Nutritional and Microbial Quality of Edible Insect Powder from Plant-Based Industrial By-Product and Fish Biowaste Diets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cricket Breeding Practices and Cricket Powder Production Methods
2.1.1. Feed Formulation
2.1.2. Diets and Cricket Powders Assessment
2.2. Analytical Procedures
2.2.1. Moisture Content, aw, Proximate Composition, and Caloric Value
2.2.2. Crude Fibre
2.2.3. Bioactive Composition
2.2.4. Mineral Content
2.2.5. Amino Acid Quantification
2.2.6. Fatty Acids Profile
2.2.7. Fatty Acids Nutritional Indices
2.3. Microbial Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Insect Diet Formulations
3.1.1. Proximal Analysis, Crude Fibre, and Caloric Value
3.1.2. Mineral Content
3.2. Insect Powder Composition
3.2.1. Proximal Analysis, aw, Crude Fibre, and Caloric Value
3.2.2. Amino Acid Profile
3.2.3. Fatty Acids Profile
3.2.4. Fatty Acids Nutritional Indices
3.2.5. Bioactive Composition
3.2.6. Mineral Content
3.2.7. Microbial Evaluation
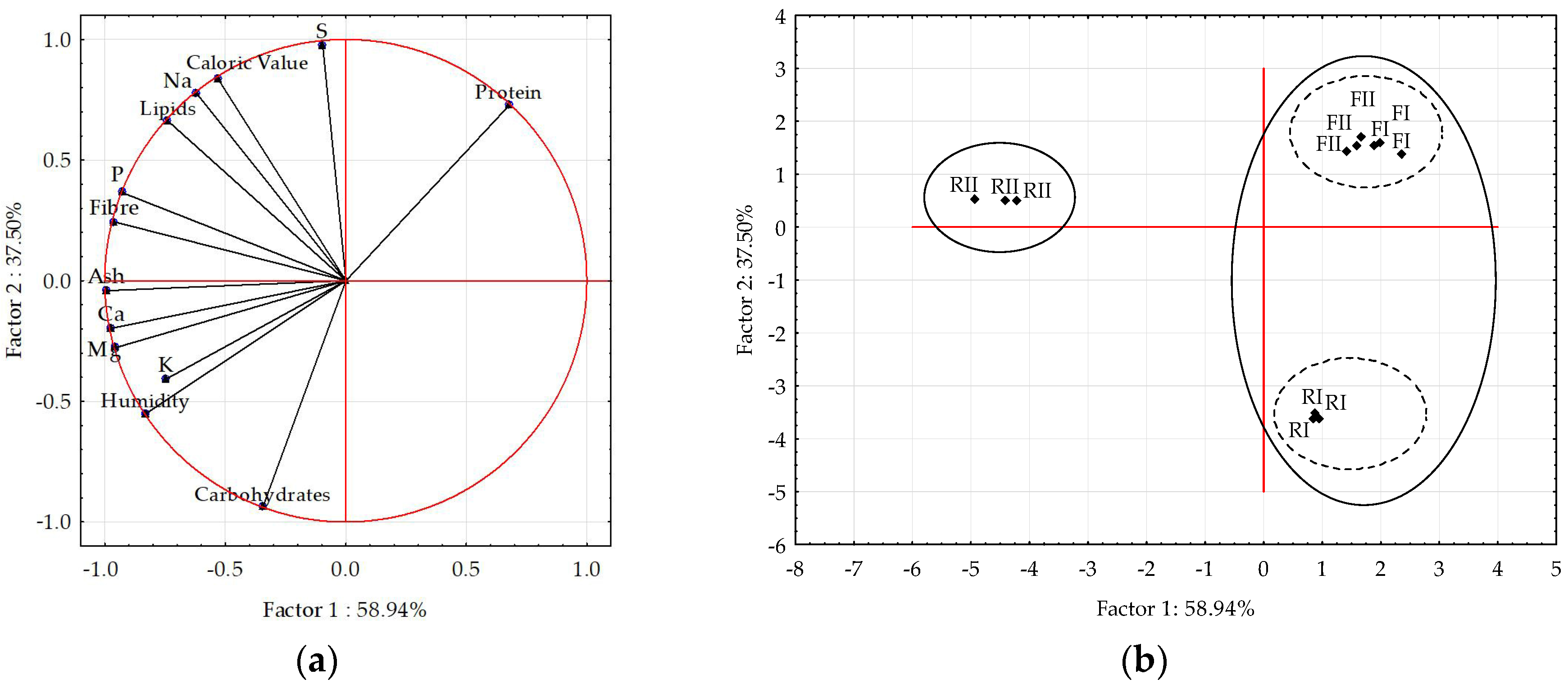
3.3. Relationship Between Cricket Diet and Insect Powder Quality
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; Volume 171, ISBN 9789251075951. [Google Scholar]
- Aguilera, Y.; Pastrana, I.; Rebollo-Hernanz, M.; Benitez, V.; Álvarez-Rivera, G.; Viejo, J.L.; Martín-Cabrejas, M.A. Investigating Edible Insects as a Sustainable Food Source: Nutritional Value and Techno-Functional and Physiological Properties. Food Funct. 2021, 12, 6309–6322. [Google Scholar] [CrossRef] [PubMed]
- Rumpold, B.A.; Schlüter, O.K. Nutritional Composition and Safety Aspects of Edible Insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef] [PubMed]
- Pilco-Romero, G.; Chisaguano-Tonato, A.M.; Herrera-Fontana, M.E.; Chimbo-Gándara, L.F.; Sharifi-Rad, M.; Giampieri, F.; Battino, M.; Vernaza, M.G.; Álvarez-Suárez, J.M. House Cricket (Acheta domesticus): A Review Based on Its Nutritional Composition, Quality, and Potential Uses in the Food Industry. Trends Food Sci. Technol. 2023, 142, 104226. [Google Scholar] [CrossRef]
- Ruggeri, M.; Bianchi, E.; Vigani, B.; Sánchez-Espejo, R.; Spano, M.; Fila, C.T.; Mannina, L.; Viseras, C.; Rossi, S.; Sandri, G. Nutritional and Functional Properties of Novel Italian Spray-Dried Cricket Powder. Antioxidants 2023, 12, 112. [Google Scholar] [CrossRef]
- Scaffardi, L.; Formici, G. Novel Foods and Edible Insects in the European Union: An Interdisciplinary Analysis; Springer: Cham, Switzerland, 2022; Volume 1st, ISBN 978-3-031-13494-4. [Google Scholar]
- European Parliament. Parliamentary Question E-000581/2023: Insects in Food. Available online: https://www.europarl.europa.eu/doceo/document/E-9-2023-000581_EN.html (accessed on 9 March 2025).
- Amoah, I.; Cobbinah, J.C.; Yeboah, J.A.; Essiam, F.A.; Lim, J.J.; Tandoh, M.A.; Rush, E. Edible Insect Powder for Enrichment of Bakery Products—A Review of Nutritional, Physical Characteristics and Acceptability of Bakery Products to Consumers. Future Foods 2023, 8, 100251. [Google Scholar] [CrossRef]
- Bashir, M.; Gupta, N.; Bhat, A.; Singh, J.; Bandral, J.D.; Sood, M.; Rafiq, N. Emerging Technologies in Fruit By-Products Valorization: A Review. Chem. Sci. Rev. Lett. 2022, 11, 349–355. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; Horwitz, W., Ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000; ISBN 0-935584-67-6. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; Horwitz, W., Ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2005; ISBN 0-935584-77-3. [Google Scholar]
- Akbar, E.; Yaakob, Z.; Kamarudin, S.K.; Salimon, J. Characteristic and Composition of Jatropha curcas Oil Seed from Malaysia and Its Potential as Biodiesel Feedstock Feedstock. Eur. J. Sci. Res. 2009, 29, 396–403. [Google Scholar]
- European Parliament and Council. Regulation (EU) No. 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32011R1169 (accessed on 9 March 2025).
- Henneberg, W.; Stohmann, F. Uber Das Erhaltungsfutter Volljahrigen Rindviehs. J. Landwirtsch 1859, 3, 485–551. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 15th ed.; Helrich, K., Ed.; The Association of Official Analytical Chemists: Arlington, VA, USA, 1990; ISBN 0-935584-42-0. [Google Scholar]
- Swain, T.; Hillis, W.E. The Phenolic Constituents of Prunus domestica. I.—The Quantitative Analysis of Phenolic Constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The Hydrophilic and Lipophilic Contribution to Total Antioxidant Activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC Assays for Estimating Antioxidant Activity from Guava Fruit Extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. SW-846 Test Method 7473: Mercury in Solids and Solutions by Thermal Decomposition, Amalgamation and Atomic Absorption Spectrometry. Available online: https://www.epa.gov/hw-sw846/sw-846-test-method-7473-mercury-solids-and-solutions-thermal-decomposition-amalgamation (accessed on 9 March 2025).
- ISO 5508:1990; Animal and Vegetable Fats and Oils—Analysis by Gas Chromatography of Methyl Esters of Fatty Acids. ISO: Geneva, Switzerland, 1990; pp. 1–9.
- ISO 5509:2000; Animal and Vegetable Fats and Oils—Preparation of Methyl Esters of Fatty Acids. ISO: Geneva, Switzerland, 2000; pp. 1–12.
- Omri, B.; Chalghoumi, R.; Izzo, L.; Ritieni, A.; Lucarini, M.; Durazzo, A.; Abdouli, H.; Santini, A. Effect of Dietary Incorporation of Linseed Alone or Together with Tomato-Red Pepper Mix on Laying Hens’ Egg Yolk Fatty Acids Profile and Health Lipid Indexes. Nutrients 2019, 11, 813. [Google Scholar] [CrossRef] [PubMed]
- Attia, Y.A.; Al-Harthi, M.A.; Korish, M.A.; Shiboob, M.M. Fatty Acid and Cholesterol Profiles and Hypocholesterolemic, Atherogenic, and Thrombogenic Indices of Table Eggs in the Retail Market. Lipids Health Dis. 2015, 14, 136. [Google Scholar] [CrossRef] [PubMed]
- ISO 4833-1:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 °C by the Pour Plate Technique. ISO: Geneva, Switzerland, 2013; pp. 1–9.
- ISO 21527-1:2008; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 1: Colony Count Technique in Products with Water Activity Greater than 0.95. ISO: Geneva, Switzerland, 2008; pp. 1–9.
- StatSoft, Inc. STATISTICA (Data Analysis Software System), Version 8.0; StatSoft, Inc.: Tulsa, OK, USA, 2007.
- Freeman, J. A User’s Guide to Principal Components. J. Oper. Res. Soc. 1992, 43, 641. [Google Scholar] [CrossRef]
- Larrigaudière, C.; Lentheric, I.; Puy, J.; Pintó, E. Biochemical Characterisation of Core Browning and Brown Heart Disorders in Pear by Multivariate Analysis. Postharvest Biol. Technol. 2004, 31, 29–39. [Google Scholar] [CrossRef]
- Patton, R.L. Oligidic Diets for Acheta domesticus (Orthoptera: Gryllidae). Ann. Entomol. Soc. Am. 1967, 60, 1238–1242. [Google Scholar] [CrossRef]
- Hanboonsong, A.; Durst, P. Guidance on Sustainable Cricket Farming—A Practical Manual for Farmers and Inspectors; Food and Agriculture Organization of the United Nations: Bangkok, Thailand, 2020; Volume 1, ISBN 978-92-5-133739-4. [Google Scholar]
- Oonincx, D.G.A.B.; Van Broekhoven, S.; Van Huis, A.; Van Loon, J.J.A. Feed Conversion, Survival and Development, and Composition of Four Insect Species on Diets Composed of Food by-Products. PLoS ONE 2015, 10, e0144601. [Google Scholar] [CrossRef]
- Collavo, A.; Glew, R.H.; Huang, Y.-S.; Chuang, L.T.; Bosse, R.; Paoletti, M.G. House Cricket Small-Scale Farming. Ecol. Implic. Minilivestock Potential Insects Rodents Frogs Snails 2005, 27, 515–540. [Google Scholar]
- Bawa, M.; Songsermpong, S.; Kaewtapee, C.; Chanput, W. Effect of Diet on the Growth Performance, Feed Conversion, and Nutrient Content of the House Cricket. J. Insect Sci. 2021, 20, 10. [Google Scholar] [CrossRef]
- Visanuvimol, L.; Bertram, S.M. How Dietary Phosphorus Availability during Development Influences Condition and Life History Traits of the Cricket. Acheta Domesticus. J. Insect Sci. 2011, 11, 63. [Google Scholar] [CrossRef]
- European Parliament and Council. Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on Undesirable Substances in Animal Feed. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32002L0032 (accessed on 9 March 2025).
- Udomsil, N.; Imsoonthornruksa, S.; Gosalawit, C.; Ketudat-Cairns, M. Nutritional Values and Functional Properties of House Cricket (Acheta Domesticus) and Field Cricket (Gryllus bimaculatus). Food Sci. Technol. Res. 2019, 25, 597–605. [Google Scholar] [CrossRef]
- Tapia, M.S.; Alzamora, S.M.; Chirife, J. Effects of Water Activity (Aw) on Microbial Stability: As a Hurdle in Food Preservation. In Water Activity in Foods: Fundamentals and Applications; Blackwell Publishing Ltd.: Oxford, UK, 2020; pp. 323–355. ISBN 9781118768310. [Google Scholar]
- Magara, H.J.O.; Niassy, S.; Ayieko, M.A.; Mukundamago, M.; Egonyu, J.P.; Tanga, C.M.; Kimathi, E.K.; Ongere, J.O.; Fiaboe, K.K.M.; Hugel, S.; et al. Edible Crickets (Orthoptera) Around the World: Distribution, Nutritional Value, and Other Benefits—A Review. Front. Nutr. 2021, 7, 537915. [Google Scholar] [CrossRef] [PubMed]
- Mokrane, H.; Amoura, H.; Belhaneche-Bensemra, N.; Courtin, C.M.; Delcour, J.A.; Nadjemi, B. Assessment of Algerian Sorghum Protein Quality [Sorghum bicolor (L.) Moench] Using Amino Acid Analysis and in Vitro Pepsin Digestibility. Food Chem. 2010, 121, 719–723. [Google Scholar] [CrossRef]
- Ghosh, S.; Lee, S.M.; Jung, C.; Meyer-Rochow, V.B. Nutritional Composition of Five Commercial Edible Insects in South Korea. J. Asia-Pac. Entomol. 2017, 20, 686–694. [Google Scholar] [CrossRef]
- Kurpad, A.V. Protein: Quality and Sources. In Encyclopedia of Human Nutrition; Elsevier Inc.: Amsterdam, The Netherlands, 2012; Volume 4, pp. 123–130. ISBN 9780123848857. [Google Scholar]
- Sanz, J.M.M.; Navarro, A.N.; García, E.S.; López, I.S. An Overview on Essential Amino Acids and Branched Chain Amino Acids. Nutr. Enhanc. Sports Perform. 2019, 2, 509–519. [Google Scholar] [CrossRef]
- Skolnik, P.; Eaglstein, W.H.; Ziboh, V.A. Human Essential Fatty Acid Deficiency: Treatment by Topical Application of Linoleic Acid. Arch. Dermatol. 1977, 113, 939–941. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, M.; Song, L. A Review of Fatty Acids Influencing Skin Condition. J. Cosmet. Dermatol. 2020, 19, 3199–3204. [Google Scholar] [CrossRef]
- Durst, P.B.; Johnson, D.V.; Leslie, R.N.; Shono, K. Forest Insects as Food: Humans Bite Back, Proceedings of a Workshop on Asia-Pacific Resources and Their Potential for Development, Chiang Mai, Thailand, 19–21 February 2008; Food and Agriculture Organization of the United Nations: Bangkok, Thailand, 2010; Volume 2, ISBN 9789251064887. [Google Scholar]
- Perez-Santaescolastica, C.; de Pril, I.; van de Voorde, I.; Fraeye, I. Fatty Acid and Amino Acid Profiles of Seven Edible Insects: Focus on Lipid Class Composition and Protein Conversion Factors. Foods 2023, 12, 4090. [Google Scholar] [CrossRef]
- Lawal, K.G.; Kavle, R.R.; Akanbi, T.O.; Mirosa, M.; Agyei, D. Lipid Nutritional Indices, Regioisomeric Distribution, and Thermal Properties of Tenebrio molitor and Hermetia illucens Larvae Fat. J. Asia Pac. Entomol. 2022, 25, 101951. [Google Scholar] [CrossRef]
- Ulbritch, T.L.V.; Southgate, D.A.T. Coronary Heart Disease: Seven Dietary Factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Garaffo, M.A.; Vassallo-Agius, R.; Nengas, Y.; Lembo, E.; Rando, R.; Maisano, R.; Dugo, G.; Giuffrida, D. Fatty Acids Profile, Atherogenic (IA) and Thrombogenic (IT) Health Lipid Indices, of Raw Roe of Blue Fin Tuna (Thunnus thynnus L.) and Their Salted Product “Bottarga”. Food Nutr. Sci. 2011, 2, 736–743. [Google Scholar] [CrossRef]
- Elias, M.; Laranjo, M.; Potes, M.E.; Agulheiro-Santos, A.C.; Fernandes, M.J.; Garcia, R.; Fraqueza, M.J. Impact of a 25% Salt Reduction on the Microbial Load, Texture, and Sensory Attributes of a Traditional Dry-Cured Sausage. Foods 2020, 9, 554. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef] [PubMed]
- Tilami, S.K.; Kouřimská, L. Assessment of the Nutritional Quality of Plant Lipids Using Atherogenicity and Thrombogenicity Indices. Nutrients 2022, 14, 3795. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.C.; Romero, A.M.; Doval, M.M.; Judis, M.A. Nutritional Value and Fatty Acid Composition of Some Traditional Argentinean Meat Sausages. Food Sci. Technol. 2013, 33, 161–166. [Google Scholar] [CrossRef]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An Online Comprehensive Database on Polyphenol Contents in Foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The Total Antioxidant Content of More than 3100 Foods, Beverages, Spices, Herbs and Supplements Used Worldwide. Nutr. J. 2010, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Nino, M.C.; Reddivari, L.; Ferruzzi, M.G.; Liceaga, A.M. Targeted Phenolic Characterization and Antioxidant Bioactivity of Extracts from Edible Acheta domesticus. Foods 2021, 10, 2295. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Implementing Regulation (EU) 2022/188 of 10 February 2022 Authorising the Placing on the Market of Frozen, Dried and Powder Forms of Acheta domesticus as a Novel Food Under Regulation (EU) 2015/2283 of the European Parliament and of the Council, and Amending Commission Implementing Regulation (EU) 2017/2470. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32022R0188 (accessed on 9 March 2025).
- European Commission. Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32023R0915 (accessed on 9 March 2025).
- Kolakowski, B.M.; Johaniuk, K.; Zhang, H.; Yamamoto, E. Analysis of Microbiological and Chemical Hazards in Edible Insects Available to Canadian Consumers. J. Food Prot. 2021, 84, 1575–1581. [Google Scholar] [CrossRef]
- National Academies of Sciences, E. and M. Dietary Reference Intakes for Sodium and Potassium; Stallings, V.A., Harrison, M., Oria, M., Eds.; The National Academies Press: Washington, DC, USA, 2019; ISBN 978-0-309-48834-1. [Google Scholar]
- U.S. Department of Agriculture. FoodData Central Food Details: Chicken, Broiler or Fryers, Breast, Skinless, Boneless, Meat Only, Cooked, Braised (SR Legacy, 171140). Available online: https://fdc.nal.usda.gov/food-details/171140/nutrients (accessed on 9 March 2025).
- U.S. Department of Agriculture. FoodData Central Food Details: Pork, Fresh, Loin, Tenderloin, Separable Lean Only, Raw (SR Legacy, 168249). Available online: https://fdc.nal.usda.gov/food-details/168249/nutrients (accessed on 9 March 2025).
| Parameters | RI | RII |
|---|---|---|
| Moisture content (%, FW) | 53.3 ± 0.1 b | 72.5 ± 0.8 a |
| Protein (%, DW) | 8.9 ± 0.8 a | 9.1 ± 0.1 a |
| Lipids (%, DW) | 3.7 ± 0.1 b | 26.1 ± 0.3 a |
| Carbohydrates (%, DW) | 81.4 ± 0.9 a | 52.7 ± 0.6 b |
| Ash (%, DW) | 6.0 ± 0.2 b | 12.1 ± 0.3 a |
| Crude fibre (%, DW) | 6.2 ± 1.0 b | 11.7 ± 0.4 a |
| Caloric value (kcal) | 394.4 ± 1.3 b | 482.3 ± 0.5 a |
| Minerals (mg/kg DW) | RI | RII |
|---|---|---|
| Sodium—Na | 1880.8 ± 38.7 b | 5372.7 ± 293.2 a |
| Potassium—K | 12,012.7 ± 217.3 a | 12,604.4 ± 621.2 a |
| Calcium—Ca | 9590.9 ± 1063.5 b | 23,995.2 ± 2481.4 a |
| Magnesium—Mg | 1831.5 ± 52.1 b | 2655.3 ± 205.4 a |
| Phosphorus—P | 7000.6 ± 100.8 b | 22,978.8 ± 1594.6 a |
| Sulfur—S | 2729.6 ± 56.6 b | 5434.6 ± 331.8 a |
| Chromium—Cr | 4.5 ± 0.1 a | 4.1 ± 0.3 a |
| Copper—Cu | 14.3 ± 1.9 a | 10.0 ± 0.9 b |
| Zinc—Zn | 64.1 ± 3.4 a | 76.2 ± 9.5 a |
| Iron—Fe | 108.6 ± 8.2 b | 198.9 ± 27.1 a |
| Manganese—Mn | 61.5 ± 0.1 a | 46.2 ± 4.2 b |
| Cadmium—Cd | 0.1 ± 0.0 b | 0.4 ± 0.0 a |
| Lead—Pb | <1.0 | <1.0 |
| Arsenic—As | <1.5 | <1.5 |
| Nickel—Ni | <1.5 | <1.5 |
| Mercury—Hg | <0.004 | 0.015 ± 0.00 |
| Parameters | FI | FII |
|---|---|---|
| aw | 0.4 ± 0.0 a | 0.3 ± 0.1 a |
| Moisture content (%, FW) | 5.0 ± 0.2 a | 4.3 ± 0.6 a |
| Protein (%, DW) | 57.4 ± 0.6 b | 60.8 ± 1.1 a |
| Lipids (%, DW) | 14.1 ± 2.0 a | 13.4 ± 0.3 a |
| Carbohydrates (%, DW) | 19.1 ± 1.9 a | 16.8 ± 0.6 a |
| Ash (%, DW) | 4.4 ± 0.2 b | 4.8 ± 0.1 a |
| Crude Fibre (%, DW) | 14.8 ± 1.7 a | 16.4 ± 0.4 a |
| Caloric value (kcal) | 433.2 ± 8.4 a | 430.6 ± 4.6 a |
| Amino Acid (g/100 g DW) | FI | FII |
|---|---|---|
| Histidine | 2.47 ± 0.47 a | 2.33 ± 0.47 a |
| Threonine | 2.37 ± 0.8 a | 1.27 ± 0.03 a |
| Methionine | 2.09 ± 0.83 a | 1.86 ± 0.22 a |
| Valine | 3.78 ± 0.18 a | 2.57 ± 0.64 b |
| Phenylalanine | 2.87 ± 0.5 a | 2.68 ± 0.97 a |
| Isoleucine | 3.19 ± 0.48 a | 1.76 ± 0.54 b |
| Leucine | 3.68 ± 0.1 a | 2.93 ± 0.46 a |
| Lysine | 3.19 ± 0.93 a | 2.98 ± 0.55 a |
| Tryptophan | 0.36 ± 0.04 a | 0.28 ± 0.04 a |
| Aspartic acid | 1.85 ± 0.75 a | 1.97 ± 0.27 a |
| Glutamic acid | 3.38 ± 1.07 a | 3.45 ± 0.62 a |
| Serine | 2.87 ± 0.56 a | 1.31 ± 0.11 b |
| Glycine | 2.02 ± 0.42 a | 1.19 ± 0.16 b |
| Arginine | 4.37 ± 1.43 a | 3.32 ± 0.66 a |
| Alanine | 3.45 ± 0.6 a | 1.93 ± 0.28 b |
| Tyrosine | 3.49 ± 0.33 a | 3.0 ± 0.83 a |
| ∑EAA | 24.00 ± 0.81 a | 18.70 ± 0.74 b |
| ∑NEAA | 21.43 ± 2.52 a | 16.17 ± 0.92 b |
| Fatty Acid (g/100 g of Fat) | FI | FII |
|---|---|---|
| Myristic acid C14 | 0.81 ± 0.03 b | 1.54 ± 0.29 a |
| Pentadecanoic acid C15 | 0.07 ± 0.01 b | 0.27 ± 0.07 a |
| Palmitic acid C16 | 28.03 ± 0.55 a | 25.32 ± 0.58 b |
| 7-Hexadecenoic acid C16:1 (7) | 0.39 ± 0.01 a | 0.34 ± 0.09 a |
| 11-Hexadecenoic acid C16:1 (11) | 0.05 ± 0.01 b | 0.21 ± 0.06 a |
| Palmitoleic acid C16:1 (9) | 0.86 ± 0.04 b | 2.17 ± 0.34 a |
| Margaric acid C17 | 0.27 ± 0.01 b | 0.69 ± 0.14 a |
| Heptadecenoic acid C17:1 | 0.07 ± 0.03 b | 0.37 ± 0.09 a |
| Stearic acid C18 | 9.09 ± 0.07 a | 7.41 ± 0.04 b |
| Petroselinic acid C18:1 (6) | 0.15 ± 0.07 b | 0.37 ± 0.12 a |
| Oleic acid C18:1 (9) | 23.69 ± 0.64 a | 24.02 ± 0.65 a |
| Vaccenic acid C18:1 (11) | 0.24 ± 0.13 b | 1.34 ± 0.39 a |
| Trans-Linoleic acid C18:2 (9 t, 12 t) | 0.37 ± 0.06 a | 0.12 ± 0.04 b |
| Trans-Linoleic acid C18:2 trans | ND | 0.3 ± 0.04 |
| Linoleic acid C18:2 (9, 12) | 30.91 ± 0.61 a | 26.12 ± 2.58 b |
| Linolenic acid C18:3 (9, 12, 15) | 0.6 ± 0.04 b | 1.26 ± 0.06 a |
| Octadecatetraenoic acid C18:4 | ND | 0.44 ± 0.07 |
| Arachidic acid C20 | 0.32 ± 0.01 a | 0.29 ± 0.02 a |
| Gadoleic acid C20:1 (9) | 0.09 ± 0.02 b | 0.39 ± 0.11 a |
| Eicosenoic acid C20:1 (11) | 0.03 ± 0.02 b | 0.68 ± 0.32 a |
| Behenic acid C20:2 | 0.1 ± 0.05 a | 0.09 ± 0.02 a |
| Eicosatetraenoic acid C20:4 w3 | ND | 0.19 ± 0.03 |
| Arachidonic acid C20:4 w6 | 0.01 ± 0.03 b | 0.54 ± 0.09 a |
| Eicosatrienoic acid (n-3) C20:3 w3 | 1.0 ± 0.07 | ND |
| Eicosatrienoic acid (n-6) C20:3 (11, 14, 17) | ND | 0.55 ± 0.07 |
| Eicosapentaenoic acid C20:5 EPA | ND | 1.38 ± 0.21 |
| Erucic acid C22:1 | 0.34 ± 0.13 a | 0.17 ± 0.05 a |
| Docosapentaenoic acid C22:5 | ND | 0.14 ± 0.01 |
| Docosahexaenoic acid C22:6 DHA | ND | 0.48 ± 0.05 |
| Nervonic acid C24:1 | 0.32 ± 0.17 | ND |
| Lignoceric acid C24 | 0.16 ± 0.07 a | 0.11 ± 0.09 a |
| Fatty Acid Types | FI | FII |
|---|---|---|
| SFA (%) (saturated fatty acids) | 39.1 ± 0.7 a | 36.5 ± 1.1 b |
| MUFA (%) (monounsaturated fatty acids) | 26.3 ± 0.5 b | 31.3 ± 1.3 a |
| PUFA (%) (polyunsaturated fatty acids) | 33.1 ± 0.7 a | 29.6 ± 2.5 a |
| Nutritional Indices (Lipids) | FI | FII |
|---|---|---|
| IA (index of atherogenicity) | 0.53 ± 0.02 a | 0.51 ± 0.04 a |
| IT (index of thrombogenicity) | 1.10 ± 0.06 a | 0.83 ± 0.03 b |
| H/H (ratio hypocholesterolemic and hypercholesterolemic fatty acids) | 1.93 ± 0.09 a | 1.99 ± 0.17 a |
| ω6/ω3 (ratio omega-6 and omega-3 fatty acids) | 16.44 ± 1.98 a | 6.80 ± 1.27 b |
| Index Food Source | IA | IT | H/H | References |
|---|---|---|---|---|
| Cricket powders FI and FII | 0.51–0.53 | 0.83–1.10 | 1.93–1.99 | data from the present study |
| Salmo trutta | 0.64–0.72 | 0.21–0.30 | 1.88–2.16 | [52] |
| Heifer (Limousin heifer) | 0.50–0.57 | 1.10–1.34 | 1.27–1.87 | [52] |
| Chicken | 0.37–0.39 | 0.76–0.78 | 2.66–2.79 | [52] |
| Milk of Jersey cow | 2.48–3.44 | 3.98–4.66 | 0.41–0.57 | [52] |
| Olive oil | 0.16 | 0.39 | 6.01 | [53] |
| Samples Id | AOx (DPPH) (µmol TE/100 g DW) | AOx (FRAP) (mmol FeSO4·7H2O/100 g DW) | TPC (mg GAE/100 g DW) |
|---|---|---|---|
| FI | 5055.0 ± 2.3 a | 72.9 ± 1.9 a | 212.0 ± 24.8 a |
| FII | 4928.5 ± 21.7 b | 43.0 ± 3.8 b | 120.9 ± 5.5 b |
| Minerals (mg/kg DW) | FI | FII |
|---|---|---|
| Sodium—Na | 3819.7 ± 116.0 a | 4174.4 ± 230.1 a |
| Potassium—K | 10,254.9 ± 478.1 b | 11,452.8 ± 443.0 a |
| Calcium—Ca | 2193.4 ± 117.8 a | 2382.5 ± 55.9 a |
| Magnesium—Mg | 1167.4 ± 15.6 b | 1316.7 ± 36.3 a |
| Phosphorus—P | 9956.6 ± 223.5 b | 10,965.5 ± 233.8 a |
| Sulfur—S | 5482.7 ± 127.0 b | 6098.4 ± 208.6 a |
| Copper—Cu | 26.7 ± 1.4 a | 27.0 ± 1.5 a |
| Zinc—Zn | 196.0 ± 5.2 a | 187.5 ± 10.2 a |
| Iron—Fe | 69.0 ± 9.7 a | 53.2 ± 10.9 a |
| Manganese—Mn | 27.7 ± 3.5 a | 16.6 ± 7.3 a |
| Chromium—Cr | 3.12 ± 0.1 a | 3.00 ± 0.1 b |
| Cadmium—Cd | <1.0 | <1.0 |
| Lead—Pb | <1.0 | <1.0 |
| Arsenic—As | <1.5 | <1.5 |
| Nickel—Ni | <1.5 | <1.5 |
| Mercury—Hg | 0.021 ± 0.01 b | 0.228 ± 0.07 a |
| Microbiological Parameter (log10 CFU/g) | FI | FII |
|---|---|---|
| Microorganisms at 30 °C | 3.6 ± 0.4 a | 3.2 ± 0.3 a |
| Molds and Yeasts | 1.0 ± 1.0 a | 0.3 ± 0.6 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, R.; Martins, L.L.; Mourato, M.P.; Lourenço, H.; Ramos, A.C.; Roseiro, C.; Pereira, N.; Costa, G.J.; Lucas, R.; Alvarenga, N.; et al. Nutritional and Microbial Quality of Edible Insect Powder from Plant-Based Industrial By-Product and Fish Biowaste Diets. Foods 2025, 14, 1242. https://doi.org/10.3390/foods14071242
Andrade R, Martins LL, Mourato MP, Lourenço H, Ramos AC, Roseiro C, Pereira N, Costa GJ, Lucas R, Alvarenga N, et al. Nutritional and Microbial Quality of Edible Insect Powder from Plant-Based Industrial By-Product and Fish Biowaste Diets. Foods. 2025; 14(7):1242. https://doi.org/10.3390/foods14071242
Chicago/Turabian StyleAndrade, Rafaela, Luisa Louro Martins, Miguel Pedro Mourato, Helena Lourenço, Ana Cristina Ramos, Cristina Roseiro, Nelson Pereira, Gonçalo J. Costa, Raphael Lucas, Nuno Alvarenga, and et al. 2025. "Nutritional and Microbial Quality of Edible Insect Powder from Plant-Based Industrial By-Product and Fish Biowaste Diets" Foods 14, no. 7: 1242. https://doi.org/10.3390/foods14071242
APA StyleAndrade, R., Martins, L. L., Mourato, M. P., Lourenço, H., Ramos, A. C., Roseiro, C., Pereira, N., Costa, G. J., Lucas, R., Alvarenga, N., Reis, J., Neves, A., Oliveira, M., Dias, I., & Abreu, M. (2025). Nutritional and Microbial Quality of Edible Insect Powder from Plant-Based Industrial By-Product and Fish Biowaste Diets. Foods, 14(7), 1242. https://doi.org/10.3390/foods14071242










