New Insight into Microbial Exploitation to Produce Bioactive Molecules from Agrifood and By-Products’ Fermentation
Abstract
1. Introduction
2. Bioactive Compounds from Fermented Products
2.1. Food Fermentation
2.1.1. Dairy Products
2.1.2. Vegetables or Plant-Derived Products
| Sector | Matrix | Starter Cultures | Main Effects | References |
|---|---|---|---|---|
Dairy products | Lben | L. lactis subsp. lactis and L. mesenteroides subsp. mesenteroides | Increased angiotensin-converting enzyme (ACE), Inhibitory activity γ-aminobutyric acid (GABA) | [34] |
| Goat’s milk cheese | L. lactis subsp. lactis, L. lactis subsp. cremoris and L. bulgaricus | Increased angiotensin-converting enzyme (ACE) inhibitory activity | [35] | |
| Yogurt | S. thermophilus and L. delbrueckii subsp. bulgaricus | Increased peptides production | [42] | |
| Camel milk | L. plantarum ZFM55 and L. paracasei ZFM54 and L. lactis | Increased amino acids and carbohydrate content Increased the metabolite content Increased bioactive content | [36] | |
| Milk | L. plantarum L3 | Improved taste and nutritional qualities of milk | [47] | |
| Fresh cheese | L. fermentum B44 (CGMCC 17321) L. rhamnosus KF7 (CGMCC 6430) L. rhamnosus B6 (CGMCC 13310) | Increased peptide production | [48] | |
| Vegetables, fruits and derivatives  | Cocoa bean | Pichia kudriavzevii in single culture or in combination with S. cerevisiae | Increased phenolic compounds | [53] |
| Broccoli juice | Pediococcus pentosaceus | Increased composition of bioactive compounds | [54] | |
| Vegetable juice | Companilactobacillus allii and L. lactis | In vitro inhibition of lipid accumulation | [55] | |
| Vegetable juice | L. paracasei SP5 and P ediococcus pentosaceus SP2 | Increased phenolic compounds, total phenols, zeaxanthin, lutein, β-carotene content | [56] | |
| Curly kale | L. sakei and L. plantarum | Increased content of riboflavin and pyridoxine and pyridoxine and decreased riboflavin content | [57] | |
| Fermented broccoli | L. brevis and L. lactis | Increased phenol content and antioxidant activity in Caco2 | [58] | |
| Wheat bran | Eurotium cristatum | Increased dietary fiber content and bioactive molecules | [60] | |
| Fermented soya | Bacillus spp. L. delbrueckii subsp. bulgaricus and Wickerhamomyces anomalous | Increased peptide production, total phenol and total flavonoid content, antioxidant activity, organic acids and biological amines | [62] | |
| Sourdough | L. sanfranciscensis, Leuconostoc citreum and Candida milleri | Increased phenolic and antioxidant content | [63] |
2.2. Agrifood By-Product Fermentation
2.3. Engineered Microorganisms for Bio-Production of Active Compounds
3. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guiné, R.P.F.; Florença, S.G.; Barroca, M.J.; Anjos, O. The Link between the Consumer and the Innovations in Food Product Development. Foods 2020, 9, 1317. [Google Scholar] [CrossRef] [PubMed]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef] [PubMed]
- Owolabi, I.O.; Akinmosin, B.O.; Kupoluyi, A.O.; Olatunde, O.O.; Petchkongkaew, A.; Coker, O.J.; Olajide, A.M. Packaging and packaging technology for indigenous fermented foods in the tropics: Challenges and opportunities. Indig. Fermented Foods Trop. 2023, 563–575. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef]
- Pereira, G.V.M.; De Carvalho Neto, D.P.; Junqueira, A.C.D.O.; Karp, S.G.; Letti, L.A.; Magalhães Júnior, A.I.; Soccol, C.R. A review of selection criteria for starter culture development in the food fermentation industry. Food Rev. Int. 2020, 36, 135–167. [Google Scholar] [CrossRef]
- Hammami, R.; Fliss, I.; Corsetti, A. Application of protective cultures and bacteriocins for food biopreservation. Front. Microbiol. 2019, 10, 1561. [Google Scholar] [CrossRef] [PubMed]
- Tufariello, M.; Durante, M.; Veneziani, G.; Taticchi, A.; Servili, M.; Bleve, G.; Mita, G. Pâté olive cake: Possible exploitation of a by-product for food applications. Front. Nutr. 2019, 6, 3. [Google Scholar] [CrossRef]
- García-Díez, J.; Saraiva, C. Use of Starter Cultures in Foods from Animal Origin to Improve Their Safety. Int. J. Environ. Res. Public Health 2021, 18, 2544. [Google Scholar] [CrossRef]
- Marsh, A.J.; Hill, C.; Ross, R.P.; Cotter, P.D. Fermented beverages with health-promoting potential: Past and future perspectives. Trends Food Sci. Technol. 2014, 38, 113–124. [Google Scholar] [CrossRef]
- Daniel, N.; Nachbar, R.T.; Tran, T.T.T.; Ouellette, A.; Varin, T.V.; Cotillard, A.; Quinquis, L.; Gagné, A.; St-Pierre, P.; Trottier, J.; et al. Gut microbiota and fermentation-derived branched chain hydroxy acids mediate health benefits of yogurt consumption in obese mice. Nat. Commun. 2022, 13, 1343. [Google Scholar] [CrossRef]
- Gaggìa, F.; Baffoni, L.; Galiano, M.; Nielsen, D.S.; Jakobsen, R.R.; Castro-Mejía, J.L.; Bosi, S.; Truzzi, F.; Musumeci, F.; Dinelli, G.; et al. Kombucha Beverage from Green, Black and Rooibos Teas: A Comparative Study Looking at Microbiology, Chemistry and Antioxidant Activity. Nutrients 2019, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.; Alian Samakkhah, S.; Bahadori, A.; Jafari, S.M.; Ziaee, M.; Khodayari, M.T.; Pourjafar, H. Health-promoting properties of Saccharomyces cerevisiae var. boulardii as a probiotic; characteristics, isolation, and applications in dairy products. Crit. Rev. Food Sci. Nutr. 2023, 63, 457–485. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.Y.; Jeong, Y.; Kang, C.-H. Antioxidant Activity and Probiotic Properties of Lactic Acid Bacteria. Fermentation 2022, 8, 29. [Google Scholar] [CrossRef]
- Abid, Y.; Azabou, S. Exopolysaccharides from Lactic Acid Bacteria. In Polysaccharides of Microbial Origin: Biomedical Applications; Oliveira, J., Radhouani, H., Reis, R.L., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–23. [Google Scholar] [CrossRef]
- Mukherjee, A.; Breselge, S.; Dimidi, E.; Marco, M.L.; Cotter, P.D. Fermented foods and gastrointestinal health: Underlying mechanisms. Nat. Rev. Gastroenterol. Hepatol. 2023, 21, 248–266. [Google Scholar] [CrossRef]
- Bader, J.; Mast-Gerlach, E.; Popovic, M.K.; Bajpai, R.; Stahl, U. Relevance of microbial coculture fermentations in biotechnology. J. Appl. Microbiol. 2010, 109, 371–387. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Remize, F.; Poucheret, P. Fruits and vegetables, asasource of nutritional compounds and phytochemicals: Changes in bioactive compounds during lactic fermentation. Food Res. Int. 2018, 104, 86–99. [Google Scholar] [CrossRef]
- Fessard, A.; Kapoor, A.; Patche, J.; Assemat, S.; Hoarau, M.; Bourdon, E.; Bahorun, T.; Remize, F. Lactic Fermentation as an Efficient Tool to Enhance the Antioxidant Activity of Tropical Fruit Juices and Teas. Microorganisms 2017, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Parajuli, P.; Koffas, M.; Sohng, J. Microbial production of natural and non-natural flavonoids: Pathway engineering, directed evolution and systems/synthetic biology. Biotechnol. Adv. 2016, 34, 634. [Google Scholar] [CrossRef]
- Aman Mohammadi, M.; Maximiano, M.R.; Hosseini, S.M.; Franco, O.L. CRISPR-Cas Engineering in Food Science and Sustainable Agriculture: Recent Advancements and Applications. Bioprocess Biosyst. Eng. 2023, 46, 483–497. [Google Scholar] [CrossRef]
- Qi, D.D.; Jin, J.; Liu, D.; Jia, B.; Yuan, Y.J. In vitro and in vivo recombination of heterologous modules for improving biosynthesis of astaxanthin in yeast. Microb. Cell Factories 2020, 19, 103. [Google Scholar] [CrossRef]
- Hanlon, P.; Sewalt, V. GEMs: Genetically engineered microorganisms and the regulatory oversight of their uses in modern food production. Crit. Rev. Food Sci. Nutr. 2021, 61, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.; García-Vaquero, M. Bioactive Compounds from Fermented Food Products. In Novel Food Fermentation Technologies; Food Engineering Series; Springer: Berlin/Heidelberg, Germany, 2016; pp. 293–310. [Google Scholar]
- Bourrie, B.C.T.; Willing, B.P.; Cotter, P.D. The microbiota and health promoting characteristics of the fermented beverage kefir. Front. Microbiol. 2016, 7, 647. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ahmed, M.; Ha, V.; Jefferson, K.; Malik, V.; Ribeiro, P.; Zuchinali, P.; Drouin-Chartier, J.P. Dairy product consumption and cardiovascular health: A systematic review and meta-analysis of prospective cohort studies. Adv. Nutr. 2021, 13, 439–454. [Google Scholar] [CrossRef]
- Pino, A.; Van Hoorde, K.; Pitino, I.; Russo, N.; Carpino, S.; Caggia, C.; Randazzo, C.L. Survival of potential probiotic lactobacilli used as adjunct cultures on Pecorino Siciliano cheese ripening and passage through the gastrointestinal tract of healthy volunteers. Int. J. Food Microbiol. 2017, 252, 42–52. [Google Scholar] [CrossRef]
- Pino, A.; Russo, N.; Van Hoorde, K.; De Angelis, M.; Sferrazzo, G.; Randazzo, C.L.; Caggia, C. Piacentinu Ennese PDO Cheese as reservoir of promising probiotic bacteria. Microorganisms 2019, 7, 254. [Google Scholar] [CrossRef] [PubMed]
- Valero-Cases, E.; Cerdá-Bernad, D.; Pastor, J.-J.; Frutos, M.-J. Non-Dairy Fermented Beverages as Potential Carriers to Ensure Probiotics, Prebiotics, and Bioactive Compounds Arrival to the Gut and Their Health Benefits. Nutrients 2020, 12, 1666. [Google Scholar] [CrossRef]
- Caggia, C.; De Angelis, M.; Pitino, I.; Pino, A.; Randazzo, C.I. Probiotic features of Lactobacillus strains isolated from Ragusano and Pecorino Siciliano cheeses. Food Microbiol. 2015, 50, 109–117. [Google Scholar] [CrossRef]
- García-Burgos, M.; Moreno-Fernández, J.; Alférez, M.J.M.; Díaz-Castro, J.; López-Aliaga, I. New perspectives in fermented dairy products and their health relevance. J. Funct. Foods 2020, 72, 104059. [Google Scholar] [CrossRef]
- Agolino, G.; Pino, A.; Vaccalluzzo, A.; Cristofolini, M.; Solieri, L.; Caggia, C.; Randazzo, C.L. Bile salt hydrolase: The complexity behind its mechanism in relation to lowering-cholesterol Lactobacilli probiotics. J. Funct. Foods 2024, 120, 106357. [Google Scholar] [CrossRef]
- Agolino, G.; Cristofolini, M.; Vaccalluzzo, A.; Tagliazucchi, D.; Cattivelli, A.; Pino, A.; Caggia, C.; Solieri, L.; Randazzo, C.L. Genome Mining and Characterization of Two Novel Lacticaseibacillus rhamnosus Probiotic Candidates with Bile Salt Hydrolase Activity. Biomolecules 2025, 15, 86. [Google Scholar] [CrossRef]
- González-González, F.; Delgado, S.; Ruiz, L.; Margolles, A.; Ruas-Madiedo, P. Functional bacterial cultures for dairy applications: Towards improving safety, quality, nutritional and health benefit aspects. J. Appl. Microbiol. 2022; ahead of print. [Google Scholar] [CrossRef]
- Sarhir, S.T.; Belkhou, R.; Bouseta, A.; Hayaloglu, A.A. An optimization approach on the determination of volatile composition, bioactive compounds and sensory properties of Lben: Effect of fermentation conditions, starter system and substrate composition. J. Food Compos. Anal. 2024, 125, 105778. [Google Scholar] [CrossRef]
- Kocak, A.; Sanli, T.; Anli, E.A.; Hayaloglu, A.A. Role of Using Adjunct Cultures in Release of Bioactive Peptides in White-Brined Goat-Milk Cheese. LWT 2020, 123, 109127. [Google Scholar] [CrossRef]
- Redruello, B.; Saidi, Y.; Sampedro, L.; Ladero, V.; del Rio, B.; Alvarez, M.A. GABA-Producing Lactococcus lactis Strains Isolated from Camel’s Milk as Starters for the Production of GABA-Enriched Cheese. Foods 2021, 10, 633. [Google Scholar] [CrossRef]
- Flutto, T.; Merlet, M.; Thedy, L.; Pramotton, R.; Zenato, S.; Vernetti-Prot, L.; Valentini, S. Biochemical characterization of YoAlp®: A sheep-fermented milk obtained with autochthonous starter cultures. Eur. Food Res. Technol. 2024, 250, 1755–1760. [Google Scholar] [CrossRef]
- Andrada, E.; Marquez, A.; Russo, M.; Gauffin-Cano, P.; Medina, R. Fermented Goat Milk as a Functional Food for Obesity Prevention or Treatment: A Narrative Review. Front. Food Sci. Technol. 2024, 3, 1329037. [Google Scholar] [CrossRef]
- Chai, K.F.; Voo, A.Y.H.; Chen, W.N. Bioactive peptides from food fermentation: A comprehensive review of their sources, bioactivities, applications, and future development. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3825–3885. [Google Scholar] [CrossRef]
- Tonolo, F.; Fiorese, F.; Moretto, L.; Folda, A.; Scalcon, V.; Grinzato, A.; Ferro, S.; Arrigoni, G.; Bindoli, A.; Feller, E.; et al. Identification of New Peptides from Fermented Milk Showing Antioxidant Properties: Mechanism of Action. Antioxidants 2020, 9, 117. [Google Scholar] [CrossRef]
- Savaiano, D.A.; Hutkins, R.W. Yogurt, cultured fermented milk, and health: A systematic review. Nutr. Rev. 2021, 79, 599–614. [Google Scholar] [CrossRef]
- Nielsen, S.D.; Jakobsen, L.M.A.; Geiker, N.R.W.; Bertram, H.C. Chemically Acidified, Live and Heat-Inactivated Fermented Dairy Yoghurt Show Distinct Bioactive Peptides, Free Amino Acids and Small Compounds Profiles. Food Chem. 2022, 376, 131919. [Google Scholar] [CrossRef]
- Meng, X.; Huang, Y.J.; Xiong, J.Y.; Cheng, Z.R.; Yang, T.T.; Li, Z.; Tuo, R.; Zhang, Z.H.; Wang, G.; Gu, Q. Lactiplantibacillus plantarum ZFM55 improves texture and flavor of yogurt, increases beneficial metabolites, and the co-fermented yogurt promotes humangut microbiota health. LWT-Food Sci. Technol. 2024, 198, 115929. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, Z.; Zhang, X.; Chen, L.; Gu, Q.; Li, P. Lactobacillus paracasei ZFM54 Alters the Metabolomic Profiles of Yogurt and the Co-Fermented Yogurt Improves the Gut Microecology of Human Adults. J. Dairy Sci. 2024, 107, 5280–5300. [Google Scholar] [CrossRef] [PubMed]
- Li, K.J.; Burton-Pimentel, K.J.; Vergères, G.; Feskens, E.J.M.; Brouwer-Brolsma, E.M. Fermented foods and cardiometabolic health: Definitions, current evidence, and future perspectives. Front. Nutr. 2022, 9, 976020. [Google Scholar] [CrossRef]
- Melini, F.; Melini, V.; Luziatelli, F.; Ficca, A.G.; Ruzzi, M. Health-Promoting Components in Fermented Foods: An Up-to-Date Systematic Review. Nutrients 2019, 11, 1189. [Google Scholar] [CrossRef]
- Wang, T.; Wei, G. Integrated metabolomics and peptidomics to delineate characteristic metabolites in milk fermented with novel Lactiplantibacillus plantarum L3. Food Chem. 2023, 18, 100732. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, Y.; Liu, Z.; Su, M.; Wu, Z.; Zhang, H.; Xu, X. Insights into characteristic metabolites and potential bioactive peptides profiles of fresh cheese fermented with three novel probiotics-based metabolomics and peptidomics. Food Chem. X 2024, 21, 101147. [Google Scholar] [CrossRef]
- Bellaver, E.; Redin, E.; da Costa, I.; Moroni, L.; Kempka, A. Food peptidomic analysis of bovine milk fermented by Lacticaseibacillus caseiLBC 237: In silico prediction of bioactive peptides and anticancer potential. Food Res. Int. 2024, 180, 114060. [Google Scholar] [CrossRef]
- Filannino, P.; Bai, Y.; Di Cagno, R.; Gobbetti, M.; Gänzle, M.G.; Di Cagno, R. Metabolism of phenolic compounds by Lactobacillus spp. during fermentation of cherry juice and broccoli puree. Food Microbiol. 2015, 46, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.; Verón, H.; Contreras, L.; Isla, M.I. An overview of plant-autochthonous microorganisms and fermented vegetable foods. Food Sci. Hum. Wellness 2020, 9, 112–123. [Google Scholar] [CrossRef]
- Dominguez-Perez, L.A.; Beltran-Barrientos, L.M.; Gonzalez-Cordova, A.F.; Hernandez-Mendoza, A.; Vallejo-Cordoba, B. Artisanal cocoa bean fermentation: From cocoa bean proteins to bioactive peptides with potential health benefits. J. Funct. Foods 2020, 73, 104134. [Google Scholar] [CrossRef]
- Junior, G.C.C.; Ferreira, N.R.; Andrade, E.H.; Nascimento, L.D.; Siqueira, F.C.; Lopes, A.S. Profile of Volatile Compounds of On-Farm Fermented and Dried Cocoa Beans Inoculated with Saccharomyces cerevisiae KY794742 and Pichia kudriavzevii KY794725. Molecules 2021, 26, 344. [Google Scholar] [CrossRef]
- Xu, X.; Bi, S.; Lao, F.; Chen, F.; Liao, X.; Wu, J. Comprehensive investigation on volatile and non-volatile metabolites in broccoli juices fermented by animal-and plant-derived Pediococcus pentosaceus. Food Chem. 2021, 341, 128118. [Google Scholar] [CrossRef]
- Lee, M.; Yun, Y.; Choi, E.J.; Song, J.H.; Kang, J.Y.; Kim, D.; Lee, K.W.; Chang, J.Y. Anti-obesity effect of vegetable juice fermented with lactic acid bacteria isolated from kimchi in C57BL/6J mice and human mesenchymal stem cells. Food Funct. 2023, 14, 1349–1656. [Google Scholar] [CrossRef]
- Mantzourani, I.; Nikolaou, A.; Kourkoutas, Y.; Alexopoulos, A.; Dasenaki, M.; Mastrotheodoraki, A.; Proestos, C.; Thomaidis, N.; Plessas, S. Chemical Profile Characterization of Fruit and Vegetable Juices after Fermentation with Probiotic Strains. Foods 2024, 13, 1136. [Google Scholar] [CrossRef]
- Szutowska, J.; Gwiazdowska, D.; Rybicka, I.; Pawlak-Lemanska, K.; Bieganska-Marecik, R.; Gliszczynska-Swigło, A. Controlled Fermentation of Curly Kale Juice with the Use of Autochthonous Starter Cultures. Food Res. Int. 2021, 149, 110674. [Google Scholar] [CrossRef] [PubMed]
- Iga-Buitrón, D.; Torres-Maravilla, E.; Bermúdez-Humaran, L.G.; Ascacio-Valdes, J.A.; Rodríguez-Herrera, R.; Aguilar, C.N.; Flores-Gallegos, A.C. Lactic Fermentation of Broccoli (Brassica oleracea var. italica) to Enhance the Antioxidant and Antiproliferative Activities. Fermentation 2023, 9, 122. [Google Scholar] [CrossRef]
- Kayitesi, E.; Onojakpor, O.; Moyo, S.M. Highlighting the Impact of Lactic-Acid-Bacteria-Derived Flavours or Aromas on Sensory Perception of African Fermented Cereals. Fermentation 2023, 9, 111. [Google Scholar] [CrossRef]
- Lu, X.; Jing, Y.; Li, Y.; Zhang, N.; Cao, Y. Eurotium cristatum produced β-hydroxy acid metabolite of monacolin K and improved bioactive compound contents as well as functional properties in fermented wheat bran. LWT 2022, 158, 113088. [Google Scholar] [CrossRef]
- Zhang, L.; Kang, W.; Lu, X.; Ma, S.; Dong, L.; Zou, B. Weighted gene co-expression network analysis and connectivity map identifies lovastatin as a treatment option of gastric cancer by inhibiting HDAC2. Gene 2019, 681, 15–25. [Google Scholar] [CrossRef]
- Cui, J.; Xia, P.; Zhang, L.; Hu, Y.; Xie, Q.; Xiang, H. A Novel Fermented Soybean, Inoculated with Selected Bacillus, Lactobacillus and Hansenula Strains, Showed Strong Antioxidant and Anti-Fatigue Potential Activity. Food Chem. 2020, 333, 127527. [Google Scholar] [CrossRef]
- Sidari, R.; Martorana, A.; Zappia, C.; Mincione, A.; Giuffrè, A.M. Persistence and Effect of a Multistrain Starter Culture on Antioxidant and Rheological Properties of Novel Wheat Sourdoughs and Bread. Foods 2020, 9, 1258. [Google Scholar] [CrossRef]
- Rațu, R.N.; Veleșcu, I.D.; Stoica, F.; Usturoi, A.; Arsenoaia, V.N.; Crivei, I.C.; Postolache, A.N.; Lipșa, F.D.; Filipov, F.; Florea, A.M.; et al. Application of Agri-Food By-Products in the Food Industry. Agriculture 2023, 13, 1559. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, J.; Gao, R.; Ye, F.; Zhao, G. Sustainable valorisation of tomato pomace: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 172–187. [Google Scholar] [CrossRef]
- Valenti, F.; Porto, S.M.C.; Selvaggi, R.; Pecorino, B. Co-digestion of by-products and agricultural residues: Abioeconomy perspective for a Mediterranean feedstock mixture. Sci. Total Environ. 2020, 700, 134440. [Google Scholar] [CrossRef] [PubMed]
- Sabater, C.; Ruiz, L.; Delgado, S.; Ruas-Madiedo, P.; Margolles, A. Valorization of Vegetable Food Waste and By-Products Through Fermentation Processes. Front. Microbiol. 2020, 11, 581997. [Google Scholar] [CrossRef] [PubMed]
- RuizRodríguez, L.G.; Zamora Gasga, V.M.; Pescuma, M.; Van Nieuwenhove, C.; Mozzi, F.; Sánchez Burgos, J.A. Fruits and fruit by-products as sources of bioactive compounds. Benefits and trends of lactic acid fermentation in the development of novel fruit-based functional beverages. Food Res. Int. 2021, 140, 109854. [Google Scholar] [CrossRef]
- Wuyts, S.; Van Beeck, W.; Allonsius, C.N.; Broek, M.F.v.D.; Lebeer, S. Applications of plant-based fermented foods and their microbes. Curr. Opin. Biotechnol. 2020, 61, 45–52. [Google Scholar] [CrossRef]
- Ricci, A.; Diaz, A.B.; Caro, I.; Bernini, V.; Galaverna, G.; Lazzi, C.; Blandino, A. Orange peels: From by-product to resource through lactic acid fermentation. J. Sci. Food Agric. 2019, 99, 6761–6767. [Google Scholar] [CrossRef]
- Mladenovic, D.; Pejin, J.; Kocic-Tanackov, S.; Djukic-Vukovic, A.; Mojovic, L. Enhanced Lactic Acid Production by Adaptive Evolution of Lactobacillus Paracasei on Agro-Industrial Substrate. Appl. Biochem. Biotechnol. 2019, 187, 753–769. [Google Scholar] [CrossRef]
- Goto, M.; Kuda, T.; Shikano, A.; Charrouf, Z.; Yamauchi, K.; Yokozawa, M.; Takahashi, H.; Kimura, B. Induction of superoxide anion radical-scavenging capacity in an argan press cake-suspension by fermentation using Lactobacillus plantarum Argan-L1. LWT 2019, 100, 56–61. [Google Scholar] [CrossRef]
- Bahry, H.; Rawa, A.; Pons, A.; Taha, S.; Vial, C. Optimization of lactic acid production using immobilized Lactobacillus rhamnosus and carob pod waste from the Lebanese food industry. J. Biotechnol. 2019, 306, 81–88. [Google Scholar] [CrossRef]
- Sarris, D.; Rapti, A.; Papafotis, N.; Koutinas, A.A.; Papanikolaou, S. Production of Added-Value Chemical Compounds through Bioconversions of Olive-Mill Wastewaters Blended with Crude Glycerol by a Yarrowia lipolytica Strain. Molecules 2019, 24, 222. [Google Scholar] [CrossRef] [PubMed]
- Romeo, F.V.; Granuzzo, G.; Foti, P.; Ballistreri, G.; Caggia, C.; Rapisarda, P. Microbial Application to Improve Olive Mill Wastewater Phenolic Extracts. Molecules 2021, 26, 1944. [Google Scholar] [CrossRef] [PubMed]
- Lazzaroli, C.; Sordini, B.; Daidone, L.; Veneziani, G.; Esposto, S.; Urbani, S.; Selvaggini, R.; Servili, M.; Taticchi, A. Recovery and Valorization of Food Industry By-Products through the Application of Olea europaea L. Leaves in Kombucha Tea Manufacturing. Food Biosci. 2023, 53, 102551. [Google Scholar] [CrossRef]
- Foti, P.; Occhipinti, P.S.; Russo, N.; Scilimati, A.; Miciaccia, M.; Caggia, C.; Perrone, M.G.; Randazzo, C.L.; Romeo, F.V. Olive Mill Wastewater Fermented with Microbial Pools as a New Potential Functional Beverage. Molecules 2023, 28, 646. [Google Scholar] [CrossRef]
- Foti, P.; Randazzo, C.L.; Russo, M.; Sanzo, R.D.; Romeo, F.V.; Scilimati, A.; Miciaccia, M.; Perrone, M.G.; Caggia, C. Effect of microbial fermentation on functional traits and volatiloma profile of pâté olive cake. Food Res. Int. 2023, 174, 1113510. [Google Scholar] [CrossRef]
- Cheng, Y.; Wu, T.; Chu, X.; Tang, S.; Cao, W.; Liang, F.; Fang, Y.; Pan, S.; Xu, X. Fermented blueberry pomace with antioxidant properties improves fecal microbiota community structure and short chain fatty acids production in an in vitro mode. LWT Food Sci. Technol. 2020, 125, 109260. [Google Scholar] [CrossRef]
- Falah, F.; Vasiee, A.; Tabatabaei-Yazdi, F.; Moradi, S.; Sabahi, S. Optimization of gamma-aminobutyric acid (GABA) production by Lactobacillus spp. from agro-food waste. Biomass Convers. Biorefin. 2022, 14, 3425–3437. [Google Scholar] [CrossRef]
- Aruna, T.E. Production of value-added product from pineapple peels using solid state fermentation. Innov. Food Sci. Emerg. Technol. 2019, 57, 102193. [Google Scholar] [CrossRef]
- Luo, J.W.; Xiao, S.; Suo, H.; Wang, B.; Cai, Y.X.; Wang, J.H. Dynamics of Nutrients, Sensory Quality and Microbial Communities and Their Interactions during Co-Fermentation of Pineapple by-Products and Whey Protein. Food Chem. X 2024, 22, 101254. [Google Scholar] [CrossRef]
- Cantatore, V.; Filannino, P.; Giuseppe, G.; De Pasquale, I.; Pan, S.; Gobbetti, M.; Di Cagno, R. Lactic Acid Fermentation to Re-Cycle Apple By-Products for Wheat Bread Fortification. Front. Microbiol. 2019, 10, 2574. [Google Scholar] [CrossRef]
- Li, J.; Ye, F.; Zhou, Y.; Lei, L.; Chen, J.; Li, S.; Zhao, G. Tailoring the Composition, Antioxidant Activity, and Prebiotic Potential of Apple Peel by Aspergillus oryzae Fermentation. Food Chem. X 2024, 21, 101134. [Google Scholar] [CrossRef] [PubMed]
- Bortolini, D.G.; Benvenutti, L.; Demiate, I.M.; Nogueira, A.; Alberti, A.; Zielinski, A.A.F. A New Approach to the Use of Apple Pomace in Cider Making for the Recovery of Phenolic Compounds. LWT 2020, 126, 109316. [Google Scholar] [CrossRef]
- Dulf, F.V.; Vodnar, D.C.; Dulf, E.H. Solid-State Fermentation with Zygomycetes Fungi as a Tool for Biofortification of Apple Pomace with γ-Linolenic Acid, Carotenoid Pigments and Phenolic Antioxidants. Food Res. Int. 2023, 173, 113448. [Google Scholar] [CrossRef] [PubMed]
- Pontonio, E.; Dingeo, C.; Gobbetti, M.; Rizzello, C.G. Maize Milling By-Products: From Food Wastes to Functional Ingredients Through Lactic Acid Bacteria Fermentation. Front. Microbiol. 2019, 10, 561. [Google Scholar] [CrossRef]
- Schettino, R.; Pontonio, E.; Rizzello, C.G. Use of Fermented Hemp, Chickpea and Milling By-Products to Improve the Nutritional Value of Semolina Pasta. Foods 2019, 8, 604. [Google Scholar] [CrossRef]
- Lücke, F.K.; Fritz, V.; Tannhäuser, K.; Arya, A. Controlled fermentation of rapeseed presscake by Rhizopus, and its effect on some components with relevance to human nutrition. Food Res. Int. 2019, 120, 726–732. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Villela-Castrejón, J.; Perez-Carrillo, E.; Gómez-Sánchez, C.E.; Gutiérrez-Uribe, J.A. Effects of Solid-State Fungi Fermentation on Phenolic Content, Antioxidant Properties and Fiber Composition of Lime Cooked Maize by-Product (Nejayote). J. Cereal Sci. 2019, 90, 102837. [Google Scholar] [CrossRef]
- Chebaibi, S.; Grandchamp, M.L.; Burgé, G.; Clément, T.; Allais, F.; Laziri, F. Improvement of Protein Content and Decrease of Anti-nutritional Factors in Olive Cake by Solid-State Fermentation: A Way to Valorize This Industrial By-Product in Animal Feed. J. Biosci. Bioeng. 2019, 128, 384–390. [Google Scholar] [CrossRef]
- Pyne, M.E.; Narcross, L.; Martin, V.J.J. Engineering plant secondary metabolism in microbial systems. Plant Physiol. 2019, 179, 844–861. [Google Scholar] [CrossRef]
- Ding, W.; Zhang, Y.; Shi, S. Development and Application of CRISPR/Cas in Microbial Biotechnology. Front. Bioeng. Biotechnol. 2020, 8, 711. [Google Scholar] [CrossRef]
- Leavell, M.D.; Singh, A.H.; Kaufmann-Malaga, B.B. High-throughput screening for improved microbial cell factories, perspective and promise. Curr. Opin. Biotechnol. 2020, 62, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guleria, S.; Koffas, M.A.G.; Yan, Y. Microbial Production of Value-Added Nutraceuticals. Curr. Opin. Biotechnol. 2016, 37, 97–104. [Google Scholar] [CrossRef]
- Wu, X.; Zha, J.; Koffas, M.A.G. Microbial production of bioactive chemicals for human health. Curr. Opin. Food Sci. 2020, 32, 9–16. [Google Scholar] [CrossRef]
- Abid, R.; Waseem, H.; Ali, J.; Ghazanfar, S.; Muhammad Ali, G.; Elasbali, A.M.; Alharethi, S.H. Probiotic Yeast Saccharomyces: Back to Nature to Improve Human Health. J. Fungi 2022, 8, 444. [Google Scholar] [CrossRef]
- Guo, H.; Jacobsen, S.A.B.; Walter, K.; Lewandowski, A.; Czarnotta, E.; Knuf, C.; Polakowski, T.; Maury, J.; Lang, C.; Förster, J.; et al. Triterpenoid production with a minimally engineered Saccharomyces cerevisiae chassis. bioRxiv 2022. [Google Scholar] [CrossRef]
- Gowers, G.F.; Chee, S.M.; Bell, D.; Suckling, L.; Kern, M.; Tew, D.; McClymont, D.W.; Ellis, T. Improved betulinic acid biosynthesis using synthetic yeast chromosome recombination and semi-automated rapid LC-MS screening. Nat. Commun. 2020, 11, 868. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, J.; Zhao, G.; Yan, X.; Zhou, Z. Systematic optimization of the yeast cell factory for sustainable and high efficiency production of bioactive ginsenoside compound K. Synth. Syst. Biotechnol. 2021, 6, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Gao, J.; Yu, W.; Chen, X.; Zhai, X.; Chen, Y.; Zhang, L.; Zhou, Y.J. Engineering cofactor supply and recycling to drive phenolic acid biosynthesis in yeast. Nat. Chem. Biol. 2022, 18, 520–529. [Google Scholar] [CrossRef]
- Yuan, S.; Alper, H.S. Metabolic Engineering of Microbial Cell Factories for Production of Nutraceuticals. Microb. Cell Fact. 2019, 18, 46. [Google Scholar] [CrossRef]
- Peng, Y.F.; Chen, W.C.; Xiao, K.; Xu, L.; Wang, L.; Wan, X. DHA production in Escherichia coli by expressing reconstituted key genes of polyketide synthase pathway from marine bacteria. PLoS ONE 2016, 11, e0162861. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.; Li, X.; Liu, D.; Dong, X.T.; Li, F.F.; Wang, E.X.; Li, B.Z.; Yuan, Y.J. Production of naringenin from D-xylose with co-culture of E. coli and S. cerevisiae. Eng. Life Sci. 2017, 17, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Zaragoza, J.M.; Hernández-Chávez, G.; Moreno-Avitia, F.; Ramírez-Iñiguez, R.; Martínez, A.; Bolívar, F.; Gosset, G. Engineering of a microbial coculture of Escherichia coli strains for the biosynthesis of resveratrol. Microb. Cell Fact. 2016, 15, 163. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Binkley, R.M.; Kim, W.J.; Lee, M.H.; Lee, S.Y. Metabolic engineering of Escherichia coli for high-level astaxanthin production with high productivity. Metab. Eng. 2018, 49, 105–115. [Google Scholar] [CrossRef]
- Soma, Y.; Fujiwara, Y.; Nakagawa, T.; Tsuruno, K.; Hanai, T. Reconstruction of a Metabolic Regulatory Network in Escherichia coli for Purposeful Switching from Cell Growth Mode to Production Mode in Direct GABA Fermentation from Glucose. Metab. Eng. 2017, 43, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.H. Genome-Editing Technologies: Concept, Pros, and Cons of Various Genome-Editing Techniques and Bioethical Concerns for Clinical Application. Mol. Ther. Nucleic Acids 2019, 16, 326–334. [Google Scholar] [CrossRef]
- Ayanoglu, F.B.; Elçin, A.E.; Elçin, Y.M. Bioethical issues in genome editing by CRISPR-Cas9 technology. Turk. J. Biol. 2020, 44, 110–120. [Google Scholar] [CrossRef]
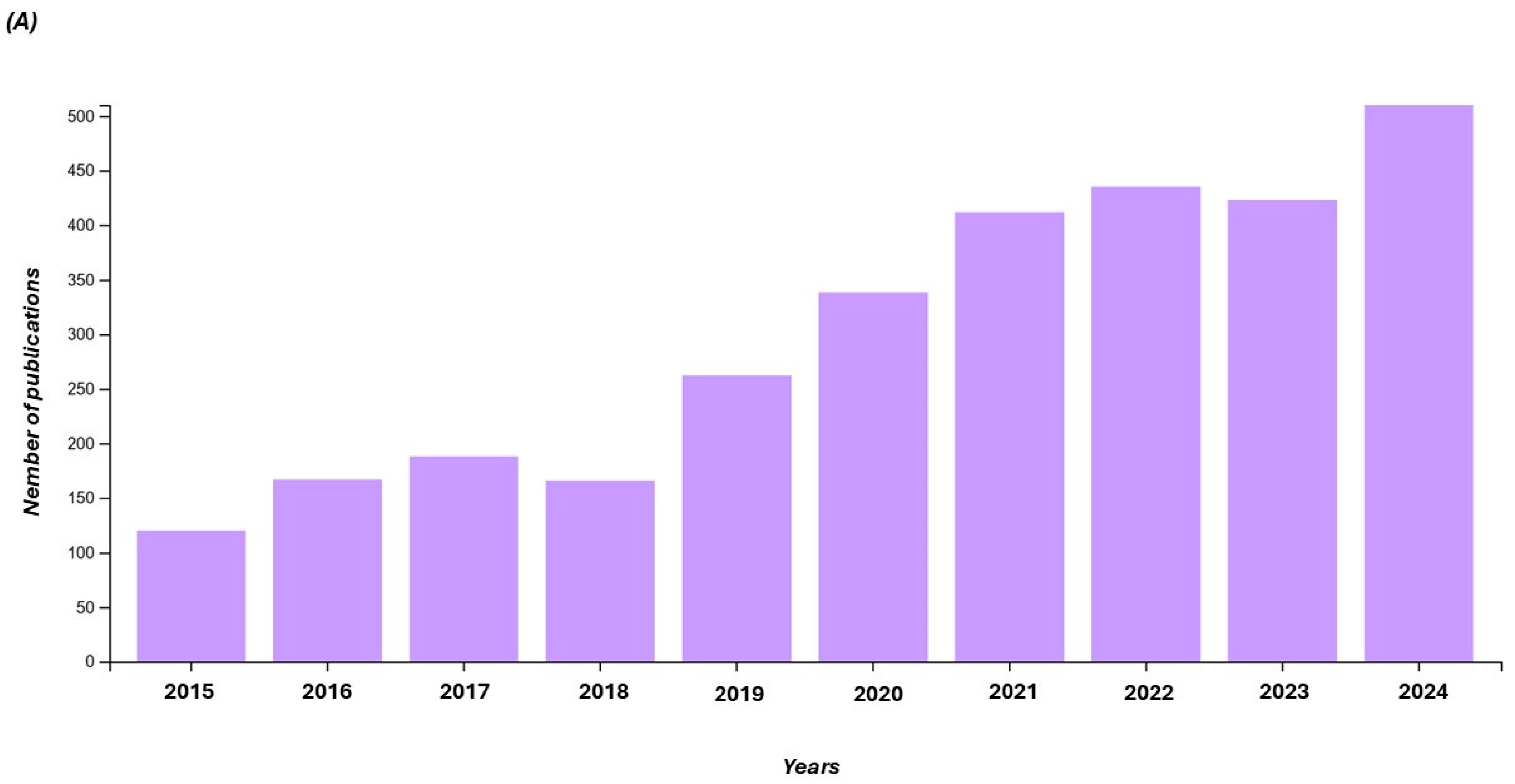
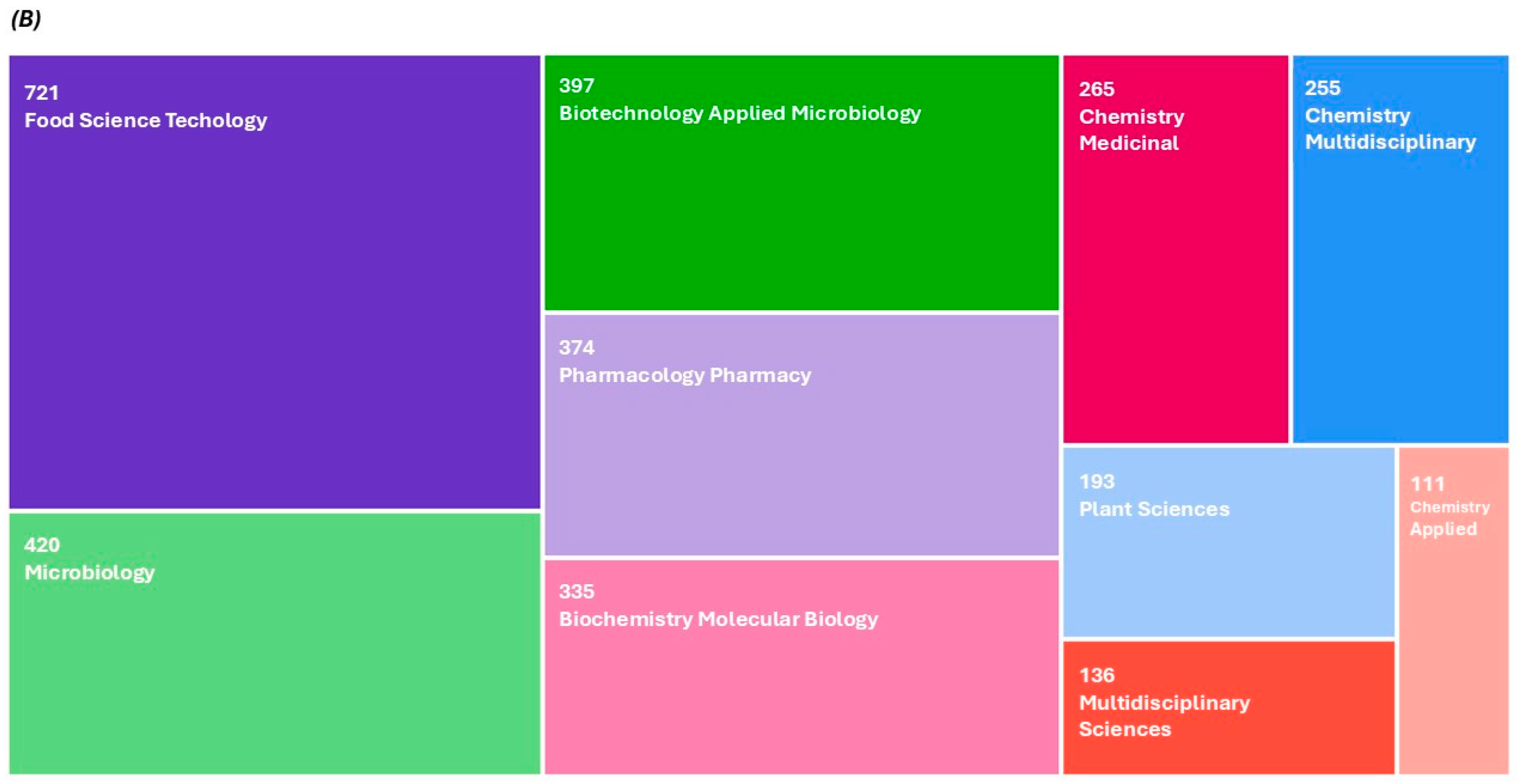
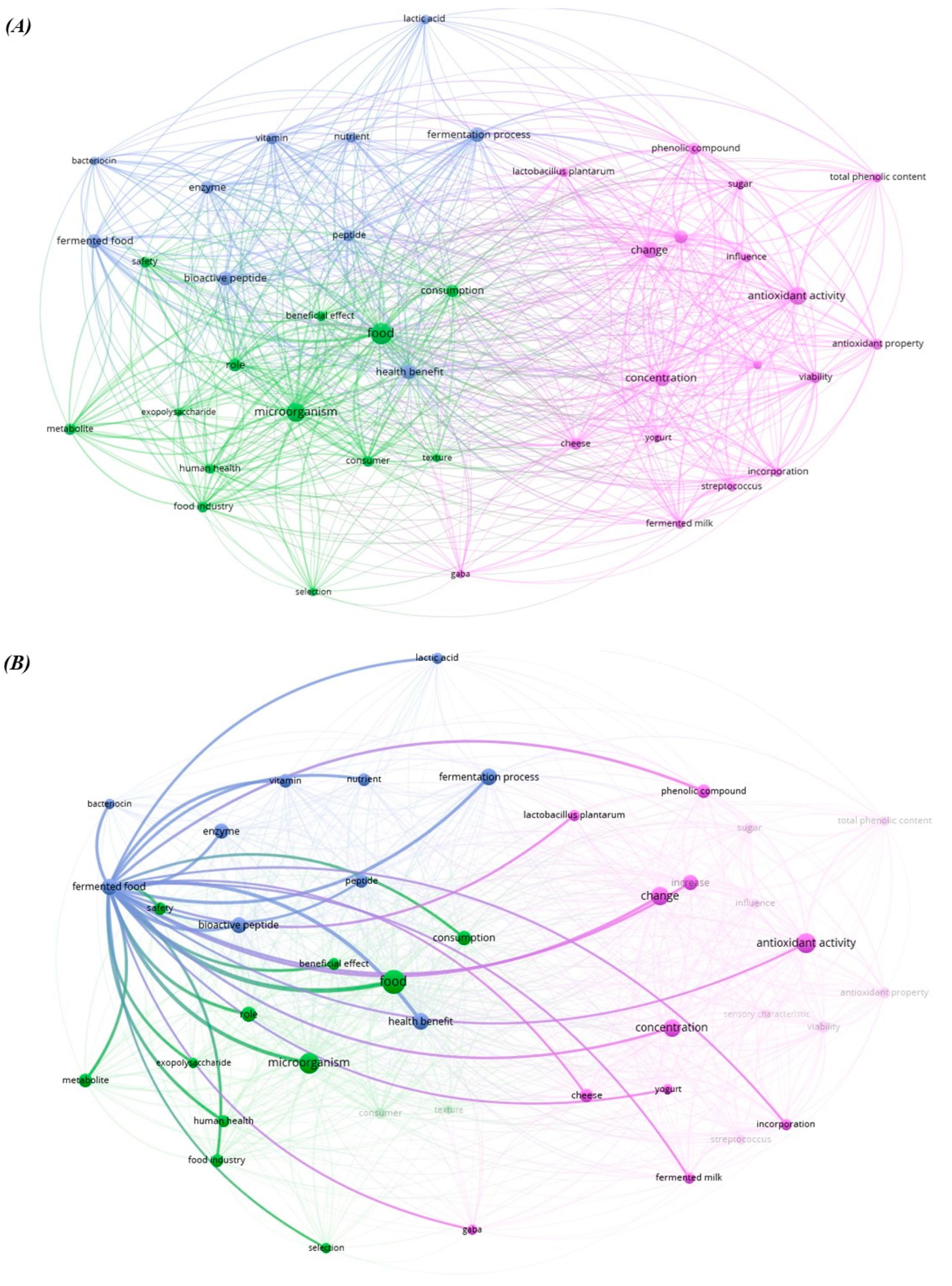
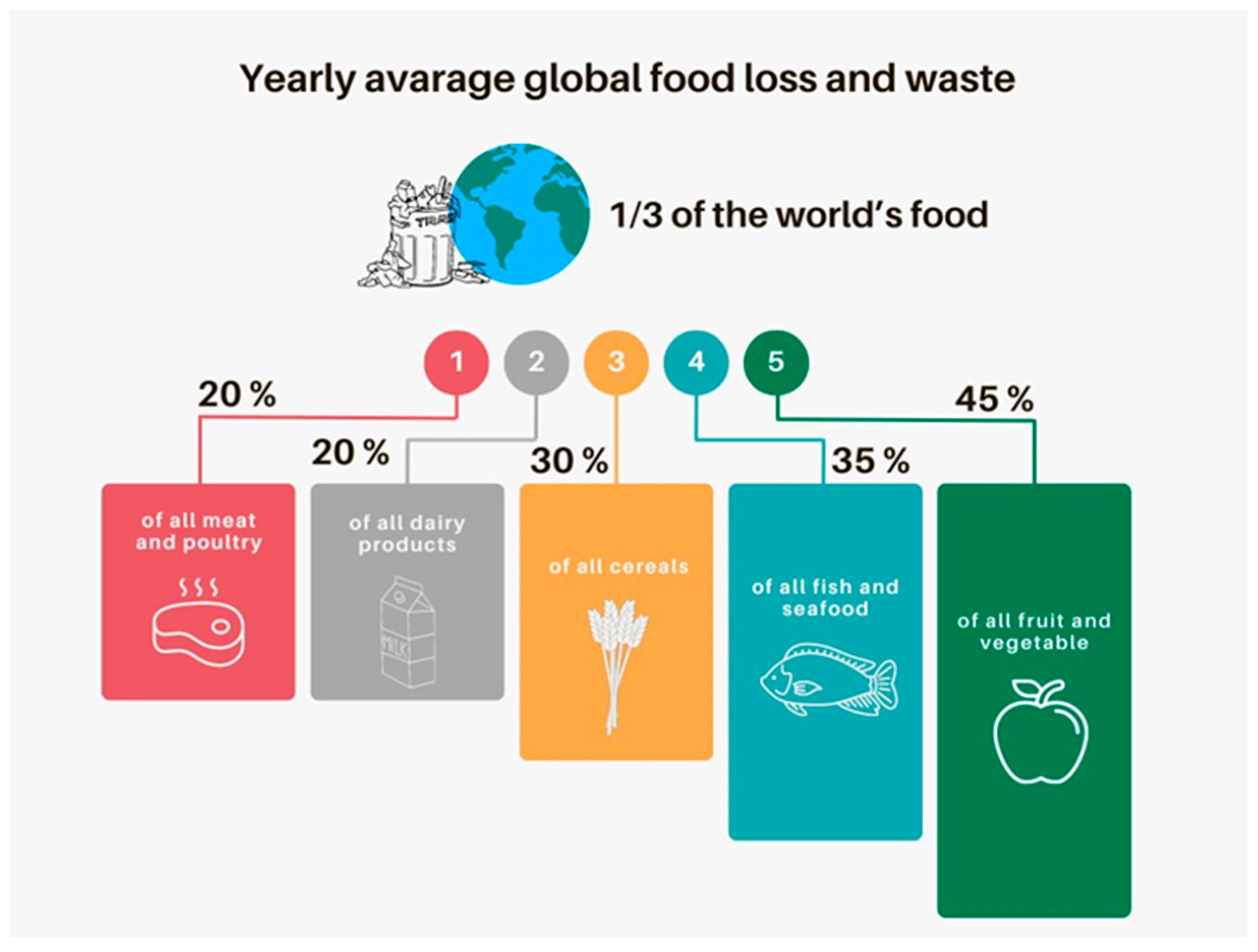
| Agro-Food By-Products | Starter Cultures | Main Results | References |
|---|---|---|---|
| Orange peels | L. casei 2246 (single culture) L. plantarum 285 and L. paracasei 4186 (mixed cultures) | Lactic acid production in solid state fermentation | [70] |
| Molasses | L. paracasei NRRL B-4564 | Lactic acid production in solid state fermentation | [71] |
| Argan press cake | L. plantarum Argan-L1 | Increased antioxidant activity related to lactic acid and protein content | [72] |
| Carob pods | L. rhamnosus ATCC 53103 | Improved lactic acid production and yield | [73] |
| Olive mill wastewater | Yarrowia lipolytica | Production of citric and oleic acid | [74] |
| L. plantarum and W. anomalus | Increased hydroxyditorosol content | [75] | |
| L. plantarum, W. anomalus and C. boidinii | Increased hydroxyditorosol content and biological activity | [77] | |
| Olive leaves | Commercial SCOBY | Increased hydroxyditorosol and oleuropein content | [76] |
| Blueberry pomace | L. casei | Increased antioxidant activity and polyphenol content | [79] |
| Molasses, dairy sludge, and soybean meal | L. brevis PML1, L. fermentum a L. plantarum 1058 | Production of valuable medicinal and bioactive compounds | [80] |
| Pineapple peels | Trichoderma | High protein production | [81] |
| Pineapple core | Lactococcus lactis LA5, Hanseniaspora opuntiaeSA2 | Production of new fermented food with high fiber and amino acid content | [82] |
| Apple by-products | Weissella cibaria PEP23F Saccharomyces cerevisiae AN6Y19 | Production of a functional ingredient for wheat bread | [83] |
| Aspergillus oryzae | Production of a functional ingredient with antioxidant and prebiotic activity | [84] | |
| Saccharomyces cerevisiae | Production of new fermented beverages with antioxidant activity | [85] | |
| Zygomycetes fungi | Production of a functional ingredient with a high level of phenol | [86] | |
| Maize milling by-products | L. plantarum T6B10 and Weissella confusa BAN8 | Production of a functional ingredient for bread | [87] |
| Hemp, chickpea, and milling by-products | L. plantarum LB1 and L. rossiae LB5 | Production of a functional ingredient for pasta | [88] |
| Rapeseed press cake | Rhizopus microsporus var. oligosporus | Production of a functional ingredient | [89] |
| Lime-cooked maize | Aspergillus oryzae, Pleurotus ostreatus, and Hericium erinaceus | Increased free phenolic compounds, antioxidant capacity, and soluble dietary fiber | [90] |
| Olive cake | Fusarium flocciferum, Rhizodiscina cf. lignyota | Production of efficient enzymes leading to nutritional enhancement of this by-product | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foti, P.; Caggia, C.; Romeo, F.V. New Insight into Microbial Exploitation to Produce Bioactive Molecules from Agrifood and By-Products’ Fermentation. Foods 2025, 14, 1439. https://doi.org/10.3390/foods14081439
Foti P, Caggia C, Romeo FV. New Insight into Microbial Exploitation to Produce Bioactive Molecules from Agrifood and By-Products’ Fermentation. Foods. 2025; 14(8):1439. https://doi.org/10.3390/foods14081439
Chicago/Turabian StyleFoti, Paola, Cinzia Caggia, and Flora Valeria Romeo. 2025. "New Insight into Microbial Exploitation to Produce Bioactive Molecules from Agrifood and By-Products’ Fermentation" Foods 14, no. 8: 1439. https://doi.org/10.3390/foods14081439
APA StyleFoti, P., Caggia, C., & Romeo, F. V. (2025). New Insight into Microbial Exploitation to Produce Bioactive Molecules from Agrifood and By-Products’ Fermentation. Foods, 14(8), 1439. https://doi.org/10.3390/foods14081439









