Effects of Dairy Matrix on the Intestinal, Liver, and Bone Transcriptome of Healthy Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Rat Intervention Study
2.2. RNA Extraction and Transcriptome Analysis
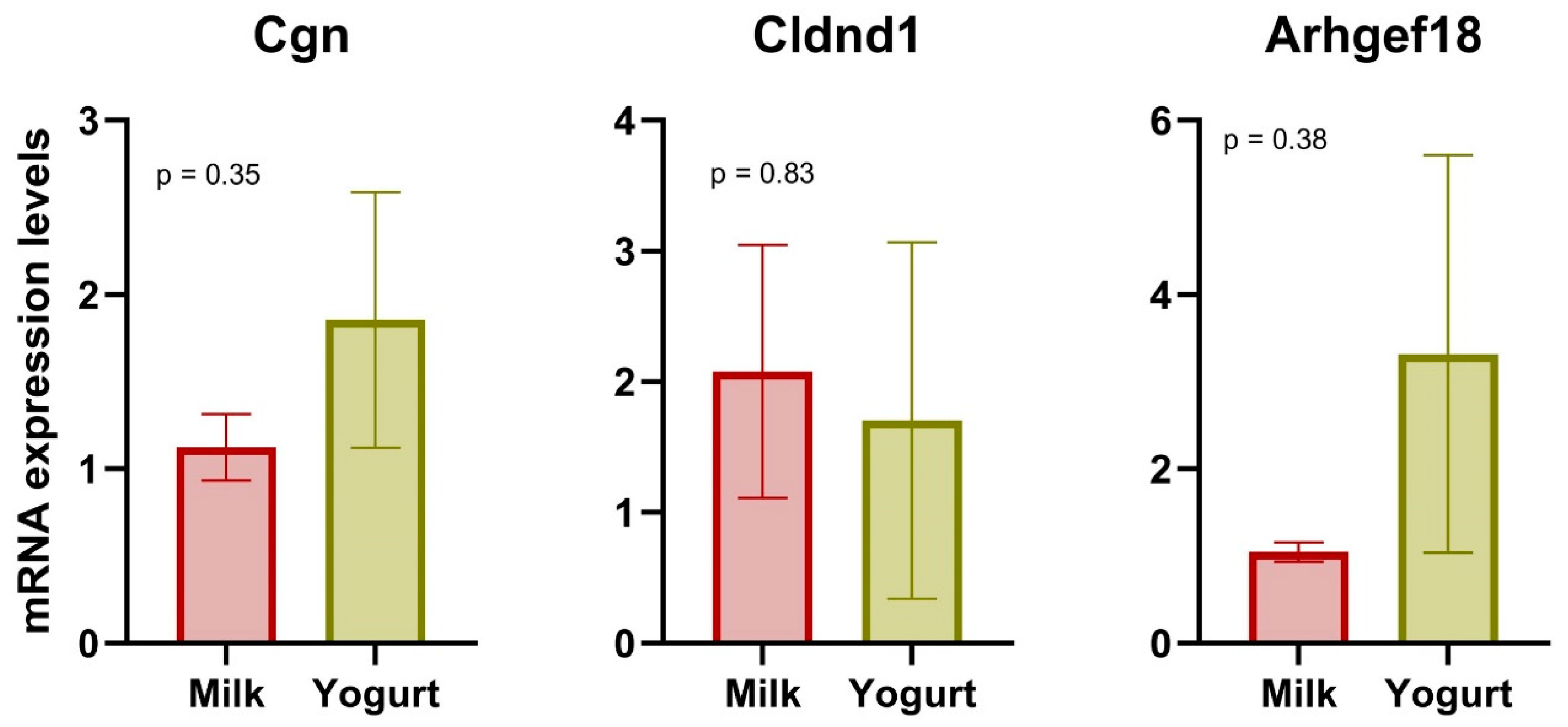
2.3. qPCR
2.4. Statistical Analysis
3. Results
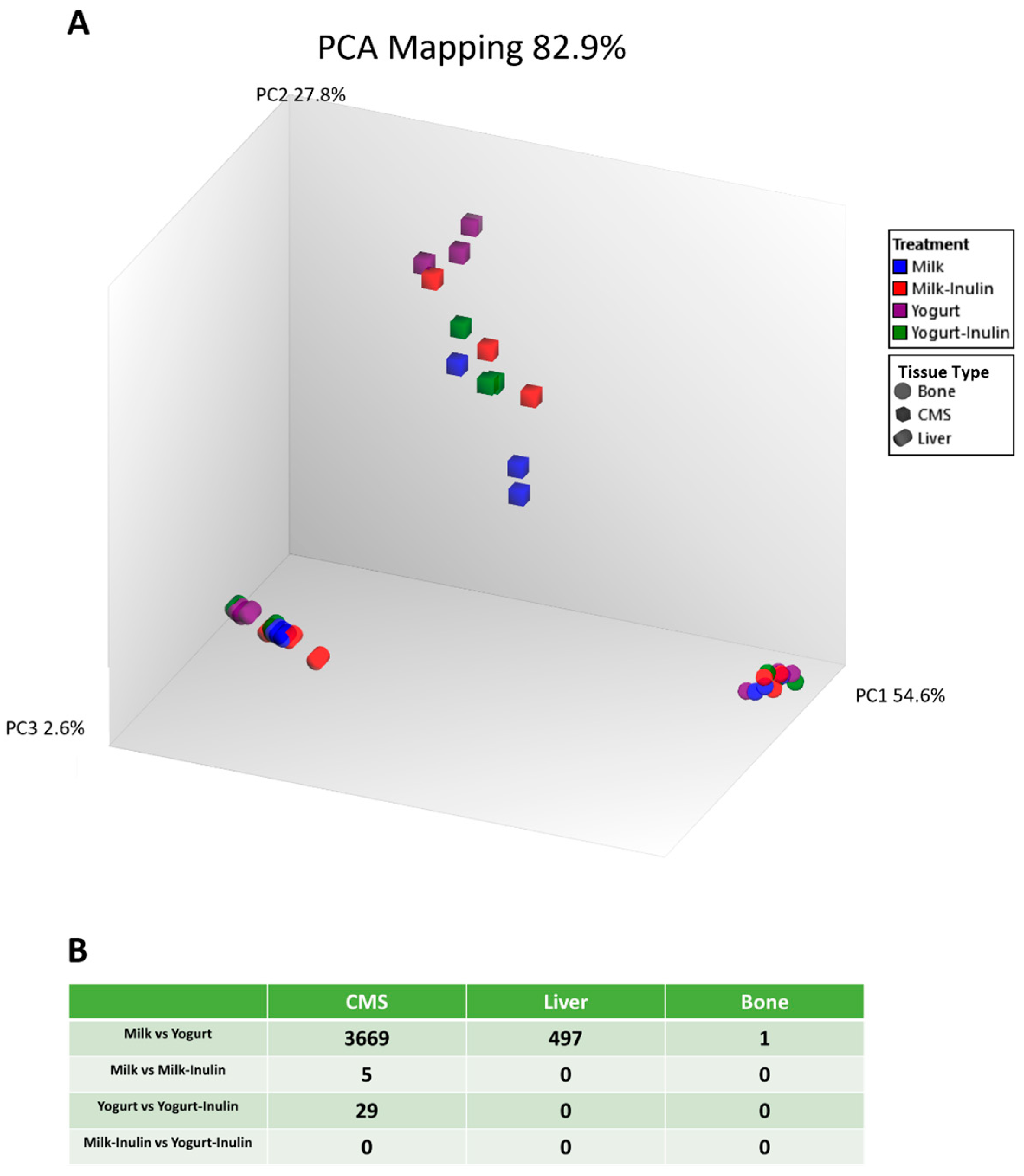
3.1. Distinct Transcriptome Features of Different Tissue Types
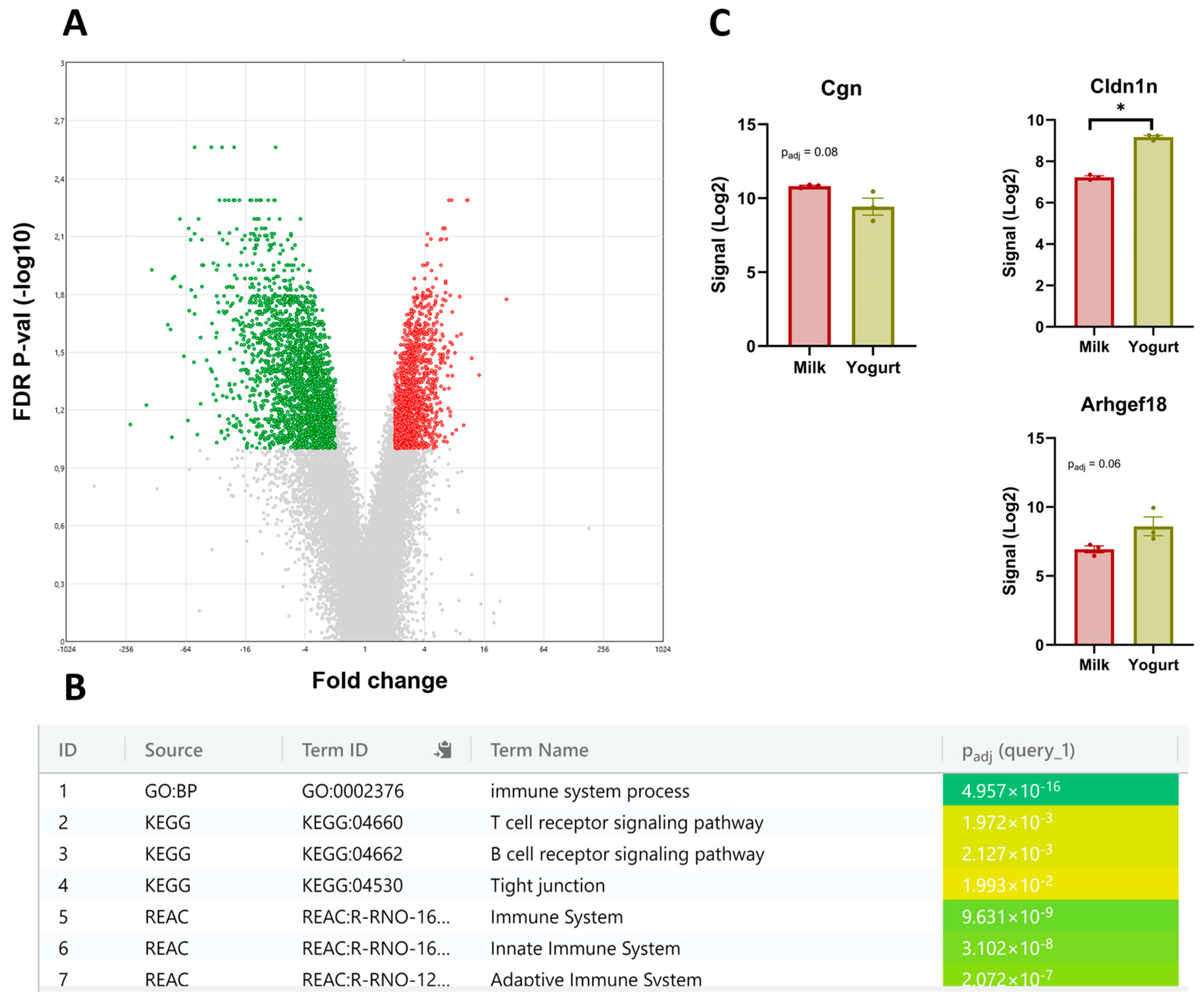
3.2. The CMS Transcriptome
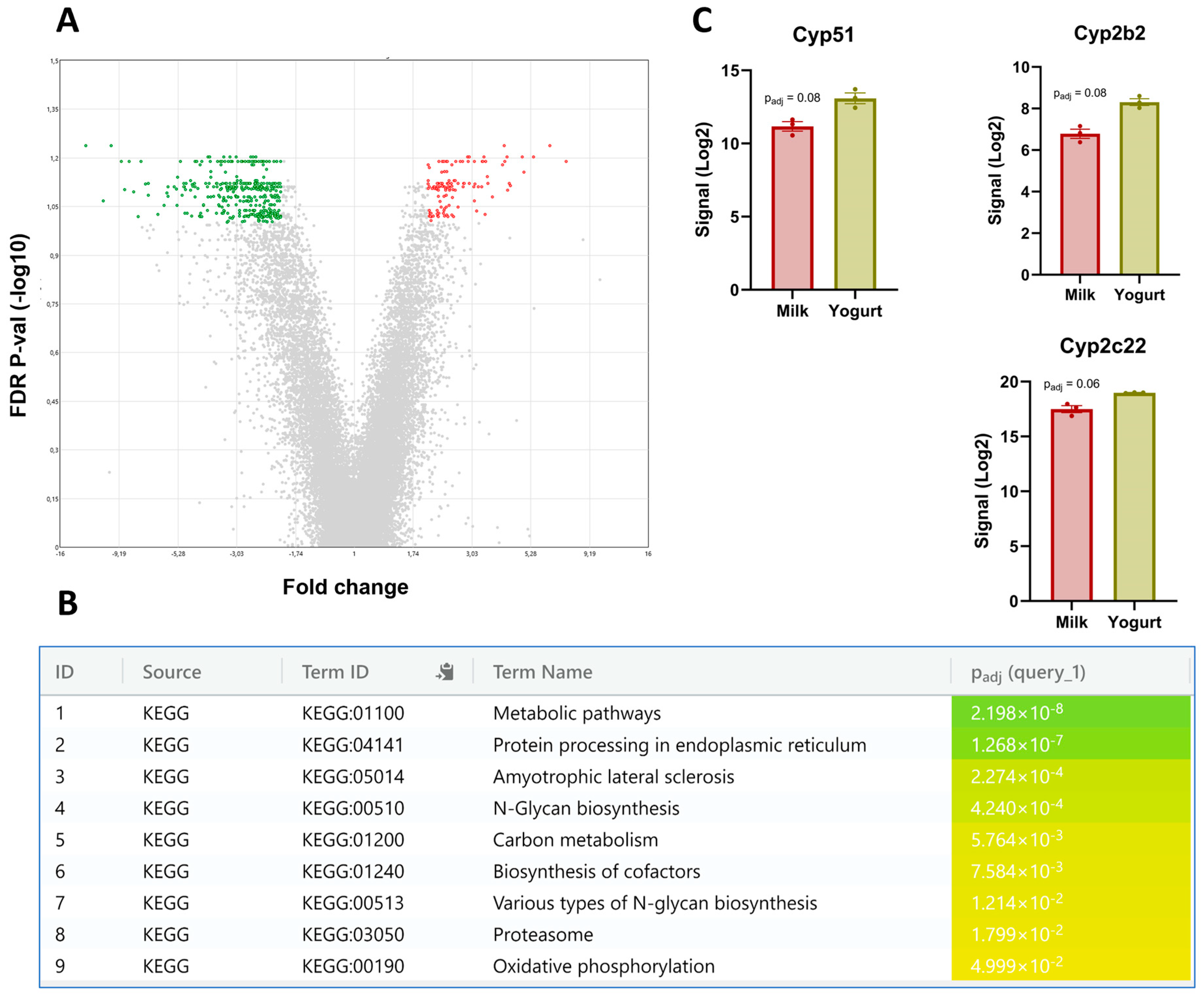
3.3. The Liver Transcriptome
3.4. The Bone Transcriptome
4. Discussion
4.1. Tissue Type
4.2. Gene Expression in CMS
4.3. Gene Expression in Liver
4.4. Gene Expression in Bone
4.5. Inulin Effects
4.6. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CMS | Colon mucosa scrubs |
| DEG | Differential expressed genes |
| GO | Gene ontology |
| KEGG | Kyoto encyclopedia of genes and genomes |
| PCA | Principal component analysis |
References
- Visioli, F.; Strata, A. Milk, Dairy Products, and Their Functional Effects in Humans: A Narrative Review of Recent Evidence. Adv. Nutr. 2014, 5, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Rettedal, E.A.; Altermann, E.; Roy, N.C.; Dalziel, J.E. The Effects of Unfermented and Fermented Cow and Sheep Milk on the Gut Microbiota. Front. Microbiol. 2019, 10, 458. [Google Scholar] [CrossRef] [PubMed]
- Asbjornsdottir, B.; Sigurdsson, S.; Miranda-Ribera, A.; Fiorentino, M.; Konno, T.; Lan, J.; Gudmundsson, L.S.; Gottfredsson, M.; Lauth, B.; Birgitsdottir, B.E.; et al. Evaluating Prophylactic Effect of Bovine Colostrum on Intestinal Barrier Function in Zonulin Transgenic Mice: A Transcriptomic Study. Int. J. Mol. Sci. 2023, 24, 14730. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Y.; Zhao, Q.; Chen, B.; Wang, N.; Zhang, T.; Jiang, Y.; Wu, Y.; He, N.; Zhao, G.; et al. Dairy products intake and prevalence, incidence, and recovery of non-alcoholic fatty liver disease in Chinese population. Hepatol. Int. 2024, 18, 529–539. [Google Scholar] [CrossRef]
- Dai, J.Y.; Yin, T.; Cao, L.Y. Dairy consumption and liver cancer risk: A meta-analysis of observational studies. Oncol. Lett. 2024, 27, 108. [Google Scholar] [CrossRef]
- Romulo, A.; Surya, R. Tempe: A traditional fermented food of Indonesia and its health benefits. Int. J. Gastron. Food Sci. 2021, 26, 100413. [Google Scholar] [CrossRef]
- Illikoud, N.; Mantel, M.; Rolli-Derkinderen, M.; Gagnaire, V.; Jan, G. Dairy starters and fermented dairy products modulate gut mucosal immunity. Immun. Lett. 2022, 251–252, 91–102. [Google Scholar]
- He, W.; Xie, Z.; Wittig, N.K.; Zachariassen, L.F.; Andersen, A.; Andersen, H.J.; Birkedal, H.; Nielsen, D.S.; Hansen, A.K.; Bertram, H. Yogurt Benefits Bone Mineralization in Ovariectomized Rats with Concomitant Modulation of the Gut Microbiome. Mol. Nutr. Food Res. 2022, 66, 2200174. [Google Scholar] [CrossRef]
- Kruse, H.P.; Kleessen, B.; Blaut, M. Effects of inulin on faecal bifidobacteria in human subjects. Br. J. Nutr. 1999, 82, 375–382. [Google Scholar] [CrossRef]
- Cardarelli, H.R.; Saad, S.M.I.; Gibson, G.R.; Vulevic, J. Functional petit-suisse cheese: Measure of the prebiotic effect. Anaerobe 2007, 13, 200–207. [Google Scholar] [CrossRef]
- Dong, Z.C.; Chen, Y. Transcriptomics: Advances and approaches. Sci. China-Life Sci. 2013, 56, 960–967. [Google Scholar] [CrossRef]
- Roberts, M.D.; Mobley, C.B.; Toedebush, R.G.; Heese, A.J.; Zhu, C.; Krieger, A.E.; Cruthirds, C.L.; Lockwood, C.M.; Hofheins, J.C.; Wiedmeyer, C.E.; et al. Western diet-induced hepatic steatosis and alterations in the liver transcriptome in adult Brown-Norway rats. BMC Gastroenterol. 2015, 15, 151. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Yang, Y.; Luo, Z.; Guan, L.; Zhu, W. The Colonic Microbiome and Epithelial Transcriptome Are Altered in Rats Fed a High-Protein Diet Compared with a Normal-Protein Diet. J. Nutr. 2016, 146, 474–483. [Google Scholar] [CrossRef]
- Zhao, X.R.; He, W.; Jakobsen, L.M.A.; Zachariassen, L.F.; Hansen, A.K.; Rasmusen, M.K.; Bertram, H.C. Inulin Supplementation Modulates the Hepatic Transcriptome, Metabolome, and Ferritin Content in Ovariectomized Rats. Mol. Nutr. Food Res. 2023, 67, 2300372. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, S.; Asha; Sharma, K.K. Gut-organ axis: A microbial outreach and networking. Lett. Appl. Microbiol. 2021, 72, 636–668. [Google Scholar] [CrossRef]
- Zhao, X.R.; He, W.; Jakobsen, L.M.A.; Panah, F.; Correia, B.; Nielsen, D.S.; Hansen, A.K.; Bertram, H.C. Influence of dairy matrix on the prebiotic effects of inulin related to gut metabolic activity and bone health. Food Func. 2024, 15, 11129. [Google Scholar] [CrossRef] [PubMed]
- Nance, R.; Agarwal, P.; Sandey, M.; Starenki, D.; Koehler, J.; Sajib, A.M.; Smith, B.F. A method for isolating RNA from canine bone. Biotechniques 2020, 68, 311–317. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Thøgersen, R.; Lindholm, P.H.; Bertram, H.C.; Pilegaard, H. Hepatic PGC-1α has minor regulatory effect on the liver transcriptome and metabolome during high fat high fructose diet and exercise training. Gene 2023, 851, 147030. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Zamaratskaia, G.; Ekstrand, E. Gender-related Differences in Cytochrome P450 in Porcine Liver—Implication for Activity, Expression and Inhibition by Testicular Steroids. Reprod. Domest. Anim. 2011, 46, 616–623. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Bertholdt, L.; Gudiksen, A.; Pilegaard, H.; Knudsen, J.G. Impact of fasting followed by short-term exposure to interleukin-6 on cytochrome P450 mRNA in mice. Toxicol. Lett. 2018, 282, 93–99. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Bolling, B.W. Yogurt consumption for improving immune health. Curr. Opin. Food Sci. 2023, 51, 101017. [Google Scholar] [CrossRef]
- El-Abbadi, N.H.; Dao, M.C.; Meydani, S.N. Yogurt: Role in healthy and active aging. Am. J. Clin. Nutr. 2014, 99, 1263S–1270S. [Google Scholar] [CrossRef]
- Maukonen, J.; Saarela, M. Human gut microbiota: Does diet matter? Proc. Nutr. Soc. 2015, 74, 23–36. [Google Scholar] [CrossRef]
- Matsuoka, H.; Tamura, A.; Kinehara, M.; Shima, A.; Uda, A.; Tahara, H.; Michihara, A. Levels of tight junction protein CLDND1 are regulated by microRNA-124 in the cerebellum of stroke-prone spontaneously hypertensive rats. Biochem. Biophys. Res. Comm. 2018, 498, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Beal, R.; Fernandz, A.A.-C.; Grammatopoulos, D.K.; Matter, K.; Balda, M.S. ARHGEF18/p114RhoGEF Coordinates PKA/CREB Signaling and Actomyosin Remodeling to Promote Trophoblast Cell-Cell Fusion During Placenta Morphogenesis. Front. Cell Dev. Biol. 2021, 9, 658006. [Google Scholar] [CrossRef] [PubMed]
- Van Itallie, C.M.; Anderson, J.M. Architecture of tight junctions and principles of molecular composition. Semin. Cell Dev. Biol. 2014, 36, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Pei, R.; Martin, D.A.; DiMarco, D.M.; Bolling, B.W. Evidence for the effects of yogurt on gut health and obesity. Crit. Rev. Food Sci. Nutr. 2017, 57, 1569–1583. [Google Scholar] [CrossRef]
- Bajic, S.S.; Djokic, J.; Dinic, M.; Veljovic, K.; Golic, N.; Mihajlovic, S.; Tolinacki, M. GABA-Producing Natural Dairy Isolate From Artisanal Zlatar Cheese Attenuates Gut Inflammation and Strengthens Gut Epithelial Barrier in vitro. Front. Microbiol. 2019, 10, 527. [Google Scholar]
- Popovic, N.; Brdaric, E.; Dokic, J.; Dinic, M.; Veljovic, K.; Golic, N.; Terzic-Vidojevic, A. Yogurt Produced by Novel Natural Starter Cultures Improves Gut Epithelial Barrier In Vitro. Microorganisms 2020, 8, 1586. [Google Scholar] [CrossRef]
- Popovic, N.; Dokic, J.; Brdaric, E.; Dinic, M.; Terzic-Vidojevic, A.; Veljovic, K. The Influence of Heat-Killed Enterococcus faecium BGPAS1-3 on the Tight Junction Protein Expression and Immune Function in Differentiated Caco-2 Cells Infected with Listeria monocytogenes ATCC 19111. Front. Microbiol. 2019, 10, 412. [Google Scholar] [CrossRef]
- Abdulqadir, R.; Engers, J.; Al-Sadi, R. Role of Bifidobacterium in Modulating the Intestinal Epithelial Tight Junction Barrier: Current Knowledge and Perspectives. Curr. Dev. Nutr. 2023, 7, 102026. [Google Scholar] [CrossRef]
- Holmberg, S.M.; Feeney, R.H.; Prasoodanan, P.K.V.; Puértolas-Balint, F.; Singh, D.K.; Wongkuna, S.; Zandbergen, L.; Hauner, H.; Brandl, B.; Nieminen, A.I.; et al. The gut commensal Blautia maintains colonic mucus function under low-fiber consumption through secretion of short-chain fatty acids. Nat. Commun. 2024, 15, 3502. [Google Scholar] [CrossRef] [PubMed]
- Nusrat, A.; von Eichel-Streiber, C.; Turner, J.R.; Verkade, P.; Madara, J.L.; Parkos, C.A. Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins. Infect. Immunity 2001, 69, 1329–1336. [Google Scholar] [CrossRef]
- Putt, K.K.; Pei, R.S.; White, H.M.; Bolling, B.W. Yogurt inhibits intestinal barrier dysfunction in Caco-2 cells by increasing tight junctions. Food Func. 2017, 8, 406–414. [Google Scholar] [CrossRef]
- Wen, L.; Duffy, A. Factors Influencing the Gut Microbiota, Inflammation, and Type 2 Diabetes. J. Nutr. 2017, 147, 1468S–1475S. [Google Scholar] [CrossRef] [PubMed]
- Usui, Y.; Kimura, Y.; Satoh, T.; Takemura, N.; Ouchi, Y.; Hiroko, O.; Kobayashi, K.; Suzuki, H.; Koyama, H.; Hagiwara, S.; et al. Effects of long-term intake of a yogurt fermented with Lactobacillus delbrueckii subsp bulgaricus 2038 and Streptococcus thermophilus 1131 on mice. Int. Immunol. 2018, 30, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.M.; Jeong, J.-J.; Woo, K.H.; Han, M.J.; Kim, D.-H. Lactobacillus sakei OK67 ameliorates high-fat diet-induced blood glucose intolerance and obesity in mice by inhibiting gut microbiota lipopolysaccharide production and inducing colon tight junction protein expression. Nutr. Res. 2016, 36, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, A.; Chanez-Paredes, S.D.; Haest, X.; Turner, J.R. Paracellular permeability and tight junction regulation in gut health and disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 417–432. [Google Scholar] [CrossRef]
- Companys, J.; Pla-Pagà, L.; Calderón-Pérez, L.; Llauradó, E.; Solà, R.; Pedret, A.; Valls, R.M. Fermented Dairy Products, Probiotic Supplementation, and Cardiometabolic Diseases: A Systematic Review and Meta-analysis. Adv. Nutr. 2020, 11, 834–863. [Google Scholar] [CrossRef]
- Song, J.X.; Zhang, S.Z.; Lu, L. Fungal cytochrome P450 protein Cyp51: What we can learn from its evolution, regulons and Cyp51-based azole resistance. Fungal Biol. Rev. 2018, 32, 131–142. [Google Scholar] [CrossRef]
- Lee, C.M.; Lee, B.S.; Arnold, S.L.; Isoherranen, N.; Morgan, E.T. Nitric Oxide and Interleukin-1β Stimulate the Proteasome-Independent Degradation of the Retinoic Acid Hydroxylase CYP2C22 in Primary Rat Hepatocytes. J. Pharmacol. Exp. Ther. 2014, 348, 141–152. [Google Scholar] [CrossRef]
- Audet-Walsh, E.; Lachaud, A.A.; Anderson, A. The CYP2B2 5′ flank contains a complex glucocorticoid response unit. Biochem. Pharmacol. 2008, 76, 1298–1306. [Google Scholar] [CrossRef]
- Tarasenko, T.N.; McGuire, P.J. The liver is a metabolic and immunologic organ: A reconsideration of metabolic decompensation due to infection in inborn errors of metabolism (IEM). Mol. Gene Metab. 2017, 121, 283–288. [Google Scholar] [CrossRef]
- Schnabl, B.; Brenner, D.A. Interactions Between the Intestinal Microbiome and Liver Diseases. Gastroenterology 2014, 146, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Lyytikäinen, A.; Kröger, H.; Lamberg-Allardt, C.; Alén, M.; Koistinen, A.; Wang, Q.J.; Suuriniemi, M.; Suominen, H.; Mahonen, A.; et al. Effects of calcium, dairy product, and vitamin D supplementation on bone mass accrual and body composition in 10–12-y-old girls: A 2-y randomized trial. Am. J. Clin. Nutr. 2005, 82, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Manios, Y.; Moschonis, G.; Trovas, G.; Lyritis, G.P. Changes in biochemical indexes of bone metabolism and bone mineral density after a 12-mo dietary intervention program: The Postmenopausal Health Study. Am. J. Clin. Nutr. 2007, 86, 781–789. [Google Scholar] [CrossRef]
- McCabe, L.; Britton, R.A.; Parameswaran, N. Prebiotic and Probiotic Regulation of Bone Health: Role of the Intestine and its Microbiome. Curr. Osteoporos. Rep. 2015, 13, 363–371. [Google Scholar] [CrossRef]
- Sevane, N.; Bialade, F.; Velasco, S.; Rebole, A.; Rodriguez, M.L.; Ortiz, L.T. Dietary inulin supplementation modifies significantly the liver transcriptomic profile of broiler chickens. PLoS ONE 2014, 9, 98942. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.C.; Jia, H.; Aw, W.; Saito, K.; Hasegawa, S.; Kato, H. Beneficial effects of soluble dietary Jerusalem artichoke (Helianthus tuberosus in the prevention of the onset of type 2 diabetes and non-alcoholic fatty liver disease in high-fructose diet-fed rats. Br. J. Nutr. 2014, 112, 709–717. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Mu, T.; Cao, J.; Liu, X.; Yang, X.; Ren, D.; Zhao, K. Response of gut microbiota and ileal transcriptome to inulin intervention in HFD induced obese mice. Int. J. Biol. Mol. 2023, 225, 861–872. [Google Scholar] [CrossRef]
- Bornholdt, J.; Broholm, C.; Chen, Y.; Rago, A.; Sloth, S.; Hendel, J.; Melsæther, C.; Müller, C.V.; Nilesen, M.J.; Strickertsson, J.; et al. Personalized B cell response to the Lactobacillus rhamnosus GG probiotic in healthy human subjects: A randomized trial. Gut Microbiota 2020, 12, 1–14. [Google Scholar] [CrossRef]
- van Baarlen, P.; Troost, F.; van der Meer, C.; Hooiveld, G.; Boekschoten, M.; Brummer, R.J.; Kleerebezem, M. Human mucosal in vivo transcriptome responses to three lactobacilli indicate how probiotics may modulate human cellular pathways. Proc. Natl. Acad. Sci. USA 2011, 108, 4562–4569. [Google Scholar] [CrossRef] [PubMed]
| Name | Forward (5′-3′) | Reverse (5′-3′) | TaqMan Probe (5′-3′) |
|---|---|---|---|
| Cgn | CCTGCCTTGCTGCTTTACTC | GGCTGGTCTTTGGAGAGGTATG | TCAGTGCCTTACCAGTGTGCGA |
| Arhgef18 | GAATGCTGAGTCTGTTTTCATAGAAGA | CCTCAAACTCATGGGCATCTG | TGAGGTGTGAGATCGAA |
| Cldnd1 | CGTGACTGCTCAGGCCATCT | CGGTGCTTTGCGAAACG | AGGGACTGTGGATGTC |
| Eeflal | AGCAAAAATGACCCACCAATG | GATCTGGCCTGGATGGTTCA | CAGCTGGCTTCACTGCTCAGGTGATTATC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Rasmussen, M.K.; Hansen, A.K.; Bertram, H.C. Effects of Dairy Matrix on the Intestinal, Liver, and Bone Transcriptome of Healthy Rats. Foods 2025, 14, 1375. https://doi.org/10.3390/foods14081375
Zhao X, Rasmussen MK, Hansen AK, Bertram HC. Effects of Dairy Matrix on the Intestinal, Liver, and Bone Transcriptome of Healthy Rats. Foods. 2025; 14(8):1375. https://doi.org/10.3390/foods14081375
Chicago/Turabian StyleZhao, Xiaorui, Martin Krøyer Rasmussen, Axel Kornerup Hansen, and Hanne Christine Bertram. 2025. "Effects of Dairy Matrix on the Intestinal, Liver, and Bone Transcriptome of Healthy Rats" Foods 14, no. 8: 1375. https://doi.org/10.3390/foods14081375
APA StyleZhao, X., Rasmussen, M. K., Hansen, A. K., & Bertram, H. C. (2025). Effects of Dairy Matrix on the Intestinal, Liver, and Bone Transcriptome of Healthy Rats. Foods, 14(8), 1375. https://doi.org/10.3390/foods14081375








