Effects of Perilla Seed Oil on Blood Lipids, Oxidative Stress, and Inflammation in Hyperlipidemic Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Perilla Seed Oil
2.2. Animals
2.3. Experimental Design
2.4. Blood Lipid Measurement
2.5. Malondialdehyde (MDA) Measurement Using the TBARs Method
2.6. Interleukin-6 (IL-6) Measurement
2.7. Statistical Analysis
3. Results and Discussion
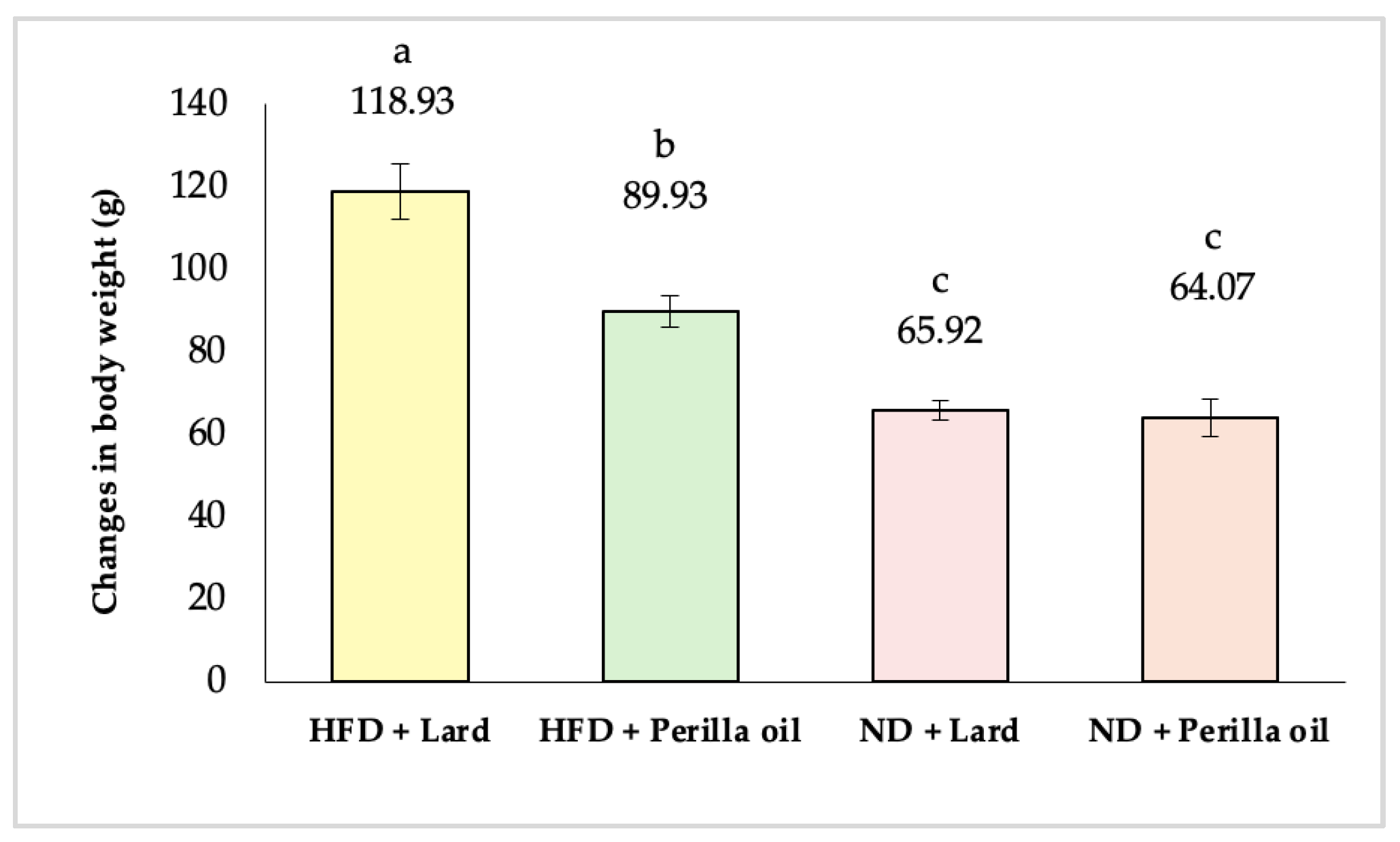
3.1. Effects on Body Weight
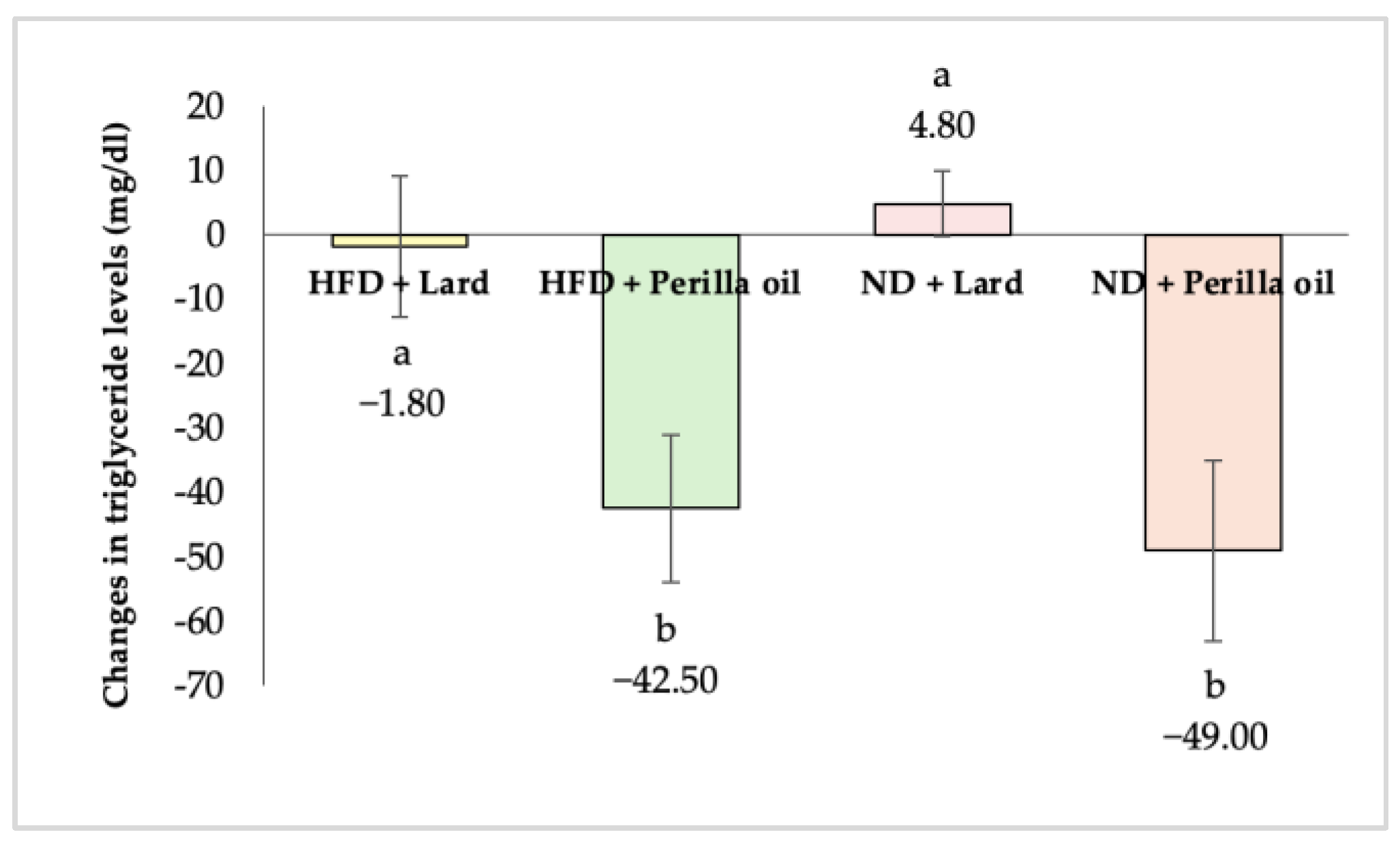
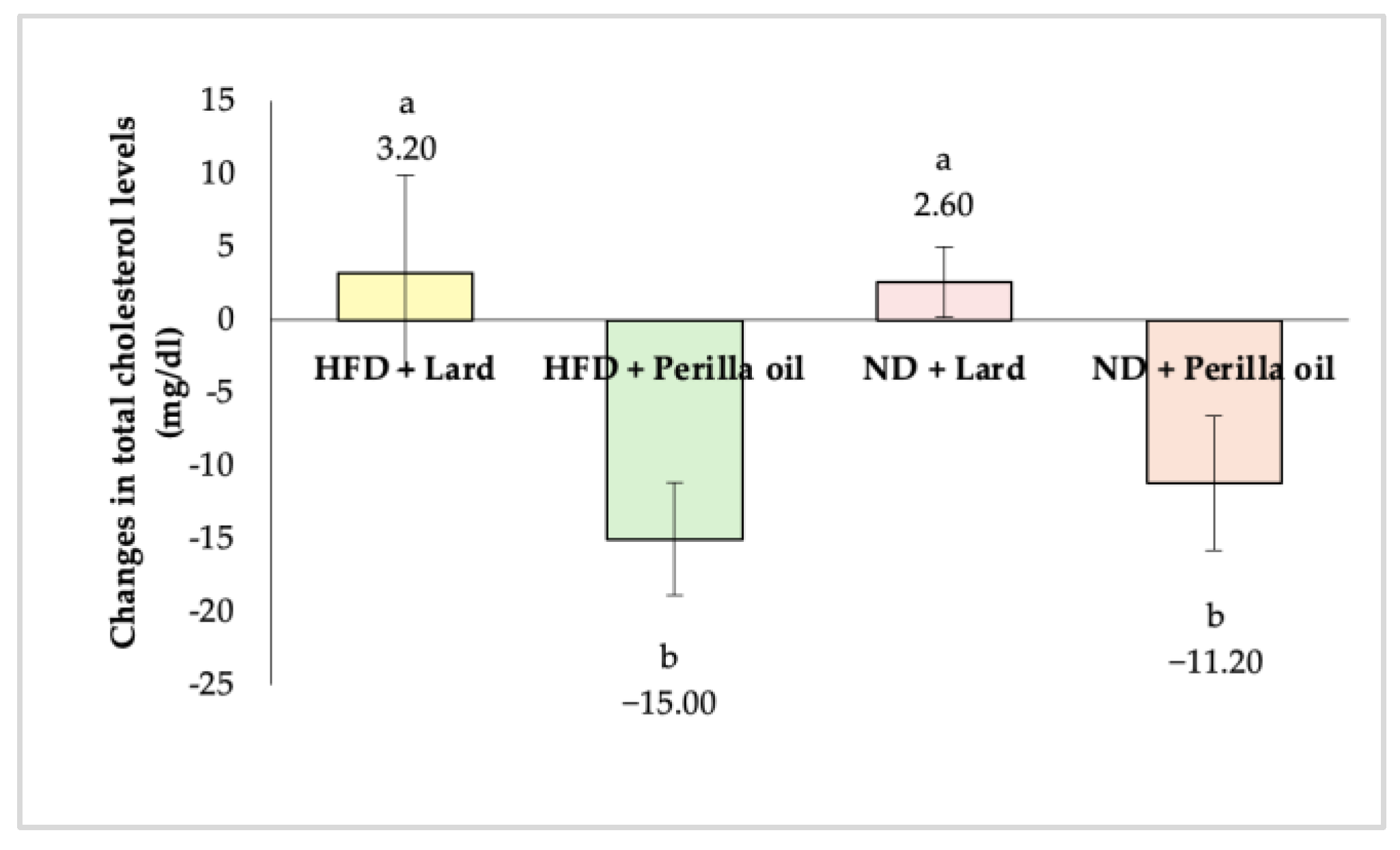
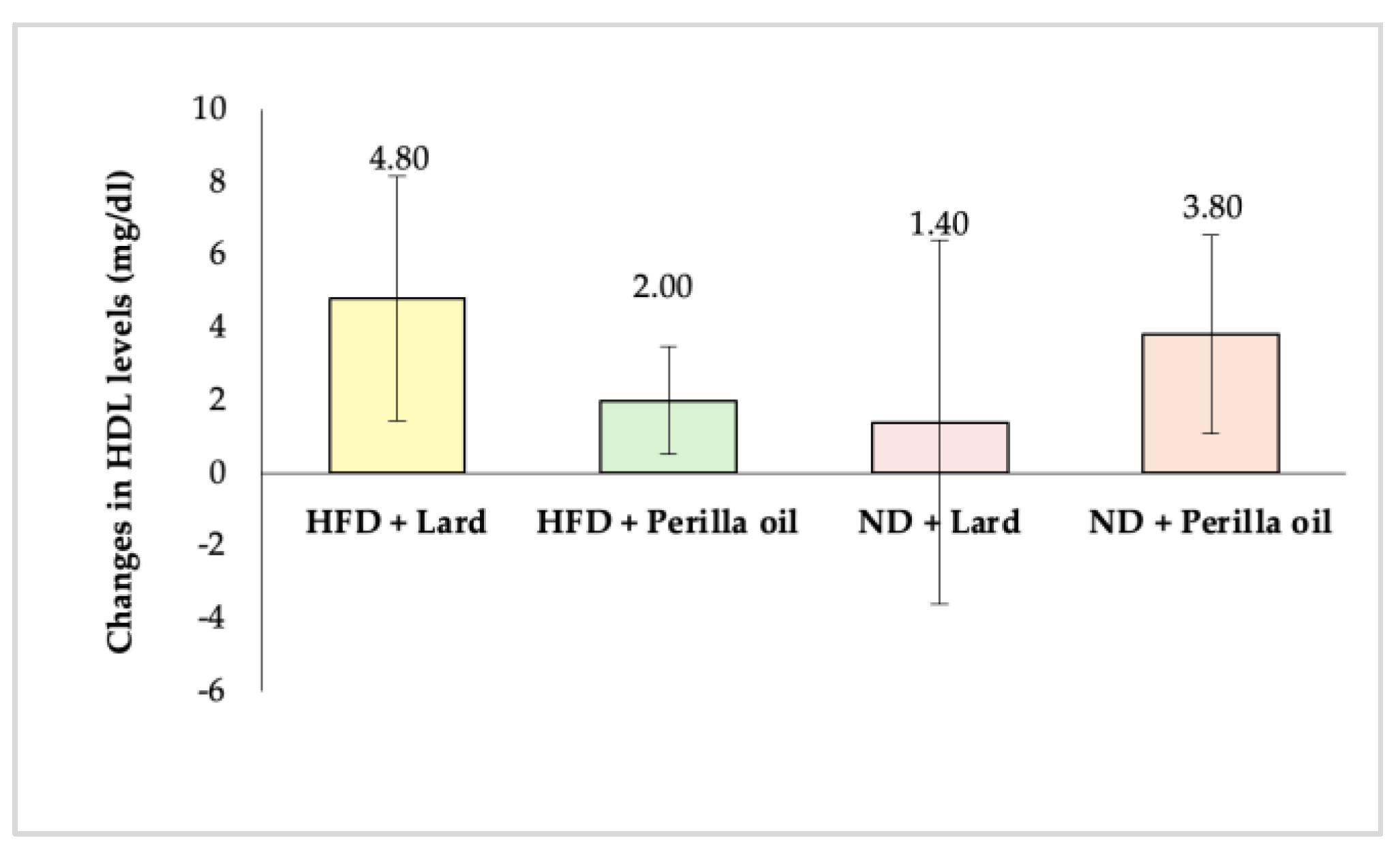
3.2. Effects on Blood Lipid Levels
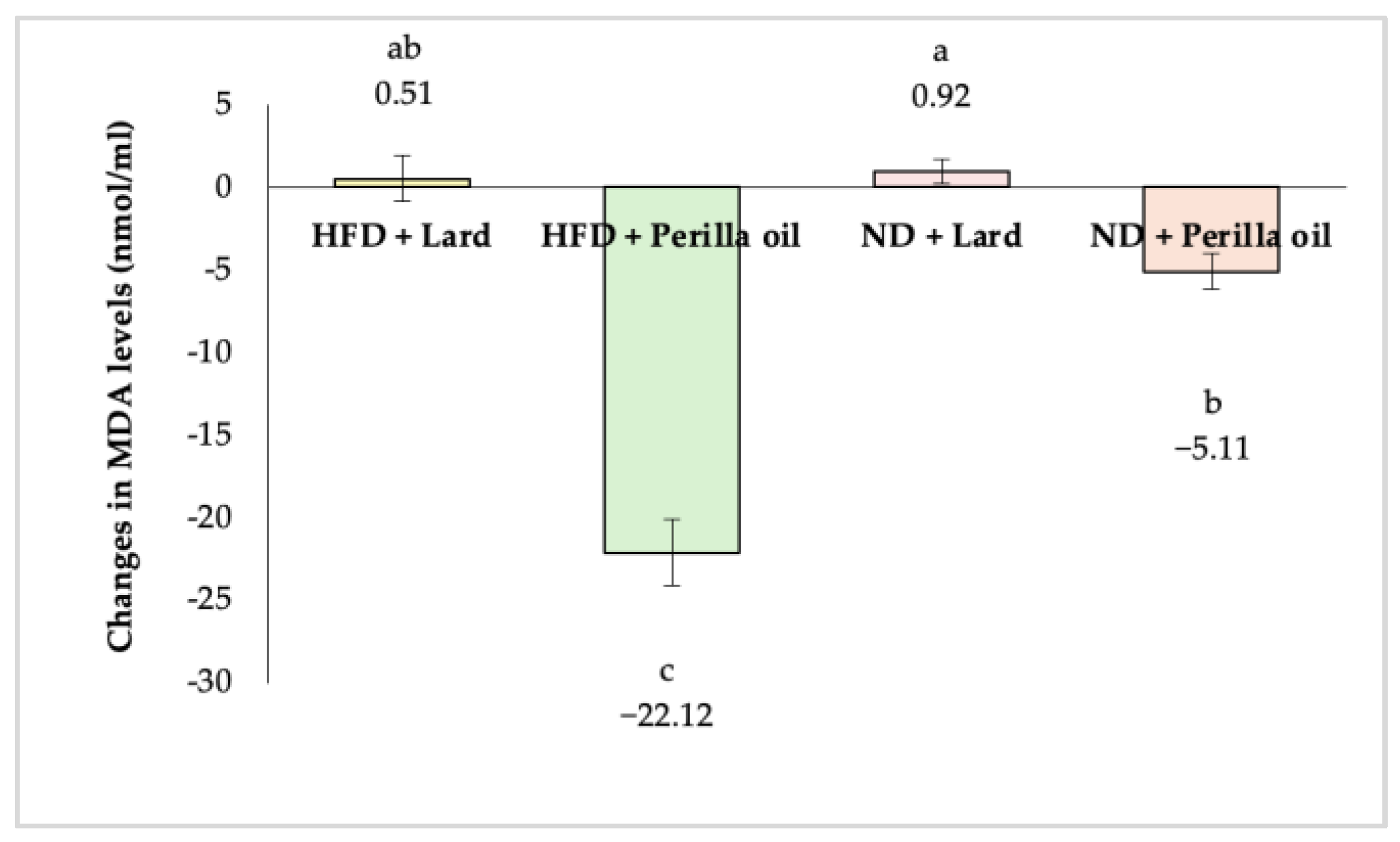
3.3. Antioxidant Activity Assessment
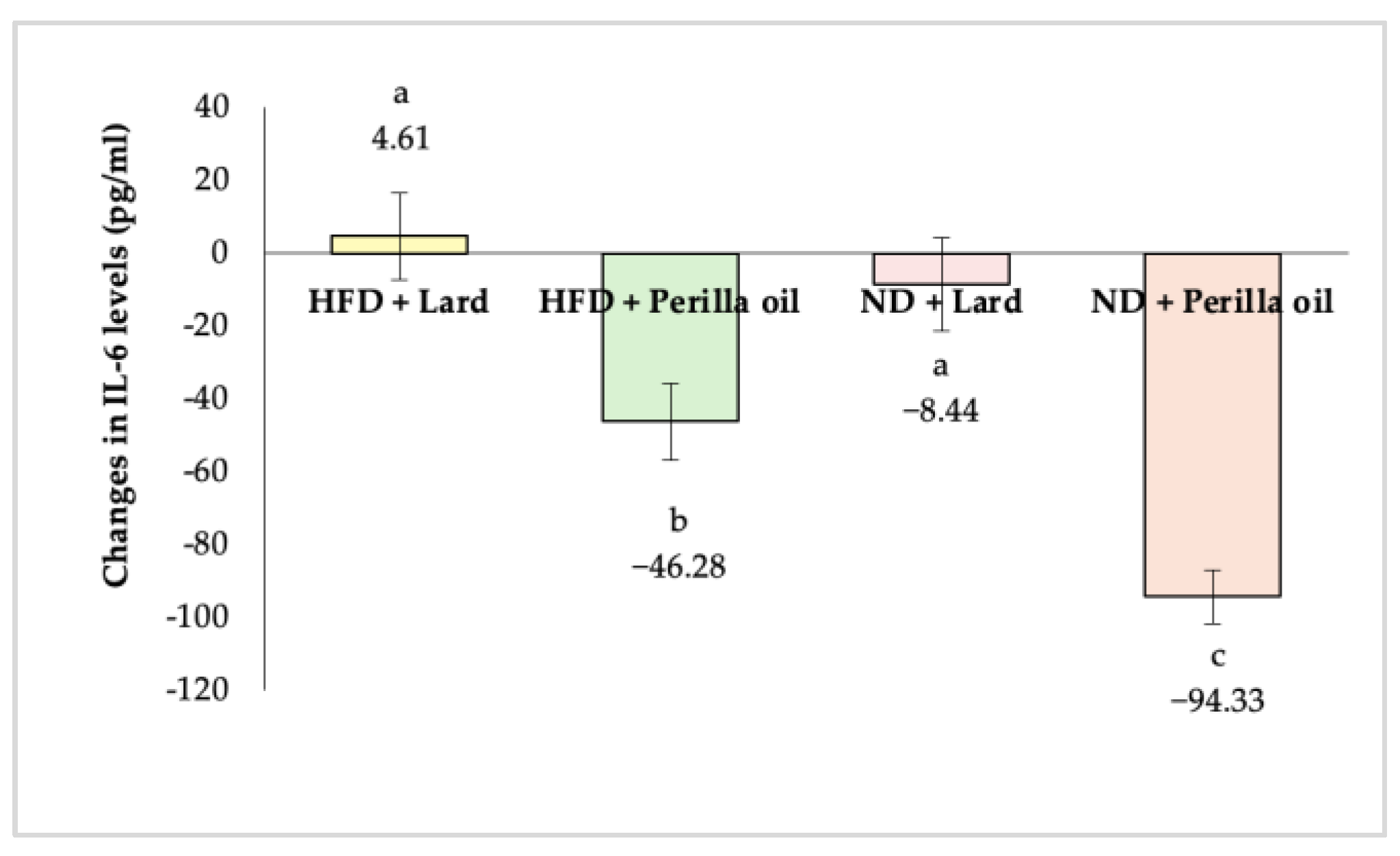
3.4. Anti-Inflammatory Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALA | Alpha-linolenic acid |
| HDL | High-density lipoprotein |
| LDL | Low-density lipoprotein |
| MDA | Malondialdehyde |
References
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Capó, X.; Bouzas, C.; Mateos, D.; Pons, A.; Tur, J.A.; Sureda, A. Metabolic Syndrome is Associated with Oxidative Stress and Proinflammatory State. Antioxidants 2020, 9, 236. [Google Scholar] [CrossRef]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef]
- Albracht-Schulte, K.; Kalupahana, N.S.; Ramalingam, L.; Wang, S.; Rahman, S.M.; Robert-McComb, J.; Moustaid-Moussa, N. Omega-3 fatty acids in obesity and metabolic syndrome: A mechanistic update. J. Nutr. Biochem. 2018, 58, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bishehkolaei, M.; Pathak, Y. Influence of omega n-6/n-3 ratio on cardiovascular disease and nutritional interventions. Hum. Nutr. Metab. 2024, 37, 200275. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J. The Importance of Maintaining a Low Omega-6/Omega-3 Ratio for Reducing the Risk of Autoimmune Diseases, Asthma, and Allergies. Mo. Med. 2021, 118, 453–459. [Google Scholar]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Khan, S.U.; Lone, A.N.; Khan, M.S.; Virani, S.S.; Blumenthal, R.S.; Nasir, K.; Miller, M.; Michos, E.D.; Ballantyne, C.M.; Boden, W.E.; et al. Effect of omega-3 fatty acids on cardiovascular outcomes: A systematic review and meta-analysis. EClinicalMedicine 2021, 38, 100997. [Google Scholar] [CrossRef]
- Jain, A.P.; Aggarwal, K.K.; Zhang, P.Y. Omega-3 fatty acids and cardiovascular disease. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 441–445. [Google Scholar]
- Poudyal, H.; Panchal, S.K.; Diwan, V.; Brown, L. Omega-3 fatty acids and metabolic syndrome: Effects and emerging mechanisms of action. Prog. Lipid Res. 2011, 50, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Yashodhara, B.; Umakanth, S.; Pappachan, J.; Bhat, S.; Kamath, R.; Choo, B. Omega-3 fatty acids: A comprehensive review of their role in health and disease. Postgrad. Med. J. 2009, 85, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Tousoulis, D.; Plastiras, A.; Siasos, G.; Oikonomou, E.; Verveniotis, A.; Kokkou, E.; Maniatis, K.; Gouliopoulos, N.; Miliou, A.; Paraskevopoulos, T.; et al. Omega-3 PUFAs improved endothelial function and arterial stiffness with a parallel antiinflammatory effect in adults with metabolic syndrome. Atherosclerosis 2014, 232, 10–16. [Google Scholar] [CrossRef]
- Lima Rocha, J.É.; Mendes Furtado, M.; Mello Neto, R.S.; da Silva Mendes, A.V.; Brito, A.K.d.S.; Sena de Almeida, J.O.C.; Rodrigues Queiroz, E.I.; de Sousa França, J.V.; Silva Primo, M.G.; de Cunha Sales, A.L.; et al. Effects of Fish Oil Supplementation on Oxidative Stress Biomarkers and Liver Damage in Hypercholesterolemic Rats. Nutrients 2022, 14, 426. [Google Scholar] [CrossRef] [PubMed]
- Pothinam, S.; Siriwoharn, T.; Jirarattanarangsri, W. Optimization of perilla seed oil extraction using supercritical CO2. Qual. Assur. Saf. Crops Foods 2025, 17, 14–29. [Google Scholar] [CrossRef]
- Fleming, J.A.; Kris-Etherton, P.M. The Evidence for α-Linolenic Acid and Cardiovascular Disease Benefits: Comparisons with Eicosapentaenoic Acid and Docosahexaenoic Acid. Adv. Nutr. 2014, 5, 863S–876S. [Google Scholar] [CrossRef]
- Asif, M. Health effects of omega-3,6,9 fatty acids: Perilla frutescens is a good example of plant oils. Orient. Pharm. Exp. Med. 2011, 11, 51–59. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, K.H.; Lee, M.-H.; Kim, H.-T.; Seo, W.D.; Kim, J.Y.; Baek, I.-Y.; Jang, D.S.; Ha, T.J. Identification, characterisation, and quantification of phenolic compounds in the antioxidant activity-containing fraction from the seeds of Korean perilla (Perilla frutescens) cultivars. Food Chem. 2013, 136, 843–852. [Google Scholar] [CrossRef]
- Morrison, W.R.; Smith, L.M. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride–methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar] [CrossRef]
- Thomas, S.S.; Cha, Y.-S.; Kim, K.-A. Perilla oil alleviates high-fat diet-induced inflammation in the colon of mice by suppressing nuclear factor-kappa B activation. J. Med. Food 2020, 23, 818–826. [Google Scholar] [CrossRef]
- Pratchayasakul, W.; Kerdphoo, S.; Petsophonsakul, P.; Pongchaidecha, A.; Chattipakorn, N.; Chattipakorn, S.C. Effects of high-fat diet on insulin receptor function in rat hippocampus and the level of neuronal corticosterone. Life Sci. 2011, 88, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wu, W.; Chen, J.; Wang, X.; Wang, Y. AMP-activated protein kinase is required for the anti-adipogenic effects of alpha-linolenic acid. Nutr. Metab. 2015, 12, 10. [Google Scholar] [CrossRef]
- Zhang, H.-X.; Tian, Y.-H.; Guan, J.; Xie, Q.-M.; Zhao, Y.-Q. The anti-tussive, anti-inflammatory effects and sub-chronic toxicological evaluation of perilla seed oil. J. Sci. Food Agric. 2021, 101, 1419–1427. [Google Scholar] [CrossRef]
- Han, K.; Li, X.-Y.; Zhang, Y.-Q.; He, Y.-L.; Hu, R.; Lu, X.-L.; Li, Q.-J.; Hui, J. Chia Seed Oil Prevents High Fat Diet Induced Hyperlipidemia and Oxidative Stress in Mice. Eur. J. Lipid Sci. Technol. 2020, 122, 1900443. [Google Scholar] [CrossRef]
- Elimam, H.; Kamal, B. Comparative Study of the Possible Prophylactic and Curative Effects of Flaxseed Oil on the Lipid Profile and Antioxidant Status of Hyperlipidaemic Rats. J. Appl. Pharm. 2018, 10, 257. [Google Scholar] [CrossRef]
- Viecili, P.R.N.; da Silva, B.; Hirsch, G.E.; Porto, F.G.; Parisi, M.M.; Castanho, A.R.; Wender, M.; Klafke, J.Z. Chapter One—Triglycerides Revisited to the Serial. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 80, pp. 1–44. [Google Scholar]
- Farnier, M.; Zeller, M.; Masson, D.; Cottin, Y. Triglycerides and risk of atherosclerotic cardiovascular disease: An update. Arch. Cardiovasc. Dis. 2021, 114, 132–139. [Google Scholar] [CrossRef]
- Harris, W.S.; Bulchandani, D. Why do omega-3 fatty acids lower serum triglycerides? Curr. Opin. Lipidol. 2006, 17, 387–393. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H.Y. Omega-3 Fatty Acids and Cardiovascular Disease: Effects on Risk Factors, Molecular Pathways, and Clinical Events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef]
- Morise, A.; Mourot, J.; Riottot, M.; Weill, P.; Fénart, E.; Hermier, D. Dose effect of alpha-linolenic acid on lipid metabolism in the hamster. Reprod. Nutr. Dev. 2005, 45, 405–418. [Google Scholar] [CrossRef]
- Wang, H.H.; Garruti, G.; Liu, M.; Portincasa, P.; Wang, D.Q.H. Cholesterol and Lipoprotein Metabolism and Atherosclerosis: Recent Advances in Reverse Cholesterol Transport. Ann. Hepatol. 2017, 16, S27–S42. [Google Scholar] [CrossRef] [PubMed]
- Huff, T.; Boyd, B.; Jialal, I. Physiology, Cholesterol. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Bird, J.K.; Calder, P.C.; Eggersdorfer, M. The Role of n-3 Long Chain Polyunsaturated Fatty Acids in Cardiovascular Disease Prevention, and Interactions with Statins. Nutrients 2018, 10, 775. [Google Scholar] [CrossRef]
- Kim, S.R.; Je, J.; Jeong, K.; Kim, S.J.; Lee, K.-Y.; Choi, S.-G.; Kim, H.; Park, S.W. Perilla Oil Decreases Aortic and Hepatic Lipid Accumulation by Modulating Lipogenesis and Lipolysis in High-Fat Diet-Fed Mice. J. Med. Food 2018, 22, 14–21. [Google Scholar] [CrossRef]
- Cha, Y.; Jang, J.; Ban, Y.-H.; Guo, H.; Shin, K.; Kim, T.-S.; Lee, S.-P.; Choi, J.; An, E.-S.; Seo, D.-W.; et al. Anti-atherosclerotic effects of perilla oil in rabbits fed a high-cholesterol diet. Lab. Anim. Res. 2016, 32, 171–179. [Google Scholar] [CrossRef]
- Lewis, G.F.; Rader, D.J. New Insights Into the Regulation of HDL Metabolism and Reverse Cholesterol Transport. Circ. Res. 2005, 96, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Ouimet, M.; Barrett, T.J.; Fisher, E.A. HDL and Reverse Cholesterol Transport. Circ. Res. 2019, 124, 1505–1518. [Google Scholar] [CrossRef]
- Packard, C.; Caslake, M.; Shepherd, J. The role of small, dense low density lipoprotein (LDL): A new look. Int. J. Cardiol. 2000, 74, S17–S22. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, S.; Mahmoodi, M.S.; Komaki, A.; Sadeghian, R. The comparison of omega-3 and flaxseed oil on serum lipids and lipoproteins in hyperlipidemic male rats. Heliyon 2022, 8, e09662. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, S.; Li, W.; Ma, L.; Ding, M.; Li, R.; Liu, Y. High-fat diet from perilla oil induces insulin resistance despite lower serum lipids and increases hepatic fatty acid oxidation in rats. Lipids Health Dis. 2014, 13, 15. [Google Scholar] [CrossRef]
- Singh, Z.; Karthigesu, I.; Singh, P.; Kaur, R. Use of Malondialdehyde as a Biomarker for Assessing Oxidative Stress in Different Disease Pathologies: A Review (OPEN ACCESS). Iran. J. Public Health 2014, 43, 7–16. [Google Scholar]
- Kasote, D. Flaxseed phenolics as natural antioxidants. Int. Food Res. J. 2013, 20, 27–34. [Google Scholar]
- Alam, S.-I.; Kim, M.-W.; Shah, F.A.; Saeed, K.; Ullah, R.; Kim, M.-O. Alpha-Linolenic Acid Impedes Cadmium-Induced Oxidative Stress, Neuroinflammation, and Neurodegeneration in Mouse Brain. Cells 2021, 10, 2274. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.J.; Kim, J.E.; Choi, H.J.; Choi, Y.J.; Lee, S.J.; Gong, J.E.; Seo, S.; Yang, S.Y.; An, B.-S.; Lee, H.S.; et al. α-Linolenic Acid-Enriched Cold-Pressed Perilla Oil Suppress High-Fat Diet-Induced Hepatic Steatosis through Amelioration of the ER Stress-Mediated Autophagy. Molecules 2020, 25, 2662. [Google Scholar] [CrossRef]
- Han, H.; Qiu, F.; Zhao, H.; Tang, H.; Li, X.; Shi, D. Dietary Flaxseed Oil Prevents Western-Type Diet-Induced Nonalcoholic Fatty Liver Disease in Apolipoprotein-E Knockout Mice. Oxid. Med. Cell Longev. 2017, 2017, 3256241. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar] [CrossRef]
- Danesh, J.; Kaptoge, S.; Mann, A.G.; Sarwar, N.; Wood, A.; Angleman, S.B.; Wensley, F.; Higgins, J.P.T.; Lennon, L.; Eiriksdottir, G.; et al. Long-Term Interleukin-6 Levels and Subsequent Risk of Coronary Heart Disease: Two New Prospective Studies and a Systematic Review. PLOS Med. 2008, 5, e78. [Google Scholar] [CrossRef]
- Al-Madhagy, S.; Ashmawy, N.S.; Mamdouh, A.; Eldahshan, O.A.; Farag, M.A. A comprehensive review of the health benefits of flaxseed oil in relation to its chemical composition and comparison with other omega-3-rich oils. Eur. J. Med. Res. 2023, 28, 240. [Google Scholar] [CrossRef]
- Noeman, S.A.; Hamooda, H.E.; Baalash, A.A. Biochemical Study of Oxidative Stress Markers in the Liver, Kidney and Heart of High Fat Diet Induced Obesity in Rats. Diabetol. Metab. Syndr. 2011, 3, 17. [Google Scholar] [CrossRef]
- Phillips, C.M.; Kesse-Guyot, E.; McManus, R.; Hercberg, S.; Lairon, D.; Planells, R.; Roche, H.M. High dietary saturated fat intake accentuates obesity risk associated with the fat mass and obesity-associated gene in adults. J. Nutr. 2012, 142, 824–831. [Google Scholar] [CrossRef]
- Wali, J.A.; Jarzebska, N.; Raubenheimer, D.; Simpson, S.J.; Rodionov, R.N.; O’Sullivan, J.F. Cardio-Metabolic Effects of High-Fat Diets and Their Underlying Mechanisms—A Narrative Review. Nutrients 2020, 12, 1505. [Google Scholar] [CrossRef]
- Farhangi, M.A.; Mesgari-Abbasi, M.; Hajiluian, G.; Nameni, G.; Shahabi, P. Adipose Tissue Inflammation and Oxidative Stress: The Ameliorative Effects of Vitamin D. Inflammation 2017, 40, 1688–1697. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pothinam, S.; Putpim, C.; Siriwoharn, T.; Jirarattanarangsri, W. Effects of Perilla Seed Oil on Blood Lipids, Oxidative Stress, and Inflammation in Hyperlipidemic Rats. Foods 2025, 14, 1380. https://doi.org/10.3390/foods14081380
Pothinam S, Putpim C, Siriwoharn T, Jirarattanarangsri W. Effects of Perilla Seed Oil on Blood Lipids, Oxidative Stress, and Inflammation in Hyperlipidemic Rats. Foods. 2025; 14(8):1380. https://doi.org/10.3390/foods14081380
Chicago/Turabian StylePothinam, Suwajee, Chaochetdhapada Putpim, Thanyaporn Siriwoharn, and Wachira Jirarattanarangsri. 2025. "Effects of Perilla Seed Oil on Blood Lipids, Oxidative Stress, and Inflammation in Hyperlipidemic Rats" Foods 14, no. 8: 1380. https://doi.org/10.3390/foods14081380
APA StylePothinam, S., Putpim, C., Siriwoharn, T., & Jirarattanarangsri, W. (2025). Effects of Perilla Seed Oil on Blood Lipids, Oxidative Stress, and Inflammation in Hyperlipidemic Rats. Foods, 14(8), 1380. https://doi.org/10.3390/foods14081380




