Advances in Food Aroma Analysis: Extraction, Separation, and Quantification Techniques
Abstract
:1. Introduction
2. Developing Trends in Aroma Analysis
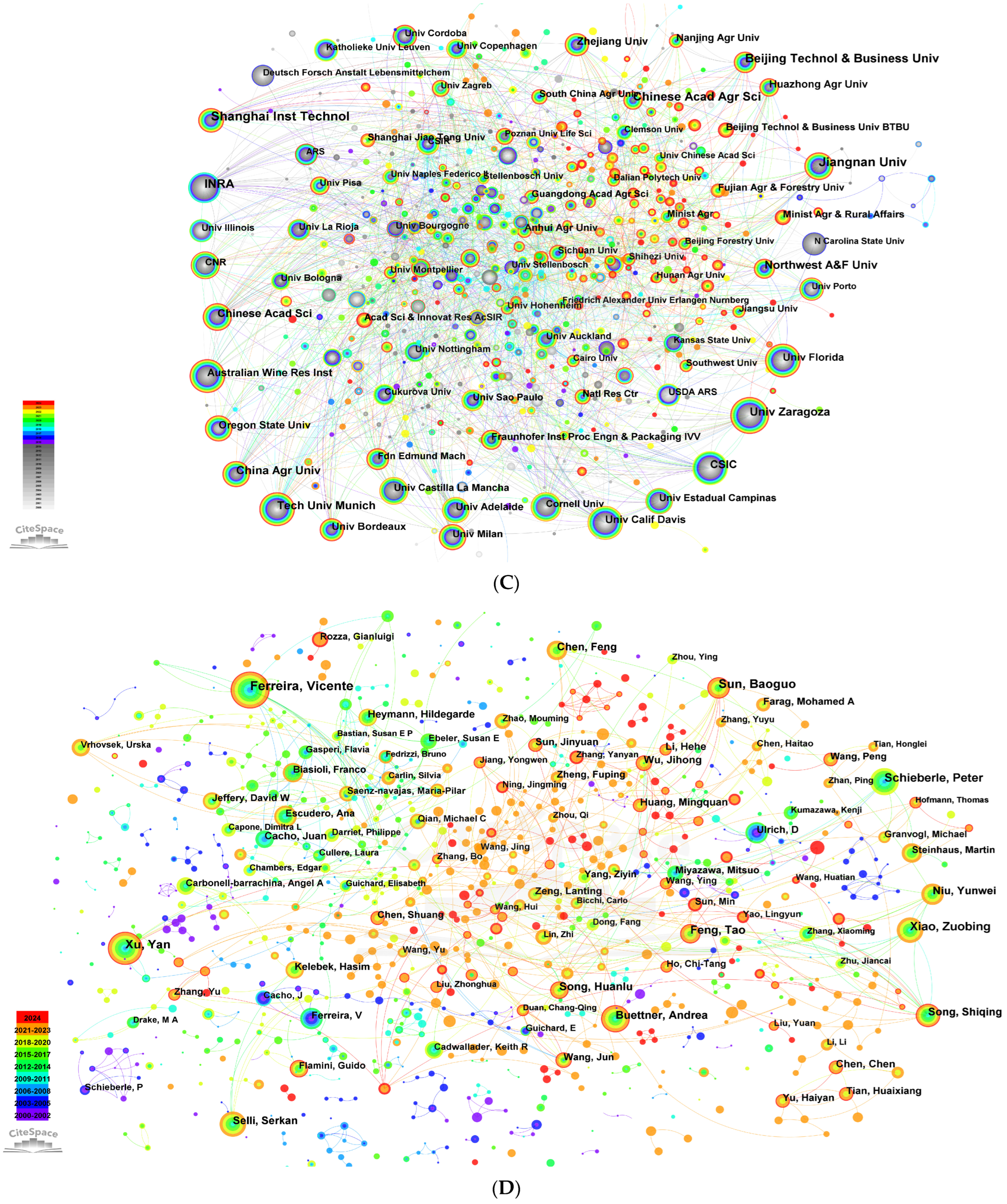
2.1. Trends in Aroma Analysis Literature
2.2. Evolutionary Patterns of Aroma Analysis
3. Aroma Analytical Methods
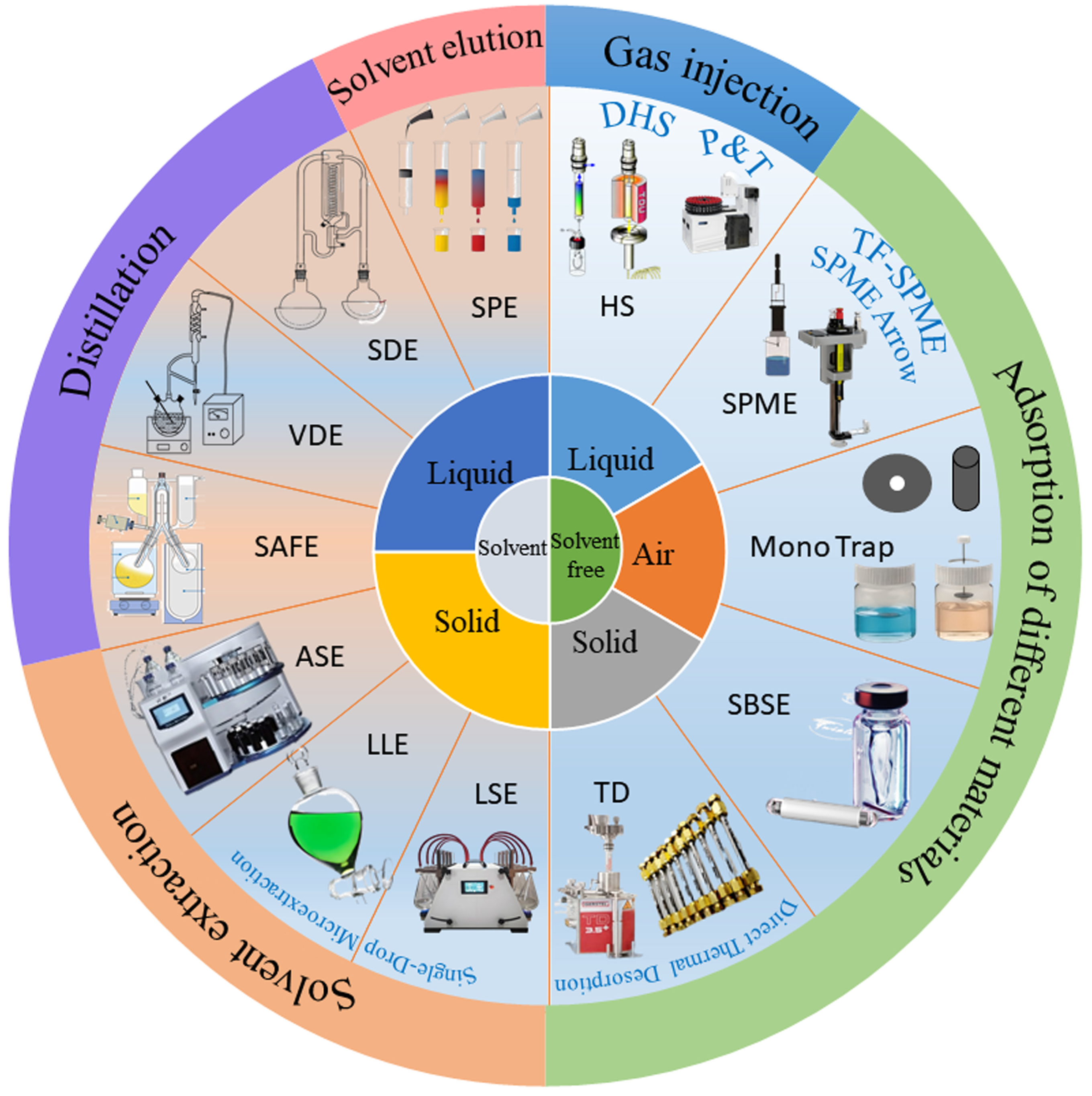
3.1. Isolation of Aroma Compounds
3.1.1. Solvent-Free Aroma Extraction Methods
3.1.2. Solvent Aroma Extraction Methods
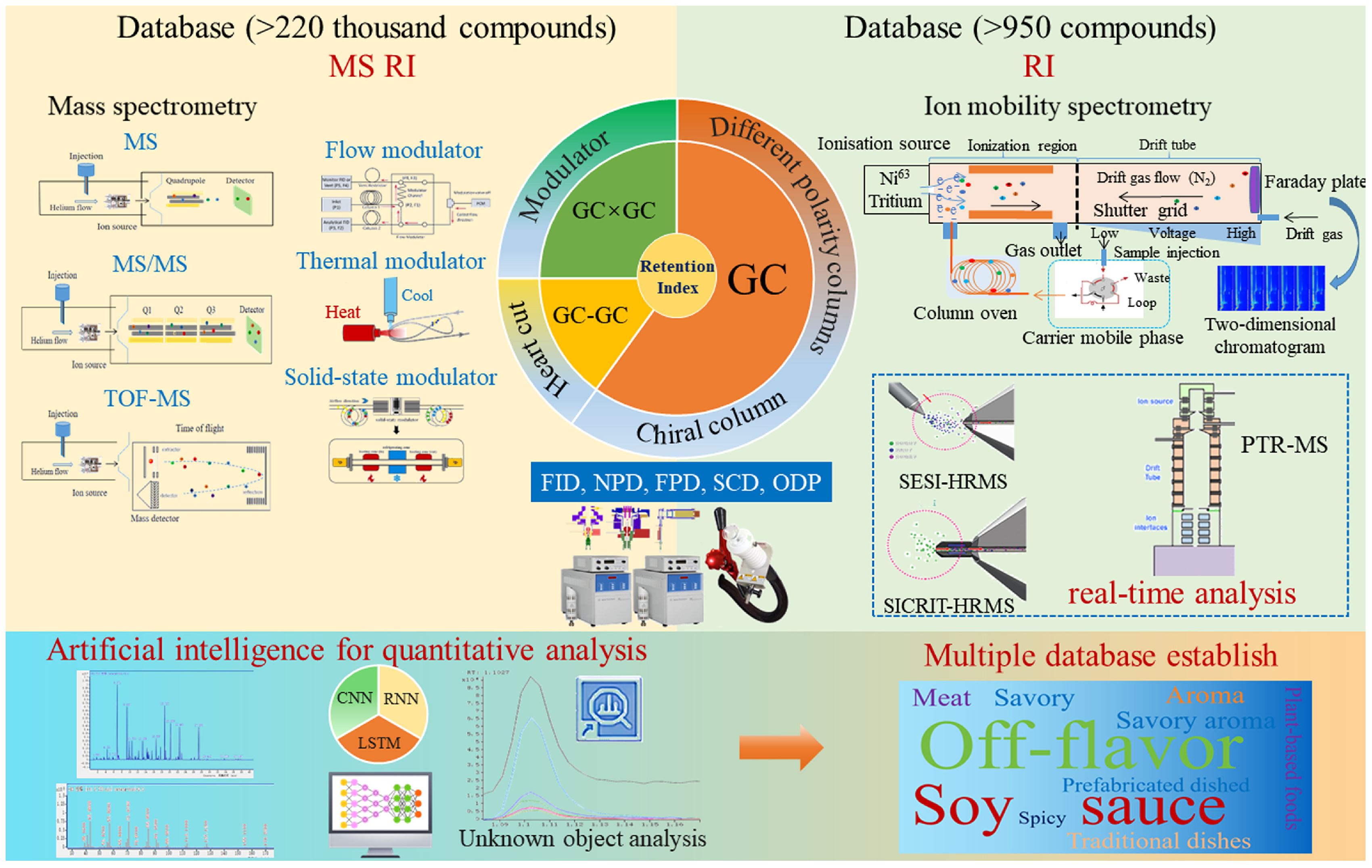
3.2. Qualitative Analysis of Aroma Compounds
3.2.1. Chromatographic Separation Technology
3.2.2. Instrumental Detector for Aroma Identification
3.2.3. Artificial Intelligence for Qualitative Analysis
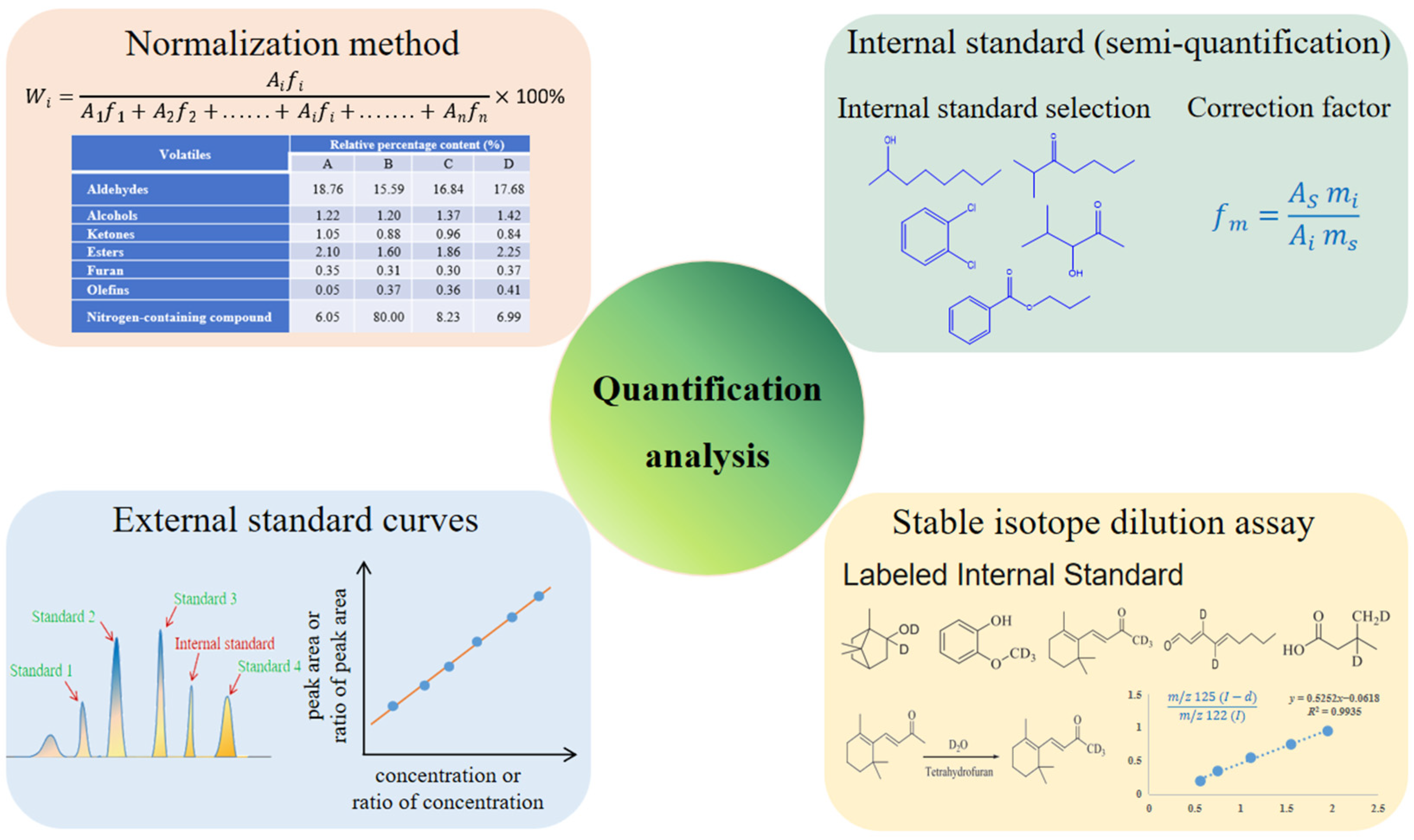
3.3. Quantification Analysis of Aroma Compounds
3.3.1. Normalization Method
3.3.2. Internal Standard Method
3.3.3. External Standard and Internal Standard Curve Methods
3.3.4. Stable Isotope Dilution Analysis (SIDA)
4. Flavoromics
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaczmarska, K.; Taylor, M.; Piyasiri, U.; Frank, D. Flavor and metabolite profiles of meat, meat substitutes, and traditional plant-based high-protein food products available in Australia. Foods 2021, 10, 801. [Google Scholar] [CrossRef] [PubMed]
- Dunkel, A.; Steinhaus, M.; Kotthoff, M.; Nowak, B.; Krautwurst, D.; Schieberle, P.; Hofmann, T. Nature’s chemical signatures in human olfaction: A foodborne perspective for future biotechnology. Angew. Chem. Int. Ed. 2014, 53, 7124–7143. [Google Scholar] [CrossRef] [PubMed]
- Genovese, A.; Caporaso, N.; di Bari, V.; Yang, N.; Fisk, I. Effect of olive oil phenolic compounds on the aroma release and persistence from O/W emulsion analysed in vivo by APCI-MS. Food Res. Int. 2019, 126, 108686. [Google Scholar] [CrossRef] [PubMed]
- Starowicz, M.; Zieliński, H. How Maillard reaction influences sensorial properties (color, flavor and texture) of food products? Food Rev. Int. 2019, 35, 707–725. [Google Scholar] [CrossRef]
- Pastorelli, S.; Valzacchi, S.; Rodriguez, A.; Simoneau, C. Solid-phase microextraction method for the determination of hexanal in hazelnuts as an indicator of the interaction of active packaging materials with food aroma compounds. Food Addit. Contam. 2016, 23, 1236–1241. [Google Scholar] [CrossRef]
- Wang, F.; Rui, H. Overview of Food Aroma Sources. Chin. Condiments 2008, 7, 27–29. [Google Scholar]
- Chen, C. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J. Am. Soc. Inf. Sci. Technol. 2006, 57, 359–377. [Google Scholar] [CrossRef]
- Grosch, W. Detection of potent odorants in foods by aroma extract dilution analysis. Trends Food Sci. Technol. 1993, 4, 68–73. [Google Scholar] [CrossRef]
- Steinhaus, P.; Schieberle, P. Characterization of the key aroma compounds in soy sauce using approaches of molecular sensory science. J. Agric. Food Chem. 2007, 55, 6262–6269. [Google Scholar] [CrossRef]
- Hirst, M.B.; Richter, C.L. Review of aroma formation through metabolic pathways of Saccharomyces cerevisiae in beverage fermentations. Am. J. Enol. Vitic. 2016, 67, 361–370. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Z.; Zou, S.; Dong, L.; Lin, X.; Chen, Y.; Liang, H. Chemical composition and flavor characteristics of cider fermented with Saccharomyces cerevisiae and non-Saccharomyces cerevisiae. Foods 2023, 12, 3565. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Chen, X.; Feng, S.; Bi, P.; Han, J.; Li, S.; Guo, J. Effect of sequential fermentation with indigenous non-Saccharomyces cerevisiae combinations and Saccharomyces cerevisiae on the chemical composition and aroma compounds evolution of kiwifruit wine. Food Chem. 2024, 460, 140758. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, L.; Song, Y.; Zhang, P.; Chen, D.; Guan, L.; Liu, S. Comprehensive study of volatile compounds and transcriptome data providing genes for grape aroma. BMC Plant Biol. 2023, 23, 171. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ren, G.; Deng, B.; Di, J.; Wang, Y. Unveiling the mechanisms of aroma metabolism in selenium-treated broccoli through transcriptome sequencing analyses. Sci. Hortic. 2023, 314, 111930. [Google Scholar] [CrossRef]
- Yakubu, H.G.; Kovacs, Z.; Toth, T.; Bazar, G. Trends in artificial aroma sensing by means of electronic nose technologies to advance dairy production–a review. Crit. Rev. Food Sci. Nutr. 2022, 63, 234–248. [Google Scholar] [CrossRef]
- Giannetti, V.; Biancolillo, A.; Marini, F.; Mariani, M.B.; Livi, G. Characterization of the aroma profile of edible flowers using HS-SPME/GC–MS and chemometrics. Food Res. Int. 2024, 178, 114001. [Google Scholar] [CrossRef]
- Rodinkov, O.V.; Bugaichenko, A.S.; Moskvin, L.N. Static headspace analysis and its current. J. Anal. Chem. 2020, 75, 1–17. [Google Scholar] [CrossRef]
- Snow, N. From Gas to Gas: Fundamentals of Static Headspace Extraction-Gas Chromatography. LCGC Int. 2024, 1, 22–25. [Google Scholar] [CrossRef]
- Rouseff, R.; Cadwallader, K. Headspace techniques in foods, fragrances and flavors: An overview. In Headspace Analysis of Foods and Flavors: Theory and Practice; Springer: Berlin/Heidelberg, Germany, 2001; pp. 1–8. [Google Scholar]
- Belardi, R.P.; Pawliszyn, J.B. The application of chemically modified fused silica fibers in the extraction of organics from water matrix samples and their rapid transfer to capillary columns. Water Qual. Res. J. 1989, 24, 179–191. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Szczepańska, N.; de La Guardia, M.; Namieśnik, J. Miniaturized solid-phase extraction techniques. TrAC Trends Anal. Chem. 2015, 73, 19–38. [Google Scholar] [CrossRef]
- Silva, E.A.S.; Risticevic, S.; Pawliszyn, J. Recent trends in SPME concerning sorbent materials, configurations and in vivo applications. TrAC Trends Anal. Chem. 2013, 43, 24–36. [Google Scholar] [CrossRef]
- Souza-Silva, E.A.; Risticevic, S.; Pawliszyn, J. Immersive solid-phase microextraction: A comprehensive review. J. Chromatogr. A 2016, 1469, 11–25. [Google Scholar]
- Kremser, A.; Jochmann, M.A.; Schmidt, T.C. PAL SPME Arrow—Evaluation of a novel solid-phase microextraction device for freely dissolved PAHs in water. Anal. Bioanal. Chem. 2016, 408, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Asadi, S.; Maddah, B. Rapid screening of chemical warfare agents (nerve agents) using dimethyl methylphosphonate as simulant substances in beverages by hollow fiber membrane-protected solid phase microextraction followed by corona discharge ion mobility spectrometry. J. Chromatogr. A 2022, 1661, 462704. [Google Scholar] [CrossRef]
- Li, S.; Feng, S.; Van Schepdael, A.; Wang, X. Hollow fiber membrane-protected amino/hydroxyl bifunctional microporous organic network fiber for solid-phase microextraction of bisphenols A, F, S, and triclosan in breast milk and infant formula. Food Chem. 2022, 390, 133217. [Google Scholar] [CrossRef]
- Mirnaghi, F.S.; Chen, Y.; Sidisky, L.M.; Pawliszyn, J. Optimization of the coating procedure for a high-throughput 96-blade solid phase microextraction system coupled with LC–MS/MS for analysis of complex samples. Anal. Chem. 2011, 83, 6018–6025. [Google Scholar] [CrossRef]
- Rajska, A.; Raczak-Gutknecht, J.; Struck-Lewicka, W.; Buszewska-Forajta, M.; Wityk, P.; Verding, P.; Markuszewski, M.J. Determination of urinary androgens in women with polycystic ovary syndrome using LC-QqQ/MS and the application of thin film solid-phase microextraction (TF-SPME). J. Chromatogr. A 2024, 1718, 464735. [Google Scholar] [CrossRef]
- Herrington, J.S.; Gómez-Ríos, G.A.; Myers, C.; Stidsen, G.; Bell, D.S. Hunting molecules in complex matrices with spme arrows: A review. Separations 2020, 7, 12. [Google Scholar] [CrossRef]
- Sato, A.; Sotomaru, K.; Takeda, M.A. A Novel Approach for Aroma Components Analysis Using a Monolithic Hybrid Adsorbent as a New Generation Medium “MonoTrap”. 2009. Available online: https://www.glsciences.eu/monotrap/poster_monotrap_ISEO2009.pdf (accessed on 25 September 2019).
- Wang, X.; Meng, Q.; Song, H. Characterization of odor-active compounds in high-salt liquid-state soy sauce after cooking. Food Chem. 2022, 373, 131460. [Google Scholar] [CrossRef]
- Liu, C.; Liu, X.; Tian, X.; Zhang, J.; Zhang, Z.; Shi, J.; Xu, J.; Ren, X. Determination of volatile profiles inside apple fruit storage facilities using Monotrap™ monolithic silica adsorbent and GC–MS. Hortic. Plant J. 2021, 7, 267–274. [Google Scholar] [CrossRef]
- Baltussen, E.; Sandra, P.; David, F.; Cramers, C. Stir bar sorptive extraction (SBSE), a novel extraction technique for aqueous samples: Theory and principles. J. Microcolumn Sep. 1999, 11, 737–747. [Google Scholar] [CrossRef]
- Yao, X.; Zhou, Z.; He, M.; Chen, B.; Liang, Y.; Hu, B. One-pot polymerization of monolith coated stir bar for high efficient sorptive extraction of perfluoroalkyl acids from environmental water samples followed by high performance liquid chromatography-electrospray tandem mass spectrometry detection. J. Chromatogr. A 2018, 1553, 7–15. [Google Scholar] [CrossRef] [PubMed]
- David, F.; Ochiai, N.; Sandra, P. Two decades of stir bar sorptive extraction: A retrospective and future outlook. TrAC Trends Anal. Chem. 2019, 112, 102–111. [Google Scholar] [CrossRef]
- Ma, L.; Meng, Q.; Chen, F.; Gao, W. SAFE and SBSE combined with GC-MS and GC-O for characterization of flavor compounds in Zhizhonghe Wujiapi medicinal liquor. J. Food Sci. 2022, 87, 939–956. [Google Scholar] [CrossRef]
- de Siqueira, A.C.P.; Sandes, R.D.D.; Nogueira, J.P.; Araujo, H.C.S.; de Jesus, M.S.; Rajkumar, G.; Neta, M.T.S.L.; Narain, N. Volatile profiles of Murcott and Ponkan mandarins obtained by stir bar sorptive extraction technique and their contributions to the fruit aroma. J. Food Sci. 2024, 89, 4823–4838. [Google Scholar] [CrossRef] [PubMed]
- Analytical Methods Committee AMCTB No. 97. Thermal desorption part 1: Introduction and instrumentation. Anal. Methods 2020, 12, 3425–3428. [Google Scholar]
- Woolfenden, E. Thermal desorption gas chromatography. In Gas Chromatography, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 267–323. [Google Scholar]
- Chen, Z.; Chen, Z.; Li, Y.; Zhang, R.; Liu, Y.; Hui, A.; Cao, W.; Liu, J.; Bai, H.; Song, J. A review on remediation of chlorinated organic contaminants in soils by thermal desorption. J. Ind. Eng. Chem. 2023, 133, 112–121. [Google Scholar] [CrossRef]
- Chappuis, C.J.F.; Niclass, Y.; Vuilleumier, C.; Starkenmann, C. Quantitative headspace analysis of selected odorants from latrines in Africa and India. Environ. Sci. Technol. 2015, 49, 6134–6140. [Google Scholar] [CrossRef]
- Meng, R.; Pu, D.; Xu, Z.; Liu, J.; Zhang, Q.; Xu, M.; Zhang, Y. Decoding the aroma changes of stir-fried shredded potatoes with different soy sauces using thermal desorption combined with gas chromatography–mass spectrometry and sensory evaluation. Food Chem. 2025, 467, 142252. [Google Scholar] [CrossRef]
- Pérez-Jiménez, M.; Muñoz-González, C.; Pozo-Bayón, M.A. Oral release behavior of wine aroma compounds by using in-mouth headspace sorptive extraction (HSSE) method. Foods 2021, 10, 415. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, S.; Tian, H.; Sun, B. Research progress in the use of liquid-liquid extraction for food flavour analysis. Trends Food Sci. Technol. 2023, 132, 138–149. [Google Scholar] [CrossRef]
- Hammad, S.F.; Abdallah, I.A.; Bedair, A.; Mansour, F.R. Homogeneous liquid–liquid extraction as an alternative sample preparation technique for biomedical analysis. J. Sep. Sci. 2022, 45, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Darıcı, M.; Bergama, D.; Cabaroglu, T. Effect of triple pot still distillation on the volatile compositions during the Rakı production. J. Food Process. Preserv. 2019, 43, e13864. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Lavilla, I.; Bendicho, C. Liquid-phase microextraction techniques within the framework of green chemistry. TrAC Trends Anal. Chem. 2010, 29, 617–628. [Google Scholar] [CrossRef]
- Kannouma, R.E.; Hammad, M.A.; Kamal, A.H.; Mansour, F.R. Miniaturization of Liquid-Liquid extraction; the barriers and the enablers. Microchem. J. 2022, 182, 107863. [Google Scholar] [CrossRef]
- Richter, B.E.; Jones, B.A.; Ezzell, J.L.; Porter, N.L.; Avdalovic, N.; Pohl, C. Accelerated solvent extraction: A technique for sample preparation. Anal. Chem. 1996, 68, 1033–1039. [Google Scholar] [CrossRef]
- Shen, J.; Shao, X. A comparison of accelerated solvent extraction, Soxhlet extraction, and ultrasonic-assisted extraction for analysis of terpenoids and sterols in tobacco. Anal. Bioanal. Chem. 2005, 383, 1003–1008. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, X.; Zhao, M.; Liu, X.; Pang, Y.; Zhang, M. Characterization of the key aroma constituents in fried tilapia through the sensorics concept. Foods 2022, 11, 494. [Google Scholar] [CrossRef]
- Likens, S.T.; Nickerson, G.B. Detection of certain hop oil constituents in brewing products. In Proceedings. Annual meeting—American Society of Brewing Chemists; Taylor & Francis: Abingdon, UK, 1964; Volume 22, pp. 5–13. [Google Scholar]
- Peng, F.; Sheng, L.; Liu, B.; Tong, H.; Liu, S. Comparison of different extraction methods: Steam distillation, simultaneous distillation and extraction and headspace co-distillation, used for the analysis of the volatile components in aged flue-cured tobacco leaves. J. Chromatogr. A 2004, 1040, 1–17. [Google Scholar] [CrossRef]
- Pu, D.; Zhang, H.; Zhang, Y.; Sun, B.; Ren, F.; Chen, H.; Tang, Y. Characterization of the key aroma compounds in traditional hunan smoke-cured pork leg (Larou, THSL) by aroma extract dilution analysis (AEDA), odor activity value (OAV), and sensory evaluation experiments. Foods 2020, 9, 413. [Google Scholar] [CrossRef]
- Ribeiro, B.S.; Ferreira, M.D.F.; Moreira, J.L.; Santos, L. Simultaneous Distillation–Extraction of Essential Oils from Rosmarinus officinalis L. Cosmetics 2021, 8, 117. [Google Scholar] [CrossRef]
- Maignial, L.; Pibarot, P.; Bonetti, G.; Chaintreau, A.; Marion, J.P. Simultaneous distillation-extraction under static vacuum: Isolation of volatile compounds at room temperature. J. Chromatogr. A 1992, 606, 87–94. [Google Scholar] [CrossRef]
- Wu, X.; Du, Z.; Ma, R.; Zhang, X.; Yang, D.; Liu, H.; Zhang, Y. A novel application of the vacuum distillation technology in extracting Origanum vulgare L. Essent. Oils. Ind. Crops Prod. 2019, 139, 111516. [Google Scholar] [CrossRef]
- Majors, R.E. Sample preparation for HPLC and gas chromatography using solid-phase extraction. LC-GC 1986, 4, 972–984. [Google Scholar]
- Castro, R.; Natera, R.; Durán, E.; García-Barroso, C. Application of solid phase extraction techniques to analyse volatile compounds in wines and other enological products. Eur. Food Res. Technol. 2008, 228, 1–18. [Google Scholar] [CrossRef]
- Ötles, S.; Kartal, C. Solid-Phase Extraction (SPE): Principles and applications in food samples. Acta Sci. Pol. Technol. Aliment. 2016, 15, 5–15. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, C.; Gao, X.; Kang, Y.; Huang, M.; Wu, J.; Liu, Y.; Zhang, J.; Li, H.; Zhang, Y. Characterization of key aroma compounds in Huangjiu from northern China by sensory-directed flavor analysis. Food Res. Int. 2020, 134, 109238. [Google Scholar] [CrossRef]
- Pu, D.; Shan, Y.; Zhang, L.; Sun, B.; Zhang, Y. Identification and inhibition of the key off-odorants in duck broth by means of the sensomics approach and binary odor mixture. J. Agric. Food Chem. 2022, 70, 13367–13378. [Google Scholar] [CrossRef]
- Engel, W.; Bahr, W.; Schieberle, P. Solvent assisted flavour evaporation–a new and versatile technique for the careful and direct isolation of aroma compounds from complex food matrices. Eur. Food Res. Technol. 1999, 209, 237–241. [Google Scholar] [CrossRef]
- Sun, J.; Ma, M.; Sun, B.; Ren, F.; Chen, H.; Zhang, N.; Zhang, Y. Identification of characteristic aroma components of butter from Chinese butter hotpot seasoning. Food Chem. 2021, 338, 127838. [Google Scholar] [CrossRef]
- Schlumpberger, P.; Stübner, C.A.; Steinhaus, M. Development and evaluation of an automated solvent-assisted flavour evaporation (aSAFE). Eur. Food Res. Technol. 2022, 248, 2591–2602. [Google Scholar] [CrossRef]
- Pu, D.; Cao, B.; Xu, Z.; Zhang, L.; Meng, R.; Chen, J.; Sun, B.; Zhang, Y. Decoding of the enhancement of saltiness perception by aroma-active compounds during Hunan Larou (smoke-cured bacon) oral processing. Food Chem. 2025, 463, 141029. [Google Scholar] [CrossRef] [PubMed]
- Bartle, K.D.; Myers, P. History of gas chromatography. TrAC Trends Anal. Chem. 2002, 21, 547–557. [Google Scholar] [CrossRef]
- Phillips, J.B.; Luu, D.; Pawliszyn, J.B.; Carle, G.C. Multiplex gas chromatography by thermal modulation of a fused silica capillary column. Anal. Chem. 1985, 57, 2779–2787. [Google Scholar] [CrossRef]
- Marriott, P.J.; Chin, S.T.; Maikhunthod, B.; Schmarr, H.G.; Bieri, S. Multidimensional gas chromatography. TrAC Trends Anal. Chem. 2012, 34, 1–21. [Google Scholar] [CrossRef]
- Seeley, J.V.; Schimmel, N.E.; Seeley, S.K. Influence of modulator injection width on comprehensive two-dimensional gas chromatography peak dimensions. Anal. Bioanal. Chem. 2023, 415, 2399–2409. [Google Scholar] [CrossRef]
- Zanella, D.; Focant, J.F.; Franchina, F.A. 30th Anniversary of comprehensive two-dimensional gas chromatography: Latest advances. Anal. Sci. Adv. 2021, 2, 213–224. [Google Scholar] [CrossRef]
- Yu, M.; Li, T.; Song, H. Characterization of key aroma-active compounds in four commercial oyster sauce by SGC/GC×GC–O–MS, AEDA, and OAV. J. Food Compos. Anal. 2022, 107, 104368. [Google Scholar] [CrossRef]
- Chauhan, A.; Goyal, M.K.; Chauhan, P. GC-MS technique and its analytical applications in science and technology. Anal. Bioanal. Tech. 2014, 5, 1000222. [Google Scholar] [CrossRef]
- Pu, D.; Shan, Y.; Wang, J.; Sun, B.; Xu, Y.; Zhang, W.; Zhang, Y. Recent trends in aroma release and perception during food oral processing: A review. Crit. Rev. Food Sci. Nutr. 2024, 64, 3441–3457. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.P.; Blank, I.; Li, F.; Li, C.; Liu, Y. GC×GC-ToF-MS and GC-IMS based volatile profile characterization of the Chinese dry-cured hams from different regions. Food Res. Int. 2021, 142, 110222. [Google Scholar] [CrossRef]
- Zhao, M.; Li, T.; Yang, F.; Cui, X.; Zou, T.; Song, H.; Liu, Y. Characterization of key aroma-active compounds in Hanyuan Zanthoxylum bungeanum by GC-O-MS and switchable GC×GC-O-MS. Food Chem. 2022, 385, 132659. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Cai, Y.; Wu, X.; Gai, S.; Wang, B.; Liu, D. Characterization of selected commercially available grilled lamb shashliks based on flavor profiles using GC-MS, GC×GC-TOF-MS, GC-IMS, E-nose and E-tongue combined with chemometrics. Food Chem. 2023, 423, 136257. [Google Scholar] [CrossRef]
- Warren, C.R. Use of chemical ionization for GC–MS metabolite profiling. Metabolomics 2013, 9, 110–120. [Google Scholar] [CrossRef]
- Capellades, J.; Junza, A.; Samino, S.; Brunner, J.S.; Schabbauer, G.; Vinaixa, M.; Yanes, O. Exploring the use of gas chromatography coupled to chemical ionization mass spectrometry (GC-CI-MS) for stable isotope labeling in metabolomics. Anal. Chem. 2020, 93, 1242–1248. [Google Scholar] [CrossRef]
- Hansen, M.; Jacobsen, N.W.; Nielsen, F.K.; Björklund, E.; Styrishave, B.; Halling-Sørensen, B. Determination of steroid hormones in blood by GC–MS/MS. Anal. Bioanal. Chem. 2011, 400, 3409–3417. [Google Scholar] [CrossRef]
- Ferrer, I.; Thurman, M.E. Advanced Techniques in Gas Chromatography-Mass Spectrometry (GC-MS-MS and GC-TOF-MS) for Environmental Chemistry; Newnes: Boston, UK, 2013. [Google Scholar]
- Król, S.; Zabiegała, B.; Namieśnik, J. Monitoring and analytics of semivolatile organic compounds (SVOCs) in indoor air. Anal. Bioanal. Chem. 2011, 400, 1751–1769. [Google Scholar] [CrossRef] [PubMed]
- Pu, D.; Zhang, H.; Zhang, Y.; Sun, B.; Ren, F.; Chen, H. Characterization of the key aroma compounds in white bread by aroma extract dilution analysis, quantitation, and sensory evaluation experiments. J. Food Process. Preserv. 2019, 43, e13933. [Google Scholar] [CrossRef]
- Hill, H.H., Jr.; Siems, W.F.; St Louis, R.H. Ion mobility spectrometry. Anal. Chem. 1990, 62, 1201–1209. [Google Scholar] [CrossRef]
- Pu, D.; Duan, W.; Huang, Y.; Zhang, Y.; Sun, B.; Ren, F.; Zhang, H.; Chen, H.; Tang, Y. Characterization of the key odorants contributing to retronasal olfaction during bread consumption. Food Chemistr. 2020, 318, 126520. [Google Scholar] [CrossRef]
- Parastar, H.; Weller, P. Towards greener volatilomics: Is GC-IMS the new Swiss army knife of gas phase analysis? TrAC Trends Anal. Chem. 2023, 170, 117438. [Google Scholar] [CrossRef]
- Song, H.; Liu, J. GC-O-MS technique and its applications in food flavor analysis. Food Res. Int. 2018, 114, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Fuller, G.H.; Steltenkamp, R.; Tisserand, G.A. The gas chromatograph with human sensor: Perfumer model. Ann. New York Acad. Sci. 1964, 116, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Xie, Q.; Song, H.; Wang, L.; Sun, H.; Jiang, S.; Zhang, Y.; Zheng, C. Characterization of the odor compounds in human milk by DHS/GC×GC-O-MS: A feasible and efficient method. Food Res. Int. 2023, 174, 113597. [Google Scholar] [CrossRef]
- Wang, H.; Yang, P.; Liu, C.; Song, H.; Pan, W.; Gong, L. Characterization of key odor-active compounds in thermal reaction beef flavoring by SGC×GC-O-MS, AEDA, DHDA, OAV and quantitative measurements. J. Food Compos. Anal. 2022, 114, 104805. [Google Scholar] [CrossRef]
- McDaniel, M.R.; Miranda-Lopez, R.; Watson, B.T.; Micheals, N.J.; Libbey, L.M. Pinot Noir Aroma: A Sensory/Gas Chromatographic Approach; Developments in Food Science: Amsterdam, The Netherlands, 1990. [Google Scholar]
- Ulrich, F.; Grosch, W. Identification of the most intense volatile flavour compounds formed during autoxidation of linoleic acid. Z. Lebensm. Unters. Forsch 1987, 184, 277–282. [Google Scholar] [CrossRef]
- Acree, T.E.; Barnard, J.; Cunningham, D.G. A procedure for the sensory analysis of gas chromatographic effluents. Food Chem. 1984, 14, 273–286. [Google Scholar] [CrossRef]
- Barba, C.; Beno, N.; Guichard, E.; Thomas-Danguin, T. Selecting odorant compounds to enhance sweet flavor perception by gas chromatography/olfactometry-associated taste (GC/O-AT). Food Chem. 2018, 257, 172–181. [Google Scholar] [CrossRef]
- Zhou, T.; Feng, Y.; Thomas-Danguin, T.; Zhao, M. Enhancement of saltiness perception by odorants selected from Chinese soy sauce: A gas chromatography/olfactometry-associated taste study. Food Chem. 2021, 335, 127664. [Google Scholar] [CrossRef]
- Chen, Y.P.; Wang, M.; Fang, X.; Liya, A.; Zhang, H.; Blank, I.; Liu, Y. Odorants identified in Chinese dry-cured ham contribute to salty taste enhancement. J. Agric. Food Chem. 2023, 72, 613–624. [Google Scholar] [CrossRef]
- Rincón, C.A.; De Guardia, A.; Couvert, A.; Wolbert, D.; Le Roux, S.; Soutrel, I.; Nunes, G. Odor concentration (OC) prediction based on odor activity values (OAVs) during composting of solid wastes and digestates. Atmos. Environ. 2019, 201, 1–12. [Google Scholar] [CrossRef]
- Song, W.; Sun, M.; Lu, H.; Wang, S.; Wang, R.; Shang, X.; Feng, T. Variations in key aroma compounds and aroma profiles in yellow and white cultivars of flammulina filiformis based on gas chromatography-mass spectrometry-olfactometry, aroma recombination, and omission experiments coupled with odor threshold concentrations. Foods 2024, 13, 684. [Google Scholar] [CrossRef] [PubMed]
- Pu, D.; Shi, Y.; Meng, R.; Yong, Q.; Shi, Z.; Shao, D.; Zhang, Y. Decoding the different aroma-active compounds in soy sauce for cold dishes via a multiple sensory evaluation and instrumental analysis. Foods 2023, 12, 3693. [Google Scholar] [CrossRef]
- Kim, S.H.; Hwang, J.H.; Lee, K.G. Analysis of acrylamide using gas chromatography-nitrogen phosphorus detector (GC-NPD). Food Sci. Biotechnol. 2011, 20, 835–839. [Google Scholar] [CrossRef]
- Firor, R.L.; Quimby, B.D. A Comparison of Sulfur Selective Detectors for Low Level Analysis in Gaseous Streams; Agilent Technologies: Santa Clara, CA, USA, 2001. [Google Scholar]
- Yan, X. Unique selective detectors for gas chromatography: Nitrogen and sulfur chemiluminescence detectors. J. Sep. Sci. 2006, 29, 1931–1945. [Google Scholar] [CrossRef]
- Barallat-Pérez, C.; Pedrotti, M.; Oliviero, T.; Martins, S.; Fogliano, V.; de Jong, C. Drivers of the In-Mouth Interaction between Lupin Protein Isolate and Selected Aroma Compounds: A Proton Transfer Reaction–Mass Spectrometry and Dynamic Time Intensity Analysis. J. Agric. Food Chem. 2024, 72, 8731–8741. [Google Scholar] [CrossRef] [PubMed]
- Pu, D.; Zhang, H.; Zhang, Y.; Sun, B.; Ren, F.; Chen, H.; Xie, J. Characterization of the oral breakdown, sensory properties, and volatile release during mastication of white bread. Food Chem. 2019, 298, 125003. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, Y.; Chen, X.; Zhang, Y.; Li, H.; Zhao, D.; Wang, B.; Ye, X.; Sun, B.; Sun, J. Peanut Pairing Baijiu: To Enhance Retronasal Aroma Intensity while Reducing Baijiu Aftertaste. J. Agric. Food Chem. 2024, 72, 14851–14864. [Google Scholar] [CrossRef]
- Gisler, A.; Lan, J.; Singh, K.D.; Usemann, J.; Frey, U.; Zenobi, R.; Sinues, P. Real-time breath analysis of exhaled compounds upon peppermint oil ingestion by secondary electrospray ionization-high resolution mass spectrometry: Technical aspects. J. Breath Res. 2020, 14, 046001. [Google Scholar] [CrossRef]
- Wang, K.; Xu, Z. Comparison of freshly squeezed, Non-thermally and thermally processed orange juice based on traditional quality characters, untargeted metabolomics, and volatile overview. Food Chem. 2022, 373, 131430. [Google Scholar] [CrossRef]
- Ding, C.; Ierapetritou, M. Machine learning-based optimization of a multi-step ion exchange chromatography for ternary protein separation. Comput. Chem. Eng. 2024, 184, 108642. [Google Scholar] [CrossRef]
- Matyushin, D.D.; Sholokhova, A.Y.; Buryak, A.K. Deep learning driven GC-MS library search and its application for metabolomics. Anal. Chem. 2020, 92, 11818–11825. [Google Scholar] [CrossRef]
- Bi, K.; Zhang, D.; Qiu, T.; Huang, Y. GC-MS fingerprints profiling using machine learning models for food flavor prediction. Processes 2019, 8, 23. [Google Scholar] [CrossRef]
- Wu, X.; Du, Z.; Ma, R.; Zhang, X.; Yang, D.; Liu, H.; Zhang, Y. Qualitative and quantitative studies of phthalates in extra virgin olive oil (EVOO) by surface-enhanced Raman spectroscopy (SERS) combined with long short term memory (LSTM) neural network. Food Chem. 2024, 433, 137300. [Google Scholar] [CrossRef]
- Lee, B.K.; Mayhew, E.J.; Sanchez-Lengeling, B.; Wei, J.N.; Qian, W.W.; Little, K.A.; Andres, M.; Nguyen, B.B.; Moloy, T.; Yasonik, J.; et al. A principal odor map unifies diverse tasks in olfactory perception. Science 2023, 381, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.; Brintrup, A. Aiding food security and sustainability efforts through graph neural network-based consumer food ingredient detection and substitution. Sci. Rep. 2023, 13, 18809. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.; Poll, L.; Callesen, O.; Lewis, M. Relations between the content of aroma compounds and the sensory evaluation of 10 raspberry varieties (Rubus idaeus L). Acta Agric. Scand. 1991, 41, 447–454. [Google Scholar] [CrossRef]
- Preininger, M. Quantitation of potent food aroma compounds by using stable isotope labeled and unlabeled internal standard methods. In Developments in Food Science; Elsevier: Amsterdam, The Netherlands, 1998; pp. 87–97. [Google Scholar]
- Pu, D.; Zhang, H.; Zhang, Y.; Sun, B.; Ren, F.; Chen, H.; He, J. Characterization of the aroma release and perception of white bread during oral processing by gas chromatography-ion mobility spectrometry and temporal dominance of sensations analysis. Food Res. Int. 2019, 123, 612–622. [Google Scholar] [CrossRef]
- Barata, A.; Campo, E.; Malfeito-Ferreira, M.; Loureiro, V.; Cacho, J.; Ferreira, V. Analytical and sensorial characterization of the aroma of wines produced with sour rotten grapes using GC-O and GC-MS: Identification of key aroma compounds. J. Agric. Food Chem. 2011, 59, 2543–2553. [Google Scholar] [CrossRef]
- Shan, Y.; Pu, D.; Cao, B.; Shi, Y.; Li, P.; Xiong, J.; Li, K.; Sun, B.; Zhang, Y. Elucidating salt-reduction mechanisms of aroma-active compounds from yeast extracts through sensomics approaches and electroencephalography. Food Chem. X 2024, 22, 101339. [Google Scholar] [CrossRef]
- George, J.; Nguyen, T.T.; Sanewski, G.; Hardner, C.; Smyth, H.E. Stable isotope dilution assay and HS-SPME-GCMS quantification of key aroma volatiles of Australian pineapple (Ananas comosus) cultivars. Food Chem. 2024, 455, 139956. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Yang, W.; Huang, J.; Liu, Y.; Huang, M.; Sun, B.; Li, C. Characterization of potent aroma compounds in preserved egg yolk by gas chromatography–olfactometry, quantitative measurements, and odor activity value. J. Agric. Food Chem. 2018, 66, 6132–6141. [Google Scholar] [CrossRef] [PubMed]
- Schieberle, P.; Grosch, W. Quantitative analysis of aroma compounds in wheat and rye bread crusts using a stable isotope dilution assay. J. Agric. Food Chem. 1987, 35, 252–257. [Google Scholar] [CrossRef]
- Wang, J.; Liu, N.; Yang, S.; Qiu, G.; Tian, H.; Sun, B. Research progress in the synthesis of stable isotopes of food flavour compounds. Food Chem. 2023, 435, 137635. [Google Scholar] [CrossRef]
- Qin, X.; Xiong, T.; Xiong, S.; Liu, Z.; Xie, M.; Guan, Q. Metatranscriptomics unravel the formation mechanism of key flavors during the natural fermentation of suansun, a Chinese traditional fermented bamboo shoot. Food Biosci. 2024, 57, 103436. [Google Scholar] [CrossRef]
- Chen, D.; Zheng, Z.; Zhou, Z.; Liu, Y.; Chen, Z.; Lin, G.; Xue, T. 4D-FastDIA-based quantitative proteomics reveals protein biomarkers linked to flavor traits between triploid and diploid oysters (Crassostrea angulata). LWT 2024, 211, 116907. [Google Scholar] [CrossRef]
- Ji, H.; Pu, D.; Yan, W.; Zhang, Q.; Zuo, M.; Zhang, Y. Recent advances and application of machine learning in food flavor prediction and regulation. Trends Food Sci. Technol. 2023, 138, 738–751. [Google Scholar] [CrossRef]


| No. | Keywords | Year | Strength a | Begin | End | 2000–2024 |
|---|---|---|---|---|---|---|
| 1 | Solid-phase microextraction | 2000 | 90.83 | 2003 | 2012 | ▃▃▃▃▃▃▃▃▃▃▃▃▃▃▂▂▂▂▂▂▂▂▂▂▂ |
| 2 | Potent odorant | 2000 | 43.98 | 2000 | 2009 | ▃▃▃▃▃▃▃▃▃▃▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂ |
| 3 | Headspace analysis | 2000 | 38.56 | 2000 | 2014 | ▃▃▃▃▃▃▃▃▃▃▃▃▃▃▃▂▂▂▂▂▂▂▂▂▂ |
| 4 | Impact odorant | 2002 | 34.43 | 2002 | 2016 | ▂▂▃▃▃▃▃▃▃▃▃▃▃▃▃▃▃▂▂▂▂▂▂▂▂ |
| 5 | Quantitative determination | 2000 | 32.84 | 2000 | 2015 | ▃▃▃▃▃▃▃▃▃▃▃▃▃▃▃▃▂▂▂▂▂▂▂▂▂ |
| 6 | Red wine | 2001 | 30.2 | 2007 | 2016 | ▂▂▂▂▂▂▂▃▃▃▃▃▃▃▃▃▃▂▂▂▂▂▂▂▂ |
| 7 | Flavor compound | 2000 | 29.88 | 2000 | 2008 | ▃▃▃▃▃▃▃▃▃▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂ |
| 8 | Character impact odorant | 2000 | 29.46 | 2000 | 2009 | ▃▃▃▃▃▃▃▃▃▃▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂ |
| 9 | Food analysis | 2000 | 29.29 | 2000 | 2005 | ▃▃▃▃▃▃▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂ |
| 10 | Gas chromatography-olfactometry | 2001 | 27.58 | 2001 | 2012 | ▂▃▃▃▃▃▃▃▃▃▃▃▃▂▂▂▂▂▂▂▂▂▂▂▂ |
| 11 | Correlation analysis | 2020 | 25.92 | 2021 | 2024 | ▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▃▃▃▃ |
| 12 | Aroma extract dilution analysis | 2000 | 24.1 | 2000 | 2006 | ▃▃▃▃▃▃▃▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂ |
| 13 | Component | 2000 | 24.04 | 2000 | 2008 | ▃▃▃▃▃▃▃▃▃▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂ |
| 14 | Microbial community | 2019 | 21.74 | 2020 | 2024 | ▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▃▃▃▃▃ |
| 15 | Headspace | 2001 | 21.31 | 2001 | 2013 | ▂▃▃▃▃▃▃▃▃▃▃▃▃▃▂▂▂▂▂▂▂▂▂▂▂ |
| 16 | White wine | 2000 | 21.14 | 2005 | 2015 | ▂▂▂▂▂▃▃▃▃▃▃▃▃▃▃▃▂▂▂▂▂▂▂▂▂ |
| 17 | Dynamic headspace | 2000 | 20.84 | 2000 | 2008 | ▃▃▃▃▃▃▃▃▃▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂ |
| 18 | Constituent | 2000 | 20.18 | 2000 | 2013 | ▃▃▃▃▃▃▃▃▃▃▃▃▃▃▂▂▂▂▂▂▂▂▂▂▂ |
| 19 | Cheddar cheese | 2000 | 19.95 | 2000 | 2008 | ▃▃▃▃▃▃▃▃▃▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂▂ |
| 20 | Volatile thiol | 2002 | 17.62 | 2008 | 2017 | ▂▂▂▂▂▂▂▂▃▃▃▃▃▃▃▃▃▃▂▂▂▂▂▂▂ |
| 21 | Volatile constituent | 2000 | 15.87 | 2000 | 2015 | ▃▃▃▃▃▃▃▃▃▃▃▃▃▃▃▃▂▂▂▂▂▂▂▂▂ |
| 22 | Solid phase extraction | 2006 | 15.00 | 2006 | 2014 | ▂▂▂▂▂▂▃▃▃▃▃▃▃▃▃▂▂▂▂▂▂▂▂▂▂ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, D.; Xu, Z.; Sun, B.; Wang, Y.; Xu, J.; Zhang, Y. Advances in Food Aroma Analysis: Extraction, Separation, and Quantification Techniques. Foods 2025, 14, 1302. https://doi.org/10.3390/foods14081302
Pu D, Xu Z, Sun B, Wang Y, Xu J, Zhang Y. Advances in Food Aroma Analysis: Extraction, Separation, and Quantification Techniques. Foods. 2025; 14(8):1302. https://doi.org/10.3390/foods14081302
Chicago/Turabian StylePu, Dandan, Zikang Xu, Baoguo Sun, Yanbo Wang, Jialiang Xu, and Yuyu Zhang. 2025. "Advances in Food Aroma Analysis: Extraction, Separation, and Quantification Techniques" Foods 14, no. 8: 1302. https://doi.org/10.3390/foods14081302
APA StylePu, D., Xu, Z., Sun, B., Wang, Y., Xu, J., & Zhang, Y. (2025). Advances in Food Aroma Analysis: Extraction, Separation, and Quantification Techniques. Foods, 14(8), 1302. https://doi.org/10.3390/foods14081302







