36 ± 0.01 ab | 15.97 ± 0.15 c | 45.93 ± 0.31 cd | 0.89 ± 0.02 ab |
| 2017 | 320.80 ± 54.61 ab | 207.67 ± 7.97 a | 12.53 ± 2.16 a | 24.49 ± 1.98 a | 100.58 ± 9.85 a | 2.45 ± 0.60 ab | 266.13 ± 33.14 abc | 9.64 ± 2.10 abc | 3.41 ± 0.01 ab | 16.40 ± 0.0 a | 47.80 ± 0.20 b | 0.88 ± 0.03 b | |
| Madrecuishe | 2016 | 338.70 ± 29.77 b | 239.97 ± 28.86 a | 20.69 ± 6.80 a | 27.64 ± 4.47 a | 71.39 ± 20.35 b | 2.05 ± 0.72 ab | 408.76 ± 82.88 a | 14.62 ± 2.95 a | 3.26 ± 0.29 b | 16.30 ± 0.10 ab | 47.23 ± 0.15 b | 0.92 ± 0.01 ab |
| 2018–2020 | 372.77 ± 47.30 b | 175.06 ± 27.13 a | 19.80 ± 4.68 a | 31.77 ± 5.28 a | 102.15 ± 18.64 a | 3.57 ± 0.59 ab | 335.65 ± 29.73 ab | 13.28 ± 2.13 ab | 3.34 ± 0.25 ab | 16.23 ± 0.06 abc | 46.67 ± 0.21 bc | 0.92 ± 0.01 ab | |
| 2021 | 214.76 ± 16.92 b | 157.82 ± 12.46 a | 7.48 ± 1.92 a | 16.03 ± 4.31 a | 108.06 ± 12.90 a | 1.02 ± 0.37 b | 228.35 ± 30.51 abc | 5.57 ± 1.44 bcd | 3.38 ± 0.01 ab | 15.33 ± 0.06 d | 50.33 ± 0.99 a | 0.89 ± 0.03 ab | |
| Tobasiche | 2021 | 178.96 ± 4.02 b | 124.82 ± 5.05 a | 20.22 ± 10.77 a | 23.52 ± 10.79 a | 141.97 ± 31.19 a | 0.00 b | 74.74 ± 9.31 c | 0.31 ± 0.14 cd | 3.37 ± 0.01 ab | 16.00 ± 0.01 bc | 45.43 ± 1.10 d | 0.91 ± 0.01 ab |
| A. angustifolia Haw. | |||||||||||||
| Espadín | 2021 | 190.78 ± 32.02 b | 211.94 ± 53.34 a | 28.15 ± 12.76 a | 23.55 ± 10.01 a | 127.98 ± 48.31 a | 1.02 ± 0.51b | 142.66 ± 58.56 bc | 1.55 ± 0.48 cd | 3.62 ± 0.40 a | 15.97 ± 0.06 c | 45.67 ± 0.06 cd | 0.89 ± 0.04 ab |
| A. marmorata Roezl. | |||||||||||||
| Tepeztate | 2021 | 296.41 ± 31.35 ab | 120.43 ± 40.70 a | 30.44 ± 10.47 a | 23.02 ± 11.83 a | 113.24 ± 29.74 a | 1.06 ± 0.71 b | 174.47 ± 35.79 bc | 3.25 ± 1.35 cd | 3.37 ± 0.01 ab | 16.20 ± 0.40 abc | 47.83 ± 0.25 b | 0.90 ± 0.02 ab |
| Compound | Bicuishe 2017 | Espadín 2021 | Tobasiche 2021 | Bicuishe 2015 | Tepeztate 2021 | Tobalá 2015 | Madrecuishe 2021 | Madrecuishe 2018–2020 | Madrecuishe 2016 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/L | mg/100 mL A.A. | mg/L | mg/100 mL A.A. | mg/L | mg/100 mL A.A. | mg/L | mg/100 mL A.A. | mg/L | mg/100 mL A.A. | mg/L | mg/100 mL A.A. | mg/L | mg/100 mL A.A. | mg/L | mg/100 mL A.A. | mg/L | mg/100 mL A.A. | |
| Ethyl acetate | 1681.45 ± 286.21 | 320.80 ± 54.61 | 1029.11 ± 172.71 | 190.78 ± 32.02 | 907.86 ± 20.40 | 178.96 ± 4.02 | 1425.79 ± 327.06 | 263.16 ± 60.37 | 1575.96 ±166.69 | 296.41 ± 31.35 | 2504.01 ± 821.99 | 503.54 ± 165.30 | 1166.87 ± 91.92 | 214.76 ± 16.92 | 1876.71 ± 238.11 | 372.77 ± 47.30 | 1758.02 ± 154.52 | 338.70 ± 29.77 |
| Acetaldehyde diethyl acetal | 65.70 ± 11.33 | 12.53 ± 2.16 | 151.86 ± 68.82 | 28.15 ± 12.76 | 102.60 ± 54.62 | 20.22 ± 10.77 | 143.56 ± 64.73 | 26.50 ± 11.95 | 161.84 ± 55.65 | 30.44 ± 10.47 | 205.61 ± 182.53 | 41.35 ± 36.71 | 40.63 ± 10.44 | 7.48 ± 1.92 | 99.70 ± 23.57 | 19.80 ± 4.68 | 107.38 ± 35.31 | 20.69 ± 6.80 |
| Methyl alcohol | 1088.51 ± 41.76 | 207.67 ± 7.97 | 1143.27 ± 287.72 | 211.94 ± 53.34 | 633.20 ± 25.63 | 124.82 ± 5.05 | 1720.49 ± 992.17 | 317.55 ± 183.12 | 853.76 ± 43.54 | 120.43 ± 40.70 | 1282.03 ± 127.03 | 257.81 ± 25.54 | 857.50 ± 67.67 | 157.82 ± 12.46 | 881.33 ± 136.57 | 175.06 ± 27.13 | 1245.58 ± 149.81 | 239.97 ± 28.86 |
| 1-propanol | 128.34 ± 10.36 | 24.49 ± 1.98 | 127.05 ± 54.00 | 23.55 ± 10.01 | 119.34 ± 54.72 | 23.52 ± 10.79 | 87.80 ± 53.73 | 16.21 ± 9.92 | 163.16 ± 58.61 | 23.02 ± 11.83 | 212.99 ± 17.94 | 42.83 ± 3.61 | 87.08 ± 23.43 | 16.03 ± 4.31 | 159.92 ± 26.58 | 31.77 ± 5.28 | 143.46 ±23.23 | 27.64 ± 4.47 |
| 2-methyl-1-propanol | 527.18 ± 51.62 | 100.58 ± 9.85 | 690.35 ± 260.60 | 127.98 ± 48.31 | 720.22 ± 158.24 | 141.97 ± 31.19 | 477.64 ± 180.92 | 88.16 ± 33.39 | 602.09 ± 158.14 | 113.24 ± 29.74 | 592.41 ± 10.74 | 119.13 ± 2.16 | 587.13 ± 70.12 | 108.06 ± 12.90 | 514.28 ± 93.84 | 102.15 ± 18.64 | 370.53 ± 105.64 | 71.39 ± 20.35 |
| 1-butanol | 12.83 ± 3.14 | 2.45 ± 0.60 | 8.24 ± 0.41 | 1.02 ± 0.51 | NQ | NQ | 14.34 ± 6.62 | 1.99 ± 1.20 | 11.24 ± 3.40 | 1.06 ± 0.71 | 43.10 ± 9.26 | 8.67 ± 1.86 | 7.38 ± 0.97 | 1.02 ± 0.37 | 17.97 ± 2.97 | 3.57 ± 0.59 | 10.64 ± 3.75 | 2.05 ± 0.72 |
| Acetic acid | 1394.94 ± 173.72 | 266.13 ± 33.14 | 769.56 ± 315.87 | 142.66 ± 58.56 | 379.16 ± 47.22 | 74.74 ± 9.31 | 677.30 ± 144.34 | 125.01 ± 26.64 | 927.62 ± 190.31 | 174.47 ± 35.79 | 1584.45 ± 130.78 | 318.62 ± 26.30 | 1240.67 ± 165.77 | 228.35 ± 30.51 | 1689.85 ± 149.69 | 335.65 ± 29.73 | 2121.69 ± 430.20 | 408.76 ± 82.88 |
| Furfural | 50.54 ± 10.99 | 9.64 ± 2.10 | 8.38 ± 2.61 | 1.55 ± 0.48 | 2.08 ± 0.60 | 0.31 ± 0.14 | 12.98 ± 9.98 | 2.40 ± 1.84 | 17.30 ± 7.18 | 3.25 ± 1.35 | 32.61 ± 4.51 | 6.56 ± 0.91 | 30.28 ± 7.83 | 5.57 ± 1.44 | 66.88 ± 10.74 | 13.28 ± 2.13 | 75.86 ± 15.31 | 14.62 ± 2.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Fernández, R.E.; Pérez-López, A.; Morales-Solis, A.; Manilla-Tellez, Y.; Reyes-Carmona, E.D.; Avila-Uribe, G. Chemistry of Mezcal: Volatile Profile of Artisanal Mezcal Made from Wild Agaves of Oaxaca. Foods 2025, 14, 1222. https://doi.org/10.3390/foods14071222
Sánchez-Fernández RE, Pérez-López A, Morales-Solis A, Manilla-Tellez Y, Reyes-Carmona ED, Avila-Uribe G. Chemistry of Mezcal: Volatile Profile of Artisanal Mezcal Made from Wild Agaves of Oaxaca. Foods. 2025; 14(7):1222. https://doi.org/10.3390/foods14071222
Chicago/Turabian StyleSánchez-Fernández, Rosa Elvira, Artemio Pérez-López, Anabel Morales-Solis, Yesenia Manilla-Tellez, Erika Daniela Reyes-Carmona, and Graciela Avila-Uribe. 2025. "Chemistry of Mezcal: Volatile Profile of Artisanal Mezcal Made from Wild Agaves of Oaxaca" Foods 14, no. 7: 1222. https://doi.org/10.3390/foods14071222
APA StyleSánchez-Fernández, R. E., Pérez-López, A., Morales-Solis, A., Manilla-Tellez, Y., Reyes-Carmona, E. D., & Avila-Uribe, G. (2025). Chemistry of Mezcal: Volatile Profile of Artisanal Mezcal Made from Wild Agaves of Oaxaca. Foods, 14(7), 1222. https://doi.org/10.3390/foods14071222











