Effect of Two-Stage Water Addition on Consistency of Processed Cheese: Physicochemical, Mechanical, Thermal, and Organoleptic Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ingredients Used for the Manufacture of the Processed Cheese Samples
2.2. Manufacture of the Processed Cheese Samples
2.3. Basic Physicochemical Analysis of the Processed Cheese Samples
2.4. Determination of Processed Cheese Samples’ Viscoelastic Properties
2.5. Texture Profile Analysis of the Processed Cheese Samples
2.6. Tribological Analysis of the Processed Cheese Samples
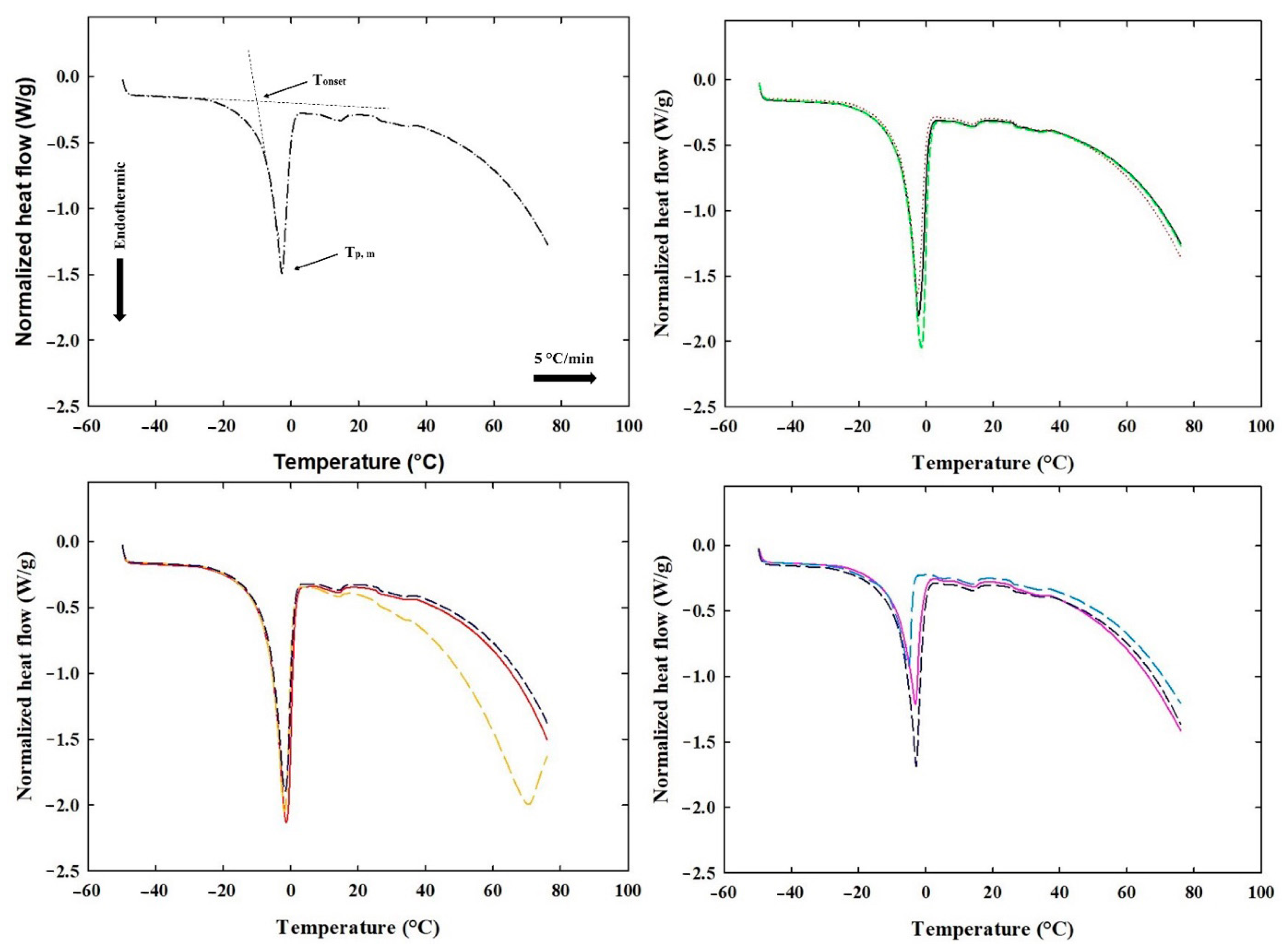
2.7. Differential Scanning Calorimetry of the Processed Cheese Samples
2.8. Instrumental Determination of the Color of the Processed Cheese Samples
2.9. Sensory Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Basic Physicochemical Analysis and Water Activity of the Processed Cheese Samples
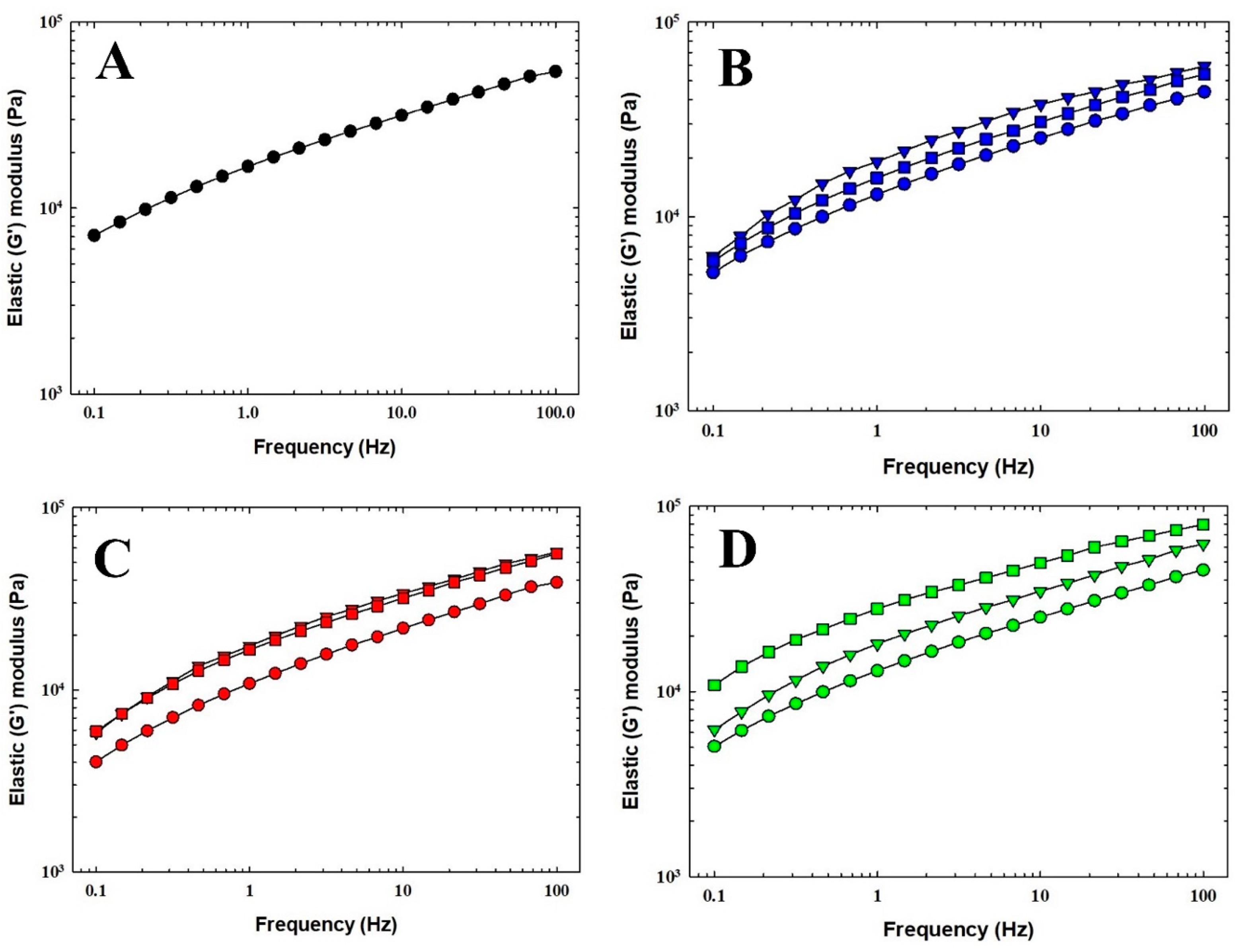
3.2. Viscoelastic Properties of the Processed Cheese Samples
3.3. Texture Profile Analysis of the Processed Cheese Samples
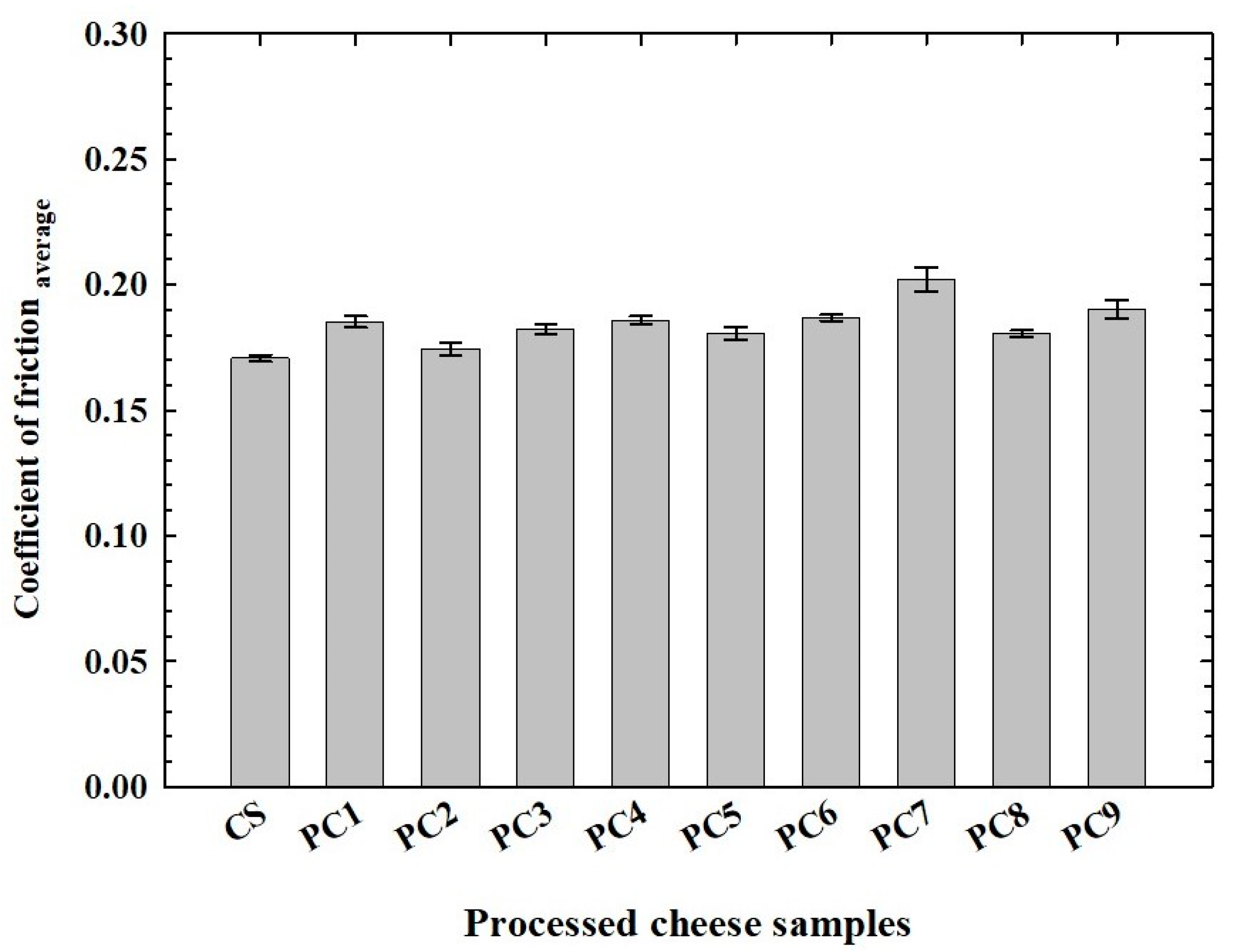
3.4. Tribological Analysis of the Processed Cheese Samples
3.5. DSC of the Processed Cheese Samples
3.6. Instrumental Color Analysis of the Processed Cheese Samples
3.7. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, L.; Liu, H. Effect of emulsifying salts on the physicochemical properties of processed cheese made from Mozzarella. J. Dairy Sci. 2012, 95, 4823–4830. [Google Scholar] [CrossRef] [PubMed]
- Tamime, A.Y. Processed cheese and analogues. In Society of Dairy Technology Series; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2011; pp. 1–24. [Google Scholar] [CrossRef]
- El-Bakry, M.; Mehta, B.M. Overview of processed cheese and its products. In Processed Cheese Science and Technology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–28. [Google Scholar] [CrossRef]
- Kowalska, M.; Janas, S.; Woźniak, M. Innovative application of the moisture analyzer for determination of dry mass content of processed cheese. Heat Mass Transf. 2018, 54, 3071–3080. [Google Scholar] [CrossRef]
- Joyner, H.S. Explaining food texture through rheology. Curr. Opin. Food Sci. 2018, 21, 7–14. [Google Scholar] [CrossRef]
- Schädle, C.N.; Bader-Mittermaier, S.; Sanahuja, S. The effect of corn dextrin on the rheological, tribological, and aroma release properties of a reduced-fat model of processed cheese spread. Molecules 2022, 27, 1864. [Google Scholar] [CrossRef]
- Lee, S.K.; Klostermeyer, H. The Effect of pH on the Rheological Properties of Reduced-fat Model Processed Cheese Spreads. LWT-Food Sci. Technol. 2001, 34, 288–292. [Google Scholar] [CrossRef]
- Salek, R.N.; Černíková, M.; Lorencová, E.; Pachlová, V.; Kůrová, V.; Šenkýřová, J.; Buňka, F. The impact of Cheddar or white brined cheese with various maturity degrees on the processed cheese consistency: A comparative study. Int. Dairy J. 2020, 111, 104816. [Google Scholar] [CrossRef]
- Prakash, S.; Tan, D.D.Y.; Chen, J. Applications of Tribology in Studying Food Oral Processing and Texture Perception. Food Res. Int. 2013, 54, 1627–1635. [Google Scholar] [CrossRef]
- Kapoor, R.; Metzger, L.E. Process cheese: Scientific and technological. aspects. A review. Compr. Rev. Food Sci. Food Saf. 2008, 7, 194–214. [Google Scholar] [CrossRef]
- Monteiro, R.R.; Tavares, D.Q.; Kindstedt, P.S.; Gigante, M.L. Effect of pH on microstructure and characteristics of cream cheese. J. Food Sci. 2009, 74, C112–C117. [Google Scholar] [CrossRef]
- Kim, J.; Watkinson, P.; Matia-Merino, L.; Smith, J.R.; Golding, M. Evaluation of formulation design on the physical and structural properties of commercial cream cheeses. Int. J. Food Sci. Technol. 2022, 57, 6422–6434. [Google Scholar] [CrossRef]
- Gliguem, H.; Ghorbel, D.; Grabielle-Madelmont, C.; Goldschmidt, B.; Lesieur, S.; Attia, H.; Ollivon, M.; Lesieur, P. Water behavior in processed cheese spreads. J. Therm. Anal. Calorim. 2009, 98, 73–82. [Google Scholar] [CrossRef]
- Marchesseau, S.; Cuq, J.L. Water-holding capacity and characterization of protein interactions in processed cheese. J. Dairy Res. 1995, 62, 479–489. [Google Scholar] [CrossRef]
- ISO Standard, No. 5534; Cheese and Processed Cheese. Determination of the Total Solid Content (Reference Method). ISO: Geneva, Switzerland, 2004.
- Ruiz-Ramírez, J.; Arnau, J.; Serra, X.; Gou, P. Effect of Ph(24), NaCl content and proteolysis index on the relationship between water content and texture parameters in biceps femoris and semimembranosus muscles in dry-cured ham. Meat Sci. 2006, 72, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Habig, K.H.; Woydt, M. Tribologie. In Dubbel Taschenbuch für den Maschinenbau 1: Grundlagen und Tabellen; Bender, B., Göhlich, D., Eds.; Springer Vieweg: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Glumac, M.; Bosc, V.; Menut, P.; Ramaioli, M.; Restagno, F.; Mariot, S.; Mathieu, V. Signal analysis to study the impact of tongue roughness on oral friction mechanisms with a custom-built tribometer. Biotribology 2023, 35–36, 100257. [Google Scholar] [CrossRef]
- Šantová, K.; Salek, R.N.; Kůrová, V.; Mizera, A.; Lapčíková, B.; Vincová, A.; Zálešáková, L.; Kratochvílová, A.; Lorencová, E.; Vinter, Š.; et al. Potassium-based emulsifying salts in processed cheese: A rheological, textural, tribological, and thermal approach. J. Dairy Sci. 2024, 107, 7704–7717. [Google Scholar] [CrossRef]
- Tylewicz, U.; Aganovic, K.; Vannini, M.; Toeofl, S.; Bortolotti, V.; Rosa, M.D.; Oey, I.; Heinz, V. Effect of pulsed electric field treatment on water distribution of freeze-dried apple tissue evaluated with DSC and TD-NMR techniques. Innov. Food Sci. Emerg. Technol. 2016, 37, 352–358, ISSN 14668564. [Google Scholar] [CrossRef]
- Du, X.; Li, Y.; Xia, Y.T.; Ai, S.M.; Liang, J.; Sang, P.; Ji, X.L.; Liu, S.Q. Insights into Protein-Ligand Interactions: Mechanisms, Models, and Methods. Int. J. Mol. Sci. 2016, 17, 144. [Google Scholar] [CrossRef]
- Yudianti, R.; Karina, M.; Sakamoto, M.; Azuma, J.-I. DSC analysis on water state of salvia hydrogels. Macromol. Res. 2009, 17, 1015–1020, ISSN 1598-5032. [Google Scholar] [CrossRef]
- Wang, H.H.; Sun, D.W. Assessment of Cheese Browning Affected by Baking Conditions Using Computer Vision. J. Food Eng. 2003, 56, 339–345. [Google Scholar] [CrossRef]
- ISO Standard No. 8586; Sensory Analysis: General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. ISO: Geneva, Switzerland, 2012.
- ISO Standard No. 8589; Sensory Analysis: General Guidance for the Design of Test Rooms. ISO: Geneva, Switzerland, 2007.
- ISO Standard No. 5479; Statistical Interpretation of Data—Tests for Departure from the Normal Distribution for the Small Amount of the Data. ISO: Geneva, Switzerland, 1997.
- Guinee, T.P.; Carić, M.; Kaláb, M. Pasteurized processed cheese and substitute/imitation cheese products. In Cheese: Chemistry, Physics and Microbiology, 3rd ed.; Fox, P.H., Ed.; Elsevier: London, UK, 2004; Volume 2 Major Cheese Groups; pp. 349–394. [Google Scholar] [CrossRef]
- Weiserová, E.; Doudová, L.; Galiová, L.; Žák, L.; Michálek, J.; Janiš, R.; Buňka, F. The effect of combinations of sodium phosphates in binary mixtures on selected texture parameters of processed cheese spreads. Int. Dairy J. 2011, 21, 979–986. [Google Scholar] [CrossRef]
- Salek, R.N.; Černíková, M.; Nagyová, G.; Kuchař, D.; Bačová, H.; Minarčíková, L.; Buňka, F. The effect of composition of ternary mixtures containing phosphate and citrate emulsifying salts on selected textural properties of spreadable processed cheese. Int. Dairy J. 2015, 44, 37–43. [Google Scholar] [CrossRef]
- Glass, K.; Doyle, M.E. Safety of Processed Cheese. Ph.D. Thesis, FRI Briefings, Food Research Institute, University of Wisconsin, Madison, WI, USA, 2005. [Google Scholar]
- Winter, H.H.; Chambon, F. Analysis of linear viscoelasticity of a crosslinking polymer at the gel point. J. Rheol. 1986, 30, 367–382. [Google Scholar] [CrossRef]
- Lee, S.K.; Buwalda, R.J.; Euston, S.R.; Foegeding, E.A.; McKenna, A.B. Changes in the rheology and microstructure of process cheese during cooking. LWT-Food Sci. Technol. 2003, 36, 339–345. [Google Scholar] [CrossRef]
- Sołowiej, B.G.; Nastaj, M.; Szafrańska, J.O.; Muszyński, S.; Gustaw, W.; Tomczyńska-Mleko, M.; Mleko, S. Effect of emulsifying salts replacement with polymerised whey protein isolate on textural, rheological and melting properties of acid casein model processed cheeses. Int. Dairy J. 2020, 105, 104694. [Google Scholar] [CrossRef]
- Dimitreli, G.; Thomareis, A.S. Texture evaluation of block-type processed cheese as a function of chemical composition and in relation to its apparent viscosity. J. Food Eng. 2007, 79, 1364–1373. [Google Scholar] [CrossRef]
- Nagyová, G.; Buňka, F.; Salek, R.N.; Černíková, M.; Mančík, P.; Grůber, T.; Kuchař, D. Use of sodium polyphosphates with different linear lengths in the production of spreadable processed cheese. J. Dairy Sci. 2014, 97, 111–122. [Google Scholar] [CrossRef]
- Ningtyas, D.W.; Bhandari, B.; Bansal, N.; Prakash, S. A tribological analysis of cream cheeses manufactured with different fat content. Int. Dairy J. 2017, 73, 155–165. [Google Scholar] [CrossRef]
- Brune, W.H. First Law of Thermodynamics; E-Education Institute, College of Earth and Mineral Sciences: State College, PA, USA, 2020. [Google Scholar]
- Anonymus. Thermal Analysis to Determine Various Forms of Water Present in Hydrogels; TA Instruments: New Castle, DE, USA, 2022. [Google Scholar]
- Clausse, D. Differential thermal analysis, differential scanning calorimetry, and emulsions. J. Therm. Anal. Calorim. 2010, 101, 1071–1077, ISSN 1388-6150. [Google Scholar] [CrossRef]
- Van der Sman, R.G.M.; BOER, E. Predicting the initial freezing point and water activity of meat products from composition data. J. Food Eng. 2005, 66, 469–475, ISSN 02608774. [Google Scholar] [CrossRef]
- Wolfe, J.; Bryant, G.; Koster, K.L. What is ‘unfreezable water’, how unfreezable is it and how much is there? CryoLetters 2002, 23, 157–166. [Google Scholar]
- Kristensen, D.; Hansen, E.; Arndal, A.; Trinderup, R.A.; Skibsted, L.H. Influence of light and temperature on the color and oxidative stability of processed cheese. Int. Dairy J. 2001, 11, 837–843. [Google Scholar] [CrossRef]
- Milovanovic, B.; Djekic, I.; Miocinovic, J.; Djordjevic, V.; Lorenzo, J.M.; Barba, F.J.; Mörlein, D.; Tomasevic, I. What Is the Color of Milk and Dairy Products and How Is It Measured? Foods 2020, 9, 1629. [Google Scholar] [CrossRef] [PubMed]
- Osthoff, G.; Slabber, E.; Kneifel, W.; Dürrschmid, K. Flavours and flavourants, colours and pigment. In Processed Cheese and Analogues; Tamime, A.Y., Ed.; Wiley-Blackwell: West Sussex, UK, 2011. [Google Scholar] [CrossRef]
- León, K.; Mery, D.; Pedreschi, F.; León, J. Color measurement in L* a* b* units from RGB digital images. Food Res. Int. 2006, 39, 1084–1091. [Google Scholar] [CrossRef]
- Kůrová, V.; Salek, R.N.; Vašina, M.; Vinklárková, K.; Zálešáková, L.; Gál, R.; Adámek, R.; Buňka, F. The effect of homogenization and addition of polysaccharides on the viscoelastic properties of processed cheese sauce. J. Dairy Sci. 2022, 105, 6563–6577. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Anema, S.; Klostermeyer, H. The influence of moisture content on the rheological properties of processed cheese spreads. Int. J. Food Sci. Technol. 2004, 39, 763–771. [Google Scholar] [CrossRef]
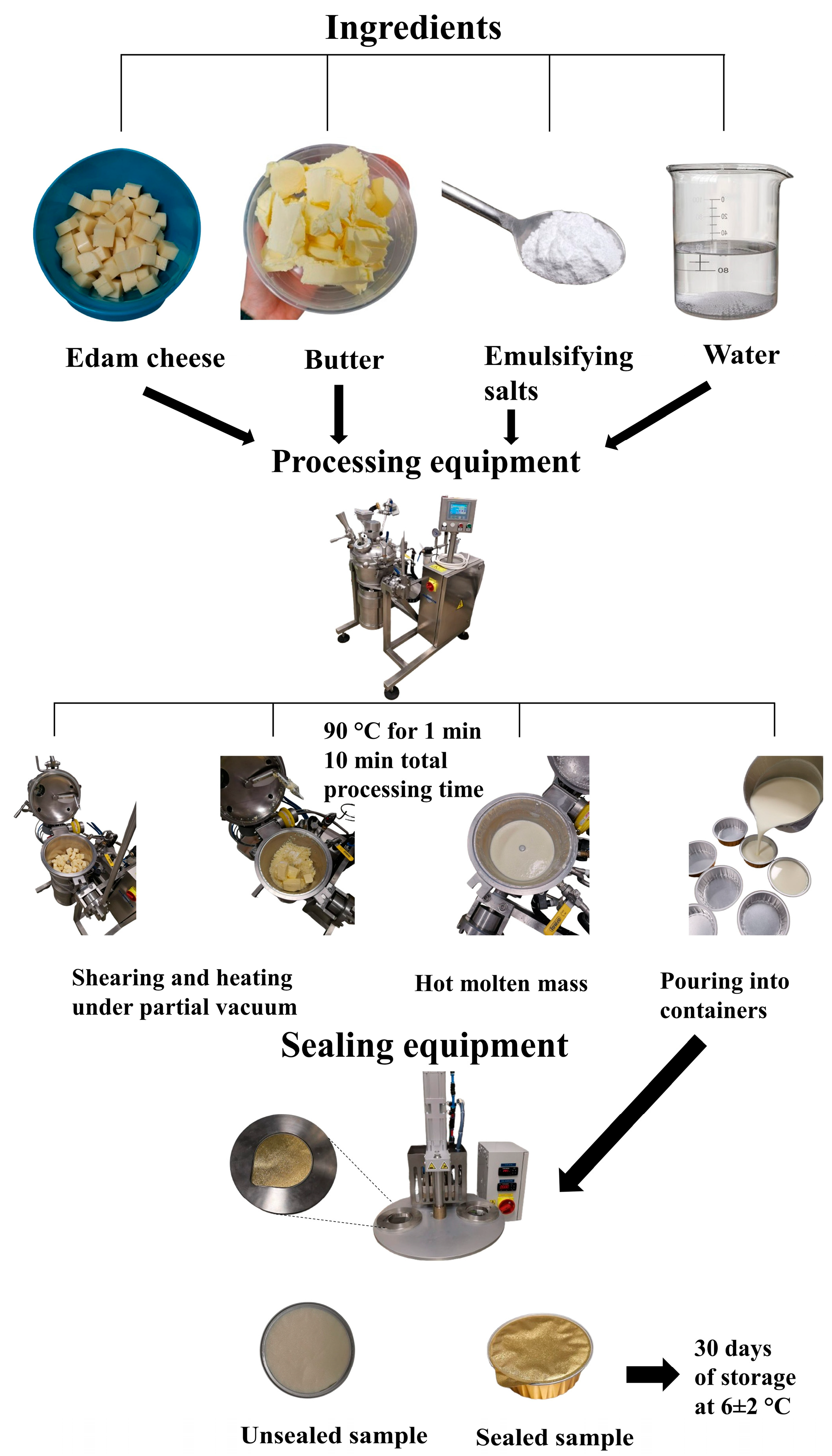
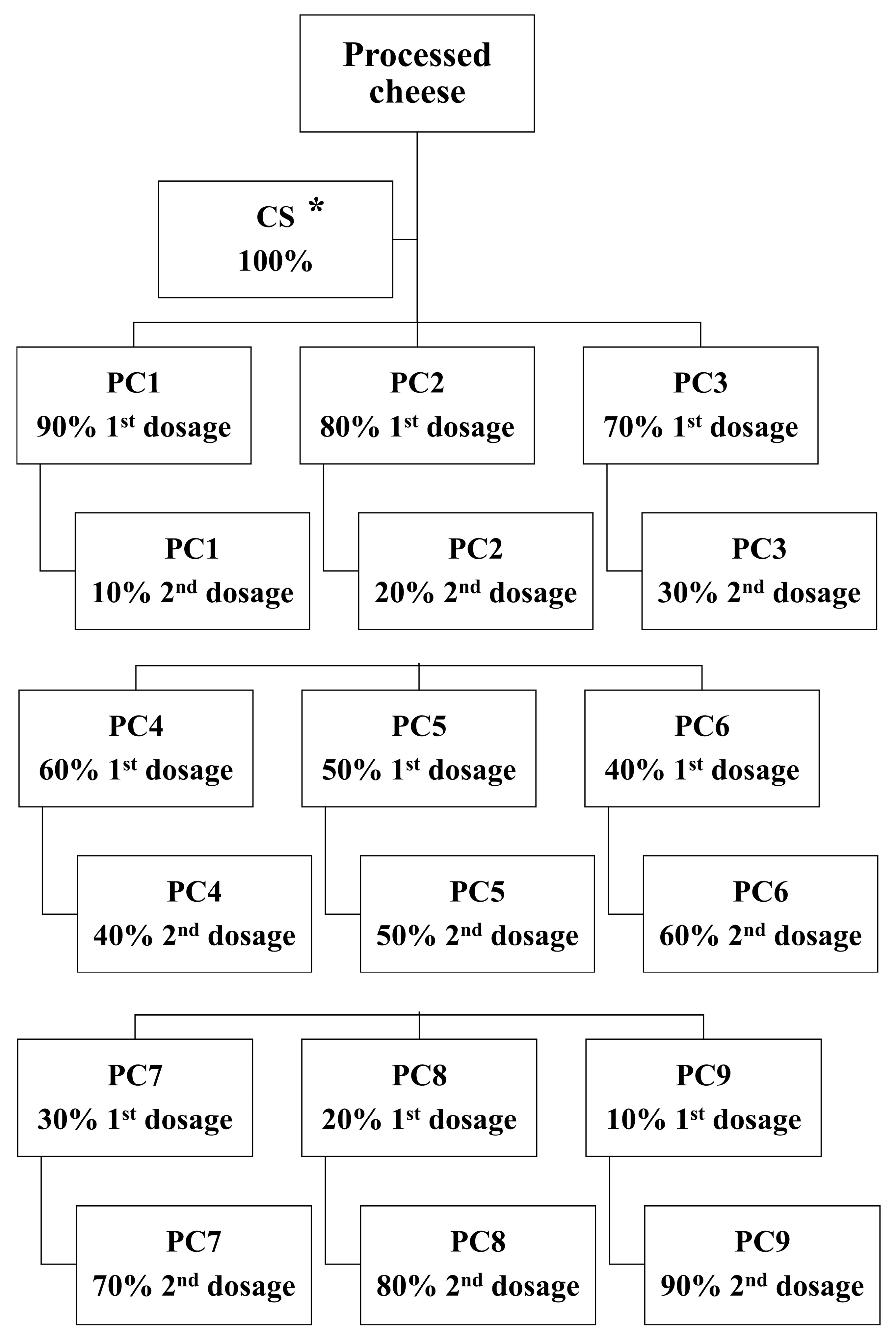

| Raw Materials | Ingredient Composition (% w/w) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CS * | PC 1 | PC 2 | PC 3 | PC 4 | PC 5 | PC 6 | PC 7 | PC 8 | PC 9 | |
| Edam cheese 1 | 50.60 | 50.60 | 50.60 | 50.60 | 50.60 | 50.60 | 50.60 | 50.60 | 50.60 | 50.60 |
| Butter | 13.70 | 13.70 | 13.70 | 13.70 | 13.70 | 13.70 | 13.70 | 13.70 | 13.70 | 13.70 |
| Water addition | 33.40 | |||||||||
| First stage | 30.06 | 26.72 | 23.38 | 20.04 | 16.70 | 13.36 | 10.02 | 6.68 | 3.34 | |
| Second stage | 3.34 | 6.68 | 10.02 | 13.36 | 16.70 | 20.04 | 23.38 | 26.72 | 30.06 | |
| Amount of emulsifying salt | 2.30 | 2.30 | 2.30 | 2.30 | 2.30 | 2.30 | 2.30 | 2.30 | 2.30 | 2.30 |
| Na2HPO4 | 39.00 | 39.00 | 39.00 | 39.00 | 39.00 | 39.00 | 39.00 | 39.00 | 39.00 | 39.00 |
| NaH2PO4 | 18.00 | 18.00 | 18.00 | 18.00 | 18.00 | 18.00 | 18.00 | 18.00 | 18.00 | 18.00 |
| Na4P2O7 | 21.00 | 21.00 | 21.00 | 21.00 | 21.00 | 21.00 | 21.00 | 21.00 | 21.00 | 21.00 |
| P20 | 22.00 | 22.00 | 22.00 | 22.00 | 22.00 | 22.00 | 22.00 | 22.00 | 22.00 | 22.00 |
| Physical Properties | Component A | Component B | Mixture |
|---|---|---|---|
| Composition | UR 58300 POLYOL | UR 5803 ISO | |
| Mixing ratio (by weight) | 100 | 10 | |
| State | Liquid | Liquid | Liquid |
| Color | Beige | Amber | Beige |
| Viscosity (mPa.s) | 6900 | 2000 | 4000 |
| Density (kg/L) | 1.37 | 1.16 | 1.35 |
| Processing time (25 °C) | 15–20 min |
| Experiment | Panelist | n | Gender (%) | Age (Years) | |||
|---|---|---|---|---|---|---|---|
| Female | Male | Total | Female | Male | |||
| PC samples | Experienced | 4 | 50 | 50 | 44.00 ± 4.24 | 43.50 ± 0.71 | 44.50 ± 7.78 |
| Trained | 8 | 75 | 25 | 32.67 ± 6.31 | 28.33 ± 4.13 | 37.00 ± 8.49 | |
| Parameter | Processed Cheese | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CS * | PC 1 | PC 2 | PC 3 | PC 4 | PC 5 | PC 6 | PC 7 | PC 8 | PC 9 | |
| DM content (% w/w) | 46.22 ± 1.30 a | 44.82 ± 0.84 a | 45.22 ± 1.01 a | 45.59 ± 1.48 a | 45.54 ± 0.98 a | 46.22 ± 0.84 a | 45.88 ± 1.00 a | 46.03 ± 0.60 a | 46.58 ± 0.68 a | 45.49 ± 1.03 a |
| Fat content (% w/w) | 19.98 | 19.78 | 19.88 | 19.75 | 19.81 | 19.92 | 19.91 | 19.88 | 19.89 | 19.95 |
| pH (-) | 5.51 ± 0.01 a | 5.51 ± 0.04 a | 5.48 ± 0.01 a | 5.46 ± 0.02 a | 5.52 ± 0.03 a | 5.52 ± 0.05 a | 5.45 ± 0.02 a | 5.56 ± 0.05 a | 5.47 ± 0.01 a | 5.59 ± 0.03 a |
| Water activity (aw) (-) | 0.975 ± 0.01 a | 0.975 ± 0.01 a | 0.977 ± 0.01 a | 0.977 ± 0.01 a | 0.976 ± 0.01 a | 0.977 ± 0.01 a | 0.978 ± 0.01 a | 0.978 ± 0.01 a | 0.978 ± 0.01 a | 0.978 ± 0.02 a |
| Lightness (L*) | 90.83 ± 0.34 a | 91.39 ± 0.17 b | 91.60 ± 0.20 b | 91.69 ± 0.02 b | 91.65 ± 0.22 b | 91.49 ± 0.28 b | 91.50 ± 0.10 b | 91.66 ± 0.11 b | 91.77 ± 0.12 b | 91.73 ± 0.19 b |
| Chromaticity on a green-to-red axis (a*) | −0.44 ± 0.01 a | −0.48 ± 0.03 b | −0.52 ± 0.07 c | −0.45 ± 0.02 a | −0.47 ± 0.03 b | −0.40 ± 0.15 d | −0.49 ± 0.02 b | −0.45 ± 0.03 a | −0.39 ± 0.06 a | −0.45 ± 0.01 b |
| Chromaticity on a blue-to-yellow axis (b*) | 15.50 ± 0.64 a | 13.63 ± 0.21 b | 12.78 ± 0.04 b | 12.92 ± 0.13 b | 12.73 ± 0.28 b | 12.69 ± 0.17 b | 13.20 ± 0.52 a | 12.53 ± 0.21 b | 13.04 ± 0.17 a | 13.14 ± 0.04 a |
| Hue angle (h*; °) | −1.54 ± 0.02 a | −1.54 ± 0.03 a | −1.53 ± 0.01 a | −1.54 ± 0.01 a | −1.53 ± 0.02 a | −1.54 ± 0.01 a | −1.54 ± 0.01 a | −1.54 ± 0.01 a | −1.54 ± 0.01 a | −1.54 ± 0.01 a |
| Chroma (C*) | 15.50 ± 0.64 a | 13.64 ± 0.21 b | 12.79 ± 0.04 c | 12.93 ± 0.13 c | 12.74 ± 0.28 c | 12.70 ± 0.17 c | 13.21 ± 0.52 b | 12.54 ± 0.21 c | 13.05 ± 0.17 b | 13.15 ± 0.04 b |
| Total color difference (∆E12) | - | 1.94 ± 0.47 a | 2.82 ± 0.63 b | 2.72 ± 0.61 b | 2.88 ± 0.39 b | 2.88 ± 0.50 b | 2.39 ± 0.28 b | 3.08 ± 0.50 c | 2.63 ± 0.53 b | 2.52 ± 0.62 b |
| Whiteness index (WI) | 81.99 ± 0.34 a | 83.87 ± 0.17 b | 84.70 ± 0.19 c | 84.63 ± 0.02 c | 84.76 ± 0.22 c | 84.71 ± 0.28 c | 84.29 ± 0.09 c | 84.94 ± 0.11 c | 84.57 ± 0.12 c | 84.47 ± 0.19 c |
| Complex modulus G* (Pa) | 25,381.1 a | 14,606.0 b | 21,012.3 c | 22,025.3 c | 21,273.9 c | 21,675.6 c | 18,277.8 d | 30,171.6 e | 20,013.0 c | 30,181.7 e |
| Loss tangent tan δ (-) | 0.426 a | 0.511 b | 0.451 c | 0.445 c | 0.434 c | 0.439 c | 0.462 c | 0.409 d | 0.473 e | 0.399 f |
| Hardness | 10.78 ± 0.07 a | 8.49 ± 0.24 b | 10.02 ± 0.13 c | 11.90 ± 0.15 d | 11.33 ± 0.27 d | 12.10 ± 0.28 e | 14.19 ± 0.15 f | 18.42 ± 0.02 h | 11.67 ± 0.32 d | 13.41 ± 0.07 g |
| Elasticity | 10.82 ± 0.07 a | 11.05 ± 0.15 b | 10.95 ± 0.03 a | 10.86 ± 0.01 a | 10.82 ± 0.01 a | 10.88 ± 0.13 a | 10.81 ± 0.01 a | 10.80 ± 0.04 a | 10.85 ± 0.08 a | 10.82 ± 0.03 a |
| Cohesiveness | 0.58 ± 0.01 a | 0.61 ± 0.01 b | 0.53 ± 0.01 c | 0.59 ± 0.02 a | 0.49 ± 0.06 d | 0.55 ± 0.02 c | 0.61 ± 0.04 b | 0.61 ± 0.04 b | 0.59 ± 0.05 a | 0.59 ± 0.05 a |
| Relative adhesiveness | 0.10 ± 0.04 a | 0.10 ± 0.02 a | 0.10 ± 0.06 a | 0.12 ± 0.48 a | 0.10 ± 0.04 a | 0.10 ± 0.04 a | 0.08 ± 0.02 a | 0.12 ± 0.08 a | 0.08 ± 0.02 a | 0.03 ± 0.01 a |
| Sample * | Cooling Cycle | Heating Cycle | ||||
|---|---|---|---|---|---|---|
| Tp,cryst (°C) | ∆Hcryst (J·g−1) | Tonset (°C) | Tp,m (°C) | ∆Hfus (J·g−1) | Wf,s (%) | |
| CS | −19.36 ± 1.24 a | 71.64 ± 2.00 a | −9.05 ± 1.47 a | −3.26 ± 0.91 a | 72.65 ± 1.57 a | 21.78 ± 0.47 a |
| PC 1 | −15.82 ± 2.62 b | 105.66 ± 1.68 a | −7.19 ± 0.47 b | −2.55 ± 0.46 b | 109.50 ± 0.89 b | 32.83 ± 0.27 b |
| PC 2 | −21.17 ± 2.09 c | 54.328 ± 1.61 b | −10.71 ± 0.09 a | −4.60 ± 0.77 c | 56.02 ± 0.22 a | 16.80 ± 0.07 c |
| PC 3 | −17.80 ± 1.32 d | 72.45 ± 0.86 c | −8.56 ± 0.25 a | −2.94 ± 0.13 b | 74.20 ± 0.63 a | 22.25 ± 0.19 a |
| PC 4 | −16.24 ± 0.37 e | 98.81 ± 0.90 d | −6.80 ± 0.47 b | −2.23 ± 0.23 b | 104.55 ± 1.63 b | 31.35 ± 0.49 b |
| PC 5 | −16.01 ± 2.83 e | 100.40 ± 1.41 a | −6.87 ± 0.74 b | −2.40 ± 0.28 b | 100.02 ± 1.70 b | 29.99 ± 0.51 d |
| PC 6 | −13.61 ± 0.66 f | 117.65 ± 0.35 a | −6.32 ± 0.02 b | −1.95 ± 0.02 b | 122.03 ± 1.15 b | 36.59 ± 0.35 e |
| PC 7 | −14.48 ± 1.48 h | 104.028 ± 1.63 a | −7.06 ± 0.95 b | −2.28 ± 0.52 b | 106.21 ± 1.10 b | 31.85 ± 0.33 b |
| PC 8 | −15.55 ± 0.69 b | 75.918 ± 1.83 a | −7.70 ± 1.03 b | −3.14 ± 0.91 a | 77.92 ± 1.36 a | 23.36 ± 0.41 a |
| PC 9 | −14.69 ± 0.18 i | 107.95 ± 0.99 a | −6.68 ± 0.33 b | −1.93 ± 0.64 b | 117.19 ± 0.64 b | 35.14 ± 0.19 e |
| Sample * | Appearance | Consistency | Hardness | Spreadability | Flavor | Off-Flavor |
|---|---|---|---|---|---|---|
| CS | 1 | 4 a | 4 a | 4 a | 1 a | 1 a |
| PC 1 | 1 | 3 b | 2 b | 3 b | 1 a | 1 a |
| PC 2 | 1 | 3 b | 3 c | 3 b | 1 a | 1 a |
| PC 3 | 1 | 3 b | 4 a | 4 a | 1 a | 1 a |
| PC 4 | 1 | 3 b | 4 a | 4 a | 1 a | 1 a |
| PC 5 | 1 | 2 c | 3 c | 3 b | 1 a | 1 a |
| PC 6 | 1 | 4 a | 4 a | 4 a | 1 a | 1 a |
| PC 7 | 1 | 6 d | 6 d | 6 c | 1 a | 1 a |
| PC 8 | 1 | 4 d | 4 a | 4 a | 1 a | 1 a |
| PC 9 | 1 | 3 b | 6 d | 5 d | 1 a | 1 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vincová, A.; Polášková, M.; Stěnička, M.; Šantová, K.; Kůrová, V.; Lapčíková, B.; Gryger, T.; Lorencová, E.; Lazárková, Z.; Pluta-Kubica, A.; et al. Effect of Two-Stage Water Addition on Consistency of Processed Cheese: Physicochemical, Mechanical, Thermal, and Organoleptic Approach. Foods 2025, 14, 1361. https://doi.org/10.3390/foods14081361
Vincová A, Polášková M, Stěnička M, Šantová K, Kůrová V, Lapčíková B, Gryger T, Lorencová E, Lazárková Z, Pluta-Kubica A, et al. Effect of Two-Stage Water Addition on Consistency of Processed Cheese: Physicochemical, Mechanical, Thermal, and Organoleptic Approach. Foods. 2025; 14(8):1361. https://doi.org/10.3390/foods14081361
Chicago/Turabian StyleVincová, Anna, Martina Polášková, Martin Stěnička, Kristýna Šantová, Vendula Kůrová, Barbora Lapčíková, Tomáš Gryger, Eva Lorencová, Zuzana Lazárková, Agnieszka Pluta-Kubica, and et al. 2025. "Effect of Two-Stage Water Addition on Consistency of Processed Cheese: Physicochemical, Mechanical, Thermal, and Organoleptic Approach" Foods 14, no. 8: 1361. https://doi.org/10.3390/foods14081361
APA StyleVincová, A., Polášková, M., Stěnička, M., Šantová, K., Kůrová, V., Lapčíková, B., Gryger, T., Lorencová, E., Lazárková, Z., Pluta-Kubica, A., Pětová, M., Novosad, O., & Salek, R. N. (2025). Effect of Two-Stage Water Addition on Consistency of Processed Cheese: Physicochemical, Mechanical, Thermal, and Organoleptic Approach. Foods, 14(8), 1361. https://doi.org/10.3390/foods14081361









