Advances in Multifunctional Nanoagents and SERS-Based Multimodal Sensing for Biotoxin in Foods
Abstract
:1. Introduction
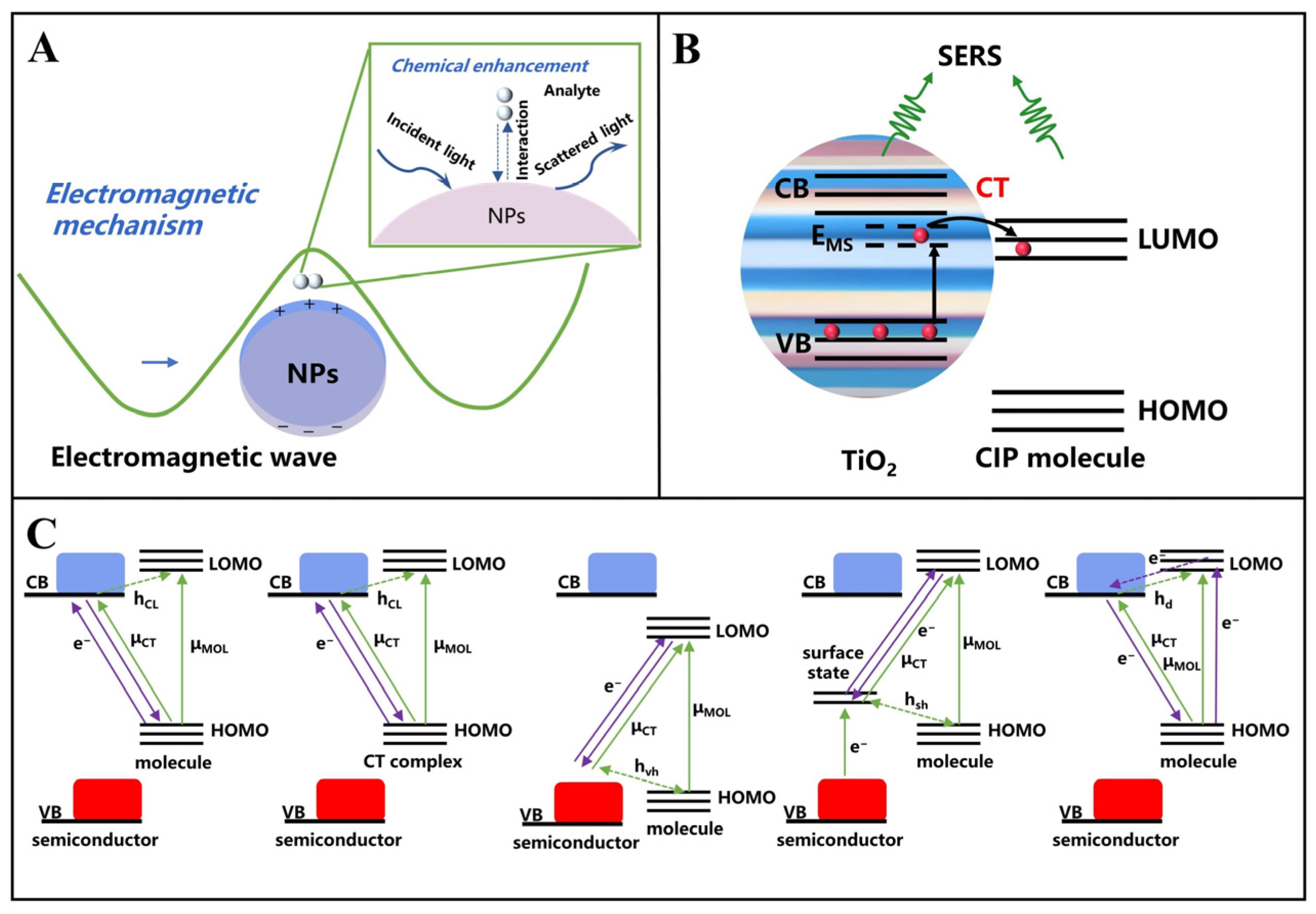
2. SERS
2.1. Raman Spectroscopy
2.2. SERS Substrate
3. Nanomaterials for SERS Sensors
3.1. Metal Nanomaterials
3.2. Metal-Organic Framework (MOF)
3.3. Covalent-Organic Framework (COF)
3.4. Semiconductors
3.5. Two-Dimensional Materials
| Material | Synthesis | Material Diameter (nm) | Target | AEF | Linear Range | LOD | Ref. |
|---|---|---|---|---|---|---|---|
| cDNA-AuNCs AgNPs/MPDA-Apt-TAMRA | Covalent Conjugation Thiol-Metal Covalent Conjugation | 3.06 ± 0.70 (AuNCs) 27.40 ± 2.48 (AgNPs) | DON | 2.1 × 106 | 0.1–100 ng/mL | 0.06 ng/mL | [49] |
| aptamers@papain@AuNCs | Biotemplated Synthesis Thiol-Metal Covalent Conjugation | 10.17 ± 1.21 | Escherichia coli O157:H7 | / | 10–106 cfu/mL | 39 cfu/mL | [50] |
| AgNCs Zr-MOF | Chemical Reduction Synthesis Solvothermal Synthesis | 2–5 100–200 | Hg2+ | / | 10–500 ng/mL | 1.8 ng/mL | [51] |
| Fe3O4@SiO2@Ag-Apt AuNPs-cDNA | Seed-Mediated Growth Thiol-Metal Covalent Conjugation | 500 50 | OTA | 1.57 × 107 | 0.01–5 ng/mL | 0.0074 ng/mL | [54] |
| MBs-apt Mn/Fe-MIL (53)@AuNSs-MBA-cDNA | Biotin-Avidin System Electrostatic Self-Assembly | / | Stx2 | / | 0.05–1000 ng/mL | 0.026 ng/mL | [57] |
| DdBd AuNPs | Solvothermal Polycondensation Nanocatalytic Analysis | 70 30 | OTC | 3.67 × 106 | 0.0012–0.028 ng/mL | 3.6 × 10−4 ng/mL | [61] |
| B–TiO2-AR-PEG-FA | Solid-phase synthesis | 25 (B–TiO2 NPs) | CTC | / | / | 2 cells/mL | [64] |
| 2D BiOI | Hydrothermal | 120 | TCP | 107 | 1.97–32,770 ng/mL | 0.0197 ng/mL | [65] |
| Ag@Cu2O NPs MXene NSs | Chemical Reduction Chemical Etching | 80.1 | TTX | / | 0.1–104 ng/mL | 0.0316 ng/mL | [70] |
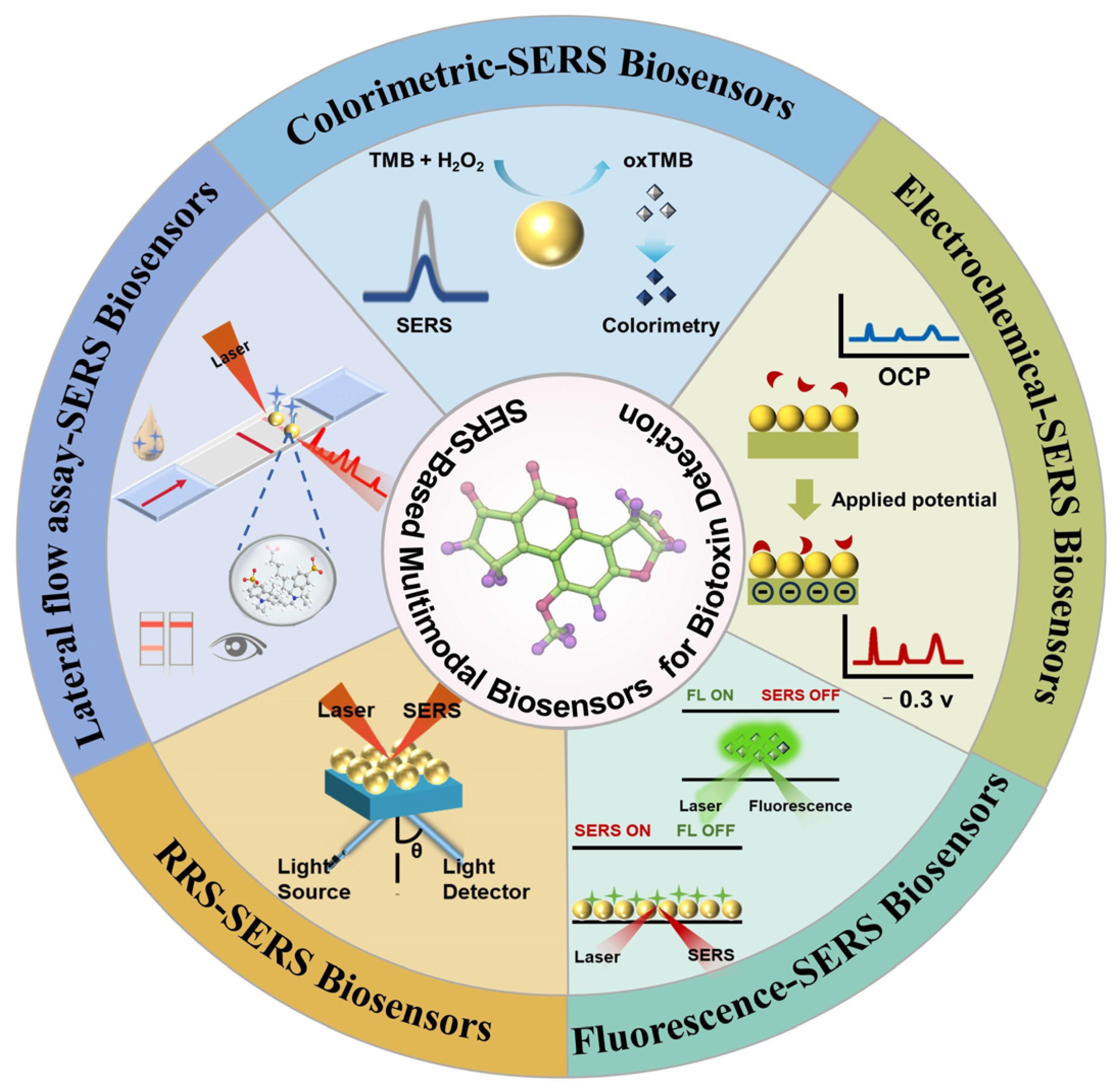
4. Applications of SERS-Based Multimodal Sensors
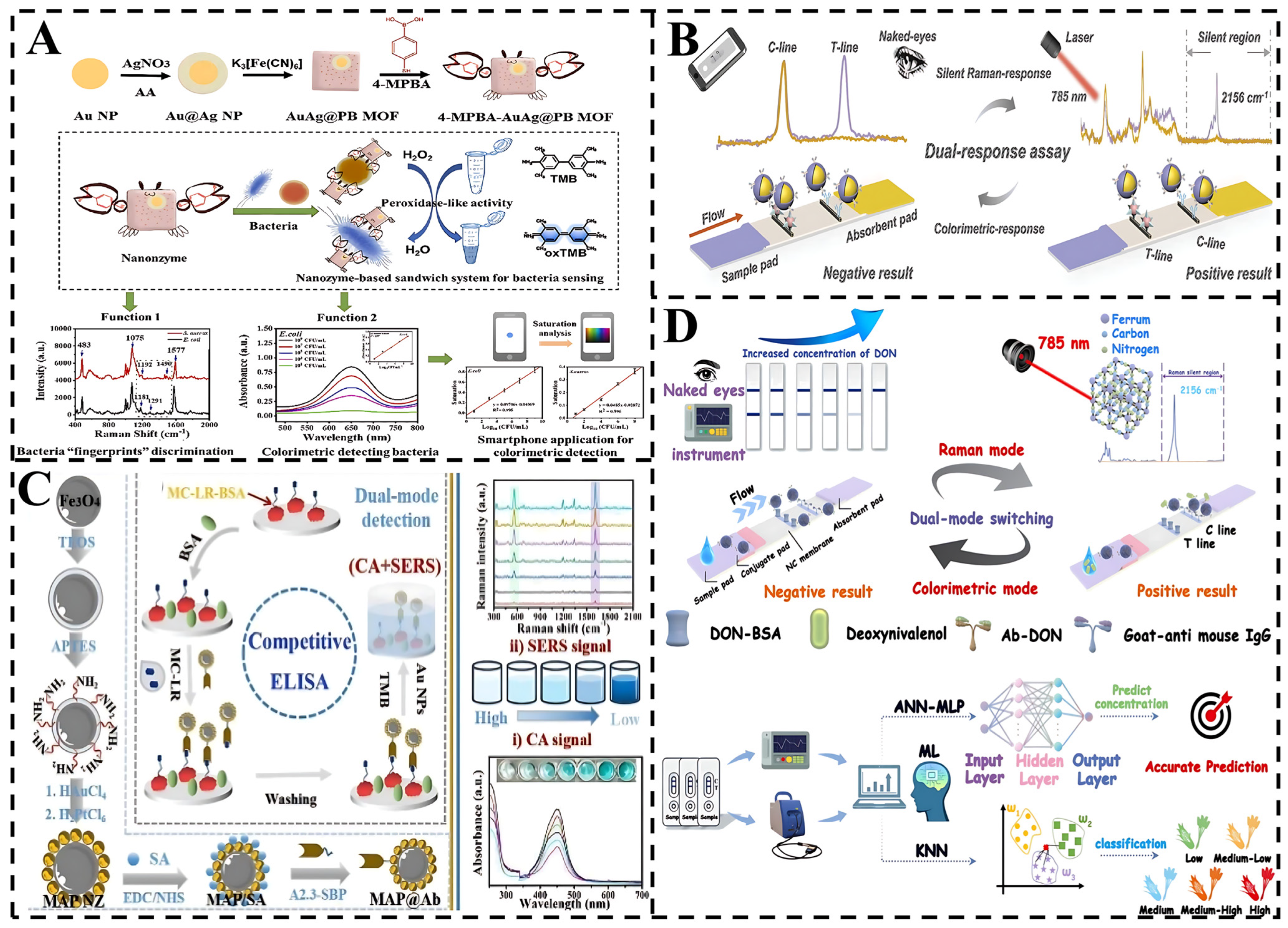
4.1. SERS-Colorimetric Sensors
4.2. SERS-Fluorescence Assay
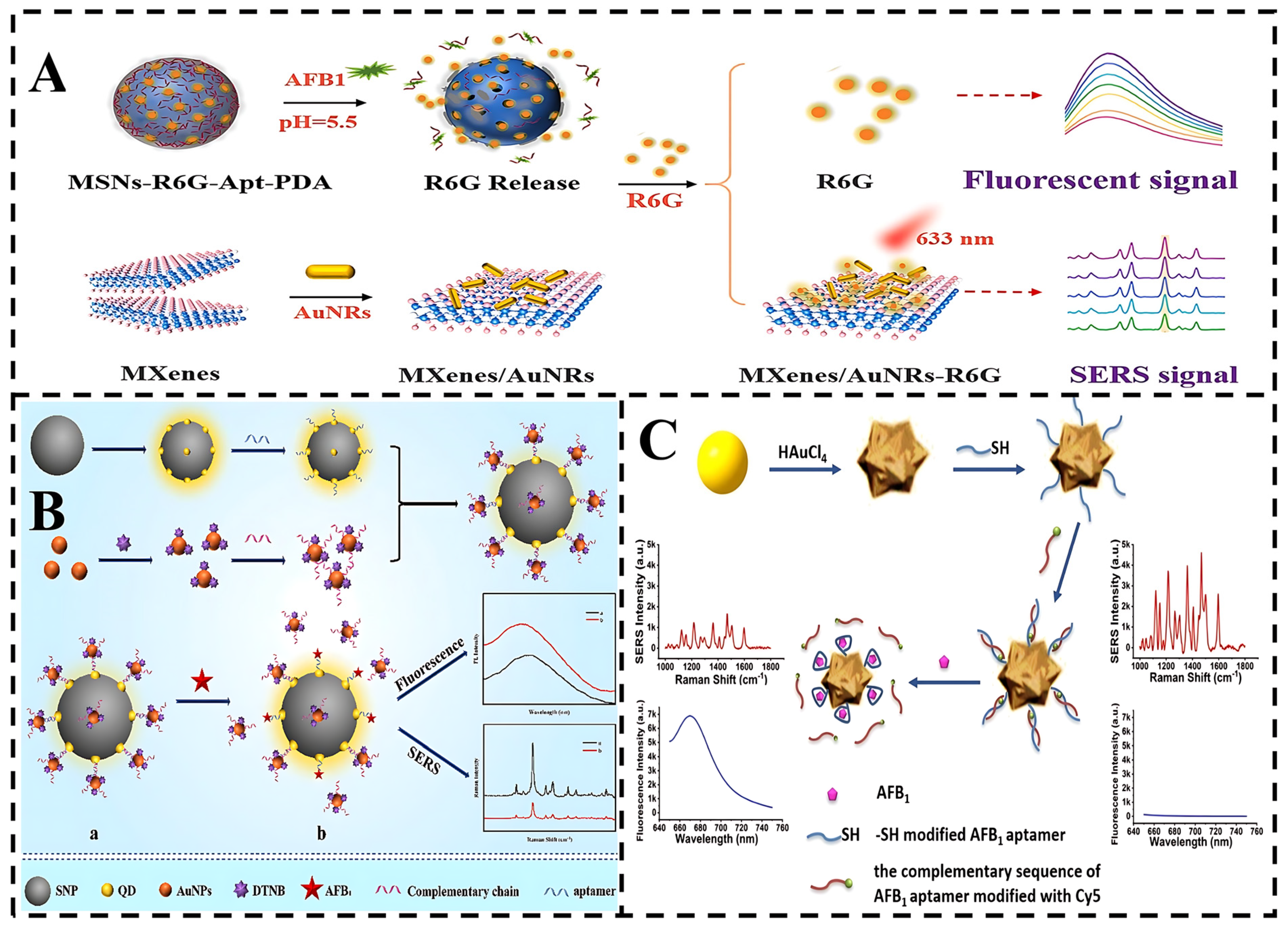
4.3. EC-SERS Detection
4.4. SERS-RRS Technique
4.5. Other SERS-Based Techniques
4.6. SERS Multimodal Sensing
5. Challenges and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, X.; Zuo, J.; Yang, C.; Jiang, J.; Zhang, Q.; Ping, J.; Li, P. Current trends in biosensors for biotoxins (mycotoxins, marine toxins, and bacterial food toxins): Principles, application, and perspective. TrAC Trends Anal. Chem. 2023, 165, 117144. [Google Scholar] [CrossRef]
- Bryła, M.; Pierzgalski, A.; Zapaśnik, A.; Uwineza, P.A.; Ksieniewicz-Woźniak, E.; Modrzewska, M.; Waśkiewicz, A. Recent research on Fusarium mycotoxins in maize—A review. Foods 2022, 11, 3465. [Google Scholar] [CrossRef]
- Peng, Z.; Chen, L.; Zhu, Y.; Huang, Y.; Hu, X.; Wu, Q.; Nüssler, A.K.; Liu, L.; Yang, W. Current major degradation methods for aflatoxins: A review. Trends Food Sci. Technol. 2018, 80, 155–166. [Google Scholar] [CrossRef]
- Rawson, A.M.; Dempster, A.W.; Humphreys, C.M.; Minton, N.P. Pathogenicity and virulence of Clostridium botulinum. Virulence 2023, 14, 2205251. [Google Scholar] [CrossRef] [PubMed]
- Morabito, S.; Silvestro, S.; Faggio, C. How the marine biotoxins affect human health. Nat. Prod. Res. 2018, 32, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, K.; Bodini, S.F.; Fabiani, L.; Micheli, L.; Porchetta, A.; Piermarini, S.; Volpe, G.; Pasquazzi, F.M.; Sanfilippo, L.; Moscetta, P. Re-modeling ELISA kits embedded in an automated system suitable for on-line detection of algal toxins in seawater. Sens. Actuators B Chem. 2019, 283, 865–872. [Google Scholar] [CrossRef]
- Chibudike, E.C.; Chibudike, H.O.; Odega, N.E.; Njokanma, E.E.; Adeyoju, O.A.; Ngige, C.O. Design of automated database system for storage and management of reports on mycotoxins contaminated agricultural products in Sub-Saharan Africa. World J. Adv. Res. Rev. 2022, 13, 763–770. [Google Scholar] [CrossRef]
- Brondz, I. Structure elucidation of a new toxin from the mushroom Cortinarius rubellus using gas chromatography-mass spectrometry (GC-MS). Int. J. Anal. Mass Spectrom. Chromatogr. 2013, 1, 109. [Google Scholar] [CrossRef]
- Quintanilla-Villanueva, G.E.; Sánchez-Álvarez, A.; Núñez-Salas, R.E.; Rodríguez-Delgado, M.M.; Luna-Moreno, D.; Villarreal-Chiu, J.F. Recent Advances in Monitoring Microbial Toxins in Food Samples by HPLC-Based Techniques: A Review. Analytica 2024, 5, 512–537. [Google Scholar] [CrossRef]
- Li, Y.; Luo, X.; Yang, S.; Cao, X.; Wang, Z.; Shi, W.; Zhang, S. High specific monoclonal antibody production and development of an ELISA method for monitoring T-2 toxin in rice. J. Agric. Food Chem. 2014, 62, 1492–1497. [Google Scholar] [CrossRef]
- GONG, A.-J. Research progress on ELISA and GICA for aflatoxin determination in Chinese materia medica. Chin. Tradit. Herb. Drugs 2018, 24, 2195–2202. [Google Scholar]
- Lu, Y.; Shi, Z.; Liu, Q. Smartphone-based biosensors for portable food evaluation. Curr. Opin. Food Sci. 2019, 28, 74–81. [Google Scholar] [CrossRef]
- Kundu, M.; Krishnan, P.; Kotnala, R.; Sumana, G. Recent developments in biosensors to combat agricultural challenges and their future prospects. Trends Food Sci. Technol. 2019, 88, 157–178. [Google Scholar] [CrossRef]
- You, R.; Wang, H.; Wang, C.; Huang, J.; Zhu, H.; Liu, Y.; Zhang, J.-H.; Liu, J.; Yu, X.; Lu, Y. Bacterial cellulose loaded with silver nanoparticles as a flexible, stable and sensitive SERS-active substrate for detection of the shellfish toxin DTX-1. Food Chem. 2023, 427, 136692. [Google Scholar] [CrossRef]
- Rippa, M.; Sagnelli, D.; Vestri, A.; Marchesano, V.; Munari, B.; Carnicelli, D.; Varrone, E.; Brigotti, M.; Tozzoli, R.; Montalbano, M. Plasmonic metasurfaces for specific SERS detection of Shiga toxins. ACS Appl. Mater. Interfaces 2022, 14, 4969–4979. [Google Scholar] [CrossRef]
- Dou, L.; Luo, L.; Zhang, X.; Zhang, W.; Liu, L.; Yin, X.; Liu, S.; Sun, J.; Zhang, D.; Wang, J. Biomimetic cell model for fluorometric and smartphone colorimetric dual-signal readout detection of bacterial toxin. Sens. Actuators B Chem. 2020, 312, 127956. [Google Scholar] [CrossRef]
- Khosropour, H.; Keramat, M.; Laiwattanapaisal, W. A dual action electrochemical molecularly imprinted aptasensor for ultra-trace detection of carbendazim. Biosens. Bioelectron. 2024, 243, 115754. [Google Scholar] [CrossRef]
- Cao, L.; Ye, Q.; Ren, Y.; Gao, B.; Wu, Y.; Zhao, X.; Ling, N.; Chen, M.; Ye, Y.; Wu, Q. Nanomaterial-mediated self-calibrating biosensors for ultra-precise detection of food hazards: Recent advances and new horizons. Coord. Chem. Rev. 2025, 522, 216204. [Google Scholar] [CrossRef]
- Liu, B.; Zhuang, J.; Wei, G. Recent advances in the design of colorimetric sensors for environmental monitoring. Environ. Sci. Nano 2020, 7, 2195–2213. [Google Scholar] [CrossRef]
- Sahoo, H. Fluorescent labeling techniques in biomolecules: A flashback. RSC Adv. 2012, 2, 7017–7029. [Google Scholar] [CrossRef]
- Monzó, J.; Insua, I.; Fernandez-Trillo, F.; Rodriguez, P. Fundamentals, achievements and challenges in the electrochemical sensing of pathogens. Analyst 2015, 140, 7116–7128. [Google Scholar] [CrossRef] [PubMed]
- Welch, E.C.; Powell, J.M.; Clevinger, T.B.; Fairman, A.E.; Shukla, A. Advances in biosensors and diagnostic technologies using nanostructures and nanomaterials. Adv. Funct. Mater. 2021, 31, 2104126. [Google Scholar] [CrossRef]
- Cai, Y.Y.; Choi, Y.C.; Kagan, C.R. Chemical and Physical Properties of Photonic Noble-Metal Nanomaterials. Adv. Mater. 2023, 35, 2108104. [Google Scholar] [CrossRef] [PubMed]
- Joudeh, N.; Linke, D. Nanoparticle classification, physicochemical properties, characterization, and applications: A comprehensive review for biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef]
- Miller, A.C.; Singh, I.; Koehler, E.; Polgreen, P.M. A smartphone-driven thermometer application for real-time population-and individual-level influenza surveillance. Clin. Infect. Dis. 2018, 67, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Wang, X.; Yi, F.; Zhou, Y.S.; Wang, Z.L. A universal self-charging system driven by random biomechanical energy for sustainable operation of mobile electronics. Nat. Commun. 2015, 6, 8975. [Google Scholar] [CrossRef]
- Rong, D.; Wang, H.; Ying, Y.; Zhang, Z.; Zhang, Y. Peach variety detection using VIS-NIR spectroscopy and deep learning. Comput. Electron. Agric. 2020, 175, 105553. [Google Scholar] [CrossRef]
- Wei, Q.; Zhu, X.; Zhang, D.; Liu, H.; Sun, B. Innovative nanomaterials drive dual and multi-mode sensing strategies in food safety. Trends Food Sci. Technol. 2024, 151, 104636. [Google Scholar] [CrossRef]
- Chowdhury, P.; Lawrance, R.; Lu, Z.-Y.; Lin, H.-C.; Chan, Y.-H. Recent Progress in Dual/Multi-Modal Detection Modes for Improving Sensitivity and Specificity of Lateral Flow Immunoassays Applied for Point-of-Care Diagnostics. TrAC Trends Anal. Chem. 2024, 177, 117798. [Google Scholar] [CrossRef]
- Chen, M.; Qileng, A.; Liang, H.; Lei, H.; Liu, W.; Liu, Y. Advances in immunoassay-based strategies for mycotoxin detection in food: From single-mode immunosensors to dual-mode immunosensors. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1285–1311. [Google Scholar] [CrossRef]
- Zada, L.; Leslie, H.A.; Vethaak, A.D.; Tinnevelt, G.H.; Jansen, J.J.; de Boer, J.F.; Ariese, F. Fast microplastics identification with stimulated Raman scattering microscopy. J. Raman Spectrosc. 2018, 49, 1136–1144. [Google Scholar] [CrossRef]
- Conti, C.; Botteon, A.; Colombo, C.; Pinna, D.; Realini, M.; Matousek, P. Advances in Raman spectroscopy for the non-destructive subsurface analysis of artworks: Micro-SORS. J. Cult. Herit. 2020, 43, 319–328. [Google Scholar] [CrossRef]
- Pu, H.; Xiao, W.; Sun, D.-W. SERS-microfluidic systems: A potential platform for rapid analysis of food contaminants. Trends Food Sci. Technol. 2017, 70, 114–126. [Google Scholar] [CrossRef]
- Chen, X.; Xie, L.; He, Y.; Guan, T.; Zhou, X.; Wang, B.; Feng, G.; Yu, H.; Ji, Y. Fast and accurate decoding of Raman spectra-encoded suspension arrays using deep learning. Analyst 2019, 144, 4312–4319. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, J.; Lin, T.; Ying, Y. Food and agro-product quality evaluation based on spectroscopy and deep learning: A review. Trends Food Sci. Technol. 2021, 112, 431–441. [Google Scholar] [CrossRef]
- Jin, J.; Guo, Z.; Fan, D.; Zhao, B. Spotting the driving forces for SERS of two-dimensional nanomaterials. Mater. Horiz. 2023, 10, 1087–1104. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xu, J.; Shao, D.; Liu, Y.; Qu, X.; Hu, S.; Dong, B. SERS-Based Local Field Enhancement in Biosensing Applications. Molecules 2024, 30, 105. [Google Scholar] [CrossRef]
- Li, B.; Liu, S.; Huang, L.; Jin, M.; Wang, J. Nanohybrid SERS substrates intended for food supply chain safety. Coord. Chem. Rev. 2023, 494, 215349. [Google Scholar] [CrossRef]
- Yu, X.; Song, H.; Huang, J.; Chen, Y.; Dai, M.; Lin, X.; Xie, Z. An aptamer@ AuNP-modified POSS–polyethylenimine hybrid affinity monolith with a high aptamer coverage density for sensitive and selective recognition of ochratoxin A. J. Mater. Chem. B 2018, 6, 1965–1972. [Google Scholar] [CrossRef]
- Lie, J.; Huang, J.; You, R.; Lu, Y. Preparation and Application of Magnetic Molecularly Imprinted Plasmonic SERS Composite Nanoparticles. Crit. Rev. Anal. Chem. 2024, 54, 2940–2959. [Google Scholar] [CrossRef]
- Singh, H.; Bamrah, A.; Bhardwaj, S.K.; Deep, A.; Khatri, M.; Brown, R.J.; Bhardwaj, N.; Kim, K.-H. Recent advances in the application of noble metal nanoparticles in colorimetric sensors for lead ions. Environ. Sci. Nano 2021, 8, 863–889. [Google Scholar] [CrossRef]
- Mayer, K.M.; Hafner, J.H. Localized surface plasmon resonance sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L. Nanostructures for surface plasmons. Adv. Opt. Photonics 2012, 4, 157–321. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Yi, J.; Li, J.-F.; Ren, B.; Wu, D.-Y.; Panneerselvam, R.; Tian, Z.-Q. Nanostructure-based plasmon-enhanced Raman spectroscopy for surface analysis of materials. Nat. Rev. Mater. 2016, 1, 16021. [Google Scholar] [CrossRef]
- Ding, S.-Y.; You, E.-M.; Tian, Z.-Q.; Moskovits, M. Electromagnetic theories of surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 2017, 46, 4042–4076. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, Y.; Liu, Y.; Hu, X.; Chen, H. Current strategies of plasmonic nanoparticles assisted surface-enhanced Raman scattering toward biosensor studies. Biosens. Bioelectron. 2023, 228, 115231. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, Y.; Zeng, T.; Aleisa, R.; Qiu, Z.; Chen, Y.; Huang, J.; Wang, D.; Yan, Z.; Yin, Y. Anisotropic plasmonic nanostructures for colorimetric sensing. Nano Today 2020, 32, 100855. [Google Scholar] [CrossRef]
- Fraire, J.C.; Pérez, L.A.; Coronado, E.A. Cluster size effects in the surface-enhanced Raman scattering response of Ag and Au nanoparticle aggregates: Experimental and theoretical insight. J. Phys. Chem. C 2013, 117, 23090–23107. [Google Scholar] [CrossRef]
- Yu, W.; Lin, X.; Duan, N.; Wang, Z.; Wu, S. A fluorescence and surface-enhanced Raman scattering dual-mode aptasensor for sensitive detection of deoxynivalenol based on gold nanoclusters and silver nanoparticles modified metal-polydopamine framework. Anal. Chim. Acta 2023, 1244, 340846. [Google Scholar] [CrossRef]
- Song, Y.; Wang, L.; Zhao, J.; Li, H.; Yang, X.; Fu, S.; Qin, X.; Chen, Q.; Jiang, Y.; Man, C. A novel colorimetric sensor using aptamers to enhance peroxidase-like property of gold nanoclusters for detection of Escherichia coli O157: H7 in milk. Int. Dairy J. 2022, 128, 105318. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; Hu, X.; Shi, Y.; Xin, W.; Liang, N.; Shen, T.; Xiao, J.; Daglia, M.; Zou, X. Dual modes of fluorescence sensing and smartphone readout for sensitive and visual detection of mercury ions in Porphyra. Anal. Chim. Acta 2022, 1226, 340153. [Google Scholar] [CrossRef]
- Amendola, V.; Bakr, O.M.; Stellacci, F. A study of the surface plasmon resonance of silver nanoparticles by the discrete dipole approximation method: Effect of shape, size, structure, and assembly. Plasmonics 2010, 5, 85–97. [Google Scholar] [CrossRef]
- Dai, X.; Fu, W.; Chi, H.; Mesias, V.S.D.; Zhu, H.; Leung, C.W.; Liu, W.; Huang, J. Optical tweezers-controlled hotspot for sensitive and reproducible surface-enhanced Raman spectroscopy characterization of native protein structures. Nat. Commun. 2021, 12, 1292. [Google Scholar] [CrossRef]
- Yin, L.; Cai, J.; Jayan, H.; Yosri, N.; Majeed, U.; Guo, Z.; Zou, X. Assembly of stabilized dendritic magnetic ferric-silver nanocomposite with gold nanoparticles for sensitive detection of ochratoxin A using SERS aptasensor. Food Control 2024, 165, 110704. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to metal–organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Kuppler, R.J.; Timmons, D.J.; Fang, Q.-R.; Li, J.-R.; Makal, T.A.; Young, M.D.; Yuan, D.; Zhao, D.; Zhuang, W.; Zhou, H.-C. Potential applications of metal-organic frameworks. Coord. Chem. Rev. 2009, 253, 3042–3066. [Google Scholar] [CrossRef]
- Ren, K.; Duan, M.; Su, T.; Ying, D.; Wu, S.; Wang, Z.; Duan, N. A colorimetric and SERS dual-mode aptasensor for the detection of Shiga toxin type II based on Mn/Fe-MIL (53)@ AuNSs. Talanta 2024, 270, 125636. [Google Scholar] [CrossRef]
- Wang, Y.; He, D.; Du, Z.; Xu, E.; Jin, Z.; Wu, Z.; Cui, B. Ultrasensitive detection of staphylococcal enterotoxin B with an AuNPs@ MIL-101 nanohybrid-based dual-modal aptasensor. Food Anal. Methods 2022, 15, 1368–1376. [Google Scholar] [CrossRef]
- Ma, J.-X.; Li, J.; Chen, Y.-F.; Ning, R.; Ao, Y.-F.; Liu, J.-M.; Sun, J.; Wang, D.-X.; Wang, Q.-Q. Cage based crystalline covalent organic frameworks. J. Am. Chem. Soc. 2019, 141, 3843–3848. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.-Q.; Zhao, Y.-H.; Zhang, Q.-P.; Sun, Y.-L.; Yang, B.-B.; Bu, J.-H.; Zhang, C. Highly covalent molecular cage based porous organic polymer: Pore size control and pore property enhancement. RSC Adv. 2022, 12, 16486–16490. [Google Scholar] [CrossRef]
- Liang, A.; Zhi, S.; Liu, Q.; Li, C.; Jiang, Z. A new covalent organic framework of dicyandiamide-benzaldehyde nanocatalytic amplification SERS/RRS aptamer assay for ultratrace oxytetracycline with the nanogold indicator reaction of polyethylene glycol 600. Biosensors 2021, 11, 458. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yang, Y.; Zhang, C.-Y. Toward biocompatible semiconductor quantum dots: From biosynthesis and bioconjugation to biomedical application. Chem. Rev. 2015, 115, 11669–11717. [Google Scholar] [CrossRef]
- Du, X.; Liu, D.; An, K.; Jiang, S.; Wei, Z.; Wang, S.; Ip, W.F.; Pan, H. Advances in oxide semiconductors for surface enhanced Raman scattering. Appl. Mater. Today 2022, 29, 101563. [Google Scholar] [CrossRef]
- Xu, X.; Lin, J.; Guo, Y.; Wu, X.; Xu, Y.; Zhang, D.; Zhang, X.; Yujiao, X.; Wang, J.; Yao, C. TiO2-based Surface-Enhanced Raman Scattering bio-probe for efficient circulating tumor cell detection on microfilter. Biosens. Bioelectron. 2022, 210, 114305. [Google Scholar] [CrossRef] [PubMed]
- Balaji, R.; Maheshwaran, S.; Chen, S.-M.; Tamilalagan, E.; Chandrasekar, N.; Ethiraj, S.; Samuel, M.S. Fabricating BiOI nanostructures armed catalytic strips for selective electrochemical and SERS detection of pesticide in polluted water. Environ. Pollut. 2022, 296, 118754. [Google Scholar] [CrossRef]
- Lv, Q.; Lv, R. Two-dimensional heterostructures based on graphene and transition metal dichalcogenides: Synthesis, transfer and applications. Carbon 2019, 145, 240–250. [Google Scholar] [CrossRef]
- Gogotsi, Y. The future of MXenes. Chem. Mater. 2023, 35, 8767–8770. [Google Scholar] [CrossRef]
- Cai, X.; Luo, Y.; Liu, B.; Cheng, H.-M. Preparation of 2D material dispersions and their applications. Chem. Soc. Rev. 2018, 47, 6224–6266. [Google Scholar] [CrossRef]
- Yang, X.; Peng, J.; Zhao, L.; Zhang, H.; Li, J.; Yu, P.; Fan, Y.; Wang, J.; Liu, H.; Dou, S. Insights on advanced g-C3N4 in energy storage: Applications, challenges, and future. Carbon Energy 2024, 6, e490. [Google Scholar] [CrossRef]
- Yao, J.; Jin, Z.; Zhao, Y. Electroactive and SERS-active Ag@ Cu2O NP-programed aptasensor for dual-mode detection of tetrodotoxin. ACS Appl. Mater. Interfaces 2023, 15, 10240–10249. [Google Scholar] [CrossRef]
- Zhang, D.; Pu, H.; Huang, L.; Sun, D.-W. Advances in flexible surface-enhanced Raman scattering (SERS) substrates for nondestructive food detection: Fundamentals and recent applications. Trends Food Sci. Technol. 2021, 109, 690–701. [Google Scholar] [CrossRef]
- Yoo, S.C.; Lee, D.; Ryu, S.W.; Kang, B.; Ryu, H.J.; Hong, S.H. Recent progress in low-dimensional nanomaterials filled multifunctional metal matrix nanocomposites. Prog. Mater. Sci. 2023, 132, 101034. [Google Scholar] [CrossRef]
- Han, Q.; Wang, H.; Wang, J. Multi-mode/signal biosensors: Electrochemical integrated sensing techniques. Adv. Funct. Mater. 2024, 34, 2403122. [Google Scholar] [CrossRef]
- Pardehkhorram, R.; Alshawawreh, F.A.; Gonçales, V.R.; Lee, N.A.; Tilley, R.D.; Gooding, J.J. Functionalized gold nanorod probes: A sophisticated design of sers immunoassay for biodetection in complex media. Anal. Chem. 2021, 93, 12954–12965. [Google Scholar] [CrossRef]
- Tan, R.; Zeng, M.; Huang, Q.; Zhou, N.; Deng, M.; Li, Y.; Luo, X. Dual-mode SERS/colorimetric sensing of nitrite in meat products based on multifunctional au NPs@ COF composite. Food Chem. 2024, 457, 140166. [Google Scholar] [CrossRef]
- Tan, E.X.; Zhong, Q.-Z.; Ting Chen, J.R.; Leong, Y.X.; Leon, G.K.; Tran, C.T.; Phang, I.Y.; Ling, X.Y. Surface-Enhanced Raman Scattering-Based Multimodal Techniques: Advances and Perspectives. ACS Nano 2024, 18, 32315–32334. [Google Scholar] [CrossRef]
- Cai, J.; Lin, Y.; Yu, X.; Yang, Y.; Hu, Y.; Gao, L.; Xiao, H.; Du, J.; Wang, H.; Zhong, X. Multifunctional AuAg-doping prussian blue-based MOF: Enhanced colorimetric catalytic activities and amplified SERS signals for bacteria discrimination and detection. Sens. Actuators B Chem. 2023, 394, 134279. [Google Scholar] [CrossRef]
- Liu, S.; Luo, X.; Shu, R.; Liao, Y.; Dou, L.; Bu, T.; Wang, S.; Li, Y.; Sun, J.; Zhang, D. Engineered Core–Shell Multifunctional Nano-Tracer in Raman-Silent Region with Highly Retained Affinity to Enhance Lateral Flow Immunoassays. Small 2022, 18, 2204859. [Google Scholar] [CrossRef]
- Wu, L.; Jiao, L.; Xue, D.; Li, Y.; Han, Y.; Ouyang, W.; Chen, Q. Nanozyme and bifunctional nanobody-based colorimetric-SERS dual-mode Immunosensor for microcystin-LR detection. Food Chem. 2025, 464, 141574. [Google Scholar] [CrossRef]
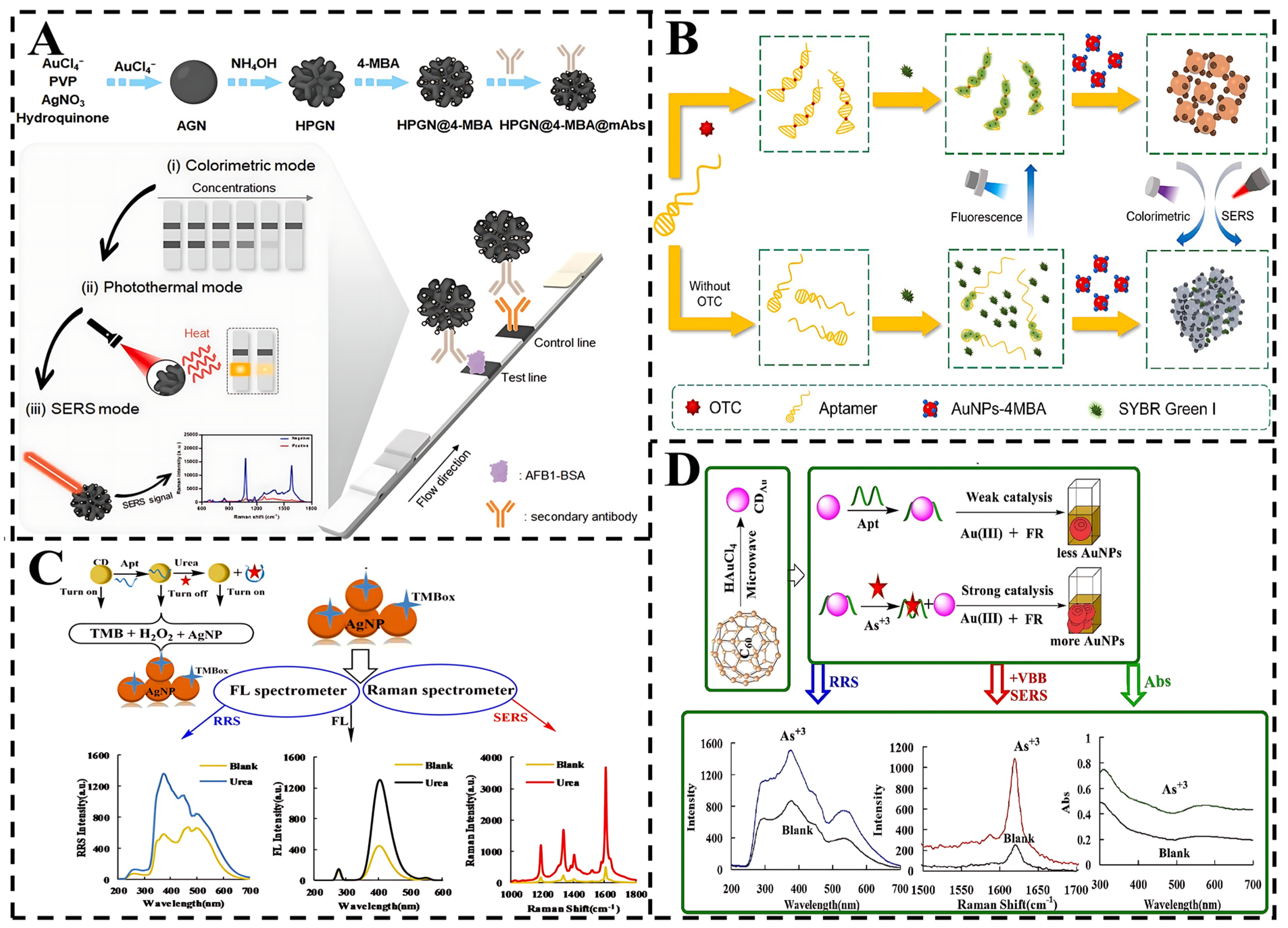
- Sun, B.; Wu, H.; Fang, T.; Wang, Z.; Xu, K.; Yan, H.; Cao, J.; Wang, Y.; Wang, L. Dual-Mode Colorimetric/SERS Lateral Flow Immunoassay with Machine Learning-Driven Optimization for Ultrasensitive Mycotoxin Detection. Anal. Chem. 2025, 97, 4824–4831. [Google Scholar] [CrossRef]
- Tian, Z.; Li, A.D. Photoswitching-enabled novel optical imaging: Innovative solutions for real-world challenges in fluorescence detections. Acc. Chem. Res. 2013, 46, 269–279. [Google Scholar] [CrossRef]
- Itoh, T.; Procházka, M.; Dong, Z.-C.; Ji, W.; Yamamoto, Y.S.; Zhang, Y.; Ozaki, Y. Toward a new era of SERS and TERS at the nanometer scale: From fundamentals to innovative applications. Chem. Rev. 2023, 123, 1552–1634. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, D.-W.; Pu, H.; Wei, Q. A dual signal-on biosensor based on dual-gated locked mesoporous silica nanoparticles for the detection of Aflatoxin B1. Talanta 2023, 253, 124027. [Google Scholar] [CrossRef]
- Wei, J.; He, Y.; Song, Z.; Khan, I.M.; Wang, Z.; Jiang, C.; Ma, X. Satellite nanostructures composed of CdTe quantum dots and DTNB-labeled AuNPs used for SERS-fluorescence dual-signal detection of AFB1. Food Control 2024, 156, 110112. [Google Scholar] [CrossRef]
- Yang, J.; Peng, Y.; Li, S.; Mu, J.; Huang, Z.; Ma, J.; Shi, Z.; Jia, Q. Metal nanocluster-based hybrid nanomaterials: Fabrication and application. Coord. Chem. Rev. 2022, 456, 214391. [Google Scholar] [CrossRef]
- Song, Z.; Wang, X.; Chen, P.; Wang, Z.; Ma, X. A gold nanoflower based dual mode aptasensor for aflatoxin B1 detection using SERS and fluorescence effect simultaneously. Spectrochim. Acta Part A 2023, 301, 122963. [Google Scholar] [CrossRef] [PubMed]
- Markin, A.V.; Arzhanukhina, A.I.; Markina, N.E.; Goryacheva, I.Y. Analytical performance of electrochemical surface-enhanced Raman spectroscopy: A critical review. TrAC Trends Anal. Chem. 2022, 157, 116776. [Google Scholar] [CrossRef]
- Juang, R.-S.; Wang, K.-S.; Chen, Y.-C.; Chu, Y.-J.; Lin, Y.-J.; Liu, S.-H.; Lin, D.-Z.; Liu, T.-Y. Electrochemically deposited Au nano-island on laser-scribed graphene substrates as EC-SERS biochips for uremic toxins detection. J. Taiwan Inst. Chem. Eng. 2024, 163, 105657. [Google Scholar] [CrossRef]
- Dai, Q.; Huang, J.; Qiu, X.; Chen, X.; Li, Y. SERS/EC dual-mode sensing system for the assay of SARS-CoV-2 RNA by using single gold nanowires as a platform. ACS Appl. Nano Mater. 2024, 7, 6369–6379. [Google Scholar] [CrossRef]
- Lv, X.; Liao, L.; Chen, S.; Xiao, Y.; Jiang, Z.; Wen, G. A cholesterol benzoate RRS probe for the determination of trace ammonium ions. Spectrochim. Acta Part A 2022, 272, 120945. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Shi, J.; Wen, G.; Liang, A.; Jiang, Z. A novel aptamer RRS assay platform for ultratrace melamine based on COF-loaded Pd nanocluster catalytic amplification. J. Hazard. Mater. 2022, 423, 127263. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Abtahi, S.M.H.; Vikesland, P.J. Plasmonic colorimetric and SERS sensors for environmental analysis. Environ. Sci. Nano 2015, 2, 120–135. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, Z.; Liang, A.; Wen, G.; Jiang, Z. A new N/Fe doped carbon dot nanosurface molecularly imprinted polymethacrylate nanoprobe for trace fipronil with SERS/RRS dimode technique. Talanta 2024, 269, 125417. [Google Scholar] [CrossRef]
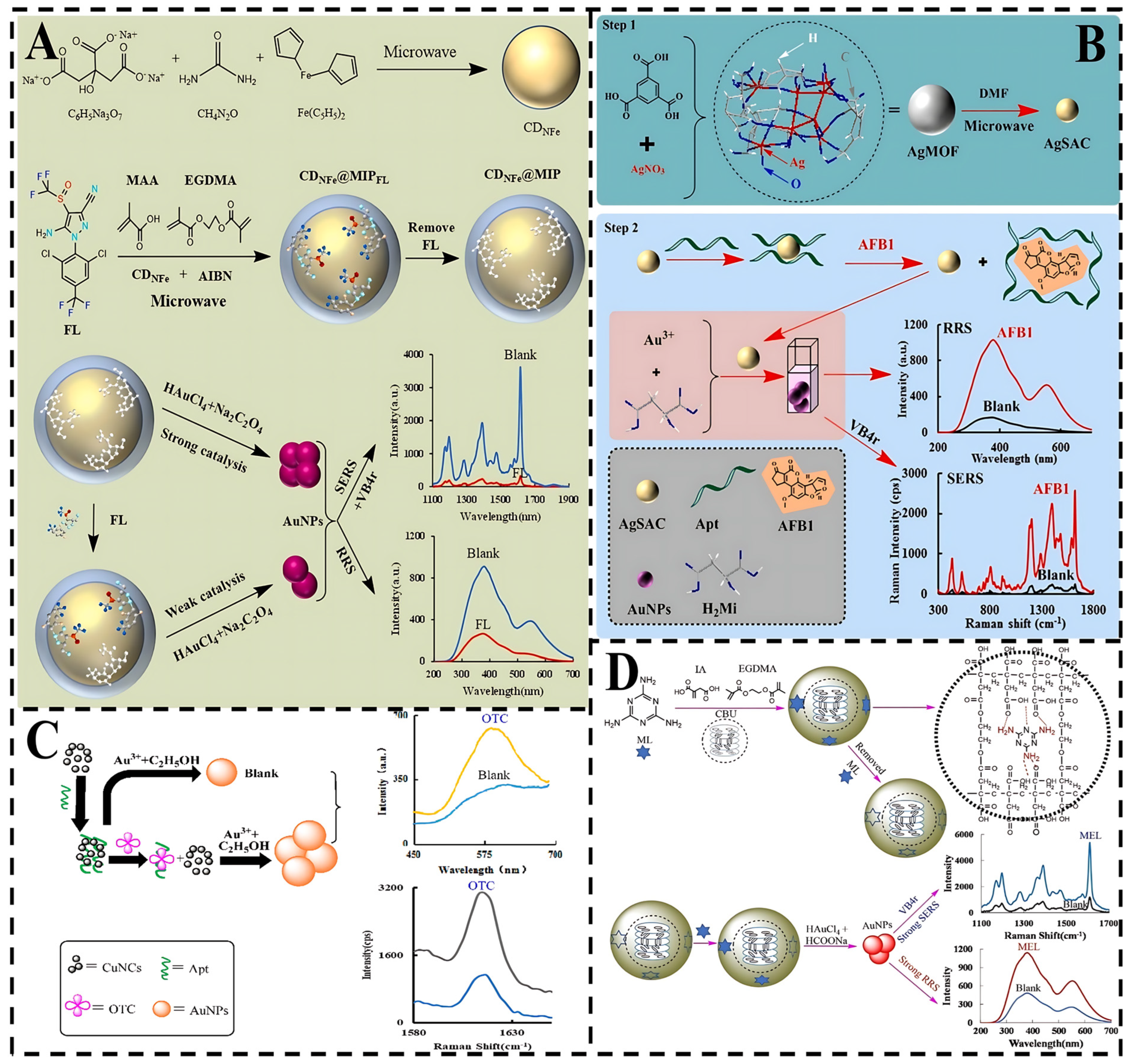
- Lv, X.; Liu, Y.; Qin, Z.; Jiang, Z.; Wen, G. A novel highly active AgMOF-based silver single-atom catalyst and its application to the aptamer SERS/RRS for the determination of aflatoxin B1. Talanta 2024, 269, 125419. [Google Scholar] [CrossRef]
- Chen, S.; Lv, X.; Shen, J.; Pan, S.; Jiang, Z.; Xiao, Y.; Wen, G. Sensitive aptamer SERS and RRS assays for trace oxytetracycline based on the catalytic amplification of CuNCs. Nanomaterials 2021, 11, 2501. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Liang, A.; Wen, G.; Jiang, Z. A new difunctional liquid crystal nanosurface molecularly imprinted polyitaconic acid nanoprobe for SERS/RRS determination of ultratrace melamine. Food Chem. 2024, 436, 137716. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Lu, L.; Rong, Z.; Wang, C.; Peng, Y.; Wang, F.; Wang, J.; Sun, M.; Dong, J.; Wang, D. Portable and multiplexed lateral flow immunoassay reader based on SERS for highly sensitive point-of-care testing. Biosens. Bioelectron. 2020, 168, 112524. [Google Scholar] [CrossRef]
- Meyer, S.A.; Le Ru, E.C.; Etchegoin, P.G. Combining surface plasmon resonance (SPR) spectroscopy with surface-enhanced Raman scattering (SERS). Anal. Chem. 2011, 83, 2337–2344. [Google Scholar] [CrossRef]
- Tian, Y.; Yin, X.; Li, J.; Dou, L.; Wang, S.; Jia, C.; Li, Y.; Chen, Y.; Yan, S.; Wang, J. A dual-mode lateral flow immunoassay by ultrahigh signal-to background ratio SERS probes for nitrofurazone metabolites ultrasensitive detection. Food Chem. 2024, 441, 138374. [Google Scholar] [CrossRef]
- Cao, H.; Liang, D.; Tang, K.; Sun, Y.; Xu, Y.; Miao, M.; Zhao, Y. SERS and MRS signals engineered dual-mode aptasensor for simultaneous distinguishment of aflatoxin subtypes. J. Hazard. Mater. 2024, 462, 132810. [Google Scholar] [CrossRef]
- Tian, M.; Wang, J.; Li, C.; Wang, Z.; Liu, G.; Lv, E.; Zhao, X.; Li, Z.; Cao, D.; Liu, H. Qualitative and quantitative detection of microcystin-LR based on SERS-FET dual-mode biosensor. Biosens. Bioelectron. 2022, 212, 114434. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Liang, R. Integrating artificial intelligence with smartphone-based imaging for cancer detection in vivo. Biosens. Bioelectron. 2025, 271, 116982. [Google Scholar] [CrossRef]
- Xie, G.; Liu, L.; Gong, Y.; Zhang, G.; Huang, J.; Xu, H.; Wang, J. Development of tri-mode lateral flow immunoassay based on tailored porous gold nanoflower for sensitive detection of aflatoxin B1. Food Biosci. 2024, 61, 104700. [Google Scholar] [CrossRef]
- Shao, Y.; Qi, X.; Wang, H.; Tang, B.; Cheng, Y.; Zhang, Z.; Zhang, X.; Zhu, M. Aptamer-based tri-mode sensing for detecting oxytetracycline mediated by SYBR Green I and functionalized Au nanoparticles. Biosens. Bioelectron. 2025, 270, 116930. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, J.; Liang, A.; Wen, G.; Jiang, Z. Aptamer turn-on SERS/RRS/fluorescence tri-mode platform for ultra-trace urea determination using Fe/N-doped carbon dots. Front. Chem. 2021, 9, 613083. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Z.; Chen, C.; Liang, A.; Jiang, Z. Fullerene carbon dot catalytic amplification-aptamer assay platform for ultratrace As+3 utilizing SERS/RRS/Abs trifunctional Au nanoprobes. J. Hazard. Mater. 2021, 403, 123633. [Google Scholar] [CrossRef]
- Li, Q.; Han, Q.; Yang, D.; Li, K.; Wang, Y.; Chen, D.; Yang, Y.; Li, H. Methylmercury-sensitized “turn on” SERS-active peroxidase-like activity of carbon dots/Au NPs nanozyme for selective detection of ochratoxin A in coffee. Food Chem. 2024, 434, 137440. [Google Scholar] [CrossRef]
- He, H.; Yang, Z.; Peng, L.; Jiang, F.; Xu, Y.; Yang, Y.; Wang, Y.; Chen, Q.; Chen, Q.; Jiao, T. Colorimetric/SERS dual-mode detection for chloramphenicol through MIL-101/Au ETHH NR based catalysis and structural degradation. Food Biosci. 2025, 65, 106032. [Google Scholar] [CrossRef]
- Zhang, R.; Xie, S.; Yang, X.; Tang, Y.; Liu, J.; Liao, M.; Wang, M.; He, Y. A SERS and colorimetric dual-mode biosensor based on stimulus-responsive DNA/MOF-bound bimetallic nanozyme for the ultrasensitive detection of chloramphenicol in food. Microchim. Acta 2025, 192, 62. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, R.; Lai, H.; Lai, L.; Liu, Y.; Fan, Y.; Zhang, Y.; Shi, J.; Yang, M. A MXene-Au nanosheets-based fluorescent-SERS dual-mode biosensor integrated with CRISPR/Cas12a system for endotoxin detection. Sens. Actuators B Chem. 2025, 427, 137120. [Google Scholar] [CrossRef]
- Gao, X.; Liu, Y.; Wei, J.; Wang, Z.; Ma, X. A facile dual-mode SERS/fluorescence aptasensor for AFB1 detection based on gold nanoparticles and magnetic nanoparticles. Spectrochim. Acta Part A 2024, 315, 124268. [Google Scholar] [CrossRef]
- Ma, X.; Wei, J.; Jiang, C.; Wang, Z. A SERS and Fluorescence Dual-Signal Aptasensor for AFB1 Detection Based on DTNB Labeled AuNPs and CdTe Quantum Dots. Food Anal. Methods 2024, 18, 293–304. [Google Scholar] [CrossRef]
- Khan, I.M.; Niazi, S.; Pasha, I.; Khan, M.K.I.; Yue, L.; Ye, H.; Mohsin, A.; Shoaib, M.; Zhang, Y.; Wang, Z. Novel metal enhanced dual-mode fluorometric and SERS aptasensor incorporating a heterostructure nanoassembly for ultrasensitive T-2 toxin detection. J. Mater. Chem. B 2023, 11, 441–451. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Sun, D.-W.; Pu, H.; Wu, Z. A SERS-Fluorescence dual-signal aptasensor for sensitive and robust determination of AFB1 in nut samples based on Apt-Cy5 and MNP@ Ag-PEI. Talanta 2023, 253, 123962. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, B.; Ye, Y.; Qi, X.; Zhang, Y.; Xia, X.; Wang, X.; Zhou, N. A fluorescence and surface-enhanced Raman scattering dual-mode aptasensor for rapid and sensitive detection of ochratoxin A. Biosens. Bioelectron. 2022, 207, 114164. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y.; Wang, X.; Zhou, X.; Qiu, Y.; Qin, W.; ShenTu, X.; Wang, S.; Yu, X.; Ye, Z. Dosage-sensitive and simultaneous detection of multiple small-molecule pollutants in environmental water and agriproducts using portable SERS-based lateral flow immunosensor. Sci. Total Environ. 2024, 912, 169440. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, S.; Meng, S.; Gong, Q.; Li, W.; Liu, D.; Yang, X.; You, T. In-situ photoelectrochemical surface-enhanced Raman scattering based on Au@ Ag/WO3 hollow microsphere for dual-mode sensing of microcystin-LR. Sens. Actuators B Chem. 2025, 422, 136707. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, M.; Xu, D.; Jiang, T.; Zhang, J. Quantitative detection of microcystin-LR in Bellamya aeruginosa by thin-layer chromatography coupled with surface-enhanced Raman spectroscopy based on in-situ ZIF-67/Ag NPs/Au NWs composite substrate. Food Chem. 2024, 452, 139481. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, D.-W.; Pu, H. CRISPR/Cas12a and G-quadruplex DNAzyme-driven multimodal biosensor for visual detection of Aflatoxin B1. Spectrochim. Acta Part A 2023, 302, 123121. [Google Scholar] [CrossRef]
- Wu, Z.; He, D.; Cui, B.; Jin, Z.; Xu, E. Triple-mode aptasensor for sensitive and reliable determination of staphylococcal enterotoxin B. Food Anal. Methods 2020, 13, 1255–1261. [Google Scholar] [CrossRef]


| Material | Target | Mode | Linear Range | LOD | Detection Time (T)/ Reproducibility (RSD or CV)/ Stability (S) | Real Samples | Recovery (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| Colorimetric-SERS | ||||||||
| Au@HgNPs | OTA | Colorimetry | 0.5–10.25 μg/L | 0.29 µg/L | / | coffee | 95.9–104.0 | [107] |
| SERS | 0.25–18.75 μg/L | 0.16 µg/L | / | 96.7–108.9 | ||||
| MIL-101/Au ETHH NRs | CAP | Colorimetry | 32.3–3231.3 μg/L | 0.48 μg/L | / | fish | 95.64–110.62 | [108] |
| SERS | 0.03–323.1 μg/L | 3.23 ng/L | RSD: 6.7% | 98.5–102.2 | ||||
| Fe3O4@MOF-GNS@CP | CAP | Colorimetry | 3.23–80,782.5 μg/L | 6.69 μg/L | / | milk, honey, mineral | 95.7–103.7 | [109] |
| SERS | 0.32–323.13 μg/L | 0.25 μg/L | RSD: 4.23% | 91.4–103.6 | ||||
| Fluorescence-SERS | ||||||||
| MXene-Au | Endotoxins | Fluorescence | 0.1–2500 ng/mL | 0.26 ng/mL | T: 30 min RSD: 1.6% | Water, milk | 93.5–105.5 | [110] |
| SERS | 0.01–2500 ng/mL | 0.04 ng/mL | T: 30 min RSD: 4.8% | 104.0–112.1 | ||||
| AuNPs-4MBA/cDNA@MNPs-Cy5/AFB1 | AFB1 | Fluorescence | 0.10–100 ng/mL | 5.81 pg/mL | / | peanut | 91.4–92.9 | [111] |
| SERS | 0.10–100 ng/mL | 0.01 ng/mL | / | 91.7–95.6 | ||||
| DTNB-AuNPs, QD@SiO2 | AFB1 | Fluorescence | 0.0001–100 ng/mL | 0.100 pg/mL | / | peanuts, peanut oil | 95.22–105.80 | [112] |
| SERS | 0.0001–1000 ng/mL | 0.087 pg/mL | / | 96.30–101.36 | ||||
| AgNPs/MPDA-apt-TAMRA | DON | Fluorescence | 0.1–100 ng/mL | 0.08 ng/mL | RSD: 2.59% | wheat flour | 98.00–104.1 | [49] |
| SERS | 0.1–100 ng/mL | 0.06 ng/mL | RSD: 1.37% | 99.88–105.4 | ||||
| AuNPs@PVP@RITC@SiO2NPs/ rGO-AuNS | T-2 toxin | Fluorescence | 0.001–500 ng/mL | 0.85 pg/mL | T: 25 min | corn, wheat | 88.03–100.3 | [113] |
| SERS | 0.001–500 ng/mL | 0.12 pg/mL | T: 25 min | 88.4–102.9 | ||||
| MNP@Ag-PEI | AFB1 | Fluorescence | 0.2–20,000 ng/mL | 0.135 ng/mL | / | peanut, walnut, almond | 94.7–109.7 | [114] |
| SERS | 0.001–1000 ng/mL | 0.45 pg/mL | RSD: 3.60% S: 60 days | 95.2–108.6 | ||||
| cDNA-AuNPs, Apt-AuNSs | OTA | Fluorescence | 1–100 ng/mL | 0.17 ng/mL | / | coffee, wine | 99.14–100.96 | [115] |
| SERS | 5–250 pg/mL | 1.03 pg/mL | / | 99.85–118.08 | ||||
| LFIA-SERS | ||||||||
| Au4-MBA@AgNPs | IMI, PYR, AFB1 | SERS | 0.025–1, 1–100, 0.025–0.25 ng/mL | 8.6, 97.4, 8.9 pg/mL | T: 8 min RSD: 4.83%, 5.32%, 6.15% S: 28 days | Pu’er tea, black tea, surface water | 91.40–112.50, 86.16–113.08, 90.40–115.00 | [116] |
| PEC-SERS | ||||||||
| Au@Ag/H-WO3 | MC-LR | SERS | 1–100 ng/mL | 0.13 ng/mL | RSD: 3.5% S: 7 days | lake water | 90.0–96.0 | [117] |
| PEC | 0.3–50 ng/mL | 0.06 ng/mL | RSD: 2.3 % S: 7 days | 97.0–98.0 | ||||
| TLC-SERS | ||||||||
| ZIF-67/Ag NPs/Au NWs | MC-LR | SERS | 4.976–497.6 ng/mL | 2.259104 ng/mL | RSD: 10.4% | bellamya aeruginosa | 93.28–101.66 | [118] |
| Colourimetric/SERS/Fluorescent | ||||||||
| CRISPR/Cas12a, G4-DNAzyme | AFB1 | Colourimetric | 0.001–0.1 ng/mL | 0.85 pg/mL | peanut, maize, badam | 83.1–108.3 | [119] | |
| SERS | 0.001–0.1 ng/mL | 0.79 pg/mL | RSD: 7.52% S: 7 days | 85.9–106.8 | ||||
| Fluorescent | 0.001–0.1 ng/mL | 1.65 pg/mL | 84.0–108.5 | |||||
| SERS/Fluorescence/CD | ||||||||
| UCNPs | SEB | SERS | 1–750 pg/mL | 0.1 pg/mL | / | milk | 80.93–105.45 | [120] |
| Fluorescent | 1–750 pg/mL | 0.1 pg/mL | / | 85.27–106.26 | ||||
| CD | 2–500 pg/mL | 0.3 pg/mL | / | 89.21–108.33 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; Zhang, S.; Li, B.; Wu, L. Advances in Multifunctional Nanoagents and SERS-Based Multimodal Sensing for Biotoxin in Foods. Foods 2025, 14, 1393. https://doi.org/10.3390/foods14081393
Jiang H, Zhang S, Li B, Wu L. Advances in Multifunctional Nanoagents and SERS-Based Multimodal Sensing for Biotoxin in Foods. Foods. 2025; 14(8):1393. https://doi.org/10.3390/foods14081393
Chicago/Turabian StyleJiang, Huan, Sihang Zhang, Bei Li, and Long Wu. 2025. "Advances in Multifunctional Nanoagents and SERS-Based Multimodal Sensing for Biotoxin in Foods" Foods 14, no. 8: 1393. https://doi.org/10.3390/foods14081393
APA StyleJiang, H., Zhang, S., Li, B., & Wu, L. (2025). Advances in Multifunctional Nanoagents and SERS-Based Multimodal Sensing for Biotoxin in Foods. Foods, 14(8), 1393. https://doi.org/10.3390/foods14081393







