Composition and Biological Activity of Colored Rice—A Comprehensive Review
Abstract
1. Introduction
2. Nutritional Composition of Colored Rice
2.1. Carbohydrates, Protein, and Fat
| Nutrient | Purple-Black Rice | White Rice | Red-Brown Rice | References | |
|---|---|---|---|---|---|
| Brown Rice | Red Rice | ||||
| Energy/kcal | 341 | 345–349 | 367 | 408.6 | https://www.boohee.com https://www.foodwake.cn |
| Protein/g | 9.4 | 7.7–7.9 | 7.54 | 9.1 | |
| Carbohydrate/g | 72.2 | 77.4–78.3 | 76.25 | 87.2 | |
| Fat/g | 2.5 | 0.6 | 3.2 | 2.6 | |
| Dietary fiber/g | 3.9 | 0.6–0.8 | 3.6 | 4.4 | |
| Carbohydrate Profile | |||||
| Amylose (%) | 1.9–13.8 | 14.3 | 2.8–25 | [75,76,77] | |
| Starch (%) | 73.5–79.6 | 78.7 | 74.7–76.7 | ||
| Amylopectin | 89.87 | 70–100 | 75 | ||
| Protein Profile | |||||
| Albumin (Alb) g/100 g | 1.208 | 0.67–2.0 | 0.9–2.3 | [78,79,80] | |
| Globulin (Glo) g/100 g | 0.327 | 0.652–2.0 | 0.67–2.3 | ||
| Prolamin (Pro) g/100 g | 0.119 | 0.20–2.3 | 0.28–2.73 | ||
| Glutelin (Glu) g/100 g | 7.929 | 1.684–5.258 | 2.0–6.18 | ||
| Amino acid Profile (ng/mg) | |||||
| beta-Alanine | 611.72 | 1120.07 | 676.04 | https://www.foodwake.cn; [54] | |
| gamma-Aminobutyric acid | 4924.82 | 24,773.64 | 56,672.55 | ||
| L-Alanine | 50,803.14 | 30,287.82 | 43,769.23 | ||
| L-Arginine | 39,126.49 | 22,084.74 | 15,158.64 | ||
| L-Asparagine | 149,503.2 | 85,589.82 | 61,915.03 | ||
| L-Aspartic acid | 122,281.8 | 167,794.52 | 80,174.63 | ||
| L-Glutamic acid | 227,473.6 | 112,713.66 | 53,711.18 | ||
| L-Glutamine | 23,746.48 | 98,415.37 | 86,887.77 | ||
| L-Histidine | 4766.91 | 8966.76 | 20,289.02 | ||
| L-Isoleucine | 18,844.8 | 31,887.29 | 30,977.34 | ||
| L-Leucine | 2147.2 | 5393.29 | 6076.29 | ||
| L-Lysine | 8572.17 | 11,177.98 | 16,866.32 | ||
| L-Methionine | 1963.14 | 967.09 | 832.6 | ||
| L-Ornithine | 1372.53 | 1024.82 | 938.33 | ||
| L-Phenylalanine | 11,641.85 | 12,563.72 | 10,775.23 | ||
| L-Proline | 17,804.19 | 10,371.11 | 20,058.6 | ||
| L-Serine | 27,620.62 | 42,558.51 | 33,615.54 | ||
| L-Threonine | 7630.53 | 15,770.47 | 19,250.02 | ||
| L-Tryptophan | 45,506.56 | 13,171.08 | 24,495.74 | ||
| L-Tyrosine | 1503.94 | 2772.80 | 5209.25 | ||
| L-Valine | 7372.66 | 14,789.27 | 18,103.1 | ||
| Taurine | 1208.35 | 800.74 | 1588.84 | ||
| Fatty Acids Profile (% of total fatty acids) | |||||
| Palmitic | 19 | 21 | 20.96 | [35,81,82] | |
| Palmitoleic | 0.21 | 0.27 | 0.18 | ||
| Stearic | 2.5 | 3.76 | 2.80 | ||
| Oleic | 40 | 39.19 | 38.60 | ||
| Linoleic | 36 | 33.99 | 35.88 | ||
| Arachidic | 0.6 | 0.76 | 0.54 | ||
| α-Linolenic | 1.50 | 1.18 | 1.35 | ||
| Behenic | 0.30 | 0.38 | 0.28 | ||
| Lignoceric | 0.60 | 0.79 | 0.55 | ||
| Total lipids (%) | 1.40 | 0.90 | 2.10 | ||
| Total γ-oryzanol µg/mg | 63 | 79.00 | 8.2 | ||
2.2. Minerals and Vitamins
| Minerals | ||||
|---|---|---|---|---|
| Nutrient/Vitamin | Black Rice | White Rice | Brown-Red Rice | References |
| Calcium/mg | 12 | 11–12 | 9 | https://www.foodwake.cn; [86,87,88,89] |
| Magnesium/mg | 147 | 28–34 | 116 | |
| Phosphorus/mg | 356 | 112–121 | 311 | |
| Zinc/mg | 3.80 | 1.45–1.47 | 2.13 | |
| Sodium/mg | 7.1 | 1.7 | 5 | |
| Potassium/mg | 256 | 97–109 | 250 | |
| Sulfur/mg | 177.62 | 66–37-81.7 | 64.1749 | |
| Chlorine/mg | 10.95 | 2.62–3.7 | 7.71 | |
| Iron | 1.6 | 1.1–1.6 | 1.29 | |
| Selenium/µg | 3.2 | 1.99–2.5 | 17.1 | |
| Copper/mg | 0.15 | 0.19–0.29 | 0.302 | |
| Manganese/mg | 1.72 | 1.27–1.36 | 2.853 | |
| Iodine/µg | NR | 2.8 | NR | |
| Vitamins | ||||
| Vitamin A | ND | ND | ND | https://www.foodwake.cn; [35,92,94,97,98,99] |
| Vitamin C | ND | ND | ND | |
| Vitamin D | ND | ND | ND | |
| Vitamin E | 43.92 | 38.55 | 29.77 | |
| α-tocopherol/mg/Kg | 3.32–16.47 1 35.40–62.10 2 | 2.56–4.90 1 23.27–54.56 2 | 2.82–14.10 1 27.66–103.73 2 | |
| β-tocopherol/mg/Kg | 0.35–1.09 1 2.43–2.68 2 | 0.27–0.41 1 0.83–2.24 2 | 0.34–0.89 1 0.92–3.26 2 | |
| γ-tocopherol/mg/Kg | 3.20–7.69 1 10.55–39.22 2 | 3.49–6.13 1 15.51–33.38 2 | 1.43–7.00 6.09–37.68 2 | |
| δ-tocopherol/mg/Kg | 0.28–0.66 1 1.10–3.27 2 | 0.20–0.43 1 1.34–1.67 2 | 0.18–0.54 1 0.26–3.48 2 | |
| α-tocotrienol/mg/Kg | 1.63–6.22 1 12.52–43.56 2 | 1.03–2.21 1 12.52–43.56 2 | 1.50–7.95 1 8.44–67.01 2 | |
| β-tocotrienol/mg/Kg | ND 1 0.48–0.73 2 | ND 1 0.06 2 | ND 1 0.25–5.74 2 | |
| γ-tocotrienol/mg/Kg | 11.52–27.35 1 97.79–130.64 2 | 11.29–23.67 1 75.98–120.26 2 | 15.34–31.14 1 68.52–151.09 2 | |
| δ-tocotrienol/mg/Kg | 0.65–1.90 1 1.68–2.16 2 | 0.52–0.68 1 1.45–1.78 2 | 0.44–1.51 1 1.34–3.54 2 | |
| Vitamin K/µg | NR | 0.1 | 0.6 | |
| Vitamin B1 (Thiamine)/mg | 0.67 | 0.65–1.76 | 0.29–1.38 | |
| Vitamin B2 (Riboflavin)/mg | 0.04 | 0.02 | 0.04–0.3 | |
| Vitamin B3 (Niacin)/mg | 0.19 | 0.05 | 3.5–5.3 | |
| Vitamin B6 (pyridoxine) | 0.105–0.129 | 0.112 | 0.28 | |
| Vitamin B7 (Biotin) | 0.024 | ND | ND | |
| Vitamin B9 (Folate) | 0.0391 | 0.016 | 0.0329 | |
3. Phytochemistry of Colored Rice
3.1. Flavonoids
| Phenolics | Flavonoids | Anthocyanin | Proanthocyanidin | Antioxidant Capacity | Cultivar | Country of Origin | Reference |
|---|---|---|---|---|---|---|---|
| Black-Purple Rice | |||||||
| 7.6 mg/g FW | 2.6 mg/g FW | ~31.0 µmol Trolox/g ABTS value | Yangzi 6 hao | China | [19] | ||
| ~7.6 Trolox/g DDPH value | |||||||
| ~5.8 Trolox/g FRAP value | |||||||
| 172 mg CE/100 g | 125.60–155.45 mg CE/100 g | 7.21 µm TE/g DPPH | Binhei and Heixiangdao | China | [121] | ||
| 4.83 µm TE/g ABTS | Binhei and Heixiangdao | China | [121] | ||||
| 95.33 µg/100 g | 28 mg RE/100 g DW | 51 mg CyGE/100 g | 61.00% | Riceberry | Thailand | [122] | |
| 78.72 µg/100 g | 29 mg RE/100 g DW | 62 mg CyGE/100 g | 63.00% | Hom Nil | Thailand | [122] | |
| 361–436 GAE mg/100 g | 359–405 QE mg/100 g | 189–239 C3G mg/100 g | 76–146 CAE mg/100 g | 35.14–41.25% Free Phenolics DPPH | Chakhao poireiton, Farmer collection | India | [120] |
| 65.92–75.14% Bound Phenolics DPPH | |||||||
| 3–8 µM TE/g Free Phenolics ABTS | |||||||
| 6–12 µM TE/g Bound Phenolics ABTS | |||||||
| 5267.20 µg/g | 3276–5045.6 µg/g | 32.10 µg/g | 22 TAC, μmol/g | Minenomurasaki | Japan | [28] | |
| Red Rice | |||||||
| 1.2 mg/g | 2.0 mg/g | 0.6 mg/g | 6 µmol Trolox/g ABTS value | Hongnuo | China | [19] | |
| 2.0 Trolox/g DDPH value | |||||||
| 1.3 Trolox/g FRAP value | |||||||
| 61–68.06 mg CE/100 g | 205.46–249.40 mg CE/100 g | 4.28–6.26 µm TE/g DPPH | Caofeihong and Yanzhidao | China | [121] | ||
| 4.70–5.27 µm TE/g ABTS | |||||||
| 50.42 µg/100 g | 20 mg RE/100 g DW | 22 mg CyGE/100 g | 60% | Mali Dang | Thailand | [122] | |
| 48.04 µg/100 g | 16 mg RE/100 g DW | 13 mg CyGE/100 g | 41% | Mun Poo | Thailand | [122] | |
| 93.5 | 3060.60 | 5 | Yuyakemochi | Japan | [28] | ||
| 215–439 GAE mg/100 g | 258–399 QE mg/100 g | 2.5–3.9 C3G mg/100 g | 77–204 CAE mg/100 g | 25.60–37.60% Free Phenolics DPPH | Ghaselu, Dumai, Kammra, Bakung, Maintinmolosty, Yangkum | India | [120] |
| 61.29–79.54% Bound Phenolics DPPH | |||||||
| 0.16–1.3 µM TE/g Free Phenolics ABTS | |||||||
| 2.4–3.1 µM TE/g Bound Phenolics ABTS | |||||||
| White Rice | |||||||
| 0.5 mg/g | 1.2 mg/g | <0.1 mg/g | 7 µmol Trolox/g ABTS value | Yangchannuo 1 hao | China | [19] | |
| 2.0 Trolox/g DDPH value | |||||||
| 1.3 Trolox/g FRAP value | |||||||
| 45.75 µg/100 g | 17 mg RE/100 g DW | ND | 39% | KDML105 | Thailand | [122] | |
| 59.58 µg/100 g | 10 mg RE/100 g DW | ND | 20% | Sung Yod | Thailand | [122] | |
| 59–80.39 mg CE/100 g | 29.20–40.72 mg CE/100 g | 1.95–2.85 µm TE/g DPPH | Nongken2021 and Zhongzao35 | China | [121] | ||
| 2.53–2.87 µm TE/g ABTS | Nongken2021 and Zhongzao35 | China | [121] | ||||
| 61–120 GAE mg/100 g | 89–133 QE mg/100 g | 0.4–2.98 C3G mg/100 g | 0.1–0.44 CAE mg/100 g | 19.40–48.21% Free Phenolics DPPH | PR I14, I21, I26, Basmati 370, Punjab Basmati 7, Pusa Basmati 1121 | India | [120] |
| 38.11–70.98% Bound Phenolics DPPH | |||||||
| 0.06–0.14 µM TE/g Free Phenolics ABTS | |||||||
| 1.1–2.2 µM TE/g Bound Phenolics ABTS | |||||||
3.2. Lipids
3.3. Amino Acids and Derivatives
3.4. Phenolic Acids
3.5. Organic Acids and Alkaloids
3.6. Other Metabolites
3.7. Volatile Aroma Compounds
4. Biological Activities of Colored Rice
4.1. Antioxidant Activity
4.2. Anti-Inflammatory Activity
4.3. Antitumor (Anticancer) Activity
4.4. Antimicrobial (Antibacterial and Antiviral) Activities
4.5. Hypoglycemic Activities
4.6. Anti-Aging Properties
4.7. Neuroprotective Properties
4.8. Clinical Trials
| Country | Study Design | Number Completed | Sex | Age | Health Status | Intervention | Dose | Experimental Duration | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Italy | Crossover randomized controlled clinical trial | 19 | Male and Female | 24.7 ± 3.8 | Healthy | Black rice | 100 g serving | 30, 60, 120, and 180 min | ↑Plasma total phenol index Venera had higher than Artemide. ↑Total flavonoids ↑DPPH ↑ABTS | [171] |
| Korea | Placebo-controlled preliminary clinical trial | 86 | Female | 56.91 ± 5.71 57.32 ± 5.45 | Obese postmenopausal women | Black rice extract | 1 g capsules (twice a day) | 12 weeks | ↓Trunk fat, ↓total fat, ↓total body fat % Non-significant differences in anthropometric measures | [257] |
| Indonesia | Clinical trail | Female | 18–25 | Health | Black rice bran extracts | 0.5 g 10% black rice extract containing lotion—twice daily | 14 days | ↓Melatonin index. ↓Erythema index | [258] | |
| Iran | Open label randomized controlled trial | 50 | Male | 65–74 | Suffering from metabolic syndrome | Brown rice bran powder | 15 g brown rice bran powder | 8 weeks | ↓BMI ↓Waist circumference, ↓Total cholesterol ↓Blood sugar ↓Triglyceride glucose-BMI index ↑Antioxidant enzyme activities | [259] |
| Thailand | Intervention trial | 120 | Male | 65–74 | Healthy | Black rice germ and bran supplement | 10 g black rice powder + exercise | 24 weeks | ↓Inflammatory biomarkers (c-reactive protein and interleukin-6 levels) ↑Insuline-like growth factor-1 level | [260] |
| Thailand | Randomized placebo-controlled clinical trial | 60 | Male and female | Type 2 diabetes mellitus | Dietary fibre (from Jerusalem artichoke and white rice bran) and anthocyanin (from riceberry rice) | 1.26 g/day of each dietary fibre (from Jerusalem artichoke and white rice bran) and 0.28 g/day of anthocyanin (from riceberry rice) | 60 days | ↓Glucose and lipid profiles ↑Kidney’s function glomerular filtration rate | [261] | |
| Japan | Open Label Test | 65 | Male and female | 23.8 ± 8.8 22.0 ± 1.2 (CK) | Healthy | De-waxed brown rice vs. polished rice | >150 g per day, once a day | One month | Improved skin age, Improve wrinkles and porphyrin levels in Female subjects | [262] |
| Italy | Placebo-controlled preliminary clinical trial | 90 | 30–75 | Moderately hypercholesterolemic | Combined nutraceutical (phytosterols (800 mg) and red yeast rice) | One tablet after dinner (phytoesterols 800 mg + red yeast rice having 5 mg monacolins + niacin 27 mg + policosanols 10 mg) | 8 weeks | Reduced the plasma levels of LDL-C, TC, non-HDL-C, ApoB, TC/HDL-C and LDL-C/HDL-C ratios in mildly hypercholesterolemic patients | [263] | |
| Japan | Multi-centre randomized trial | 18 | Male and female | 20–90 | Mild dyslipidaemia | Red yeast rice | 200 mg/day red yeastrice, containing 2 mg monacolin K, once a day with water after dinner | 8 Weeks | ↓Low-density lipoprotein cholesterol, ↓Total cholesterol, ↓Apolipoprotein B, ↓Blood pressure | [264] |
| India | Dietary-intervention | 40 | Female | 25–50 | Prediabetic women | Germinated brown rice | 120 g daily | 120 days | ↓Fasting blood glucose, ↓average glycated hemoglobin, ↓total cholesterol, ↓Triglycerides, ↓Low-density lipoprotein cholesterol | [265] |
| Thailand | Randomized crossover | 19 | Male and female | 18–40 | Healthy | Anthocyanins from Riceberry rice | 350 g whole milk, skimmed milk powder (3% w/w), and sucrose (5% w/w), with or without the anthocyanin-rich extract of riceberry rice (0.25% w/w). | 15, 30, 60, 90, 120, 150, and 180 min | ↑Plasma ferric reducing ability of plasma, Trolox ↑equivalent antioxidant capacity, ↑oxygen radical absorbance capacity, ↓Decrease in iAUC for plasma malondialdehyde | [266] |
| Thailand | Randomized control trial | 24 | Not specified | 45–70 | Healthy | Germinated Black Rice | 1000 mg/day | 8 Weeks | Improved working memory, (Decreased response time, attentional control, memory speed) ↓Acetylcholinesterase, ↓Monoamine oxidase | [267] |
| Chinese | Randomized control trial | 24 | Not specified | 21–65 | Healthy | Black rice anthocyanin extract | 2% and 4% Black rice extract per 100 g of wheat flour | 2–4 h | Improved plasma HDL-c, Apo-A1, Apo-B, modified LDL and HDL subfractions, remodelled lipid distributions in HDL and LDL particles | [268] |
5. Conclusions
6. Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdel-Aal, E.-S.M.; Young, J.C.; Rabalski, I. Anthocyanin composition in black, blue, pink, purple, and red cereal grains. J. Agric. Food Chem. 2006, 54, 4696–4704. [Google Scholar] [CrossRef] [PubMed]
- Paiva, F.F.; Vanier, N.L.; Berrios, J.D.J.; Pinto, V.Z.; Wood, D.; Williams, T.; Pan, J.; Elias, M.C. Polishing and parboiling effect on the nutritional and technological properties of pigmented rice. Food Chem. 2016, 191, 105–112. [Google Scholar] [CrossRef]
- Rathna Priya, T.; Eliazer Nelson, A.R.L.; Ravichandran, K.; Antony, U. Nutritional and functional properties of coloured rice varieties of South India: A review. J. Ethn. Foods 2019, 6, 11. [Google Scholar] [CrossRef]
- Pratiwi, R.; Purwestri, Y.A. Black rice as a functional food in Indonesia. Funct. Foods Health Dis. 2017, 7, 182–194. [Google Scholar] [CrossRef]
- Tsuzuki, W.; Komba, S.; Kotake-Nara, E. Diversity in γ-oryzanol profiles of Japanese black-purple rice varieties. J. Food Sci. Technol. 2019, 56, 2778–2786. [Google Scholar] [CrossRef]
- Kushwaha, U. Black Rice Cultivation. In Black Rice: Research, History and Development; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 115–150. [Google Scholar]
- Ito, V.C.; Lacerda, L.G. Black rice (Oryza sativa L.): A review of its historical aspects, chemical composition, nutritional and functional properties, and applications and processing technologies. Food Chem. 2019, 301, 125304. [Google Scholar] [CrossRef] [PubMed]
- Batubara, I.; Maharni, M.; Sadiah, S. The potency of white rice (Oryza sativa), black rice (Oryza sativa L. indica), and red rice (Oryza nivara) as antioxidant and tyrosinase inhibitor. J. Phys. Conf. Ser. 2017, 824, 01207, IOP Publishing: Bristol, UK. [Google Scholar]
- Finocchiaro, F.; Ferrari, B.; Gianinetti, A.; Dall’Asta, C.; Galaverna, G.; Scazzina, F.; Pellegrini, N. Characterization of antioxidant compounds of red and white rice and changes in total antioxidant capacity during processing. Mol. Nutr. Food Res. 2007, 51, 1006–1019. [Google Scholar] [CrossRef]
- Li, P.; Wang, Q.; Chen, K.; Zou, S.; Shu, S.; Lu, C.; Wang, S.; Jiang, Y.; Fan, C.; Luo, Y. Red yeast rice for hyperlipidemia: A meta-analysis of 15 high-quality randomized controlled trials. Front. Pharmacol. 2022, 12, 819482. [Google Scholar] [CrossRef]
- Mendoza-Sarmiento, D.; Mistades, E.V.; Hill, A.M. Effect of Pigmented Rice Consumption on Cardiometabolic Risk Factors: A Systematic Review of Randomized Controlled Trials. Curr. Nutr. Rep. 2023, 12, 797–812. [Google Scholar] [CrossRef]
- Mendoza-Sarmiento, D.S.; Hill, A.M. The Role of Pigmented Rice in Reducing Cardiovascular Disease Risk: A Mini-Review of Animal and Human Studies. J. Med. Univ. St. Tomas 2023, 7, 1310–1316. [Google Scholar] [CrossRef]
- Mbanjo, E.G.N.; Kretzschmar, T.; Jones, H.; Ereful, N.; Blanchard, C.; Boyd, L.A.; Sreenivasulu, N. The genetic basis and nutritional benefits of pigmented rice grain. Front. Genet. 2020, 11, 229. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Zhou, H.; Wang, Y.; Li, P.; Fu, P.; Wu, B.; He, Y. How rice organs are colored: The genetic basis of anthocyanin biosynthesis in rice. Crop J. 2021, 9, 598–608. [Google Scholar] [CrossRef]
- Veni, B.K. Nutrition profiles of different colored rice: A review. J. Pharmacogn. Phytochem. 2019, 8, 303–305. [Google Scholar]
- Samyor, D.; Das, A.B.; Deka, S.C. Pigmented rice a potential source of bioactive compounds: A review. Int. J. Food Sci. Technol. 2017, 52, 1073–1081. [Google Scholar] [CrossRef]
- Verma, D.K.; Srivastav, P.P. Bioactive compounds of rice (Oryza sativa L.): Review on paradigm and its potential benefit in human health. Trends Food Sci. Technol. 2020, 97, 355–365. [Google Scholar] [CrossRef]
- Kumar, A.; Mosa, K.A.; Ji, L.; Kage, U.; Dhokane, D.; Karre, S.; Madalageri, D.; Pathania, N. Metabolomics-assisted biotechnological interventions for developing plant-based functional foods and nutraceuticals. Crit. Rev. Food Sci. Nutr. 2018, 58, 1791–1807. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, R.; Zhang, Y.; Lu, Y.; Cai, S.; Xiong, Q. Metabolomics reveals antioxidant metabolites in colored rice grains. Metabolites 2024, 14, 120. [Google Scholar] [CrossRef]
- Catena, S.; Rakotomanomana, N.; Zunin, P.; Boggia, R.; Turrini, F.; Chemat, F. Solubility study and intensification of extraction of phenolic and anthocyanin compounds from Oryza sativa L.‘Violet Nori’. Ultrason. Sonochemistry 2020, 68, 105231. [Google Scholar] [CrossRef]
- Dittgen, C.L.; Hoffmann, J.F.; Chaves, F.C.; Rombaldi, C.V.; Colombari Filho, J.M.; Vanier, N.L. Discrimination of genotype and geographical origin of black rice grown in Brazil by LC-MS analysis of phenolics. Food Chem. 2019, 288, 297–305. [Google Scholar] [CrossRef]
- Schaub, P.; Al-Babili, S.; Drake, R.; Beyer, P. Why is golden rice golden (yellow) instead of red? Plant Physiol. 2005, 138, 441–450. [Google Scholar] [CrossRef]
- Dubock, A. Golden rice: To combat vitamin A deficiency for public health. In Vitamin A; InTechOpen: London, UK, 2019; pp. 1–21. [Google Scholar] [CrossRef]
- Zhu, Q.; Yu, S.; Zeng, D.; Liu, H.; Wang, H.; Yang, Z.; Xie, X.; Shen, R.; Tan, J.; Li, H. Development of “purple endosperm rice” by engineering anthocyanin biosynthesis in the endosperm with a high-efficiency transgene stacking system. Mol. Plant 2017, 10, 918–929. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Lee, J. Volatile and sensory profiles of different black rice (Oryza sativa L.) cultivars varying in milling degree. Food Res. Int. 2021, 141, 110150. [Google Scholar] [CrossRef]
- Das, M.; Dash, U.; Mahanand, S.S.; Nayak, P.K.; Kesavan, R.K. Black rice: A comprehensive review on its bioactive compounds, potential health benefits and food applications. Food Chem. Adv. 2023, 3, 100462. [Google Scholar] [CrossRef]
- Tai, L.; Huang, G. Extraction and properties of black rice pigment. Pharm. Chem. J. 2021, 54, 1051–1056. [Google Scholar] [CrossRef]
- Hosoda, K.; Sasahara, H.; Matsushita, K.; Tamura, Y.; Miyaji, M.; Matsuyama, H. Anthocyanin and proanthocyanidin contents, antioxidant activity, and in situ degradability of black and red rice grains. Asian-Australas. J. Anim. Sci. 2018, 31, 1213. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Lee, S.Y.; Chu, S.M.; Lim, S.H.; Suh, S.-C.; Lee, Y.-T.; Cho, H.S.; Ha, S.-H. Variation and correlation analysis of flavonoids and carotenoids in Korean pigmented rice (Oryza sativa L.) cultivars. J. Agric. Food Chem. 2010, 58, 12804–12809. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Watanabe, S.; Crozier, A.; Fujimura, T.; Yokota, T.; Ashihara, H. Phytochemical profile of a Japanese black–purple rice. Food Chem. 2013, 141, 2821–2827. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Cros, G.; Yokota, T.; Crozier, A. Phytochemical profiles of black, red, brown, and white rice from the Camargue region of France. J. Agric. Food Chem. 2013, 61, 7976–7986. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Z.; Chen, C.; Wu, W.; Ren, N.; Jiang, C.; Yu, J.; Zhao, Y.; Zheng, X.; Yang, Q. The C–S–A gene system regulates hull pigmentation and reveals evolution of anthocyanin biosynthesis pathway in rice. J. Exp. Bot. 2018, 69, 1485–1498. [Google Scholar] [CrossRef]
- Chen, T.; Xie, L.; Wang, G.; Jiao, J.; Zhao, J.; Yu, Q.; Chen, Y.; Shen, M.; Wen, H.; Ou, X. Anthocyanins-natural pigment of colored rice bran: Composition and biological activities. Food Res. Int. 2023, 175, 113722. [Google Scholar] [CrossRef]
- Lamberts, L.; Delcour, J.A. Carotenoids in raw and parboiled brown and milled rice. J. Agric. Food Chem. 2008, 56, 11914–11919. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Kim, D.-E.; Keum, Y.-S.; Saini, R.K. Metabolite profiling and antioxidant activities of white, red, and black rice (Oryza sativa L.) grains. J. Food Meas. Charact. 2018, 12, 2484–2492. [Google Scholar] [CrossRef]
- Chaudhari, P.R.; Tamrakar, N.; Singh, L.; Tandon, A.; Sharma, D. Rice nutritional and medicinal properties: A review article. J. Pharmacogn. Phytochem. 2018, 7, 150–156. [Google Scholar]
- Zhang, L.; Zhao, L.; Zhang, J.; Cai, X.; Liu, Q.; Wei, C. Relationships between transparency, amylose content, starch cavity, and moisture of brown rice kernels. J. Cereal Sci. 2019, 90, 102854. [Google Scholar] [CrossRef]
- Farooq, M.A.; Murtaza, M.A.; Aadil, R.M.; Arshad, R.; Rahaman, A.; Siddique, R.; Hassan, S.; Akhtar, H.M.S.; Manzoor, M.F.; Karrar, E. Investigating the structural properties and in vitro digestion of rice flours. Food Sci. Nutr. 2021, 9, 2668–2675. [Google Scholar] [CrossRef]
- Huang, M.; Li, X.; Hu, L.; Xiao, Z.; Chen, J.; Cao, F. Comparing texture and digestion properties between white and brown rice of indica cultivars preferred by Chinese consumers. Sci. Rep. 2021, 11, 19054. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Huo, Y.; Liu, H.; Kamal, G.M.; Yang, J.; Zeng, Y.; Zhao, S.; Liu, Y. Fast nutritional characterization of different pigmented rice grains using a combination of NMR and decision tree analysis. CyTA-J. Food 2019, 17, 128–136. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, B.; Zeng, X.; Tang, Q.; Shu, Z.; Wang, P. Physicochemical and digestible properties of parboiled black rice with different amylose contents. Front. Nutr. 2022, 9, 934209. [Google Scholar] [CrossRef]
- Meera, K.; Smita, M.; Haripriya, S.; Sen, S. Varietal influence on antioxidant properties and glycemic index of pigmented and non-pigmented rice. J. Cereal Sci. 2019, 87, 202–208. [Google Scholar] [CrossRef]
- Veni, B.K.; Raja, D.S.; Sridevi, P.; Tushara, M. Impact of starch profile on glycemic index of coloured and non-pigmented genotypes of rice (Oryza sativa L.). Electron. J. Plant Breed. 2024, 15, 700–707. [Google Scholar]
- Andriani, A.; Abd Hamid, M.H.N.; Sahrom, M.S.; Yusof, M.S.M.; Yamin, B.M. Physical properties and glycemic index of organic and conventional black rice grown in Bali, Indonesia. Int. J. Adv. Sci. Eng. Inf. Technol. 2020, 10, 1688–1695. [Google Scholar] [CrossRef]
- Jukanti, A.K.; Pautong, P.A.; Liu, Q.; Sreenivasulu, N. Low glycemic index rice—A desired trait in starchy staples. Trends Food Sci. Technol. 2020, 106, 132–149. [Google Scholar] [CrossRef]
- Zafar, M.I.; Mills, K.E.; Zheng, J.; Regmi, A.; Hu, S.Q.; Gou, L.; Chen, L.-L. Low-glycemic index diets as an intervention for diabetes: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2019, 110, 891–902. [Google Scholar] [CrossRef]
- Dhanalakshmi, T.; Shashidhara, N.; Santosh, D.; Quadri, S.S.; Vilas, G. Breeding Low Glycemic Index Rice (Oryza sativa L.) Cultivars: Progress, Benefits, and Challenges. Int. J. Bioresour. Sci. 2023, 10, 79–88. [Google Scholar]
- Shimoda, H.; Aitani, M.; Tanaka, J.; Hitoe, S. Purple rice extract exhibits preventive activities on experimental diabetes models and human subjects. J. Rice Res. 2015, 3, 137. [Google Scholar]
- Prasantha, B.D.R. Glycemic index of four traditional red pigmented rice. Integr. Food Nutr. Metab 2018, 5, 1–3. [Google Scholar] [CrossRef]
- Tiozon, R.N.; Lenaerts, B.; Kor, S.; Demont, M.; Fernie, A.R.; Sreenivasulu, N. Low glycemic index rice: A healthier diet for countering diabetes epidemic in Asia. Trends Plant Sci. 2024, in press. [Google Scholar] [CrossRef]
- Selvaraj, R.; Singh, A.K.; Singh, V.K.; Abbai, R.; Habde, S.V.; Singh, U.M.; Kumar, A. Superior haplotypes towards development of low glycemic index rice with preferred grain and cooking quality. Sci. Rep. 2021, 11, 10082. [Google Scholar] [CrossRef]
- Fitzgerald, M.; Rahman, S.; Resurreccion, A.; Concepcion, J.; Daygon, V.; Dipti, S.; Kabir, K.; Klingner, B.; Morell, M.; Bird, A. Identification of a major genetic determinant of glycaemic index in rice. Rice 2011, 4, 66–74. [Google Scholar] [CrossRef]
- Badoni, S.; Pasion-Uy, E.A.; Kor, S.; Kim, S.-R.; Tiozon Jr, R.N.; Misra, G.; Buenafe, R.J.Q.; Labarga, L.M.; Ramos-Castrosanto, A.R.; Pratap, V. Multiomics of a rice population identifies genes and genomic regions that bestow low glycemic index and high protein content. Proc. Natl. Acad. Sci. USA 2024, 121, e2410598121. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, X.; Li, A.; Wang, R.; Ni, X.; Hu, J.; Wei, H.; Zhang, H.; Xiong, Q. The main nutritional components in colored rice grains. LWT 2024, 191, 115663. [Google Scholar] [CrossRef]
- Saleh, A.S.; Wang, P.; Wang, N.; Yang, L.; Xiao, Z. Brown rice versus white rice: Nutritional quality, potential health benefits, development of food products, and preservation technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1070–1096. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, A.; Pandey, V.K.; Dar, A.H.; Fayaz, U.; Dash, K.K.; Shams, R.; Ahmad, S.; Bashir, I.; Fayaz, J.; Singh, P. Rice bran: Nutritional, phytochemical, and pharmacological profile and its contribution to human health promotion. Food Chem. Adv. 2023, 2, 100296. [Google Scholar] [CrossRef]
- Senarathna, S.; Mel, R.; Malalgoda, M. Utilization of cereal-based protein ingredients in food applications. J. Cereal Sci. 2024, 116, 103867. [Google Scholar] [CrossRef]
- Pandarinathan, S. Studies on the Protein Status of White and Brown Rice Grain in Selected Varieties Cultivated in and around Tiruchirappalli District of Tamil Nadu. Int. Res. J. Pure Appl. Chem. 2020, 21, 71–83. [Google Scholar] [CrossRef]
- Amagliani, L.; O’Regan, J.; Kelly, A.L.; O’Mahony, J.A. The composition, extraction, functionality and applications of rice proteins: A review. Trends Food Sci. Technol. 2017, 64, 1–12. [Google Scholar] [CrossRef]
- Chen, D.-G.; Guo, J.; Chen, K.; Ye, C.-J.; Liu, J.; Chen, Y.-D.; Zhou, X.-Q.; Liu, C.-G. Pyramiding breeding of low-glutelin-content indica rice with good quality and resistance. Plants 2023, 12, 3763. [Google Scholar] [CrossRef]
- Xinkang, L.; Chunmin, G.; Lin, W.; Liting, J.; Xiangjin, F.; Qinlu, L.; Zhengyu, H.; Chun, L. Rice storage proteins: Focus on composition, distribution, genetic improvement and effects on rice quality. Rice Sci. 2023, 30, 207–221. [Google Scholar] [CrossRef]
- Suen, P.K.; Zheng, L.; Yang, Q.-q.; Mak, W.S.; Pak, W.Y.; Mo, K.Y.; Chan, M.-l.; Liu, Q.-q.; Qin, L.; Sun, S.S.-M. Lysine-rich rice partially enhanced the growth and development of skeletal system with better skeletal microarchitecture in young rats. Nutr. Res. 2024, 121, 67–81. [Google Scholar] [CrossRef]
- Satmalee, P.; Surojanametakul, V.; Lowithun, N.; Mungkung, R.; Dangsiri, S. Development of ready-to-eat color rice product enriched with natural amino acids. J. Agric. Sci. 2019, 11, 56–63. [Google Scholar] [CrossRef]
- Mojumder, M.N.; Mahmud, Z.; Khan, I.; Tamanna, S.; Rahman, M.R.; Ferdous, N.; Alauddin, M.; Howlader, M.Z.H. Antioxidant, antidiabetic, and antihypertensive activities of defatted pigmented rice bran protein hydrolysates. Discov. Appl. Sci. 2024, 6, 628. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, D.; Yuan, M.; Shu, X. Functional characteristics of lipids in rice and its’ biological regulation. J. Nucl. Agric. Sci. 2019, 33, 1105–1115. [Google Scholar]
- Samaranayake, M.D.W.; Abeysekera, W.K.S.M.; Hewajulige, I.G.N.; Somasiri, H.P.P.S.; Mahanama, K.R.R.; Senanayake, D.M.J.B.; Premakumara, G.A.S. Fatty acid profiles of selected traditional and new improved rice varieties of Sri Lanka. J. Food Compos. Anal. 2022, 112, 104686. [Google Scholar] [CrossRef]
- Hu, E.A.; Pan, A.; Malik, V.; Sun, Q. White rice consumption and risk of type 2 diabetes: Meta-analysis and systematic review. Bmj 2012, 344, e1454. [Google Scholar] [CrossRef]
- Saneei, P.; Larijani, B.; Esmaillzadeh, A. Rice consumption, incidence of chronic diseases and risk of mortality: Meta-analysis of cohort studies. Public Health Nutr. 2017, 20, 233–244. [Google Scholar] [CrossRef]
- Izadi, V.; Azadbakht, L. Is there any association between rice consumption and some of the cardiovascular diseases risk factors? A systematic review. ARYA Atheroscler. 2015, 11, 109. [Google Scholar]
- Liu, L.; Waters, D.L.; Rose, T.J.; Bao, J.; King, G.J. Phospholipids in rice: Significance in grain quality and health benefits: A review. Food Chem. 2013, 139, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-H.; Lim, S.-Y.; Rehman, A.; Farooq, M.; Lee, D.-J. Characterization and quantification of γ-oryzanol in Korean rice landraces. J. Cereal Sci. 2019, 88, 150–156. [Google Scholar] [CrossRef]
- Britz, S.J.; Prasad, P.; Moreau, R.A.; Allen, L.H.; Kremer, D.F.; Boote, K.J. Influence of growth temperature on the amounts of tocopherols, tocotrienols, and γ-oryzanol in brown rice. J. Agric. Food Chem. 2007, 55, 7559–7565. [Google Scholar] [CrossRef]
- Harakotr, B.; Prompoh, K.; Suriharn, K.; Lertrat, K. Genotype by environment interaction effects on nutraceutical lipid compounds of pigmented rice (Oryza sativa L. ssp. indica). Int. J. Agron. 2021, 2021, 8880487. [Google Scholar] [CrossRef]
- Tung, Y.H.; Ng, L.T. Effects of Soil Salinity on Tocopherols, Tocotrienols, and γ-Oryzanol Accumulation and their Relation to Oxidative Stress in Rice Plants. Crop Sci. 2016, 56, 3143–3151. [Google Scholar] [CrossRef]
- Thilakarathna, G.; Navarathne, S.; Wickramasinghe, I. Identification of important physical properties and amylose content in commercially available improved and traditional rice varieties in Sri Lanka. Int. J. Adv. Eng. Res. Sci. 2017, 4, 186–194. [Google Scholar] [CrossRef]
- da Silva, L.R.; de Carvalho, C.W.P.; Velasco, J.I.; Fakhouri, F.M. Extraction and characterization of starches from pigmented rice. Int. J. Biol. Macromol. 2020, 156, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.L.J.; dos Santos Pereira, T.; de Andrade Freire, V.; Santiago, Â.M.; Oliveira, H.M.L.; de Sousa Conrado, L.; de Gusmão, R.P. Influence of enzymatic hydrolysis on the properties of red rice starch. Int. J. Biol. Macromol. 2019, 141, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, Y.; Gao, L.; Zhou, X.; Ma, P.; Wang, Q. Effect of different carbohydrates on the functional properties of black rice glutelin (BRG) modified by the maillard reaction. J. Cereal Sci. 2020, 93, 102979. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Liang, M.; Yang, L. Glutelin and prolamin, different components of rice protein, exert differently in vitro antioxidant activities. J. Cereal Sci. 2016, 72, 108–116. [Google Scholar] [CrossRef]
- Ning, H.; Qiao, J.; Liu, Z.; Lin, Z.; Li, G.; Wang, Q.; Wang, S.; Ding, Y. Distribution of proteins and amino acids in milled and brown rice as affected by nitrogen fertilization and genotype. J. Cereal Sci. 2010, 52, 90–95. [Google Scholar] [CrossRef]
- Massaretto, I.L.; Meza, S.L.R.; Sinnecker, P.; Schmiele, M.; Noldin, J.A.; Wickert, E.; Marquez, U.M.L. Chemical, nutritional and sensory profiles of different pigmented rice varieties impacted by cooking process. Res. Soc. Dev. 2022, 11, e24411931799. [Google Scholar] [CrossRef]
- Sompong, R.; Siebenhandl-Ehn, S.; Linsberger-Martin, G.; Berghofer, E. Physicochemical and antioxidative properties of red and black rice varieties from Thailand, China and Sri Lanka. Food Chem. 2011, 124, 132–140. [Google Scholar] [CrossRef]
- Quintaes, K.D.; Diez-Garcia, R.W. The importance of minerals in the human diet. In Handbook of Mineral Elements in Food; Wiley: Hoboken, NJ, USA, 2015; pp. 1–21. [Google Scholar]
- Shrestha, J.; Kandel, M.; Subedi, S.; Shah, K.K. Role of nutrients in rice (Oryza sativa L.): A review. Agrica 2020, 9, 53–62. [Google Scholar] [CrossRef]
- Jiang, S.; Wu, J.; Feng, Y.; Yang, X.; Shi, C. Correlation analysis of mineral element contents and quality traits in milled rice (Oryza stavia L.). J. Agric. Food Chem. 2007, 55, 9608–9613. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Lin, S.; Huang, W.; Zhao, W.; Tan, J.; Wang, M. Comprehensive analysis of the metabolic nutritional components and exploration of health-promoting small molecules in japonica rice and indica rice seeds. Food Saf. Health 2024, 2, 380–392. [Google Scholar] [CrossRef]
- Govarethinam, B.; Koki, I.B.; Low, K.H. Exploring the elemental variations in commercial non-glutinous brown and white rice from Malaysia by chemometrics. Braz. J. Dev. 2024, 10, e70040. [Google Scholar] [CrossRef]
- Verma, D.K.; Srivastav, P.P. Proximate composition, mineral content and fatty acids analyses of aromatic and non-aromatic Indian rice. Rice Sci. 2017, 24, 21–31. [Google Scholar] [CrossRef]
- Hurtada, W.; Barrion, A.; Nguyen-Orca, M. Mineral content of dehulled and well-milled pigmented and non-pigmented rice varieties in the Philippines. Int. Food Res. J. 2018, 25, 2063–2067. [Google Scholar]
- Yoshida, H.; Tomiyama, Y.; Mizushina, Y. Lipid components, fatty acids and triacylglycerol molecular species of black and red rices. Food Chem. 2010, 123, 210–215. [Google Scholar] [CrossRef]
- Ghosh, S.; Datta, K.; Datta, S.K. Rice vitamins. In Rice; Elsevier: Amsterdam, The Netherlands, 2019; pp. 195–220. [Google Scholar]
- Shammugasamy, B.; Ramakrishnan, Y.; Ghazali, H.M.; Muhammad, K. Tocopherol and tocotrienol contents of different varieties of rice in Malaysia. J. Sci. Food Agric. 2015, 95, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Sudtasarn, G.; Homsombat, W.; Chotechuen, S.; Chamarerk, V. Quantification of tocopherols, tocotrienols and γ-oryzanol contents of local rice varieties in northeastern Thailand. J. Nutr. Sci. Vitaminol. 2019, 65, S125–S128. [Google Scholar] [CrossRef]
- Huang, S.-H.; Ng, L.-T. Quantification of tocopherols, tocotrienols, and γ-oryzanol contents and their distribution in some commercial rice varieties in Taiwan. J. Agric. Food Chem. 2011, 59, 11150–11159. [Google Scholar] [CrossRef]
- Sridevi, J.; Kowsalya, S. Macro and micro nutrient content in raw and cooked forms of black rice and white rice. Indian J. Nutr. Diet. 2020, 57, 116–126. [Google Scholar]
- Roy, P.; Deb, D.; Pradeep, T.; Talai-Mukhopadhyay, S.; Sinha, A.K.; Saha, T. Comparative analyses of the nutraceutical potentialities of selected Indian traditional black rice (Oryza sativa L.) landraces. Oryza-An Int. J. Rice 2021, 58, 295–309. [Google Scholar] [CrossRef]
- Choi, S.-R.; Song, E.-J.; Song, Y.-E.; Choi, M.-K.; Han, H.-A.; Lee, I.-S.; Shin, S.-H.; Lee, K.-K.; Choi, Y.-M.; Kim, H.-R. Determination of vitamin B 6 content using HPLC in agricultural products cultivated in local areas in Korea. Korean J. Food Nutr. 2017, 30, 710–718. [Google Scholar]
- Nguyen, T.T.L.; Pham, T.M.N.; Ho, T.B.; Ly-Nguyen, B. Optimization of Vitamin B1, B2, and B6 Absorption in Nang Tay Dum Floating Rice Grains. Foods 2024, 13, 2650. [Google Scholar] [CrossRef]
- Wu, X.; Guo, T.; Luo, F.; Lin, Q. Brown rice: A missing nutrient-rich health food. Food Sci. Hum. Wellness 2023, 12, 1458–1470. [Google Scholar] [CrossRef]
- Ren, C.-Y.; Lu, S.-W.; Guan, L.-J.; Bin, H.; Zhang, Y.-L.; Huang, W.-G.; Bo, L.; Wei, L.; Lu, W.-H. The metabolomics variations among rice, brown rice, wet germinated brown rice, and processed wet germinated brown rice. J. Integr. Agric. 2022, 21, 2767–2776. [Google Scholar] [CrossRef]
- Oikawa, A.; Matsuda, F.; Kusano, M.; Okazaki, Y.; Saito, K. Rice metabolomics. Rice 2008, 1, 63–71. [Google Scholar] [CrossRef]
- Liang, C.; Guan, Z.; Wei, K.; Yu, W.; Wang, L.; Chen, X.; Wang, Y. Characteristics of antioxidant capacity and metabolomics analysis of flavonoids in the bran layer of green glutinous rice (Oryza sativa L. var. Glutinosa Matsum). Sci. Rep. 2023, 13, 16372. [Google Scholar] [CrossRef]
- Moon, H.-S.; Thiruvengadam, M.; Chi, H.-Y.; Kim, B.; Prabhu, S.; Chung, I.-M.; Kim, S.-H. Comparative study for metabolomics, antioxidant activity, and molecular docking simulation of the newly bred Korean red rice accessions. Food Chem. 2024, 458, 140277. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, X.; Shen, J.; Yang, D.; Lu, Q.; Long, C.; Liu, R. Widely targeted metabolomics analysis of nutrient changes in a new black rice variety ‘Huamoxiang No. 3’(Oryza sativa L. var. Glutinosa Matsum) under different cooking modes. Cereal Chem. 2024, 101, 1106–1117. [Google Scholar] [CrossRef]
- Ren, C.-y.; Lu, S.-w.; Hong, B.; Zhang, Y.-l.; Guan, L.-j.; Li, B.; Huang, W.-g.; Lu, W.-h. Non-targeted metabolomic analysis of brown rice and white rice. Shipin Kexue/Food Sci. 2022, 43, 183–190. [Google Scholar]
- Zhang, L.; Cui, D.; Ma, X.; Han, B.; Han, L. Comparative analysis of rice reveals insights into the mechanism of colored rice via widely targeted metabolomics. Food Chem. 2023, 399, 133926. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhai, L.; Tang, Q.; Ren, J.; Zhou, S.; Wang, H.; Yun, Y.; Yang, Q.; Yan, X.; Xing, F. Comparative Metabolic Profiling of Different Colored Rice Grains Reveals the Distribution of Major Active Compounds and Key Secondary Metabolites in Green Rice. Foods 2024, 13, 1899. [Google Scholar] [CrossRef] [PubMed]
- Surh, J.; Koh, E. Effects of four different cooking methods on anthocyanins, total phenolics and antioxidant activity of black rice. J. Sci. Food Agric. 2014, 94, 3296–3304. [Google Scholar] [CrossRef] [PubMed]
- Chmiel, T.; Saputro, I.E.; Kusznierewicz, B.; Bartoszek, A. The impact of cooking method on the phenolic composition, total antioxidant activity and starch digestibility of rice (Oryza sativa L.). J. Food Process. Preserv. 2018, 42, e13383. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Y.; Yang, X.; Zhu, G.; Lu, X.; Jia, F.; Diao, B.; Yu, S.; Ali, A.; Zhang, H. Investigation of flavonoid components and their associated antioxidant capacity in different pigmented rice varieties. Food Res. Int. 2022, 161, 111726. [Google Scholar] [CrossRef]
- Sholikhah, U.; Handoyo, T.; Yunus, A. Anthocyanin Content in Some Black Rice Cultivars. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021. [Google Scholar]
- Constantin, O.E.; Istrati, D.I. Extraction, quantification and characterization techniques for anthocyanin compounds in various food matrices—A review. Horticulturae 2022, 8, 1084. [Google Scholar] [CrossRef]
- Sanghamitra, P.; Sah, R.P.; Bagchi, T.B.; Sharma, S.G.; Kumar, A.; Munda, S.; Sahu, R.K. Evaluation of variability and environmental stability of grain quality and agronomic parameters of pigmented rice (O. sativa L.). J. Food Sci. Technol. 2018, 55, 879–890. [Google Scholar] [CrossRef]
- Shao, Y.; Xu, F.; Chen, Y.; Huang, Y.; Beta, T.; Bao, J. Analysis of genotype, environment, and their interaction effects on the phytochemicals and antioxidant capacities of red rice (Oryza sativa L.). Cereal Chem. 2015, 92, 204–210. [Google Scholar] [CrossRef]
- Thitipramote, N.; Pradmeeteekul, P.; Nimkamnerd, J.; Chaiwut, P.; Pintathong, P.; Thitilerdecha, N. Bioactive compounds and antioxidant activities of red (Brown Red Jasmine) and black (Kam Leum Pua) native pigmented rice. Int. Food Res. J. 2016, 23, 410. [Google Scholar]
- Murphy, K.J.; Walker, K.M.; Dyer, K.A.; Bryan, J. Estimation of daily intake of flavonoids and major food sources in middle-aged Australian men and women. Nutr. Res. 2019, 61, 64–81. [Google Scholar] [CrossRef] [PubMed]
- Justino, J. Flavonoids: From Biosynthesis to Human Health; InTechOpen: London, UK, 2017. [Google Scholar]
- Thompson, A.S.; Jennings, A.; Bondonno, N.P.; Tresserra-Rimbau, A.; Parmenter, B.H.; Hill, C.; Perez-Cornago, A.; Kühn, T.; Cassidy, A. Higher habitual intakes of flavonoids and flavonoid-rich foods are associated with a lower incidence of type 2 diabetes in the UK Biobank cohort. Nutr. Diabetes 2024, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Avinash, G.; Sharma, N.; Prasad, K.R.; Kaur, R.; Singh, G.; Pagidipala, N.; Thulasinathan, T. Unveiling the distribution of free and bound phenolic acids, flavonoids, anthocyanins, and proanthocyanidins in pigmented and non-pigmented rice genotypes. Front. Plant Sci. 2024, 15, 1324825. [Google Scholar] [CrossRef]
- Yu, J.; Zheng, X.; Zhu, D.; Xu, Q.; Xu, F.; Chen, M.; Meng, L.; Shao, Y. Changes of polyphenols and their antioxidant activities in non-pigmented, red and black rice during in vitro digestion. Food Chem. X 2024, 24, 101821. [Google Scholar] [CrossRef] [PubMed]
- Ratseewo, J.; Warren, F.J.; Meeso, N.; Siriamornpun, S. Effects of Far-Infrared Radiation Drying on Starch Digestibility and the Content of Bioactive Compounds in Differently Pigmented Rice Varieties. Foods 2022, 11, 4079. [Google Scholar] [CrossRef]
- Zhou, Z.; Robards, K.; Helliwell, S.; Blanchard, C. Composition and functional properties of rice. Int. J. Food Sci. Technol. 2002, 37, 849–868. [Google Scholar] [CrossRef]
- Kim, N.-H.; Sohn, J.-K.; Kim, K.-M. Physicochemical characteristics and QTL mapping associated with the lipid content of high-lipid rice. Am. J. Plant Sci. 2013, 4, 1949. [Google Scholar] [CrossRef]
- Khatun, A.; Waters, D.L.; Liu, L. The impact of rice lipid on in vitro rice starch digestibility. Foods 2022, 11, 1528. [Google Scholar] [CrossRef]
- Kraithong, S.; Theppawong, A.; Bunyameen, N.; Zhang, X.; Huang, R. Advancements in understanding low starch hydrolysis in pigmented rice: A comprehensive overview of mechanisms. Food Chem. 2024, 439, 138079. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, Y.; Jiang, Y. Amino acids in rice grains and their regulation by polyamines and phytohormones. Plants 2022, 11, 1581. [Google Scholar] [CrossRef]
- Tyagi, A.; Lim, M.-J.; Kim, N.-H.; Barathikannan, K.; Vijayalakshmi, S.; Elahi, F.; Ham, H.-J.; Oh, D.-H. Quantification of amino acids, phenolic compounds profiling from nine rice varieties and their antioxidant potential. Antioxidants 2022, 11, 839. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-O.; Oh, J.-H.; Lee, K.-T.; Im, J.-G.; Kim, S.-S.; Suh, H.-S.; Choi, S.-W. Chemical compositions and antioxidant activity of the colored rice cultivars. Food Sci. Preserv. 2008, 15, 118–124. [Google Scholar]
- Liyanaarachchi, G.; Mahanama, K.; Somasiri, H.; Punyasiri, P.; Ranatunga, M.; Wijesena, K.; Weerasinghe, W. Impact of seasonal, geographical and varietal variations on amino acid profile of Sri Lankan rice varieties (Oryza sativa L.). J. Food Compos. Anal. 2022, 109, 104494. [Google Scholar] [CrossRef]
- Ciulu, M.; Cádiz-Gurrea, M.d.l.L.; Segura-Carretero, A. Extraction and analysis of phenolic compounds in rice: A review. Molecules 2018, 23, 2890. [Google Scholar] [CrossRef]
- Shao, Y.; Hu, Z.; Yu, Y.; Mou, R.; Zhu, Z.; Beta, T. Phenolic acids, anthocyanins, proanthocyanidins, antioxidant activity, minerals and their correlations in non-pigmented, red, and black rice. Food Chem. 2018, 239, 733–741. [Google Scholar] [CrossRef]
- Jun, H.I.; Song, G.S.; Yang, E.I.; Youn, Y.; Kim, Y.S. Antioxidant activities and phenolic compounds of pigmented rice bran extracts. J. Food Sci. 2012, 77, C759–C764. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Shi, Q.; Sun, C.; Hu, J.; Zhou, N.; Wei, H.; He, H.; Zhou, D.; Zhang, H.; Xiong, Q. Processing affects (decreases or increases) metabolites, flavonoids, black rice pigment, and total antioxidant capacity of purple glutinous rice grains. Food Chem. X 2022, 16, 100492. [Google Scholar] [CrossRef]
- Thuengtung, S.; Ogawa, Y. Comparative study of conventional steam cooking and microwave cooking on cooked pigmented rice texture and their phenolic antioxidant. Food Sci. Nutr. 2020, 8, 965–972. [Google Scholar] [CrossRef]
- Shi, Y.; Pu, D.; Zhou, X.; Zhang, Y. Recent progress in the study of taste characteristics and the nutrition and health properties of organic acids in foods. Foods 2022, 11, 3408. [Google Scholar] [CrossRef]
- Yotmanee, S. Identification of the Characteristic Taste, Aroma Compounds and the Corresponding Precursors in Pigmented Rice Wine. Doctoral Dissertation, University of Reading, Reading, UK, 2018. [Google Scholar]
- Akhatou, I.; González-Domínguez, R.; Fernández-Recamales, Á. Investigation of the effect of genotype and agronomic conditions on metabolomic profiles of selected strawberry cultivars with different sensitivity to environmental stress. Plant Physiol. Biochem. 2016, 101, 14–22. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, D.; Zhao, L.; Liu, J.; Shang, B.; Yang, W.; Duan, X.; Sun, H. Metabolomic analysis reveals insights into deterioration of rice quality during storage. Foods 2022, 11, 1729. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Y.; Dang, P.; Zhao, S.; Lai, D.; Zhou, L. Rice secondary metabolites: Structures, roles, biosynthesis, and metabolic regulation. Molecules 2018, 23, 3098. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, J.; Bai, L.; Liu, J.; Li, H.; Hua, J.; Luo, S. Chemical Structure Diversity and Extensive Biological Functions of Specialized Metabolites in Rice. Int. J. Mol. Sci. 2023, 24, 17053. [Google Scholar] [CrossRef] [PubMed]
- Kusano, M.; Yang, Z.; Okazaki, Y.; Nakabayashi, R.; Fukushima, A.; Saito, K. Using metabolomic approaches to explore chemical diversity in rice. Mol. Plant 2015, 8, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.; Sharma, R.; Bharti, R. Pharmacological activities and toxicities of alkaloids on human health. Mater. Today Proc. 2022, 48, 1407–1415. [Google Scholar] [CrossRef]
- Chung, H.S.; Shin, J.C. Characterization of antioxidant alkaloids and phenolic acids from anthocyanin-pigmented rice (Oryza sativa cv. Heugjinjubyeo). Food Chem. 2007, 104, 1670–1677. [Google Scholar] [CrossRef]
- Vichit, W.; Saewan, N. Antioxidant activities and cytotoxicity of Thai pigmented rice. Int. J. Pharm. Pharm. Sci. 2015, 7, 329–334. [Google Scholar]
- Witte, C.-P.; Herde, M. Nucleotides and nucleotide derivatives as signal molecules in plants. J. Exp. Bot. 2024, 75, erae377. [Google Scholar] [CrossRef]
- Sanchez-Pozo, A.; Gil, A. Nucleotides as semiessential nutritional components. Br. J. Nutr. 2002, 87, S135–S137. [Google Scholar] [CrossRef]
- Li, K.; Wang, D.; Gong, L.; Lyu, Y.; Guo, H.; Chen, W.; Jin, C.; Liu, X.; Fang, C.; Luo, J. Comparative analysis of metabolome of rice seeds at three developmental stages using a recombinant inbred line population. Plant J. 2019, 100, 908–922. [Google Scholar] [CrossRef]
- Yang, C.; Shen, S.; Zhou, S.; Li, Y.; Mao, Y.; Zhou, J.; Shi, Y.; An, L.; Zhou, Q.; Peng, W. Rice metabolic regulatory network spanning the entire life cycle. Mol. Plant 2022, 15, 258–275. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Fan, W.; Gao, Y.-N.; Wu, S.-F.; Wang, S.-X. Study on volatile compounds in rice by HS-SPME and GC-MS. Jul. -Kühn-Arch. 2010, 425, 125. [Google Scholar]
- Routray, W.; Rayaguru, K. 2-Acetyl-1-pyrroline: A key aroma component of aromatic rice and other food products. Food Rev. Int. 2018, 34, 539–565. [Google Scholar] [CrossRef]
- Tansawat, R.; Jindawatt, S.; Ekkaphan, P.; Ruengphayak, S.; Vanavichit, A.; Suttipanta, N.; Vimolmangkang, S.; De-Eknamkul, W. Metabolomics approach to identify key volatile aromas in Thai colored rice cultivars. Front. Plant Sci. 2023, 14, 973217. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Zhang, Z.; Zhao, S.; Liu, J.; Gao, R.; Jiang, P. Characterization of volatile organic compounds of different pigmented rice after puffing based on gas chromatography-ion migration spectrometry and chemometrics. Food Res. Int. 2023, 169, 112879. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.S.; Lee, K.-S.; Jeong, O.-Y.; Kim, K.-J.; Kays, S.J. Characterization of volatile aroma compounds in cooked black rice. J. Agric. Food Chem. 2008, 56, 235–240. [Google Scholar] [CrossRef]
- Yang, D.S.; Lee, K.S.; Kays, S.J. Characterization and discrimination of premium-quality, waxy, and black-pigmented rice based on odor-active compounds. J. Sci. Food Agric. 2010, 90, 2595–2601. [Google Scholar] [CrossRef]
- Sirilertpanich, P.; Ekkaphan, P.; Andriyas, T.; Leksungnoen, N.; Ruengphayak, S.; Vanavichit, A.; De-Eknamkul, W.; Tansawat, R. Metabolomics study on the main volatile components of Thai colored rice cultivars from different agricultural locations. Food Chem. 2024, 434, 137424. [Google Scholar] [CrossRef]
- Nadhifah, A.M.a.; Fibri, D.L.N.; Handoko, D.D.; Wahyudi, D.; Budijanto, S.; Shirakawa, H. The Volatile Compounds and Aroma Profile of some Pigmented Rice Brans After Fermentation. Curr. Res. Nutr. Food Sci. 2022, 10, 145. [Google Scholar] [CrossRef]
- Goufo, P.; Trindade, H. Rice antioxidants: Phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, γ-oryzanol, and phytic acid. Food Sci. Nutr. 2014, 2, 75–104. [Google Scholar] [CrossRef]
- Cano, A.; Maestre, A.B.; Hernández-Ruiz, J.; Arnao, M.B. ABTS/TAC methodology: Main milestones and recent applications. Processes 2023, 11, 185. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH radical scavenging assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Shah, P.; Modi, H. Comparative study of DPPH, ABTS and FRAP assays for determination of antioxidant activity. Int. J. Res. Appl. Sci. Eng. Technol 2015, 3, 636–641. [Google Scholar]
- Fujita, A.; Fujitake, H.; Kawakami, K.; Nomura, M. Antioxidant activity of colored rice bran obtained at different milling yields. J. Oleo Sci. 2010, 59, 563–568. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ghasemzadeh, A.; Karbalaii, M.T.; Jaafar, H.Z.; Rahmat, A. Phytochemical constituents, antioxidant activity, and antiproliferative properties of black, red, and brown rice bran. Chem. Cent. J. 2018, 12, 17. [Google Scholar] [CrossRef]
- Aguilar-Garcia, C.; Gavino, G.; Baragaño-Mosqueda, M.; Hevia, P.; Gavino, V.C. Correlation of tocopherol, tocotrienol, γ-oryzanol and total polyphenol content in rice bran with different antioxidant capacity assays. Food Chem. 2007, 102, 1228–1232. [Google Scholar] [CrossRef]
- Rebeira, S.; Jayatilake, D.; Prasantha, R.; Kariyawasam, T.; Suriyagoda, L. Assessment of antioxidant properties in selected pigmented and non-pigmented rice (Oryza sativa L.) germplasm and determination of its association with Rc gene haplotypes. BMC Plant Biol. 2024, 24, 884. [Google Scholar] [CrossRef]
- Radix AP Jati, I.; Nohr, D.; Konrad Biesalski, H. Nutrients and antioxidant properties of Indonesian underutilized colored rice. Nutr. Food Sci. 2014, 44, 193–203. [Google Scholar] [CrossRef]
- Callcott, E.T.; Blanchard, C.L.; Snell, P.; Santhakumar, A.B. The anti-inflammatory and antioxidant effects of acute consumption of pigmented rice in humans. Food Funct. 2019, 10, 8230–8239. [Google Scholar] [CrossRef]
- Wu, F.; Yang, N.; Touré, A.; Jin, Z.; Xu, X. Germinated brown rice and its role in human health. Crit. Rev. Food Sci. Nutr. 2013, 53, 451–463. [Google Scholar] [CrossRef]
- Cho, D.-H.; Lim, S.-T. Germinated brown rice and its bio-functional compounds. Food Chem. 2016, 196, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.I.; Ham, T.-h.; Kang, M.Y. Effect of germinated pigmented rice “Superjami” on the glucose level, antioxidant defense system, and bone metabolism in menopausal rat model. Nutrients 2019, 11, 2184. [Google Scholar] [CrossRef] [PubMed]
- Vitalini, S.; Sardella, A.; Fracassetti, D.; Secli, R.; Tirelli, A.; Lodi, G.; Carrassi, A.; Varoni, E.M.; Iriti, M. Polyphenol bioavailability and plasma antiradical capacity in healthy subjects after acute intake of pigmented rice: A crossover randomized controlled clinical trial. J. Clin. Med. 2020, 9, 3209. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Han, P.; Zhang, M.; Xia, M.; Zhu, H.; Ma, J.; Hou, M.; Tang, Z.; Ling, W. Supplementation of black rice pigment fraction improves antioxidant and anti-inflammatory status in patients with coronary heart disease. Asia Pac. J. Clin. Nutr. 2007, 16, 295–301. [Google Scholar]
- Eviana, R.; Widyastiti, N.; Mahati, E.; Syauqy, A.; Al-Baarri, A. Black rice (Oryza sativa L. indica) bran ethanolic extract improved insulin levels and total antioxidant capacity in type 2 diabetic rats. Food Res. 2023, 7, 361–367. [Google Scholar]
- Callcott, E.T.; Blanchard, C.L.; Snell, P.; Santhakumar, A.B. The anti-inflammatory and antioxidant effects of pigmented rice consumption in an obese cohort. Food Funct. 2019, 10, 8016–8025. [Google Scholar] [CrossRef]
- Ling, W.H.; Wang, L.L.; Ma, J. Supplementation of the black rice outer layer fraction to rabbits decreases atherosclerotic plaque formation and increases antioxidant status. J. Nutr. 2002, 132, 20–26. [Google Scholar] [CrossRef]
- Ling, W.H.; Cheng, Q.X.; Ma, J.; Wang, T. Red and black rice decrease atherosclerotic plaque formation and increase antioxidant status in rabbits. J. Nutr. 2001, 131, 1421–1426. [Google Scholar] [CrossRef]
- Schmid-Schönbein, G.W. Analysis of inflammation. Annu. Rev. Biomed. Eng. 2006, 8, 93–151. [Google Scholar] [CrossRef]
- Kumar Dhingra, A.; Chopra, B.; Dass, R.; Kumar Mittal, S. An update on anti-inflammatory compounds: A review. Anti-Inflamm. Anti-Allergy Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Inflamm. Anti-Allergy Agents) 2015, 14, 81–97. [Google Scholar] [CrossRef]
- Pattananandecha, T.; Sirithunyalug, J.; Sirithunyalug, B.; Thiankhanithikun, K.; Khanongnuch, C.; Saenjum, C. Bioactive compounds constituent and anti-inflammatory activity of natural rice bran oil produced from colored and non-pigmented rice in northern Thailand. J. Pharm. Nutr. Sci. 2019, 9, 205–212. [Google Scholar] [CrossRef]
- Choi, E.-Y.; Jang, Y.-A.; Lee, J.-T. The anti-inflammatory effect of ethanol extracts of colored rice varieties against lipopolysaccharide-stimulated RAW 264.7 cells. J. Korean Soc. Food Sci. Nutr. 2018, 47, 870–875. [Google Scholar] [CrossRef]
- Saenjum, C.; Chaiyasut, C.; Chansakaow, S.; Suttajit, M.; Sirithunyalug, B. Antioxidant and anti-inflammatory activities of gamma-oryzanol rich extracts from Thai purple rice bran. J. Med. Plants Res 2012, 6, 1070–1077. [Google Scholar]
- Pattananandecha, T.; Apichai, S.; Sirilun, S.; Julsrigival, J.; Sawangrat, K.; Ogata, F.; Kawasaki, N.; Sirithunyalug, B.; Saenjum, C. Anthocyanin profile, antioxidant, anti-inflammatory, and antimicrobial against foodborne pathogens activities of purple rice cultivars in Northern Thailand. Molecules 2021, 26, 5234. [Google Scholar] [CrossRef]
- Deeseenthum, S.; Luang-In, V.; Chunchom, S. Characteristics of Thai pigmented rice milk kefirs with potential as antioxidant and anti-inflammatory foods. Pharmacogn. J. 2018, 10, 154–161. [Google Scholar] [CrossRef]
- Min, S.-W.; Ryu, S.-N.; Kim, D.-H. Anti-inflammatory effects of black rice, cyanidin-3-O-β-d-glycoside, and its metabolites, cyanidin and protocatechuic acid. Int. Immunopharmacol. 2010, 10, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Okonogi, S.; Kaewpinta, A.; Junmahasathien, T.; Yotsawimonwat, S. Effect of rice variety and modification on antioxidant and anti-inflammatory activities. Drug Discov. Ther. 2018, 12, 206–213. [Google Scholar] [CrossRef]
- Bhawamai, S.; Lin, S.-H.; Hou, Y.-Y.; Chen, Y.-H. Thermal cooking changes the profile of phenolic compounds, but does not attenuate the anti-inflammatory activities of black rice. Food Nutr. Res. 2016, 60, 32941. [Google Scholar] [CrossRef]
- Callcott, E.T.; Thompson, K.; Oli, P.; Blanchard, C.L.; Santhakumar, A.B. Coloured rice-derived polyphenols reduce lipid peroxidation and pro-inflammatory cytokines ex vivo. Food Funct. 2018, 9, 5169–5175. [Google Scholar] [CrossRef]
- Zhao, M.; Huang, C.; Mao, Q.; Zhou, Z.; Xiang, Y.; Zhang, Q.; Lin, Y.; Chen, H. How anthocyanin biosynthesis affects nutritional value and anti-inflammatory effect of black rice. J. Cereal Sci. 2021, 101, 103295. [Google Scholar] [CrossRef]
- Munhoz, R.R.; Postow, M.A. Recent advances in understanding antitumor immunity. F1000Research 2016, 5, 2545. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, W.; Kumari, A.; Rahman, N.; Mandave, P. Anticancer activities of plant secondary metabolites: Rice callus suspension culture as a new paradigm. Rice Sci. 2021, 28, 13–30. [Google Scholar] [CrossRef]
- Koide, T.; Kamei, H.; Hashimoto, Y.; Kojima, T.; Hasegawa, M. Antitumor effect of hydrolyzed anthocyanin from grape rinds and red rice. Cancer Biother. Radiopharm. 1996, 11, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Lu, H.; Shen, M.; Yu, Q.; Chen, Y.; Wen, H.; Xie, J. Phytochemical composition, antioxidant activities and immunomodulatory effects of pigment extracts from Wugong Mountain purple red rice bran. Food Res. Int. 2022, 157, 111493. [Google Scholar] [CrossRef]
- Baek, J.-A.; Chung, N.-J.; Choi, K.-C.; Hwang, J.-M.; Lee, J.-C. Hull extracts from pigmented rice exert antioxidant effects associated with total flavonoid contents and induce apoptosis in human cancer cells. Food Sci. Biotechnol. 2015, 24, 241–247. [Google Scholar] [CrossRef]
- Lee, K.H.; Kwon, K.S.; Hwang, W.S.; Lee, W.Y.; Kim, J.; Lee, S.J.; Kim, S.P.; Friedman, M. Bioprocessed Black Rice Bran Potentiates the Growth Inhibitory Activity of an Immune Checkpoint Inhibitor against Murine Colon Carcinoma. Food Nutr. Sci. 2023, 14, 1149–1171. [Google Scholar] [CrossRef]
- Hartati, F.K.; Djauhari, A.B. Potential of black rice (Oryza sativa L.) as anticancer through mortalin-p53 complex inhibitors. Biointerface Res. Appl. Chem 2020, 10, 6174–6181. [Google Scholar]
- Chung SooIm, C.S.; Lee SangChul, L.S.; Yi SeongJoon, Y.S.; Kang MiYoung, K.M. Antioxidative and antiproliferative activities of ethanol extracts from pigmented giant embryo rice (Oryza sativa L. cv. Keunnunjami) before and after germination. Nutr. Res. Pract. 2018, 12, 365–370. [Google Scholar]
- Park, S.-H.; Cho, I.-J.; Kim, Y.-S.; Ha, T.-Y. Effect of methanol extracts of red colored rices on antioxidant activity and growth inhibitory activities of cancer cells. J. Korean Soc. Food Sci. Nutr. 2007, 36, 1365–1370. [Google Scholar] [CrossRef]
- Suttiarporn, P.; Chumpolsri, W.; Mahatheeranont, S.; Luangkamin, S.; Teepsawang, S.; Leardkamolkarn, V. Structures of phytosterols and triterpenoids with potential anti-cancer activity in bran of black non-glutinous rice. Nutrients 2015, 7, 1672–1687. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, J.; He, H.; Wang, Y.; Zhao, Y.; Lu, Q.; Qin, Y.; Ke, Y.; Peng, Y. Green synthesis of silver nanoparticles with black rice (Oryza sativa L.) extract endowing carboxymethyl chitosan modified cotton with high anti-microbial and durable properties. Cellulose 2021, 28, 1827–1842. [Google Scholar] [CrossRef]
- Sasaki, D.; Suzuki, H.; Kusamori, K.; Itakura, S.; Todo, H.; Nishikawa, M. Development of rice bran-derived nanoparticles with excellent anti-cancer activity and their application for peritoneal dissemination. J. Nanobiotechnology 2024, 22, 114. [Google Scholar] [CrossRef] [PubMed]
- Almand, E.A.; Moore, M.D.; Jaykus, L.-A. Virus-bacteria interactions: An emerging topic in human infection. Viruses 2017, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Wijayanti, E.D.; Safitri, A.; Siswanto, D.; Triprisila, L.F.; Fatchiyah, F. Antimicrobial activity of ferulic acid in Indonesian purple rice through toll-like receptor signaling. Makara J. Sci. 2021, 25, 7. [Google Scholar]
- Chandramouli, B.; Latha, M.M.; Narendra, K.; Mallikarjuna, K. Phytochemical and antimicrobial investigations of methanolic seed extract of black rice (Oryza sativa L.) mentioned in an ancient palm leaf manuscript (talapatra). World J. Pharm. Res. 2017, 7, 598–616. [Google Scholar]
- Marhamah, H.A.; Handayani, H.; Horax, S.; Fajriani, N.H.G.B.; Arief, S.M. Inhibition of Oral Bacterial Growth (Streptococcus Mutans and Porphyromonas Gingivalis) using Black Rice Bran Ethanol Extract (Oryza sativa L.) as A Natural Mouthwash. Syst. Rev. Pharm. 2020, 11, 667–668. [Google Scholar]
- Kim, S.-H.; Woo, H.J.; Lee, M.H.; Park, M.; Nagendran, T.; Rhee, K.-J.; Lee, D.; Jin, Y.-B.; Choi, S.-W.; Seo, W.D. Antimicrobial effects of black rice extract on Helicobacter pylori infection in Mongolian gerbil. J. Cereal Sci. 2019, 85, 1–5. [Google Scholar] [CrossRef]
- Khairi, N.; Hapiwaty, S.; Indrisari, M.; Marwati, M. Activity, formulation and effectiveness of black rice extract (Oryza sativa L.) gel against staphylococcus aures and escherichia coli bacteria. J. Penelit. Pendidik. IPA 2023, 9, 4273–4278. [Google Scholar] [CrossRef]
- Lai, S.-F.; Chen, Y.-W.; Lee, S.-M.; Huang, H.-Y.; Huang, Y.-H.; Lu, Y.-C.; Chen, C.-W. Development and Optimization of Black Rice (Oryza sativa L.) Sourdough Fermented by Levilactobacillus brevis LUC 247 for Physicochemical Characteristics and Antioxidant Capacity. Foods 2023, 12, 1389. [Google Scholar] [CrossRef]
- Santoso, I.; Fadhilah, Q.G.; Maryanto, A.E.; Dwiranti, A.; Wang, P.; Al-Rais, M.F.; Sigar, I.M. Characteristics of isolated lactic acid bacilli bacteria from black glutinous rice (Oryza sativa L.) tapai and its antimicrobial activity in mung bean (Vigna radiata L.) milk. Kuwait J. Sci. 2024, 51, 100161. [Google Scholar] [CrossRef]
- Ninfali, P.; Antonelli, A.; Magnani, M.; Scarpa, E.S. Antiviral properties of flavonoids and delivery strategies. Nutrients 2020, 12, 2534. [Google Scholar] [CrossRef] [PubMed]
- O Moustafa, G. Synthesis of Dibenzofurans Possessing Anti-Allergy, Antioxidant, Anti-Inflammatory, Antimalarial and Treatment of Skin Conditions. Egypt. J. Chem. 2021, 64, 2539–2556. [Google Scholar] [CrossRef]
- Raman, A.P.S.; Singh, M.B.; Vishvakarma, V.K.; Jain, P.; Kumar, A.; Sachdeva, S.; Kumari, K.; Singh, P. An investigation for the interaction of gamma oryzanol with the Mpro of SARS-CoV-2 to combat COVID-19: DFT, molecular docking, ADME and molecular dynamics simulations. J. Biomol. Struct. Dyn. 2023, 41, 1919–1929. [Google Scholar] [CrossRef]
- Antonopoulou, I.; Sapountzaki, E.; Rova, U.; Christakopoulos, P. Ferulic acid from plant biomass: A phytochemical with promising antiviral properties. Front. Nutr. 2022, 8, 777576. [Google Scholar] [CrossRef]
- Semmarath, W.; Mapoung, S.; Umsumarng, S.; Arjsri, P.; Srisawad, K.; Thippraphan, P.; Yodkeeree, S.; Dejkriengkraikul, P. Cyanidin-3-O-glucoside and peonidin-3-O-glucoside-rich fraction of black rice germ and bran suppresses inflammatory responses from SARS-CoV-2 spike glycoprotein S1-induction in vitro in A549 lung cells and THP-1 macrophages via inhibition of the NLRP3 inflammasome pathway. Nutrients 2022, 14, 2738. [Google Scholar] [CrossRef] [PubMed]
- Suantai, B.; Jantakee, K.; Kaewkod, T.; Sangboonruang, S.; Chitov, T.; Tragoolpua, Y. Anthocyanins in red jasmine rice (Oryza sativa L.) extracts and efficacy on inhibition of herpes simplex virus, free radicals and cancer cell. Nutrients 2022, 14, 1905. [Google Scholar] [CrossRef]
- Suantai, B.; Tragoolpua, Y. Biological properties of extracts from rice berry and black rice for inhibition of herpes simplex viruses, phytochemical compounds, and antioxidant activities. In Proceedings of the RSU International Research Conference; Rangsit University: Pathum, Thailand, 2020; pp. 646–653. [Google Scholar]
- Duckworth, W.C. Hyperglycemia and cardiovascular disease. Curr. Atheroscler. Rep. 2001, 3, 383–391. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. A comprehensive review on anti-diabetic property of rice bran. Asian Pac. J. Trop. Biomed. 2018, 8, 79–84. [Google Scholar] [CrossRef]
- Agustin, A.T.; Safitri, A.; Fatchiyah, F. Java red rice (Oryza sativa L.) nutritional value and anthocyanin profiles and its potential role as antioxidant and anti-diabetic. Indones. J. Chem. 2021, 21, 968–978. [Google Scholar] [CrossRef]
- Ogunyemi, O.M.; Gyebi, G.A.; Saheed, A.; Paul, J.; Nwaneri-Chidozie, V.; Olorundare, O.; Adebayo, J.; Koketsu, M.; Aljarba, N.; Alkahtani, S. Inhibition mechanism of alpha-amylase, a diabetes target, by a steroidal pregnane and pregnane glycosides derived from Gongronema latifolium Benth. Front. Mol. Biosci. 2022, 9, 866719. [Google Scholar] [CrossRef]
- Almundarij, T.I.; Zaki, A.K.A.; Albarrak, S.M.; Alharbi, Y.M.; Almuzaini, S.A.; Abo-Aziza, F.A. Evaluation of the anti-diabetic activities of colored rice varieties in streptozotocin-induced diabetic rats. Syst. Rev. Pharm 2020, 11, 1424–1433. [Google Scholar]
- Kim, H.-Y.; Kim, J.-H.; Lee, S.; Ryu, S.-N.; Han, S.-J.; Hong, S.-G. Antioxidative and anti-diabetic activity of C3GHi, novel black rice breed. Korean J. Crop Sci. 2010, 55, 38–46. [Google Scholar]
- Cho, E.-K.; Jung, K.-I.; Choi, Y.-J. Anti-inflammatory and antidiabetic effects of brown rice (Oryza sativa L.) extracts. J. Life Sci. 2012, 22, 126–131. [Google Scholar] [CrossRef]
- Aalim, H.; Wang, D.; Luo, Z. Black rice (Oryza sativa L.) processing: Evaluation of physicochemical properties, in vitro starch digestibility, and phenolic functions linked to type 2 diabetes. Food Res. Int. 2021, 141, 109898. [Google Scholar] [CrossRef]
- Boue, S.M.; Daigle, K.W.; Chen, M.-H.; Cao, H.; Heiman, M.L. Antidiabetic potential of purple and red rice (Oryza sativa L.) bran extracts. J. Agric. Food Chem. 2016, 64, 5345–5353. [Google Scholar] [CrossRef]
- Ghatak, S.B.; Panchal, S.S. Anti-diabetic activity of oryzanol and its relationship with the anti-oxidant property. Int. J. Diabetes Dev. Ctries. 2012, 32, 185–192. [Google Scholar] [CrossRef]
- Song, L.; Zhang, S. Anti-aging activity and modes of action of compounds from natural food sources. Biomolecules 2023, 13, 1600. [Google Scholar] [CrossRef]
- Lipsky, M.S.; King, M. Biological theories of aging. Dis.-A-Mon. 2015, 61, 460–466. [Google Scholar] [CrossRef]
- Choi, M.J.; Kim, H.Y.; Cho, E.J. Anti-aging effect of black rice against H2O2-induced premature senescence. J. Med. Plants Res. 2012, 6, 3672–3680. [Google Scholar]
- Wijayanti, E.D.; Safitri, A.; Siswanto, D.; Fatchiyah, F. Indonesian purple rice ferulic acid as a candidate for anti-aging through the inhibition of collagenase and tyrosinase activities. Indones. J. Chem. 2023, 23, 475–488. [Google Scholar] [CrossRef]
- Poomanee, W.; Wattananapakasem, I.; Panjan, W.; Kiattisin, K. Optimizing anthocyanins extraction and the effect of cold plasma treatment on the anti-aging potential of purple glutinous rice (Oryza sativa L.) extract. Cereal Chem. 2021, 98, 571–582. [Google Scholar] [CrossRef]
- Yodkeeree, S.; Thippraphan, P.; Punfa, W.; Srisomboon, J.; Limtrakul, P. Skin anti-aging assays of proanthocyanidin rich red rice extract, oryzanol and other phenolic compounds. Nat. Prod. Commun. 2018, 13, 1934578X1801300812. [Google Scholar] [CrossRef]
- Saewan, N.; Vichit, W.; Prinyarux, T. Anti-aging efficacy of Thai red rice callus cosmetic product. J. Appl. Sci. Emerg. Technol. 2018, 17, 63–72. [Google Scholar]
- Vitchit, W.; Saewan, N. Anti-Oxidant and Anti-Aging Activities of Callus Culture from Three Rice Varieties. Cosmetics 2022, 9, 79. [Google Scholar] [CrossRef]
- Choi, E.-Y.; Lee, J.-T. The effects of antioxidant and anti-aging treatment of UVB-irradiated human HaCaT keratinocytes with ethanol extracts of colored rice varieties. Korean J. Food Sci. Technol. 2018, 50, 653–659. [Google Scholar]
- Kanlayavattanakul, M.; Lourith, N.; Chaikul, P. Jasmine rice panicle: A safe and efficient natural ingredient for skin aging treatments. J. Ethnopharmacol. 2016, 193, 607–616. [Google Scholar] [CrossRef]
- Chaikul, P.; Kanlayavattanakul, M.; Khongkow, M.; Jantimaporn, A.; Lourith, N. Anti-skin ageing activities of rice (Oryza sativa) bran soft and hard waxes in cultured skin cells. Int. J. Cosmet. Sci. 2024, 46, 162–174. [Google Scholar] [CrossRef]
- González-Fuentes, J.; Selva, J.; Moya, C.; Castro-Vázquez, L.; Lozano, M.V.; Marcos, P.; Plaza-Oliver, M.; Rodríguez-Robledo, V.; Santander-Ortega, M.J.; Villaseca-González, N. Neuroprotective natural molecules, from food to brain. Front. Neurosci. 2018, 12, 721. [Google Scholar] [CrossRef]
- Rojas-García, A.; Fernández-Ochoa, Á.; Cádiz-Gurrea, M.d.l.L.; Arráez-Román, D.; Segura-Carretero, A. Neuroprotective effects of agri-food by-products rich in phenolic compounds. Nutrients 2023, 15, 449. [Google Scholar] [CrossRef]
- Singh, D.; Hembrom, S.; Raj, A. Neuroprotective effect of flavonoids: A systematic review. J. Pharmacogn. Phytochem. 2019, 8, 699–707. [Google Scholar]
- Li, P.; Feng, D.; Yang, D.; Li, X.; Sun, J.; Wang, G.; Tian, L.; Jiang, X.; Bai, W. Protective effects of anthocyanins on neurodegenerative diseases. Trends Food Sci. Technol. 2021, 117, 205–217. [Google Scholar] [CrossRef]
- Pannangrong, W.; Wattanathorn, J.; Muchimapura, S.; Tiamkao, S.; Tong-Un, T. Purple rice berry is neuroprotective and enhances cognition in a rat model of Alzheimer’s disease. J. Med. Food 2011, 14, 688–694. [Google Scholar] [CrossRef]
- Thummayot, S.; Tocharus, C.; Pinkaew, D.; Viwatpinyo, K.; Sringarm, K.; Tocharus, J. Neuroprotective effect of purple rice extract and its constituent against amyloid beta-induced neuronal cell death in SK-N-SH cells. Neurotoxicology 2014, 45, 149–158. [Google Scholar] [CrossRef]
- Vargas, C.G.; da Silva Junior, J.D.; Rabelo, T.K.; Moreira, J.C.F.; Gelain, D.P.; Rodrigues, E.; Augusti, P.R.; de Oliveira Rios, A.; Flôres, S.H. Bioactive compounds and protective effect of red and black rice brans extracts in human neuron-like cells (SH-SY5Y). Food Res. Int. 2018, 113, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Chompoopong, S.; Jarungjitaree, S.; Punbanlaem, T.; Rungruang, T.; Chongthammakun, S.; Kettawan, A.; Taechowisan, T. Neuroprotective effects of germinated brown rice in rotenone-induced Parkinson’s-like disease rats. Neuromol. Med. 2016, 18, 334–346. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, P. The beneficial effect of rice bran extract against rotenone-induced experimental Parkinson’s disease in rats. Curr. Mol. Pharmacol. 2021, 14, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.E.; Kim, J.Y.; Song, W.T.; Lee, S.H.; Hong, J.H.; Lee, C.K.; Kang, S.G. Neuroprotective effect of rice bran extract supplemented with ferulic acid in the rat model of ischemic brain injury. Anim. Cells Syst. 2014, 18, 93–100. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Yin, L.; Huang, L.; Tekliye, M.; Xia, X.; Li, J.; Dong, M. Composition, antioxidant activity, and neuroprotective effects of anthocyanin-rich extract from purple highland barley bran and its promotion on autophagy. Food Chem. 2021, 339, 127849. [Google Scholar] [CrossRef]
- Srisuwan, S.; Arkaravichien, T.; Mahatheeranont, S.; Puangsombat, P.; Seekhaw, P.; Noenplab, A.N.L.; Sattayasai, J. Effects of aqueous extract of unpolished dark purple glutinous rice, Var Luem Pua, on ROS in SK-N-SH cells and scopolamine-induced memory deficit in mice. Trop. J. Pharm. Res. 2015, 14, 1635–1641. [Google Scholar] [CrossRef]
- Kelsey, N.; Hulick, W.; Winter, A.; Ross, E.; Linseman, D. Neuroprotective effects of anthocyanins on apoptosis induced by mitochondrial oxidative stress. Nutr. Neurosci. 2011, 14, 249–259. [Google Scholar] [CrossRef]
- Rungtip, S.-a.; Sarawut, J.; Surin, K.; Sompong, L.; Wanphen, K.; Supranee, U. Antioxidative and neuroprotective activities of the pre-germinated brown rice extract. Food Nutr. Sci. 2012, 3, 135–140. [Google Scholar]
- Pramoolsilpa, G.; Nudmamud-Thanoi, S.; Khongsombat, O. The neuroprotective effects of germinated black glutinous rice diet on Aβ25-35 peptide induced learning and memory deficits in male rats. Thai. J. Pharmacol. 2017, 39, 49. [Google Scholar]
- Yadav, S.; Appukuttan, J.P. Inhibition of LPS induced neurochemical imbalance and oxidative stress by pigmented and non-pigmented rice bran extracts. J. Food Biochem. 2019, 43, e12735. [Google Scholar] [CrossRef]
- Golzarand, M.; Toolabi, K.; Eskandari Delfan, S.; Mirmiran, P. The effect of brown rice compared to white rice on adiposity indices, lipid profile, and glycemic markers: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2022, 62, 7395–7412. [Google Scholar] [CrossRef]
- Sungthong, B.; Yoothaekool, C.; Promphamorn, S.; Phimarn, W. Efficacy of red yeast rice extract on myocardial infarction patients with borderline hypercholesterolemia: A meta-analysis of randomized controlled trials. Sci. Rep. 2020, 10, 2769. [Google Scholar] [CrossRef]
- Rahmani, P.; Melekoglu, E.; Tavakoli, S.; Malekpour Alamdari, N.; Rohani, P.; Sohouli, M.H. Impact of red yeast rice supplementation on lipid profile: A systematic review and meta-analysis of randomized-controlled trials. Expert Rev. Clin. Pharmacol. 2023, 16, 73–81. [Google Scholar] [CrossRef]
- Xu, G.; Lin, M.; Dai, X.; Hu, J. Comparing the effectiveness of Chinese patent medicines containing red yeast rice on hyperlipidaemia: A network meta-analysis of randomized controlled trials. Endocrinol. Diabetes Metab. 2022, 5, e00314. [Google Scholar] [CrossRef]
- Jung, A.J.; Sharma, A.; Lee, S.-H.; Lee, S.-J.; Kim, J.-H.; Lee, H.-J. Efficacy of black rice extract on obesity in obese postmenopausal women: A 12-week randomized, double-blind, placebo-controlled preliminary clinical trial. Menopause 2021, 28, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Jufri, M.; Vardhani, A.; Purwaningsih, E. Evaluating the efficacy of lotion containing black rice bran (Oryza sativa L. indica) extract as skin brightening agent: A clinical trial. Jundishapur J. Nat. Pharm. Prod 2021, 16, e114152. [Google Scholar]
- Ghorbani, Z.; Shoaibinobarian, N.; Zamani, E.; Salari, A.; Mahdavi-Roshan, M.; Porteghali, P.; Ahmadnia, Z. Supplementing the standard diet with brown rice bran powder might effectively improve the metabolic syndrome characteristics and antioxidant status: An open label randomized controlled trial. Food Funct. 2025, 16, 750–762. [Google Scholar] [CrossRef]
- Seesen, M.; Semmarath, W.; Yodkeeree, S.; Sapbamrer, R.; Ayood, P.; Malasao, R.; Ongprasert, K.; Chittrakul, J.; Siviroj, P.; Limtrakul, P. Combined black rice germ, bran supplement and exercise intervention modulate aging biomarkers and improve physical performance and lower-body muscle strength parameters in aging population. Int. J. Environ. Res. Public Health 2020, 17, 2931. [Google Scholar] [CrossRef]
- Teparak, C.; Uriyapongson, J.; Phoemsapthawee, J.; Tunkamnerdthai, O.; Aneknan, P.; Tong-un, T.; Panthongviriyakul, C.; Leelayuwat, N.; Alkhatib, A. Diabetes Therapeutics of Prebiotic Soluble Dietary Fibre and Antioxidant Anthocyanin Supplement in Patients with Type 2 Diabetes: Randomised Placebo-Controlled Clinical Trial. Nutrients 2025, 17, 1098. [Google Scholar] [CrossRef] [PubMed]
- Yonei, Y.; Uenaka, S.; Yagi, M.; Wickramasinghe, U.P.P.; Kawakami, M.; Yamaguchi, R.; Nishiyama, N.; Keiji, S.; Ogura, M. Effects on skin by dewaxed brown rice: An open label test. Glycative Stress Res. 2021, 8, 29–38. [Google Scholar]
- Cicero, A.F.; D’Addato, S.; Borghi, C. A randomized, double-blinded, placebo-controlled, clinical study of the effects of a nutraceutical combination (LEVELIP DUO®) on LDL cholesterol levels and lipid pattern in subjects with sub-optimal blood cholesterol levels (NATCOL study). Nutrients 2020, 12, 3127. [Google Scholar] [CrossRef] [PubMed]
- Minamizuka, T.; Koshizaka, M.; Shoji, M.; Yamaga, M.; Hayashi, A.; Ide, K.; Ide, S.; Kitamoto, T.; Sakamoto, K.; Hattori, A. Low dose red yeast rice with monacolin K lowers LDL cholesterol and blood pressure in Japanese with mild dyslipidemia: A multicenter, randomized trial. Asia Pac. J. Clin. Nutr. 2021, 30, 424–435. [Google Scholar]
- Ganapathyswamy, H.; Subramani, T.; Kalaivanan, K.; Pannerselvam, A.; Sundararajan, A. Effect of Germinated Brown Rice Supplementation on Prediabetes Markers: A 120-Day Dietary Intervention Study in Selected Female Groups. Rice Sci. 2025, 32, 11. [Google Scholar] [CrossRef]
- Anuyahong, T.; Chusak, C.; Thilavech, T.; Adisakwattana, S. Postprandial effect of yogurt enriched with anthocyanins from riceberry rice on glycemic response and antioxidant capacity in healthy adults. Nutrients 2020, 12, 2930. [Google Scholar] [CrossRef]
- Pongnaratorn, P.; Kaewsoongnern, T.; Nukulkit, C.; Pakdee, N.; Sriset, Y.; Ohnon, W. The Effect of an 8-Week Germinated Black Rice Extract Consumption on Cognitive Functions in Healthy Middle-Aged and Elderly Volunteers: A Randomized Controlled Trial. Trends Sci. 2024, 21, 7981. [Google Scholar] [CrossRef]
- Ou, S.J.L.; Yang, D.; Pranata, H.P.; Tai, E.S.; Liu, M.H. Postprandial glycemic and lipidemic effects of black rice anthocyanin extract fortification in foods of varying macronutrient compositions and matrices. npj Sci. Food 2023, 7, 59. [Google Scholar] [CrossRef]
- Cosomn, U.; Hettiarachchi, P.; Wanigasuriya, K.; Perera, R.; Jayewardenepura, G.; Lanka, S. Glycemic and Lipid Metabolic Markers in Type 2 Diabetes Mellitus Patients after Consuming Red Pigmented Parboiled Rice as a Staple—A Clinical Trial. Food Sci. Nutr. Stud. 2017, 1, 122–134. [Google Scholar] [CrossRef]
- Khusnutdinov, E.; Sukhareva, A.; Panfilova, M.; Mikhaylova, E. Anthocyanin biosynthesis genes as model genes for genome editing in plants. Int. J. Mol. Sci. 2021, 22, 8752. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zuo, Z.; Yang, Z. Toward breeding pigmented rice balancing nutrition and yield. Trends Plant Sci. 2024, 29, 504–506. [Google Scholar] [CrossRef] [PubMed]
- Sedeek, K.; Zuccolo, A.; Fornasiero, A.; Weber, A.M.; Sanikommu, K.; Sampathkumar, S.; Rivera, L.F.; Butt, H.; Mussurova, S.; Alhabsi, A. Multi-omics resources for targeted agronomic improvement of pigmented rice. Nat. Food 2023, 4, 366–371. [Google Scholar] [CrossRef] [PubMed]
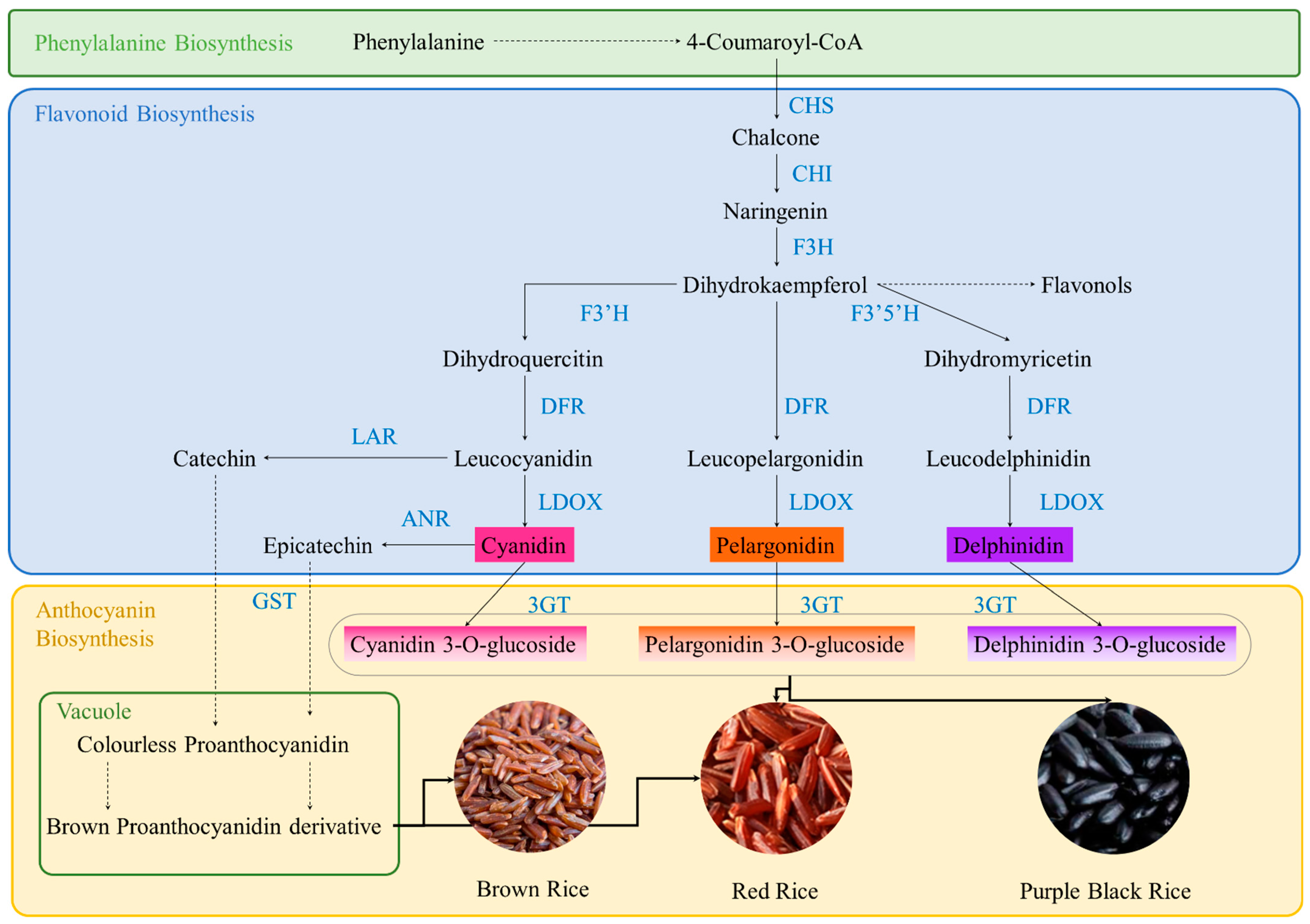
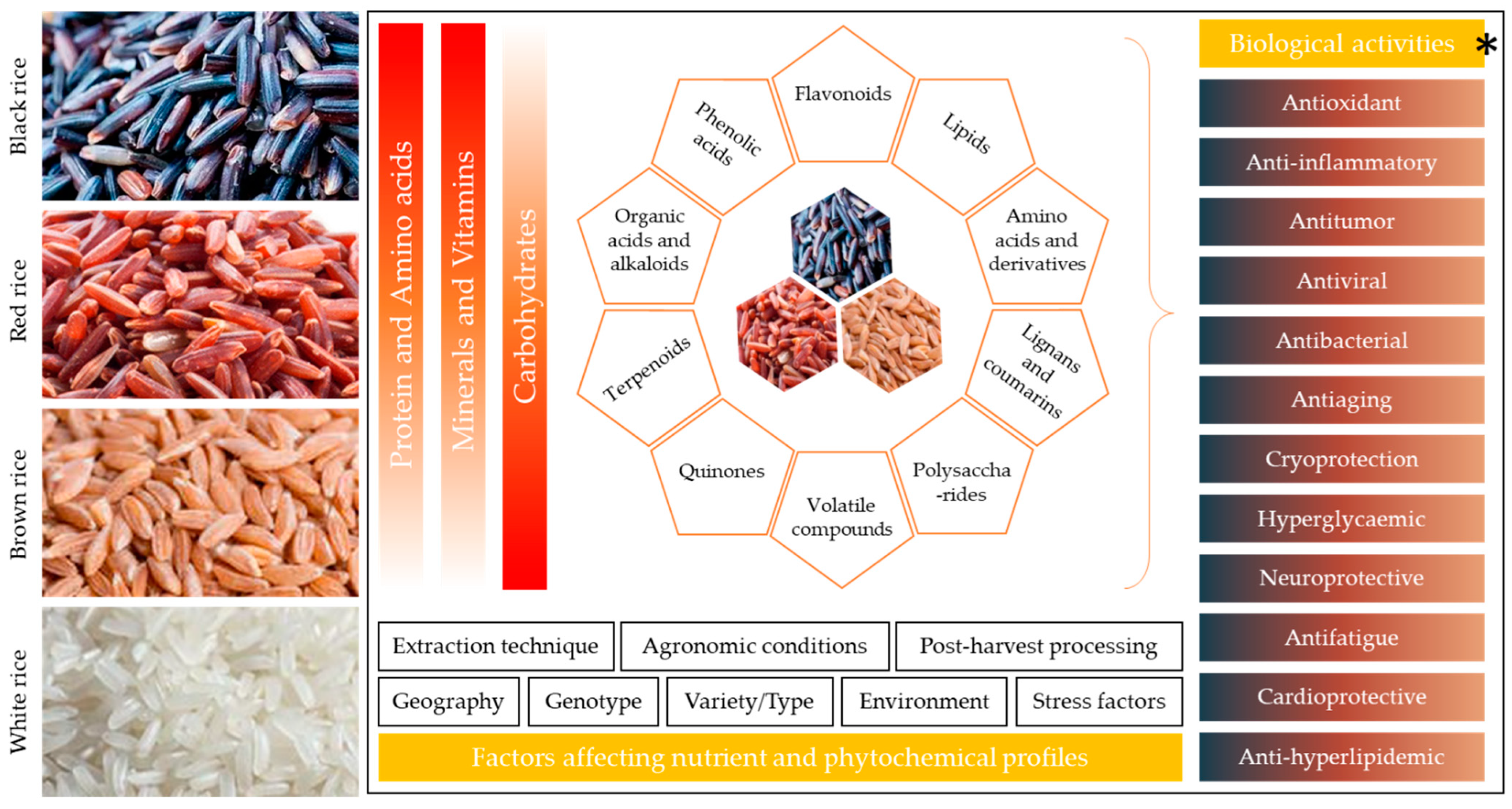
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Xiao, X.; Jin, D.; Zhai, L.; Li, Y.; Yang, Q.; Xing, F.; Qiao, W.; Yan, X.; Tang, Q. Composition and Biological Activity of Colored Rice—A Comprehensive Review. Foods 2025, 14, 1394. https://doi.org/10.3390/foods14081394
Zhao M, Xiao X, Jin D, Zhai L, Li Y, Yang Q, Xing F, Qiao W, Yan X, Tang Q. Composition and Biological Activity of Colored Rice—A Comprehensive Review. Foods. 2025; 14(8):1394. https://doi.org/10.3390/foods14081394
Chicago/Turabian StyleZhao, Mingchao, Xiaorong Xiao, Dingsha Jin, Linan Zhai, Yapeng Li, Qingwen Yang, Funeng Xing, Weihua Qiao, Xiaowei Yan, and Qingjie Tang. 2025. "Composition and Biological Activity of Colored Rice—A Comprehensive Review" Foods 14, no. 8: 1394. https://doi.org/10.3390/foods14081394
APA StyleZhao, M., Xiao, X., Jin, D., Zhai, L., Li, Y., Yang, Q., Xing, F., Qiao, W., Yan, X., & Tang, Q. (2025). Composition and Biological Activity of Colored Rice—A Comprehensive Review. Foods, 14(8), 1394. https://doi.org/10.3390/foods14081394










