Bioactive Lipid Compounds and Nutritional Potential of Glyceride Oils from Flower Buds and Fruits of Lagerstroemia indica L. Cultivar ‘Hopi’ Grown in Bulgaria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Chemical Composition
2.3. Lipid Composition
2.3.1. Fatty Acid Composition
2.3.2. Index of Atherogenicity (IA), Index of Thrombogenicity (IT), Hypocholesterolemic/Hypercholesterolemic Ratio (HH)
2.3.3. Sterols
2.3.4. Tocopherols
2.3.5. Phospholipids
2.4. Refractive Index and Iodine Value
2.5. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Editorial Committee of Chinese Botany, Chinese Academy of Sciences. Flora Reipublicae Popularis Sinicae; Science Press: Beijing, China, 1983; Volume 52, p. 94. [Google Scholar]
- Ghahreman, A. Chromite Iran; University Publication Center: Tehran, Iran, 1993; Volume 2. [Google Scholar]
- Chang, M.; Ahmed, A.F.; Cui, L. The hypoglycemic effect of Lagerstroemia indica L. and Lagerstroemia indica L. f. alba (Nichols.) Rehd. in vitro and in vivo. J. Future Foods 2023, 3, 273–277. [Google Scholar] [CrossRef]
- Yoziev, L.K. Introduction of Lagerstroemia indica in the conditions of Southern Uzbekistan. In Introduction and Acclimatization of Plants; Fan: Tashkent, Uzbekistan, 1996; pp. 68–71. [Google Scholar]
- Muratgeldiev, N. Lagerstroemia indica in Ashgabat. Bull. Head. Nerd. Sada 1967, 64, 13–15. [Google Scholar]
- Kuznetsova, V.M. Collection of Lagerstroemia indica in the Nikitsky Botanical Garden. Bull. State Nikit. Bot. Gard. 1987, 8, 34–38. [Google Scholar]
- Sharopova, M.A. Characteristics of growth and development of Lagerstroemia and Cesalpinia in different phases of ontogenesis. J. Biol. Uzbekistan 2005, 1, 43–49. [Google Scholar]
- Noori Naeiji, M.; Vahdat, S.M. A study on the antioxidant and antimicrobial properties of the aerial parts extract of Lagerstroemia indica L. J. Med. Plants By-Prod. 2024, 13, 825–834. [Google Scholar] [CrossRef]
- Sharopova, M.A.; Uzakov, Z.Z.; Xaitov, I.Y. Preliminary results on the chemical components of Lagerstroemia indica L. in the conditions of southern Uzbekistan. E3S Web Conf. 2024, 510, 03032. [Google Scholar] [CrossRef]
- Jianmin, H.; Boxin, H.; Zhili, S.; Qiangfa, Y. The fatty acid composition of Lagerstroemia indica seed oil determined by GC/MS. J. Agric. 2013, 3, 52–56. [Google Scholar]
- Zhang, D.; Ni, G.; Tang, Y.J. Chemical constituents in stem-leaves of Lagerstroemia indica. Chin. Tradit. Herbal Drugs 2005, 46, 2209–2211. [Google Scholar] [CrossRef]
- Labib, R.M.; Ayoub, N.A.; Singab, A.B.; Al-Azizi, M.M.; Sleem, A. Chemical constituents and pharmacological studies of Lagerstroemia indica. Phytopharmacology 2013, 4, 373–389. [Google Scholar]
- Chen, Y.; Li, S.-W.; Yin, F.-Z.; Yang, M.; Huan, X.-J.; Miao, Z.-H.; Wang, X.-M.; Guo, Y.-W. Lagerindicine, a new pyrrole alkaloid isolated from the flowers of Lagerstroemia indica Linnaeus. Nat. Prod. Bioprospect. 2021, 11, 73–79. [Google Scholar] [CrossRef]
- Sharopova, M.A. On the Ecological Characteristics of Acclimatized Lagerstroemia indica in South Uzbekistan, Tashkent. 2000; 73–75. [Google Scholar]
- Niranjan, M.H.; Sudarshana, M.S. Preliminary phytochemical studies of Lagerstroemia indica Linn. J. Pharm. Res. 2010, 3, 216–218. [Google Scholar]
- Gunstone, F.D. Food Applications of Lipids. In Food Lipids; CRC Press: Boca Raton, FL, USA, 2002; pp. 748–769. [Google Scholar]
- Kris-Etherton, P.M. Monounsaturated fatty acids and risk of cardiovascular disease. Circulation 1999, 100, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Moreau, R.A.; Whitaker, B.D.; Hicks, K.B. Phytosterols, phytostanols, and their conjugates in foods: Structural diversity, quantitative analysis, and health-promoting uses. Prog. Lipid Res. 2002, 41, 457–500. [Google Scholar] [CrossRef] [PubMed]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Ulbricht, T.; Southgate, D. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- ISO 659:2009; Oilseeds—Determination of Oil Content (Reference Method). ISO: Geneva, Switzerland, 2014.
- AOAC—Association of Official Analytical Chemists. Official Methods of Analysis, 20th ed.; AOAC: Arlington, VA, USA, 2016; Method 976.06. [Google Scholar]
- FAO. Food Energy—Methods of Analysis and Conversion Factors; Report of a Technical Workshop, FAO Food and Nutrition Paper 77; FAO: Rome, Italy, 2003. [Google Scholar]
- ISO 12966-1:2014; Animal and Vegetable Fats and Oils. Gas Chromatography of Fatty Acid Methyl Esters—Part 1: Guidelines on Modern Gas Chromatography of Fatty Acid Methyl Esters. ISO: Geneva, Switzerland, 2014.
- ISO 12966-2:2017; Animal and Vegetable Fat and Oils. Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of Methyl Esters of Fatty Acids. ISO: Geneva, Switzerland, 2017.
- Santos-Silva, J.; Bessa, R.J.B.; Santos-Silva, F. Effect of genotype, feeding system and slaughter weight on the quality of light lambs: Fatty and composition of meat. Livest. Prod. Sci. 2002, 77, 187–194. [Google Scholar] [CrossRef]
- ISO 18609:2000; Animal and Vegetable Fats and Oils. Determination of Unsaponifiable Matter. Method Using Hexane Extraction. ISO: Geneva, Switzerland, 2000.
- Ivanov, S.; Bitcheva, P.; Konova, B. Méthode de détermination chromatographyque et colorimétrique des phytosterols dans les huiles végétales et les concentrates steroliques. Rev. Fr. Corps Gras 1972, 19, 177–180. [Google Scholar]
- ISO 12228-1:2014; Animal and Vegetable Fats and Oils. Determination of Individual and Total Sterols Contents. Gas Chromatographic Method. ISO: Geneva, Switzerland, 2014.
- ISO 9936:2016; Animal and Vegetable Fats and Oils. Determination of Tocopherol and Tocotrienol Contents by High-Performance Liquid Chromatography. ISO: Geneva, Switzerland, 2016.
- Schneiter, R.; Daum, G. Analysis of yeast lipids. In Methods in Molecular Biology, 2nd ed.; Xiao, W., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2006; pp. 75–84. [Google Scholar]
- ISO 10540-1:2014; Animal and Vegetable Fats and Oils. Determination of Phosphorus Content. Part 1: Colorimetric Method. ISO: Geneva, Switzerland, 2014.
- Basu, S.; Kundu, P.; Sinhababu, A. Characterization of fatty acid and sterol composition of seed lipid of Lagerstroemia speciosa Pers. Res. Chem. Intermed. 2015, 41, 6511–6522. [Google Scholar] [CrossRef]
- Tranbarger, T.J.; Dussert, S.; Joët, T.; Argout, X.; Summo, M.; Champion, A.; Cros, D.; Omore, A.; Nouy, B.; Morcillo, F. Regulatory Mechanisms Underlying Oil Palm Fruit Mesocarp Maturation, Ripening, and Functional Specialization in Lipid and Carotenoid Metabolism. Plant Physiol. 2011, 156, 564–584. [Google Scholar] [CrossRef]
- Kumari, R.; Hamal, U.; Sharma, N. Resource Allocation in Flowering Plants: Concept and Implications. In Resource Allocation in Flowering Plants: Concept and Implications; Tandon, R., Shivanna, K., Koul, M., Eds.; Springer: Singapore, 2020; pp. 157–171. [Google Scholar] [CrossRef]
- Famiani, F.; Bonghi, C.; Chen, Z.H.; Drincovich, M.F.; Farinelli, D.; Lara, M.V.; Proietti, S.; Rosati, A.; Vizzotto, G.; Walker, R.P. Stone Fruits: Growth and Nitrogen and Organic Acid Metabolism in the Fruits and Seeds—A Review. Front. Plant Sci. 2020, 11, 572601. [Google Scholar] [CrossRef]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar Sensing and Signaling in Plants: Conserved and Novel Mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef] [PubMed]
- Nestorova, V. Food Hygiene and Food Legislation; Matkom: Bulgaria, 2014; p. 96. [Google Scholar]
- Tian, J.; Gong, Y.; Li, J. Nutritional attributes and phenolic composition of flower and bud of Sophora japonica L. and Robinia pseudoacacia L. Molecules 2022, 27, 8932. [Google Scholar] [CrossRef] [PubMed]
- Teneva, O.; Petkova, Z.; Antova, G.; Angelova-Romova, M.; Stoyanov, P.; Todorov, K.; Mladenova, T.; Radoukova, T.; Mladenov, R.; Petkov, V.; et al. Chemical composition and lipid bioactive components of Centaurea thracica dwelling in Bulgaria. Molecules 2024, 29, 3282. [Google Scholar] [CrossRef] [PubMed]
- Bansal, M.; Poonia, A.; Kolluri, S.R.P.; Vasundhara. Introduction on Bioactive Compounds, Sources and Their Potential Applications; Thakur, M., Belwal, T., Eds.; Bioactive Components; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Schaller, H. The Role of Sterols in Plant Growth and Development. Prog. Lipid Res. 2003, 42, 163–175. [Google Scholar] [CrossRef]
- Grunwald, C. Function of sterols. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1978, 284, 541–558. [Google Scholar] [CrossRef]
- Lusby, W.R.; Oliver, J.E.; McKibben, G.H.; Thompson, M.J. Free and esterified sterols of cotton buds and anthers. Lipids 1987, 22, 80–83. [Google Scholar] [CrossRef]
- Yang, J.; Li, C.; Zhang, Y. Engineering of Saccharomyces cerevisiae for 24-methylene-cholesterol production. Biomolecules 2021, 11, 1710. [Google Scholar] [CrossRef]
- Zienkiewicz, A. Phosphatidylcholine in plants: Functional diversity. In Encyclopedia of Lipidomics; Wenk, M., Ed.; Springer: Dordrecht, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Ischebeck, T. Phosphatidic acid in plants: Functional diversity. In Encyclopedia of Lipidomics; Wenk, M., Ed.; Springer: Dordrecht, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Kelle, H.I.; Udeozo, I.P. Characterization of the chemical properties of some selected refined vegetable oils commonly sold in Nigeria. Br. J. Appl. Sci. Technol. 2015, 5, 538. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Codex-Stan 210: Codex Standard for Named Vegetable Oils; FAO: Rome, Italy, 2008. [Google Scholar]
- Assempoor, R.; Daneshvar, M.S.; Taghvaei, A. Atherogenic index of plasma and coronary artery disease: A systematic review and meta-analysis of observational studies. Cardiovasc. Diabetol. 2025, 24, 35. [Google Scholar] [CrossRef]
- Fehily, A.M.; Pickering, J.E.; Yarnell, J.W.G. Dietary indices of atherogenicity and thrombogenicity and ischaemic heart disease risk: The Caerphilly Prospective Study. Br. J. Nutr. 1994, 71, 249–257. [Google Scholar] [CrossRef]
- Bhatnagar, D.; Soran, H.; Durrington, P.N. Hypercholesterolaemia and its management. BMJ 2008, 337, a993. [Google Scholar] [CrossRef]
- Petkova, Z.; Teneva, O.; Antova, G.; Angelova-Romova, M.; Gecheva, G.; Dimitrova-Dyulgerova, I. Chemical composition, lipid-soluble bioactive compounds, and potential health benefits of the moss Hypnum cupressiforme Hedw. Plants 2023, 12, 4190. [Google Scholar] [CrossRef] [PubMed]
| Compounds | Flower Buds | Fruits |
|---|---|---|
| Glyceride oil, % | 3.3 ± 0.2 a* | 14.8 ± 0.4 b |
| Protein, % | 8.7 ± 0.2 a | 15.7 ± 0.2 b |
| Carbohydrates, % | 72.0 ± 0.5 a | 53.1 ± 0.6 b |
| Crude fiber, % | 31.6 ± 0.8 a | 28.3 ± 0.8 b |
| Ash, % | 6.3 ± 0.1 a | 8.2 ± 0.1 b |
| Energy value, kcal/100 g | 352.9 a | 408.2 b |
| Biologically Active Components | Flower Buds | Fruits |
|---|---|---|
| Unsaponifiable matter, % | 19.6 ± 0.2 a* | 4.6 ± 0.1 b |
| Total sterols, % | 4.1 ± 0.1 a | 1.6 ± 0.1 b |
| Cholesterol, % (of total sterols) | 0.4 ± 0.0 a* | 0.4 ± 0.1 a |
| Campesterol, % (of total sterols) | 9.3 ± 0.2 a | 9.2 ± 0.1 a |
| 24-Methylenecholesterol, % (of total sterols) | 10.3 ± 0.2 a | 2.4 ± 0.1 b |
| Stigmasterol, % (of total sterols) | 2.8 ± 0.1 a | 13.4 ± 0.4 b |
| β—Sitosterol, % (of total sterols) | 76.5 ± 0.5 a | 74.3 ± 0.3 b |
| Δ7—Campesterol, % (of total sterols) | 0.5 ± 0.0 a | 0.3 ± 0.0 b |
| Δ7—Avenasterol, % (of total sterols) | 0.2 ± 0.0 | nd ** |
| α—Tocopherol, mg/kg | 80 ± 4 a | nd |
| γ—Tocopherol, mg/kg | nd | 56 ± 2 a |
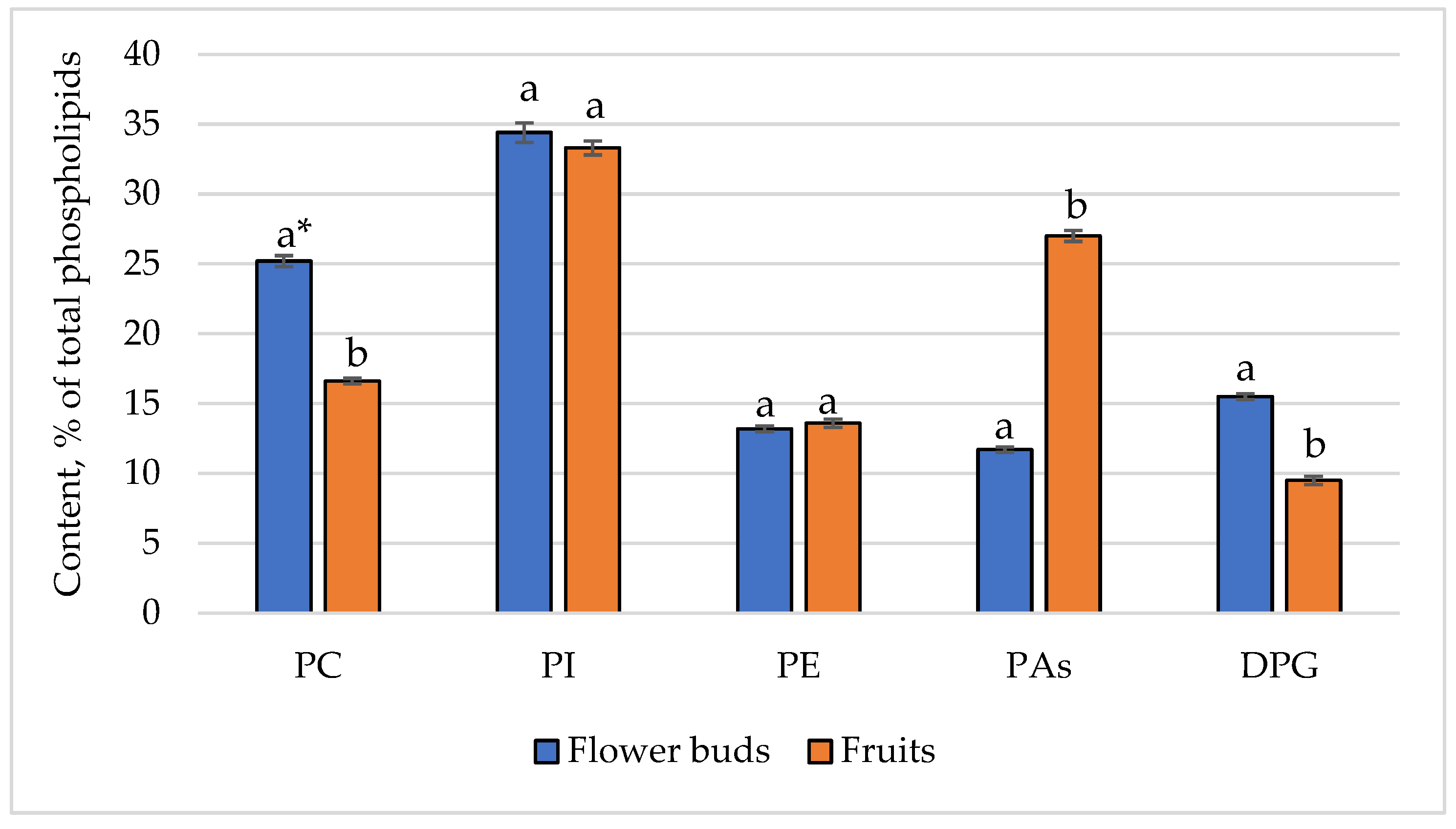
| Phospholipids, % | 2.8 ± 0.1 a | 0.8 ± 0.0 b |
| Fatty Acids (FAs) *, % | L. indica Cultivar ‘Hopi’ | L. indica Amabilis [10] | L. indica Rubra [10] | L. indica Alba [10] | ||
|---|---|---|---|---|---|---|
| Flower Buds | Fruits | Seeds | Seeds | Seeds | ||
| C8:0 | Caprylic | 0.3 ± 0.0 a* | 0.1 ± 0.0 b | |||
| C10:0 | Capric | 0.4 ± 0.0 a | 0.1 ± 0.0 b | |||
| C11:0 | Undecanoic | 0.1 ± 0.0 | nd ** | |||
| C14:0 | Myristic | 0.2 ± 0.0 | nd | |||
| C15:0 | Pentadecanoic | 1.2 ± 0.1 a | 0.1 ± 0.0 b | |||
| C15:1 | Pentadecenoic | 0.3 ± 0.0 | nd | |||
| C16:0 | Palmitic | 8.1 ± 0.2 a | 5.8 ± 0.1 b | 8.94 | 9.54 | 9.60 |
| C16:1 | Palmitoleic | 0.2 ± 0.0 | nd | |||
| C17:0 | Margaric | 0.4 ± 0.0 a | 0.1 ± 0.0 b | |||
| C17:1 | Heptadecenoic | 0.5 ± 0.0 | nd | |||
| C18:0 | Stearic | 1.2 ± 0.0 a | 1.1 ± 0.1 a | 2.31 | 2.96 | 2.76 |
| C18:1 trans | Elaidic | 0.7 ± 0.0 | nd | |||
| C18:1 | Oleic | 6.1 ± 0.1 a | 5.6 ± 0.1 b | 13.74 | 12.76 | 11.52 |
| C18:2 trans | trans-Linoleic | 0.8 ± 0.0 | nd | |||
| C18:2 (n-6) | Linoleic | 77.3 ± 0.2 a | 86.0 ± 0.3 b | 71.62 | 70.91 | 72.87 |
| C18:3 (n-6) | γ-Linolenic | 0.1 ± 0.0 | nd | |||
| C18:3 (n-3) | α-Linolenic | 0.6 ± 0.0 a | 0.3 ± 0.0 b | 0.55 | 0.69 | 0.49 |
| C20:0 | Arachidic | 0.5 ± 0.0 a | 0.2 ± 0.0 b | |||
| C20:1 | Gadoleic | 0.2 ± 0.0 a | 0.3 ± 0.0 b | |||
| C20:2 (n-6) | Eicosadienoic | 0.1 ± 0.0 a | 0.1 ± 0.0 a | |||
| C20:3 (n-6) | Dihomo-γ-linolenic acid | 0.1 ± 0.0 | nd | 0.12 | 0.10 | 0.10 |
| C22:0 | Behenic | 0.4 ± 0.0 a | 0.1 ±0.0 b | |||
| C22:1 | Erucic | 0.1 ± 0.0 a | 0.1 ±0.0 a | |||
| C22:6 (n-3) | Docosahexaenoic | 0.1 ± 0.0 | nd | |||
| Saturated FAs | 12.8 | 7.6 | 13.05 | 14.38 | 14.00 | |
| Unsaturated FAs | 87.2 | 92.4 | 86.57 | 85.21 | 85.57 | |
| Monounsaturated FAs | 8.1 | 6.0 | ||||
| Polyunsaturated FAs | 79.1 | 86.4 | ||||
| Physicochemical characteristics | ||||||
| Iodine value, g I2/100 g | 147 ± 1 a | 162 ± 3 b | ||||
| Refractive index | 1.4802 ± 0.0002 a | 1.4825 ± 0.0009 b | ||||
| Lipid indices | ||||||
| Index of atherogenicity (IA) | 0.1021 ± 0.0013 | 0.0628 ± 0.0008 | ||||
| Index of thrombogenicity (IT) | 1.1008 ± 0.0021 | 0.9928 ± 0.0014 | ||||
| Hypocholesterolemic/hypercholesterolemic ratio (HH) | 10.1566 ± 0.0053 | 15.8621 ± 0.0061 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teneva, O.; Petkova, Z.; Angelova-Romova, M.; Antova, G. Bioactive Lipid Compounds and Nutritional Potential of Glyceride Oils from Flower Buds and Fruits of Lagerstroemia indica L. Cultivar ‘Hopi’ Grown in Bulgaria. Foods 2025, 14, 1449. https://doi.org/10.3390/foods14091449
Teneva O, Petkova Z, Angelova-Romova M, Antova G. Bioactive Lipid Compounds and Nutritional Potential of Glyceride Oils from Flower Buds and Fruits of Lagerstroemia indica L. Cultivar ‘Hopi’ Grown in Bulgaria. Foods. 2025; 14(9):1449. https://doi.org/10.3390/foods14091449
Chicago/Turabian StyleTeneva, Olga, Zhana Petkova, Maria Angelova-Romova, and Ginka Antova. 2025. "Bioactive Lipid Compounds and Nutritional Potential of Glyceride Oils from Flower Buds and Fruits of Lagerstroemia indica L. Cultivar ‘Hopi’ Grown in Bulgaria" Foods 14, no. 9: 1449. https://doi.org/10.3390/foods14091449
APA StyleTeneva, O., Petkova, Z., Angelova-Romova, M., & Antova, G. (2025). Bioactive Lipid Compounds and Nutritional Potential of Glyceride Oils from Flower Buds and Fruits of Lagerstroemia indica L. Cultivar ‘Hopi’ Grown in Bulgaria. Foods, 14(9), 1449. https://doi.org/10.3390/foods14091449





