Evaluation of White Grape Marc Extract as an Additive to Extend the Shelf-Life of Fish Fillets
Abstract
1. Introduction
2. Materials and Methods
2.1. Additives
2.2. GME Total Phenolic Content
2.3. GME Ferric Reducing Antioxidant Power (FRAP)
2.4. GME Radical Scavenging Activity Determination (DPPH)
2.5. Experimental Design
2.6. Total Viable Counts
2.7. Lipid Oxidation
2.8. pH and Water Holding Capacity (WHC)
2.9. Texture Profile Analysis (TPA)
2.10. Instrumental Color
2.11. Statistics
3. Results
3.1. Grape Marc Extract (GME) Total Phenolics and Antioxidant Activities
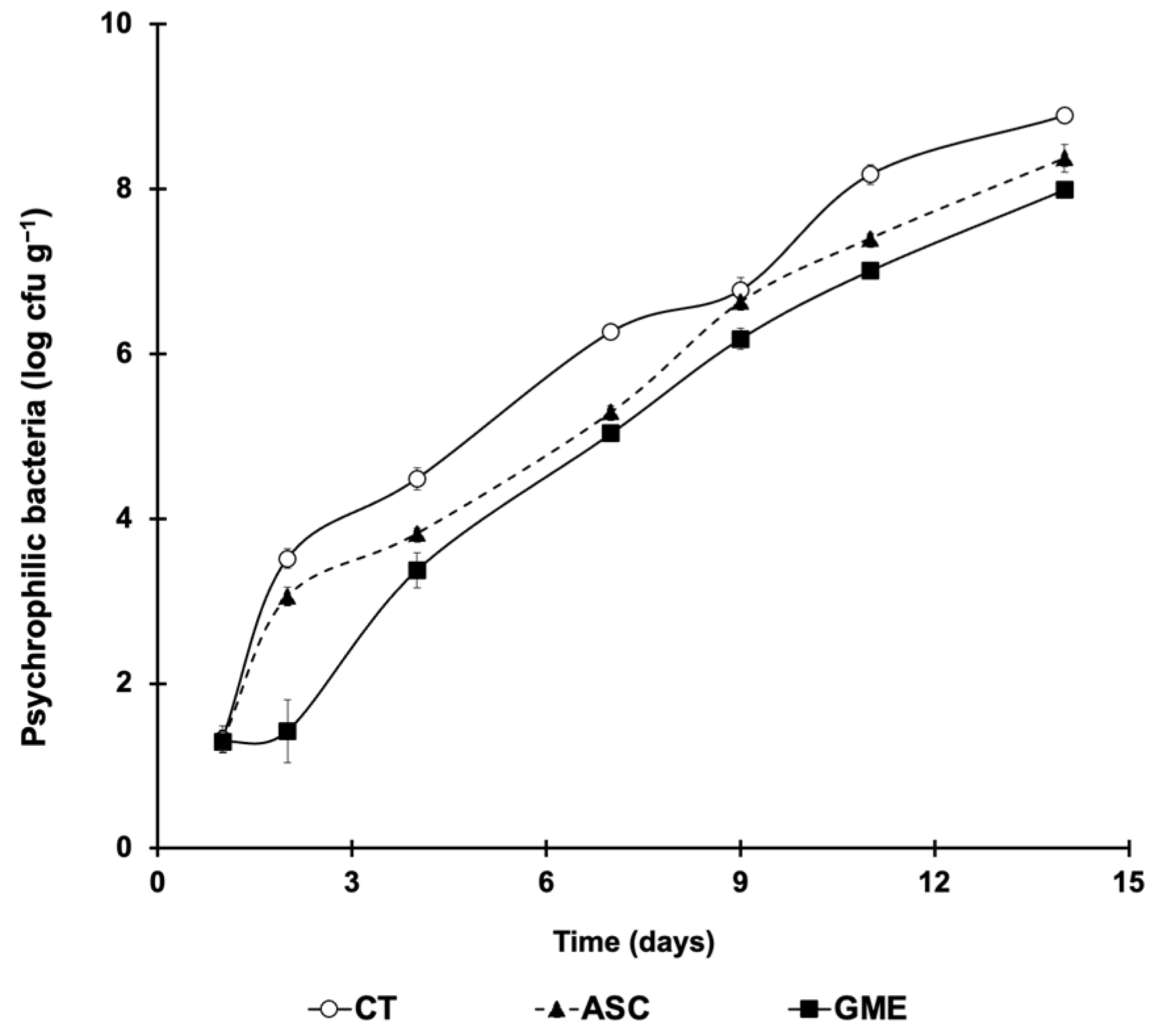
3.2. Total Viable Counts
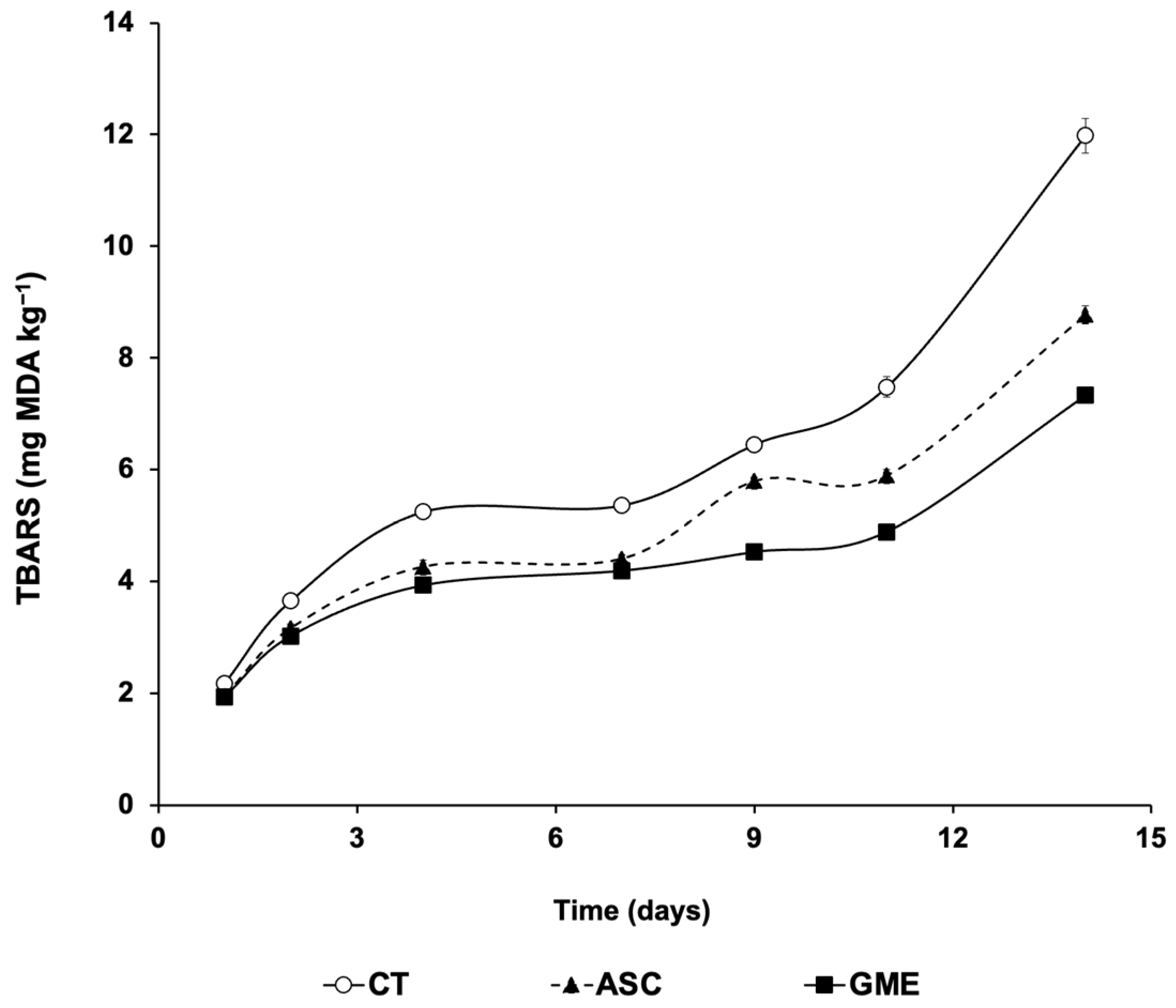
3.3. Lipid Oxidation
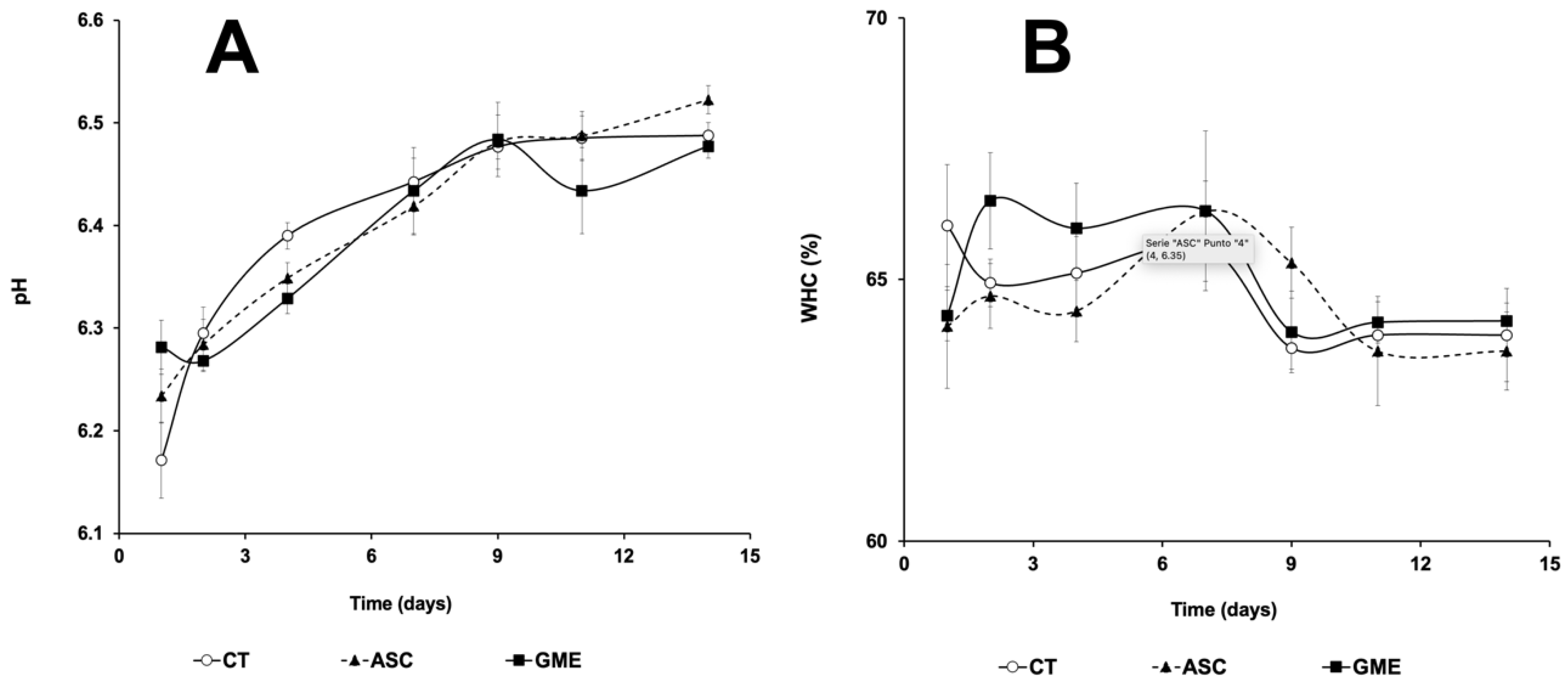
3.4. Fillet pH and Water Holding Capacity (WHC)
3.5. Fillet Texture Profile Analysis (TPA)
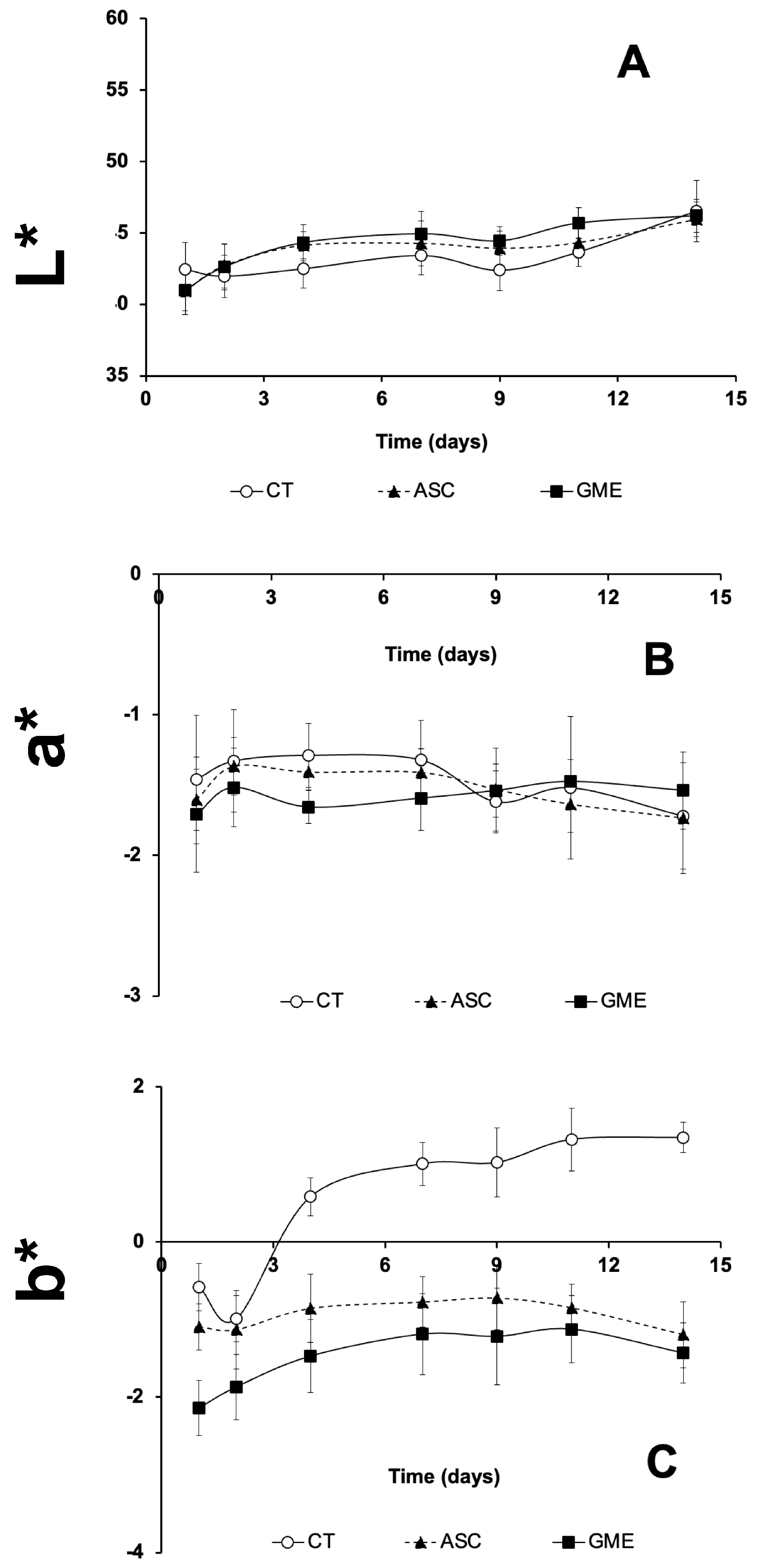
3.6. Instrumental Color
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dawood, M.A.O.; Habotta, O.A.E.; Elsabagh, M.; Azra, M.N.; Van Doan, H.; Kari, Z.A.; Sewilam, H. Fruit processing by-products in the aquafeed industry: A feasible strategy for aquaculture sustainability. Rev. Aquac. 2022, 14, 1945–1965. [Google Scholar] [CrossRef]
- Habotta, O.A.; Dawood, M.A.O.; Kari, Z.A.; Tapingkae, W.; Van Doan, H. Antioxidative and Immunostimulant Potential of fruit derived biomolecules in aquaculture. Fish. Shellfish. Immunol. 2022, 130, 317–322. [Google Scholar] [CrossRef] [PubMed]
- EUROSTAT. Vineyards in the EU-Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Vineyards_in_the_EU_-_statistics (accessed on 10 March 2025).
- Câmara, J.S.; Lourenço, S.; Silva, C.; Lopes, A.; Andrade, C.; Perestrelo, R. Exploring the potential of wine industry by-products as source of additives to improve the quality of aquafeed. Microchem. J. 2020, 155, 104758. [Google Scholar] [CrossRef]
- Barros, A.; Gironés-Vilaplana, A.; Texeira, A.; Baenas, N.; Domínguez-Perles, R. Grape stems as a source of bioactive compounds: Application towards added-value commodities and significance for human health. Phytochem. Rev. 2015, 14, 921–931. [Google Scholar] [CrossRef]
- Bordiga, M.; Travaglia, F.; Locatelli, M. Valorisation of grape pomace: An approach that is increasingly reaching its maturity—A review. Int. J. Food Sci. Technol. 2019, 54, 933–942. [Google Scholar] [CrossRef]
- Guerra-Rivas, C.; Vieira, C.; Rubio, B.; Martínez, B.; Gallardo, B.; Mantecón, A.R.; Lavín, P.; Manso, T. Effects of grape pomace in growing lamb diets compared with vitamin e and grape seed extract on meat shelf life. Meat Sci. 2016, 116, 221–229. [Google Scholar] [CrossRef]
- Chedea, V.S.; Pelmus, R.S.; Lazar, C.; Pistol, G.C.; Calin, L.G.; Toma, S.M.; Dragomir, C.; Taranu, I. Effects of a diet containing dried grape pomace on blood metabolites and milk composition of dairy cows. J. Sci. Food Agric. 2017, 97, 2516–2523. [Google Scholar] [CrossRef]
- Peña, E.; Badillo-Zapata, D.; Viana, M.T.; Correa-Reyes, G. Use of grape pomace in formulated feed for the rainbow trout fry, Oncorhynchus mykiss (Walbaum, 1792). J. World Aquac. Soc. 2020, 51, 542–550. [Google Scholar] [CrossRef]
- Mousavi, S.; Sheikhzadeh, N.; Hamidian, G.; Mardani, K.; Oushani, A.K.; Firouzamandi, M.; Esteban, M.Á.; Shohreh, P. Changes in rainbow trout (Oncorhynchus mykiss) growth and mucosal immune parameters after dietary administration of grape (Vitis vinifera) seed extract. Fish. Physiol. Biochem. 2021, 47, 547–563. [Google Scholar] [CrossRef]
- Mehrinakhi, Z.; Ahmadifar, E.; Sheikhzadeh, N.; Moghadam, M.S.; Dawood, M.A.O. Extract of grape seed enhances the growth performance, humoral and mucosal immunity, and resistance of common carp (Cyprinus carpio) against Aeromonas Hydrophila. Ann. Anim. Sci. 2021, 21, 217–232. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Meneghetti, B.B.; Soares, I.H.B.T.; Soares, C.T.; Bevilaqua, G.; Fakhouri, F.M.; de Oliveira, R.A. Multipurpose arrowroot starch films with anthocyanin-rich grape pomace extract: Color migration for food simulants and monitoring the freshness of fish meat. Int. J. Biol. Macromol. 2024, 265, 130934. [Google Scholar] [CrossRef] [PubMed]
- Aresta, A.M.; De Vietro, N.; Gubitosa, J.; Rizzi, V.; De Pasquale, I.; Fini, P.; Cosma, P.; Curri, M.L.; Zambonin, C. Effect of a composite alginate/grape pomace extract packaging material for improving meat storage. Int. J. Mol. Sci. 2023, 24, 15958. [Google Scholar] [CrossRef] [PubMed]
- Rathod, N.B.; Ranveer, R.C.; Benjakul, S.; Kim, S.; Pagarkar, A.U.; Patange, S.; Ozogul, F. Recent developments of natural antimicrobials and antioxidants on fish and fishery food products. Compr. Rev. Food Sci. Food Safe 2021, 20, 4182–4210. [Google Scholar] [CrossRef] [PubMed]
- EU Regulation. Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on Food Additives. Off. J. Eur. Union. 2008, L354, 16–33. [Google Scholar]
- Van Haute, S.; Raes, K.; Van Der Meeren, P.; Sampers, I. The effect of cinnamon, oregano and thyme essential oils in marinade on the microbial shelf life of fish and meat products. Food Control 2016, 68, 30–39. [Google Scholar] [CrossRef]
- Ozogul, Y.; Kuley Boğa, E.; Akyol, I.; Durmus, M.; Ucar, Y.; Regenstein, J.M.; Köşker, A.R. Antimicrobial activity of thyme essential oil nanoemulsions on spoilage bacteria of fish and food-borne pathogens. Food Biosci. 2020, 36, 100635. [Google Scholar] [CrossRef]
- Fontana, A.R.; Antoniolli, A.; Bottini, R. Grape pomace as a sustainable source of bioactive compounds: Extraction, characterization, and biotechnological applications of phenolics. J. Agric. Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M. Functional components of grape pomace: Their composition, biological properties and potential applications. Int. J. Food Sci. Technol. 2013, 48, 221–237. [Google Scholar] [CrossRef]
- Sánchez-Alonso, I.; Jiménez-Escrig, A.; Saura-Calixto, F.; Borderías, A.J. Antioxidant protection of white grape pomace on restructured fish products during frozen storage. LWT Food Sci. Technol. 2008, 41, 42–50. [Google Scholar] [CrossRef]
- Singh, R.P.; Chidambara Murthy, K.N.; Jayaprakasha, G.K. Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J. Agric. Food Chem. 2002, 50, 81–86. [Google Scholar] [CrossRef]
- Hajimahmoodi, M.; Faramarzi, M.A.; Mohammadi, N.; Soltani, N.; Oveisi, M.R.; Nafissi-Varcheh, N. Evaluation of antioxidant properties and total phenolic contents of some strains of microalgae. J. Appl. Phycol. 2010, 22, 43–50. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Sáez, M.I.; Martínez, T.F.; Cárdenas, S.; Suárez, M.D. Effects of different preservation strategies on microbiological counts, lipid oxidation and color of cultured meagre (Argyrosomus regius, L.) fillets: Preservation methods in cold-stored meagre fillets. J. Food Process Preserv. 2015, 39, 768–775. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. [30]Microsomal lipid peroxidation. In Methods in enzymology; Elsevier: Amsterdam, The Netherlands, 1978; Volume 52, pp. 302–310. ISBN 978-0-12-181952-1. [Google Scholar]
- Molina, B.; Sáez, M.I.; Martínez, T.F.; Guil-Guerrero, J.L.; Suárez, M.D. Effect of ultraviolet light treatment on microbial contamination, some textural and organoleptic parameters of cultured sea bass fillets (Dicentrarchus labrax). Innov. Food Sci. Emerg. Technol. 2014, 26, 205–213. [Google Scholar] [CrossRef]
- Suárez, M.D.; Martínez, T.F.; Sáez, M.I.; Morales, A.E.; García-Gallego, M. Effects of dietary restriction on post-mortem changes in white muscle of sea bream (Sparus aurata). Aquaculture 2010, 307, 49–55. [Google Scholar] [CrossRef]
- Bourne, M.C. Texture profile analysis. Food Technol. 1978, 32, 62–66. [Google Scholar]
- Commission Internationale de l’Eclairage, CIE. Colorimetry. In CIE Pub. No. 15.2; CIE: Vienna, Austria, 1986. [Google Scholar]
- Ferreira, D.; Marais, J.P.J.; Coleman, C.M.; Slade, D. Proanthocyanidins: Chemistry and biology. In Comprehensive Natural Products II; Elsevier: Amsterdam, The Netherlands, 2010; pp. 605–661. ISBN 978-0-08-045382-8. [Google Scholar]
- Gil-Sánchez, I.; Cueva, C.; Sanz-Buenhombre, M.; Guadarrama, A.; Moreno-Arribas, M.V.; Bartolomé, B. Dynamic gastrointestinal digestion of grape pomace extracts: Bioaccessible phenolic metabolites and impact on human gut microbiota. J. Food Compost. Anal. 2018, 68, 41–52. [Google Scholar] [CrossRef]
- Moro, K.I.B.; Bender, A.B.B.; Da Silva, L.P.; Penna, N.G. Green extraction methods and microencapsulation technologies of phenolic compounds from grape pomace: A review. Food Bioprocess. Technol. 2021, 14, 1407–1431. [Google Scholar] [CrossRef]
- Alvarez-Casas, M.; Pájaro, M.; Lores, M.; Garcia-Jares, C. Characterization of grape marcs from native and foreign white varieties grown in northwestern Spain by their polyphenolic composition and antioxidant activity. Eur. Food Res. Technol. 2016, 242, 655–665. [Google Scholar] [CrossRef]
- Sáez, M.I.; Suárez, M.D.; Alarcón, F.J.; Martínez, T.F. Assessing the potential of algae extracts for extending the shelf life of rainbow trout (Oncorhynchus mykiss) fillets. Foods 2021, 10, 910. [Google Scholar] [CrossRef]
- Pazos, M.; Gallardo, J.M.; Torres, J.L.; Medina, I. Activity of grape polyphenols as inhibitors of the oxidation of fish lipids and frozen fish muscle. Food Chem. 2005, 92, 547–557. [Google Scholar] [CrossRef]
- Wang, R.; Hu, X.; Agyekumwaa, A.K.; Li, X.; Xiao, X.; Yu, Y. Synergistic effect of kojic acid and tea polyphenols on bacterial inhibition and quality maintenance of refrigerated sea bass (Lateolabrax japonicus) fillets. LWT Food Sci. Technol. 2021, 137, 110452. [Google Scholar] [CrossRef]
- Fan, W.; Chi, Y.; Zhang, S. The Use of a tea polyphenol dip to extend the shelf life of silver carp (Hypophthalmicthys molitrix) during storage in ice. Food Chem. 2008, 108, 148–153. [Google Scholar] [CrossRef]
- Huang, J.; Wang, L.; Zhu, Z.; Zhang, Y.; Xiong, G.; Li, S. Three phenolic extracts regulate the physicochemical properties and microbial community of refrigerated channel catfish fillets during storage. Foods 2023, 12, 765. [Google Scholar] [CrossRef]
- Erkan, N.; Özden, Ö. Gutted and un-gutted sea bass (Dicentrarchus labrax) stored in ice: Influence on fish quality and shelf-life. Int. J. Food Prop. 2006, 9, 331–345. [Google Scholar] [CrossRef]
- Li, Y.; Zhuang, S.; Liu, Y.; Zhang, L.; Liu, X.; Cheng, H.; Liu, J.; Shu, R.; Luo, Y. Effect of grape seed extract on quality and microbiota community of container-cultured snakehead (Channa argus) fillets during chilled storage. Food Microbiol. 2020, 91, 103492. [Google Scholar] [CrossRef]
- Negro, C.; Tommasi, L.; Miceli, A. Phenolic compounds and antioxidant activity from red grape marc extracts. Bioresour. Technol. 2003, 87, 41–44. [Google Scholar] [CrossRef]
- Guan, W.; Ren, X.; Li, Y.; Mao, L. The beneficial effects of grape seed, sage and oregano extracts on the quality and volatile flavor component of hairtail fish balls during cold storage at 4 °C. LWT Food Sci. Technol. 2019, 101, 25–31. [Google Scholar] [CrossRef]
- Raeisi, M.; Tajik, H.; Aliakbarlu, J.; Valipour, S. Effect of carboxymethyl cellulose edible coating containing zataria multiflora essential oil and grape seed extract on chemical attributes of rainbow trout meat. Vet. Res. Forum 2014, 5, 89–93. [Google Scholar]
- Hasani, S.; Hasani, M. Antimicrobial properties of grape extract on common carp (Cyprinus carpio) fillet during storage in 4 °C. Int. J. Fish. Aquat. Stud. 2014, 1, 130–136. [Google Scholar]
- Trošt, K.; Klančnik, A.; Mozetič Vodopivec, B.; Sternad Lemut, M.; Jug Novšak, K.; Raspor, P.; Smole Možina, S. Polyphenol, antioxidant and antimicrobial potential of six different white and red wine grape processing leftovers. J. Sci. Food Agric. 2016, 96, 4809–4820. [Google Scholar] [CrossRef] [PubMed]
- International Union of Microbiological Societies (ICMSF). Sampling for Microbiological Analysis: Principles and Specific Applications, 2nd ed; University of Toronto Press: Toronto, ON, Canada, 1986; ISBN 978-0-8020-5693-1. [Google Scholar]
- Zhao, X.; Chen, L.; Wongmaneepratip, W.; He, Y.; Zhao, L.; Yang, H. Effect of vacuum impregnated fish gelatin and grape seed extract on moisture state, microbiota composition, and quality of chilled seabass fillets. Food Chem. 2021, 354, 129581. [Google Scholar] [CrossRef] [PubMed]
- Huss, H.H. El Pescado Fresco: Su Calidad y Cambios de su Calidad; FAO: Rome, Italy, 1998; ISBN 978-92-5-303507-6. [Google Scholar]
- Skipnes, D.; Østby, M.L.; Hendrickx, M.E. A method for characterising cook loss and water holding capacity in heat treated cod (Gadus morhua) muscle. J. Food Eng. 2007, 80, 1078–1085. [Google Scholar] [CrossRef]
- Albashr, T.K.M.; Khidhir, Z.K.; Mahmood, A.B.; Khidhir, B.; Jaffar, H.; Muhamed, V.; Hussein, S. Uses of grape seed extract as a preservative for fish fillets under refrigeration storage. Ann. Rom. Soc. Cell Biol. 2021, 25, 3818–3822. [Google Scholar]
- Zhao, W.; Yu, D.; Xia, W. Vacuum impregnation of chitosan coating combined with water-soluble polyphenol extracts on sensory, physical state, microbiota composition and quality of refrigerated grass carp slices. Int. J. Biol. Macromol. 2021, 193, 847–855. [Google Scholar] [CrossRef]
- Lin, R.; Cheng, S.; Wang, S.; Tan, M.; Zhu, B. Influence of refrigerated storage on water status, protein oxidation, microstructure, and physicochemical qualities of atlantic mackerel (Scomber scombrus). Foods 2021, 10, 214. [Google Scholar] [CrossRef]
- Silva-Brito, F.; Alexandrino, D.A.M.; Jia, Z.; Mo, Y.; Kijjoa, A.; Abreu, H.; Carvalho, M.F.; Ozório, R.; Magnoni, L. Fish performance, intestinal bacterial community, digestive function and skin and fillet attributes during cold storage of gilthead seabream (Sparus aurata) fed diets supplemented with Gracilaria by-products. Aquaculture 2021, 541, 736808. [Google Scholar] [CrossRef]
- Sáez, M.I.; Suárez, M.D.; Martínez, T.F. Effects of alginate coating enriched with tannins on shelf life of cultured rainbow trout (Oncorhynchus mykiss) fillets. LWT Food Sci. Technol. 2020, 118, 108767. [Google Scholar] [CrossRef]


| TPC 1 | 5.48 ± 0.17 |
| FRAP 2 | 21.99 ± 0.57 |
| DPPH 3 | 7.6 ± 0.75 |
| Diets | ||||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | dpm | CT | ASC | GME | p | Treat. | Time | Treat. × Time |
| Springiness (mm) | 1 | 0.71 ± 0.02 1 | 0.69 ± 0.02 | 0.71 ± 0.02 1 | 0.547 | 0.249 | ≤0.001 | 0.099 |
| 2 | 0.75 ± 0.01 123 | 0.76 ± 0.04 | 0.74 ± 0.01 12 | 0.919 | ||||
| 4 | 0.79 ± 0.03 3 | 0.75 ± 0.01 | 0.77 ± 0.01 2 | 0.176 | ||||
| 7 | 0.74 ± 0.01 12 | 0.74 ± 0.01 | 0.74 ± 0.01 12 | 0.882 | ||||
| 9 | 0.79 ± 0.01 b,3 | 0.77 ± 0.02 b | 0.71 ± 0.01 a,1 | ≤0.001 | ||||
| 11 | 0.76 ± 0.01 23 | 0.72 ± 0.01 | 0.75 ± 0.02 12 | 0.148 | ||||
| 14 | 0.74 ± 0.01 12 | 0.75 ± 0.02 | 0.78 ± 0.03 2 | 0.425 | ||||
| p | 0.006 | 0.064 | 0.041 | |||||
| Cohesiveness | 1 | 0.47 ± 0.01 | 0.46 ± 0.02 | 0.47 ± 0.01 12 | 0.824 | 0.689 | 0.008 | 0.461 |
| 2 | 0.47 ± 0.03 | 0.51 ± 0.01 | 0.45 ± 0.01 1 | 0.166 | ||||
| 4 | 0.47 ± 0.01 a | 0.45 ± 0.01 a | 0.50 ± 0.01 b,2 | 0.011 | ||||
| 7 | 0.47 ± 0.02 | 0.45 ± 0.02 | 0.46 ± 0.01 12 | 0.810 | ||||
| 9 | 0.50 ± 0.01 | 0.49 ± 0.02 | 0.50 ± 0.02 2 | 0.783 | ||||
| 11 | 0.48 ± 0.01 | 0.46 ± 0.02 | 0.44 ± 0.04 1 | 0.596 | ||||
| 14 | 0.51 ± 0.01 | 0.50 ± 0.01 | 0.51 ± 0.02 2 | 0.737 | ||||
| p | 0.435 | 0.135 | 0.057 | |||||
| Gumminess (N mm−2) | 1 | 8.25 ± 0.38 4 | 7.94 ± 0.40 34 | 8.05 ± 0.50 34 | 0.876 | ≤0.001 | ≤0.001 | 0.595 |
| 2 | 6.89 ± 1.14 234 | 8.46 ± 0.38 4 | 7.80 ± 0.32 234 | 0.312 | ||||
| 4 | 6.98 ± 0.19 a,34 | 7.35 ± 0.37 a,234 | 8.57 ± 0.33 b,4 | 0.005 | ||||
| 7 | 6.05 ± 0.31 123 | 7.01 ± 0.32 123 | 7.34 ± 0.60 234 | 0.108 | ||||
| 9 | 5.97 ± 0.30 123 | 7.34 ± 0.71 234 | 7.07 ± 0.33 123 | 0.126 | ||||
| 11 | 5.30 ± 0.23 1 | 6.32 ± 0.52 12 | 5.93 ± 0.47 1 | 0.245 | ||||
| 14 | 5.50 ± 0.33 12 | 5.94 ± 0.58 1 | 6.60 ± 0.45 12 | 0.252 | ||||
| p | 0.002 | 0.009 | 0.002 | |||||
| Chewiness (N mm−1) | 1 | 5.93 ± 0.42 3 | 5.45 ± 0.32 123 | 5.71 ± 0.38 23 | 0.667 | 0.009 | ≤0.001 | 0.335 |
| 2 | 5.15 ± 0.87 123 | 6.43 ± 0.47 3 | 5.80 ± 0.28 23 | 0.325 | ||||
| 4 | 5.54 ± 0.29 a,23 | 5.48 ± 0.30 a,123 | 6.65 ± 0.24 b,3 | 0.014 | ||||
| 7 | 4.45 ± 0.24 12 | 5.23 ± 0.30 12 | 5.42 ± 0.45 12 | 0.120 | ||||
| 9 | 4.71 ± 0.23 12 | 5.69 ± 0.60 23 | 5.03 ± 0.28 12 | 0.233 | ||||
| 11 | 4.04 ± 0.16 1 | 4.59 ± 0.38 12 | 4.47 ± 0.44 1 | 0.506 | ||||
| 14 | 4.08 ± 0.25 1 | 4.42 ± 0.38 1 | 5.10 ± 0.30 12 | 0.085 | ||||
| p | 0.012 | 0.019 | 0.004 | |||||
| Resilience (N mm−1) | 1 | 0.21 ± 0.01 1 | 0.20 ± 0.01 1 | 0.22 ± 0.00 12 | 0.353 | 0.036 | 0.009 | ≤0.001 |
| 2 | 0.23 ± 0.01 a,12 | 0.27 ± 0.02 b,2 | 0.21 ± 0.01 a,1 | 0.005 | ||||
| 4 | 0.23 ± 0.01 b,12 | 0.20 ± 0.01 a,1 | 0.26 ± 0.01 c,3 | ≤0.001 | ||||
| 7 | 0.23 ± 0.01 12 | 0.22 ± 0.01 1 | 0.21 ± 0.01 1 | 0.337 | ||||
| 9 | 0.24 ± 0.01 2 | 0.23 ± 0.01 1 | 0.23 ± 0.01 123 | 0.608 | ||||
| 11 | 0.25 ± 0.01 b,2 | 0.20 ± 0.01 a,1 | 0.22 ± 0.02 ab,12 | 0.017 | ||||
| 14 | 0.24 ± 0.01 2 | 0.22 ± 0.01 1 | 0.24 ± 0.01 23 | 0.075 | ||||
| p | 0.039 | ≤0.001 | 0.020 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sáez, M.I.; Sabio, J.; Galafat, A.; Vizcaíno, A.J.; Alarcón-López, F.J.; Moya, T.F.M. Evaluation of White Grape Marc Extract as an Additive to Extend the Shelf-Life of Fish Fillets. Foods 2025, 14, 1438. https://doi.org/10.3390/foods14081438
Sáez MI, Sabio J, Galafat A, Vizcaíno AJ, Alarcón-López FJ, Moya TFM. Evaluation of White Grape Marc Extract as an Additive to Extend the Shelf-Life of Fish Fillets. Foods. 2025; 14(8):1438. https://doi.org/10.3390/foods14081438
Chicago/Turabian StyleSáez, María Isabel, Javier Sabio, Alba Galafat, Antonio Jesús Vizcaíno, Francisco Javier Alarcón-López, and Tomás Francisco Martínez Moya. 2025. "Evaluation of White Grape Marc Extract as an Additive to Extend the Shelf-Life of Fish Fillets" Foods 14, no. 8: 1438. https://doi.org/10.3390/foods14081438
APA StyleSáez, M. I., Sabio, J., Galafat, A., Vizcaíno, A. J., Alarcón-López, F. J., & Moya, T. F. M. (2025). Evaluation of White Grape Marc Extract as an Additive to Extend the Shelf-Life of Fish Fillets. Foods, 14(8), 1438. https://doi.org/10.3390/foods14081438








