Metagenomics Insights into the Role of Microbial Communities in Mycotoxin Accumulation During Maize Ripening and Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
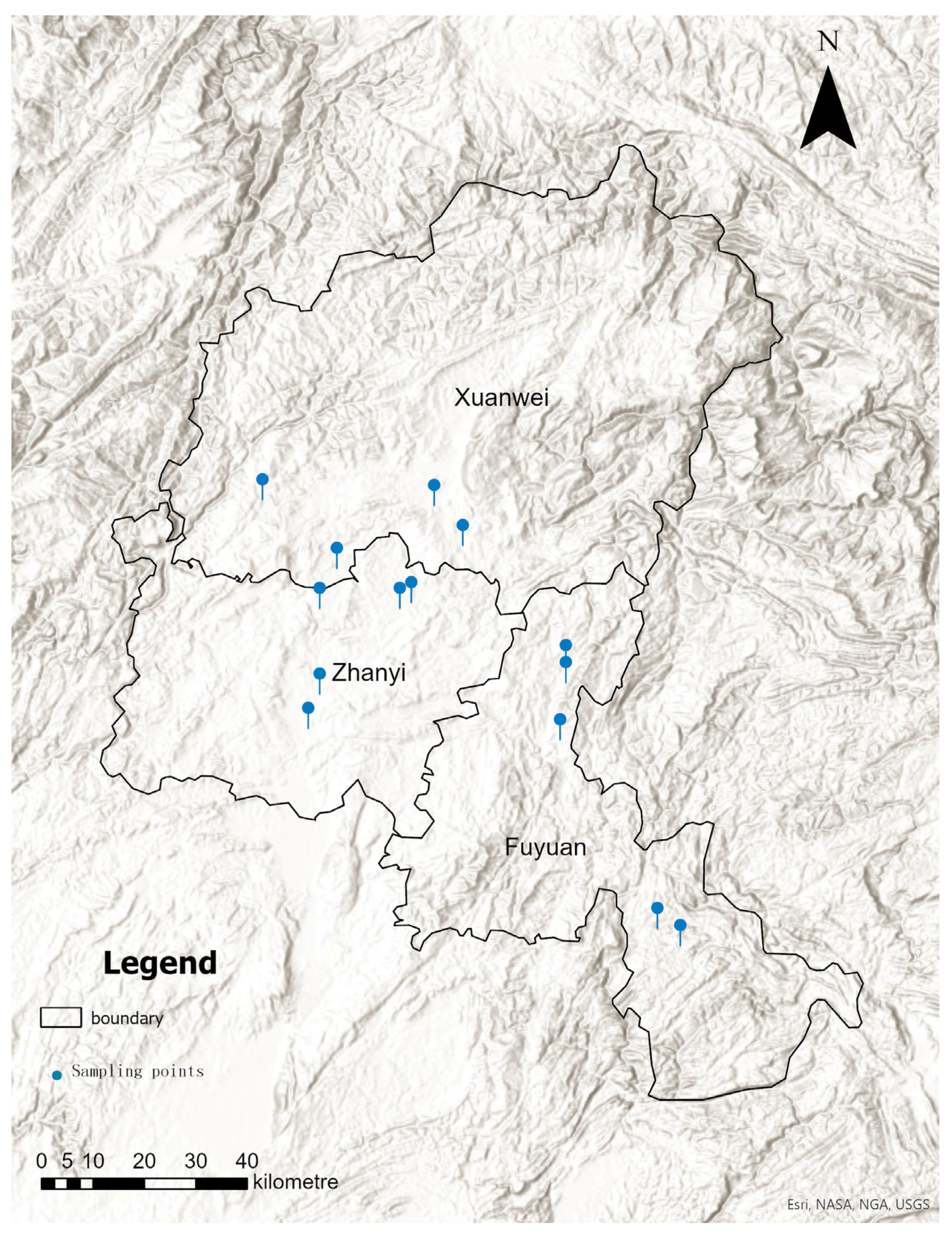
2.2. Experimental Design and Sample Collection
2.3. Extraction and Purification
2.4. Mycotoxins Determination and Apparatus
2.5. Metagenomic Data Analysis
2.5.1. Genomic DNA Extraction
2.5.2. Library Construction
2.5.3. Metagenomic Sequencing
2.5.4. Genomic Data Preprocessing
2.5.5. Gene Prediction and Abundance Analysis
2.5.6. Taxonomic and Gene Functional Annotation
2.6. Statistical Analyses
3. Results
3.1. Mycotoxin Content of Maize Samples
3.2. Metagenomic Sequencing Profiles
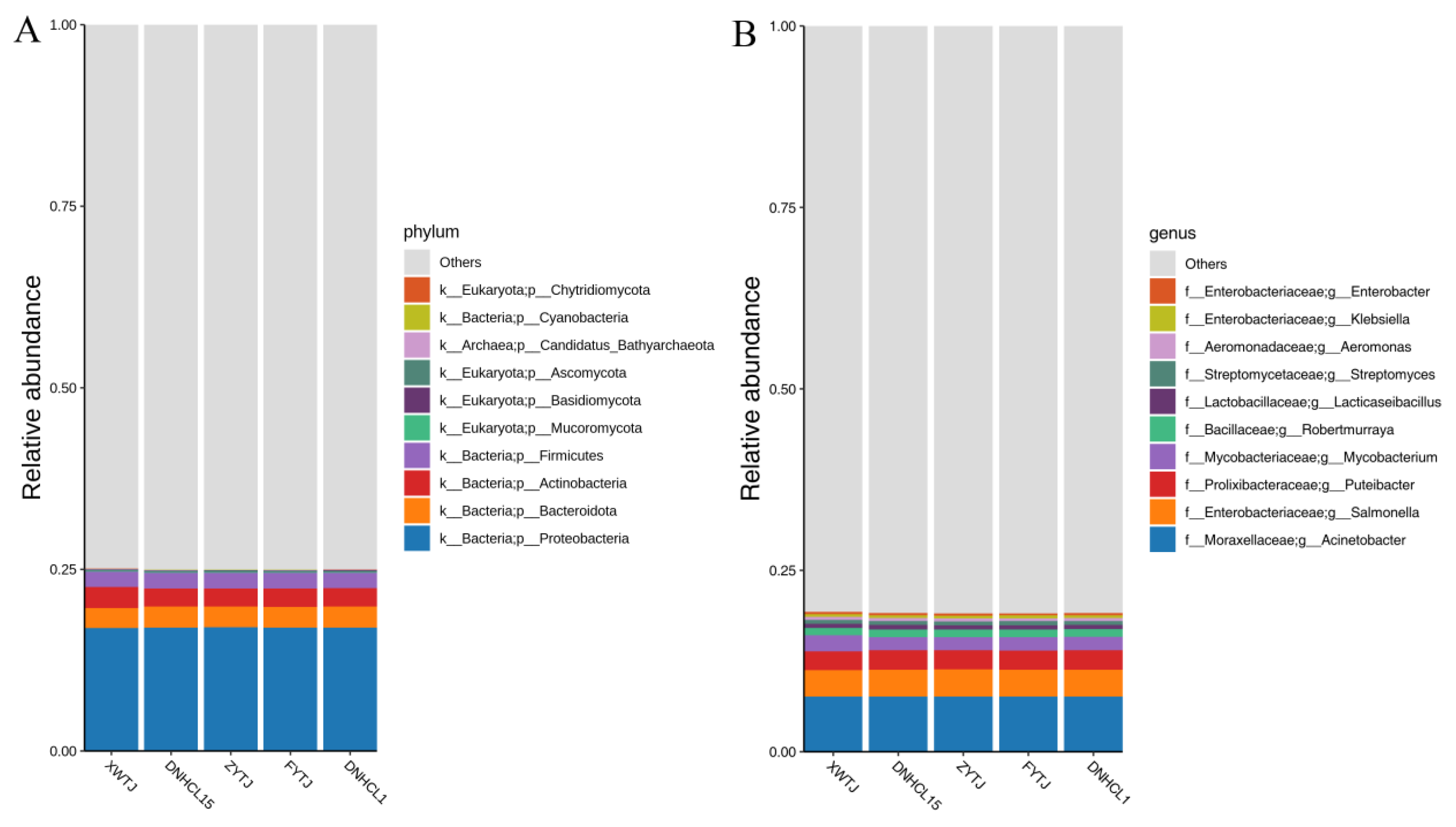
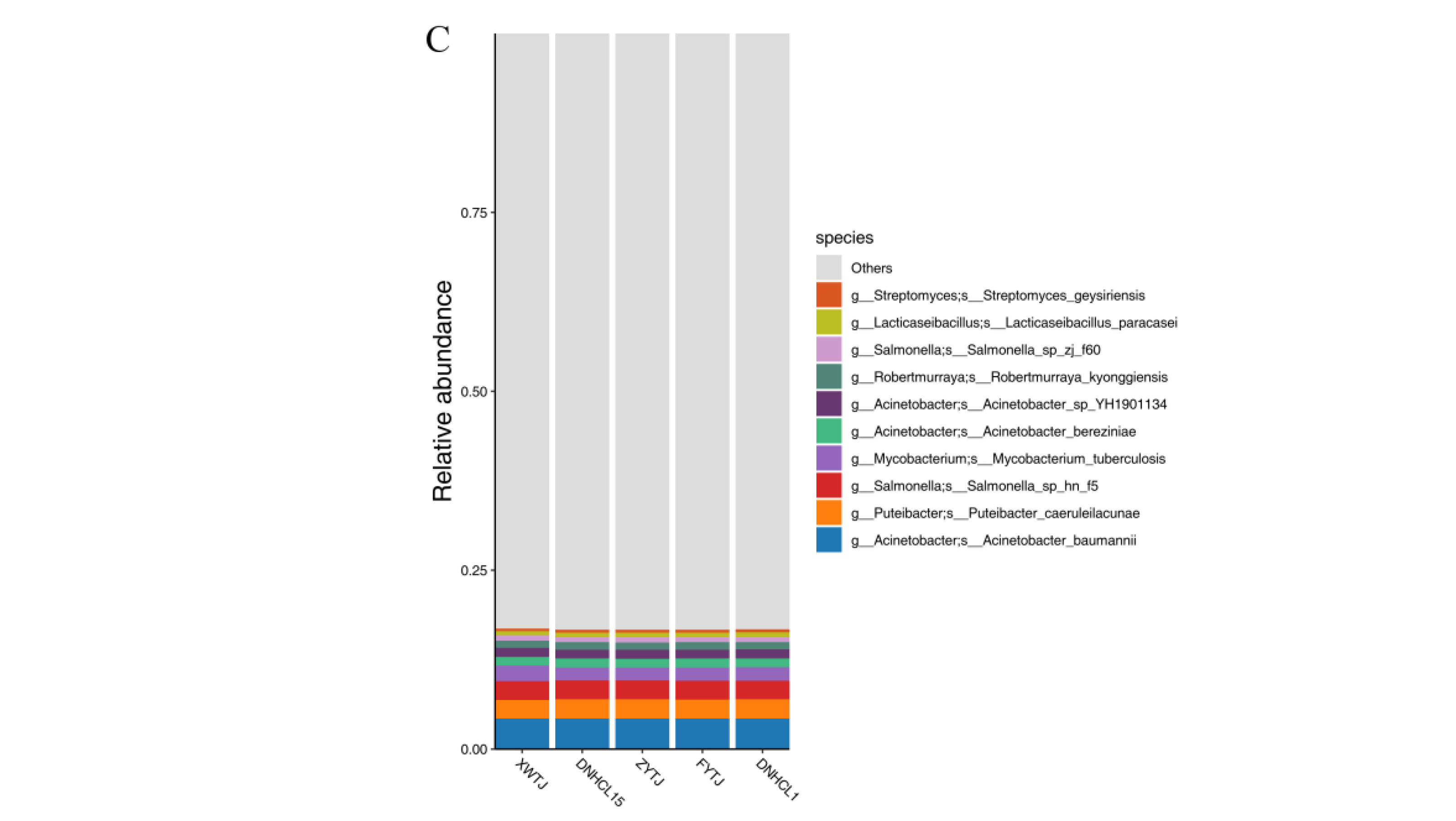
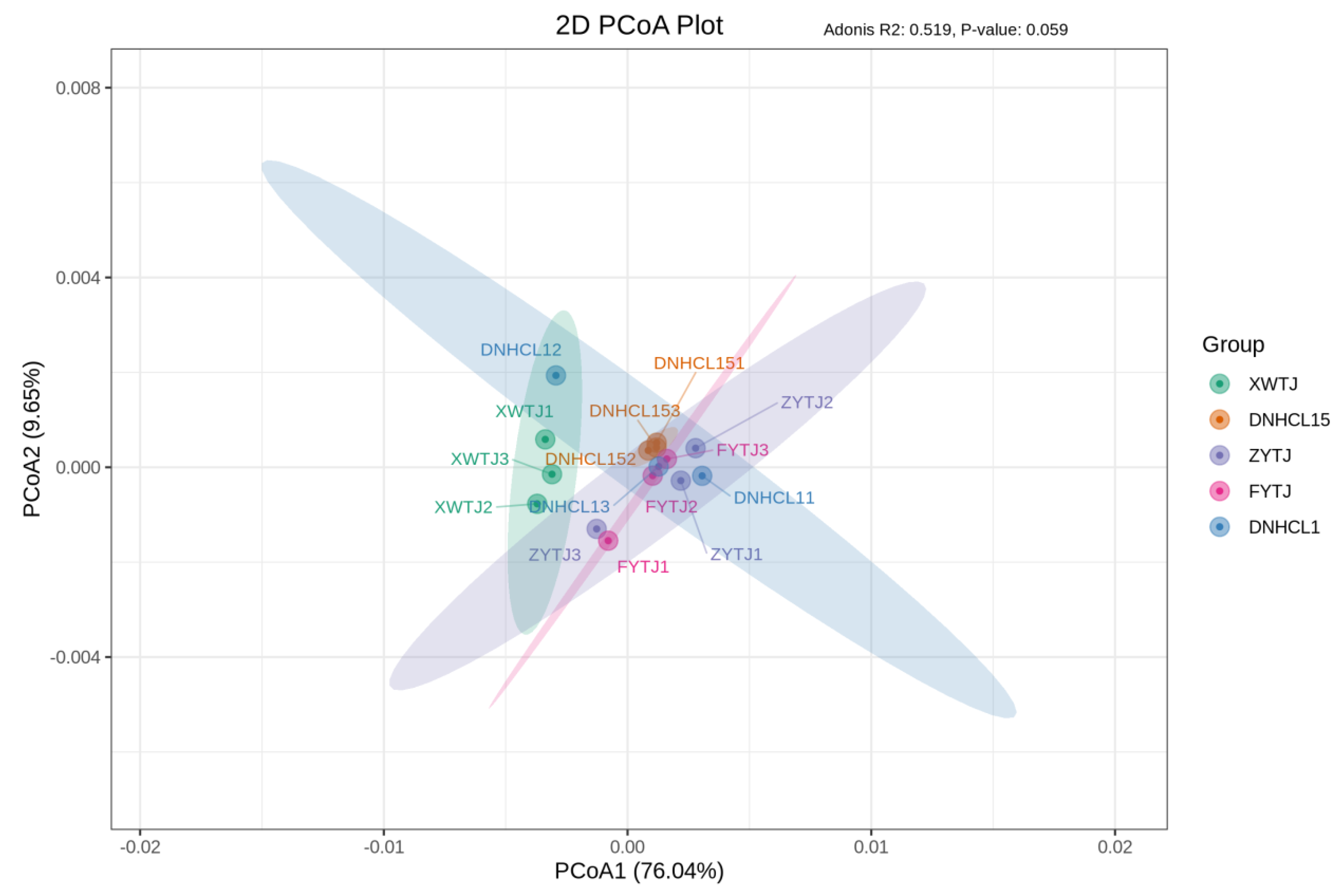
3.3. Microbial Community Structures
3.4. Microorganism Community Diversity Among Treatments
3.5. Function of Microorganism
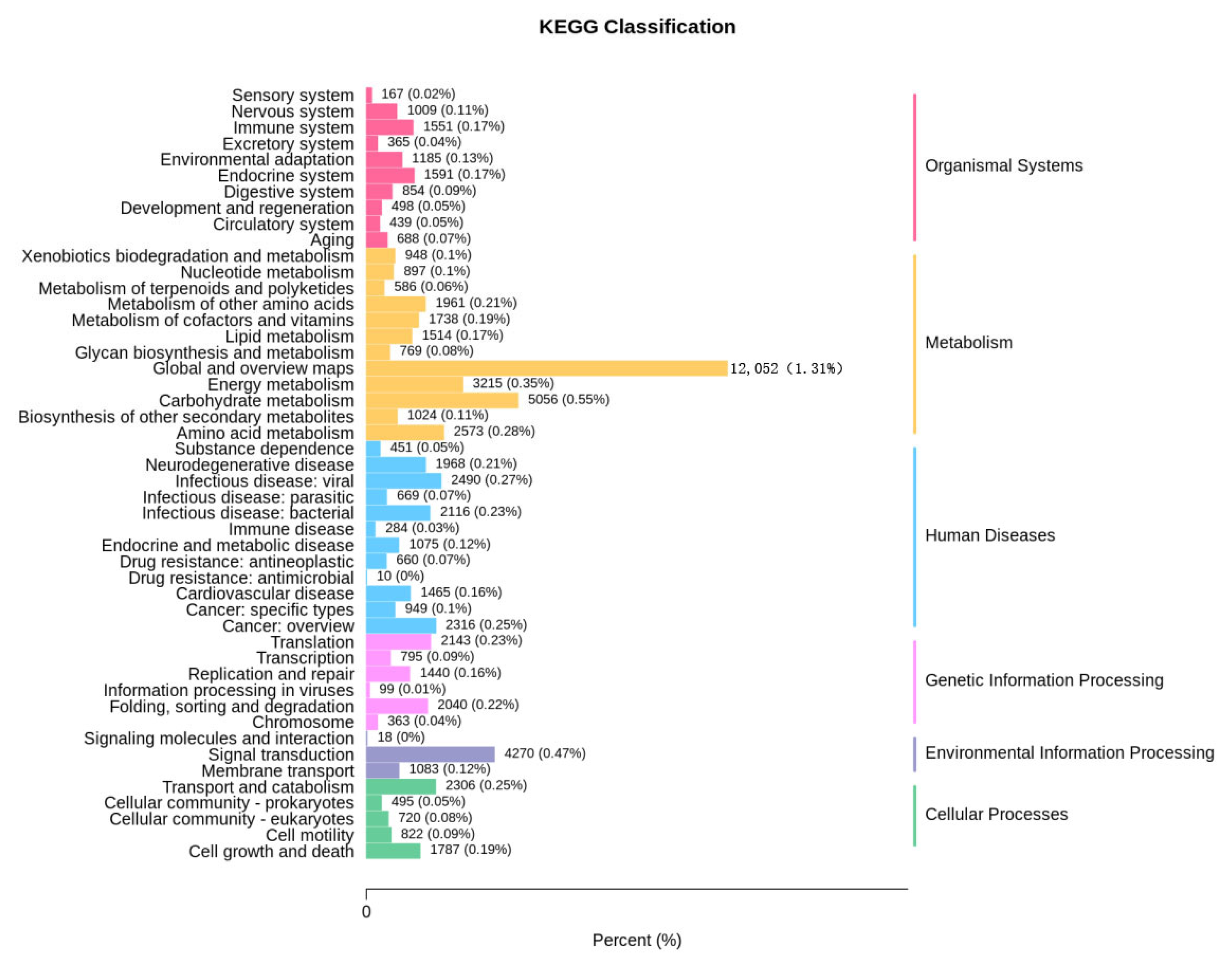
3.5.1. KEGG Functional Analysis
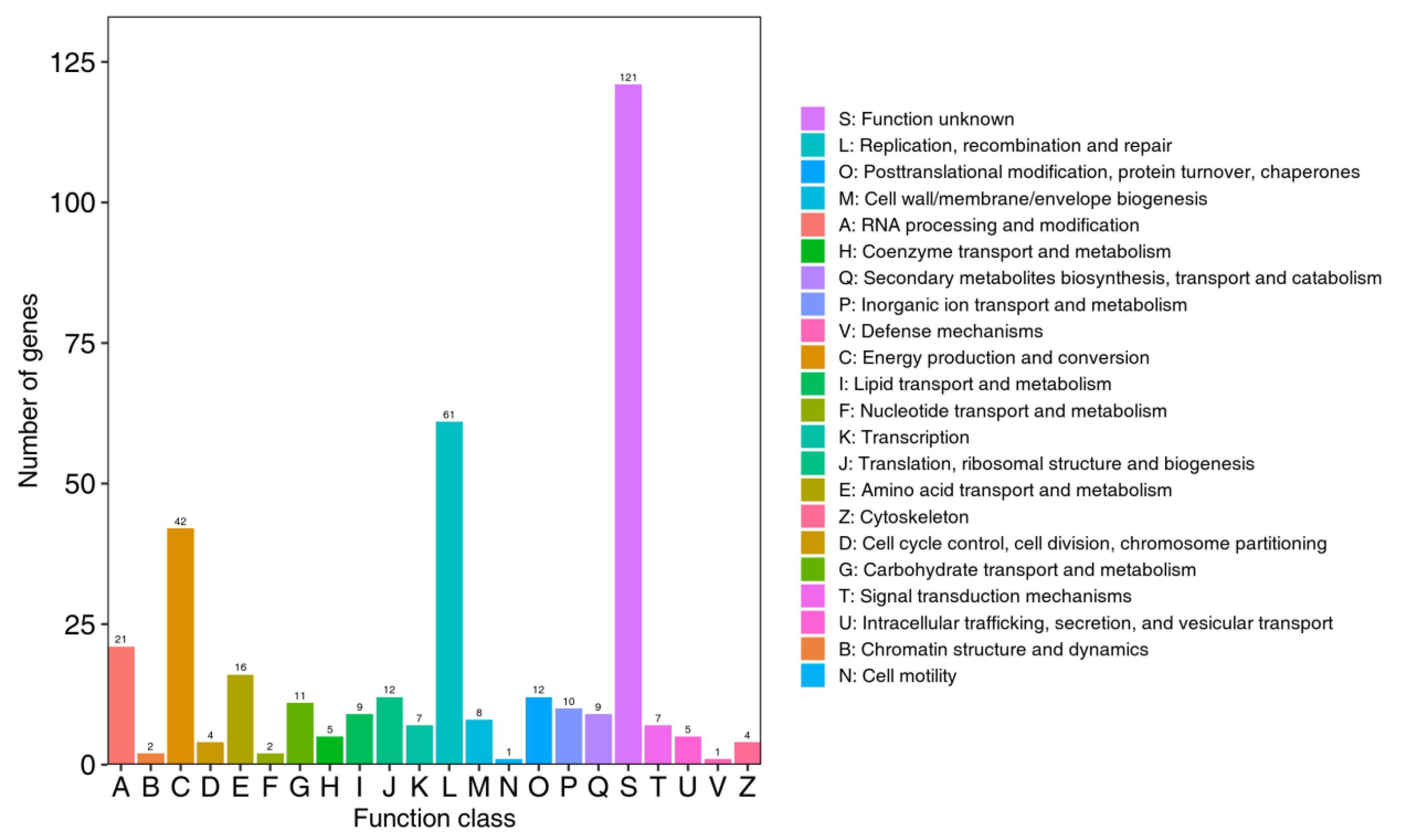
3.5.2. eggNOG Functional Analysis
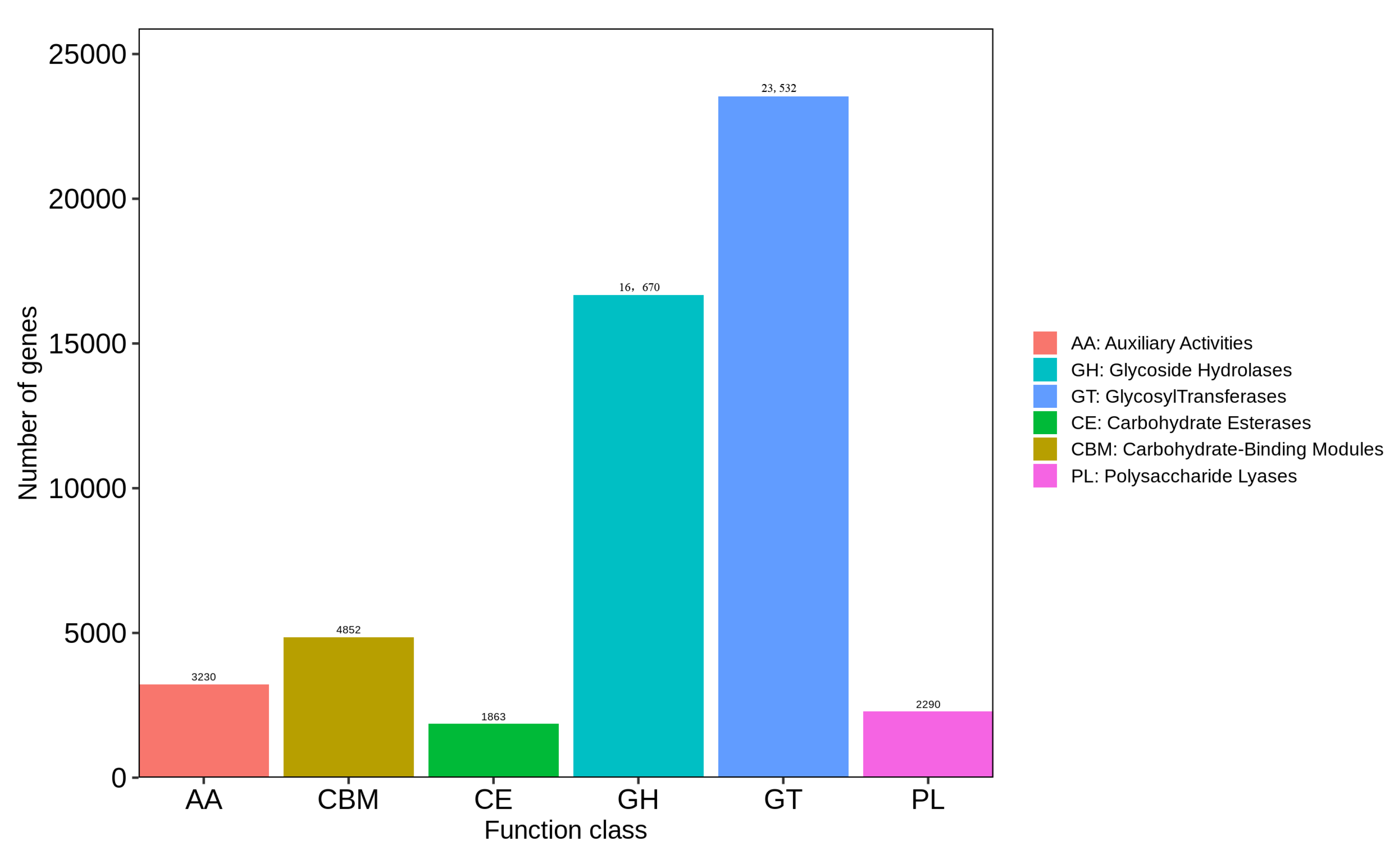
3.5.3. CAZy Functional Analysis
3.6. Microbial Profile Involved in Maize Mycotoxins
4. Discussion
4.1. Mycotoxins in Maize and Their Influencing Factors
4.2. Composition and Function of Microorganisms
4.3. The Toxigenic Fungi in Maize Samples
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pitt, J.I.; Miller, J.D. Short history of mycotoxin research. J. Agric. Food Chem. 2016, 65, 7021–7033. [Google Scholar] [CrossRef] [PubMed]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ’FAO estimate’ of 25. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Tian, L.; Zhang, H.; Lei, Y.; Tang, F. Fungal community analysis of hot spots in bulk maize under different storage conditions. LWT 2023, 182, 114819. [Google Scholar] [CrossRef]
- Carbas, B.; Simes, D.; Soares, A.; Freitas, A.; Brites, C. Occurrence of Fusarium spp. in Maize Grain Harvested in Portugal and Accumulation of Related Mycotoxins during Storage. Foods 2021, 10, 375. [Google Scholar] [CrossRef]
- Li, S.; Liu, N.; Cai, D.; Liu, C.; Ye, J.; Li, B.; Wu, Y.; Li, L.; Wang, S.; Fels-Klerx, H.J.V.D. A predictive model on deoxynivalenol in harvested wheat in China: Revealing the impact of the environment and agronomic practicing. Food Chem. 2023, 405, 134727. [Google Scholar] [CrossRef]
- Moretti, A.; Pascale, M.; Logrieco, A.F. Mycotoxin risks under a climate change scenario in Europe. Trends Food Sci. Technol. 2019, 84, 38–40. [Google Scholar] [CrossRef]
- Brett, L.; Sharma, S.; Niu, C.; Maina, A.W.; Wagacha, J.M.; Bluhm, B.H.; Woloshuk, C.P. Changes in the Fungal Microbiome of Maize During Hermetic Storage in the United States and Kenya. Front. Microbiol. 2018, 9, 2336. [Google Scholar] [CrossRef]
- Sserumaga, J.; Ortegabeltran, A.; Wagacha, J.; Mutegi, C.; Bandyopadhyay, R. Aflatoxin-producing fungi associated with pre-harvest maize contamination in Uganda. Int. J. Food Microbiol. 2020, 313, 108376. [Google Scholar] [CrossRef]
- Wan, J.; Chen, B.; Rao, J. Occurrence and preventive strategies to control mycotoxins in cereal-based food. Compr. Rev. Food Sci. Food Saf. 2020, 19, 928–953. [Google Scholar] [CrossRef]
- Cheng, S.; Feng, X.; Liu, G.; Zhao, N.; Liu, J.; Zhang, Z.; Yang, N.; Zhou, L.; Pang, M.; Tang, B.; et al. Natural Occurrence of Mycotoxins in Maize in North China. Toxins 2022, 14, 521. [Google Scholar] [CrossRef]
- Zunino, M.P.; Zygadlo, J.A.; Brito, V.D.; Achimón, F.; Pizzolitto, R.P. Fungal diversity and mycotoxins detected in maize stored in silo-bags: A review. J. Sci. Food Agric. 2022, 102, 2640–2650. [Google Scholar] [CrossRef]
- GB 5009.22-2016; Determination of Aflatoxins Group B and Group G in Food. National Standards for Food Safety: Beijing, China, 2016; p. 40.
- GB 5009.111-2016; Determination of Deoxynivalenol and Its Acetylated Derivatives in Food. National Standards for Food Safety: Beijing, China, 2016; p. 28.
- GB 5009.209-2016; Determination of Zearalenone in Food. National Standards for Food Safety: Beijing, China, 2016; p. 16.
- Chen, C.; Liu, Y.; Tian, H.; Ai, L.; Yu, H. Metagenomic analysis reveals the impact of JIUYAO microbial diversity on fermentation and the volatile profile of Shaoxing-jiu. Food Microbiol. 2023, 86, 103326. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Dinghua, L.; Chi-Man, L.; Ruibang, L.; Kunihiko, S.; Tak-Wah, L. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef]
- Wenhan, Z.; Alexandre, L.; Mark, B. Ab initio gene identification in metagenomic sequences. Nucleic Acids Res. 2010, 38, e132. [Google Scholar] [CrossRef]
- Godzik, L.A. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006, 22, 1658. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Mally, A.; Solfrizzo, M.; Degen, G.H. Biomonitoring of the mycotoxin Zearalenone: Current state-of-the art and application to human exposure assessment. Arch. Toxicol. 2016, 90, 1281–1292. [Google Scholar] [CrossRef]
- Tahir, N.I.; Hussain, S.; Javed, M.; Rehman, H.; Shahzady, T.G.; Parveen, B.; Ali, K.G. Nature of aflatoxins: Their extraction, analysis, and control. J. Food Saf. 2018, 38, 12561. [Google Scholar] [CrossRef]
- García-Díaz, M.; Gil-Serna, J.; Vázquez, C.; Botia, M.N.; Patio, B. A Comprehensive Study on the Occurrence of Mycotoxins and Their Producing Fungi during the Maize Production Cycle in Spain. Microorganisms 2020, 8, 141. [Google Scholar] [CrossRef]
- Mudili, V.; Siddaih, C.N.; Nagesh, M.; Garapati, P.; Batra, H.V. Mould incidence and mycotoxin contamination in freshly harvested maize kernels originated from India. J. Sci. Food Agric. 2014, 94, 2674–2683. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.S.; Diel, A.C.L.; Rauber, R.H.; Fontoura, F.P.; Mallmann, A.; Dilkin, P.; Mallmann, C.A. Free and hidden fumonisins in Brazilian raw maize samples. Food Control 2015, 53, 217–221. [Google Scholar] [CrossRef]
- Kos, J.; Hajnal, E.J.; Malachová, A.; Steiner, D.; Sulyok, M. Mycotoxins in maize harvested in Republic of Serbia in the period 2012–2015. Part 1: Regulated mycotoxins and its derivatives. Food Chem. 2019, 312, 126034. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J.H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public. Health 2017, 14, 632. [Google Scholar] [CrossRef] [PubMed]
- Ferrigo, D.; Raiola, A.; Causin, R. Fusarium Toxins in Cereals: Occurrence, Legislation, Factors Promoting the Appearance and Their Management. Molecules 2016, 21, 627. [Google Scholar] [CrossRef]
- Ramírez-Camejo, L.A.; Zuluaga-Montero, A.; Lázaro-Escudero, M.; Hernández-Kendall, V.; Bayman, P. Phylogeography of the cosmopolitan fungus Aspergillus flavus: Is everything everywhere? Fungal Biol. 2012, 116, 452–463. [Google Scholar] [CrossRef]
- Zinedine, A.; Soriano, J.M.; Moltó, J.C.; Ma?Es, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens—The IARC Monographs classification. Mycotoxin Res. 2016, 33, 65–73. [Google Scholar] [CrossRef]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef]
- Mwanda, O.W.; Otieno, C.F.; Omonge, E. Acute aflatoxicosis: Case report. East Afr. Med. J. 2005, 82, 320–324. [Google Scholar] [CrossRef]
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and its toxicity. Interdiscip. Toxicol. 2010, 3, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.S.; Brasel, J.M. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology 2001, 167, 101–134. [Google Scholar] [CrossRef]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef]
- Yang, R.; Wang, Y.M.; Zhang, L.; Zhao, Z.M.; Zhao, J.; Peng, S.Q. Prepubertal exposure to an oestrogenic mycotoxin zearalenone induces central precocious puberty in immature female rats through the mechanism of premature activation of hypothalamic kisspeptin-GPR54 signaling. Mol. Cell. Endocrinol. 2016, 437, 62–74. [Google Scholar] [CrossRef]
- Xing, H.Q.; Ma, J.C.; Xu, B.L.; Zhang, S.W.; Wang, J.; Cao, L.; Yang, X.M. Mycobiota of maize seeds revealed by rDNA-ITS sequence analysis of samples with varying storage times. Microbiologyopen 2018, 7, e00609. [Google Scholar] [CrossRef]
- Tringe, S.G.; Mering, C.V.; Kobayashi, A.; Salamov, A.A.; Chen, K.; Chang, H.W.; Podar, M.; Short, J.M.; Mathur, E.J.; Detter, J.C. Comparative Metagenomics of Microbial Communities. Science 2005, 308, 554–557. [Google Scholar] [CrossRef]
- Ye, S.H.; Siddle, K.J.; Park, D.J.; Sabeti, P.C. Benchmarking Metagenomics Tools for Taxonomic Classification. Cell 2019, 178, 779–794. [Google Scholar] [CrossRef]
- Riesenfeld, C.S.; Schloss, P.D.; Handelsman, J. Metagenomics: Genomic Analysis of Microbial Communities—Annual Review of Genetics. Annu. Rev. Genet. 2004, 38, 525. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, L.; Niu, Z.; Song, S.; Zhao, Y. Water stress affects the frequency of Firmicutes, Clostridiales and Lysobacter in rhizosphere soils of greenhouse grape. Agric. Water Manag. 2019, 226, 105776. [Google Scholar] [CrossRef]
- Stewart, R.D.; Auffret, M.D.; Warr, A.; Wiser, A.H.; Press, M.O.; Langford, K.W.; Liachko, I.; Snelling, T.J.; Dewhurst, R.J.; Walker, A.W. Assembly of 913 microbial genomes from metagenomic sequencing of the cow rumen. Nat. Commun. 2018, 9, 870. [Google Scholar] [CrossRef] [PubMed]
- Jose, V.L.; Appoothy, T.; More, R.P.; Arun, A.S. Metagenomic insights into the rumen microbial fibrolytic enzymes in Indian crossbred cattle fed finger millet straw. AMB Express 2017, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Lairson, L.L.; Henrissat, B.; Davies, G.J.; Withers, S.G. Glycosyltransferases: Structures, functions, and mechanisms. Annu. Rev. Biochem. 2008, 77, 521. [Google Scholar] [CrossRef] [PubMed]
- Boraston, A.B.; Bolam, D.N.; Gilbert, H.J.; Davies, G.J. Carbohydrate-binding modules: Fine-tuning polysaccharide recognition. Biochem. J. 2004, 382, 769–781. [Google Scholar] [CrossRef]
- Abrar, M.; Muhammad Anjum, F.; Sadiq Butt, M.; Pasha, I.; Atif Randhawa, M.; Farhan Saeed, K.W. Aflatoxins: Biosynthesis, occurrence, toxicity, and remedies. Crit. Rev. Food Sci. Nutr. 2013, 53, 862–874. [Google Scholar] [CrossRef]
- János, V.; Nikolett, B.; Muthusamy, C.; Csaba, V.; Sándor, K. Mycotoxin producers in the Aspergillus genus: An update. Univ. Szeged. 2015, 59, 151–167. [Google Scholar]
- Frisvad, J.; Hubka, V.; Ezekiel, C.; Hong, S.-B.; Nováková, A.; Chen, A.; Arzanlou, M.; Larsen, T.; Sklenář, F.; Mahakarnchanakul, W.; et al. Taxonomy of Aspergillus section Flavi and their production of aflatoxins, ochratoxins and other mycotoxins. Stud. Mycol. 2019, 93, 1–63. [Google Scholar] [CrossRef]
- Gao, J.; Liu, H.Q.; Zhang, Z.Z.; Liang, Z.H. Production, toxicity and biosynthesis of ochratoxin A: A review. Microbiol. China 2023, 50, 1265–1280. (In Chinese) [Google Scholar] [CrossRef]
- Bottalico, A.; Perrone, G. Toxigenic Fusarium species and Mycotoxins Associated with Head Blight in Small-Grain Cereals in Europe. Eur. J. Plant Pathol. 2002, 108, 611–624. [Google Scholar] [CrossRef]
- Barros, G.; Zanon, M.S.A.; Palazzini, J.M.; Haidukowski, M.; Pascale, M.; Chulze, S. Trichothecenes and zearalenone production by Fusarium equiseti and Fusarium semitectum species isolated from Argentinean soybean. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012, 29, 1436–1442. [Google Scholar] [CrossRef]
- Laraba, I.; Mccormick, S.P.; Vaughan, M.M.; Geiser, D.M.; O’Donnell, K. Phylogenetic diversity, trichothecene potential, and pathogenicity within Fusarium sambucinum species complex. PLoS ONE 2021, 16, e0245037. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Crit. Rev. Food Sci. Nutr. 2019, 60, 2710–2729. [Google Scholar] [CrossRef] [PubMed]
| Mycotoxin | Content (μg/kg) | ||||
|---|---|---|---|---|---|
| XWTJ | ZYTJ | FYTJ | 1DNHCL | 15DNHCL | |
| AFB1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 |
| DON | 247.33 ± 28.03 b | 219.27 ± 9.63 b | 200 ± 0 b | 662.23 ± 163.19 a | 321.13 ± 29.32 b |
| ZEN | 21 ± 2.87 bc | 47 ± 18.36 ab | 12.5 ± 1.61 bc | 46.33 ± 16.46 ab | 87 ± 30.89 a |
| Samples | Gene Numbers | Total Length | Average Length | GC/% |
|---|---|---|---|---|
| FYTJ | 146,409 ± 12,925.63 a | 38.12 ± 3.22 a | 260.44 ± 1.06 a | 49.35 ± 0.20 a |
| XWTJ | 130,407.67 ± 12,209.41 a | 33.93 ± 3.05 a | 260.21 ± 1.07 a | 48.28 ± 0.29 a |
| ZYTJ | 137,473.67 ± 13,373.03 a | 36.04 ± 2.99 a | 262.44 ± 3.84 a | 49.48 ± 0.33 a |
| 1DNHCL | 132,814.67 ± 11,180.54 a | 35.06 ± 3.08 a | 263.91 ± 1.02 a | 49.59 ± 0.20 a |
| 15DNHCL | 133,184.67 ± 6713.58 a | 35.42 ± 1.63 a | 265.97 ± 1.80 a | 49.58 ± 0.15 a |
| Samples | Chaol Indices | ACE Indices | Shannon Indices | Simpson Indices |
|---|---|---|---|---|
| FYTJ | 1415.39 ± 68.77 a | 1381.67 ± 28.36 a | 1.124 ± 0.003 a | 0.369 ± 0.001 a |
| XWTJ | 1393.32 ± 54.51 a | 1328.23 ± 12.59 a | 1.128 ± 0.001 a | 0.371 ± 0.001 a |
| ZYTJ | 1368.82 ± 181.08 a | 1313.70 ± 100.81 a | 1.122 ± 0.004 a | 0.369 ± 0.002 a |
| 1DNHCL | 1401.87 ± 87.90 a | 1387.38 ± 91.42 a | 1.124 ± 0.003 a | 0.370 ± 0.002 a |
| 15DNHCL | 1401.20 ± 71.02 a | 1401.20 ± 71.02 a | 1.124 ± 0.003 a | 0.369 ± 0.001 a |
| Species | Contents | ||||
|---|---|---|---|---|---|
| XWTJ | ZYTJ | FYTJ | 1DNHCL | 15DNHCL | |
| A. terreus | 5.33 ± 1.86 a | 11.67 ± 3.51 a | 7.33 ± 4.06 a | 10.67 ± 1.76 a | 9.33 ± 1.76 a |
| F. graminearum | 10.33 ± 3.84 a | 21.33 ± 4.81 a | 46.00 ± 35.59 a | 25.33 ± 10.74 a | 10.33 ± 3.67 a |
| F. poae | 4 ± 0 ab | 7.33 ± 1.76 a | 6.67 ± 0.67 a | 4.67 ± 0.67 ab | 2.67 ± 1.33 cb |
| F. culmorum | 10.67 ± 8.74 a | 11.33 ± 8.51 a | 24.67 ± 17.29 a | 5.33 ± 3.53 a | 8.33 ± 6.89 a |
| F. oxysporum | 10 ± 3.46 a | 15.00 ± 2.51 a | 9.00 ± 2.52 a | 15.33 ± 5.46 a | 15.33 ± 1.86 a |
| A. alternata | 12.00 ± 2.65 ab | 12.67 ± 5.04 ab | 17.33 ± 3.28 a | 5.67 ± 1.20 bc | 4.67 ± 0.67 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, X.; Chen, X.; Lu, X.; Yang, S.; Wang, X.; Liu, F.; Yang, J.; Guo, Y.; Shi, H.; Xu, H.; et al. Metagenomics Insights into the Role of Microbial Communities in Mycotoxin Accumulation During Maize Ripening and Storage. Foods 2025, 14, 1378. https://doi.org/10.3390/foods14081378
Nie X, Chen X, Lu X, Yang S, Wang X, Liu F, Yang J, Guo Y, Shi H, Xu H, et al. Metagenomics Insights into the Role of Microbial Communities in Mycotoxin Accumulation During Maize Ripening and Storage. Foods. 2025; 14(8):1378. https://doi.org/10.3390/foods14081378
Chicago/Turabian StyleNie, Xuheng, Xuefeng Chen, Xianli Lu, Shuiyan Yang, Xin Wang, Fuying Liu, Jin Yang, Ying Guo, Huirong Shi, Hui Xu, and et al. 2025. "Metagenomics Insights into the Role of Microbial Communities in Mycotoxin Accumulation During Maize Ripening and Storage" Foods 14, no. 8: 1378. https://doi.org/10.3390/foods14081378
APA StyleNie, X., Chen, X., Lu, X., Yang, S., Wang, X., Liu, F., Yang, J., Guo, Y., Shi, H., Xu, H., Zhang, X., Fang, M., Tao, Y., & Liu, C. (2025). Metagenomics Insights into the Role of Microbial Communities in Mycotoxin Accumulation During Maize Ripening and Storage. Foods, 14(8), 1378. https://doi.org/10.3390/foods14081378





