Non-Thermal Stabilization Strategies for Rice Bran: Mechanistic Insights, Technological Advances, and Implications for Industrial Applications
Abstract
1. Introduction
Review Methodology
2. Structure, Nutritional Composition, and Utilization of Rice Bran
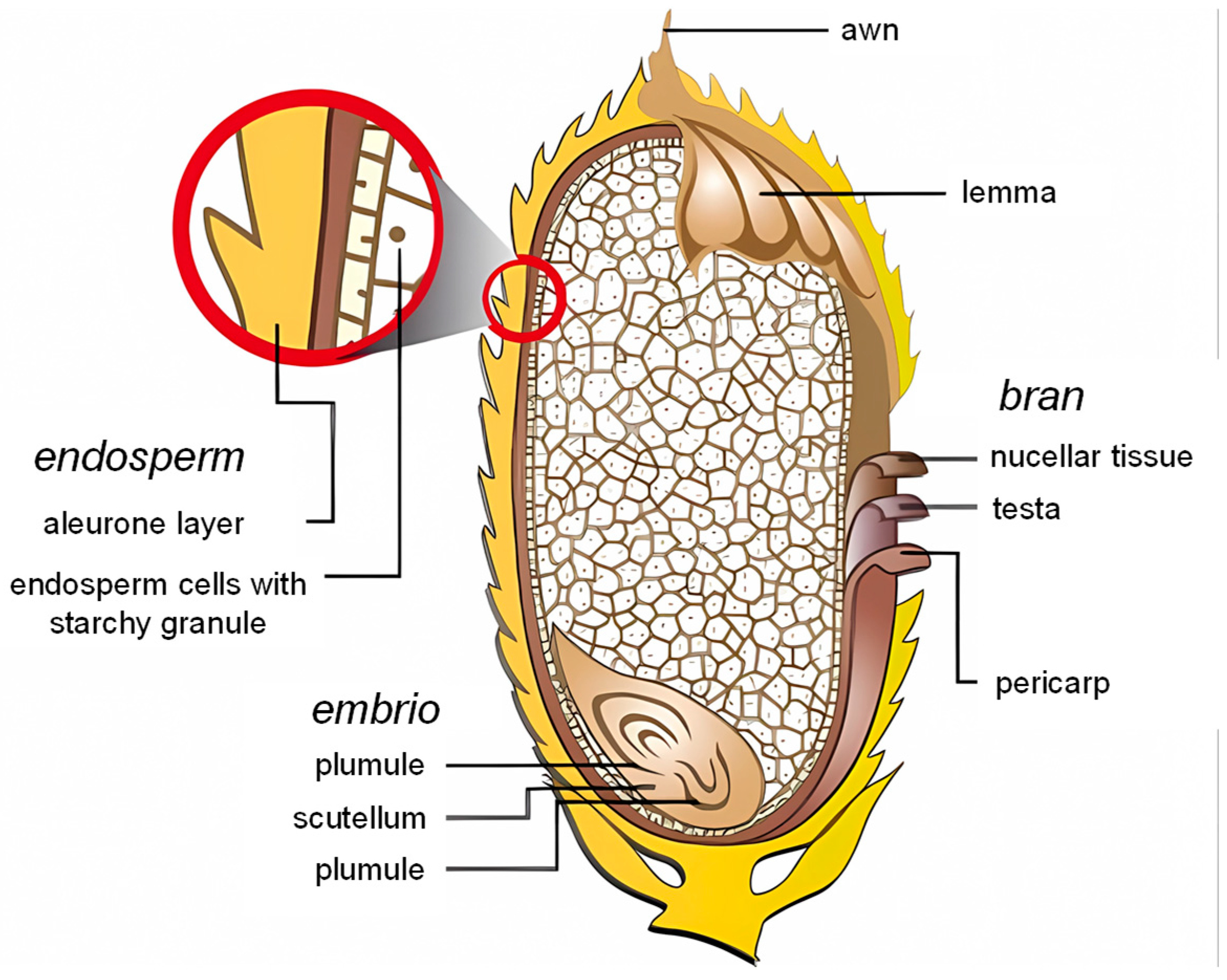
2.1. Morphological Structure of Rice Bran
2.2. Nutritional Composition of Rice Bran
2.3. Advanced Utilization Strategies of Rice Bran
2.3.1. Lipid/Protein Utilization and Nutritional Advantages
2.3.2. Phenolic Compounds and Bioactive Properties
2.3.3. Food Manufacturing Applications
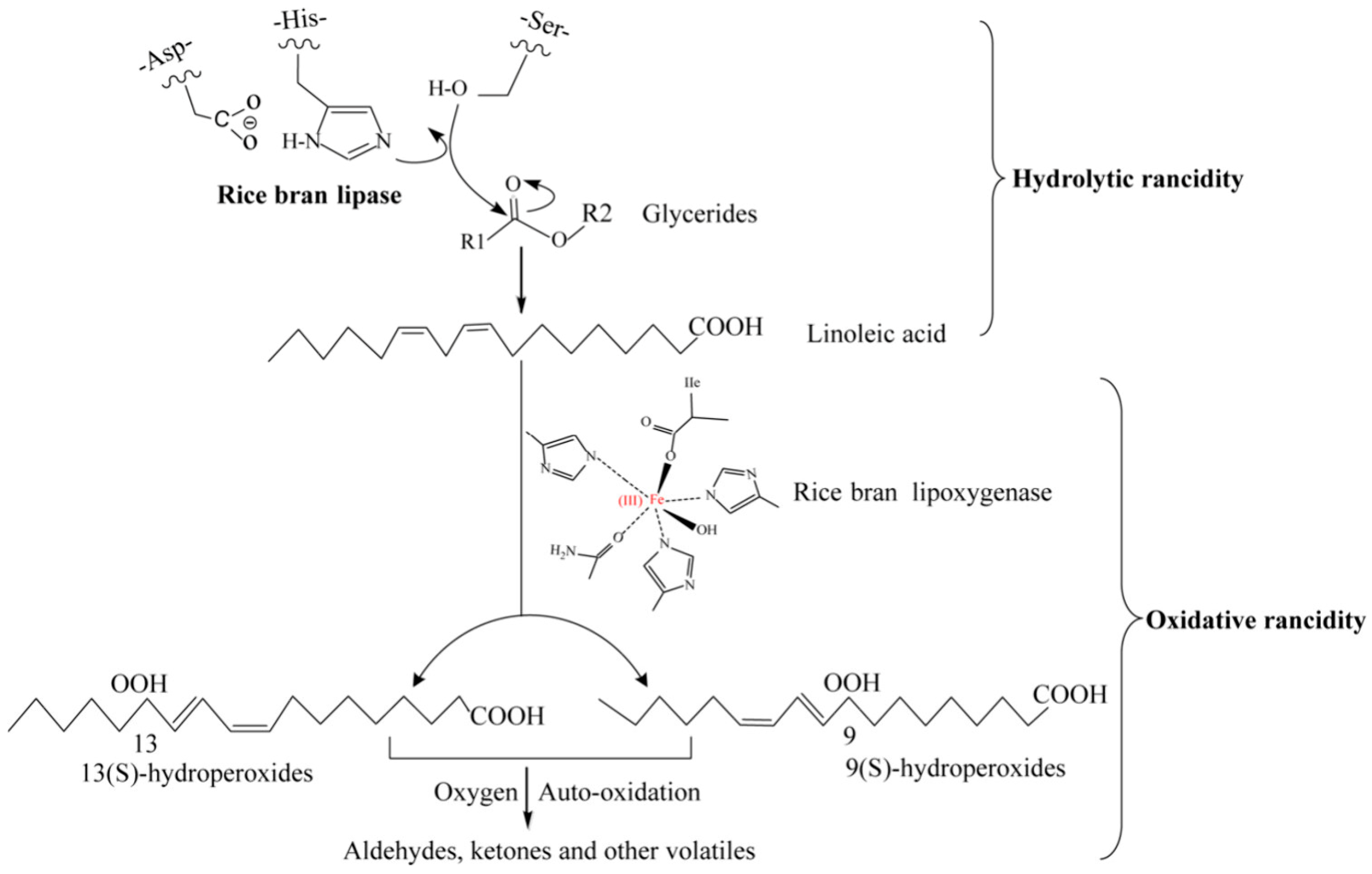
3. Mechanisms of Rice Bran Rancidity
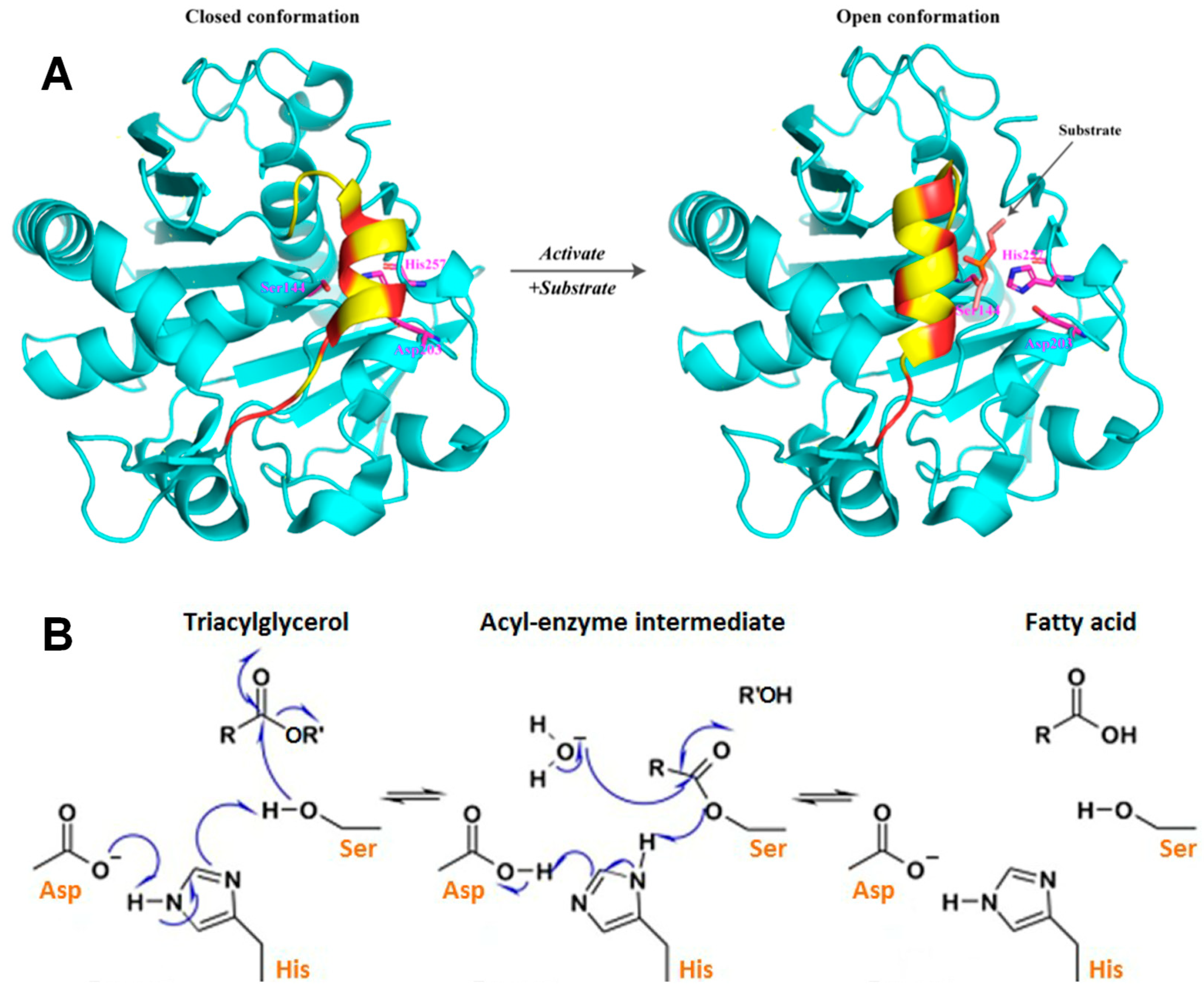
3.1. Hydrolytic Rancidity
3.2. Oxidative Rancidity
3.3. Other Factors
3.3.1. Water Activity
3.3.2. Microbial Contamination
3.3.3. Phenolic Compounds
4. Non-Thermal Stabilization Technologies for Rice Bran
4.1. Thermal Stabilization Strategies
4.2. Non-Thermal Stabilization Technologies
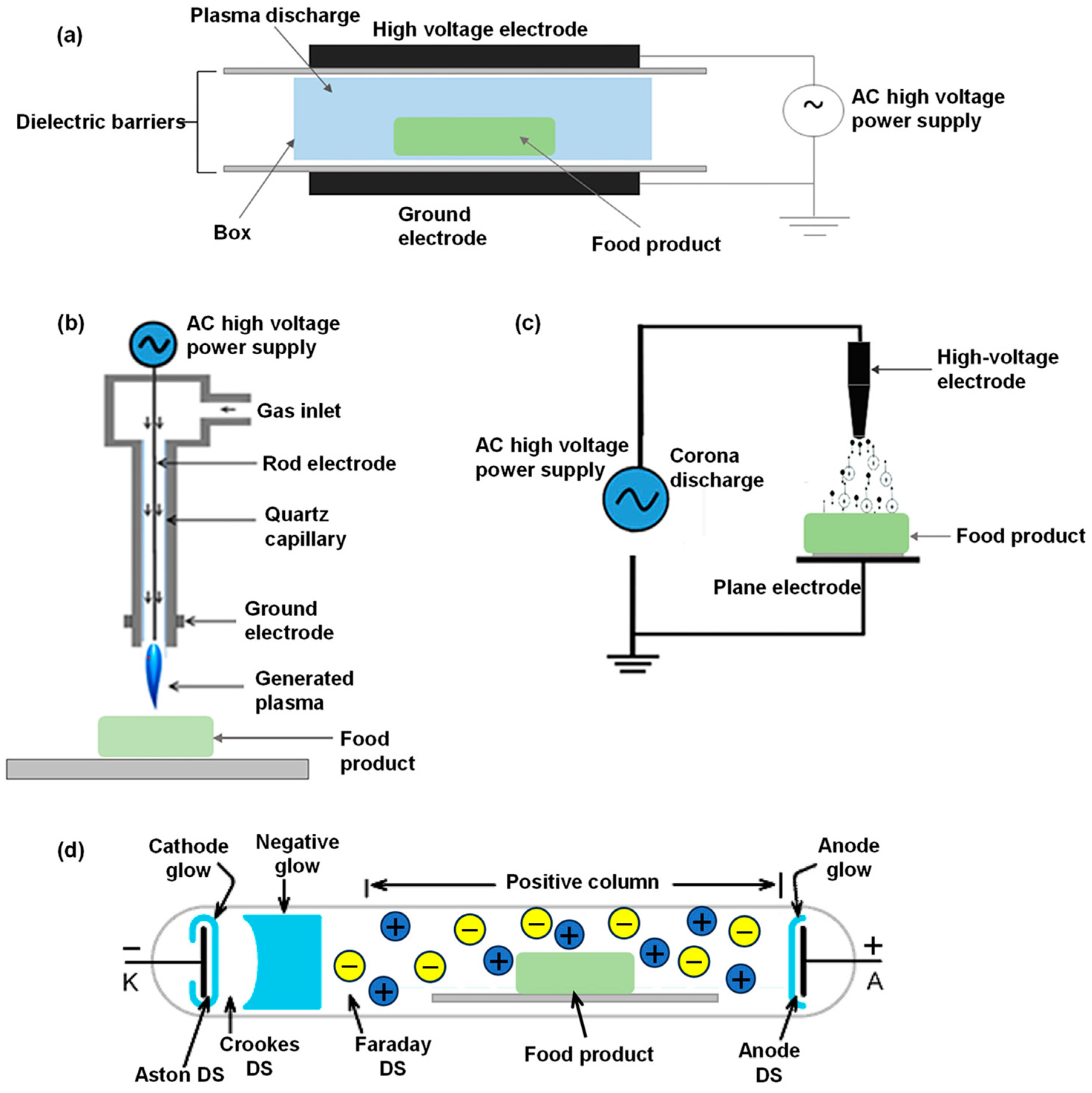
4.2.1. Low-Temperature Plasma
4.2.2. High-Energy Electron Beam (HEEB) Irradiation
4.2.3. Ultra-High Pressure Stabilization
4.2.4. Enzymatic Stabilization
5. Limitations and Future Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tufail, T.; Ain, H.B.U.; Chen, J.; Virk, M.S.; Ahmed, Z.; Ashraf, J.; Shahid, N.U.A.; Xu, B. Contemporary Views of the Extraction, Health Benefits, and Industrial Integration of Rice Bran Oil: A Prominent Ingredient for Holistic Human Health. Foods 2024, 13, 1305. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.; Jianlong, X. Recent Advances to Enhance Nutritional Quality of Rice. Rice Sci. 2023, 30, 523–536. [Google Scholar] [CrossRef]
- Bollinedi, H.; Singh, A.K.; Singh, N.; S, G.K.; Bhowmick, P.K.; K K, V.; M, N.; R K, E. Genetic and genomic approaches to address rapid rancidity of rice bran. Crit. Rev. Food Sci. Nutr. 2020, 61, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhao, L.; Xu, B.; Deng, B.; Liu, Y.; Dong, Y. Rice bran real-time stabilisation technology with flowing microwave radiation: Its impact on rancidity and some bioactive compounds. Qual. Assur. Saf. Crops Foods 2018, 10, 25–34. [Google Scholar] [CrossRef]
- Dabbour, M.; He, R.; Ma, H.; Musa, A. Optimization of ultrasound assisted extraction of protein from sunflower meal and its physicochemical and functional properties. J. Food Process Eng. 2018, 41, e12799. [Google Scholar] [CrossRef]
- Estévez, M.; Díaz-Velasco, S.; Martínez, R. Protein carbonylation in food and nutrition: A concise update. Amino Acids 2022, 54, 559–573. [Google Scholar] [CrossRef]
- Dubey, B.N. Comparative Study on the Rice Bran Stabilization Processes: A Review. Res. Dev. Mater. Sci. 2019, 11, 1147–1152. [Google Scholar] [CrossRef]
- Sethi, S.; Kaur, L.; Nath, P.; Yadav, D.N. Bioactive Compounds and Phytonutrients From Cereals. In Plant-Based Bioactive Compounds and Food Ingredients; Apple Academic Press: Palm Bay, FL, USA, 2023; pp. 155–205. [Google Scholar] [CrossRef]
- Cao, Y.W.; Zhao, J.W.; Jin, Z.Y.; Tian, Y.Q.; Zhou, X.; Long, J. Improvement of rice bran modified by extrusion combined with ball milling on the quality of steamed brown rice cake. J. Cereal Sci. 2021, 99, 103229. [Google Scholar] [CrossRef]
- Wu, N.N.; Ma, Z.Q.; Li, H.H.; Tian, X.H.; Fang, Y.; Tan, B. Nutritional and cooking quality improvement of brown rice noodles prepared with extruded rice bran. Cereal Chem. 2021, 98, 346–354. [Google Scholar] [CrossRef]
- Ayoub, W.S.; Zahoor, I.; Dar, A.H.; Anjum, N.; Pandiselvam, R.; Farooq, S.; Rusu, A.V.; Rocha, J.M.; Trif, M.; Jeevarathinam, G. Effect of incorporation of wheat bran, rice bran and banana peel powder on the mesostructure and physicochemical characteristics of biscuits. Front. Nutr. 2022, 9, 1016717. [Google Scholar] [CrossRef]
- Park, J.; Kim, H.-S. Rice-Based Gluten-Free Foods and Technologies: A Review. Foods 2023, 12, 4110. [Google Scholar] [CrossRef] [PubMed]
- Gul, K.; Yousuf, B.; Singh, A.K.; Singh, P.; Wani, A.A. Rice bran: Nutritional values and its emerging potential for development of functional food—A review. Bioact. Carbohydr. Diet. Fibre 2015, 6, 24–30. [Google Scholar] [CrossRef]
- Gao, R.; Feng, X.; Li, W.; Yuan, L.; Ge, J.; Lu, D.; Chen, B.; Yu, G. Changes in properties of white shrimp (Litopenaeus vannamei) protein during thermal denaturation. Food Sci. Biotechnol. 2016, 25, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shao, F.; Wan, X.; Zhang, H.; Cai, M.; Hu, K.; Duan, Y. Effects of rapeseed protein addition on soybean protein-based textured protein produced by low-moisture extrusion: Changes in physicochemical attributes, structural properties and barrel flow behaviors. Food Hydrocoll. 2024, 149, 109631. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Strappe, P.; Zhou, Z.K.; Blanchard, C. Impact on the nutritional attributes of rice bran following various stabilization procedures. Crit. Rev. Food Sci. Nutr. 2018, 59, 2458–2466. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Z.H.; Qi, X.; Li, L.; Zhu, J.; Brennan, C.S.; Yan, J.K. Application of nonthermal processing technologies in extracting and modifying polysaccharides: A critical review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4367–4389. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, Z.; Mintah, B.K.; Xu, H.; Dabbour, M.; Cheng, Y.; Dai, C.; He, R.; Ma, H. Effects of nonthermal physical processing technologies on functional, structural properties and digestibility of food protein: A review. J. Food Process Eng. 2022, 45, e14010. [Google Scholar] [CrossRef]
- Tolouie, H.; Mohammadifar, M.A.; Ghomi, H.; Yaghoubi, A.S.; Hashemi, M. The impact of atmospheric cold plasma treatment on inactivation of lipase and lipoxygenase of wheat germs. Innov. Food Sci. Emerg. Technol. 2018, 47, 346–352. [Google Scholar] [CrossRef]
- Huang, H.W.; Hsu, C.P.; Wang, C.Y. Healthy expectations of high hydrostatic pressure treatment in food processing industry. J. Food Drug Anal. 2020, 28, 1–13. [Google Scholar] [CrossRef]
- Li, P.; Chen, Y.-H.; Lu, J.; Zhang, C.-Q.; Liu, Q.-Q.; Li, Q.-F. Genes and Their Molecular Functions Determining Seed Structure, Components, and Quality of Rice. Rice 2022, 15, 18. [Google Scholar] [CrossRef]
- Wang, B.; Khir, R.; Pan, Z.; Wood, D.; Mahoney, N.E.; El-Mashad, H.; Wu, B.; Ma, H.; Liu, X. Simultaneous decontamination and drying of rough rice using combined pulsed light and holding treatment. J. Sci. Food Agric. 2015, 96, 2874–2881. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhou, Y.; Hu, Y.; Li, B.; Zhang, R.; Zheng, K.; Liu, J.; Wang, J.; Zuo, M.; Liu, S. Integrated Analysis of Metabolome and Volatile Profiles of Germinated Brown Rice from the Japonica and Indica Subspecies. Foods 2021, 10, 2448. [Google Scholar] [CrossRef] [PubMed]
- Shakri, A.N.A.; Kasim, K.F.; Rukunudin, I.B. Chemical Compositions and Physical Properties of Selected Malaysian Rice: A Review. IOP Conf. Ser. Earth Environ. Sci. 2021, 765, 012024. [Google Scholar] [CrossRef]
- Fan, S.; Zhang, Z.; Zhao, Y.; Daglia, M.; Zhang, J.; Zhu, Y.; Bai, J.; Zhu, L.; Xiao, X. Recent advances in targeted manipulation of the gut microbiome by prebiotics: From taxonomic composition to metabolic function. Curr. Opin. Food Sci. 2023, 49, 100959. [Google Scholar] [CrossRef]
- Zhou, K.; Luo, Z.; Huang, W.; Liu, Z.; Miao, X.; Tao, S.; Wang, J.; Zhang, J.; Wang, S.; Zeng, X. Biological Roles of Lipids in Rice. Int. J. Mol. Sci. 2024, 25, 9046. [Google Scholar] [CrossRef]
- Song, L.; Wen, S.; Ye, Q.; Lou, H.; Gao, Y.; Bajpai, V.K.; Carpena, M.; Prieto, M.-A.; Simal-Gandara, J.; Xiao, J.; et al. Advances on delta 5-unsaturated-polymethylene-interrupted fatty acids: Resources, biosynthesis, and benefits. Crit. Rev. Food Sci. Nutr. 2021, 63, 767–789. [Google Scholar] [CrossRef]
- He, W.-S.; Zhu, H.; Chen, Z.-Y. Plant Sterols: Chemical and Enzymatic Structural Modifications and Effects on Their Cholesterol-Lowering Activity. J. Agric. Food Chem. 2018, 66, 3047–3062. [Google Scholar] [CrossRef]
- Obadi, M.; Xu, B. Effect of processing methods and storage on the bioactive compounds of black rice (Oryza sativa L.): A review. Food Funct. 2023, 14, 9100–9122. [Google Scholar] [CrossRef]
- Wang, B.; Mahoney, N.E.; Pan, Z.; Khir, R.; Wu, B.; Ma, H.; Zhao, L. Effectiveness of pulsed light treatment for degradation and detoxification of aflatoxin B1 and B2 in rough rice and rice bran. Food Control 2016, 59, 461–467. [Google Scholar] [CrossRef]
- Yılmaz Tuncel, N. Stabilization of Rice Bran: A Review. Foods 2023, 12, 1924. [Google Scholar] [CrossRef]
- Liu, W.; Yang, W.; Wu, J.; Cheng, Y.; Wei, Z.; Wang, T.; Ampofo, K.A.; Ma, H.; Cui, F.; Yang, X.; et al. ARTP Mutagenesis to Improve Mycelial Polysaccharide Production of Grifola frondosa Using a Mixture of Wheat Bran and Rice Bran as Substrate. J. Food Qual. 2021, 2021, 6110743. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.Q.; Zhang, H.H.; Boateng, N.A.S.; Ma, H.L. Latest developments in polyphenol recovery and purification from plant by-products: A review. Trends Food Sci. Technol. 2020, 99, 375–388. [Google Scholar] [CrossRef]
- Kittipongpatana, O.S.; Trisopon, K.; Wattanaarsakit, P.; Kittipongpatana, N. Utilization and Evaluation of Rice Bran and Rice Bran Wax as a Tablet Lubricant. Pharmaceutics 2024, 16, 428. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, P.; Xu, Z.X.; He, Z.X.; Wang, Q. Bio-fuel oil characteristic of rice bran wax pyrolysis. Renew. Energy 2018, 119, 193–202. [Google Scholar] [CrossRef]
- Punia, S.; Kumar, M.; Siroha, A.K.; Purewal, S.S. Rice Bran Oil: Emerging Trends in Extraction, Health Benefit, and Its Industrial Application. Rice Sci. 2021, 28, 217–232. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Dai, C.; He, R.; Ma, H. Alkali extraction of rice residue protein isolates: Effects of alkali treatment conditions on lysinoalanine formation and structural characterization of lysinoalanine-containing protein. Food Chem. 2018, 261, 176–183. [Google Scholar] [CrossRef]
- Zaky, A.A.; Abd El-Aty, A.M.; Ma, A.; Jia, Y. An overview on antioxidant peptides from rice bran proteins: Extraction, identification, and applications. Crit. Rev. Food Sci. Nutr. 2020, 62, 1350–1362. [Google Scholar] [CrossRef]
- Rivero Meza, S.L.; Cañizares, L.; Dannenberg, B.; Peres, B.B.; Rodrigues, L.A.; Mardade, C.; de Leon, M.A.; Gaioso, C.A.; Egea, I.; de Oliveira, M. Sustainable rice bran protein: Composition, extraction, quality properties and applications. Trends Food Sci. Technol. 2024, 145, 104355. [Google Scholar] [CrossRef]
- Alves, J.B.; Rodrigues, M.H.P.; Duarte, F.A.; Furlong, E.B.; Christ-Ribeiro, A. Rice Bran and Its Potential To Complement the Nutritional Needs of Children and Elderly. Plant Foods Hum. Nutr. 2022, 78, 86–92. [Google Scholar] [CrossRef]
- Peanparkdee, M.; Borompichaichartkul, C.; Iwamoto, S. Bioaccessibility and antioxidant activity of phenolic acids, flavonoids, and anthocyanins of encapsulated Thai rice bran extracts during in vitro gastrointestinal digestion. Food Chem. 2021, 361, 130161. [Google Scholar] [CrossRef]
- Cai, W.-D.; Zhu, J.; Wu, L.-X.; Qiao, Z.-R.; Li, L.; Yan, J.-K. Preparation, characterization, rheological and antioxidant properties of ferulic acid-grafted curdlan conjugates. Food Chem. 2019, 300, 125221. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cai, W.D.; Yao, J.; Wu, L.X.; Li, L.; Zhu, J.; Yan, J.K. Conjugation of ferulic acid onto pectin affected the physicochemical, functional and antioxidant properties. J. Sci. Food Agric. 2020, 100, 5352–5362. [Google Scholar] [CrossRef] [PubMed]
- Ibitoye, O.B.; Ajiboye, T.O. Ferulic acid potentiates the antibacterial activity of quinolone-based antibiotics against Acinetobacter baumannii. Microb. Pathog. 2019, 126, 393–398. [Google Scholar] [CrossRef]
- Chen, H.; Niu, H.; Zhang, H.; Yun, Y.; Chen, W.; Zhong, Q.; Chen, W.; Fu, X. Preparation and properties of ferulic acid-sugar beet pulp pectin ester and its application as a physical and antioxidative stabilizer in a fish oil-water emulsion. Int. J. Biol. Macromol. 2019, 139, 290–297. [Google Scholar] [CrossRef]
- Jia, M.; Yu, Q.; Chen, J.; He, Z.; Chen, Y.; Xie, J.; Nie, S.; Xie, M. Physical quality and in vitro starch digestibility of biscuits as affected by addition of soluble dietary fiber from defatted rice bran. Food Hydrocoll. 2020, 99, 105349. [Google Scholar] [CrossRef]
- Rohfritsch, Z.; Canelli, G.; Pollien, P.; Bel-Rhlid, R. Wheat and Rice Bran as Natural Additives for the Protection of Fish Oil from Oxidation. ACS Food Sci. Technol. 2021, 1, 1160–1168. [Google Scholar] [CrossRef]
- Issara, U. Improvement of Thai Sweet Sausage (Goon Chiang) Properties by Oleogel Made of Rice Bran Wax and Rice Bran Oil: A Textural, Sensorial, and Nutritional Aspect. IOP Conf. Ser. Earth Environ. Sci. 2022, 995, 012045. [Google Scholar] [CrossRef]
- Juliano, B.O.; Tuaño, A.P.P. Gross structure and composition of the rice grain. In Rice; Elsevier: Amsterdam, The Netherlands, 2019; pp. 31–53. [Google Scholar] [CrossRef]
- Bhunia, R.K.; Sinha, K.; Kaur, R.; Kaur, S.; Chawla, K. A Holistic View of the Genetic Factors Involved in Triggering Hydrolytic and Oxidative Rancidity of Rice Bran Lipids. Food Rev. Int. 2021, 39, 441–466. [Google Scholar] [CrossRef]
- Lin, H.; Man, Z.-x.; Kang, W.-c.; Guan, B.-b.; Chen, Q.-s.; Xue, Z.-l. A novel colorimetric sensor array based on boron-dipyrromethene dyes for monitoring the storage time of rice. Food Chem. 2018, 268, 300–306. [Google Scholar] [CrossRef]
- Guan, B.; Zhao, J.; Jin, H.; Lin, H. Determination of Rice Storage Time with Colorimetric Sensor Array. Food Anal. Methods 2016, 10, 1054–1062. [Google Scholar] [CrossRef]
- Li, Q.; Liu, K.; Cai, G.; Yang, X.; Ngo, J.C.K. Developing Lipase Inhibitor as a Novel Approach to Address the Rice Bran Rancidity Issue—A Critical Review. J. Agric. Food Chem. 2024, 72, 3277–3290. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Khan, I.M.; He, W.; Li, Y.; Jin, P.; Campanella, O.H.; Zhang, H.; Huo, Y.; Chen, Y.; Yang, H.; et al. Rebuilding the lid region from conformational and dynamic features to engineering applications of lipase in foods: Current status and future prospects. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2688–2714. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Peng, B.; Luo, T.; Deng, Z. Bound lipase: An important form of lipase in rice bran (Oryza sativa). Food Sci. Hum. Wellness 2023, 12, 1779–1787. [Google Scholar] [CrossRef]
- Mu, Y.; Sun, J.; Wang, S.; Wang, L.; Xu, B. Study of the interfacial activity of wheat germ lipase. Qual. Assur. Saf. Crops Foods 2019, 11, 491–498. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Liu, C.; Yang, H.; Jia, L.; Zhao, L.; Gong, F.; Tan, C.; Tao, H.; He, W.S. Improved antioxidant activity of rutin via lipase-mediated esterification with oleic acid. J. Sci. Food Agric. 2023, 103, 3489–3500. [Google Scholar] [CrossRef] [PubMed]
- De Simone, A. Engineering the Genetic Code of Escherichia coli with Methionine Analogues and Bioorthogonal Amino Acids for Protein Immobilization; Technische Universitaet Berlin: Berlin, Germany, 2016. [Google Scholar]
- Xu, B.; Wang, L.K.; Miao, W.J.; Wu, Q.F.; Liu, Y.X.; Sun, Y.; Gao, C. Thermal versus Microwave Inactivation Kinetics of Lipase and Lipoxygenase from Wheat Germ. J. Food Process Eng. 2015, 39, 247–255. [Google Scholar] [CrossRef]
- Li, B.; Zhao, L.; Chen, H.; Sun, D.; Deng, B.; Li, J.; Liu, Y.; Wang, F. Inactivation of Lipase and Lipoxygenase of Wheat Germ with Temperature-Controlled Short Wave Infrared Radiation and Its Effect on Storage Stability and Quality of Wheat Germ Oil. PLoS ONE 2016, 11, e0167330. [Google Scholar] [CrossRef]
- Sinha, K.; Kaur, R.; Singh, N.; Kaur, S.; Rishi, V.; Bhunia, R.K. Mobilization of storage lipid reserve and expression analysis of lipase and lipoxygenase genes in rice (Oryza sativa var. Pusa Basmati 1) bran during germination. Phytochemistry 2020, 180, 112538. [Google Scholar] [CrossRef]
- He, R.; Wang, Y.; Zou, Y.; Wang, Z.; Ding, C.; Wu, Y.; Ju, X. Storage characteristics of infrared radiation stabilized rice bran and its shelf-life evaluation by prediction modeling. J. Sci. Food Agric. 2020, 100, 2638–2647. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Ma, J.; Li, P.; Geng, Z.; Wang, D.; Zhang, M.; Xu, W. Recombinant Porcine 12-Lipoxygenase Catalytic Domain: Effect of Inhibitors, Selectivity of Substrates and Specificity of Oxidation Products of Linoleic Acid. Foods 2022, 11, 980. [Google Scholar] [CrossRef]
- Zhou, C.; Zhou, Y.; Yu, H.; Li, B.; Hu, Y.; Yang, W.; Zhai, X.; Wang, X.; Liu, J.; Wang, J.; et al. Air and Argon Cold Plasma Effects on Lipolytic Enzymes Inactivation, Physicochemical Properties and Volatile Profiles of Lightly-Milled Rice. Food Chem. 2024, 445, 138699. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Jiang, H.; Lin, J.; Chen, Q.; Ali, S.; Teng, S.W.; Zuo, M. Rice Freshness Identification Based on Visible Near-Infrared Spectroscopy and Colorimetric Sensor Array. Food Anal. Methods 2021, 14, 1305–1314. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Gerothanassis, I.P. Analytical and Structural Tools of Lipid Hydroperoxides: Present State and Future Perspectives. Molecules 2022, 27, 2139. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, C.; Wang, Y.; Fan, M.; Wang, L.; Qian, H. Analysis of the aroma volatile compounds in different stabilized rice bran during storage. Food Chem. 2023, 405, 134753. [Google Scholar] [CrossRef]
- Mohammadi, F.; Marti, A.; Nayebzadeh, K.; Hosseini, S.M.; Tajdar-oranj, B.; Jazaeri, S. Effect of washing, soaking and pH in combination with ultrasound on enzymatic rancidity, phytic acid, heavy metals and coliforms of rice bran. Food Chem. 2021, 334, 127583. [Google Scholar] [CrossRef]
- Ling, B.; Lyng, J.G.; Wang, S. Effects of hot air-assisted radio frequency heating on enzyme inactivation, lipid stability and product quality of rice bran. LWT 2018, 91, 453–459. [Google Scholar] [CrossRef]
- Barden, L.; Decker, E.A. Lipid Oxidation in Low-moisture Food: A Review. Crit. Rev. Food Sci. Nutr. 2013, 56, 2467–2482. [Google Scholar] [CrossRef]
- Velasco, J.; Dobarganes, C.; Márquez-Ruiz, G. Oxidative rancidity in foods and food quality. In Chemical Deterioration and Physical Instability of Food and Beverages; Elsevier: Amsterdam, The Netherlands, 2010; pp. 3–32. [Google Scholar] [CrossRef]
- Yu, C.-w.; Luo, T.; Cao, Y.; Wei, C.-h.; Deng, Z.-y. The influence of microbial contamination on rice bran rancidity. LWT 2021, 146, 111468. [Google Scholar] [CrossRef]
- Sunli, C.; Jun, S.; Hanping, M.; Xiaohong, W.; Pei, W.; Xiaodong, Z. Non-destructive detection for mold colonies in rice based on hyperspectra and GWO-SVR. J. Sci. Food Agric. 2017, 98, 1453–1459. [Google Scholar] [CrossRef]
- Ortenero, J.R.; Choi, A.E.S.; Calingacion, M.S. A Review of the Different Sensors for the Detection of Rice Rancidity Indicators. Chem. Eng. Trans. 2024, 114, 421–426. [Google Scholar]
- Tan, Y.; Chang, S.K.C.; Zhang, Y. Comparison of α-amylase, α-glucosidase and lipase inhibitory activity of the phenolic substances in two black legumes of different genera. Food Chem. 2017, 214, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; He, L.; Hou, X.; Wei, J.; Ma, X.; Gao, Z.; Yuan, Y.; Xiao, J.; Li, P.; Yue, T. Relationships between Structure and Antioxidant Capacity and Activity of Glycosylated Flavonols. Foods 2021, 10, 849. [Google Scholar] [CrossRef] [PubMed]
- Fabroni, S.; Ballistreri, G.; Amenta, M.; Romeo, F.V.; Rapisarda, P. Screening of the anthocyanin profile and in vitro pancreatic lipase inhibition by anthocyanin-containing extracts of fruits, vegetables, legumes and cereals. J. Sci. Food Agric. 2016, 96, 4713–4723. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Yang, Y.; Hassane Hamadou, A.; Shen, Q.; Xu, B. Tempering–preservation treatment inactivated lipase in wheat bran and retained phenolic compounds. Int. J. Food Sci. Technol. 2021, 57, 2104–2112. [Google Scholar] [CrossRef]
- Zhang, Y.; He, C.; Wu, Y.; Yang, J.; Xuan, H.; Zhu, X. Effect of lipoxygenase activity and red seed coat on rice bran deterioration. J. Sci. Food Agric. 2009, 89, 1904–1908. [Google Scholar] [CrossRef]
- Chen, M.-H.; Bergman, C.J.; McClung, A.M. Hydrolytic rancidity and its association with phenolics in rice bran. Food Chem. 2019, 285, 485–491. [Google Scholar] [CrossRef]
- Raghavendra, M.P.; Kumar, P.R.; Prakash, V. Mechanism of Inhibition of Rice Bran Lipase by Polyphenols: A Case Study with Chlorogenic Acid and Caffeic Acid. J. Food Sci. 2007, 72, E412–E419. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, Z.; Zhao, S.; Liu, J.; Gao, R.; Jiang, P. Characterization of volatile organic compounds of different pigmented rice after puffing based on gas chromatography-ion migration spectrometry and chemometrics. Food Res. Int. 2023, 169, 112879. [Google Scholar] [CrossRef]
- Hu, Y.; Tang, Q.; Sun, Y.; Wu, J.; Sun, Z.; Zuo, M.; Cai, J.; Zhai, X.; Zhou, C.; Shi, J.; et al. Comparative study on physicochemical, nutritional and cooking properties of different pigmented dehusked rice varieties influenced by superheated steam treatment. J. Cereal Sci. 2024, 117, 103934. [Google Scholar] [CrossRef]
- Osae, R.; Essilfie, G.; Alolga, R.N.; Akaba, S.; Song, X.; Owusu-Ansah, P.; Zhou, C. Application of non-thermal pretreatment techniques on agricultural products prior to drying: A review. J. Sci. Food Agric. 2020, 100, 2585–2599. [Google Scholar] [CrossRef]
- Lv, S.W.; Sun, L.H.; Zhao, S.Y.; Bao, Y.M. Effect of dry heat stabilisation on the functional properties of rice bran proteins. Int. J. Food Sci. Technol. 2017, 52, 1836–1843. [Google Scholar] [CrossRef]
- Xiaofei, S.; Wenxue, Z.; Xinling, L.; Jinling, F. Effects of heat pump drying temperature and dietary fat on carrot β-carotene bioaccessibility. Int. J. Agric. Biol. Eng. 2017, 10, 234–242. [Google Scholar] [CrossRef]
- Li, Z.; Guo, D.; Li, X.; Tang, Z.; Ling, X.; Zhou, T.; Zhang, B. Heat-Moisture Treatment Further Reduces In Vitro Digestibility and Enhances Resistant Starch Content of a High-Resistant Starch and Low-Glutelin Rice. Foods 2021, 10, 2562. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-M.; Chung, H.-J.; Lim, S.-T. Effect of various heat treatments on rancidity and some bioactive compounds of rice bran. J. Cereal Sci. 2014, 60, 243–248. [Google Scholar] [CrossRef]
- Li, H.-T.; Zhang, W.; Chen, Y.; Pan, W.; Bao, Y. Physical modification of high amylose starch using electron beam irradiation and heat moisture treatment: The effect on multi-scale structure and in vitro digestibility. Food Chem. 2023, 424, 136344. [Google Scholar] [CrossRef]
- Cai, C.; Wei, B.; Tian, Y.; Ma, R.; Chen, L.; Qiu, L.; Jin, Z. Structural changes of chemically modified rice starch by one-step reactive extrusion. Food Chem. 2019, 288, 354–360. [Google Scholar] [CrossRef]
- Dang, T.T.; Vasanthan, T. Modification of rice bran dietary fiber concentrates using enzyme and extrusion cooking. Food Hydrocoll. 2019, 89, 773–782. [Google Scholar] [CrossRef]
- Mushtaq, B.S.; Al-Ansi, W.; Dhungle, A.; Haq, F.u.; Mahdi, A.A.; Walayat, N.; Manzoor, M.S.; Nawaz, A.; Fan, M.; Qian, H.; et al. Influence of pretreatments combined with extrusion on γ-amino butyric acid, nutritional composition and physicochemical properties of foxtail millet (Setaria italica). J. Cereal Sci. 2021, 102, 103359. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Wang, L.; Liu, S.; Wang, K.; Sun, J.; Xu, B. Evaluation of the possible non-thermal effect of microwave radiation on the inactivation of wheat germ lipase. J. Food Process Eng. 2016, 40, e12506. [Google Scholar] [CrossRef]
- Cao, H.; Wang, X.; Liu, J.; Sun, Z.; Yu, Z.; Battino, M.; El-Seedi, H.; Guan, X. Mechanistic insights into the changes of enzyme activity in food processing under microwave irradiation. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2465–2487. [Google Scholar] [CrossRef]
- Li, M.; Zhou, C.; Wang, B.; Zeng, S.; Mu, R.; Li, G.; Li, B.; Lv, W. Research progress and application of ultrasonic- and microwave-assisted food processing technology. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3707–3731. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, Y.; Okonkwo, C.E.; Chen, L.; Zhou, C. Multimode ultrasonic-assisted decontamination of fruits and vegetables: A review. Food Chem. 2024, 450, 139356. [Google Scholar] [CrossRef] [PubMed]
- Bei, X.; Yu, X.; Li, D.; Sun, Q.; Yu, Y.; Wang, Y.; Okonkwo, C.E.; Zhou, C. Heat source replacement strategy using catalytic infrared: A future for energy saving drying of fruits and vegetables. J. Food Sci. 2023, 88, 4827–4839. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Yu, X.; Zhou, C.; Yagoub, A.E.A.; Li, D. A Catalytic Infrared System as a Hot Water Replacement Strategy: A Future Approach for Blanching Fruits and Vegetables to Save Energy and Water. Food Rev. Int. 2023, 40, 641–657. [Google Scholar] [CrossRef]
- Wu, B.; Ma, Y.; Guo, X.; Guo, Y.; Qiu, C.; Gao, K.; Ma, H.; Pan, Z. Catalytic infrared blanching and drying of carrot slices with different thicknesses: Effects on surface dynamic crusting and quality characterization. Innov. Food Sci. Emerg. Technol. 2023, 88, 103444. [Google Scholar] [CrossRef]
- Wang, T.; Khir, R.; Pan, Z.; Yuan, Q. Simultaneous rough rice drying and rice bran stabilization using infrared radiation heating. LWT 2017, 78, 281–288. [Google Scholar] [CrossRef]
- Dhingra, D.; Chopra, S.; Rai, D.R. Stabilization of Raw Rice Bran using Ohmic Heating. Agric. Res. 2012, 1, 392–398. [Google Scholar] [CrossRef]
- Roobab, U.; Khan, A.W.; Irfan, M.; Madni, G.M.; Zeng, X.A.; Nawaz, A.; Walayat, N.; Manzoor, M.F.; Aadil, R.M. Recent developments in ohmic technology for clean label fruit and vegetable processing: An overview. J. Food Process Eng. 2022, 45, e14045. [Google Scholar] [CrossRef]
- Xiong, Z.; Shi, T.; Jin, W.; Bao, Y.; Monto, A.R.; Yuan, L.; Gao, R. Gel performance of surimi induced by various thermal technologies: A review. Crit. Rev. Food Sci. Nutr. 2022, 64, 3075–3090. [Google Scholar] [CrossRef]
- Bárdos, L.; Baránková, H. Cold atmospheric plasma: Sources, processes, and applications. Thin Solid Films 2010, 518, 6705–6713. [Google Scholar] [CrossRef]
- Umair, M.; Sultana, T.; Xun, S.; Jabbar, S.; Riaz Rajoka, M.S.; Albahi, A.; Abid, M.; Ranjha, M.M.A.N.; El-Seedi, H.R.; Xie, F.; et al. Advances in the application of functional nanomaterial and cold plasma for the fresh-keeping active packaging of meat. Food Sci. Nutr. 2023, 11, 5753–5772. [Google Scholar] [CrossRef]
- Jia, W.; Wang, X.; Zhang, R.; Shi, Q.; Shi, L. Irradiation role on meat quality induced dynamic molecular transformation: From nutrition to texture. Food Rev. Int. 2022, 39, 4442–4464. [Google Scholar] [CrossRef]
- Mir, S.A.; Dar, B.N.; Mir, M.M.; Sofi, S.A.; Shah, M.A.; Sidiq, T.; Sunooj, K.V.; Hamdani, A.M.; Mousavi Khaneghah, A. Current strategies for the reduction of pesticide residues in food products. J. Food Compos. Anal. 2022, 106, 104274. [Google Scholar] [CrossRef]
- Tchabo, W.; Ma, Y.; Kwaw, E.; Zhang, H.; Li, X.; Afoakwah, N.A. Effects of Ultrasound, High Pressure, and Manosonication Processes on Phenolic Profile and Antioxidant Properties of a Sulfur Dioxide-Free Mulberry (Morus nigra) Wine. Food Bioprocess Technol. 2017, 10, 1210–1223. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Zhen, L.; Wang, Y.; Wang, Y.; Qin, Y.; Zhang, Z.; Zhao, T.; Cao, J.; Liu, Y.; et al. Ultra-High Hydrostatic Pressure Pretreatment on White Que Zui Tea: Chemical Constituents, Antioxidant, Cytoprotective, and Anti-Inflammatory Activities. Foods 2023, 12, 628. [Google Scholar] [CrossRef]
- Wei, B.; Cai, C.; Xu, B.; Jin, Z.; Tian, Y. Disruption and molecule degradation of waxy maize starch granules during high pressure homogenization process. Food Chem. 2018, 240, 165–173. [Google Scholar] [CrossRef]
- Yu, C.w.; Hu, Q.r.; Wang, H.w.; Deng, Z.y. Comparison of 11 rice bran stabilization methods by analyzing lipase activities. J. Food Process. Preserv. 2020, 44, e14370. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Keener, K.M. Cold plasma: Background, applications and current trends. Curr. Opin. Food Sci. 2017, 16, 49–52. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, C.; Cui, H.; Lin, L. Feasibility of cold plasma for the control of biofilms in food industry. Trends Food Sci. Technol. 2020, 99, 142–151. [Google Scholar] [CrossRef]
- Liu, C.; Li, M.; Wang, J.; Zhou, X.; Guo, Q.; Yan, J.; Li, Y. Plasma methods for preparing green catalysts: Current status and perspective. Chin. J. Catal. 2016, 37, 340–348. [Google Scholar] [CrossRef]
- Kumar, A.; Škoro, N.; Gernjak, W.; Puač, N. Cold atmospheric plasma technology for removal of organic micropollutants from wastewater—A review. Eur. Phys. J. D 2021, 75, 283. [Google Scholar] [CrossRef]
- Cui, H.; Bai, M.; Yuan, L.; Surendhiran, D.; Lin, L. Sequential effect of phages and cold nitrogen plasma against Escherichia coli O157:H7 biofilms on different vegetables. Int. J. Food Microbiol. 2018, 268, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Liao, X.; Li, C.; Abdel-Samie, M.A.; Cui, H. Inhibitory effect of cold nitrogen plasma on Salmonella Typhimurium biofilm and its application on poultry egg preservation. LWT 2020, 126, 109340. [Google Scholar] [CrossRef]
- Lin, L.; Liao, X.; Li, C.; Abdel-Samie, M.A.; Siva, S.; Cui, H. Cold nitrogen plasma modified cuminaldehyde/β-cyclodextrin inclusion complex and its application in vegetable juices preservation. Food Res. Int. 2021, 141, 110132. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, Y.; Aalim, H.; Cao, Y.; Peng, L.; Dou, J.; Ma, Y.; Zhai, X.; Guo, Z.; Cai, J.; et al. Cold plasma-assisted pretreatment for fabrication and characterization of rice starch-stearic acid complexes. Food Biosci. 2024, 60, 104492. [Google Scholar] [CrossRef]
- Zhou, C.; Zhou, Y.; Tang, Q.; Sun, Y.; Ji, F.; Wu, J.; Yu, H.; Liu, T.; Yang, W.; Liu, S.; et al. Impact of argon dielectric barrier discharge cold plasma on the physicochemical and cooking properties of lightly-milled rice. Innov. Food Sci. Emerg. Technol. 2024, 92, 103580. [Google Scholar] [CrossRef]
- Li, B.; Peng, L.; Cao, Y.; Liu, S.; Zhu, Y.; Dou, J.; Yang, Z.; Zhou, C. Insights into Cold Plasma Treatment on the Cereal and Legume Proteins Modification: Principle, Mechanism, and Application. Foods 2024, 13, 1522. [Google Scholar] [CrossRef]
- Shen, C.; Chen, W.; Aziz, T.; Khojah, E.; Al-Asmari, F.; Alamri, A.S.; Alhomrani, M.; Cui, H.; Lin, L. Drying kinetics and moisture migration mechanism of yam slices by cold plasma pretreatment combined with far-infrared drying. Innov. Food Sci. Emerg. Technol. 2024, 95, 103730. [Google Scholar] [CrossRef]
- Yang, X.; Ma, L.; Zheng, J.; Qiao, Y.; Bai, J.; Cai, J. Effects of atmospheric pressure plasma treatment on the quality and cellulose modification of brown rice. Innov. Food Sci. Emerg. Technol. 2024, 96, 103744. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Shi, T.; Xiong, Z.; Jin, W.; Bao, Y.; Monto, A.R.; Yuan, L.; Gao, R. Mechanism of plasma-activated water promoting the heat-induced aggregation of myofibrillar protein from silver carp (Aristichthys Nobilis). Innov. Food Sci. Emerg. Technol. 2024, 91, 103555. [Google Scholar] [CrossRef]
- Wei, W.; Yang, S.; Yang, F.; Hu, X.; Wang, Y.; Guo, W.; Yang, B.; Xiao, X.; Zhu, L. Cold Plasma Controls Nitrite Hazards by Modulating Microbial Communities in Pickled Radish. Foods 2023, 12, 2550. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, X.; Qin, X.; Wang, Z.; Zou, Y.; Wang, D.; Xu, W. Restructured ground chicken quality study by ultrasound combined with plasma protein treatment. Food Biosci. 2023, 56, 103289. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Zhang, Y.; Hu, H.; Luo, S.; Zhang, L.; Zhou, H.; Li, P. Effects of in-package atmospheric cold plasma treatment on the qualitative, metabolic and microbial stability of fresh-cut pears. J. Sci. Food Agric. 2021, 101, 4473–4480. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Ma, C.; Lin, L. Synergetic antibacterial efficacy of cold nitrogen plasma and clove oil against Escherichia coli O157:H7 biofilms on lettuce. Food Control 2016, 66, 8–16. [Google Scholar] [CrossRef]
- Tolouie, H.; Mohammadifar, M.A.; Ghomi, H.; Hashemi, M. Argon and nitrogen cold plasma effects on wheat germ lipolytic enzymes: Comparison to thermal treatment. Food Chem. 2021, 346, 128974. [Google Scholar] [CrossRef]
- Saberi, M.; Modarres-Sanavy, S.A.M.; Zare, R.; Ghomi, H. Amelioration of Photosynthesis and Quality of Wheat under Non-thermal Radio Frequency Plasma Treatment. Sci. Rep. 2018, 8, 11655. [Google Scholar] [CrossRef]
- Los, A.; Ziuzina, D.; Akkermans, S.; Boehm, D.; Cullen, P.J.; Van Impe, J.; Bourke, P. Improving microbiological safety and quality characteristics of wheat and barley by high voltage atmospheric cold plasma closed processing. Food Res. Int. 2018, 106, 509–521. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, C.; Cui, H.; Lin, L. Plasma enhanced-nutmeg essential oil solid liposome treatment on the gelling and storage properties of pork meat batters. J. Food Eng. 2020, 266, 109696. [Google Scholar] [CrossRef]
- Bai, J.-W.; Li, D.-D.; Abulaiti, R.; Wang, M.; Wu, X.; Feng, Z.; Zhu, Y.; Cai, J. Cold Plasma as a Novel Pretreatment to Improve the Drying Kinetics and Quality of Green Peas. Foods 2025, 14, 84. [Google Scholar] [CrossRef]
- Coutinho, N.M.; Silveira, M.R.; Guimarães, J.T.; Fernandes, L.M.; Pimentel, T.C.; Silva, M.C.; Borges, F.O.; Fernandes, F.A.N.; Rodrigues, S.; Freitas, M.Q.; et al. Are consumers willing to pay for a product processed by emerging technologies? The case of chocolate milk drink processed by cold plasma. LWT 2021, 138, 110772. [Google Scholar] [CrossRef]
- Lung, H.-M.; Cheng, Y.-C.; Chang, Y.-H.; Huang, H.-W.; Yang, B.B.; Wang, C.-Y. Microbial decontamination of food by electron beam irradiation. Trends Food Sci. Technol. 2015, 44, 66–78. [Google Scholar] [CrossRef]
- Li, T.; Wang, L.; Chen, Z.; Li, C.; Li, X.; Sun, D. Structural changes and enzymatic hydrolysis yield of rice bran fiber under electron beam irradiation. Food Bioprod. Process. 2020, 122, 62–71. [Google Scholar] [CrossRef]
- Pan, L.; Xing, J.; Zhang, H.; Luo, X.; Chen, Z. Electron beam irradiation as a tool for rice grain storage and its effects on the physicochemical properties of rice starch. Int. J. Biol. Macromol. 2020, 164, 2915–2921. [Google Scholar] [CrossRef]
- Luo, X.; Li, Y.; Yang, D.; Xing, J.; Li, K.; Yang, M.; Wang, R.; Wang, L.; Zhang, Y.; Chen, Z. Effects of electron beam irradiation on storability of brown and milled rice. J. Stored Prod. Res. 2019, 81, 22–30. [Google Scholar] [CrossRef]
- Mousavi Khaneghah, A.; Hashemi Moosavi, M.; Oliveira, C.A.F.; Vanin, F.; Sant’Ana, A.S. Electron beam irradiation to reduce the mycotoxin and microbial contaminations of cereal-based products: An overview. Food Chem. Toxicol. 2020, 143, 111557. [Google Scholar] [CrossRef]
- Wang, C.; He, G.; Meng, J.; Wang, S.; Kong, Y.; Jiang, J.; Hu, R.; Zhou, G. Improved lignocellulose saccharification of a Miscanthus reddish stem mutant induced by heavy-ion irradiation. GCB Bioenergy 2020, 12, 1066–1077. [Google Scholar] [CrossRef]
- Wang, B.; Mahoney, N.E.; Khir, R.; Wu, B.; Zhou, C.; Pan, Z.; Ma, H. Degradation kinetics of aflatoxin B1 and B2 in solid medium by using pulsed light irradiation. J. Sci. Food Agric. 2018, 98, 5220–5224. [Google Scholar] [CrossRef]
- Xu, B.; Ren, A.; Chen, J.; Li, H.; Wei, B.; Wang, J.; Azam, S.M.R.; Bhandari, B.; Zhou, C.; Ma, H. Effect of multi-mode dual-frequency ultrasound irradiation on the degradation of waxy corn starch in a gelatinized state. Food Hydrocoll. 2021, 113, 106440. [Google Scholar] [CrossRef]
- Mohammadi Shad, Z.; Steen, E.; Devlieghere, F.; Mauromoustakos, A.; Atungulu, G.G. Biochemical changes associated with electron beam irradiation of rice and links to kernel discoloration during storage. Cereal Chem. 2019, 96, 824–835. [Google Scholar] [CrossRef]
- Li, R.; Yang, S.; Wang, D.; Liang, J.; Huang, T.; Zhang, L.; Luo, A. Electron-beam irradiation delayed the postharvest senescence of kiwifruit during cold storage through regulating the reactive oxygen species metabolism. Radiat. Phys. Chem. 2021, 189, 109717. [Google Scholar] [CrossRef]
- Aganovic, K.; Hertel, C.; Vogel, R.F.; Johne, R.; Schlüter, O.; Schwarzenbolz, U.; Jäger, H.; Holzhauser, T.; Bergmair, J.; Roth, A.; et al. Aspects of high hydrostatic pressure food processing: Perspectives on technology and food safety. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3225–3266. [Google Scholar] [CrossRef] [PubMed]
- Grau-Fuentes, E.; Garzón, R.; Rodrigo, D.; Rosell, C.M. High pressure processing at different hydration levels as a tool to enhance rice bran stability and techno-functionality. Food Res. Int. 2025, 201, 115593. [Google Scholar] [CrossRef] [PubMed]
- Engmann, F.; Ma, Y.-K.; Sanful, R. The impact of high hydrostatic pressure treatment on anthocyanins, colour, microorganisms, and enzyme activity of mulberry (Morus nigra) juice. Int. Food Res. J. 2020, 27, 88–95. [Google Scholar]
- Geng, N.; Song, J.; Zhang, K.; Dai, Z.; Li, D. Effect of dynamic high-pressure microfluidization on the physicochemical and structural properties of insoluble dietary fiber from fresh corn bract. J. Food Process. Preserv. 2021, 45, e15710. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, S.; Ramaswamy, H.S.; Hu, F.; Yu, Y. Effect of high pressure processing on rancidity of brown rice during storage. LWT 2018, 93, 405–411. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, J.; Li, P.; Wen, B.; Wang, Y.; Ma, Y.; Huang, W. Preparation of hypoglycemic anthocyanins from mulberry (Fructus mori) fruits by ultrahigh pressure extraction. Innov. Food Sci. Emerg. Technol. 2023, 84, 103255. [Google Scholar] [CrossRef]
- Ye, S.-J.; Baik, M.-Y. Physicochemical properties of amorphous granular starch (AGS) prepared by non-thermal gelatinization by high hydrostatic pressure (HHP) and spray drying. Int. J. Biol. Macromol. 2024, 260, 129508. [Google Scholar] [CrossRef]
- Laokuldilok, T.; Rattanathanan, Y. Protease Treatment for the Stabilization of Rice Bran: Effects on Lipase Activity, Antioxidants, and Lipid Stability. Cereal Chem. 2014, 91, 560–565. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, G.; Ahmad, T.; Mansoor, S.; Kaur, B. Enzyme Engineering: Current Trends and Future Perspectives. Food Rev. Int. 2019, 37, 121–154. [Google Scholar] [CrossRef]
- He, R.; Xing, H.; Wang, Z.; Ding, W.; Zhu, P.; Liu, B.; Ma, H. Establishment of an Enzymatic Membrane Reactor for Angiotensin-Converting Enzyme Inhibitory Peptides Preparation from Wheat Germ Protein Isolates. J. Food Process Eng. 2015, 39, 296–305. [Google Scholar] [CrossRef]
- Wang, F.; Xu, H.; Wang, M.; Yu, X.; Cui, Y.; Xu, L.; Ma, A.; Ding, Z.; Huo, S.; Zou, B.; et al. Application of Immobilized Enzymes in Juice Clarification. Foods 2023, 12, 4258. [Google Scholar] [CrossRef] [PubMed]
- Maghraby, Y.R.; El-Shabasy, R.M.; Ibrahim, A.H.; Azzazy, H.M.E.-S. Enzyme immobilization technologies and industrial applications. ACS Omega 2023, 8, 5184–5196. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Sehemu, R.M.; Zhang, T.; Song, B.; Yang, L.; Ren, X.; Ma, H. Immobilized enzymolysis of corn gluten meal under triple-frequency ultrasound. Int. J. Food Eng. 2018, 14, 20170347. [Google Scholar] [CrossRef]
- Shouket, S.; khurshid, S.; Khan, J.; Batool, R.; Sarwar, A.; Aziz, T.; Alhomrani, M.; Alamri, A.S.; Sameeh, M.Y.; Zubair Filimban, F. Enhancement of shelf-life of food items via immobilized enzyme nanoparticles on varied supports. A sustainable approach towards food safety and sustainability. Food Res. Int. 2023, 169, 112940. [Google Scholar] [CrossRef]
- Yu, D.; Chen, K.; Liu, J.; Pan, Z.; Jiang, L.; Wang, L.; Elfalleh, W. Application of magnetic immobilized papain on passivated rice bran lipase. Int. J. Biol. Macromol. 2020, 157, 51–59. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, T.; Ni, X.; Xia, J.; Suo, H.; Yan, L.; Zou, B. Immobilized lipase based on SBA-15 adsorption and gel embedding for catalytic synthesis of isoamyl acetate. Food Biosci. 2024, 60, 104427. [Google Scholar] [CrossRef]
- Han, J.; Feng, H.; Wu, J.; Li, Y.; Zhou, Y.; Wang, L.; Luo, P.; Wang, Y. Construction of Multienzyme Co-immobilized Hybrid Nanoflowers for an Efficient Conversion of Cellulose into Glucose in a Cascade Reaction. J. Agric. Food Chem. 2021, 69, 7910–7921. [Google Scholar] [CrossRef]
- Khan, M.R. Immobilized enzymes: A comprehensive review. Bull. Natl. Res. Cent. 2021, 45, 207. [Google Scholar] [CrossRef]
- DiCosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef]
- Douaisi, M.; Paskaleva, E.E.; Fu, L.; Grover, N.; McManaman, C.L.; Varghese, S.; Brodfuehrer, P.R.; Gibson, J.M.; de Joode, I.; Xia, K.; et al. Synthesis of bioengineered heparin chemically and biologically similar to porcine-derived products and convertible to low MW heparin. Proc. Natl. Acad. Sci. USA 2024, 121, e2315586121. [Google Scholar] [CrossRef]
- Khodadad Hosseini, E.; Derakhshi, P.; Rabbani, M.; Mooraki, N. Pollutant removal from dairy wastewater using live Azolla filiculoides in batch and continuous bioreactors. Water Environ. Res. 2021, 93, 2122–2134. [Google Scholar] [CrossRef] [PubMed]
- Araujo, T.M.; Barga, M.C.; Della-Bianca, B.E.; Basso, T.O. Yeast immobilisation for brewery fermentation. J. Inst. Brew. 2021, 127, 302–316. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Zhao, M.; Zabed, H.M.; Qi, X. Fermentative Production of Ergothioneine by Exploring Novel Biosynthetic Pathway and Remodulating Precursor Synthesis Pathways. J. Agric. Food Chem. 2024, 72, 14264–14273. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, Y.; Simpson, B. Food enzymes immobilization: Novel carriers, techniques and applications. Curr. Opin. Food Sci. 2022, 43, 27–35. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Wang, L.H.; Zeng, X.A.; Han, Z.; Brennan, C.S. Non-thermal technologies and its current and future application in the food industry: A review. Int. J. Food Sci. Technol. 2018, 54, 1–13. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Huang, X.; Chen, J.; Qin, Y.; Gao, X. Recent research and prospects of non-thermal physical technologies in green and high-efficient extraction of natural pigments: A review. Innov. Food Sci. Emerg. Technol. 2024, 92, 103593. [Google Scholar] [CrossRef]
- Hertwig, C.; Meneses, N.; Mathys, A. Cold atmospheric pressure plasma and low energy electron beam as alternative nonthermal decontamination technologies for dry food surfaces: A review. Trends Food Sci. Technol. 2018, 77, 131–142. [Google Scholar] [CrossRef]
- Lin, L.; Liao, X.; Cui, H. Cold plasma treated thyme essential oil/silk fibroin nanofibers against Salmonella Typhimurium in poultry meat. Food Packag. Shelf Life 2019, 21, 100337. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, Y.; Li, Y.; Hou, J.; Liang, Y.; Zhang, Z. Investigation of the formation of furfural compounds in apple products treated with pasteurization and high pressure processing. Food Res. Int. 2024, 190, 114546. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Wang, H.; Fan, W.; Hu, Y.; Tu, Z. Magnetic field: A non-thermal technology in food processing. Food Control 2024, 166, 110692. [Google Scholar] [CrossRef]
- Jang, W.Y.; Kim, Y.J.; Chang, J.H. Comparative Study of Enzymatic Lipolysis Using Nanofructosome-Coated CalB Lipase Encapsulated in Silica and Immobilized on Silica-Coated Magnetic Nanoparticles. ACS Omega 2025, 10, 13319–13326. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Hu, Y.; Wang, Y.; Xu, B.; Zhou, C.; Adhikari, B.; Liu, J.; Xu, T.; Wang, B. Atmosphere-controlled high-voltage electrospray for improving conductivity, flexibility, and antibacterial properties of chitosan films. Food Res. Int. 2025, 200, 115450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, J.; Huang, X.; Zhai, X.; Li, Z.; Shi, J.; Sobhy, R.; Khalifa, I.; Zou, X. Lemon-derived carbon quantum dots incorporated guar gum/sodium alginate films with enhanced the preservability for blanched asparagus active packaging. Food Res. Int. 2025, 202, 115736. [Google Scholar] [CrossRef] [PubMed]
- Vant, J.W.; Sarkar, D.; Nguyen, J.; Baker, A.T.; Vermaas, J.V.; Singharoy, A. Exploring cryo-electron microscopy with molecular dynamics. Biochem. Soc. Trans. 2022, 50, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Zhao, C.; Sun, S.; Xu, M.; Zhang, L.; Wang, P.; Fang, Y. Encrypted information reading technology at the micro/nano scale based on surface plasma-driven reactions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 281, 121607. [Google Scholar] [CrossRef]
| Component | Average Range | Component | Average Range |
|---|---|---|---|
| Crude fat (%) | 18–23 | γ-oryzanol (g/kg) | 0.5–5.5 |
| Crude protein (%) | 11–16 | ||
| Total dietary fiber (%) | 22–32 | Total Tocopherols (mg/kg) | 100–150 |
| Vitamin B1 (Thiamin) (mg/kg) | 12–40 | α-T (mg/kg) | 50–130 |
| Vitamin B2 (Riboflavin) (mg/kg) | 1–4 | β-T (mg/kg) | 2–10 |
| Vitamin B3 (Niacin) (mg/kg) | 300–800 | γ-T (mg/kg) | 10–50 |
| Vitamin B5 (Pantothenic acid) (mg/kg) | 74 | δ-T (mg/kg) | 0–2 |
| Vitamin B6 (mg/kg) | 20–40 | ||
| Ca (mg/kg) | 300–1200 | Total Tocotrienols (mg/kg) | 130–170 |
| K (mg/kg) | 5992 | α-T3 (mg/kg) | 38 |
| Fe (mg/kg) | 86–430 | β-T3 (mg/kg) | – |
| Zn (mg/kg) | 50–250 | γ-T3 (mg/kg) | 120–140 |
| P (mg/kg) | 6278 | δ-T3 (mg/kg) | 0–10 |
| Method | Principle | Advantages | Limitations | Ref |
|---|---|---|---|---|
| Dry heat | direct heating at ~120 °C to inactivate enzymes | simple and practical for commercial use; cost-effective and easy to scale | uneven heating; temperatures <100 °C ineffective; excessive heat degrades nutrients. | [84,85,86] |
| Moist heat | indirect heating using high-pressure/ambient steam | better preservation of nutrients compared to dry heat; more uniform heat distribution | requires precise moisture control; higher energy consumption | [87,88,89] |
| Extrusion | mechanical extrusion with steam injection induces structural changes and enzyme denaturation | rapid and continuous processing; stabilizes bran while improving texture | high energy consumption; expensive machinery | [90,91,92] |
| Microwave | dielectric heating via microwave absorption generates bulk heating, and synchronizing heat | fast and energy-efficient; minimal nutrient loss; | requires precise control of temperature to avoid over-heating; high initial equipment cost | [93,94,95,96] |
| Infrared | short-wavelength radiation minimizes thermal inertia and damage while suppressing enzymes activities | fast and energy-efficient; uniform heat distribution | limited material penetration depth; potential uneven heating in bulk processing | [22,97,98,99,100] |
| Ohmic Heating | Joule heating via electrical resistance converts current to thermal energy | rapid and uniform heating; minimal nutrient loss. | high equipment complexity; limited scalability for industrial use | [101,102,103] |
| Method | Principle | Advantages | Limitations | Ref |
|---|---|---|---|---|
| Low-temperature Plasma | Reactive species (ROS/RNS) disrupt enzyme structure | Rapid, nutrient retention | Limited penetration depth | [104,105] |
| HEEB Irradiation | Ionizing radiation generates free radicals to crosslink DNA/enzymes | Scalable, no chemical residues | Polymer degradation at high doses | [106,107] |
| Ultra-High Pressure | Hydrostatic pressure disrupts hydrophobic/ionic bonds | Preserves thermosensi-tive compounds | High equipment cost | [108,109,110] |
| Enzymatic Stabilization | Proteolytic degradation of enzymes using im-mobilized enzymes | Targeted action, recyclable catalysts | Requires optimization of E:S ratios | [16,111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Huang, J.; Du, Y.; Li, F.; Xu, W.; Zhou, C.; Liu, S. Non-Thermal Stabilization Strategies for Rice Bran: Mechanistic Insights, Technological Advances, and Implications for Industrial Applications. Foods 2025, 14, 1448. https://doi.org/10.3390/foods14091448
Zhou L, Huang J, Du Y, Li F, Xu W, Zhou C, Liu S. Non-Thermal Stabilization Strategies for Rice Bran: Mechanistic Insights, Technological Advances, and Implications for Industrial Applications. Foods. 2025; 14(9):1448. https://doi.org/10.3390/foods14091448
Chicago/Turabian StyleZhou, Lu, Jiangqi Huang, Yutong Du, Fanghao Li, Wenbin Xu, Chenguang Zhou, and Siyao Liu. 2025. "Non-Thermal Stabilization Strategies for Rice Bran: Mechanistic Insights, Technological Advances, and Implications for Industrial Applications" Foods 14, no. 9: 1448. https://doi.org/10.3390/foods14091448
APA StyleZhou, L., Huang, J., Du, Y., Li, F., Xu, W., Zhou, C., & Liu, S. (2025). Non-Thermal Stabilization Strategies for Rice Bran: Mechanistic Insights, Technological Advances, and Implications for Industrial Applications. Foods, 14(9), 1448. https://doi.org/10.3390/foods14091448







