Processing and Shelf-Life Prediction Models for Ready-to-Eat Crayfish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Ready-to-Eat Crayfish Typical Production Process
2.3. Single-Factor Test of the Ready-to-Eat Crayfish Production Process
2.4. Sensory Evaluation
2.5. Analysis on Physicochemical Properties of Ready-to-Eat Crayfish
2.5.1. Sample Processing
2.5.2. Determination of pH
2.5.3. Determination of TVC
2.5.4. Determination of Total Volatile Base Nitrogen (TVB-N)
2.5.5. Determination of Acid Value (AV)
2.5.6. Determination of Hardness and Elasticity
2.6. Kinetic Models Based on Freshness Index
2.7. Prediction of Shelf Life
2.8. Data Analysis
3. Results
3.1. Optimization of the Preparation Process of Ready-to-Eat Crayfish
3.2. Changes in Physicochemical Indicators of Ready-to-Eat Crayfish Under Different Storage Conditions
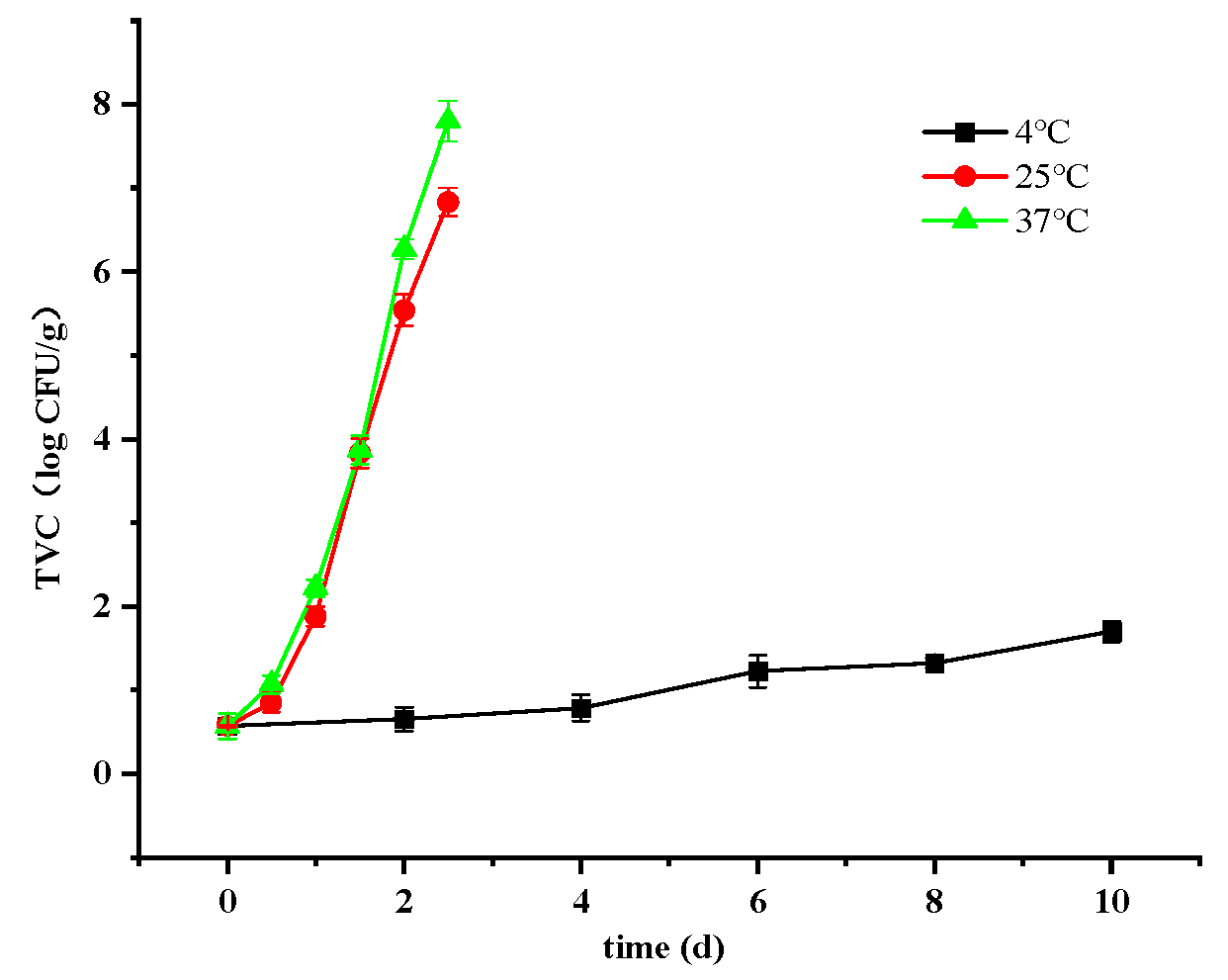
3.2.1. Changes in Total Viable Count
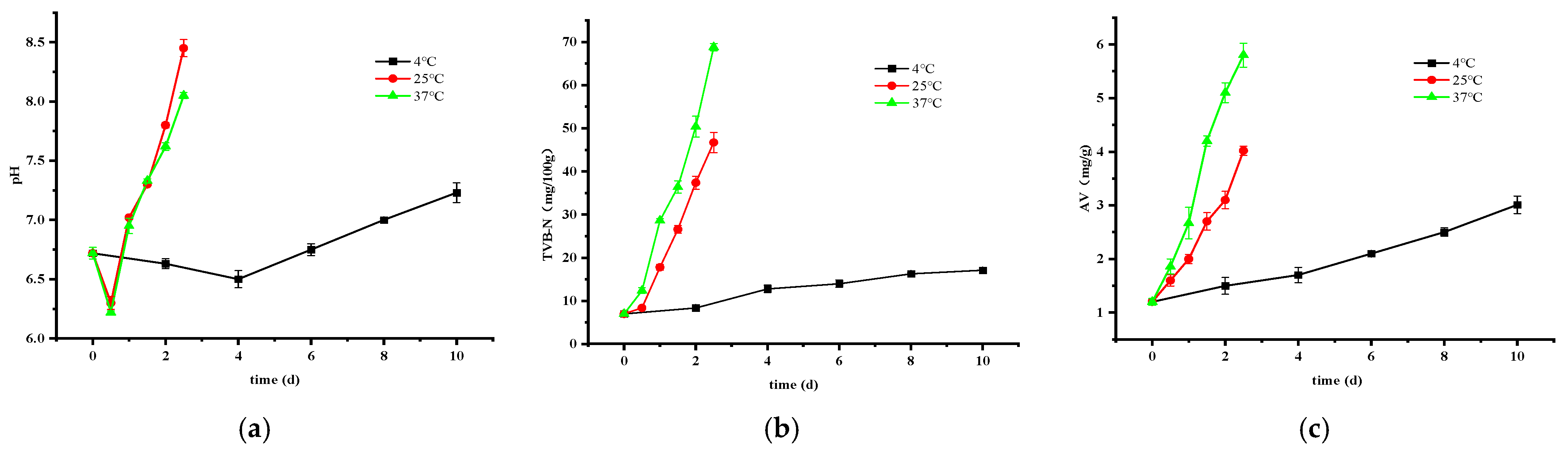
3.2.2. Changes in pH, TVB-N, and AV
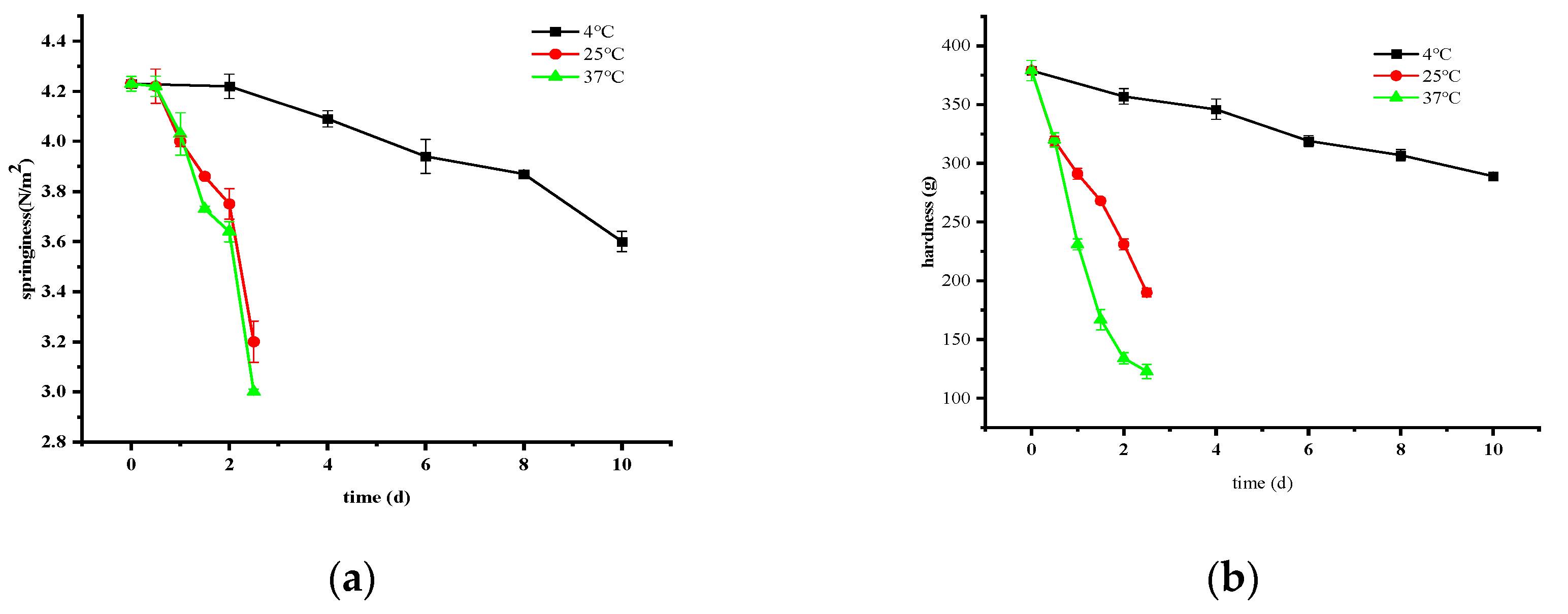
3.2.3. Changes in Springiness and Hardness
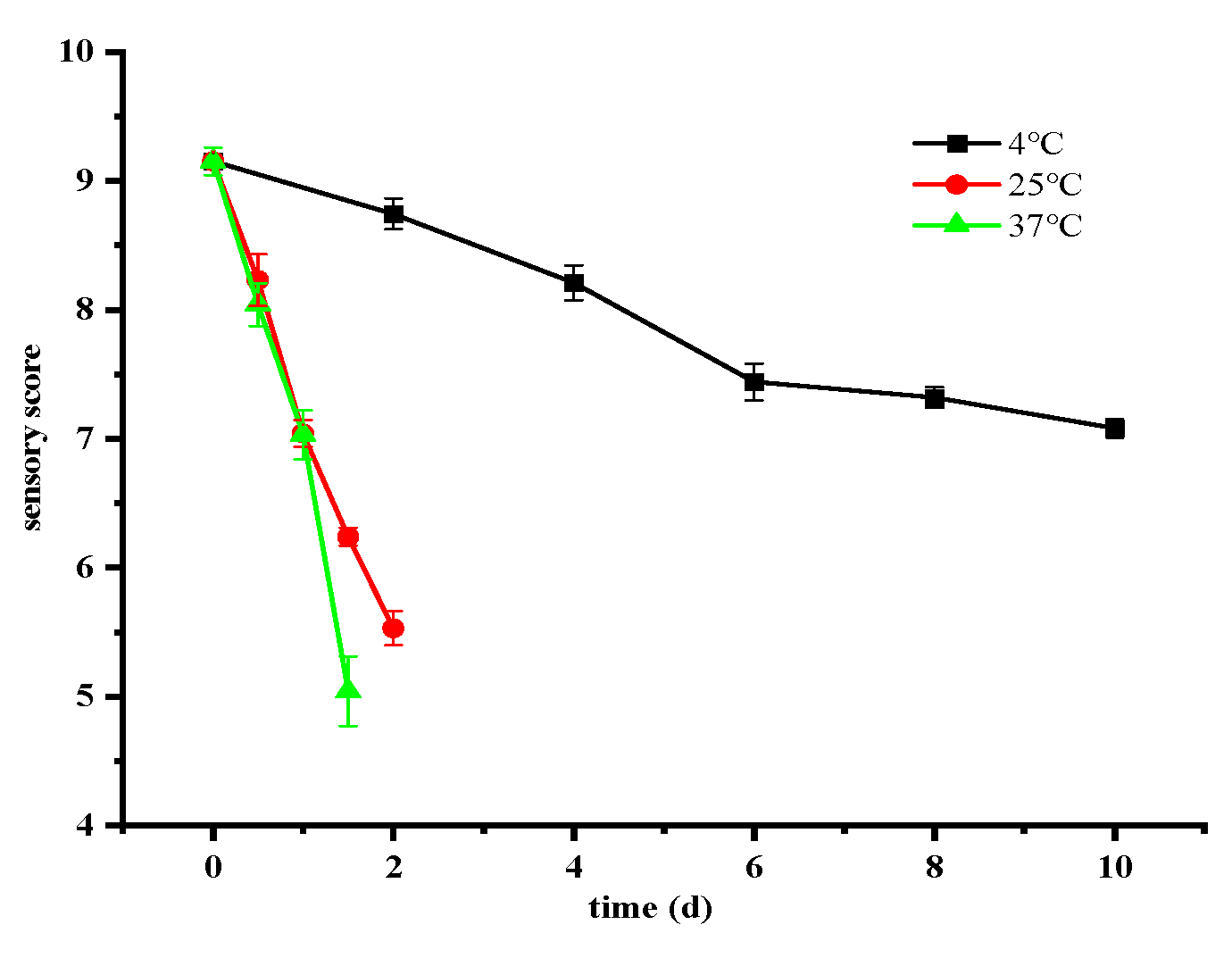
3.3. Changes in Sensory Quality of Ready-to-Eat Crayfish Under Different Storage Conditions
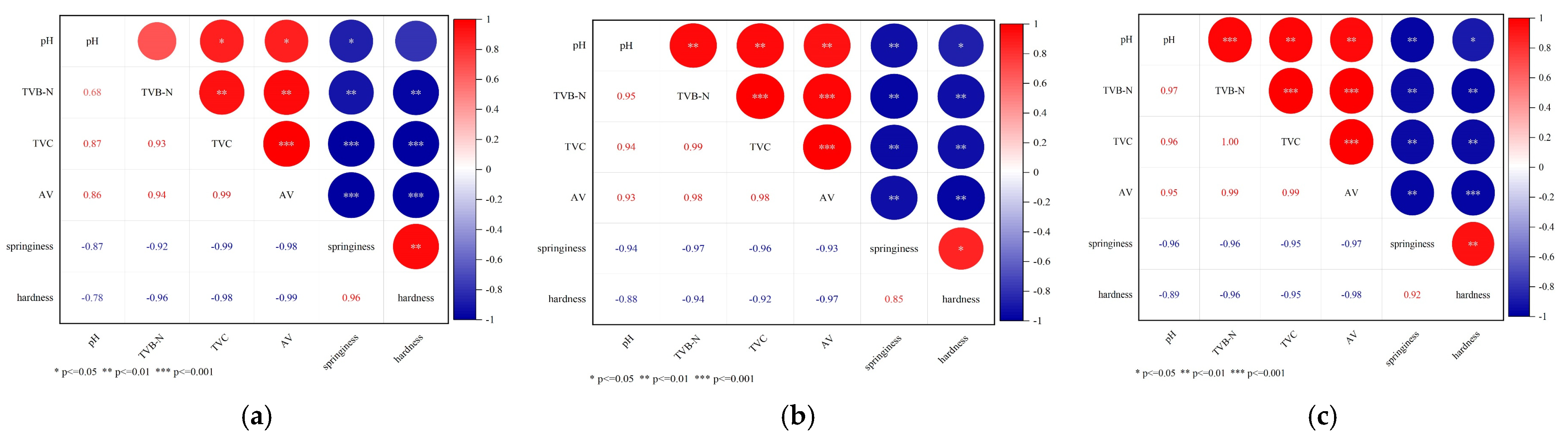
3.4. Correlation Analysis of Each Freshness Index Under Different Storage Conditions
3.5. Determination of Kinetic Parameters and Reaction Constants
3.6. Establishment of Arrhenius Equation for Quality Indicators of Ready-to-Eat Crayfish
3.7. Validation and Evaluation of Shelf-Life Prediction Models
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TVB-N | Total volatile base nitrogen |
| TVB-N | Total viable count |
| AV | Acid value |
References
- Martínez-Álvarez, Ó.; López-Caballero, M.; Gómez-Guillén, M.; Montero, P. The effect of several cooking treatments on subsequent chilled storage of thawed deepwater pink shrimp (Parapenaeus longirostris) treated with different melanosis-inhibiting formulas. LWT-Food Sci. Technol. 2009, 42, 1335–1344. [Google Scholar] [CrossRef]
- Srinivasan, P.B.; Jeyashakila, R.; Jeyasekaran, G.; Rayappan, J.B.B. Development of electronic nose (Shrimp-Nose) for the determination of perishable quality and shelf-life of cultured Pacific white shrimp (Litopenaeus vannamei). Sens. Actuators B Chem. 2020, 317, 128192. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, D.; Ren, X.; Shen, Y.; Cao, X.; Li, H.; Li, J. Quality changes and shelf-life prediction model of postharvest apples using partial least squares and artificial neural network analysis. Food Chem. 2022, 394, 133526. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Han, X.; Liu, B.; Wang, S.; Guan, W.; Wu, Z.; Theodorakis, P.E. Shelf-life prediction model of fresh-cut potato at different storage temperatures. J. Food Eng. 2022, 317, 110867. [Google Scholar] [CrossRef]
- Li, D.; Xie, H.; Liu, Z.; Li, A.; Li, J.; Liu, B.; Liu, X.; Zhou, D. Shelf life prediction and changes in lipid profiles of dried shrimp (Penaeus vannamei) during accelerated storage. Food Chem. 2019, 297, 124951. [Google Scholar] [CrossRef]
- Chen, Q.; Li, J.; Yang, H.; Qian, J. A dynamic shelf-life prediction method considering actual uncertainty: Application to fresh fruits in long-term cold storage. J. Food Eng. 2023, 349, 111471. [Google Scholar] [CrossRef]
- Zhang, W.; Luo, Z.; Wang, A.; Gu, X.; Lv, Z. Kinetic models applied to quality change and shelf life prediction of kiwifruits. LWT-Food Sci. Technol. 2020, 138, 110610. [Google Scholar] [CrossRef]
- Dabadé, D.S.; Yessoufou, N.; Adido, L.; Azokpota, P.; Hounhouigan, D.J. Quality changes, potential spoilage organisms, and shelf-life prediction of brackish river prawn (Macrobrachium macrobrachion) at different storage temperatures. Int. J. Food Microbiol. 2023, 405, 110344. [Google Scholar] [CrossRef]
- Don, S.; Xavier, K.M.; Devi, S.P.; Nayak, B.B.; Kannuchamy, N. Identification of potential spoilage bacteria in farmed shrimp (Litopenaeus vannamei): Application of Relative Rate of Spoilage models in shelf life-prediction. LWT-Food Sci. Technol. 2018, 97, 295–301. [Google Scholar] [CrossRef]
- Zaglol, N.F.; Eltadawy, F. Study on chemical quality and nutrition value of fresh water crayfish (Procambarus clarkii). J. Arab. Aquac. Soc. 2009, 4, 1–18. Available online: https://api.semanticscholar.org/CorpusID:10520086 (accessed on 1 June 2009).
- Fisheries Administration Bureau, MARA&PRC. 2023 China Fishery Statistics Yearbooks; China Agriculture Press: Beijing, China, 2023. [Google Scholar]
- Tan, Y.; Peng, B.; Wu, Y.; Xiong, L.; Sun, J.; Peng, G.; Bai, X. Human health risk assessment of toxic heavy metal and metalloid intake via consumption of red swamp crayfish (Procambarus clarkii) from rice-crayfish co-culture fields in China. Food Control 2021, 128, 108181. [Google Scholar] [CrossRef]
- Jiang, Q.; Shen, H.; Gao, P.; Yu, P.; Yang, F.; Xu, Y.; Yu, D.; Xia, W.; Wang, L. Non-enzymatic browning path analysis of ready-to-eat crayfish (Promcambarus clarkii) tails during thermal treatment and storage. Food Biosci. 2023, 51, 102334. [Google Scholar] [CrossRef]
- Delgado-Rodríguez, R.; Moreno-Padilla, M.; Moreno-Domínguez, S.; Cepeda-Benito, A. Food addiction correlates with emotional and craving reactivity to industrially prepared (ultra-processed) and home-cooked (processed) foods but not unprocessed or minimally processed foods. Food Qual. Prefer. 2023, 110, 104961. [Google Scholar] [CrossRef]
- Ebadi, Z.; Khodanazary, A.; Hosseini, S.M.; Zanguee, N. The shelf life extension of refrigerated Nemipterus japonicus fillets by chitosan coating incorporated with propolis extract. Int. J. Biol. Macromol. 2019, 139, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Temgire, S.; Borah, A.; Kumthekar, S.; Idate, A. Recent trends in ready to eat/cook food products: A review. Pharma Innov. 2021, 10, 211–217. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, G.; Hu, S. A comprehensive review on the recent technological advancements in the processing, safety, and quality control of Ready-to-Eat meals. Processes 2025, 13, 901. [Google Scholar] [CrossRef]
- Cocito, L.L.; Permigiani, S.; Tapella, F.; Lattuca, M.E.; Tomac, A.; Czerner, M.; Romero, M.C. Shelf-life of cooked meat of southern king crab (Lithodes santolla) and false king crab (Paralomis granulosa) during refrigerated storage. Heliyon 2024, 10, e36475. [Google Scholar] [CrossRef]
- Guo, X.; Yu, L.; Lu, Q.; Ding, W.; Zhong, J.; Zhang, L.; Wang, X. Quality evaluation and shelf-life prediction model establishment of frozen Chinese mitten crab (Eriocheir sinensis). LWT-Food Sci. Technol. 2023, 173, 114250. [Google Scholar] [CrossRef]
- GB 4789.2-2022; China National Standard Method for Food Safety, Microbiological Determination of Food. National Health Commission of the People’s Republic of China: Beijing, China, 2022.
- GB 5009.228-2016; China National Standard Method for Food Safety, Determination of Total Volatile Base Nitrogen in Food. National Health Commission of the People’s Republic of China: Beijing, China, 2016.
- GB 5009.229-2016; National Standard Method for Food Safety, Determination of Acid Value in Food. National Health Commission of the People’s Republic of China: Beijing, China, 2016.
- Chu, Y.; Tan, M.; Yi, Z.; Ding, Z.; Yang, D.; Xie, J. Shelf-Life Prediction of Glazed Large Yellow Croaker (Pseudosciaena crocea) during Frozen Storage Based on Arrhenius Model and Long-Short-Term Memory Neural Networks Model. Fishes 2021, 6, 39. [Google Scholar] [CrossRef]
- He, Z.; Zhang, Y.; Yang, M.; Zhang, Y.; Cui, Y.; Du, M.; Sun, H. Effect of different sweeteners on the quality, fatty acid and volatile flavor compounds of braised pork. Front. Nutr. 2022, 9, 961998. [Google Scholar] [CrossRef]
- Masniyom, P. Deterioration and shelf-life extension of fish and fishery products by modified atmosphere packaging. Songklanakarin J. Sci. Technol. 2011, 33, 181–192. Available online: https://www.thaiscience.info/journals/Article/SONG/10761805.pdf (accessed on 28 April 2011).
- Holman, B.W.; Bekhit, A.E.A.; Waller, M.; Bailes, K.L.; Kerr, M.J.; Hopkins, D.L. The association between total volatile basic nitrogen (TVB-N) concentration and other biomarkers of quality and spoilage for vacuum packaged beef. Meat Sci. 2021, 179, 108551. [Google Scholar] [CrossRef] [PubMed]
- Jay, J.M. Modern Food Microbiology; Food Science Text Series; Springer: Berlin/Heidelberg, Germany, 1995. [Google Scholar]
- Chen, Y.; Chen, H.; Gong, F.; Yang, F.; Jiang, Q.; Xu, Y.; Xia, W. A comparison of eating safety and quality of live and dead freshwater crayfish (Procambarus clarkii) at different stages. Food Res. Int. 2022, 159, 111630. [Google Scholar] [CrossRef]
- Connell, J.J. Intrinsic quality. In Control of Fish Quality; Fishing News Books: London, UK, 1995; pp. 5–36. [Google Scholar]
- Farhoosh, R.; Hoseini-Yazdi, S. Shelf-life prediction of olive oils using empirical models developed at low and high temperatures. Food Chem. 2013, 141, 557–565. [Google Scholar] [CrossRef] [PubMed]
| Spice Additions (%) | Score | Salt Addition (%) | Score | Simmering Time (min) | Score |
|---|---|---|---|---|---|
| 0.6 | 7.62 ± 0.08 c | 0.35 | 7.91 ± 0.09 d | 50 | 7.52 ± 0.11 d |
| 0.8 | 7.83 ± 0.06 c | 0.50 | 8.13 ± 0.06 c | 60 | 8.29 ± 0.07 b |
| 1.0 | 8.36 ± 0.10 b | 0.65 | 8.37 ± 0.06 b | 70 | 9.25 ± 0.10 a |
| 1.2 | 8.92 ± 0.09 a | 0.80 | 8.96 ± 0.09 a | 80 | 8.28 ± 0.12 b |
| 1.4 | 8.46 ± 0.05 b | 0.95 | 8.06 ± 0.12 cd | 90 | 7.80 ± 0.10 c |
| Indicators | Temperature (°C) | Zero-Order | First-Order | Second-Order | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2 | RSEM | ∑R2 | R2 | RSEM | ∑R2 | R2 | RSEM | ∑R2 | ||
| TVB-N | 4 | 0.956 | 0.967 | 2.906 | 0.910 | 1.382 | 2.856 | 0.873 | 1.492 | 2.556 |
| 25 | 0.972 | 2.961 | 0.972 | 2.955 | 0.886 | 4.019 | ||||
| 37 | 0.978 | 3.848 | 0.974 | 4.208 | 0.797 | 4.025 | ||||
| TVC | 4 | 0.952 | 0.108 | 2.878 | 0.970 | 0.084 | 2.888 | 0.960 | 0.105 | 2.625 |
| 25 | 0.963 | 0.551 | 0.952 | 0.534 | 0.855 | 1.278 | ||||
| 37 | 0.963 | 0.629 | 0.966 | 0.600 | 0.810 | 2.301 | ||||
| AV | 4 | 0.980 | 0.107 | 2.945 | 0.998 | 0.032 | 2.94 | 0.977 | 0.031 | 2.705 |
| 25 | 0.979 | 0.170 | 0.993 | 0.100 | 0.856 | 1.160 | ||||
| 37 | 0.986 | 0.248 | 0.949 | 0.467 | 0.872 | 1.802 | ||||
| Springiness | 4 | 0.934 | 0.070 | 2.694 | 0.924 | 0.077 | 2.639 | 0.913 | 0.505 | 2.554 |
| 25 | 0.876 | 0.151 | 0.856 | 0.161 | 0.821 | 0.813 | ||||
| 37 | 0.884 | 0.178 | 0.859 | 0.195 | 0.820 | 1.017 | ||||
| Hardness | 4 | 0.988 | 3.630 | 2.916 | 0.991 | 3.578 | 2.958 | 0.989 | 5.463 | 2.919 |
| 25 | 0.982 | 10.052 | 0.983 | 9.829 | 0.952 | 20.864 | ||||
| 37 | 0.943 | 28.032 | 0.984 | 14.868 | 0.978 | 32.768 | ||||
| Indicator | Kinetic Grade | Simultaneous Equations | R2 | RSEM | Activation Energy Ea/(KJ·mol−1) | Predisposing Factors K0 |
|---|---|---|---|---|---|---|
| TVB-N | Zero-level | lnK = 30.935 − 8507.390/T | 0.949 | 0.313 | 70.736 | 2.72 × 1013 |
| TVC | First-order | lnK =17.924 − 5527.722/T | 0.898 | 0.298 | 45.961 | 6.09 × 107 |
| AV | Zero-level | lnK = 19.695 − 5911.062/T | 0.968 | 0.172 | 49.149 | 3.58 × 108 |
| springiness | Zero-level | lnK = 17.259 − 5521.926/T | 0.939 | 0.224 | 45.913 | 4.10 × 107 |
| Hardness | First-order | lnK = 24.527 − 7776.532/T | 0.988 | 0.137 | 64.660 | 4.49 × 1010 |
| Indicator | Arrhenius Model |
|---|---|
| TVB-N | SLTVB-N = (A − A0)/[2.72×1013 exp (−8695.909/T)] |
| TVC | SLTVC = (lnA − lnA0)/[6.09×107 exp (−5650.202/T)] |
| AV | SLAV = (A − A0)/[3.58×108 exp (−6042.117/T)] |
| Springiness | SLS = (A − A0)/[4.10×107 exp (−5644.301/T)] |
| Hardness | SLH = ln(A − A0)/[4.49×1010 exp (−77,948.958/T)] |
| Quality Indicators | Storage Time (day) | Measured Value | Predicted Value | Relative Standard Deviation (%) | RSEM |
|---|---|---|---|---|---|
| TVB-N (mg/100 g) | 2 | 12.71 | 11.79 | 7.80 | |
| 4 | 17.18 | 16.57 | 3.66 | ||
| 6 | 21.09 | 21.36 | 1.27 | 0.97 | |
| 8 | 27.00 | 26.15 | 3.25 | ||
| 10 | 29.31 | 30.94 | 5.24 | ||
| TVC (log CFU/g) | 2 | 0.81 | 0.84 | 4.33 | |
| 4 | 1.21 | 1.26 | 4.22 | ||
| 6 | 2.04 | 1.88 | 8.80 | 0.18 | |
| 8 | 2.76 | 2.81 | 1.79 | ||
| 10 | 3.83 | 4.19 | 8.45 | ||
| AV (mg/g) | 2 | 1.95 | 1.81 | 7.62 | |
| 4 | 2.51 | 2.42 | 3.89 | ||
| 6 | 3.05 | 3.02 | 0.87 | 0.16 | |
| 8 | 3.42 | 3.63 | 5.78 | ||
| 10 | 4.48 | 4.24 | 5.61 | ||
| Springiness (N/m2) | 2 | 3.76 | 3.96 | 4.99 | |
| 4 | 3.73 | 3.68 | 1.43 | ||
| 6 | 3.16 | 3.40 | 7.14 | 0.15 | |
| 8 | 3.21 | 3.13 | 2.58 | ||
| 10 | 2.94 | 2.85 | 3.11 | ||
| Hardness (g) | 2 | 324.27 | 341.38 | 5.01 | |
| 4 | 335.13 | 307.49 | 8.99 | ||
| 6 | 251.84 | 276.96 | 9.07 | 21.06 | |
| 8 | 268.20 | 249.47 | 7.51 | ||
| 10 | 238.05 | 224.70 | 5.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Lei, J.; Su, K.; Chen, X.; Cheng, L.; Yang, C.; Ou, S. Processing and Shelf-Life Prediction Models for Ready-to-Eat Crayfish. Foods 2025, 14, 1296. https://doi.org/10.3390/foods14081296
Li Q, Lei J, Su K, Chen X, Cheng L, Yang C, Ou S. Processing and Shelf-Life Prediction Models for Ready-to-Eat Crayfish. Foods. 2025; 14(8):1296. https://doi.org/10.3390/foods14081296
Chicago/Turabian StyleLi, Qian, Jieyu Lei, Keying Su, Xiaoying Chen, Laihoong Cheng, Chunmin Yang, and Shiyi Ou. 2025. "Processing and Shelf-Life Prediction Models for Ready-to-Eat Crayfish" Foods 14, no. 8: 1296. https://doi.org/10.3390/foods14081296
APA StyleLi, Q., Lei, J., Su, K., Chen, X., Cheng, L., Yang, C., & Ou, S. (2025). Processing and Shelf-Life Prediction Models for Ready-to-Eat Crayfish. Foods, 14(8), 1296. https://doi.org/10.3390/foods14081296






