Purification, Structural Analysis and Bioactivity of Pueraria montana Polysaccharide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Extraction and Determination of Polysaccharides
2.3. Optimization of Polysaccharide Extraction
2.4. Experimental Design of the Response Surface Methodology (RSM)
2.5. Purification of Crude Polysaccharides
2.6. Structural Characterization of PMPS-A1
2.6.1. Molecular Weight Analysis
2.6.2. Monosaccharide Composition Analysis
2.6.3. Methylation Analysis
2.6.4. UV–Vis Spectroscopy Analysis
2.6.5. Congo Red Analysis
2.6.6. FT-IR Spectroscopy Analysis
2.6.7. Scanning Electron Microscope (SEM) Analysis
2.6.8. Thermal Analysis
2.6.9. NMR Spectroscopy Analysis
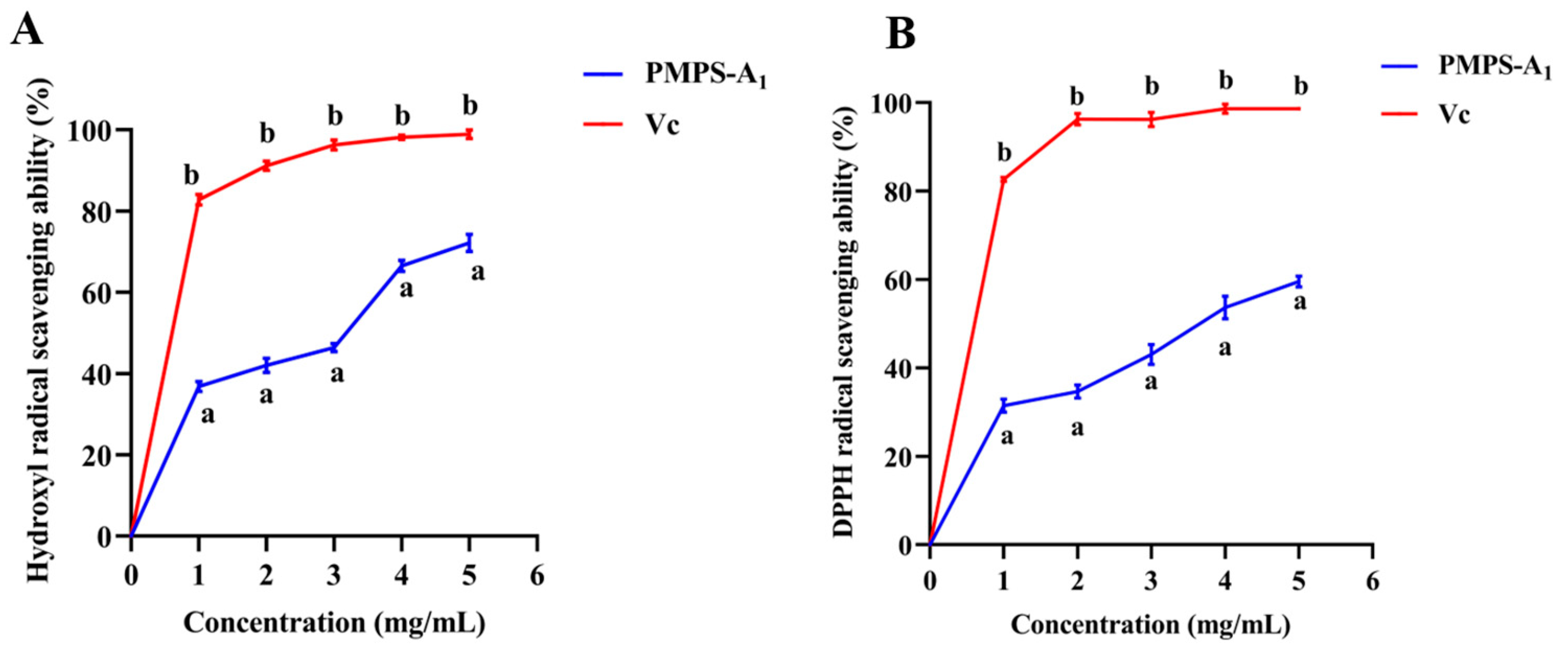
2.7. In Vitro Antioxidant Activity of PMPS-A1
2.7.1. DPPH Radical Scavenging Assay
2.7.2. Hydroxyl Radical Scavenging Assay
2.8. In Vitro Anti-Inflammatory Activity of PMPS-A1
2.8.1. Cell Viability Assay
2.8.2. Determination of Cytokines
2.9. Antibacterial Activities of PMPS-A1 Polysaccharide Extracted from Pueraria Montana
2.10. Evaluating the Impact of PMPS-A1 on Aging in C. elegans
2.10.1. Cultivation of C. elegans
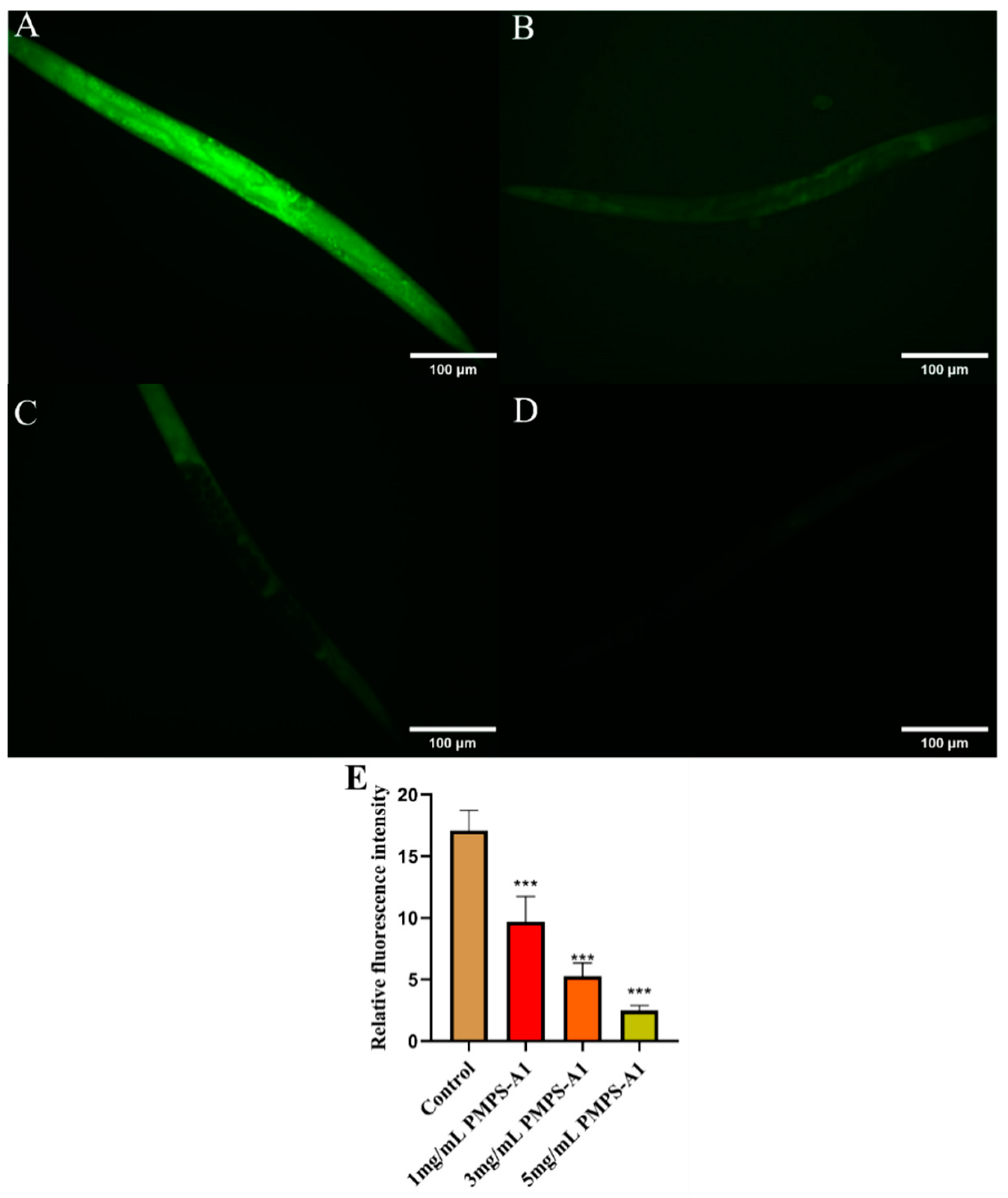
2.10.2. Determining the Levels of Reactive Oxygen Species (ROS)
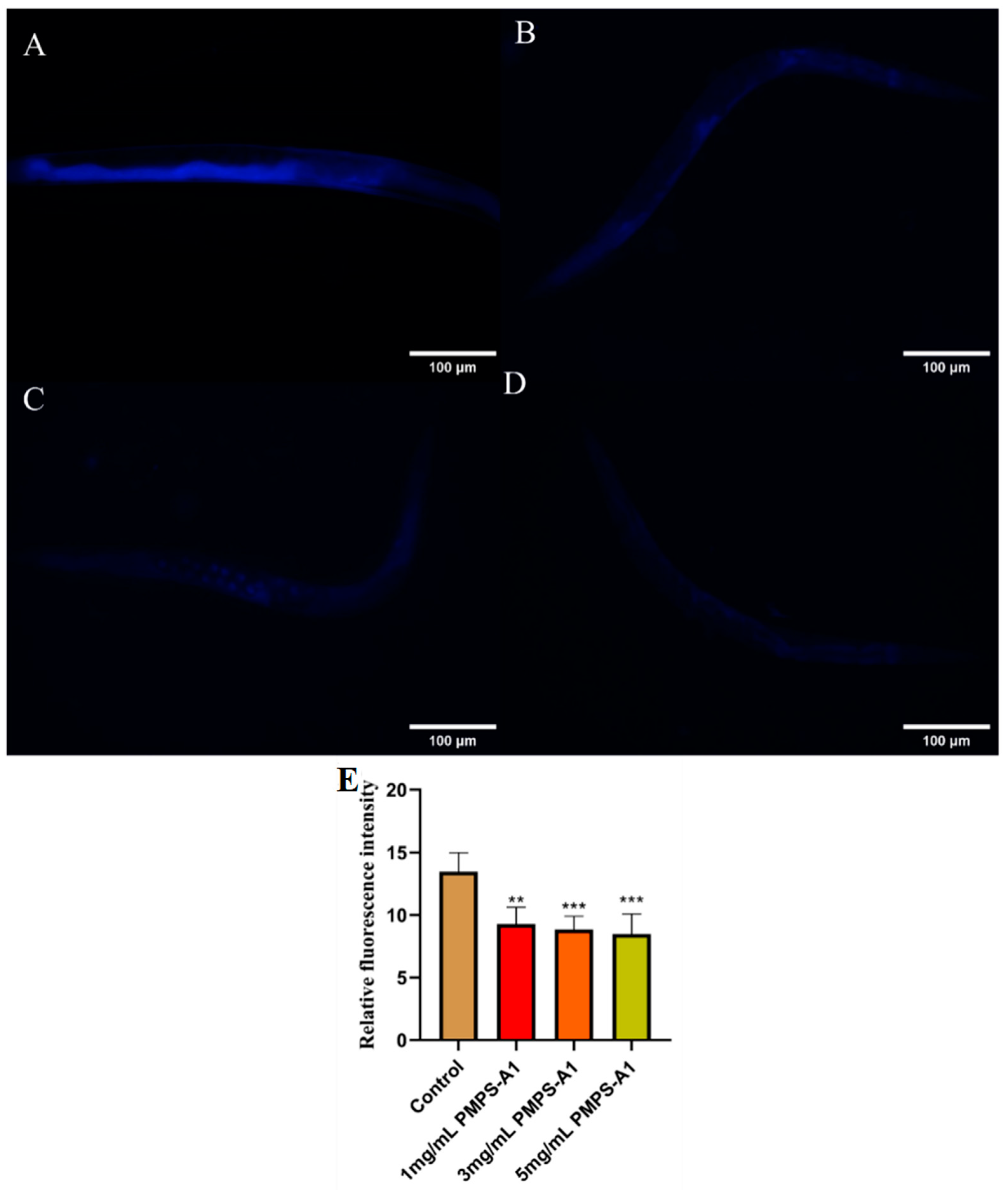
2.10.3. Lipofuscin Accumulation Assay
3. Results and Discussion
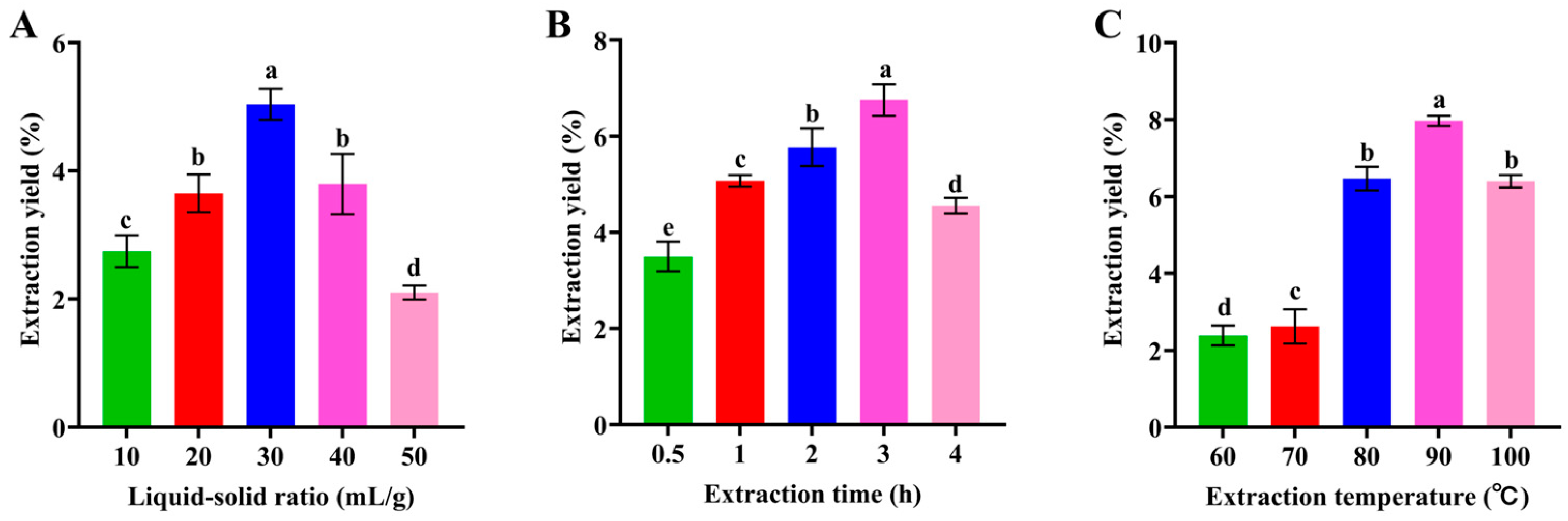
3.1. Single-Factor Experiments
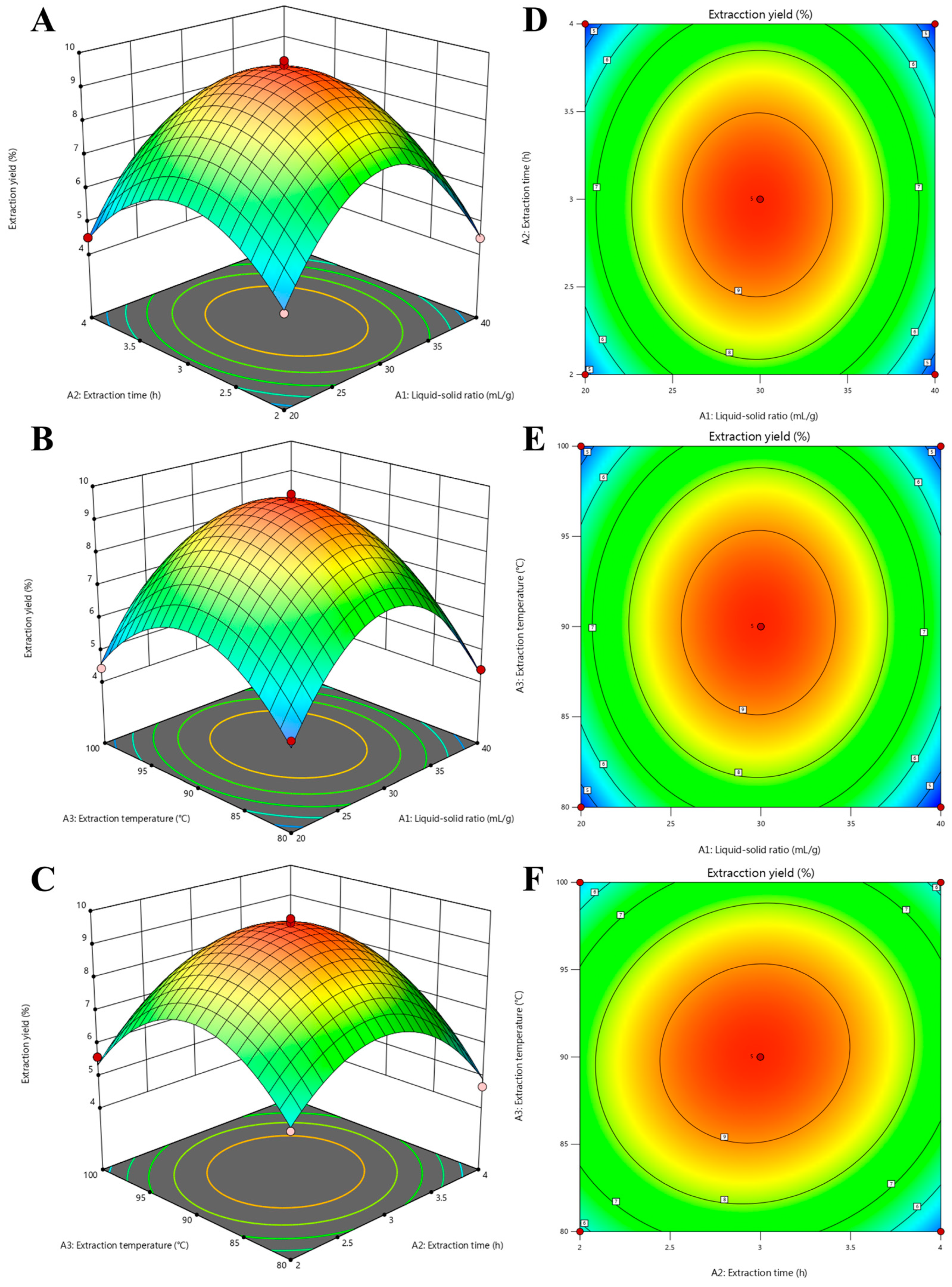
3.2. Optimization of the Polysaccharides Extraction Conditions
3.2.1. Statistical Analysis and Model Fitting
3.2.2. Response Surface Analysis
3.2.3. Optimization and Verification
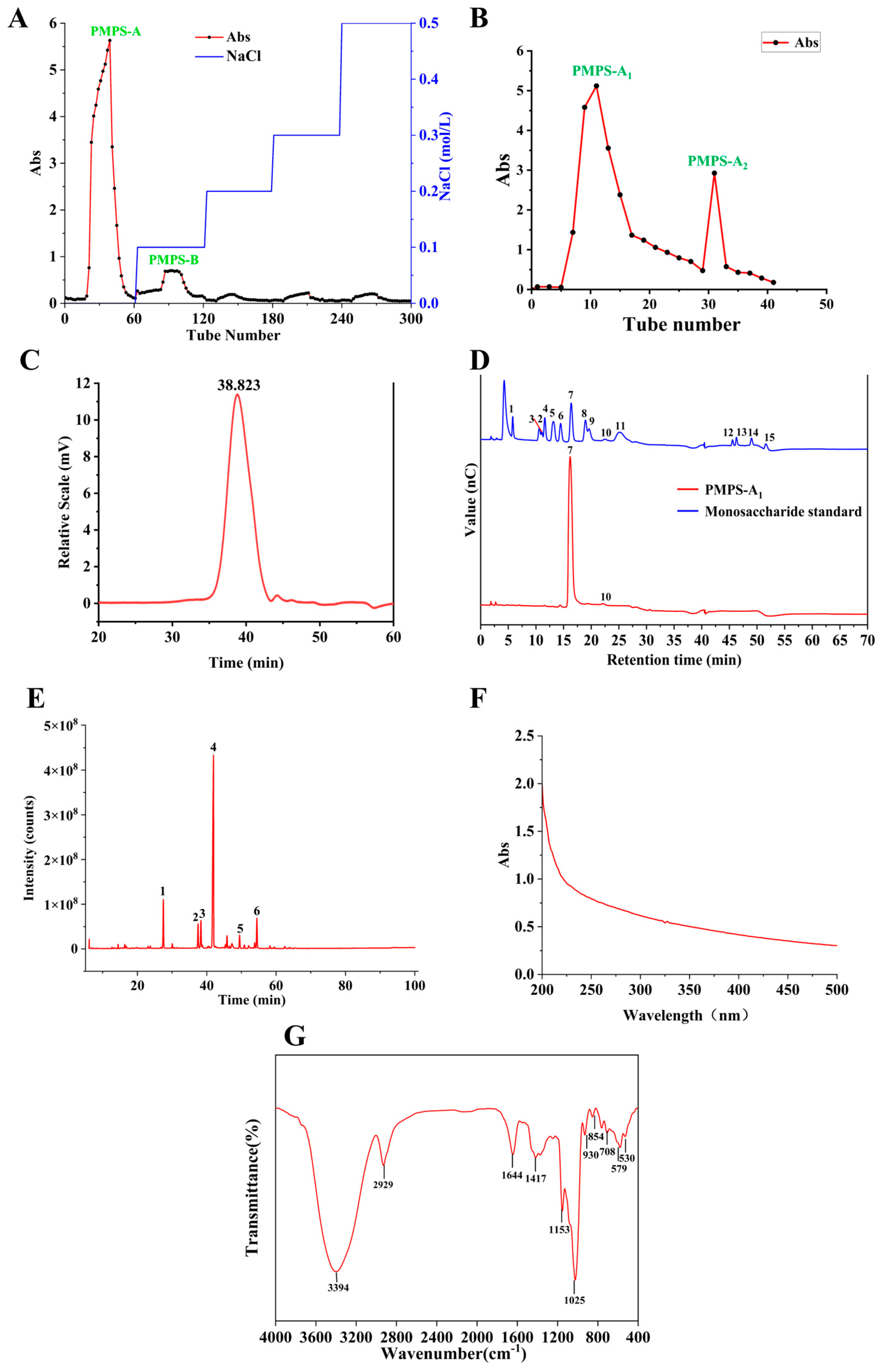
3.3. Purification of PMPS-A1
3.4. Physicochemical Properties of PMPS-A1
3.4.1. Analysis of PMPS-A1 Molecular Weight
3.4.2. Analysis of PMPS-A1 Monosaccharide Composition
3.4.3. Methylation Analysis of PMPS-A1
3.4.4. Analysis of PMPS-A1 UV-Vis and FT-IR
3.4.5. Analysis of the Thermal Properties of PMPS-A1
3.4.6. Analysis Congo Red of PMPS-A1
3.4.7. Analysis of PMPS-A1 SEM
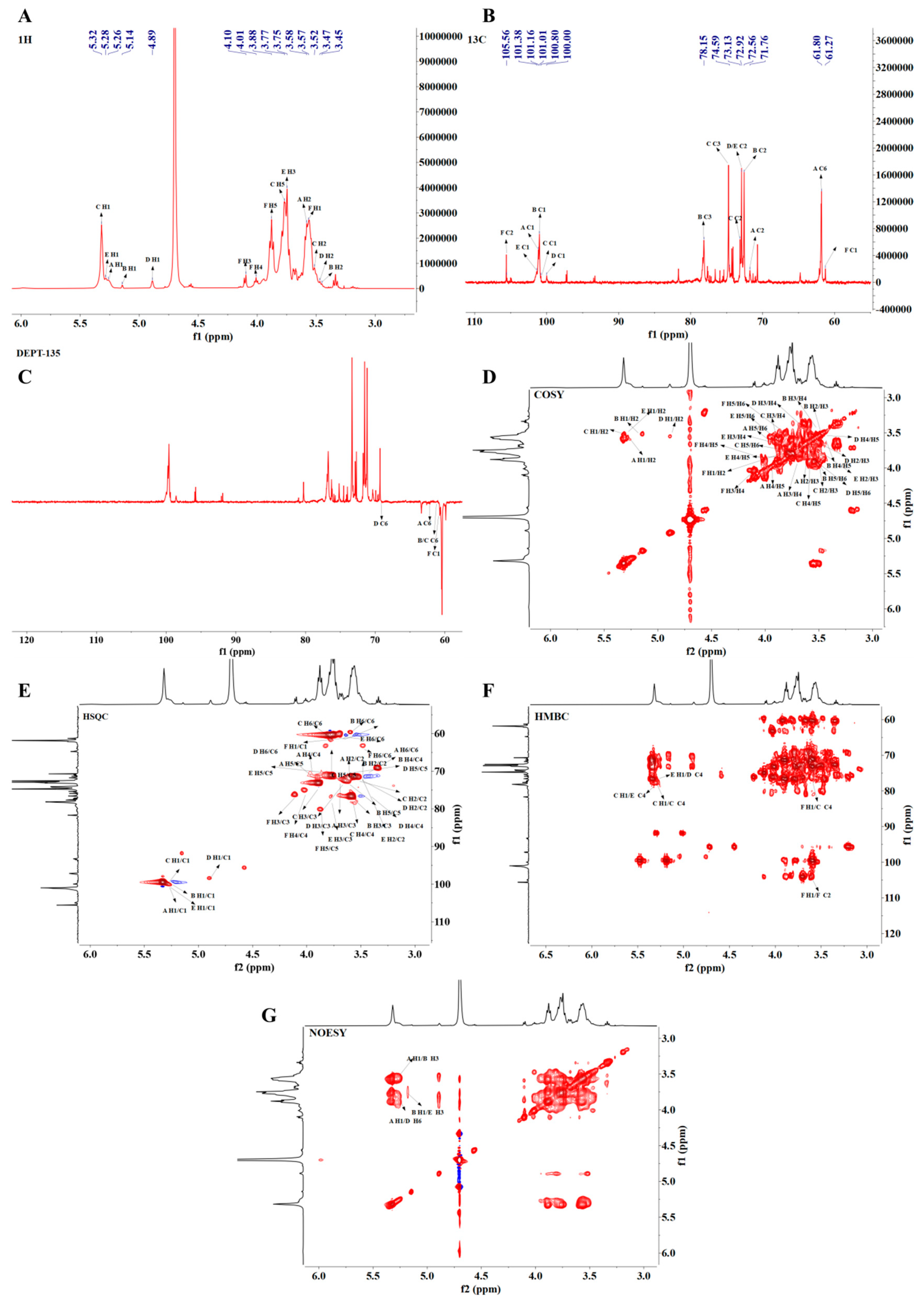
3.4.8. NMR Analysis of PMPS-A1
3.5. In Vitro Analysis of the Antioxidant Activity of PMPS-A1
3.6. In Vitro Anti-Inflammatory Activity of PMPS-A1
3.7. Antibacterial Activities of PMPS-A1
3.8. In Vivo Anti-Aging Experiment in C. elegans
3.8.1. Analysis of the Experimental Results of PMPS-A1 on the Aging of C. elegans
3.8.2. PMPS-A1 Reduce Lipofuscin Accumulation in C. elegans
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, X.; Cai, Y.; Chen, R.; Liu, Q. First report of bacterial leaf spot disease on Pueraria montana var. thomsonii caused by Robbsia andropogonis in China. Plant Dis. 2022, 106, 2985. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, W.; Zhang, H.; Chen, C.; Liu, R.; Hou, H.; Luo, Q.; Yu, Q.; Ouyang, H.; Feng, Y.; et al. alpha-D-1,3-glucan from Radix Puerariae thomsonii improves NAFLD by regulating the intestinal flora and metabolites. Carbohydr. Polym. 2023, 299, 120197. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Sun, Z.; Sun, S.Y.; Dong, J.; Li, Y.; Gao, S.; Pang, J.; Chang, Q. Effect of cortex mori on pharmacokinetic profiles of main isoflavonoids from Pueraria lobata in rat plasma. J. Ethnopharmacol. 2017, 209, 140–146. [Google Scholar] [CrossRef]
- Wang, P.; Hao, D.; Xiong, X. Anti-hypertension effect of Wuwei Jiangya decoction via ACE2/Ang1-7/MAS signaling pathway in SHR based on network degree-distribution analysis. J. Ethnopharmacol. 2024, 319, 117121. [Google Scholar] [CrossRef]
- Ma, L.; Huang, M.; Sun, G.; Lin, Y.; Lu, D.; Wu, B. Puerariae lobatae radix protects against UVB-induced skin aging via antagonism of REV-ERBα in mice. Front. Pharmacol. 2022, 13, 1088294. [Google Scholar] [CrossRef]
- Wong, K.H.; Razmovski-Naumovski, V.; Li, K.M.; Li, G.Q.; Chan, K. Comparing morphological, chemical and anti-diabetic characteristics of Puerariae lobatae Radix and Puerariae thomsonii Radix. J. Ethnopharmacol. 2015, 164, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Gao, E.; Wang, W.; Huang, Y.; Luo, Z.; Chen, B.; Xiao, S.; Li, D. ariae lobatae Radix: Progress in Extraction, Separation Methods and Pharmacological Activities Research. Separations 2024, 11, 195. [Google Scholar] [CrossRef]
- Li, W.; Zhang, M.; Zhang, R.; Huang, F.; Dong, L.; Jia, X.; Zhang, M. Structural elucidation, binding sites exploration and biological activities of bound phenolics from Radix Puerariae thomsonii. Food Chem. 2024, 450, 139323. [Google Scholar] [CrossRef]
- Li, Q.; Liu, W.; Feng, Y.; Hou, H.; Zhang, Z.; Yu, Q.; Zhou, Y.; Luo, Q.; Luo, Y.; Ouyang, H.; et al. Radix Puerariae thomsonii polysaccharide (RPP) improves inflammation and lipid peroxidation in alcohol and high-fat diet mice by regulating gut microbiota. Int. J. Biol. Macromol. 2022, 209 Pt A, 858–870. [Google Scholar] [CrossRef]
- Luo, J.-H.; Li, J.; Shen, Z.-C.; Lin, X.-F.; Chen, A.-Q.; Wang, Y.-F.; Gong, E.-S.; Liu, D.; Zou, Q.; Wang, X.-Y. Advances in health-promoting effects of natural polysaccharides: Regulation on Nrf2 antioxidant pathway. Front. Nutr. 2023, 10, 1102146. [Google Scholar] [CrossRef]
- Hu, S.; Huang, J.; Pei, S.; Ouyang, Y.; Ding, Y.; Jiang, L.; Lu, J.; Kang, L.; Huang, L.; Xiang, H.; et al. Ganoderma lucidum polysaccharide inhibits UVB-induced melanogenesis by antagonizing cAMP/PKA and ROS/MAPK signaling pathways. J. Cell. Physiol. 2019, 234, 7330–7340. [Google Scholar] [CrossRef] [PubMed]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef]
- Jedrzejewski, T.; Piotrowski, J.; Kowalczewska, M.; Wrotek, S.; Kozak, W. Polysaccharide peptide from Coriolus versicolor induces interleukin 6-related extension of endotoxin fever in rats. Int. J. Hyperth. 2015, 31, 626–634. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Y.; Gao, L.; Zhang, L.; Wang, J. Study on the Protective Effect and Mechanism of Umbilicaria esculenta Polysaccharide in DSS-Induced Mice Colitis and Secondary Liver Injury. J. Agric. Food Chem. 2024, 72, 10923–10935. [Google Scholar] [CrossRef] [PubMed]
- de Brum, T.F.C.; Camponogara da Silva Jesus, B.V.; Belke, M.; Piana, A.A.; Boligon, F.B.; Pires, S.M.; da Rosa Oliveira, M.B.; de Freitas Bauermann, L. Ethnopharmacological study and topical anti-inflammatory activity of crude extract from Poikilacanthus glandulosus (Nees) Ariza leaves. J. Ethnopharmacol. 2016, 193, 60–67. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, Z.; Yang, Y. Antioxidant and immunoregulatory activity of Ganoderma lucidum polysaccharide (GLP). Carbohydr. Polym. 2013, 95, 200–206. [Google Scholar] [CrossRef]
- Chen, J.; Liu, L.; Zhang, Y.; Jiao, Y.; Hou, J.; Liu, F.; Liu, W. Characterization, antioxidant and antibacterial activity of neutral polysaccharides from oyster (Crassostrea rivularis). LWT-Food Sci. Technol. 2024, 212, 116961. [Google Scholar] [CrossRef]
- Nakagawa, T.; Abe, H.; Gessei, T.; Takeda, K.; Igarashi, K.; Nakamura, N. Biorefinery of galacturonic acid using a biofuel cell as a reactor. React. Chem. Eng. 2022, 7, 2629–2635. [Google Scholar]
- Zhao, Y.; Chen, X.; Zhao, Y.; Jia, W.; Chang, X.; Liu, H.; Liu, N. Optimization of extraction parameters of Pleurotus eryngii polysaccharides and evaluation of the hypolipidemic effect. RSC Adv. 2020, 10, 11918–11928. [Google Scholar] [CrossRef]
- Zhang, K.; Tang, H.; Farid, M.S.; Xiang, F.; Li, B. Effect of Lactobacillus helveticus exopolysaccharides molecular weight on yogurt gel properties and its internal mechanism. Int. J. Biol. Macromol. 2024, 262 Pt 1, 130006. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, W.; Ren, Y.; Li, X.; Tang, Y.; Min, T.; Lai, F.; Wu, H. Structural Characterization of a Novel Polysaccharide from Lepidium meyenii (Maca) and Analysis of Its Regulatory Function in Macrophage Polarization In Vitro. J. Agric. Food Chem. 2017, 65, 1146–1157. [Google Scholar] [CrossRef]
- Wang, H.; Ma, S.; Mariga, A.M.; Hu, Q.; Xu, Q.; Su, A.; Ma, N.; Ma, G. Structural characterization and anti-inflammatory activities of novel polysaccharides obtained from Pleurotus eryngii. Food Sci. Hum. Wellness 2024, 13, 3031–3042. [Google Scholar] [CrossRef]
- Shen, Y.; Guo, Y.L.; Zhang, Y.; Li, Y.; Liang, J.; Kuang, H.X.; Xia, Y.G. Structure and immunological activity of an arabinan-rich acidic polysaccharide from Atractylodes lancea (Thunb.) DC. Int. J. Biol. Macromol. 2022, 199, 24–35. [Google Scholar] [CrossRef]
- Nai, J.; Zhang, C.; Shao, H.; Li, B.; Li, H.; Gao, L.; Dai, M.; Zhu, L.; Sheng, H. Extraction, structure, pharmacological activities and drug carrier applications of Angelica sinensis polysaccharide. Int. J. Biol. Macromol. 2021, 183, 2337–2353. [Google Scholar] [CrossRef]
- Sellimi, S.; Maalej, H.; Rekik, D.M.; Benslima, A.; Ksouda, G.; Hamdi, M.; Sahnoun, Z.; Li, S.; Nasri, M.; Hajji, M. Antioxidant, antibacterial and in vivo wound healing properties of laminaran purified from Cystoseira barbata seaweed. Int. J. Biol. Macromol. 2018, 119, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Zhang, Y.; Song, H.; Zhou, H.; Zhong, F.; Hu, H.; Feng, Y. Extraction optimization, characterization and antioxidant activity of polysaccharide from Gentiana scabra bge. Int. J. Biol. Macromol. 2016, 93 Pt A, 369–380. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, Z.; Peng, X.; Xu, J.; Deng, J.; Zhao, P.; Zhang, X.; Kan, H. A polysaccharide from the seed of Gleditsia japonica var. delavayi: Extraction, purification, characterization and functional properties. LWT-Food Sci. Technol. 2024, 191, 115660. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, Y.; Zhao, Z.; Yang, D.; Xu, X. Optimization of Extraction Process, Preliminary Characterization and Safety Study of Crude Polysaccharides from Morindae Officinalis Radix. Foods 2023, 12, 1590. [Google Scholar] [CrossRef]
- Qiu, X.; Geng, Y.; Cai, X.; Ou, Y.; Li, M.; Zhang, Y.; He, D.; Qian, X.; Wu, Y.; Ma, H.; et al. Anti-inflammatory activity and underlying mechanism against sepsis-induced acute lung injury of a low-molecular-weight polysaccharide from the root of Stemona tuberosa Lour. Int. J. Biol. Macromol. 2024, 282 Pt 1, 136617. [Google Scholar] [CrossRef]
- Rostami, H.; Gharibzahedi, S.M.T. Cellulase-assisted extraction of polysaccharides from Malva sylvestris: Process optimization and potential functionalities. Int. J. Biol. Macromol. 2017, 101, 196–206. [Google Scholar] [CrossRef]
- Ji, H.-Y.; Liu, C.; Dai, K.-Y.; Yu, J.; Liu, A.-J.; Chen, Y.-F. The extraction, structure, and immunomodulation activities in vivo of polysaccharides from Salvia miltiorrhiza. Ind. Crops Prod. 2021, 173, 114085. [Google Scholar] [CrossRef]
- Yan, K.; Liu, X.; Li, L.; Zhu, S.; Zheng, L.; He, S.; Jia, X.; Dong, W.; Liu, Y.; Lu, Z.; et al. Ultrasound-assisted deep eutectic solvents extraction of polysaccharides from Loquat leaf: Process optimization and bioactivity study. Int. J. Biol. Macromol. 2024, 274 Pt 1, 133308. [Google Scholar] [CrossRef]
- Qiu, J.; Xu, X.; Guo, J.; Wang, Z.; Wu, J.; Ding, H.; Xu, Y.; Wu, Y.; Ying, Q.; Qiu, J.; et al. Comparison of extraction processes, characterization and intestinal protection activity of Bletilla striata polysaccharides. Int. J. Biol. Macromol. 2024, 263 Pt 1, 130267. [Google Scholar] [CrossRef]
- Cai, G.; Wu, C.; Mao, N.; Song, Z.; Yu, L.; Zhu, T.; Peng, S.; Yang, Y.; Liu, Z.; Wang, D. Isolation, purification and characterization of Pueraria lobata polysaccharide and its effects on intestinal function in cyclophosphamide-treated mice. Int. J. Biol. Macromol. 2022, 218, 356–367. [Google Scholar] [CrossRef]
- Du, B.; Fu, Y.; Wang, X.; Jiang, H.; Lv, Q.; Du, R.; Yang, Y.; Rong, R. Isolation, purification, structural analysis and biological activities of water-soluble polysaccharide from Glehniae radix. Int. J. Biol. Macromol. 2019, 128, 724–731. [Google Scholar] [CrossRef]
- Qu, Y.; Li, C.; Zhang, C.; Zeng, R.; Fu, C. Optimization of infrared-assisted extraction of Bletilla striata polysaccharides based on response surface methodology and their antioxidant activities. Carbohydr. Polym. 2016, 148, 345–353. [Google Scholar] [CrossRef]
- Jiang, S.; Xie, H.; Zuo, Y.; Sun, J.; Wu, D.; Shu, X. Structural and functional properties of polysaccharides extracted from three Dioscorea species. Int. J. Biol. Macromol. 2024, 281 Pt 4, 136469. [Google Scholar] [CrossRef]
- Zeng, S.; Wang, B.; Lv, W.; Li, B.; Xiao, H.; Lin, R. Physicochemical properties, structure and biological activity of ginger polysaccharide: Effect of microwave infrared dual-field coupled drying. Int. J. Biol. Macromol. 2024, 281 Pt 3, 136474. [Google Scholar] [CrossRef]
- Hou, Y.; Zhao, J.; Yin, J.; Nie, S. Structural properties of Bletilla striata polysaccharide and the synergistic gelation of polysaccharide and xanthan gum. Food Hydrocoll. 2023, 142, 108843. [Google Scholar] [CrossRef]
- Rozi, P.; Abuduwaili, A.; Ma, S.; Bao, X.; Xu, H.; Zhu, J.; Yadikar, N.; Wang, J.; Yang, X.; Yili, A. Isolations, characterizations and bioactivities of polysaccharides from the seeds of three species Glycyrrhiza. Int. J. Biol. Macromol. 2020, 145, 364–371. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Ding, J.; Li, Y.; Cao, G.; Zhu, L.; Bian, Y.; Liu, Y. Extraction, structure and bioactivities of polysaccharide from root of Arctium lappa L.: A review. J. Biol. Macromol. 2024, 265 Pt 2, 131035. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Xu, W.; Wang, H.X.; Huang, F.; Wang, L.M. Natural polysaccharides experience physiochemical and functional changes during preparation: A review. Carbohydr. Polym. 2020, 234, 115896. [Google Scholar] [CrossRef]
- Paulusma, C.C.; Lamers, W.H.; Broer, S.; van de Graaf, S.F.J. Amino acid metabolism, transport and signalling in the liver revisited. Biochem. Pharmacol. 2022, 201, 115074. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Yang, W.; Huang, G.; Huang, H. The antioxidant activities of balsam pear polysaccharide. Int. J. Biol. Macromol. 2020, 142, 232–236. [Google Scholar] [CrossRef]
- Hu, S.; Yin, J.; Nie, S.; Wang, J.; Phillips, G.O.; Xie, M.; Cui, S.W. In vitro evaluation of the antioxidant activities of carbohydrates. Bioact. Carbohydr. Diet. Fibre 2016, 7, 19–27. [Google Scholar] [CrossRef]
- Machova, E.; Bystricky, S. Antioxidant capacities of mannans and glucans are related to their susceptibility of free radical degradation. Int. J. Biol. Macromol. 2013, 61, 308–311. [Google Scholar] [CrossRef]
- Cao, W.; Wu, J.; Zhao, X.; Li, Z.; Yu, J.; Shao, T.; Hou, X.; Zhou, L.; Wang, C.; Wang, G.; et al. Structural elucidation of an active polysaccharide from Radix Puerariae lobatae and its protection against acute alcoholic liver disease. Carbohydr. Polym. 2024, 325, 121565. [Google Scholar] [CrossRef]
- Hou, C.; Chen, L.; Yang, L.; Ji, X. An insight into anti-inflammatory effects of natural polysaccharides. Int. J. Biol. Macromol. 2020, 153, 248–255. [Google Scholar] [CrossRef]
- Duque, G.A.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar]
- Wei, H.; Shi, Y.; Yuan, Z.; Huang, Z.; Cai, F.; Zhu, J.; Zhang, W.; Li, J.; Xiong, Q.; Wang, Y.; et al. Isolation, Identification, and Anti-Inflammatory Activity of Polysaccharides of Typha angustifolia. Biol. Macromol. 2021, 22, 2451–2459. [Google Scholar] [CrossRef]
- Dong, Z.; Zhang, M.; Li, H.; Zhan, Q.; Lai, F.; Wu, H. Structural characterization and immunomodulatory activity of a novel polysaccharide from Pueraria lobata (Willd.) Ohwi root. Int. J. Biol. Macromol. 2020, 154, 1556–1564. [Google Scholar] [CrossRef]
- Li, X.; Ji, W.; Wu, S.; Qian, C.; Zhou, J.; Zhang, Z.; Li, D. The isolation, characterization and biological activities of the non-glucan polysaccharides from the high-starch-content plant Pueraria mirifica. Int. J. Biol. Macromol. 2024, 261 Pt 2, 129709. [Google Scholar] [CrossRef]
- Papaevgeniou, N.; Hoehn, A.; Grune, T.; Chondrogianni, N. Lipofuscin-mediated alterations effects on organismal ageing. Free Radic. Biol. Med. 2018, 120, S78–S79. [Google Scholar] [CrossRef]
- Brickute, D.; Chen, C.; Braga, M.; Barnes, C.; Wang, N.; Allott, E.O. Aboagye, Design, synthesis, and evaluation of a novel PET imaging agent targeting lipofuscin in senescent cells. RSC Adv. 2022, 12, 26372–26381. [Google Scholar] [CrossRef]
- Gao, D.; Wang, S.; Lin, Y.; Sun, Z. In vivo AAV delivery of glutathione reductase gene attenuates anti-aging gene klotho deficiency-induced kidney damage. Redox Biol. 2020, 37, 101692. [Google Scholar] [CrossRef] [PubMed]
- Strong, R.; Miller, R.A.; Astle, C.M.; Floyd, R.A.; Flurkey, K.; Hensley, K.L.; Javors, M.A.; Leeuwenburgh, C.; Nelson, J.F.; Ongini, E.; et al. Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell 2008, 7, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xin, Q.; Wei, P.; Hua, Y.; Zhang, Y.; Su, Z.; She, G.; Yuan, R. Antioxidant and anti-aging activities of Longan crude and purified polysaccharide (LP-A) in nematode Caenorhabditis elegans. Int. J. Biol. Macromol. 2024, 267, 131634. [Google Scholar] [CrossRef] [PubMed]



| Entry | Coded Variable Levels | Yield (%) | ||
|---|---|---|---|---|
| A1 a | A2 a | A3 a | ||
| 1 | −1 (1:20) | 0 (3) | −1 (80) | 4.68 |
| 2 | −1 (1:20) | −1 (2) | 0 (90) | 4.73 |
| 3 | −1 (1:20) | 0 (3) | 1 (100) | 4.43 |
| 4 | −1 (1:20) | 1 (4) | 0 (90) | 4.52 |
| 5 | 1 (1:40) | −1 (2) | 0 (90) | 4.5 |
| 6 | 1 (1:40) | 0 (3) | −1 (80) | 4.39 |
| 7 | 1 (1:40) | 0 (3) | 1 (100) | 4.31 |
| 8 | 1 (1:40) | 1 (4) | 0 (90) | 4.48 |
| 9 | 0 (1:30) | 1 (4) | −1 (80) | 4.67 |
| 10 | 0 (1:30) | 1 (4) | 1 (100) | 5.84 |
| 11 | 0 (1:30) | −1 (2) | −1 (80) | 5.69 |
| 12 | 0 (1:30) | −1 (2) | 1 (100) | 5.6 |
| 13 | 0 (1:30) | 0 (3) | 0 (90) | 9.51 |
| 14 | 0 (1:30) | 0 (3) | 0 (90) | 9.77 |
| 15 | 0 (1:30) | 0 (3) | 0 (90) | 9.47 |
| 16 | 0 (1:30) | 0 (3) | 0 (90) | 9.35 |
| 17 | 0 (1:30) | 0 (3) | 0 (90) | 9.64 |
| Source | Sum of Squares | DF | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| A1 | 0.0578 | 1 | 0.0578 | 1.03 | 0.3442 |
| A2 | 0.1275 | 1 | 0.1275 | 2.27 | 0.1756 |
| A3 | 0.0703 | 1 | 0.0703 | 1.25 | 0.3002 |
| A12 | 37.74 | 1 | 37.74 | 671.84 | <0.0001 *** |
| A22 | 16.78 | 1 | 16.78 | 298.74 | <0.0001 *** |
| A32 | 18.59 | 1 | 18.59 | 330.99 | <0.0001 *** |
| A1A2 | 0.0090 | 1 | 0.0090 | 0.1606 | 0.7005 |
| A1A3 | 0.0072 | 1 | 0.0072 | 0.1286 | 0.7305 |
| A2A3 | 0.3969 | 1 | 0.3969 | 7.06 | 0.0326 * |
| Model | 81.97 | 9 | 9.11 | 162.12 | 0.0001 *** |
| Residual | 0.3933 | 7 | 0.0562 | ||
| Lack of fit | 0.2888 | 3 | 0.0963 | 3.69 | 0.1200 |
| Pure error | 0.1045 | 4 | 0.0261 | ||
| Cor.total | 82.36 | 16 | |||
| R2 | 0.9952 | ||||
| R2adj | 0.9891 | ||||
| CV(%) | 3.82 |
| Number | Type of Linkage | Methylated Sugars | Time (min) | Molar Ratio | Mass Fragments (m/z) |
|---|---|---|---|---|---|
| 1 | Glcp-(1→ | 2,3,4,6-Me4-Glcp | 27.485 | 0.116 | 43, 71, 87, 101, 117, 129, 143, 161, 205 |
| 2 | →1)-Fruf-(2→ | 3,5,6-Me3-Manf/Glcf | 37.501 | 0.058 | 45, 71, 87, 99, 101, 129, 145, 161, 189 |
| 3 | →3)-Glcp-(1→ | 2,4,6-Me3-Glcp | 38.324 | 0.079 | 43, 87, 99, 101, 117, 129, 161, 173, 233 |
| 4 | →4)-Glcp-(1→ | 2,3,6-Me3-Glcp | 41.936 | 0.646 | 43, 87, 99, 101, 113, 117, 129, 131, 161, 173, 233 |
| 5 | →3,4)-Glcp-(1→ | 2,6-Me2-Glcp | 49.470 | 0.031 | 43, 87, 97, 117, 129, 149 |
| 6 | →4,6)-Glcp-(1→ | 2,3-Me2-Glcp | 54.456 | 0.070 | 43, 71, 85, 87, 99, 101, 117, 127, 159, 161, 201, 261 |
| Number | Glycosy Residues | Chemical Shifts (ppm) | ||||||
|---|---|---|---|---|---|---|---|---|
| H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6a/C6 | H6b | ||
| A | α-D-Glcp-1→ | 5.25 | 3.59 | 3.69 | 3.94 | 3.96 | 3.59 | 3.81 |
| 100.92 | 71.53 | 73.62 | 70.2 | 71.27 | 61.89 | |||
| B | →3)-α- D-Glcp-(1→ | 5.18 | 3.48 | 3.52 | 3.42 | 3.61 | 3.66 | 3.73 |
| 100.86 | 72.33 | 78.02 | 70.26 | 73.52 | 61.48 | |||
| C | →4-α-D-Glcp-(1→ | 5.3 | 3.51 | 3.89 | 3.57 | 3.77 | 3.75 | 3.7 |
| 100.48 | 72.5 | 74.8 | 77.9 | 72.12 | 61.48 | |||
| D | →4,6)- α-D-Glcp-(1→ | 4.87 | 3.48 | 3.66 | 3.4 | 3.56 | 3.84 | 3.85 |
| 99.48 | 72.45 | 73.61 | 76.69 | 70.28 | 69.04 | |||
| E | →3,4)-α-D-Glcp-(1→ | 5.26 | 3.49 | 3.74 | 3.73 | 3.84 | 3.65 | 3.77 |
| 100.95 | 72.45 | 78 | 78.16 | 70.76 | 61.46 | |||
| F | →1)-β-D-Fruf-(2→ | 3.58, 3.69 | nd | 4.1 | 4.01 | 3.84 | 3.46 | 3.81 |
| 61.27 | 105.62 | 77.74 | 76.59 | 70.76 | 64.81 | |||
| Microorganisms | PMPS-A1 Concentration (mg/mL) | Penicillin (mg/mL) | |||
|---|---|---|---|---|---|
| 20 | 25 | 30 | 35 | ||
| E. coli 25,922 | 10 ± 0.45 b | 11 ± 0.98 b | 12 ± 1.03 b | 13 ± 0.89 b | 25.18 ± 1.24 a |
| (MRSA) ATCC 29,213 | 11 ± 1.02 b | 12 ± 1.02 b | 13 ± 1.42 b | 14 ± 1.54 b | 26.17 ± 1.28 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Yao, H.; Qiu, X.; Xu, L.; Ou, Y.; Xin, J.; Lu, S.; Li, M.; Geng, Y.; Zhang, Y.; et al. Purification, Structural Analysis and Bioactivity of Pueraria montana Polysaccharide. Foods 2025, 14, 1359. https://doi.org/10.3390/foods14081359
Chen D, Yao H, Qiu X, Xu L, Ou Y, Xin J, Lu S, Li M, Geng Y, Zhang Y, et al. Purification, Structural Analysis and Bioactivity of Pueraria montana Polysaccharide. Foods. 2025; 14(8):1359. https://doi.org/10.3390/foods14081359
Chicago/Turabian StyleChen, Dandan, Hongliang Yao, Xiang Qiu, Lang Xu, Yanghui Ou, Jianghui Xin, Shengjia Lu, Mengjie Li, Yan Geng, Yali Zhang, and et al. 2025. "Purification, Structural Analysis and Bioactivity of Pueraria montana Polysaccharide" Foods 14, no. 8: 1359. https://doi.org/10.3390/foods14081359
APA StyleChen, D., Yao, H., Qiu, X., Xu, L., Ou, Y., Xin, J., Lu, S., Li, M., Geng, Y., Zhang, Y., Hu, M., Ren, Z., & Wu, J.-Q. (2025). Purification, Structural Analysis and Bioactivity of Pueraria montana Polysaccharide. Foods, 14(8), 1359. https://doi.org/10.3390/foods14081359





