Lactobacillus murinus ZNL-13 Modulates Intestinal Barrier Damage and Gut Microbiota in Cyclophosphamide-Induced Immunosuppressed Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Experimental Animals and Sample Collection
2.3. Body Weight and Immune Organ Weight Analysis
2.4. Blood Cell and Serological Analysis
2.5. Histological Analysis
2.6. mRNA Detection in the Intestine
2.7. Western Blot Analysis
2.8. 16S rRNA Intestinal Content Microbiota Analysis
2.9. Statistical Analysis
2.10. ARRIVE Guidelines
3. Result
3.1. L. murinus ZNL-13 Enhances the Growth Performance and Production of Immune Cells in CTX Mice
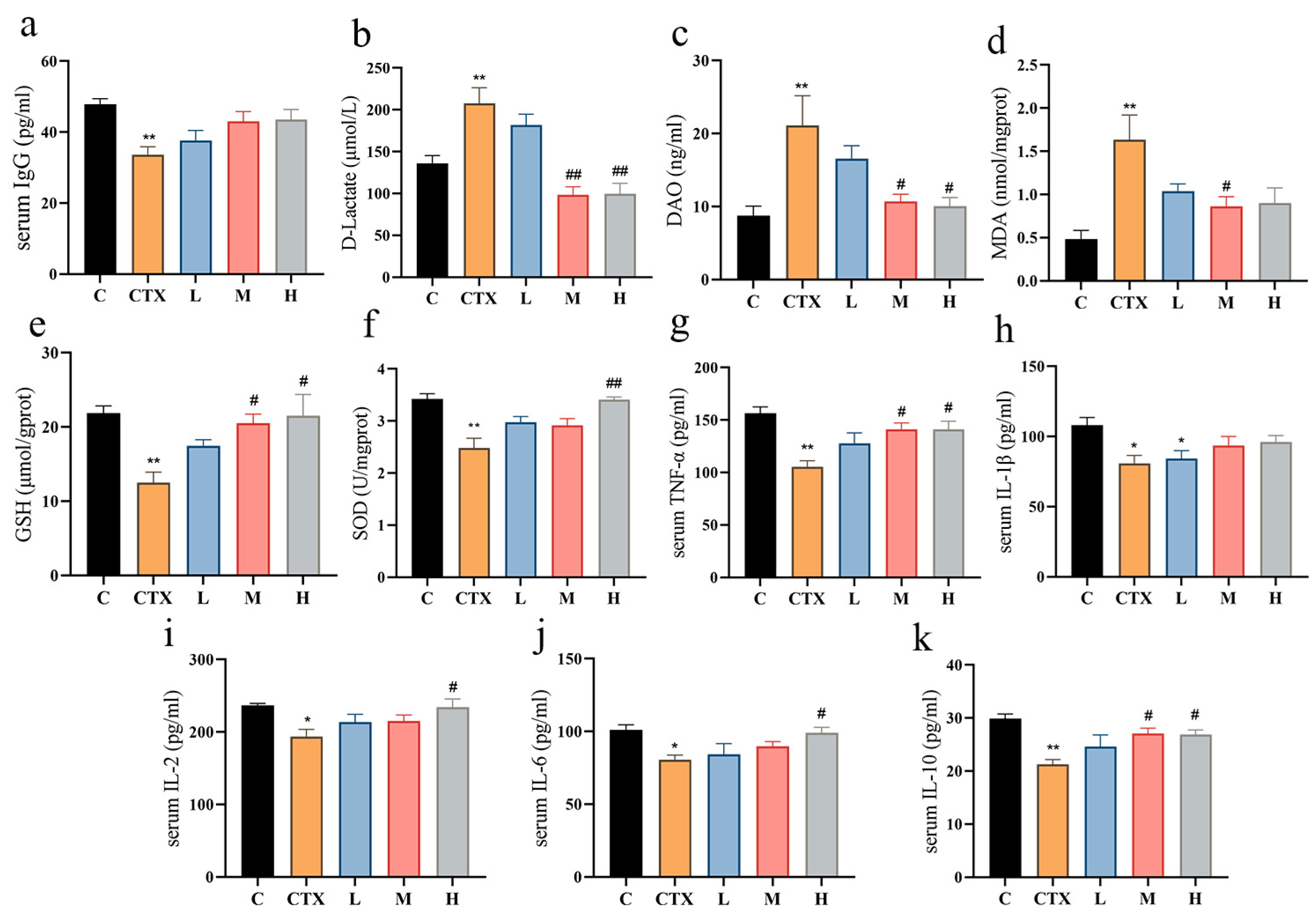
3.2. L. murinus ZNL-13 Alleviates CTX-Induced Oxidative Stress, Immune-Related Cytokines, and Enhances Intestinal Function
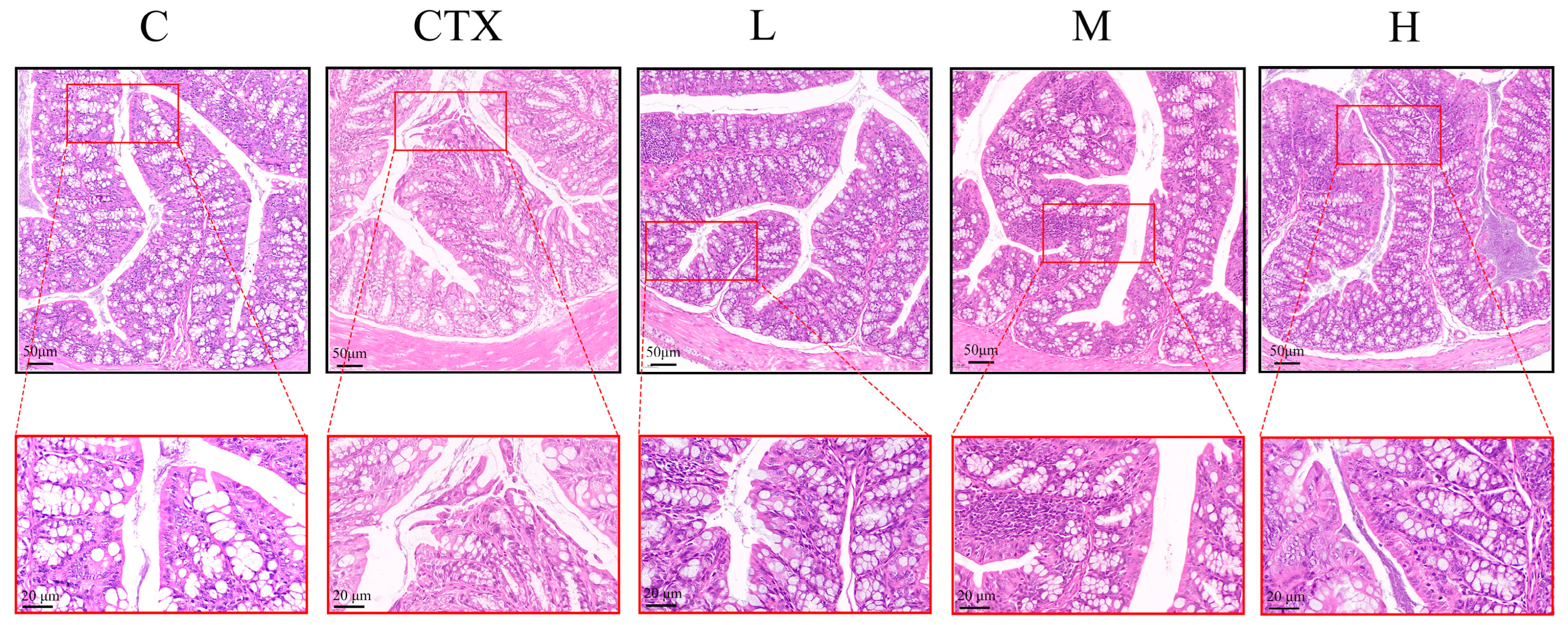
3.3. L. murinus ZNL-13 Alleviates the Damage to Colonic Tissues Induced by CTX
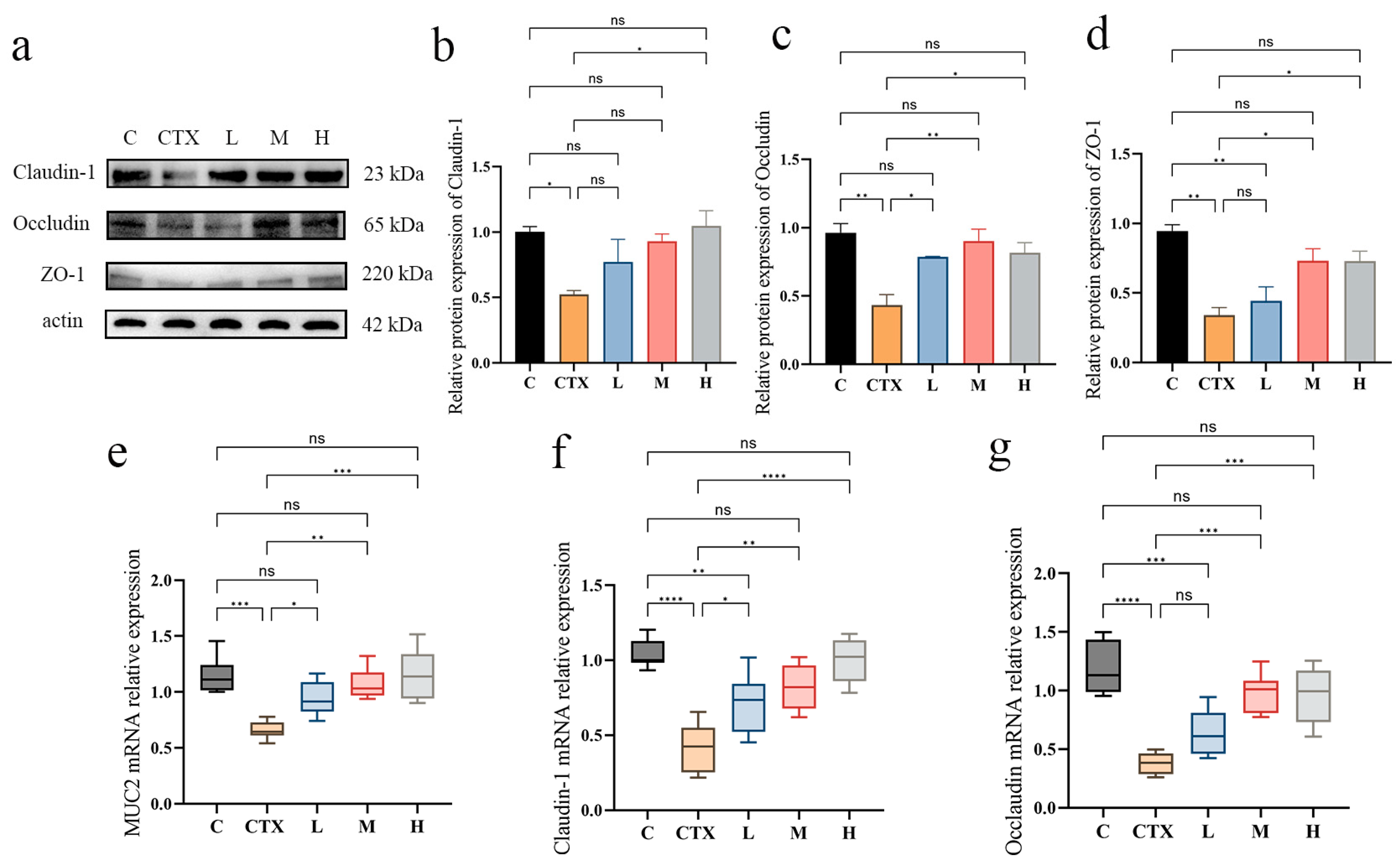
3.4. Effects of L. murinus ZNL-13 on Key Proteins in Colon of CTX Mice
3.5. L. murinus ZNL-13 Effectively Attenuates CTX-Induced Apoptosis in Intestinal Tissue Cells
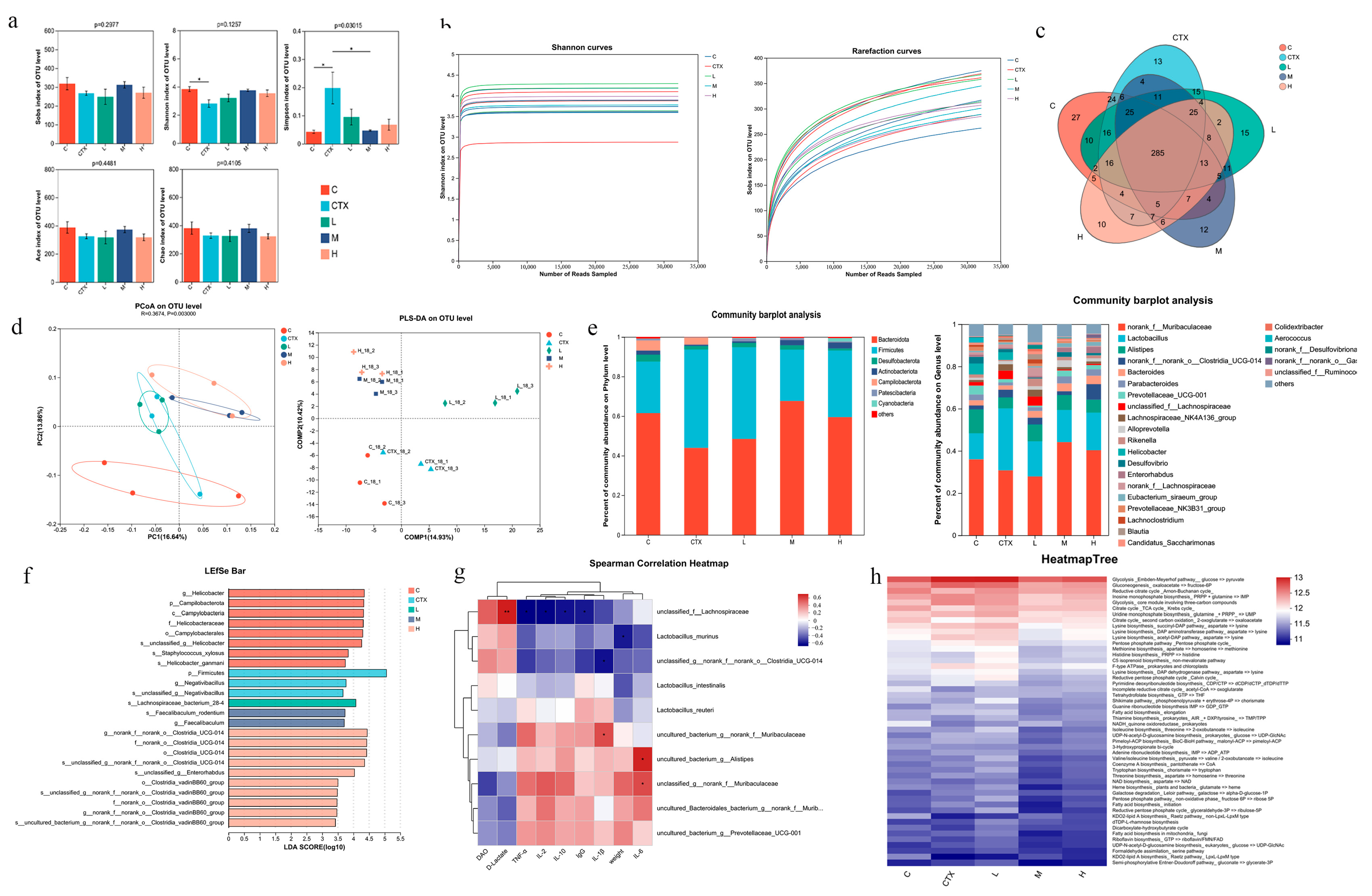
3.6. The Effect of L. murinus ZNL-13 on the Intestinal Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Emadi, A.; Jones, R.J.; Brodsky, R.A. Cyclophosphamide and cancer: Golden anniversary. Nat. Rev. Clin. Oncol. 2009, 6, 638–647. [Google Scholar] [CrossRef]
- Ahmed, A.R.; Hombal, S.M. Cyclophosphamide (Cytoxan). A review on relevant pharmacology and clinical uses. J. Am. Acad. Dermatol. 1984, 11, 1115–1126. [Google Scholar] [CrossRef]
- Hu, N.; Qu, Y.; Liu, T.Y.; Zhou, Y.; Liu, C.; Wang, J.H.; Yang, B.F.; Li, C.L. Immunomodulatory effects and mechanisms of Tiepishihu Xiyangshen granules on cyclophosphamide induced immuno-suppression via TLR4/MAPKs and PI3K/AKT/FOXO3a signal pathways. J. Ethnopharmacol. 2023, 307, 116192. [Google Scholar] [CrossRef] [PubMed]
- Brock, N.; Wilmanns, H. Effect of a cyclic nitrogen mustard-phosphamidester on experimentally induced tumors in rats; chemotherapeutic effect and pharmacological properties of B 518 ASTA. Dtsch. Med. Wochenschr. 1958, 83, 453–458. [Google Scholar] [CrossRef]
- Xie, H.; Fang, J.; Farag, M.A.; Li, Z.; Sun, P.; Shao, P. Dendrobium officinale leaf polysaccharides regulation of immune response and gut microbiota composition in cyclophosphamide-treated mice. Food Chem. X 2022, 13, 100235. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yan, Y.; Chen, D.; Ran, L.; Mi, J.; Lu, L.; Jing, B.; Li, X.; Zeng, X.; Cao, Y. Modulating effects of polysaccharides from the fruits of Lycium barbarum on the immune response and gut microbiota in cyclophosphamide-treated mice. Food Funct. 2019, 10, 3671–3683. [Google Scholar] [CrossRef]
- Zhou, R.; He, D.; Xie, J.; Zhou, Q.; Zeng, H.; Li, H.; Huang, L. The Synergistic Effects of Polysaccharides and Ginsenosides from American Ginseng (Panax quinquefolius L.) Ameliorating Cyclophosphamide-Induced Intestinal Immune Disorders and Gut Barrier Dysfunctions Based on Microbiome-Metabolomics Analysis. Front. Immunol. 2021, 12, 665901. [Google Scholar] [CrossRef] [PubMed]
- Ou, Z.; Zhu, L.; Huang, C.; Ma, C.; Kong, L.; Lin, X.; Gao, X.; Huang, L.; Wen, L.; Liang, Z.; et al. Betulinic acid attenuates cyclophosphamide-induced intestinal mucosa injury by inhibiting the NF-κB/MAPK signalling pathways and activating the Nrf2 signalling pathway. Ecotoxicol. Environ. Saf. 2021, 225, 112746. [Google Scholar] [CrossRef]
- Zhao, F.; Ma, T.; Zhang, X.; Zhao, Q.; Zhu, K.; Cao, J.; Liu, Z.; Shen, X.; Li, C. Holothuria leucospilota Polysaccharides Improve Immunity and the Gut Microbiota in Cyclophosphamide-Treated Immunosuppressed Mice. Mol. Nutr. Food Res. 2023, 67, e2200317. [Google Scholar] [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef]
- Keely, S.J.; Barrett, K.E. Intestinal secretory mechanisms and diarrhea. Am. J. Physiol. Gastrointest. Liver Physiol. 2022, 322, G405–G420. [Google Scholar] [CrossRef] [PubMed]
- Poller, W.C.; Downey, J.; Mooslechner, A.A.; Khan, N.; Li, L.; Chan, C.T.; McAlpine, C.S.; Xu, C.; Kahles, F.; He, S.; et al. Brain motor and fear circuits regulate leukocytes during acute stress. Nature 2022, 607, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Yoo, B.B.; Mazmanian, S.K. The Enteric Network: Interactions between the Immune and Nervous Systems of the Gut. Immunity 2017, 46, 910–926. [Google Scholar] [CrossRef]
- Miyauchi, E.; Shimokawa, C.; Steimle, A.; Desai, M.S.; Ohno, H. The impact of the gut microbiome on extra-intestinal autoimmune diseases. Nat. Rev. Immunol. 2023, 23, 9–23. [Google Scholar] [CrossRef]
- Spencer, J.; Bemark, M. Human intestinal B cells in inflammatory diseases. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 254–265. [Google Scholar] [CrossRef]
- Hu, H.; Sun, W.; Zhang, L.; Zhang, Y.; Kuang, T.; Qu, D.; Lian, S.; Hu, S.; Cheng, M.; Xu, Y.; et al. Carboxymethylated Abrus cantoniensis polysaccharide prevents CTX-induced immunosuppression and intestinal damage by regulating intestinal flora and butyric acid content. Int. J. Biol. Macromol. 2024, 261, 129590. [Google Scholar] [CrossRef]
- Bhunia, A.K.; Al-Sadi, R. Editorial: Intestinal epithelial barrier disruption by enteric pathogens. Front. Cell Infect. Microbiol. 2023, 13, 1134753. [Google Scholar] [CrossRef]
- Zhang, X.; Akhtar, M.; Chen, Y.; Ma, Z.; Liang, Y.; Shi, D.; Cheng, R.; Cui, L.; Hu, Y.; Nafady, A.A.; et al. Chicken jejunal microbiota improves growth performance by mitigating intestinal inflammation. Microbiome 2022, 10, 107. [Google Scholar] [CrossRef]
- Zhang, Y.; Mahmood, T.; Tang, Z.; Wu, Y.; Yuan, J. Effects of naturally oxidized corn oil on inflammatory reaction and intestinal health of broilers. Poult. Sci. 2022, 101, 101541. [Google Scholar] [CrossRef]
- Chen, V.L.; Kasper, D.L. Interactions between the intestinal microbiota and innate lymphoid cells. Gut Microbes 2014, 5, 129–140. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Wu, H.; Jia, W.; Teng, L.; Song, J.; Yang, X.; Wang, D. Sarcodon imbricatus polysaccharides protect against cyclophosphamide-induced immunosuppression via regulating Nrf2-mediated oxidative stress. Int. J. Biol. Macromol. 2018, 120, 736–744. [Google Scholar] [CrossRef]
- Sarin, S.K.; Pande, A.; Schnabl, B. Microbiome as a therapeutic target in alcohol-related liver disease. J. Hepatol. 2019, 70, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Ohland, C.L.; Macnaughton, W.K. Probiotic bacteria and intestinal epithelial barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G807–G819. [Google Scholar] [CrossRef]
- Xie, J.H.; Fan, S.T.; Nie, S.P.; Yu, Q.; Xiong, T.; Gong, D.; Xie, M.Y. Lactobacillus plantarum NCU116 attenuates cyclophosphamide-induced intestinal mucosal injury, metabolism and intestinal microbiota disorders in mice. Food Funct. 2016, 7, 1584–1592. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Huang, Z.; Yue, W.; Nawaz, S.; Chen, X.; Liu, J. Lactobacillus plantarum modulate gut microbiota and intestinal immunity in cyclophosphamide-treated mice model. Biomed. Pharmacother. 2023, 169, 115812. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Deng, F.; Zhao, B.; Lin, Z.; Sun, Q.; Yang, X.; Wu, M.; Qiu, S.; Chen, Y.; Yan, Z.; et al. Lactobacillus murinus alleviate intestinal ischemia/reperfusion injury through promoting the release of interleukin-10 from M2 macrophages via Toll-like receptor 2 signaling. Microbiome 2022, 10, 38. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, Y.; Zuo, M.; Ding, S.; Li, J.; Feng, S.; Xiao, Y.; Tao, S. Novel mechanism by which extracellular vesicles derived from Lactobacillus murinus alleviates deoxynivalenol-induced intestinal barrier disruption. Environ. Int. 2024, 185, 108525. [Google Scholar] [CrossRef]
- Obisesan, A.O.; Abiodun, O.O.; Ayeni, F.A. Lactic acid bacteria isolated from women’ breast milk and infants’ faeces have appreciable immunogenic and probiotic potentials against diarrheagenic E. coli strains. BMC Microbiol. 2024, 24, 350. [Google Scholar] [CrossRef]
- Chen, H.T.; Li, J.S.; Li, J.; Li, L.; Xu, Z.C.; Zhang, Y.; Wang, R.R. Lactobacillus murinus: A key factor in suppression of enterogenous Candida albicans infections in Compound Agrimony enteritis capsules-treated mice. J. Ethnopharmacol. 2023, 311, 116361. [Google Scholar] [CrossRef]
- Zhu, Y.; Tao, X.; Yan, T.; Cao, S.; Jiang, P.; Zhang, Z.; Li, L.; Wu, Q. Lactobacillus murinus alleviated lung inflammation induced by PAHs in mice. Ecotoxicol. Environ. Saf. 2024, 281, 116662. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Cheng, L.; Sun, Y.; Zhang, Q.; de Haan, B.J.; Zhang, H.; Faas, M.M.; de Vos, P. Lactic acid bacteria secrete toll like receptor 2 stimulating and macrophage immunomodulating bioactive factors. J. Funct. Foods 2020, 66, 103783. [Google Scholar] [CrossRef]
- Perelmuter, K.; Fraga, M.; Zunino, P. In vitro activity of potential probiotic Lactobacillus murinus isolated from the dog. J. Appl. Microbiol. 2008, 104, 1718–1725. [Google Scholar] [CrossRef]
- Ying, M.; Yu, Q.; Zheng, B.; Wang, H.; Wang, J.; Chen, S.; Nie, S.; Xie, M. Cultured Cordyceps sinensis polysaccharides modulate intestinal mucosal immunity and gut microbiota in cyclophosphamide-treated mice. Carbohydr. Polym. 2020, 235, 115957. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhao, X.; Tang, C.; Wang, X.; Zhang, X.; Xiao, L.; Li, W. Protective effect of Paecilomyces cicadae TJJ11213 exopolysaccharide on intestinal mucosa and regulation of gut microbiota in immunosuppressed mice. Food Res. Int. 2023, 165, 112477. [Google Scholar] [CrossRef]
- Gill, N.; Wlodarska, M.; Finlay, B.B. Roadblocks in the gut: Barriers to enteric infection. Cell Microbiol. 2011, 13, 660–669. [Google Scholar] [CrossRef]
- Meng, M.; Wang, H.; Li, Z.; Guo, M.; Hou, L. Protective effects of polysaccharides from Cordyceps gunnii mycelia against cyclophosphamide-induced immunosuppression to TLR4/TRAF6/NF-κB signalling in BALB/c mice. Food Funct. 2019, 10, 3262–3271. [Google Scholar] [CrossRef]
- Li, S.P.; Zhao, X.J.; Wang, J.Y. Synergy of Astragalus polysaccharides and probiotics (Lactobacillus and Bacillus cereus) on immunity and intestinal microbiota in chicks. Poult. Sci. 2009, 88, 519–525. [Google Scholar] [CrossRef]
- Yu, J.; Cong, L.; Wang, C.; Li, H.; Zhang, C.; Guan, X.; Liu, P.; Xie, Y.; Chen, J.; Sun, J. Immunomodulatory effect of Schisandra polysaccharides in cyclophosphamide-induced immunocompromised mice. Exp. Ther. Med. 2018, 15, 4755–4762. [Google Scholar] [CrossRef]
- Agúndez, J.A.; Ayuso, P.; Cornejo-García, J.A.; Blanca, M.; Torres, M.J.; Doña, I.; Salas, M.; Blanca-López, N.; Canto, G.; Rondon, C.; et al. The diamine oxidase gene is associated with hypersensitivity response to non-steroidal anti-inflammatory drugs. PLoS ONE 2012, 7, e47571. [Google Scholar] [CrossRef]
- Park, C.M.; Song, Y.-S. Luteolin and luteolin-7-O-glucoside inhibit lipopolysaccharide-induced inflammatory responses through modulation of NF-κB/AP-1/PI3K-Akt signaling cascades in RAW 264.7 cells. Nutr. Res. Pract. 2013, 7, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, Y.; Ouyang, K.; Chen, L.; Zhao, M.; Wang, W. Sulfated Cyclocarya paliurus polysaccharides improve immune function of immunosuppressed mice by modulating intestinal microbiota. Int. J. Biol. Macromol. 2022, 212, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, H.C.; He, S.B.; Zhang, X.F.; Ling, Y.H.; Li, X.Y.; Zhang, H.; Hou, D.D. The immunoenhancement effects of sea buckthorn pulp oil in cyclophosphamide-induced immunosuppressed mice. Food Funct. 2021, 12, 7954–7963. [Google Scholar] [CrossRef]
- Sun, S.; Xu, X.; Liang, L.; Wang, X.; Bai, X.; Zhu, L.; He, Q.; Liang, H.; Xin, X.; Wang, L.; et al. Lactic Acid-Producing Probiotic Saccharomyces cerevisiae Attenuates Ulcerative Colitis via Suppressing Macrophage Pyroptosis and Modulating Gut Microbiota. Front. Immunol. 2021, 12, 777665. [Google Scholar] [CrossRef]
- Awad, W.A.; Ghareeb, K.; Abdel-Raheem, S.; Böhm, J. Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult. Sci. 2009, 88, 49–56. [Google Scholar] [CrossRef]
- Emami, N.K.; Dalloul, R.A. Centennial Review: Recent developments in host-pathogen interactions during necrotic enteritis in poultry. Poult. Sci. 2021, 100, 101330. [Google Scholar] [CrossRef] [PubMed]
- Sarikaya-Bayram, Ö.; Palmer, J.M.; Keller, N.; Braus, G.H.; Bayram, Ö. One Juliet and four Romeos: VeA and its methyltransferases. Front. Microbiol. 2015, 6, 1. [Google Scholar] [CrossRef]
- Bernard-Raichon, L.; Colom, A.; Monard, S.C.; Namouchi, A.; Cescato, M.; Garnier, H.; Leon-Icaza, S.A.; Métais, A.; Dumas, A.; Corral, D.; et al. A Pulmonary Lactobacillus murinus Strain Induces Th17 and RORγt(+) Regulatory T Cells and Reduces Lung Inflammation in Tuberculosis. J. Immunol. 2021, 207, 1857–1870. [Google Scholar] [CrossRef] [PubMed]
- Singer, J.R.; Blosser, E.G.; Zindl, C.L.; Silberger, D.J.; Conlan, S.; Laufer, V.A.; DiToro, D.; Deming, C.; Kumar, R.; Morrow, C.D.; et al. Preventing dysbiosis of the neonatal mouse intestinal microbiome protects against late-onset sepsis. Nat. Med. 2019, 25, 1772–1782. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, J.; Wang, Z.; Zhang, G.; Liu, L.; Huo, G.; Li, C. Lactobacillus plantarum KLDS1.0318 Ameliorates Impaired Intestinal Immunity and Metabolic Disorders in Cyclophosphamide-Treated Mice. Front. Microbiol. 2019, 10, 731. [Google Scholar] [CrossRef]
- Frantz, A.L.; Rogier, E.W.; Weber, C.R.; Shen, L.; Cohen, D.A.; Fenton, L.A.; Bruno, M.E.; Kaetzel, C.S. Targeted deletion of MyD88 in intestinal epithelial cells results in compromised antibacterial immunity associated with downregulation of polymeric immunoglobulin receptor, mucin-2, and antibacterial peptides. Mucosal Immunol. 2012, 5, 501–512. [Google Scholar] [CrossRef]
- Johansen, F.E.; Kaetzel, C.S. Regulation of the polymeric immunoglobulin receptor and IgA transport: New advances in environmental factors that stimulate pIgR expression and its role in mucosal immunity. Mucosal Immunol. 2011, 4, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Rakoff-Nahoum, S.; Paglino, J.; Eslami-Varzaneh, F.; Edberg, S.; Medzhitov, R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 2004, 118, 229–241. [Google Scholar] [CrossRef]
- Min, F.; Hu, J.; Huang, T.; Huang, Y.; Nie, S.; Xiong, T.; Xie, M. Effects of Lactobacillus casei NCU011054 on immune response and gut microbiota of cyclophosphamide induced immunosuppression mice. Food Chem. Toxicol. 2023, 174, 113662. [Google Scholar] [CrossRef]
- Zhang, N.; Tian, Y.; Wang, Y.; Fan, Y.; Zhang, Y.; Xing, X.; Nan, B.; Ai, Z.; Li, X.; Wang, Y. Ameliorative effect of Lactobacillus plantarum Lp2 against cyclophosphamide-induced liver injury in mice. Food Chem. Toxicol. 2022, 169, 113433. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Feng, S.; Wang, Z.; He, J.; Zhang, Z.; Zou, H.; Wu, Z.; Liu, X.; Wei, H.; Tao, S. Limosilactobacillus mucosae-derived extracellular vesicles modulates macrophage phenotype and orchestrates gut homeostasis in a diarrheal piglet model. NPJ Biofilms Microbiomes 2023, 9, 33. [Google Scholar] [CrossRef]
- Tao, S.; Fan, J.; Li, J.; Wu, Z.; Yao, Y.; Wang, Z.; Wu, Y.; Liu, X.; Xiao, Y.; Wei, H. Extracellular vesicles derived from Lactobacillus johnsonii promote gut barrier homeostasis by enhancing M2 macrophage polarization. J. Adv. Res. 2025, 69, 545–563. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.J.; Chin, R.; Nachbur, U.; Silke, J.; Jia, Z.; Angus, P.W.; Torresi, J. Effect of Immunosuppressive Agents on Hepatocyte Apoptosis Post-Liver Transplantation. PLoS ONE 2015, 10, e0138522. [Google Scholar] [CrossRef]
- Liu, T.; Wu, Y.; Wang, L.; Pang, X.; Zhao, L.; Yuan, H.; Zhang, C. A More Robust Gut Microbiota in Calorie-Restricted Mice Is Associated with Attenuated Intestinal Injury Caused by the Chemotherapy Drug Cyclophosphamide. mBio 2019, 10, e02903-18. [Google Scholar] [CrossRef]
- Sherif, I.O. The effect of natural antioxidants in cyclophosphamide-induced hepatotoxicity: Role of Nrf2/HO-1 pathway. Int. Immunopharmacol. 2018, 61, 29–36. [Google Scholar] [CrossRef]
- Lv, H.; Xiao, Q.; Zhou, J.; Feng, H.; Liu, G.; Ci, X. Licochalcone A Upregulates Nrf2 Antioxidant Pathway and Thereby Alleviates Acetaminophen-Induced Hepatotoxicity. Front. Pharmacol. 2018, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, S.; Wang, M.W. Evodiamine-induced human melanoma A375-S2 cell death was mediated by PI3K/Akt/caspase and Fas-L/NF-kappaB signaling pathways and augmented by ubiquitin-proteasome inhibition. Toxicol. In Vitro 2010, 24, 898–904. [Google Scholar] [CrossRef]
- Lei, Y.; Chan, M.; Liu, H.; Lyu, W.; Chen, L.; Zhong, Y.; Gan, H.; Wang, M.; Qi, M.; Guo, Y.; et al. Evodiamine as the Active Compound of Evodiae fructus to Inhibit Proliferation and Migration of Prostate Cancer through PI3K/AKT/NF-κB Signaling Pathway. Dis. Markers 2022, 2022, 4399334. [Google Scholar] [CrossRef] [PubMed]
- Hatton, I.A.; Mazzarisi, O.; Altieri, A.; Smerlak, M. Diversity begets stability: Sublinear growth and competitive coexistence across ecosystems. Science 2024, 383, eadg8488. [Google Scholar] [CrossRef]
- Litvak, Y.; Mon, K.K.Z.; Nguyen, H.; Chanthavixay, G.; Liou, M.; Velazquez, E.M.; Kutter, L.; Alcantara, M.A.; Byndloss, M.X.; Tiffany, C.R.; et al. Commensal Enterobacteriaceae Protect against Salmonella Colonization through Oxygen Competition. Cell Host Microbe 2019, 25, 128–139.e125. [Google Scholar] [CrossRef]
- Pavel, F.M.; Vesa, C.M.; Gheorghe, G.; Diaconu, C.C.; Stoicescu, M.; Munteanu, M.A.; Babes, E.E.; Tit, D.M.; Toma, M.M.; Bungau, S. Highlighting the Relevance of Gut Microbiota Manipulation in Inflammatory Bowel Disease. Diagnostics 2021, 11, 1090. [Google Scholar] [CrossRef] [PubMed]
- Gophna, U.; Sommerfeld, K.; Gophna, S.; Doolittle, W.F.; Veldhuyzen van Zanten, S.J. Differences between tissue-associated intestinal microfloras of patients with Crohn’s disease and ulcerative colitis. J. Clin. Microbiol. 2006, 44, 4136–4141. [Google Scholar] [CrossRef]
- Shi, N.; Li, N.; Duan, X.; Niu, H. Interaction between the gut microbiome and mucosal immune system. Mil. Med. Res. 2017, 4, 14. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.; Fang, Q.; Dong, N.; Fang, Q.; Cui, S.W.; Nie, S. A polysaccharide from natural Cordyceps sinensis regulates the intestinal immunity and gut microbiota in mice with cyclophosphamide-induced intestinal injury. Food Funct. 2021, 12, 6271–6282. [Google Scholar] [CrossRef]
- Johnson, E.L.; Heaver, S.L.; Walters, W.A.; Ley, R.E. Microbiome and metabolic disease: Revisiting the bacterial phylum Bacteroidetes. J. Mol. Med. 2017, 95, 1–8. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, T.; Chen, S.; Shen, H.; Wang, J. Dietary supplementation with L-citrulline improves amino acid composition and broiler performance, and modulates gut microbiota. Front. Microbiol. 2025, 16, 1551012. [Google Scholar] [CrossRef]
- Kayama, H.; Okumura, R.; Takeda, K. Interaction Between the Microbiota, Epithelia, and Immune Cells in the Intestine. Annu. Rev. Immunol. 2020, 38, 23–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xiao, Y.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Protective effects of different Bacteroides vulgatus strains against lipopolysaccharide-induced acute intestinal injury, and their underlying functional genes. J. Adv. Res. 2022, 36, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Downes, J.; Dewhirst, F.E.; Tanner, A.C.R.; Wade, W.G. Description of Alloprevotella rava gen. nov., sp. nov., isolated from the human oral cavity, and reclassification of Prevotella tannerae Moore et al. 1994 as Alloprevotella tannerae gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2013, 63, 1214–1218. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria with Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef]
- Sakamoto, M.; Ikeyama, N.; Ogata, Y.; Suda, W.; Iino, T.; Hattori, M.; Ohkuma, M. Alistipes communis sp. nov., Alistipes dispar sp. nov. and Alistipes onderdonkii subsp. vulgaris subsp. nov., isolated from human faeces, and creation of Alistipes onderdonkii subsp. onderdonkii subsp. nov. Int. J. Syst. Evol. Microbiol. 2020, 70, 473–480. [Google Scholar] [CrossRef]
- Rau, M.; Rehman, A.; Dittrich, M.; Groen, A.K.; Hermanns, H.M.; Seyfried, F.; Beyersdorf, N.; Dandekar, T.; Rosenstiel, P.; Geier, A. Fecal SCFAs and SCFA-producing bacteria in gut microbiome of human NAFLD as a putative link to systemic T-cell activation and advanced disease. United Eur. Gastroenterol. J. 2018, 6, 1496–1507. [Google Scholar] [CrossRef]
- Takeuchi, T.; Kubota, T.; Nakanishi, Y.; Tsugawa, H.; Suda, W.; Kwon, A.T.; Yazaki, J.; Ikeda, K.; Nemoto, S.; Mochizuki, Y.; et al. Gut microbial carbohydrate metabolism contributes to insulin resistance. Nature 2023, 621, 389–395. [Google Scholar] [CrossRef]
- Tian, S.; Wang, J.; Wang, J.; Zhu, W. Differential Effects of Early-Life and Postweaning Galacto-oligosaccharide Intervention on Colonic Bacterial Composition and Function in Weaning Piglets. Appl. Environ. Microbiol. 2022, 88, e0131821. [Google Scholar] [CrossRef]
- Luise, D.; Le Sciellour, M.; Buchet, A.; Resmond, R.; Clement, C.; Rossignol, M.N.; Jardet, D.; Zemb, O.; Belloc, C.; Merlot, E. The fecal microbiota of piglets during weaning transition and its association with piglet growth across various farm environments. PLoS ONE 2021, 16, e0250655. [Google Scholar] [CrossRef]
- Gryaznova, M.V.; Solodskikh, S.A.; Panevina, A.V.; Syromyatnikov, M.Y.; Dvoretskaya, Y.D.; Sviridova, T.N.; Popov, E.S.; Popov, V.N. Study of microbiome changes in patients with ulcerative colitis in the Central European part of Russia. Heliyon 2021, 7, e06432. [Google Scholar] [CrossRef]
- Song, Z.; Qiao, Z.; Liu, J.; Han, L.; Chen, X.; Wang, Y. Sea buckthorn berries alleviate ulcerative colitis via regulating gut Faecalibaculum rodentium-mediated butyrate biosynthesis. Phytomedicine 2025, 139, 156490. [Google Scholar] [CrossRef] [PubMed]
- Ridlon, J.M.; Wolf, P.G.; Gaskins, H.R. Taurocholic acid metabolism by gut microbes and colon cancer. Gut Microbes 2016, 7, 201–215. [Google Scholar] [CrossRef]
- Yu, Z.; Xiaojia, L.; Wei, Z.; Jian, Z.; Aiting, W.; Jing, W.; Lin, Y.; Bangwei, C.; Dan, Y. Baicalin circumvents anti-PD-1 resistance by regulating the gut microbiota metabolite short-chain fatty acids. Pharmacol. Res. 2024, 199, 107033. [Google Scholar] [CrossRef]
- Duan, Y.; Guo, F.; Li, C.; Xiang, D.; Gong, M.; Yi, H.; Chen, L.; Yan, L.; Zhang, D.; Dai, L.; et al. Aqueous extract of fermented Eucommia ulmoides leaves alleviates hyperlipidemia by maintaining gut homeostasis and modulating metabolism in high-fat diet fed rats. Phytomedicine 2024, 128, 155291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lv, H.; Cai, X.; Tang, S.; Zhong, R.; Chen, L.; Zhang, H. Effects of the compound extracts of Caprifoliaceae and Scutellaria baicalensis Georgi on the intestinal microbiota and antioxidant function. Front. Microbiol. 2023, 14, 1289490. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Wang, Y.; Li, G.; Xue, S.; Zhang, G.; Dang, Y.; Wang, H. Modulating the gut microbiota is involved in the effect of low-molecular-weight Glycyrrhiza polysaccharide on immune function. Gut Microbes 2023, 15, 2276814. [Google Scholar] [CrossRef]
- Huang, B.; Yin, T.; Fu, S.; Liu, L.; Yang, C.; Zhou, L.; Liu, X.; Zhuang, H.; Cao, Z.; Hua, Z. Inflammation-oriented montmorillonite adjuvant enhanced oral delivery of anti-TNF-α nanobody against inflammatory bowel disease. Proc. Natl. Acad. Sci. USA 2024, 121, e2320482121. [Google Scholar] [CrossRef]
- Wang, H.; Ren, P.; Mang, L.; Shen, N.; Chen, J.; Zhang, Y. In vitro fermentation of novel microwave-synthesized non-digestible oligosaccharides and their impact on the composition and metabolites of human gut microbiota. J. Funct. Foods 2019, 55, 156–166. [Google Scholar] [CrossRef]
- Lee, K.; Kim, H.J.; Kim, S.A.; Park, S.D.; Shim, J.J.; Lee, J.L. Exopolysaccharide from Lactobacillus plantarum HY7714 Protects against Skin Aging through Skin-Gut Axis Communication. Molecules 2021, 26, 1651. [Google Scholar] [CrossRef]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; Ian McKenzie, C.; Hijikata, A.; Wong, C.; et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef] [PubMed]
- Ridlon, J.M.; Ikegawa, S.; Alves, J.M.; Zhou, B.; Kobayashi, A.; Iida, T.; Mitamura, K.; Tanabe, G.; Serrano, M.; De Guzman, A.; et al. Clostridium scindens: A human gut microbe with a high potential to convert glucocorticoids into androgens. J. Lipid Res. 2013, 54, 2437–2449. [Google Scholar] [CrossRef] [PubMed]
- Hang, S.; Paik, D.; Yao, L.; Kim, E.; Trinath, J.; Lu, J.; Ha, S.; Nelson, B.N.; Kelly, S.P.; Wu, L.; et al. Bile acid metabolites control T(H)17 and T(reg) cell differentiation. Nature 2019, 576, 143–148. [Google Scholar] [CrossRef]
- Dodd, D.; Spitzer, M.H.; Van Treuren, W.; Merrill, B.D.; Hryckowian, A.J.; Higginbottom, S.K.; Le, A.; Cowan, T.M.; Nolan, G.P.; Fischbach, M.A.; et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 2017, 551, 648–652. [Google Scholar] [CrossRef]
- Dagenais-Lussier, X.; Loucif, H.; Beji, C.; Telittchenko, R.; Routy, J.P.; van Grevenynghe, J. Latest developments in tryptophan metabolism: Understanding its role in B cell immunity. Cytokine Growth Factor. Rev. 2021, 59, 111–117. [Google Scholar] [CrossRef]
- Bodogai, M.; O’Connell, J.; Kim, K.; Kim, Y.; Moritoh, K.; Chen, C.; Gusev, F.; Vaughan, K.; Shulzhenko, N.; Mattison, J.A.; et al. Commensal bacteria contribute to insulin resistance in aging by activating innate B1a cells. Sci. Transl. Med. 2018, 10, eaat4271. [Google Scholar] [CrossRef] [PubMed]
- Lamas, B.; Vergnaud-Gauduchon, J.; Goncalves-Mendes, N.; Perche, O.; Rossary, A.; Vasson, M.P.; Farges, M.C. Altered functions of natural killer cells in response to L-Arginine availability. Cell Immunol. 2012, 280, 182–190. [Google Scholar] [CrossRef]
- Zagato, E.; Pozzi, C.; Bertocchi, A.; Schioppa, T.; Saccheri, F.; Guglietta, S.; Fosso, B.; Melocchi, L.; Nizzoli, G.; Troisi, J.; et al. Endogenous murine microbiota member Faecalibaculum rodentium and its human homologue protect from intestinal tumour growth. Nat. Microbiol. 2020, 5, 511–524. [Google Scholar] [CrossRef]



| Target Gene | Forward | Reverse |
|---|---|---|
| β-actin | GATATCGCTGCGCTGGTCG | CATTCCCACCATCACACCCT |
| Claudin1 | AAAGCACCGGGCAGATACAG | TCATGCCAATGGTGGACACA |
| Occludin | TCCACCTCCTTACAGACCTGA | AAGAGTACGCTGGCTGAGAG |
| MUC2 | TCCTGACCAAGAGCGAACAC | ACAGCACGACAGTCTTCAGG |
| IL-1β | AATGCCACCTTTTGACAGTGAT | ATCAGGACAGCCCAGGTCAA |
| IL-2 | GCGGCATGTTCTGGATTTGAC | CCTCAGAAAGTCCACCACAGT |
| IL-6 | TTCCTCTGGTCTTCTGGAGT | TCTGTGACTCCAGCTTATCTCTTG |
| IL-10 | CCTGGGTGAGAAGCTGAAGAC | CTTGTAGACACCTTGGTCTTGG |
| TNF-α | CCCTCACACTCACAAACCAC | ACAAGGTACAACCCATCGGC |
| Bcl-2 | CTTTGAGTTCGGTGGGGTCAT | GCCAGGAGAAATCAAACAGAGG |
| Bax | CACTAAAGTGCCCGAGCTGA | GGAGAGGAGGCCTTCCCAG |
| FAS | TGCTTGCTGGCTCACAGTTAAG | GAACCCGCCTCCTCAGCTT |
| FADD | GTGTGTGACAATGTGGGGAG | GACTCTCCCTTACCCGCTCA |
| Caspase-3 | ATGGGAGCAAGTCAGTGGAC | GTCCACATCCGTACCAGAGC |
| Caspase-8 | CGGGAAAAGGGGATGTTGGA | TCGCTCACTTCTTCTGAGAGC |
| Caspase-9 | ATCGAGGATATTCAGCAGGCA | CCTCGGGTCTCAAGGTCTGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Zhang, L.; Qiu, D.; Yao, R.; Jia, H.; Wang, H.; Zhou, L.; Zhang, J.; Zhang, N. Lactobacillus murinus ZNL-13 Modulates Intestinal Barrier Damage and Gut Microbiota in Cyclophosphamide-Induced Immunosuppressed Mice. Foods 2025, 14, 1416. https://doi.org/10.3390/foods14081416
Dong Y, Zhang L, Qiu D, Yao R, Jia H, Wang H, Zhou L, Zhang J, Zhang N. Lactobacillus murinus ZNL-13 Modulates Intestinal Barrier Damage and Gut Microbiota in Cyclophosphamide-Induced Immunosuppressed Mice. Foods. 2025; 14(8):1416. https://doi.org/10.3390/foods14081416
Chicago/Turabian StyleDong, Yihan, Luyao Zhang, Di Qiu, Renxin Yao, Haitao Jia, Haiyang Wang, Luyao Zhou, Jiantao Zhang, and Na Zhang. 2025. "Lactobacillus murinus ZNL-13 Modulates Intestinal Barrier Damage and Gut Microbiota in Cyclophosphamide-Induced Immunosuppressed Mice" Foods 14, no. 8: 1416. https://doi.org/10.3390/foods14081416
APA StyleDong, Y., Zhang, L., Qiu, D., Yao, R., Jia, H., Wang, H., Zhou, L., Zhang, J., & Zhang, N. (2025). Lactobacillus murinus ZNL-13 Modulates Intestinal Barrier Damage and Gut Microbiota in Cyclophosphamide-Induced Immunosuppressed Mice. Foods, 14(8), 1416. https://doi.org/10.3390/foods14081416





