The Valorization of Rapeseed Meal as Hydrolyzed and Lyophilized Extract to Improve the Antioxidant Properties of Refined Rapeseed Oil During Frying and Fried French Fries
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Materials
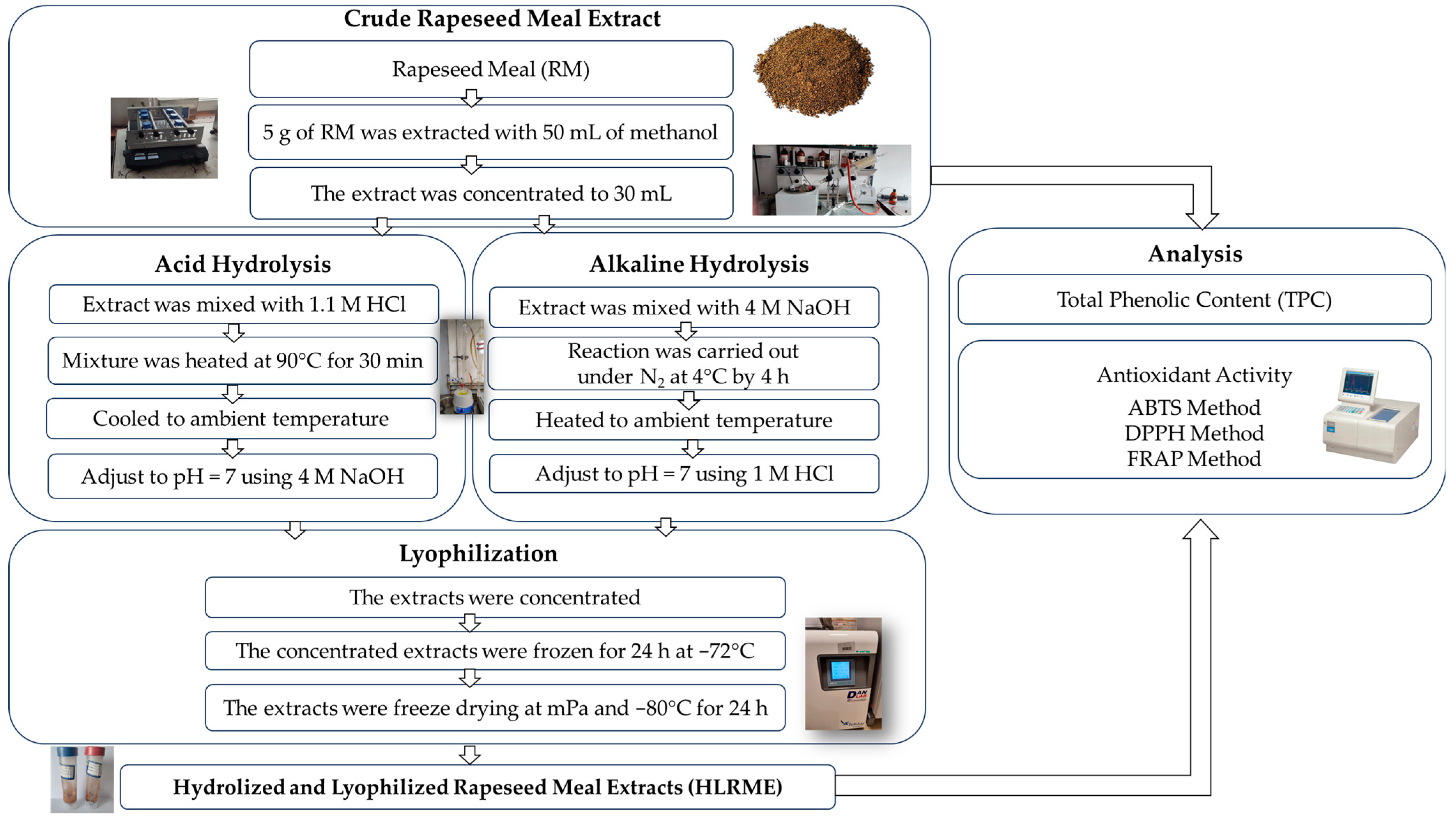
2.3. Preparation of Hydrolyzed and Lyophilized Rapeseed Meal Extracts
2.3.1. Crude Rapeseed Meal Extract
2.3.2. Alkaline Hydrolysis and Acid Hydrolysis of Rapeseed Meal Extract
2.3.3. Lyophilization of Alkaline- and Acid-Hydrolyzed Rapeseed Meal Extracts
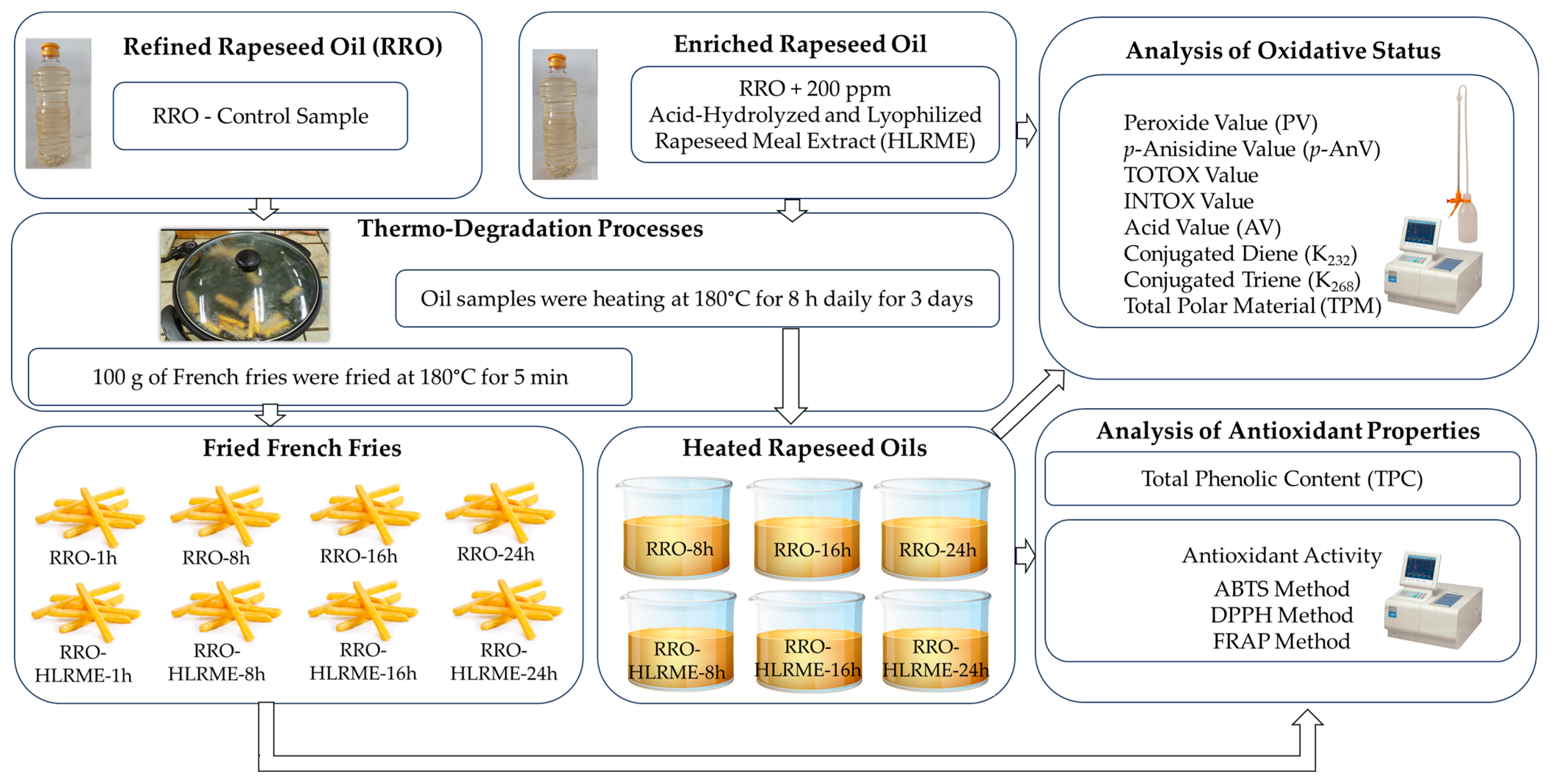
2.4. Preparation of Rapeseed Oil Enriched with Acid-Hydrolyzed Rapeseed Meal Extract
2.5. Frying Test
2.6. Determination of Oxidative Status of Oil Samples
2.7. Determination of Total Polar Material in Oil Samples
2.8. Determination of Antioxidant Activity and Total Phenolic Content
2.8.1. Preparation of Samples
2.8.2. Procedures of Analytical Methods
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effect of Chemical Hydrolysis on Antioxidant Properties of Rapeseed Meal Extract
3.2. Changes in Antioxidant Properties of Refined Rapeseed Oils Without and with Hydrolyzed and Lyophilized Rapeseed Meal Extract During Heating
3.3. Changes in Oxidative Status of Refined Rapeseed Oils Without and with Hydrolyzed and Lyophilized Rapeseed Meal Extract During Heating
3.3.1. Peroxide Value
3.3.2. Anisidine Value
3.3.3. Total Oxidation Index and Integral Oxidation Index
3.3.4. Acid Value
3.3.5. Conjugated Dienes and Conjugated Trienes
3.3.6. Total Polar Material
3.4. Effect of Hydrolyzed and Lyophilized Rapeseed Meal Extract and Heating Oil Time on Antioxidant Properties of French Fries
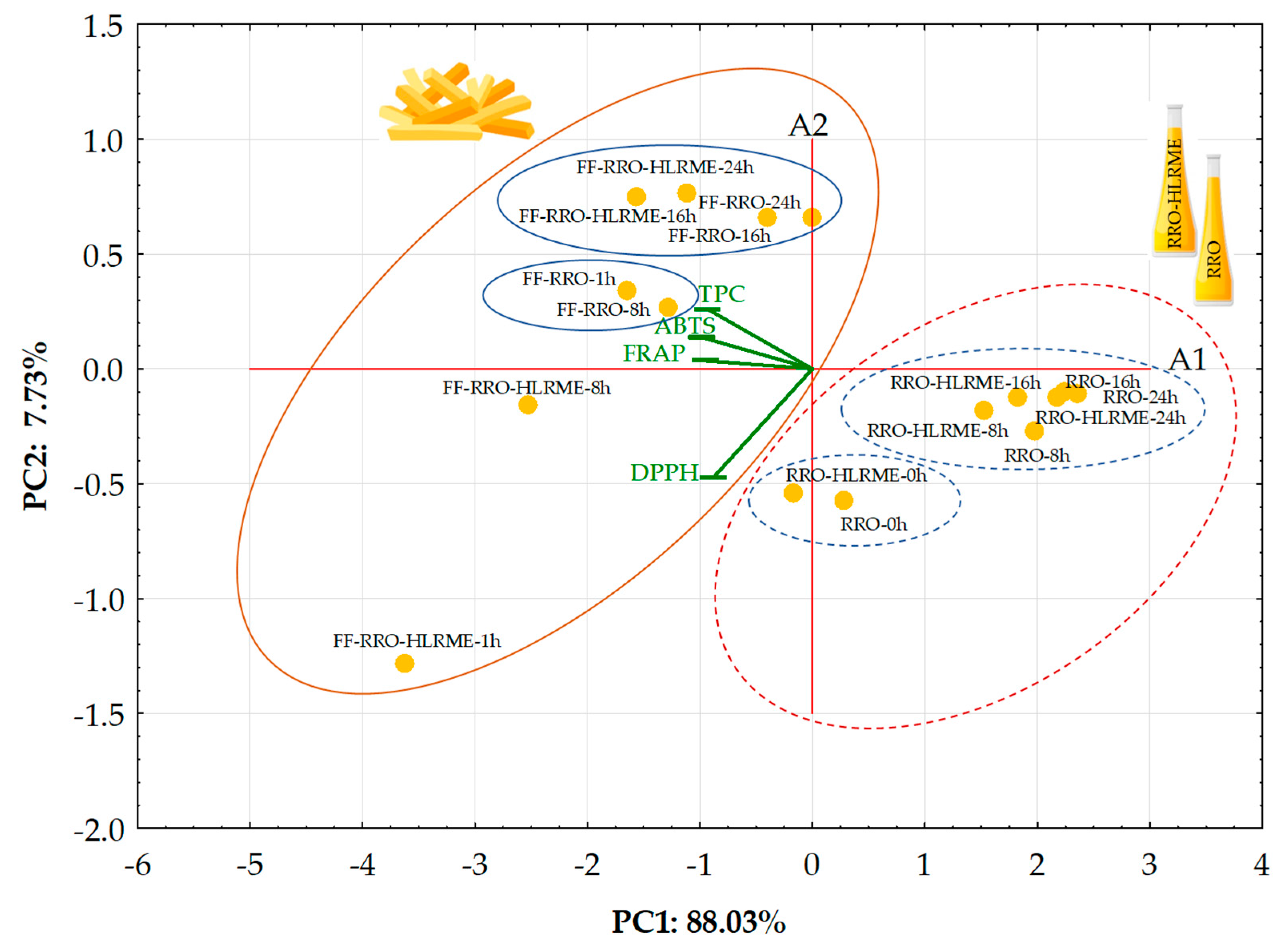
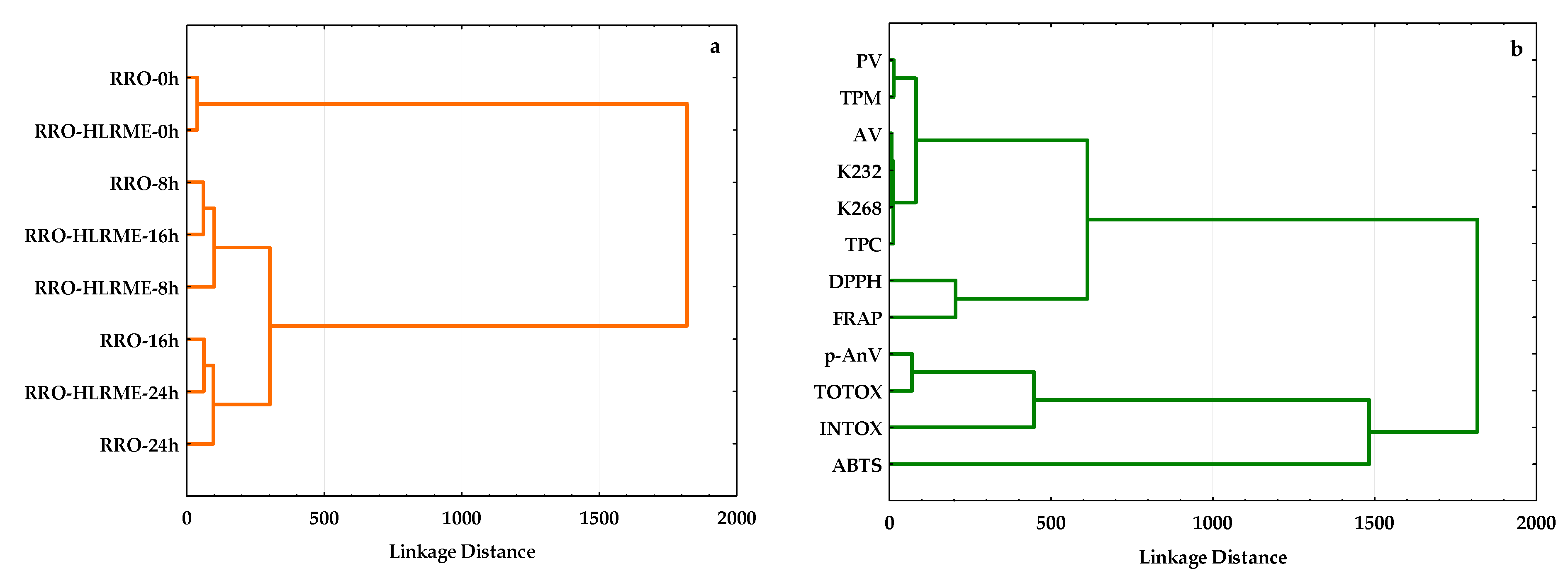
3.5. Unsupervised Multivariate Techniques
3.5.1. Principal Component Analysis
3.5.2. Hierarchical Cluster Analysis
3.5.3. Color Map of Correlations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ambrosewicz, M.; Tańska, M.; Rotkiewicz, D. Fatty Acid Composition as a Coefficient of Ways of Usage Oils from Seeds of Different Varieties of Rapeseed. Pol. J. Nat. Sci. 2011, 26, 74–83. [Google Scholar]
- Siger, A.; Kaczmarek, A.; Rudzińska, M. Antioxidant Activity and Phytochemical Content of Cold-Pressed Rapeseed Oil Obtained from Roasted Seeds. Eur. J. Lipid Sci. Technol. 2015, 117, 1225–1237. [Google Scholar] [CrossRef]
- Aladedunye, F.; Niehaus, K.; Bednarz, H.; Thiyam-Hollander, U.; Fehling, E.; Matthäus, B. Enzymatic Lipophilization of Phenolic Extract from Rowanberry (Sorbus aucuparia) and Evaluation of Antioxidative Activity in Edible Oil. LWT—Food Sci. Technol. 2015, 60, 56–62. [Google Scholar] [CrossRef]
- Abrante-Pascual, S.; Nieva-Echevarría, B.; Goicoechea-Oses, E. Vegetable Oils and Their Use for Frying: A Review of Their Compositional Differences and Degradation. Foods 2024, 13, 4186. [Google Scholar] [CrossRef]
- Aladedunye, F.; Matthäus, B. Phenolic Extracts from Sorbus aucuparia (L.) and Malus baccata (L.) Berries: Antioxidant Activity and Performance in Rapeseed Oil During Frying and Storage. Food Chem. 2014, 159, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Aladedunye, F.; Kersting, H.J.; Matthäus, B. Phenolic Extract from Wild Rose Hip with Seed: Composition, Antioxidant Activity, and Performance in Canola Oil. Eur. J. Lipid Sci. Technol. 2014, 116, 1025–1034. [Google Scholar] [CrossRef]
- Matthäus, B.; Pudel, F.; Chen, Y.; Achary, A.; Thiyam-Holländer, U. Impact of Canolol-Enriched Extract from Heat-Treated Canola Meal to Enhance Oil Quality Parameters in Deep-Frying: A Comparison with Rosemary Extract and TBHQ-Fortified Oil Systems. J. Am. Oil Chem. Soc. 2014, 91, 2065–2076. [Google Scholar] [CrossRef]
- Cao, L.; Jia, P.; Liu, H.; Kang, S.; Jiang, S.; Pang, M. Effects of High-Canolol Phenolic Extracts on Fragrant Rapeseed Oil Quality and Flavor Compounds During Frying. Foods 2023, 12, 827. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Yu, J.; Chen, N.; Zeng, W. Effects and Mechanism of Tea Polyphenols on the Quality of Oil During Frying Process. J. Food Sci. 2020, 85, 3786–3796. [Google Scholar] [CrossRef]
- Aachary, A.A.; Chen, Y.; Eskin, N.A.M.; Thiyam-Hollander, U. Crude Canolol and Canola Distillate Extracts Improve the Stability of Refined Canola Oil During Deep-Fat Frying. Eur. J. Lipid Sci. Technol. 2014, 116, 1467–1476. [Google Scholar] [CrossRef]
- Chiou, A.; Kalogeropoulos, N.; Salta, F.N.; Efstathiou, P.; Andrikopoulos, N.K. Pan-Frying of French Fries in Three Different Edible Oils Enriched with Olive Leaf Extract: Oxidative Stability and Fate of Microconstituents. LWT—Food Sci. Technol. 2009, 42, 1090–1097. [Google Scholar] [CrossRef]
- Abdo, E.M.; Shaltout, O.E.; Mansour, H.M.M. Natural Antioxidants from Agro-Wastes Enhanced the Oxidative Stability of Soybean Oil During Deep-Frying. LWT—Food Sci. Technol. 2023, 173, 114321. [Google Scholar] [CrossRef]
- Oke, E.K.; Idowu, M.A.; Sobukola, O.P.; Adeyeye, S.A.O.; Akinsola, A.O. Frying of Food: A Critical Review. J. Culin. Sci. Technol. 2018, 16, 107–127. [Google Scholar] [CrossRef]
- López, P.L.; Juncos, N.S.; Grosso, N.R.; Olmedo, R.H. Use of Humulus lupulus and Origanum vulgare as Protection Agents Against Oxidative Deterioration in “Deep-Fried Process”: Frying Model Assay with Sunflower Oil and High-Oleic Peanuts. Food Bioprocess Technol. 2024, 17, 1970–1984. [Google Scholar] [CrossRef]
- Sordini, B.; Veneziani, G.; Servili, M.; Esposto, S.; Selvaggini, R.; Lorefice, A.; Taticchi, A. A Quanti-Qualitative Study of a Phenolic Extract as a Natural Antioxidant in the Frying Processes. Food Chem. 2019, 279, 426–434. [Google Scholar] [CrossRef]
- Kedir, W.M.; Geletu, A.K.; Weldegirum, G.S.; Sima, M.F. Antioxidant Activity of Selected Plants Extract for Palm Oil Stability via Accelerated and Deep Frying Study. Heliyon 2023, 9, e17980. [Google Scholar] [CrossRef]
- Rakariyatham, K.; Teerawutgulrag, A.; Laokuldilok, T.; Osiriphun, S.; Ackcharoensuk, N.; Tanbamrung, W. Combinatorial Effects of Longan (Dimocarpus longan) Peel Extract and Lecithin on Stability of Soybean Oil and the Oxidative Stability of Fried Shrimp Crackers During Storage. LWT—Food Sci. Technol. 2024, 198, 116065. [Google Scholar] [CrossRef]
- Aydın, S.; Sayin, U.; Sezer, M.Ö.; Sayar, S. Antioxidant Efficiency of Citrus Peels on Oxidative Stability During Repetitive Deep-Fat Frying: Evaluation with EPR and Conventional Methods. J. Food Process. Preserv. 2021, 45, e15584. [Google Scholar] [CrossRef]
- Chiou, A.; Kalogeropoulos, N.; Efstathiou, P.; Papoutsi, M.; Andrikopoulos, N.K. French Fries Oleuropein Content During the Successive Deep Frying in Oils Enriched with an Olive Leaf Extract. Int. J. Food Sci. Technol. 2013, 48, 1165–1171. [Google Scholar] [CrossRef]
- Moufakkir, C.; Kharbach, Y.; Tanghort, M.; Dassouli, A.; Remmal, A. Preserving Soybean Oil for the Frying of Breaded Butterfly Shrimp Using Natural Rosemary Antioxidant. Int. J. Food Sci. 2023, 2023, 5984636. [Google Scholar] [CrossRef]
- Manzoor, S.; Masoodi, F.A.; Rashid, R.; Dar, M.M. Improving Oxidative Stability of Soyabean Oil by Apple Pomace Extract During Deep Frying of French Fries. Food Biosci. 2022, 49, 101874. [Google Scholar] [CrossRef]
- Alberdi-Cedeño, J.; Martinez-Yusta, A.; Ruiz-Aracama, A.; Goicoechea-Oses, E.; Nieva-Echevarria, B. Different Effects of Tocopherol Natural Extract on Sunflower Oil Stability under Frying and Accelerated Storage Conditions: A Comprehensive Study on the Fate of Major and Minor Components of Oil, and Added Tocopherols. Food Chem. 2025, 472, 142871. [Google Scholar] [CrossRef]
- Manzoor, S.; Masoodi, F.A.; Parvez, S.; Rashid, R.; Shah, A.R.; Kousar, M. Green Extraction of Bioactives from Apple Pomace and Olive Leaves: Characterisation and Their Effect on the Heat-Induced Trans Fatty Acid Formation in Edible Oils During Frying Process. Food Chem. Adv. 2025, 6, 100858. [Google Scholar] [CrossRef]
- Mehany, T.; González-Sáiz, J.M.; Pizarro, C. Improving the Biostability of Extra Virgin Olive Oil with Olive Fruit Extract During Prolonged Deep Frying. Foods 2025, 14, 260. [Google Scholar] [CrossRef]
- Harzalli, Z.; Nieva-Echevarria, B.; Martinez-Yusta, A.; Oueslati, I.; Medfai, W.; Mhamdi, R.; Goicoechea-Oses, E. Addition of Olive By-Product Extracts to Sunflower Oil: Study by 1H NMR on the Antioxidant Effect During Potato Deep-Frying and Further in Vitro Digestion. LWT—Food Sci. Technol. 2024, 206, 116574. [Google Scholar] [CrossRef]
- Wu, G.; Han, S.; Zhang, Y.; Liu, T.; Karrar, E.; Jin, Q.; Zhang, H.; Wang, X. Effect of Phenolic Extracts from Camellia Oleifera Seed Cake on the Formation of Polar Compounds, Core Aldehydes, and Monoepoxy Oleic Acids During Deep-Fat Frying. Food Chem. 2022, 372, 131143. [Google Scholar] [CrossRef] [PubMed]
- Metzner Ungureanu, C.-R.; Poiana, M.-A.; Cocan, I.; Lupitu, A.I.; Alexa, E.; Moigradean, D. Strategies to Improve the Thermo-Oxidative Stability of Sunflower Oil by Exploiting the Antioxidant Potential of Blueberries Processing Byproducts. Molecules 2020, 25, 5688. [Google Scholar] [CrossRef]
- Wongsirichot, P.; Gonzalez-Miquel, M.; Winterburn, J. Rapeseed Meal Biorefining: Fractionation, Valorization and Integration Approaches. Biocatal. Agric. Biotechnol. 2024, 62, 103460. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Amarowicz, R.; Szłyk, E. Antioxidant Capacity of Rapeseed Meal and Rapeseed Oils Enriched with Meal Extract. Eur. J. Lipid Sci. Technol. 2010, 112, 750–760. [Google Scholar] [CrossRef]
- Siger, A.; Czubinski, J.; Dwiecki, K.; Kachlicki, P.; Nogala-Kalucka, M. Identification and Antioxidant Activity of Sinapic Acid Derivatives in Brassica napus L. Seed Meal Extracts. Eur. J. Lipid Sci. Technol. 2013, 115, 1130–1138. [Google Scholar] [CrossRef]
- Le, T.T.; Framboisier, X.; Aymes, A.; Ropars, A.; Frippiat, J.-P.; Kapel, R. Identification and Capture of Phenolic Compounds from a Rapeseed Meal Protein Isolate Production Process By-Product by Macroporous Resin and Valorization Their Antioxidant Properties. Molecules 2021, 26, 5853. [Google Scholar] [CrossRef] [PubMed]
- Vuorela, S.; Meyer, A.S.; Heinonen, M. Quantitative Analysis of the Main Phenolics in Rapeseed Meal and Oils Processed Differently Using Enzymatic Hydrolysis and HPLC. Eur. Food Res. Technol. 2003, 217, 517–523. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, Z.; Cao, L. Biotransformation Technology and High-Value Application of Rapeseed Meal: A Review. Bioresour. Bioprocess. 2022, 9, 103. [Google Scholar] [CrossRef]
- Li, J.; Guo, Z. Complete Utilization of Rapeseed Meal to Produce Lipophilic Antioxidants, Protein, and Monosugars in a Concordant Manner. ACS Sustain. Chem. Eng. 2017, 5, 6218–6226. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Rabiej, D.; Krzemiński, M. Synthesis of Novel Octyl Sinapate to Enhance Antioxidant Capacity of Rapeseed–Linseed Oil Mixture. J. Sci. Food Agric. 2018, 98, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Szydłowska-Czerniak, A.; Rabiej, D. Effect of New Antioxidants: Phenolipids on Quality of Fried French Fries and Rapeseed Oil. J. Food Sci. Technol. 2021, 58, 2589–2598. [Google Scholar] [CrossRef]
- ISO 3960:2017; Animal and Vegetable Fats and Oils—Determination of Peroxide Value—Iodometric (Visual) Endpoint Determination. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 6885:2016; Animal and Vegetable Fats and Oils—Determination of Anisidine Value. International Organization for Standardization: Geneva, Switzerland, 2016.
- Juncos, N.S.; Cravero, C.F.; Grosso, N.R.; Olmedo, R.H. Integral Oxidation Value Used as a New Oxidation Indicator for Evaluation of Advanced Stages of Oxidative Processes: Intox Value. Microchem. J. 2024, 204, 111186. [Google Scholar] [CrossRef]
- ISO 660:2020; Animal and Vegetable Fats and Oils—Determination of Acid Value and Acidity. International Organization for Standardization: Geneva, Switzerland, 2020.
- ISO 3656:2011; Animal and Vegetable Fats and Oils—Determination of Ultraviolet Absorbance Expressed as Specific UV Extinction. International Organization for Standardization: Geneva, Switzerland, 2011.
- Szydłowska-Czerniak, A.; Łaszewska, A. Effect of Refining Process on Antioxidant Capacity, Total Phenolics and Prooxidants Contents in Rapeseed Oils. LWT—Food Sci. Technol. 2015, 64, 853–859. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Okhchlar, R.T.; Javadi, A.; Azadmard-Damirchi, S.; Torbati, M. Quality Improvement of Oil Extracted from Flaxseeds (Linum usitatissimum L.) Incorporated with Olive Leaves by Cold Press. Food Sci. Nutr. 2024, 12, 3735–3744. [Google Scholar] [CrossRef]
- Rabiej-Kozioł, D.; Szydłowska-Czerniak, A. Antioxidant Potential Evaluation at Various Stages of Black Cumin Oil Production. Foods 2024, 13, 3518. [Google Scholar] [CrossRef] [PubMed]
- Pag, A.I.; Radu, D.G.; Drăgănescu, D.; Popa, M.I.; Sĭrghie, C. Flaxseed Cake—A Sustainable Source of Antioxidant and Antibacterial Extracts. Cellulose Chem. Technol. 2014, 48, 265–273. [Google Scholar]
- Sánchez, M.; Bernal, T.; Laca, A.; Laca, A.; Díaz, M. Hydrothermal Hydrolysis of Cocoa Bean Shell to Obtain Bioactive Compounds. Processes 2024, 12, 956. [Google Scholar] [CrossRef]
- Valle, C.; Echeverría, F.; Chávez, V.; Valenzuela, R.; Bustamante, A. Deep-Frying Impact on Food and Oil Chemical Composition: Strategies to Reduce Oil Absorption in the Final Product. Food Saf. Health 2024, 2, 414–428. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Cao, P.; Liu, Y. Degradation of Edible Oil During Deep-Frying Process by Electron Spin Resonance Spectroscopy and Physicochemical Appreciation. Eur. J. Lipid Sci. Technol. 2018, 120, 1700376. [Google Scholar] [CrossRef]
- Wu, G.; Chang, C.; Hong, C.; Zhang, H.; Huang, J.; Jin, Q.; Wang, X. Phenolic Compounds as Stabilizers of Oils and Antioxidative Mechanisms under Frying Conditions: A Comprehensive Review. Trends Food Sci. Technol. 2019, 92, 33–45. [Google Scholar] [CrossRef]
- Aydeniz, B.; Yilmaz, E. Enrichment of Frying Oils with Plant Phenolic Extracts to Extend the Usage Life. Eur. J. Lipid Sci. Technol. 2012, 114, 933–941. [Google Scholar] [CrossRef]
- Abd-ElGhany, M.E.; Ammar, M.S.; Hegazy, A.E. Use of Olive Waste Cake Extract as a Natural Antioxidant for Improving the Stability of Heated Sunflower Oil. World Appl. Sci. J. 2010, 11, 106–113. [Google Scholar]
- Asadi, Y.; Farahmandfar, R. Frying Stability of Canola Oil Supplemented with Ultrasound-Assisted Extraction of Teucrium polium. Food Sci. Nutr. 2020, 8, 1187–1196. [Google Scholar] [CrossRef]
- Leitsätze Für Speisefette Und-Öle Vom 3. July 2020. (BAnz AT 18.08.2020 B3, GMBl 2020 S. 530). Available online: https://www.bmel.de/SharedDocs/Downloads/DE/_Ernaehrung/Lebensmittel-Kennzeichnung/LeitsaetzeSpeisefette.Pdf?__blob=publicationFile&v=3 (accessed on 3 February 2025).
- Rabiej-Kozioł, D.; Tymczewska, A.; Szydłowska-Czerniak, A. Changes in Quality of Cold-Pressed Rapeseed Oil with Sinapic Acid Ester-Gelatin Films During Storage. Foods 2022, 11, 3341. [Google Scholar] [CrossRef]

| Rapeseed Meal Extract | Antioxidant Features | |||
|---|---|---|---|---|
| TPC * ± SD (mg SAE/g) | ABTS * ± SD (µmol TE/g) | DPPH * ± SD (µmol TE/g) | FRAP * ± SD (µmol TE/g) | |
| Crude | 12.20 ± 0.48 a | 846.20 ± 19.91 a | 126.40 ± 1.61 a | 508.61 ± 17.20 a |
| After alkaline hydrolysis | 18.42 ± 0.10 b | 4910.54 ± 186.41 c | 981.09 ± 45.07 b | 586.47 ± 26.62 b |
| After acid hydrolysis | 24.80 ± 0.87 c | 3880.81 ± 150.26 b | 132.03 ± 6.17 a | 650.24 ± 13.48 c |
| Heating Time (h) | Antioxidant Features of Frying Medium | |||
|---|---|---|---|---|
| TPC * ± SD (mg SAE/100 g) | ABTS * ± SD (µmol TE/100 g) | DPPH * ± SD (µmol TE/100 g) | FRAP * ± SD (µmol TE/100 g) | |
| Refined Rapeseed Oil without HLRME | ||||
| 0 | 6.04 ± 0.02 f | 733.25 ± 18.36 d | 235.34 ± 5.88 f | 91.72 ± 2.99 f |
| 8 | 2.50 ± 0.06 b | 238.07 ± 4.81 b | 71.95 ± 2.72 e | 27.71 ± 1.42 c |
| 16 | 2.43 ± 0.11 b | 184.34 ± 5.34 a | 25.25 ± 1.71 b | 18.41 ± 1.11 b |
| 24 | 2.00 ± 0.10 a | 181.80 ± 8.05 a | 20.36 ± 1.19 a | 9.98 ± 0.46 a |
| Refined Rapeseed Oil with HLRME | ||||
| 0 | 8.57 ± 0.09 g | 746.24 ± 14.02 d | 256.33 ± 7.64 g | 119.66 ± 4.21 g |
| 8 | 3.15 ± 0.06 e | 297.84 ± 10.67 c | 72.26 ± 3.46 e | 62.35 ± 5.17 e |
| 16 | 2.81 ± 0.04 d | 229.01 ± 5.93 b | 46.40 ± 0.43 d | 47.83 ± 2.26 d |
| 24 | 2.70 ± 0.07 c | 230.90 ± 4.63 b | 36.02 ± 1.46 c | 17.80 ± 0.82 b |
| Heating Time (h) | Oxidation Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| PV * ± SD (meq O2/kg) | p-AnV * ± SD | TOTOX | INTOX | AV * ± SD (mg NaOH/g) | K232 * ± SD | K268 * ± SD | TPM (%) | |
| Refined Rapeseed Oil without HLRME | ||||||||
| 0 | 0.18 ± 0.04 a | 1.37 ± 0.03 a | 1.73 | 2.92 | 0.022 ± 0.005 a | 0.562 ± 0.008 a | 2.426 ± 0.028 a | |
| 8 | 14.15 ± 0.14 c | 111.41 ± 1.28 c | 139.71 | 236.97 | 0.036 ± 0.002 b | 2.789 ± 0.034 d | 2.452 ± 0.036 a | 13.0 |
| 16 | 14.23 ± 0.81 c | 148.81 ± 2.11 e | 177.27 | 311.85 | 0.141 ± 0.007 d | 2.770 ± 0.051 d | 2.441 ± 0.040 a | 19.0 |
| 24 | 17.16 ± 0.59 e | 186.78 ± 2.01 g | 221.10 | 390.72 | 0.278 ± 0.013 f | 2.814 ± 0.083 d | 2.429 ± 0.062 a | 25.5 |
| Refined Rapeseed Oil with HLRME | ||||||||
| 0 | 0.36 ± 0.03 a | 1.08 ± 0.03 a | 1.80 | 2.52 | 0.015 ± 0.01 a | 0.498 ± 0.009 a | 2.425 ± 0.051 a | |
| 8 | 8.73 ± 0.18 b | 104.88 ± 2.24 b | 122.34 | 218.49 | 0.026 ± 0.002 a,b | 2.036 ± 0.008 b | 2.455 ± 0.047 a | 11.5 |
| 16 | 13.99 ± 0.18 c | 131.47 ± 0.30 d | 159.45 | 276.93 | 0.103 ± 0.010 c | 2.100 ± 0.048 b,c | 2.374 ± 0.029 a | 17.5 |
| 24 | 16.40 ± 0.48 d | 163.38 ± 3.18 f | 196.18 | 343.16 | 0.250 ± 0.001 e | 2.144 ± 0.058 c | 2.434 ± 0.082 a | 23.5 |
| Dependent Variable | Oil Sample | Regression * | R2 | |
|---|---|---|---|---|
| Intercept (β0) | Slope (β1) | |||
| PV | RRO | 3.78 | 0.64 | 0.75 |
| RRO-HLRME | 1.86 | 0.67 | 0.94 | |
| p-AnV | RRO | 23.05 | 7.42 | 0.92 |
| RRO-HLRME | 23.18 | 6.42 | 0.89 | |
| TOTOX | RRO | 30.60 | 8.70 | 0.90 |
| RRO-HLRME | 26.91 | 7.75 | 0.90 | |
| INTOX | RRO | 49.87 | 15.48 | 0.91 |
| RRO-HLRME | 48.22 | 13.51 | 0.89 | |
| Heating Time (h) | Antioxidant Features of Fried French Fries | |||
|---|---|---|---|---|
| TPC * ± SD | ABTS * ± SD | DPPH * ± SD | FRAP * ± SD | |
| (mg SAE/100 g) | (µmol TE/100 g) | (µmol TE/100 g) | (µmol TE/100 g) | |
| Refined Rapeseed Oil without HLRME | ||||
| 1 | 28.42 ± 1.32 c | 1421.19 ± 45.27 f | 278.19 ± 8.17 f | 124.42 ± 7.13 c |
| 8 | 23.51 ± 0.85 b,c | 1307.82 ± 56.00 e | 247.54 ± 11.86 e | 123.61 ± 5.86 c |
| 16 | 19.56 ± 0.98 a,b | 1043.41 ± 32.28 b | 99.70 ± 3.36 b | 112.72 ± 7.45 b |
| 24 | 19.04 ± 0.49 a | 883.75 ± 39.55 a | 74.16 ± 5.19 a | 97.62 ± 3.05 a |
| Refined Rapeseed Oil with HLRME | ||||
| 1 | 37.57 ± 1.70 d | 1600.60 ± 27.17 g | 735.65 ± 10.79 h | 156.96 ± 4.99 e |
| 8 | 35.52 ± 0.63 d | 1424.31 ± 49.11 f | 447.60 ± 12.44 g | 144.49 ± 6.84 d |
| 16 | 35.81 ± 1.01 d | 1227.23 ± 23.67 d | 221.74 ± 8.51 d | 123.84 ± 2.32 c |
| 24 | 34.97 ± 1.43 d | 1128.50 ± 43.47 c | 194.05 ± 5.32 c | 100.25 ± 2.63 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabiej-Kozioł, D.; Szydłowska-Czerniak, A. The Valorization of Rapeseed Meal as Hydrolyzed and Lyophilized Extract to Improve the Antioxidant Properties of Refined Rapeseed Oil During Frying and Fried French Fries. Foods 2025, 14, 1444. https://doi.org/10.3390/foods14091444
Rabiej-Kozioł D, Szydłowska-Czerniak A. The Valorization of Rapeseed Meal as Hydrolyzed and Lyophilized Extract to Improve the Antioxidant Properties of Refined Rapeseed Oil During Frying and Fried French Fries. Foods. 2025; 14(9):1444. https://doi.org/10.3390/foods14091444
Chicago/Turabian StyleRabiej-Kozioł, Dobrochna, and Aleksandra Szydłowska-Czerniak. 2025. "The Valorization of Rapeseed Meal as Hydrolyzed and Lyophilized Extract to Improve the Antioxidant Properties of Refined Rapeseed Oil During Frying and Fried French Fries" Foods 14, no. 9: 1444. https://doi.org/10.3390/foods14091444
APA StyleRabiej-Kozioł, D., & Szydłowska-Czerniak, A. (2025). The Valorization of Rapeseed Meal as Hydrolyzed and Lyophilized Extract to Improve the Antioxidant Properties of Refined Rapeseed Oil During Frying and Fried French Fries. Foods, 14(9), 1444. https://doi.org/10.3390/foods14091444







