The Phytochemical Characterization of a Cili (Rosa roxburghii) Fruit Low-Temperature Extract with Hepatoprotective Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction Process
2.1.1. HHPD-Extraction of CFE
2.1.2. CS-Extraction of CFE
2.2. Chemical Characterization of CFE
2.2.1. Determination of Total Phenolics
2.2.2. Determination of Total Flavonoids
2.2.3. Determination of Total Polysaccharides
2.2.4. Measurement of SOD
2.2.5. Detection of Characteristic Compounds in CFE by HPLC
2.3. Identification of Chemical Constituents by UFLC-IT-TOF/MS in HHPD-CFE
2.4. The Anti-Liver Injury and Anti-Liver Fibrosis Activities of HHPD-CFE
2.5. Data Analysis
3. Results and Discussion
3.1. Phytochemical Characterizations of CFE
3.2. SOD Activity in CFE
3.3. Chemical Markers in CFE
3.4. Characterization of Phenolics in HHPD-CFE by UFLC-IT-TOF/MS
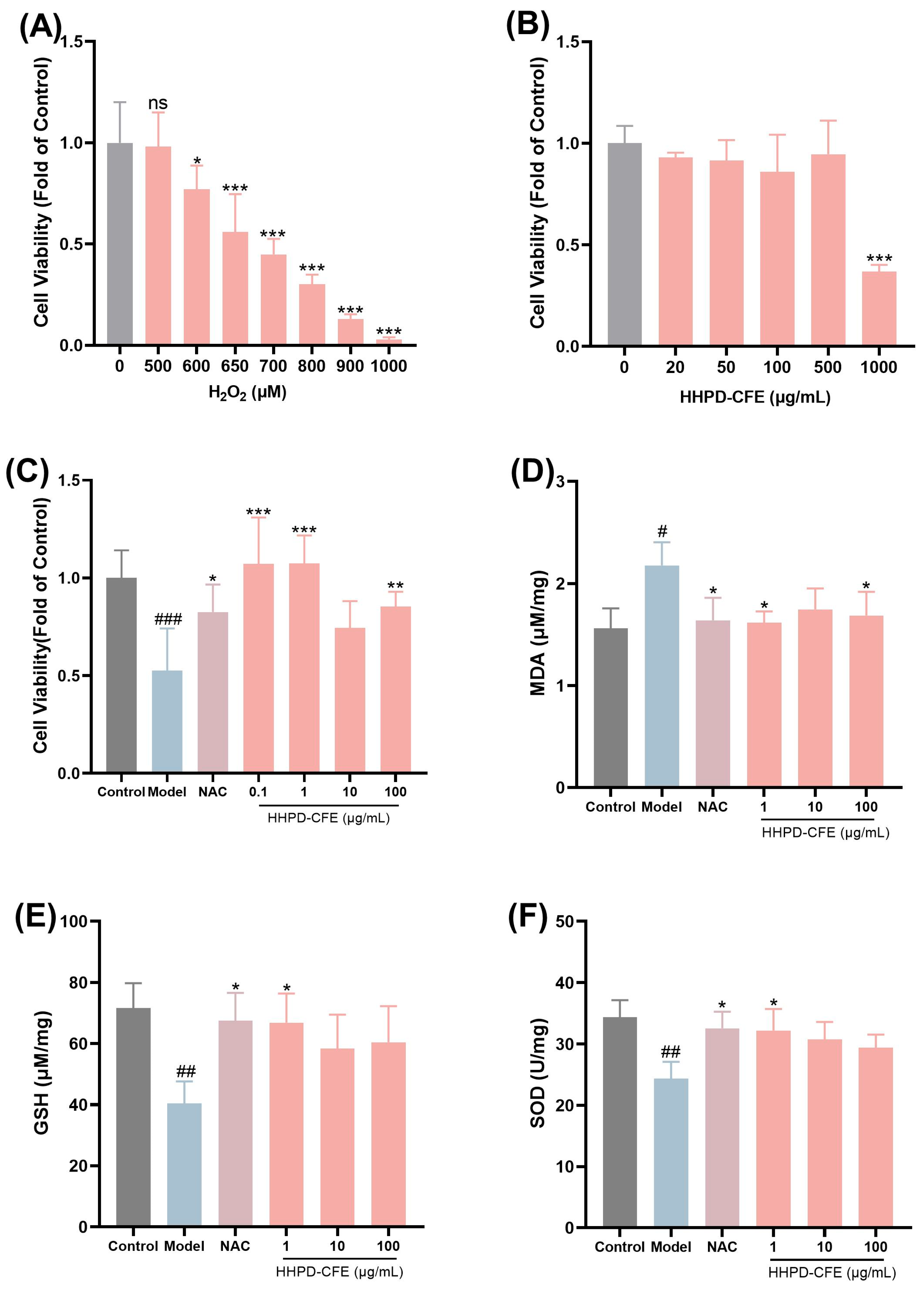
3.5. The Cytoprotective Effects of HHPD-CFE in Liver Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| HHPD | Homogenate-assisted high-pressure disruption extraction |
| SOD | Superoxide dismutase |
| HSC-T6 | Hepatic stellate cells |
| CSE | Conventional squeeze extraction |
| CFE | Cili fruit extract |
| UFLC-IT-TOF/MS | Ultrafast liquid chromatography with ion trap time-of-flight mass spectrometry |
| MDA | Malondialdehyde |
| GSH | Glutathione |
References
- Liu, M.-H.; Zhang, Q.; Zhang, Y.-H.; Lu, X.-Y.; Fu, W.-M.; He, J.-Y. Chemical Analysis of Dietary Constituents in Rosa roxburghii and Rosa sterilis Fruits. Molecules 2016, 21, 1204. [Google Scholar] [CrossRef]
- Chen, C.; Tan, S.; Ren, T.; Wang, H.; Dai, X.; Wang, H. Polyphenol from Rosaroxburghii Tratt Fruit Ameliorates the Symptoms of Diabetes by Activating the P13K/AKT Insulin Pathway in db/db Mice. Foods 2022, 11, 636. [Google Scholar] [CrossRef]
- Zhai, B.W.; Zhao, H.; Zhu, H.L.; Huang, H.; Zhang, M.Y.; Fu, Y.J. Triterpene Acids from Rosa roxburghii Tratt Fruits Exert Anti-Hepatocellular Carcinoma Activity Via Ros/Jnk Signaling Pathway-Mediated Cell Cycle Arrest and Mitochondrial Apoptosis. Phytomedicine 2023, 119, 154960. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.L.; Qin, Y.M.; Wang, Y.L.; Wang, Y.P.; Qin, Z.C. Phytochemical Profile and Antioxidant Characteristics of Bound and Free Phenolics from Rosa roxburghii Tratt. Food Biosci. 2024, 57, 103576. [Google Scholar] [CrossRef]
- Ni, H.Y.; Yu, L.; Zhao, X.L.; Wang, L.T.; Zhao, C.J.; Huang, H.; Zhu, H.L.; Efferth, T.; Gu, C.B.; Fu, Y.J. Seed Oil of Rosa roxburghii Tratt against Non-Alcoholic Fatty Liver Disease in Vivo and in Vitro through PPARα/PGC-1α-Mediated Mitochondrial Oxidative Metabolism. Phytomedicine 2022, 98, 153919. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, P.; Li, C.; Xu, F.; Chen, J. A Polysaccharide from Rosa Roxburghii Tratt Fruit Attenuates High-Fat Diet-Induced Intestinal Barrier Dysfunction and Inflammation in Mice by Modulating the Gut Microbiota. Food Funct. 2022, 13, 530–547. [Google Scholar] [CrossRef] [PubMed]
- Ordonez-Santos, L.E.; Martinez-Giron, J. Thermal degradation kinetics of carotenoids, vitamin C and provitamin A in tree tomato juice. Int. J. Food Sci. Technol. 2020, 55, 201–210. [Google Scholar] [CrossRef]
- Ni, M.; Chen, J.; Fu, M.; Li, H.; Bu, S.; Hao, X.; Gu, W. UPLC-ESI-MS/MS-Based Analysis of Various Edible Rosa Fruits Concerning Secondary Metabolites and Evaluation of Their Antioxidant Activities. Foods 2024, 13, 796. [Google Scholar] [CrossRef]
- Duan, M.H.; Fang, T.; Ma, J.F.; Shi, Q.L.; Peng, Y.; Ge, F.H.; Wang, X.L. Homogenate-Assisted High-Pressure Disruption Extraction for Determination of Phenolic Acids in Lonicerae japonicae Flos. J. Chromatogr. B 2018, 1097–1098, 119–127. [Google Scholar] [CrossRef]
- Duan, M.H.; Xu, W.J.; Yao, X.H.; Zhang, D.Y.; Zhang, Y.H.; Fu, Y.J.; Zu, Y.G. Homogenate-assisted Negative Pressure Cavitation Extraction of Active Compounds from Pyrola incarnata Fisch. and The Extraction Kinetics Study. Innov. Food Sci. Emerg. Technol. 2015, 27, 86–93. [Google Scholar] [CrossRef]
- Huang, X.Q.; Tu, Z.C.; Jiang, Y.; Xiao, H.; Zhang, Q.T.; Wang, H. Dynamic High Pressure Microfluidization-Assisted Extraction and Antioxidant Activities of Lentinan. Int. J. Biol. Macromol. 2012, 51, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Duan, M.H.; Ma, J.F.; Shi, Q.L.; Ge, F.H. Investigation into the High-Pressure Disruption Extraction of Astaxanthin from Haematococcus pluvialis. J. Chin. Med. Mater. 2018, 41, 2163–2166. (In Chinese) [Google Scholar]
- Yang, S.; Huang, X.-Y.; Zhou, N.; Wu, Q.; Liu, J.; Shi, J.-S. RNA-Seq Analysis of Protection against Chronic Alcohol Liver Injury by Rosa roxburghii Fruit Juice (Cili) in Mice. Nutrients 2022, 14, 1974. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Hou, T.; Zhu, K.; Zhang, A. Inhibition of Histone H3K18 Acetylation-Dependent Antioxidant Pathways Involved in Arsenic-Induced Liver Injury in Rats and the Protective Effect of Rosa roxburghii Tratt Juice. Toxics 2023, 11, 503. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.X.; Fu, S.F.; Bi, X.F.; Chen, F.; Liao, X.J.; Hu, X.S.; Wu, J.H. Physico-Chemical and Antioxidant Properties of Four Mango (Mangifera indica L.) Cultivars in China. Food Chem. 2013, 138, 396–405. [Google Scholar] [CrossRef]
- Xi, J.; Yan, L.G. Optimization of Pressure-Enhanced Solid-Liquid Extraction of Flavonoids from Flos sophorae and Evaluation of Their Antioxidant Activity. Sep. Purif. Technol. 2017, 175, 170–176. [Google Scholar] [CrossRef]
- Li, S.Q.; Lv, Y.M.; Yang, Q.L.; Tang, J.; Huang, Y.; Zhao, H.Y.; Zhao, F.Y. Quality Analysis and Geographical Origin Identification of Rosa roxburghii Tratt from Three Regions Based on Fourier Transform Infrared Spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 297, 122689. [Google Scholar] [CrossRef]
- Yang, J.L.; Li, X.L.; Jiang, F.L.; Jiang, F.L.; Gong, T.; Chen, J.J.; Chen, T.J.; Zhu, P. High-level soluble expression of human Cu, Zn superoxide dismutase with high activity in Escherichia coli. World J. Microbiol. Biotechnol. 2020, 36, 106. [Google Scholar] [CrossRef]
- Wang, L.T.; Zhang, S.; Fu, L.N.; Chang, Y.H.; Nie, S.M.; Fu, Y.J. Simultaneous Quantification and Quality Control of Flavor and Functional Phytochemicals in Rosa roxburghii Fruit through Multiple Reaction Monitoring Mass Spectrometry. J. Food Compos. Anal. 2023, 119, 105227. [Google Scholar] [CrossRef]
- Wang, M.H.; Lin, X.; Xu, Y.J.; Xu, B.J. The Fate of Phenolic Acids, Flavonoids, Vitamin C, Antioxidant Capacities of Cili (Rosa roxburghii) Fruits Upon Processing and Sensory Properties of the Processed Products. Food Biosci. 2023, 53, 102729. [Google Scholar] [CrossRef]
- Man, G.W.; Ma, Y.; Xu, L.; Liao, X.J.; Zhao, L. Comparison of Thermal and Non-Thermal Extraction Methods on Free and Bound Phenolics in Pomegranate Peel. Innov. Food Sci. Emerg. Technol. 2023, 84, 103291. [Google Scholar] [CrossRef]
- Blaszczak, W.; Latocha, P.; Jez, M.; Wiczkowski, W. The Impact of High-Pressure Processing on the Polyphenol Profile and Anti-Glycaemic, Anti-Hypertensive and Anti-Cholinergic Activities of Extracts Obtained from Kiwiberry (Actinidia arguta) Fruits. Food Chem. 2021, 343, 128421. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.J.; Wang, X.; Wang, T.Y.; Lin, Z.Z.; Hu, Y.J.; Huang, Z.L.; Yang, X.J.; Xu, P. Flavonoids from Rosa roxburghii Tratt Prevent Reactive Oxygen Species-Mediated DNA Damage in Thymus Cells Both Combined with and without Parp-1 Expression after Exposure to Radiation in Vivo. Aging 2020, 12, 16368–16389. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, P.; Li, L.; Huang, Y.; Pu, Y.; Hou, X.; Song, L. Identification and Antioxidant Activity of Flavonoids Extracted from Xinjiang Jujube (Ziziphus jujube Mill.) Leaves with Ultra-High Pressure Extraction Technology. Molecules 2019, 24, 122. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.Y.; Song, Q.Q.; Xie, J.H. Biological Activities and Pharmaceutical Applications of Polysaccharide from Natural Resources: A Review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Kim, Y.K.; Iwahashi, H. Properties of Polysaccharides Extracted from Phellinus linteus Using High Hydrostatic Pressure Processing and Hot Water Treatment. J. Food Process Eng. 2015, 38, 197–206. [Google Scholar] [CrossRef]
- Chen, H.; Huang, Y.Z.; Zhou, C.C.; Xu, T.L.; Chen, X.Y.; Wu, Q.Z.; Zhang, K.F.; Li, Y.; Li, D.X.; Chen, Y. Effects of Ultra-High Pressure Treatment on Structure and Bioactivity of Polysaccharides from Large Leaf Yellow Tea. Food Chem. 2022, 387, 132862. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.Q.; Zhao, L.; Wang, Y.T.; Liao, X.J. Effects of High Pressure on Activities and Properties of Superoxide Dismutase from Chestnut Rose. Food Chem. 2019, 294, 557–564. [Google Scholar] [CrossRef]
- Li, F.H.; Yang, S.H.; Liu, L.H.; Fu, H.Z.; Ming, J. Variations of Bioactive Compounds, Physicochemical and Sensory Properties of Rosa roxburghii Tratt Juice after High Pressure Processing. LWT-Food Sci. Technol. 2023, 184, 114932. [Google Scholar] [CrossRef]
- Xu, L.; Yang, H.Z.; Li, C.Z.; Liu, S.Y.; Zhao, H.D.; Liao, X.J.; Zhao, L. Composition Analysis of Free and Bound Phenolics in Chestnut Rose (Rosa roxburghii Tratt.) Fruit by UHPLC-IM-QTOF and UPLC-QQQ. LWT-Food Sci. Technol. 2023, 185, 115125. [Google Scholar] [CrossRef]
- Kanno, S.; Ishikawa, M.; Takayanagi, M.; Takayanagi, Y.; Sasaki, K. Characterization of Hydrogen Peroxide-Induced Apoptosis in Mouse Primary Cultured Hepatocytes. Biol. Pharm. Bull. 2000, 23, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Dhar, D.; Baglieri, J.; Kisseleva, T.; Brenner, D.A. Mechanisms of Liver Fibrosis and Its Role in Liver Cancer. Exp. Biol. Med. 2020, 245, 96–108. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhong, Z.; Wang, Q.; Jia, Z.; Lu, J.; Chen, J.; Liu, P. Pharmacokinetics and Tissue Distribution of Bleomycin-Induced Idiopathic Pulmonary Fibrosis Rats Treated with Cryptotanshinone. Front Pharmacol. 2023, 14, 1127219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.T.; Lu, W.T.; Zhang, X.L.; Lu, J.; Xu, S.W.; Chen, S.R.; Zhong, Z.; Zhou, T.; Wang, Q.; Chen, J.W.; et al. Cryptotanshinone Protects against Pulmonary Fibrosis through Inhibiting Smad and Stat3 Signaling Pathways. Pharmacol. Res. 2019, 147, 104307. [Google Scholar] [CrossRef]
- Wang, L.T.; Lv, M.J.; An, J.Y.; Fan, X.H.; Dong, M.Z.; Zhang, S.D.; Wang, J.D.; Wang, Y.Q.; Cai, Z.H.; Fu, Y.J. Botanical characteristics, phytochemistry and related biological activities of Rosa roxburghii Tratt fruit, and its potential use in functional foods: A review. Food Funct. 2021, 12, 1432–1451. [Google Scholar] [CrossRef]
- Zhan, J.; Liu, M.; Pan, L.; He, L.; Guo, Y. Oxidative Stress and TGF-β1/Smads Signaling Are Involved in Rosa Roxburghii Fruit Extract Alleviating Renal Fibrosis. Evid. Based Complement. Alternat. Med. 2019, 2019, 4946580. [Google Scholar] [CrossRef]
- Li, H.Z.; Qiu, P.; Wang, J.H.; Niu, C.C.; Pan, S.H. Effects of Compound Ginkgo Biloba on Intestinal Permeability in Rats with Alcohol-Induced Liver Injury. Food Funct. 2015, 6, 470–478. [Google Scholar] [CrossRef]

| Ingredient | HHPD-CFE | CS-CFE | t | p |
|---|---|---|---|---|
| Yield (wt%) | 10.2 ± 0.3 | 5.1 ± 0.4 | 23.3 | <0.001 |
| Total phenolics (g/100 g DW) | 23.6 ± 1.5 | 19.4 ± 2.1 | 3.5 | 0.01 |
| Total flavonoids (g/100 g DW) | 33.4 ± 1.8 | 23.0 ± 1.7 | 8.3 | <0.001 |
| Total polysaccharides (g/100 g DW) | 13.7 ± 0.2 | 10.2 ± 1.2 | 5.2 | <0.01 |
| SOD activity (U/g DW) | 21,194.6 ± 571.4 | 12,245.4 ± 544.7 | 15.7 | <0.001 a |
| Ingredient | HHPD-CFE | CS-CFE | t | p |
|---|---|---|---|---|
| Vitamin C (g/100 g DW) | 31.5 ± 0.6 | 24.0 ± 1.5 | 11.1 | <0.001 |
| Citric acid (g/100 g DW) | 1.1 ± 0.1 | 0.9 ± 0.1 | 2.1 | >0.05 |
| Gallic acid (g/100 g DW) | 1.5 ± 0.1 | 1.0 ± 0.1 | 5.9 | <0.001 |
| Protocatechuic acid (g/100 g DW) | 0.9 ± 0.1 | 0.7 ± 0.1 | 3.5 | 0.01 |
| Procyanidin B1 (g/100 g DW) | 0.9 ± 0.3 | 0.5 ± 0.1 | 2.3 | >0.05 |
| Catechin (g/100 g DW) | 4.2 ± 0.8 | 3.3 ± 0.2 | 2.1 | >0.05 a |
| Rutin (g/100 g DW) | 0.8 ± 0.0 | 0.5 ± 0.1 | 4.9 | <0.05 a |
| No. | Rt (min) | Formula | m/z | Adduct Type | Ion Fragmentation | Error (ppm) | Chemical Name |
|---|---|---|---|---|---|---|---|
| 1 | 2.55 | C6H14N4O2 | 175.12 | [M + H] + | 158.0216, 130.1014 | −3.6 | L-arginine |
| 2 | 3.26 | C16H18O9 | 353.09 | [M − H]− | 191.0514, 179.0623, 173.0923 | −0.6 | Chlorogenic acid |
| 3 | 3.50 | C7H12O6 | 191.05 | [M − H]− | 175.1374, 157.0557 | −1.4 | D-(-)-quinic acid |
| 4 | 3.87 | C6H8O6 | 179.04 | [M + H] + | 141.0381, 129.0066, 110.9650 | −2.6 | Ascorbic acid |
| 5 | 4.46 | C9H11NO3 | 182.08 | [M + H]+ | 147.0510, 136.0649 | 1.8 | L-(-)-tyrosine |
| 6 | 4.51 | C9H8O3 | 163.02 | [M − H]− | 136.5035, 129.0305, 111.0161 | 1.5 | p-Coumaric acid |
| 7 | 4.82 | C13H16O10 | 331.07 | [M − H]− | 271.0442, 169.0380, 125.0431 | −0.2 | Glucogallic acid |
| 8 | 4.96 | C8H8O4 | 169.05 | [M + H]+ | 158.0243, 141.0328 | 2.8 | Vanillin |
| 9 | 5.16 | C6H8O7 | 191.03 | [M − H]− | 189.0203, 173.0164 | −2.7 | Citric acid |
| 10 | 5.97 | C13H16O10 | 331.07 | [M − H]− | 271.0442, 169.0380, 125.0431 | −0.2 | Glucogallic acid |
| 11 | 6.17 | C9H11NO2 | 166.07 | [M + H]+ | 120.074 | −2.2 | Phenylalanine |
| 12 | 6.57 | C13H16O10 | 331.07 | [M − H]− | 271.0442, 169.0380, 125.0431 | −0.2 | Glucogallic acid |
| 13 | 6.79 | C7H6O5 | 169.04 | [M − H]− | 140.9893, 124.7906 | 3.9 | Gallic acid |
| 14 | 6.93 | C13H16O10 | 331.07 | [M − H]− | 271.0442, 169.0380, 125.0431 | −0.2 | Glucogallic acid |
| 15 | 11.66 | C6H14N2O2 | 147.20 | [M + H]+ | 130.0671, 112.1516 | 1.1 | L-lysine |
| 16 | 11.82 | C11H12N2O2 | 205.09 | [M + H]+ | 188.0703, 170.0418, 146.0779 | −3.1 | Tryptophan |
| 17 | 12.32 | C20H20O14 | 483.08 | [M − H]− | 331.1121, 313.0624, 271.0343 | 0.6 | b-D-glucopyranose,1,6-bis(3,4,5-trihydroxybenzoate) or isomer |
| 18 | 15.22 | C9H10O5 | 197.05 | [M − H]− | 151.0430, 125.0248 | 2.6 | Syringic acid |
| 19 | 16.51 | C7H6O4 | 155.04 | [M + H]+ | 137.0235, 109.0324 | 1.3 | Protocatechuic acid |
| 20 | 18.60 | C15H14O7 | 305.06 | [M − H]− | 247.0570, 219.0522, 178.8766, 179.0447, 163.9822 | 0.6 | (−)-Gallocatechin |
| 21 | 19.23 | C15H14O7 | 305.07 | [M − H]− | 289.0715, 279.1516, 267.0855,158.0302 | −0.1 | (−)-Epigallocatechin (EGC) |
| 22 | 19.55 | C20H20O14 | 483.08 | [M − H]− | 481.0583, 300.9917 | 0.6 | b-D-Glucopyranose,1,6-bis(3,4,5-trihydroxybenzoate) or isomer |
| 23 | 23.39 | C19H21NO6 | 360.14 | [M + H]+ | 331.1121, 313.0624, 271.0343 | −1.5 | (5R)-5-[(1S)-1,2-Dihydroxyethyl]-3,4-dihydroxy-2(5H)-furanone-1,1-diphenylmethanamine (1:1) (non-preferred name) |
| 24 | 24.51 | C15H20O4 | 265.15 | [M + H]+ | 325.0783, 279.1636, 214.0829, 208.1057, 181.0699, 158.0385 | 1.8 | Abscisic acid |
| 25 | 27.87 | C27H22O18 | 633.07 | [M − H]− | 247.1323, 217.1023, 161.1087, 158.0197 | −0.3 | Sanguiin H4 |
| 26 | 28.17 | C27H24O18 | 635.09 | [M − H]− | 481.0604, 300.9969, 275.0149 | 0.5 | 1,3,6-Tri-O-galloylglucose |
| 27 | 30.35 | C34H24O22 | 783.07 | [M − H]− | 483.0244, 295.1465, 211.0867, 193.9747, 169.8194 | 1.6 | Strictinin |
| 28 | 32.30 | C21H20O1 | 465.10 | [M + H]+ | 481.0487, 300.9965 | 0.2 | Quercetin-7-O-beta-D-glucopyranoside |
| 29 | 33.69 | C12H18O8 | 291.11 | [M + H]+ | 301.0463, 151.0014 | −2.1 | Methyl 2,3,5-tri-O-acetyl-D-ribofuranoside |
| 30 | 34.27 | C30H26O12 | 577.13 | [M − H]− | 279.1302, 214.0895, 151.0341, 123.0414 | 2.2 | Procyanidin B1 |
| 31 | 34.33 | C15H14O6 | 289.07 | [M − H]− | 427.1061, 409.0956, 291.0857, 471.1479, 425.0881, 289.0685 | −0.1 | (−)-Catechin |
| 32 | 34.62 | C30H26O12 | 577.13 | [M − H]− | 245.0912, 203.0895, 179.0449, 161.0905 | 0.9 | ProcyanidinB2 |
| 33 | 35.55 | C30H26O12 | 577.13 | [M − H]− | 453.1606, 427.0961, 409.0792, 301.0616, 291.0862, 289.0693 | 3.2 | Procyanidin B3 |
| 34 | 41.52 | C45H38O18 | 865.20 | [M − H]− | 427.1025, 409.0848, 301.0670, 291.0837, 275.0394, 425.0845, 407.0809 | 0.8 | Procyanidin C2 |
| 35 | 41.55 | C21H20O11 | 435.09 | [M + H]+ | 695.1335, 577.1316, 543.0864, 451.0935, 407.0689, 300.9994, 287.0525 | −0.6 | Quercitrin-3-O-D-xyloside |
| 36 | 42.34 | C13H8O8 | 291.02 | [M − H]− | 279.1542, 158.0262 | −0.4 | Brevifolincarboxylic acid isomer |
| 37 | 43.07 | C27H22O18 | 633.07 | [M − H]− | 247.0267, 219.0379, 203.0494, 191.1199 | 0.7 | Sanguiin H4 or isomer |
| 38 | 44.15 | C13H8O8 | 291.02 | [M − H]− | 481.0604, 300.9969, 275.0149 | −0.4 | Brevifolincarboxylic acid |
| 39 | 45.85 | C30H26O11 | 561.14 | [M − H]− | 247.0872, 175.0202, 159.1008, 147.5432 | 0.8 | Fisetinidol-(4α,8)-catechin |
| 40 | 46.22 | C15H12O5 | 273.07 | [M + H]+ | 409.1684, 391.1559, 289.0820, 245.0814, 203.1727 | −0.9 | Dihydroapigenin |
| 41 | 47.05 | C20H16O12 | 447.06 | [M − H]− | 151.0488, 123.0605 | 0.7 | Quercetin 3′-O-alpha-L-rhamnopyranoside |
| 42 | 47.17 | C20H18O9 | 401.10 | [M − H]− | 300.9967, 179.2039 | −1.0 | (Epi)catechin derivative |
| 43 | 47.39 | C30H26O11 | 561.14 | [M − H]− | 401.1233, 279.1556, 289.0508, | −0.4 | Fisetinidol-(4α,8)-catechin |
| 44 | 48.03 | C21H20O12 | 465.11 | [M + H]+ | 409.0689, 391.0705, 289.0602, 269.0557, 245.0814, 203.0631 | 3.3 | Hyperoside |
| 45 | 51.40 | C27H28O16 | 609.14 | [M + H]+ | 271.0505, 301.0723 | −0.4 | Quercetin 3-O[(X-O-3-hydroxy-3-methylglutaryl)-β-glucoside |
| 46 | 54.56 | C16H12O7 | 317.08 | [M + H]+ | 301.0155, 179.2019 | −3.8 | beta-Rhamnocitrin or isomer |
| 47 | 53.86 | C27H28O15 | 593.14 | [M + H]+ | 273.0558 | 0.6 | Kaempferol 3-O-[(X-O-3-hydroxy-3-methylglutaryl)-β-galactoside] |
| 48 | 62.20 | C27H28O6 | 447.25 | [M − H]− | 257.0426, 241.0513 | 1.8 | (6S,8S,8aS)-2-phenyl-6,7-bis(phenylmethoxy)-4,4a,6,7,8,8a-Hexahydropyrano[3,2-d][1,3]dioxin-8-ol |
| 49 | 67.30 | C21H18O13 | 479.08 | [M + H]+ | 391.2793, 279.1617, 287.1353, 214.0882 | −2.6 | Quercetin 3-O-b-D-glucuronide |
| 50 | 68.27 | C37H30O16 | 729.15 | [M − H]− | 303.0207, 158.0354 | 1.3 | Procyanidin B1 3-O-gallate |
| 51 | 80.71 | C14H6O8 | 301.00 | [M − H]− | 577.1340, 559.1054, 451.0978, 407.0765, 289.0725, 269.0910 | −0.4 | Ellagic acid |
| 52 | 81.22 | C21H20O11 | 447.10 | [M − H]− | 214.6353, 178.7923, 129.0305 | −1.1 | Quercitrin |
| 53 | 81.30 | C27H30O16 | 611.16 | [M + H]+ | 432.1673, 405.3420, 151.0657 | 3.9 | Rutin |
| 54 | 81.37 | C34H24O22 | 783.07 | [M − H]− | 303.0460, 301.0333 | 0.9 | Cornusiin C |
| 55 | 83.86 | C27H30O15 | 593.13 | [M − H]− | 633.0728, 450.9200, 300.9983 | 1.7 | Quercetin 3,7-di-O-rhamnopyranoside |
| 56 | 92.30 | C27H28O16 | 609.15 | [M + H]+ | 489.1025, 301.0355, 271.0189 | 0.1 | Kaempferol 3-O-[(X-O-3-hydroxy-3-methylglutaryl)-β-galactoside] |
| 57 | 104.23 | C30H46O3 | 455.35 | [M + H]+ | 463.0562, 301.0301 | 0.5 | Ursonic acid |
| 58 | 105.28 | C30H48O4 | 473.36 | [M + H]+ | 437.2209, 409.3996, 391.3392, 231.1277, 201.1162, 191.1023 | −0.2 | Pomolic acid |
| 59 | 105.35 | C30H48O5 | 487.34 | [M − H]− | 455.2956, 409.3509, 369.1602, 318.1010, 201.1647, 191.1897 | 0.7 | Tormentic acid |
| 60 | 105.37 | C30H48O5 | 487.34 | [M − H]− | −2.2 | Euscaphic acid | |
| 61 | 114.47 | C30H48O6 | 503.34 | [M − H]− | 427.3343, 272.9934 | 0.9 | Arjungenin |
| 62 | 115.30 | C30H48O5 | 487.34 | [M − H]− | 503.3486 | −0.8 | Asiatic acid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, R.; Lian, Z.; Cheng, Z.; Liu, Y.; Peng, X.; Wang, Y.; Ma, H.; Zhou, X.; Ge, F. The Phytochemical Characterization of a Cili (Rosa roxburghii) Fruit Low-Temperature Extract with Hepatoprotective Effects. Foods 2025, 14, 1301. https://doi.org/10.3390/foods14081301
He R, Lian Z, Cheng Z, Liu Y, Peng X, Wang Y, Ma H, Zhou X, Ge F. The Phytochemical Characterization of a Cili (Rosa roxburghii) Fruit Low-Temperature Extract with Hepatoprotective Effects. Foods. 2025; 14(8):1301. https://doi.org/10.3390/foods14081301
Chicago/Turabian StyleHe, Rifeng, Ziling Lian, Zhongjun Cheng, Yang Liu, Xiaoyan Peng, Yong Wang, Hang Ma, Xue Zhou, and Fahuan Ge. 2025. "The Phytochemical Characterization of a Cili (Rosa roxburghii) Fruit Low-Temperature Extract with Hepatoprotective Effects" Foods 14, no. 8: 1301. https://doi.org/10.3390/foods14081301
APA StyleHe, R., Lian, Z., Cheng, Z., Liu, Y., Peng, X., Wang, Y., Ma, H., Zhou, X., & Ge, F. (2025). The Phytochemical Characterization of a Cili (Rosa roxburghii) Fruit Low-Temperature Extract with Hepatoprotective Effects. Foods, 14(8), 1301. https://doi.org/10.3390/foods14081301








