Antioxidant Properties and Microbiological Stability of Yogurt Enriched with Elderberry Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. EDB Extract Procedure
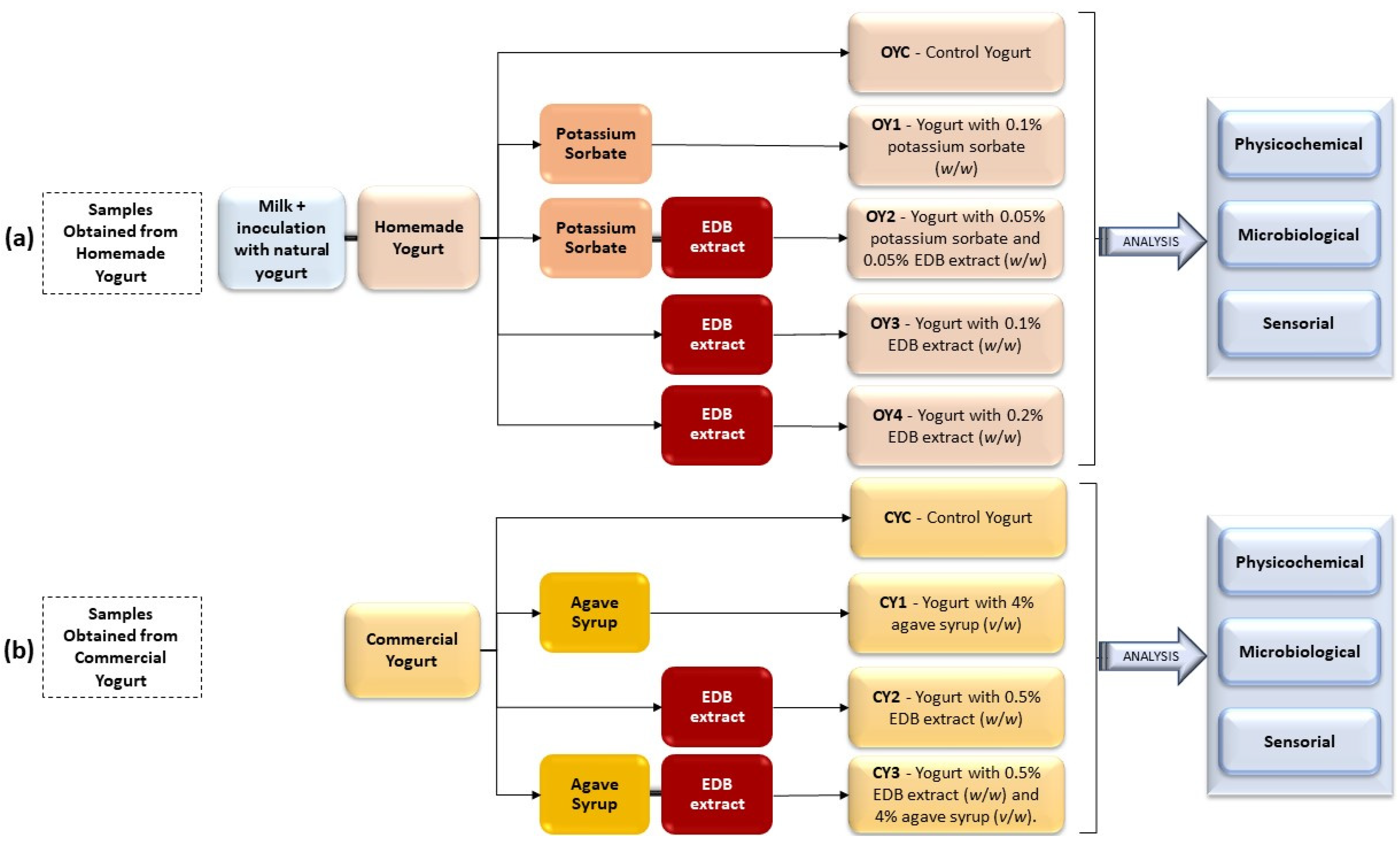
2.3. Yogurt Production
2.3.1. Samples Obtained from Homemade Yogurt
2.3.2. Samples Obtained from Commercial Yogurt
2.4. Physicochemical Analysis
2.4.1. pH Value
2.4.2. Color
2.4.3. Preparation of Hydroalcoholic Extracts of Yogurt Samples
2.4.4. Total Phenolic Content (TPC)
2.4.5. Total Flavonoid Content (TFC)
2.4.6. Free Radical-Scavenging Activity
2.5. Microbiological Analysis
2.6. Sensorial Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Chemical Analysis of EDB Extract
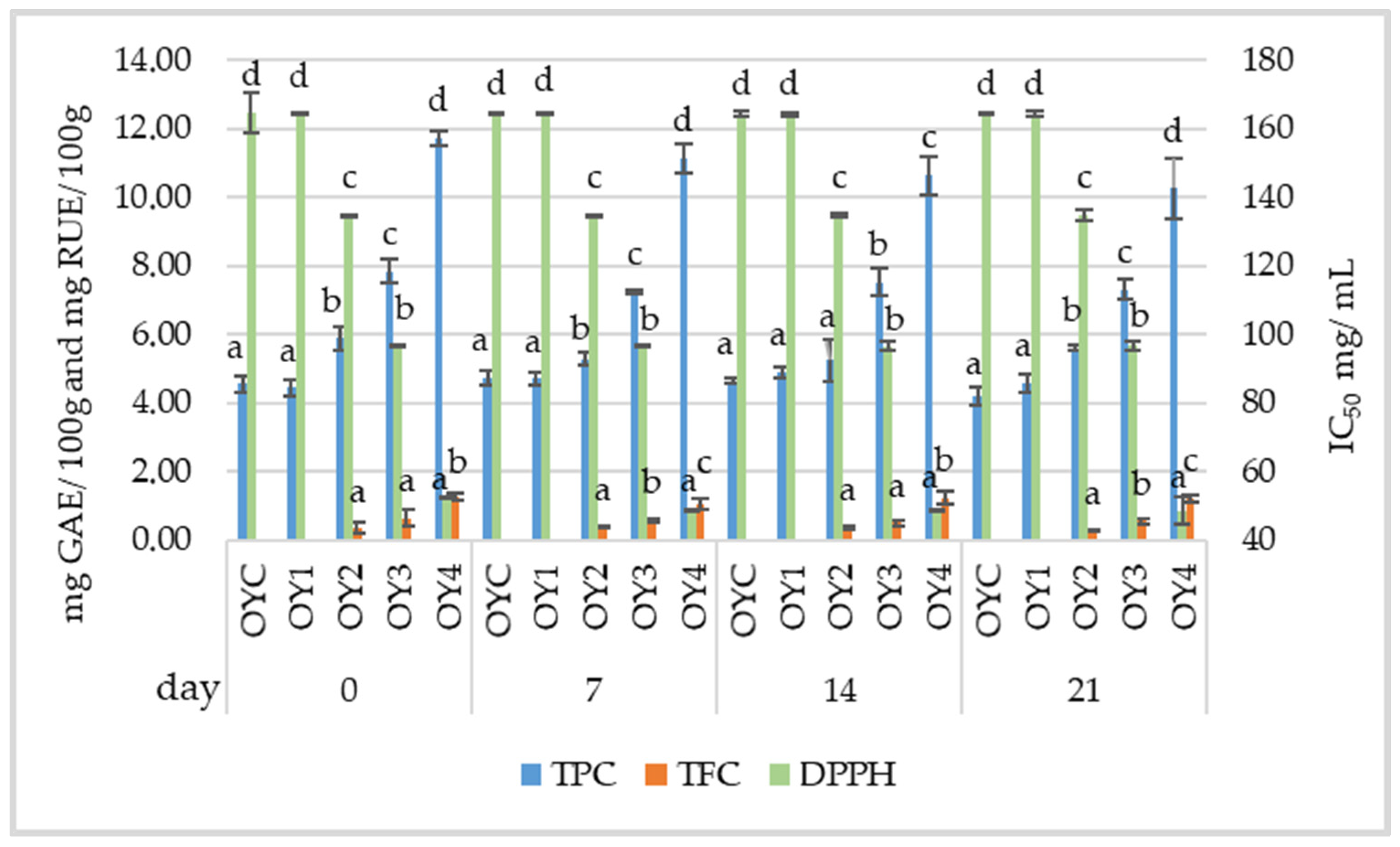
3.2. Investigation on Samples from Homemade Yogurt
3.2.1. Physicochemical Analysis of Homemade Yogurt Samples
3.2.2. Microbiological Analysis of Homemade Yogurt Samples
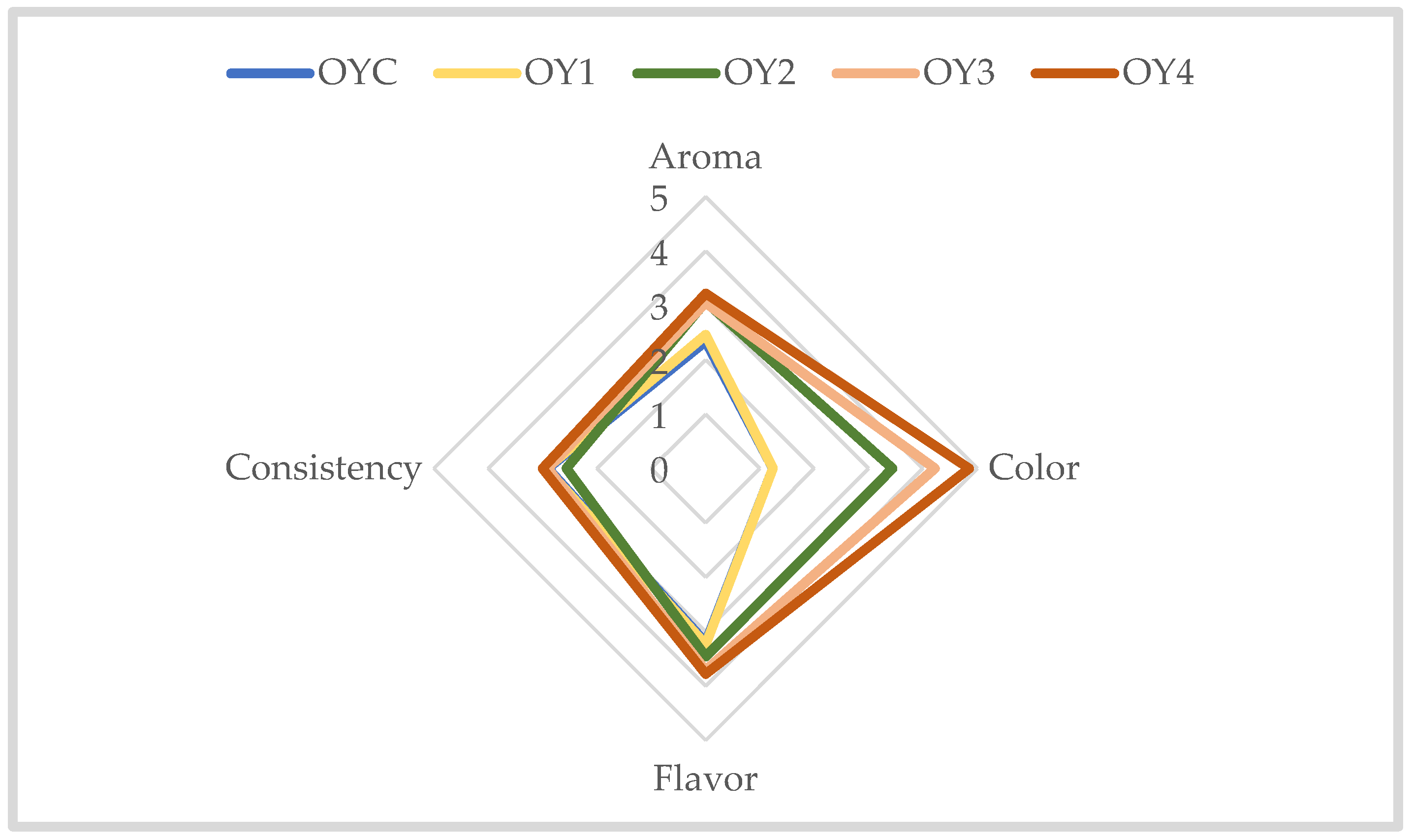
3.2.3. Sensorial Analysis of Homemade Yogurt Samples
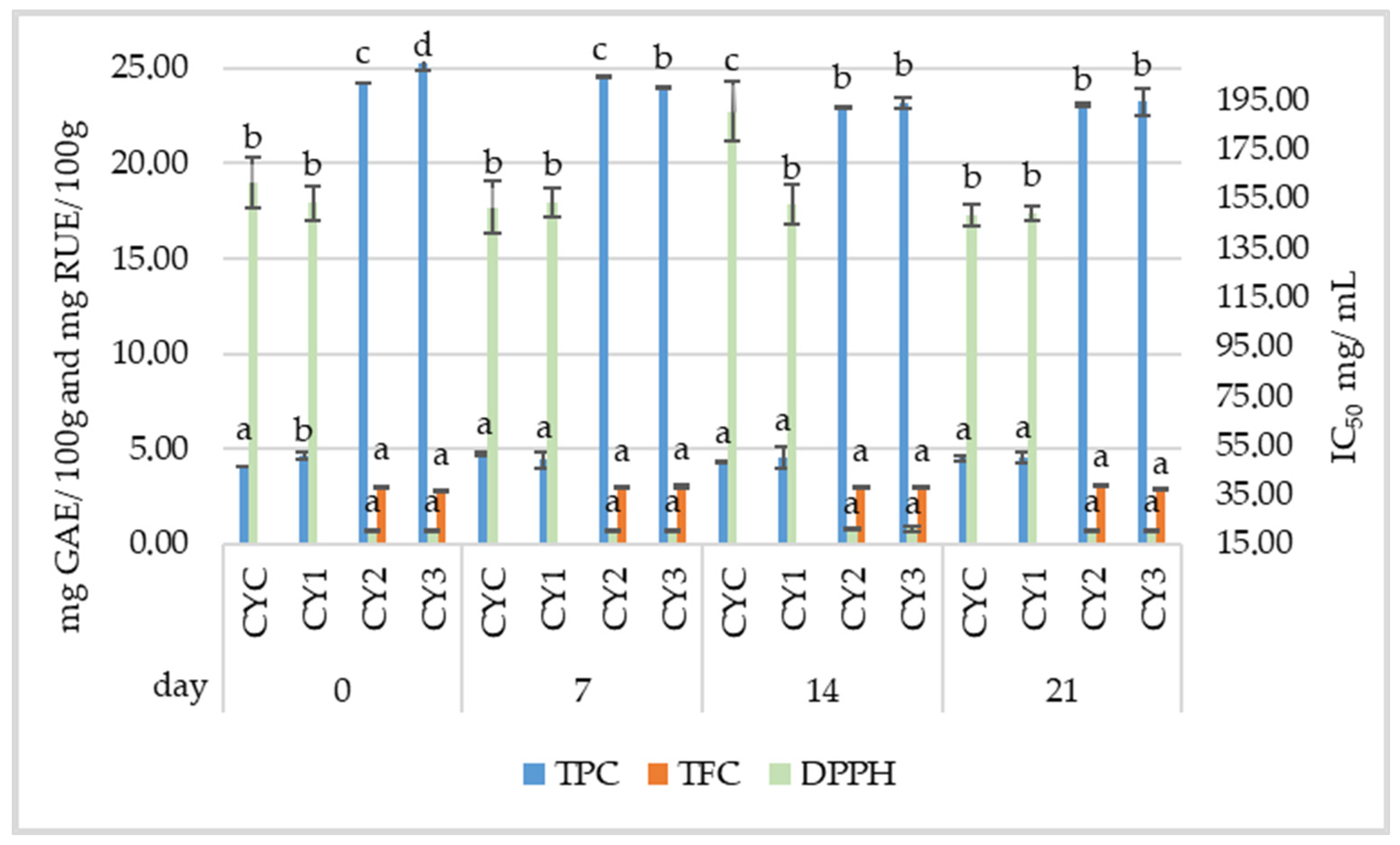
3.3. Investigation on Samples from Commercial Yogurt
3.3.1. Physicochemical Analysis of Commercial Yogurt Samples
3.3.2. Microbiological Analysis of Commercial Yogurt Samples
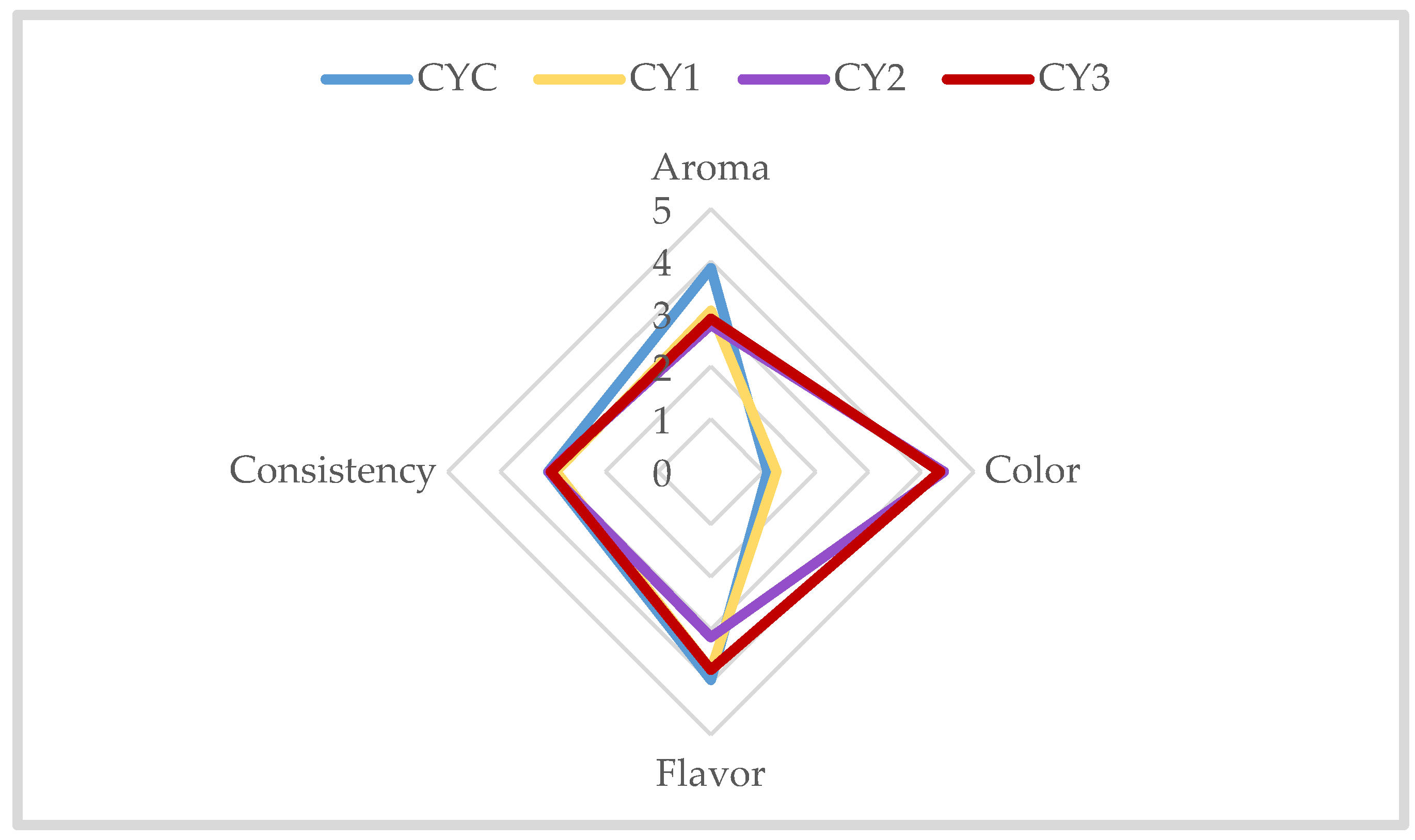
3.3.3. Sensorial Analysis of Commercial Yogurt Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atkinson, M.D.; Atkinson, E. Sambucus nigra L. J. Ecol. 2002, 90, 895–923. [Google Scholar] [CrossRef]
- Milena, V.; Tatjana, M.; Gökhan, Z.; Ivana, B.; Aleksandra, C.; Mohammad, M.F.; Marija, R. Advantages of contemporary extraction techniques for the extraction of bioactive constituents from black elderberry (Sambucus nigra L.) flowers. Ind. Crops Prod. 2019, 136, 93–101. [Google Scholar] [CrossRef]
- Mlynarczyk, K.; Walkowiak-Tomczak, D.; Lysiak, G.P. Bioactive properties of Sambucus nigra L. As a functional ingredient for food and pharmaceutical industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef]
- Toma, D.-I.; Baroi, A.M.; Din, A.; Vizitiu, D.E.; Fierascu, I.; Fierascu, R.C. Grapevine Plant Waste Utilization in Nanotechnology. AgroLife Sci. J. 2024, 13, 203–216. [Google Scholar] [CrossRef]
- Zaheer, A.H.M.; Özdemir, B.; Sevindik, M.; Eraslan, E.C.; Selamoğlu, Z.; Bal, C. Phenolic Compound and Antioxidant Potential of Hebeloma sinapizans Mushroom. AgroLife Sci. J. 2023, 12, 12–17. [Google Scholar] [CrossRef]
- Pikuli, K.; Devolli, A. Total Phenolic Content and Antioxidant Activity Evaluation of Olive Mill Pomace Extract. Sci. Bull. Ser. F Biotechnol. 2024, 28, 63–70. [Google Scholar]
- Ribeiro, A.M.; Estevinho, B.N.; Rocha, F. Microencapsulation of polyphenols–The specific case of the microencapsulation of Sambucus nigra L. extracts. Trends Foods Sci. Technol. 2020, 105, 457–467. [Google Scholar] [CrossRef]
- Stănciuc, N.; Oancea, A.M.; Aprodu, I.; Turturică, M.; Barbu, V.; Ioniţă, E.; Râpeanu, G.; Bahrim, G. Investigations on binding mechanism of bioactives from elderberry (Sambucus nigra L.) by whey proteins for efficient microencapsulation. J. Food Eng. 2017, 223, 197–207. [Google Scholar] [CrossRef]
- Nemetz, N.J.; Schieber, A.; Weber, F. Application of Crude Pomace Powder of Chokeberry, Bilberry, and Elderberry as a Coloring Foodstuff. Molecules 2021, 26, 2689. [Google Scholar] [CrossRef]
- Wang, W.D.; Xu, S.Y. Degradation kinetics of anthocyanins in blackberry juice and concentrate. J. Food Eng. 2007, 82, 271–275. [Google Scholar] [CrossRef]
- Aguiar, J.; Estevinho, B.N.; Santos, L. Microencapsulation of natural antioxidants for food application—The specific case of coffee antioxidants—A review. Trends Food Sci. Technol. 2016, 58, 21–39. [Google Scholar] [CrossRef]
- Olejnik, A.; Olkowicz, M.; Kowalska, K.; Rychlik, J.; Dembczynski, R.; Myszka, K.; Juzwa, W.; Białas, W.; Moyer, M.P. Gastrointestinal digested Sambucus nigra L. fruit extract protects in vitro cultured human colon cells against oxidative stress. Food Chem. 2016, 197, 648–657. [Google Scholar] [CrossRef]
- Salvador, Â.C.; Król, E.; Lemos, V.C.; Santos, S.A.O.; Bento, F.P.M.S.; Costa, C.P.; Almeida, A.; Szczepankiewicz, D.; Kulczyński, B.; Krejpcio, Z.; et al. Effect of elderberry (Sambucus nigra L.) extract supplementation in STZ-induced diabetic rats fed with a high-fat diet. Int. J. Mol. Sci. 2017, 18, 13. [Google Scholar] [CrossRef]
- Opriș, R.; Tatomirn, C.; Olteanu, D.; Moldovan, R.; Moldovan, B.; David, L.; Nagy, A.; Decea, N.; Kiss, M.L.; Filip, G.A. The effect of Sambucus nigra L. extract and phytosinthesized gold nanoparticles on diabetic rats. Colloids Surf. B Biointerfaces 2017, 150, 192–200. [Google Scholar] [CrossRef]
- Akduman, G.; Korkmaz, S.; Taşkın, T.; Güneş, E.F. Cytotoxicity of Sambucus nigra L. on Cancer Cell Line and In Vitro Antioxidant Properties. Clin. Exp. Health Sci. 2023, 13, 896–901. [Google Scholar] [CrossRef]
- Gleńsk, M.; Glinski, J.A.; Wlodarczyk, M.; Stefanowicz, P. Determination of ursolic and oleanolic acid in Sambucus fruits. Chem. Biodiver. 2014, 11, 1939–1944. [Google Scholar] [CrossRef] [PubMed]
- Boroduske, A.; Jekabsons, K.; Riekstina, U.; Muceniece, R.; Rostoks, N.; Nakurte, I. Wild Sambucus nigra L. from north-east edge of the species range: A valuable germplasm with inhibitory capacity against SARS-CoV2 S-protein RBD and hACE2 binding in vitro. Ind. Crops Prod. 2021, 165, 113438. [Google Scholar] [CrossRef]
- Hawkins, J.; Baker, C.; Cherry, L.; Dunne, E. Black elderberry (Sambucus nigra) supplementation effectively treats upper respiratory symptoms: A meta-analysis of randomized, controlled clinical trials. Complement. Ther. Med. 2019, 42, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Harnett, J.; Oakes, K.; Carè, J.; Leach, M.; Brown, D.; Cramer, H.; Pinder, T.A.; Steel, A.; Anheyer, D. The effects of Sambucus nigra berry on acute respiratory viral infections: A rapid review of clinical studies. Adv. Integr. Med. 2020, 7, 240–246. [Google Scholar]
- Senica, M.F.; Stampar, R.; Veberic, R.; Mikulic-Petkovsek, M. Processed elderberry (Sambucus nigra L.) products: A beneficial or harmful food alternative? LWT. Food Sci. Technol. 2016, 72, 182–188. [Google Scholar] [CrossRef]
- Schmitzer, V.; Veberic, R.; Slatnar, A.; Stampar, F. Elderberry (Sambucus nigra L.) wine: A product rich in health promoting compounds. J. Agric. Food Chem. 2010, 58, 10143–10146. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, T.; Fernandes, I.; Pinho, O.; Calhau, C.; Mateus, N.; Faria, A. Anthocyanin content in raspberry and elderberry: The impact of cooking and recipe composition. Int. J. Gastron. Food Sci. 2021, 24, 100316. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Jin, D.; Waterhouse, G.I.N. Effect of adding elderberry juice concentrate on the quality attributes, polyphenol contents and antioxidant activity of three fibre-enriched pastas. Food Res. Int. 2013, 54, 781–789. [Google Scholar] [CrossRef]
- da Silva, R.F.R.; Barreira, J.C.M.; Heleno, S.A.; Barros, L.; Calhelha, R.C.; Ferreira, I.C.F.R. Anthocyanin Profile of Elderberry Juice: A Natural-Based Bioactive Colouring Ingredient with Potential Food Application. Molecules 2019, 24, 2359. [Google Scholar] [CrossRef]
- Różyło, R.; Wójcik, M.; Dziki, D.; Biernacka, B.; Cacak-Pietrzak, G.; Gawlowski, S.; Zdybel, A. Freeze-dried elderberry and chokeberry as natural colorants for gluten-free wafer sheets. Int. Agrophysics 2019, 33, 217–225. [Google Scholar] [CrossRef]
- Jin, S.K.; Kim, G.D.; Jeong, J.Y. Evaluation of the Effect of Inhibiting Lipid Oxidation of Natural Plant Sources in a Meat Model System. J. Food Qual. 2021, 2021, 6636335. [Google Scholar] [CrossRef]
- Cordeiro, T.; Viegas, O.; Silva, M.; Martins, Z.E.; Fernandes, I.; Ferreira, I.M.L.P.V.O.; Pinho, O.; Mateus, N.; Calhau, C. Inhibitory effect of vinegars on the formation of polycyclic aromatic hydrocarbons in charcoal-grilled pork. Meat Sci. 2020, 167, 108083. [Google Scholar] [CrossRef]
- Cais-Sokolińska, D.; Walkowiak-Tomczak, D. Consumer-perception, nutritional, and functional studies of a yogurt with restructured elderberry juice. J. Dairy Sci. 2021, 104, 1318–1335. [Google Scholar] [CrossRef]
- Najgebauer-Lejko, D.; Liszka, K.; Tabaszewska, M.; Domagała, J. Probiotic Yoghurts with Sea Buckthorn, Elderberry, and Sloe Fruit Purees. Molecules 2021, 26, 2345. [Google Scholar] [CrossRef]
- Du, X.; Myracle, A.D. Development and evaluation of kefir products made with aronia or elderberry juice: Sensory and phytochemical characteristics. Int. Food Res. J. 2018, 25, 1373–1383. [Google Scholar]
- Díaz-López, A.; Bullo, M.; Martínez-González, M.A.; Corella, D.; Estruch, R.; Fito, M.; Gomez-Gracia, E.; Fiol, M.; Garcia de la Corte, F.J.; Ros, E.; et al. Dairy Product Consumption and Risk of Type 2 Diabetes in an Elderly Spanish Mediterranean Population at High Cardiovascular Risk. Eur. J. Nutr. 2016, 55, 349–360. [Google Scholar] [PubMed]
- Marette, A.; Picard-Deland, E. Yogurt Consumption and Impact on Health: Focus on Children and Cardiometabolic Risk. Am. J. Clin. Nutr. 2014, 99, 1243S–1247S. [Google Scholar]
- Cao, Y.; Miao, L. Consumer Perception of Clean Food Labels. Br. Food J. 2023, 125, 433–448. [Google Scholar] [CrossRef]
- Fernandez, M.A.; Marette, A. Potential Health Benefits of Combining Yogurt and Fruits Based on Their Probiotic and Prebiotic Properties. Adv. Nutr. 2017, 8, 155S–164S. [Google Scholar] [CrossRef]
- Masmoudi, M.; Ammar, I.; Ghribi, H.; Attia, H. Physicochemical, Radical Scavenging Activity, and Sensory Properties of a Soft Cheese Fortified with Arbutus unedo L. Extract. Food Biosci. 2020, 35, 100579. [Google Scholar] [CrossRef]
- Pascariu, O.-E.; Dias, L.G.; Israel-Roming, F. Optimization of Extraction Method of Bioactive Compounds from Elderberries (Sambucus nigra L.) and Testing Extract Stability. Horticulturae 2024, 10, 743. [Google Scholar] [CrossRef]
- Freitas, L.; Sousa-Dias, M.; Paula, V.B.; Dias, L.G.; Estevinho, L.M. Fermented Grapevine Leaves: Potential Preserving Agent in Yogurt. Foods 2024, 13, 2053. [Google Scholar] [CrossRef]
- Muncan, J.; Tei, K.; Tsenkova, R. Real-Time Monitoring of Yogurt Fermentation Process by Aquaphotomics Near-Infrared Spectroscopy. Sensors 2020, 21, 177. [Google Scholar] [CrossRef]
- Gueguim-Kana, E.B.; Oloke, J.K.; Lateef, A.; Zebaze-Kana, M.G. Novel Optimal Temperature Profile for Acidification Process of Lactobacillus bulgaricus and Streptococcus thermophilus in Yogurt Fermentation Using Artificial Neural Network and Genetic Algorithm. J. Ind. Microbiol. Biotechnol. 2007, 34, 491–496. [Google Scholar] [CrossRef]
- Othman, N.; Hamid, H.A.; Suleiman, N. Physicochemical Properties and Sensory Evaluation of Yogurt Nutritionally Enriched with Papaya. Food Res. 2019, 10, 199. [Google Scholar] [CrossRef]
- Sah, B.N.P.; Vasiljevic, T.; McKechnie, S.; Donkor, O.N. Physicochemical, textural and rheological properties of probiotic yogurt fortified with fibre-rich pineapple peel powder during refrigerated storage. LWT-Food. Sci. Technol. 2016, 65, 978–986. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and others oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–153. [Google Scholar]
- Pękal, A.; Pyrzynska, K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods. 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.M.M.; Mendes-Ferreira, A.; Benevides, C.M.J.; Melo, D.; Costa, A.S.G.; Mendes-Faia, A.; Oliveira, M.B.P.P. Effect of Controlled Microbial Fermentation on Nutritional and Functional Characteristics of Cowpea Bean Flours. Foods 2019, 8, 530. [Google Scholar] [CrossRef]
- Liofilchem. Rose Bengal CAF Agar. Selective Medium for Detection of Yeasts and Moulds from Food and Environmental Materials. 2015. Available online: http://www.liofilchem.net/login/pd/ifu/10034_IFU.pdf (accessed on 10 October 2023).
- Domínguez, R.; Zhang, L.; Rocchetti, G.; Lucini, L.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M. Elderberry (Sambucus nigra L.) as potential source of antioxidants. Characterization, optimization of extraction parameters and bioactive properties. Food Chem. 2020, 330, 127–266. [Google Scholar] [CrossRef]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Staniek, H.; Kidon, M.; Łysiak, G.P. The content of selected minerals, bioactive compounds, and the antioxidant properties of the flowers and fruit of selected cultivars and wildly growing plants of Sambucus nigra L. Molecules 2020, 25, 876. [Google Scholar] [CrossRef] [PubMed]
- Zielinska—Wasielica, J.; Olejnik, A.; Kowalska, K.; Olkowicz, M.; Dembczynski, R. Elderberry (Sambucus 838 nigra L.) fruit extract alleviates oxidative stress, insulin resistance, and inflammation in hypertrophied 839 3T3-L1 adipocytes and activated RAW 264.7 macrophages. Foods 2019, 8, 326. [Google Scholar] [CrossRef]
- Duymuş, H.G.; Göger, F.; Hüsnü Can Başer, K. In Vitro Antioxidant Properties and Anthocyanin Compositions of Elderberry Extracts. Food Chem. 2014, 155, 112–119. [Google Scholar] [CrossRef]
- Haș, I.M.; Teleky, B.-E.; Szabo, K.; Simon, E.; Ranga, F.; Diaconeasa, Z.M.; Purza, A.L.; Vodnar, D.-C.; Tit, D.M.; Nițescu, M. Bioactive Potential of Elderberry (Sambucus nigra L.): Antioxidant, Antimicrobial Activity, Bioaccessibility and Prebiotic Potential. Molecules 2023, 28, 3099. [Google Scholar] [CrossRef]
- Precup, G.; Pocol, C.B.; Teleky, B.-E.; Vodnar, D.C. Awareness, Knowledge, and Interest about Prebiotics–A Study among Romanian Consumers. Int. J. Environ. Res. Public Health 2022, 19, 1208. [Google Scholar] [CrossRef]
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2022, 14, 137. [Google Scholar]
- Fuke, N.; Nagata, N.; Suganuma, H.; Ota, T. Regulation of gut microbiota and metabolic endotoxemia with dietary factors. Nutrients 2019, 11, 2277. [Google Scholar] [CrossRef] [PubMed]
- Caleja, C.; Barros, L.; Antonio, A.L.; Carocho, M.; Oliveira, M.B.P.; Ferreira, I.C.F.R. Fortification of Yogurts with Different Antioxidant Preservatives: A Comparative Study between Natural and Synthetic Additives. Food Chem. 2016, 210, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Chouchouli, V.; Kalogeropoulos, N.; Konteles, S.J.; Karvela, E.; Makris, D.P.; Karathanos, V.T. Fortification of yoghurts with grape (Vitis vinifera) seed extracts. LWT-Food Sci. Technol. 2013, 53, 522–529. [Google Scholar]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough Study of Reactivity of Various Compound Classes Toward the Folin-Ciocalteu Reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, D.H.; Cho, W.Y.; Yeon, S.J.; Choi, S.H.; Lee, C.H. Effects of Lotus (Nelumbo nucifera) Leaf on Quality and Antioxidant Activity of Yogurt During Refrigerated Storage. Food Sci. Anim. Resour. 2019, 39, 792–803. [Google Scholar] [CrossRef]
- Anuyahong, T.; Chusak, C.; Adisakwattana, S. Incorporation of Anthocyanin-Rich Riceberry Rice in Yogurts: Effect on Physicochemical Properties, Antioxidant Activity, and In Vitro Gastrointestinal Digestion. LWT 2020, 129, 109571. [Google Scholar] [CrossRef]
- Bourassa, P.; Côté, R.; Hutchandani, S.; Samson, G.; Tajmir-Riahi, H.A. The Effect of Milk Alpha-Casein on the Antioxidant Activity of Tea Polyphenols. J. Photochem. Photobiol. B Biol. 2013, 128, 43–49. [Google Scholar]
- Hong, H.; Lim, J.M.; Kothari, D.; Kwon, S.H.; Kwon, H.C.; Han, S.G.; Kim, S.K. Antioxidant Properties and Diet-Related α-Glucosidase and Lipase Inhibitory Activities of Yogurt Supplemented with Safflower (Carthamus tinctorius L.) Petal Extract. Food Sci. Anim. Resour. 2021, 41, 122–134. [Google Scholar] [CrossRef]
- Helal, A.; Tagliazucchi, D. Impact of In-Vitro Gastro-Pancreatic Digestion on Polyphenols and Cinnamaldehyde Bioaccessibility and Antioxidant Activity in Stirred Cinnamon-Fortified Yogurt. LWT 2018, 89, 164–170. [Google Scholar] [CrossRef]
- Sik, B.; Székelyhidi, R.; Lakatos, E.; Kapcsándi, V.; Ajtony, Z. Analytical Procedures for Determination of Phenolics Active Herbal Ingredients in Fortified Functional Foods: An Overview. Eur. Food Res. Technol. 2022, 248, 329–344. [Google Scholar] [CrossRef]
- Kailasapathy, K.; Harmstorf, I.; Phillips, M. Survival of Lactobacillus acidophilus and Bifidobacterium animalis ssp. lactis in stirred fruit yogurts. LWT-Food Sci. Technol. 2008, 41, 1317–1322. [Google Scholar] [CrossRef]
- Ranadheera, C.S.; Evans, C.A.; Adams, M.C.; Baines, S.K. Probiotic Viability and Physico-Chemical and Sensory Properties of Plain and Stirred Fruit Yogurts Made from Goat’s Milk. Food Chem. 2012, 135, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Vinderola, C.G.; Bailo, N.; Reinheimer, J.A. Survival of Probiotic Microflora in Argentinian Yoghurts during Refrigerated Storage. Food Res. Int. 2000, 33, 97–102. [Google Scholar] [CrossRef]
- Rajapaksha, D.S.W.; Kodithuwakku, K.A.H.T.; Silva, K.F.S.T.; Rupasinghe, R.A.J.N.L. Evaluation of Potassium Sorbate and ε-Polylysine for Their Inhibitory Activity on Post-Acidification of Set Yoghurt under Cold Storage for 20 Days. Int. J. Sci. Res. Publ. 2018, 3, 6. Available online: http://www.ijsrp.org/research-paper-0613.php?rp=P181263 (accessed on 2 October 2023).
- Hamdan, I.Y.; Deane, D.D.; Kunsman, J.E. Effect of potassium sorbate on yogurt cultures. J. Milk Food Technol. 1971, 34, 307–311. [Google Scholar] [CrossRef]
- Lončarević, I.; Pajin, B.; Petrović, J.; Zarić, D.; Šaponjac, V.T.; Fišteš, A.; Jovanović, P. The Physical Properties, Polyphenol Content and Sensory Characteristics of White Chocolate Enriched with Black Tea Extract. Food Health Dis. Sci.-Profess. J. Nutr. Diet. 2019, 8, 83–88. [Google Scholar]
- Ren, L.; Chen, Y. Influence of Color Perception on Consumer Behavior. In HCI in Business, Government, and Organizations; Nah, F.H., Xiao, B., Eds.; HCIBGO 2018; Lecture Notes in Computer Science; Springer: Cham, Switzerland, 2018; Volume 10923, pp. 413–421. [Google Scholar]
- Roy, D.K.; Saha, T.; Akter, M.; Hosain, M.; Khatun, H.; Roy, M.C. Quality evaluation of yoghurt supplemented with fruit pulp (banana, papaya, and watermelon). Int. J. Food Sci. Nutr. 2015, 4, 695–699. [Google Scholar]
- Angelov, M.G.; Kostov, E.; Simova, D.; Beshkova, T.; Hristova, P.K. Proto-cooperation factors in yoghurt starter cultures. Rev. Genie Indust. 2009, 3, 4–12. [Google Scholar]
- Tarakci, Z.; Kucukoner, E. Physical, chemical, microbiological, and sensory characteristics of some fruit-flavored yoghurt. YYU Vet. Fak. Derg. 2003, 14, 10–14. [Google Scholar]
- Cutrim, C.S.; Cortez, M.A.S. A review on polyphenols: Classification, beneficial effects and their application in dairy products. Int. J. Dairy Technol. 2018, 71, 564–578. [Google Scholar] [CrossRef]
- Munin, A.; Edwards-Lévy, F. Encapsulation of Natural Polyphenolic Compounds: A Review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [PubMed]
- Castro-Muñoz, R.; Correa-Delgado, M.; Córdova-Almeida, R.; Lara-Nava, D.; Chávez-Muñoz, M.; Velásquez-Chávez, V.F.; Hernández-Torres, C.E.; Gontarek-Castro, E.; Ahmad, M.Z. Natural Sweeteners: Sources, Extraction and Current Uses in Foods and Food Industries. Food Chem. 2022, 370, 130991. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, M.; Romeih, E.; Huang, Z.; Enomoto, T.; Huang, L.; Li, L. Bioactive properties of probiotic set-yogurt supplemented with Siraitia grosvenorii fruit extract. Food Chem. 2019, 303, 125400. [Google Scholar] [CrossRef]
- Yousef, M.; Nateghi, L.; Azadi, E. Effect of Different Concentrations of Fruit Additives on Some Physicochemical Properties of Yoghurt During Storage. Ann. Biol. Res. 2013, 4, 244–249. [Google Scholar]
- Mhir, S.; Mejri, A.; Atrous, H.; Ayed, L. Optimization of Parameters Using Response Surface Methodology to Develop a Novel Kefir-Like Functional Beverage from Cheese Whey Enriched with Myrtle Juice. J. Chem. 2021, 2021, 2984470. [Google Scholar] [CrossRef]
- Durmus, N.; Capanoglu, E.; Kilic-Akyilmaz, M. Activity and Bioaccessibility of Antioxidants in Yoghurt Enriched with Black Mulberry as Affected by Fermentation and Stage of Fruit Addition. Int. Dairy J. 2021, 117, 105018. [Google Scholar] [CrossRef]
- Herrera, T.; Iriondo-DeHond, M.; Ramos Sanz, A.; Bautista, A.I.; Miguel, E. Effect of Wild Strawberry Tree and Hawthorn Extracts Fortification on Functional, Physicochemical, Microbiological, and Sensory Properties of Yogurt. Foods 2023, 12, 3332. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef]
- Varga, L. Effect of acacia (Robinia pseudo-acacia L.) honey on the characteristic microflora of yoghurt during refrigerated storage. Int. J. Food Microbiol. 2006, 108, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Belščak-Cvitanović, A.; Komes, D.; Durgo, K.; Vojvodić, A.; Bušić, A. Nettle (Urtica dioica L.) Extracts as Functional Ingredients for Production of Chocolates with Improved Bioactive Composition and Sensory Properties. J. Food Sci. Technol. 2015, 52, 7723–7734. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, P.; Chinnasamy, B.; Jin, L.; Clark, S. Use of Just-About-Right Scales and Penalty Analysis to Determine Appropriate Concentrations of Stevia Sweeteners for Vanilla Yogurt. J. Dairy Sci. 2014, 97, 3262–3272. [Google Scholar] [CrossRef] [PubMed]
- Barazi, Ü.; Arslan, S. Enhancement of Kefir Functionality by Adding Black Elderberry and Evaluation of Its Quality During Storage. Food Sci. Nutr. 2024, 12, 9325–9339. [Google Scholar] [CrossRef]
| Days | OYC | OY1 | OY2 | OY3 | OY4 |
|---|---|---|---|---|---|
| 0 | 4.17 a ± 0.01 | 4.18 a ± 0.01 | 4.18 a ± 0.02 | 4.17 a ± 0.02 | 4.24 b ± 0.03 |
| 7 | 4.14 a ± 0.02 | 4.15 a ± 0.01 | 4.17 b ± 0.01 | 4.16 a,b ± 0.00 | 4.25 c ± 0.01 |
| 14 | 4.20 b ± 0.02 | 4.25 c ± 0.02 | 4.11 a ± 0.01 | 4.09 a ± 0.01 | 4.09 a ± 0.01 |
| 21 | 4.24 b ± 0.04 | 4.22 b ± 0.01 | 4.11 a ± 0.01 | 4.11 c ± 0.01 | 4.10 a ± 0.01 |
| Sample | L* | a* | b* | C* | °hue |
|---|---|---|---|---|---|
| OYC | 89.10 e ± 0.10 | −2.48 a ± 0.02 | 8.72 e ± 0.05 | 9.06 b ± 0.05 | 105.85 d ± 0.08 |
| OY1 | 88.47 d ± 0.18 | −2.47 a ± 0.07 | 8.11 d ± 0.12 | 8.48 a ± 0.12 | 106.97 e ± 0.40 |
| OY2 | 78.55 c ± 0.10 | 9.23 b ± 0.02 | 2.71 c ± 0.02 | 9.63 c ± 0.01 | 16.35 c ± 0.09 |
| OY3 | 75.50 b ± 0.05 | 12.41 c ± 0.06 | 1.90 b ± 0.05 | 12.56 d ± 0.07 | 8.70 b ± 0.19 |
| OY4 | 69.09 a ± 0.07 | 17.99 d ± 0.08 | 0.64 a ± 0.02 | 18.00 e ± 0.08 | 2.03 a ± 0.05 |
| Day | Yogurt | LAB | Mesophilic Bacteria | Psychrophilic Bacteria | Yeasts and Molds | Coliform Bacteria |
|---|---|---|---|---|---|---|
| 0 | OYC | 8.74 a,b ± 0.09 | 7.02 e ± 0.08 | 2.26 ± 0.06 | 1.72 ± 0.06 | ND |
| OY1 | 8.71 a ± 0.06 | 6.28 b ± 0.05 | <1 | <1 | ND | |
| OY2 | 8.69 a ± 0.02 | 5.94 a ± 0.06 | 1.60 ± 0.03 | <1 | ND | |
| OY3 | 8.82 b ± 0.07 | 6.88 d ± 0.08 | <1 | <1 | ND | |
| OY4 | 8.78 a,b ± 0.04 | 6.59 c ± 0.09 | <1 | <1 | ND | |
| 7 | OYC | 8.33 a ± 0.10 | 7.18 b ± 0.05 | 2.30 ± 0.04 | 1.80 ± 0.02 | ND |
| OY1 | 8.32 a ± 0.06 | 6.32 a ± 0.09 | <1 | <1 | ND | |
| OY2 | 8.31 a ± 0.05 | 6.35 a ± 0.04 | <1 | <1 | ND | |
| OY3 | 8.29 a ± 0.08 | 7.59 d ± 0.07 | <1 | <1 | ND | |
| OY4 | 8.25 a ± 0.05 | 7.32 c ± 0.02 | <1 | <1 | ND | |
| 14 | OYC | 7.60 d ± 0.10 | 6.58 c ± 0.09 | 2.99 ± 0.07 | 2.04 ± 0.09 | ND |
| OY1 | 6.39 a ± 0.09 | 5.91 a ± 0.02 | <1 | <1 | ND | |
| OY2 | 6.59 b ± 0.08 | 5.89 a ± 0.09 | <1 | <1 | ND | |
| OY3 | 6.76 c ± 0.04 | 6.23 b ± 0.03 | <1 | <1 | ND | |
| OY4 | 6.81 c ± 0.06 | 5.82 a ± 0.05 | <1 | <1 | ND | |
| 21 | OYC | 6.90 d ± 0.09 | 5.43 d ± 0.07 | 3.71 ± 0.10 | 3.32 ± 0.07 | ND |
| OY1 | 6.37 b ± 0.03 | 4.62 b ± 0.04 | <1 | <1 | ND | |
| OY2 | 5.34 a ± 0.04 | 5.67 e ± 0.05 | <1 | <1 | ND | |
| OY3 | 6.49 c ± 0.07 | 5.23 c ± 0.07 | <1 | <1 | ND | |
| OY4 | 6.51 c ± 0.04 | 4.34 a ± 0.04 | <1 | <1 | ND |
| Days | CYC | CY1 | CY2 | CY3 |
|---|---|---|---|---|
| 0 | 4.51 a ± 0.02 | 4.53 a ± 0.02 | 4.50 a ± 0.01 | 4.53 a ± 0.01 |
| 7 | 4.55 a ± 0.02 | 4.57 b ± 0.01 | 4.58 b ± 0.01 | 4.54 a ± 0.01 |
| 14 | 4.61 b ± 0.02 | 4.61 b ± 0.02 | 4.62 b ± 0.01 | 4.59 a ± 0.01 |
| 21 | 4.57 c ± 0.01 | 4.54 b ± 0.01 | 4.56 c ± 0.00 | 4.53 a ± 0.01 |
| Sample | L* | a* | b* | C* | °hue |
|---|---|---|---|---|---|
| CYC | 93.45 d ± 0.01 | −1.75 a ± 0.02 | 10.04 c ± 0.03 | 10.19 a ± 0.03 | 99.87 b ± 0.08 |
| CY1 | 92.88 c ± 0.01 | −1.58 b ± 0.01 | 10.49 d ± 0.01 | 10.60 b ± 0.02 | 98.57 a ± 0.06 |
| CY2 | 67.39 b ± 0.03 | 18.85 c ± 0.03 | −2.40 a ± 0.02 | 19.00 c ± 0.03 | 352.73 c ± 0.07 |
| CY3 | 66.45 a ± 0.03 | 19.05 d ± 0.03 | −2.24 b ± 0.05 | 19.18 d ± 0.03 | 353.30 d ± 0.15 |
| Day | Yogurt | LAB | Mesophilic Bacteria | Psychrophilic Bacteria | Yeasts and Molds | Coliform Bacteria |
|---|---|---|---|---|---|---|
| 0 | CYC | 6.46 b ± 0.04 | 6.31 a ± 0.04 | <1 | <1 | ND |
| CY1 | 6.34 a ± 0.08 | 6.31 a ± 0.04 | <1 | <1 | ND | |
| CY2 | 6.30 a ± 0.07 | 6.30 a ± 0.06 | <1 | <1 | ND | |
| CY3 | 6.40 a,b ± 0.03 | 6.29 a ± 0.02 | <1 | <1 | ND | |
| 7 | CYC | 6.17 a ± 0.11 | 6.33 a ± 0.16 | <1 | <1 | ND |
| CY1 | 6.30 a ± 0.12 | 6.32 a ± 0.08 | <1 | <1 | ND | |
| CY2 | 6.34 a ± 0.06 | 6.27 a ± 0.10 | <1 | <1 | ND | |
| CY3 | 6.33 a ± 0.03 | 6.33 a ± 0.04 | <1 | <1 | ND | |
| 14 | CYC | 6.25 a ± 0.02 | 6.25 a ± 0.04 | <1 | <1 | ND |
| CY1 | 6.28 a ± 0.08 | 6.24 a ± 0.03 | <1 | <1 | ND | |
| CY2 | 6.22 a ± 0.06 | 6.31 a ± 0.06 | <1 | <1 | ND | |
| CY3 | 6.18 a ± 0.03 | 6.26 a ± 0.03 | <1 | <1 | ND | |
| 21 | CYC | 5.95 a ± 0.10 | 6.19 a ± 0.02 | <1 | <1 | ND |
| CY1 | 6.01 a ± 0.09 | 6.17 a ± 0.06 | <1 | <1 | ND | |
| CY2 | 6.00 a ± 0.11 | 6.24 a ± 0.07 | <1 | <1 | ND | |
| CY3 | 6.01 a ± 0.10 | 6.20 a ± 0.05 | <1 | <1 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascariu, O.-E.; Estevinho, L.M.; Seixas, N.L.; Dopcea, I.; Boiu-Sicuia, O.A.; Geicu-Cristea, M.; Israel-Roming, F. Antioxidant Properties and Microbiological Stability of Yogurt Enriched with Elderberry Extract. Foods 2025, 14, 1251. https://doi.org/10.3390/foods14071251
Pascariu O-E, Estevinho LM, Seixas NL, Dopcea I, Boiu-Sicuia OA, Geicu-Cristea M, Israel-Roming F. Antioxidant Properties and Microbiological Stability of Yogurt Enriched with Elderberry Extract. Foods. 2025; 14(7):1251. https://doi.org/10.3390/foods14071251
Chicago/Turabian StylePascariu, Oana-Elena, Letícia M. Estevinho, Natália L. Seixas, Ioan Dopcea, Oana A. Boiu-Sicuia, Mihaela Geicu-Cristea, and Florentina Israel-Roming. 2025. "Antioxidant Properties and Microbiological Stability of Yogurt Enriched with Elderberry Extract" Foods 14, no. 7: 1251. https://doi.org/10.3390/foods14071251
APA StylePascariu, O.-E., Estevinho, L. M., Seixas, N. L., Dopcea, I., Boiu-Sicuia, O. A., Geicu-Cristea, M., & Israel-Roming, F. (2025). Antioxidant Properties and Microbiological Stability of Yogurt Enriched with Elderberry Extract. Foods, 14(7), 1251. https://doi.org/10.3390/foods14071251









