Molecular Regulation of Carotenoid Accumulation Enhanced by Oxidative Stress in the Food Industrial Strain Blakeslea trispora
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strain
2.2. Culture and Growth Analysis of B. trispora
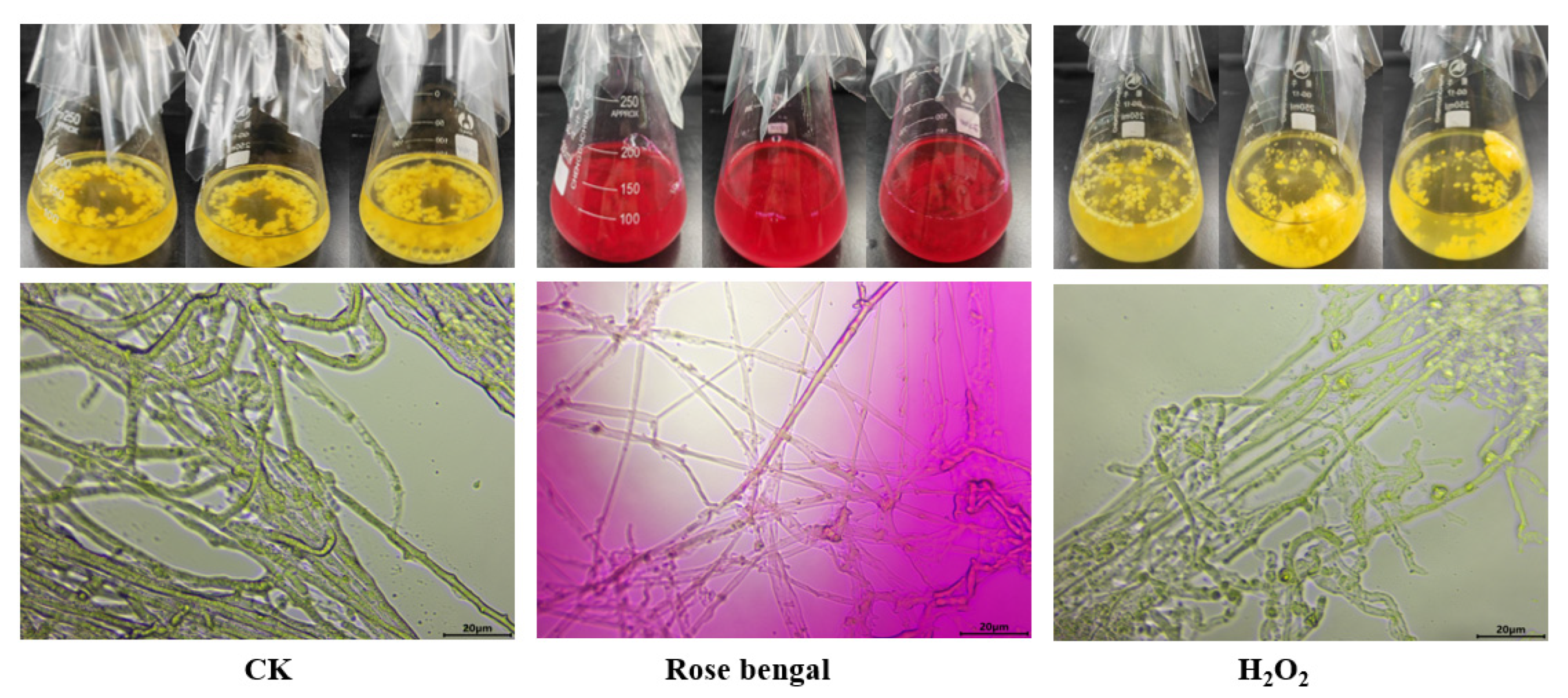
2.3. Morphological Observation of Mycelium Under Stress Conditions
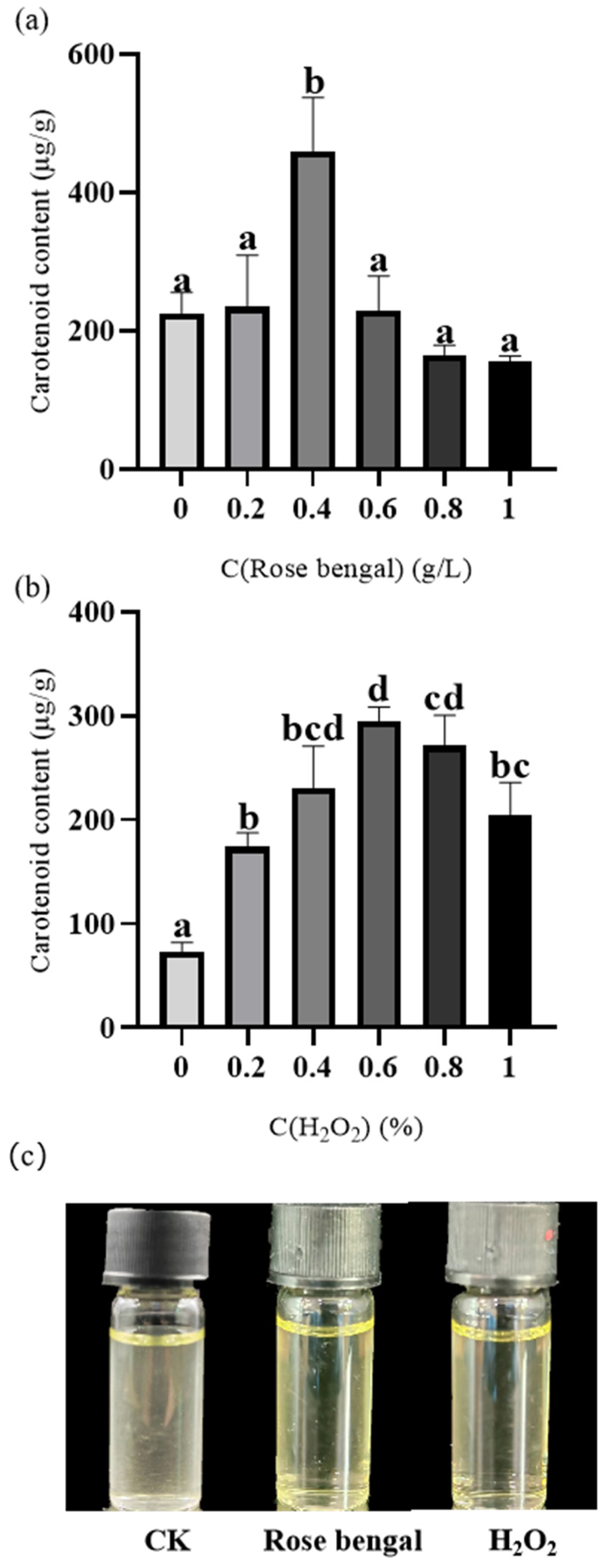
2.4. Determination of Carotenoids
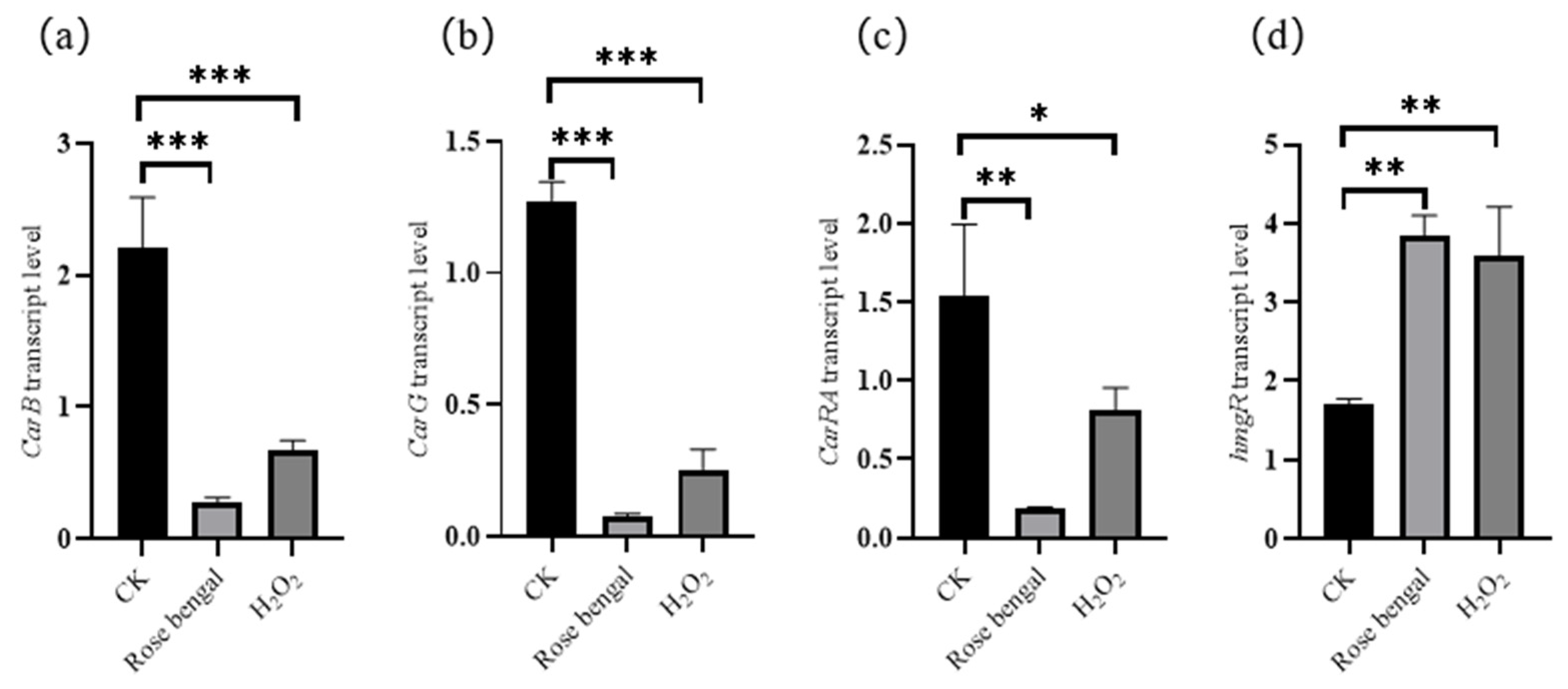
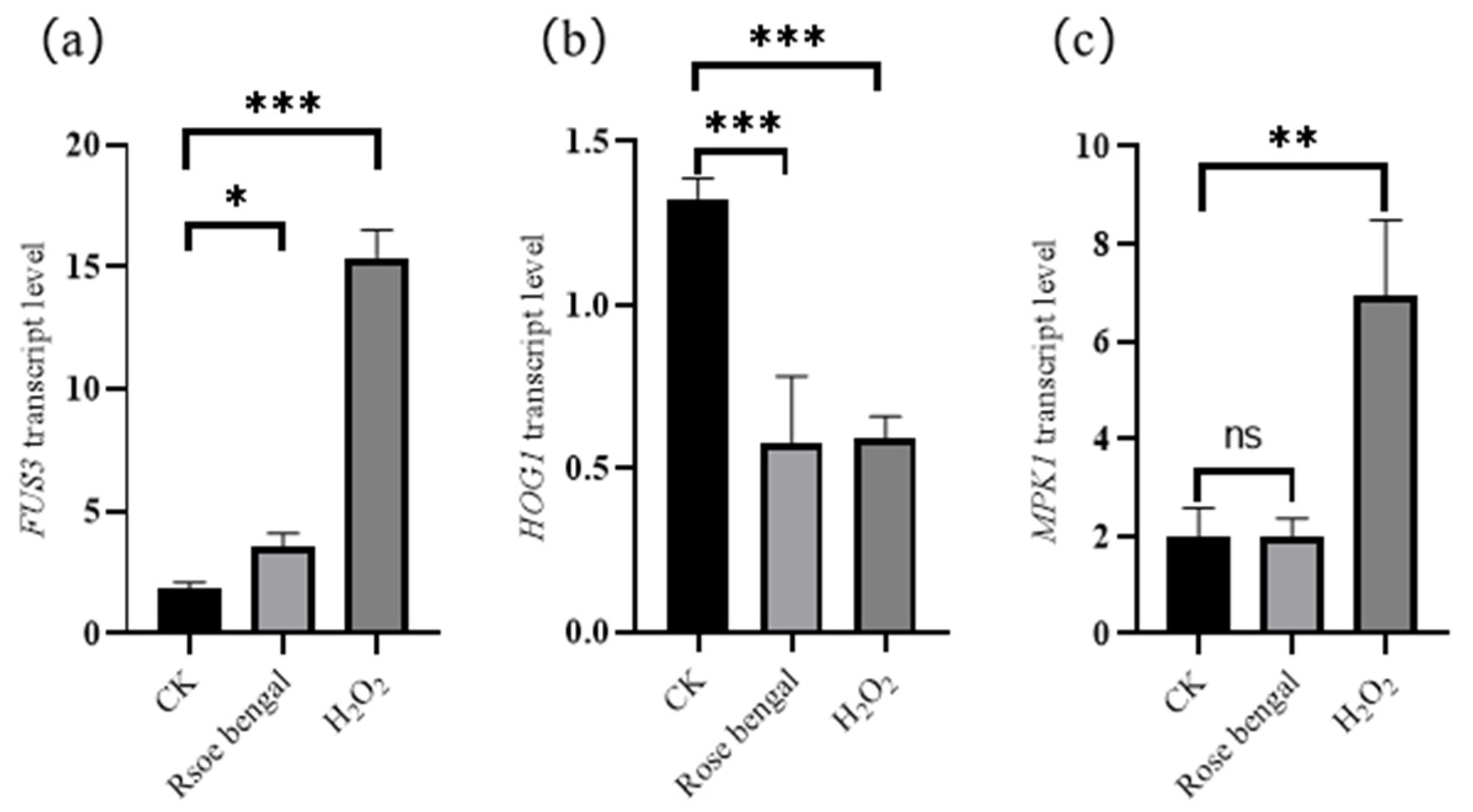
2.5. Gene Expression Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Carotenoid Content of B. trispora Under Different Stress Conditions
3.2. Changes in Mycelial Morphology Under Stress
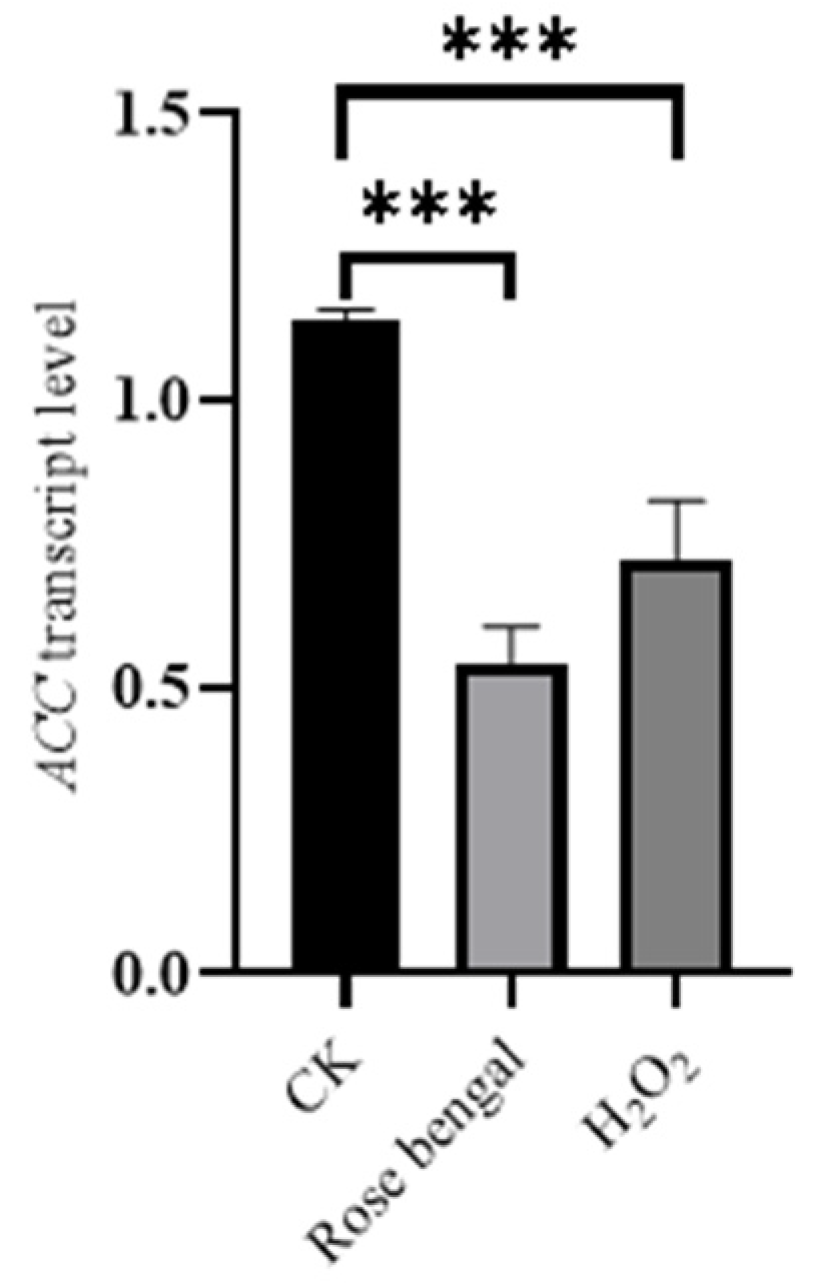
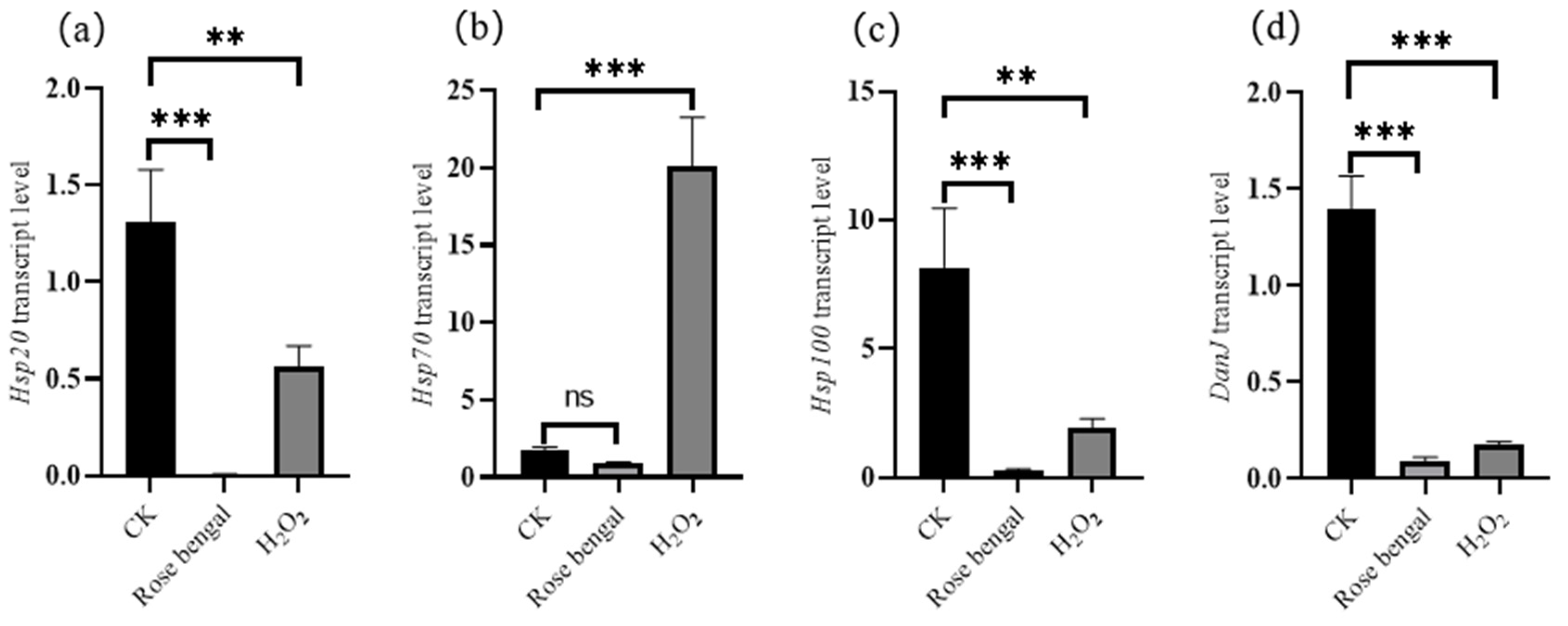
3.3. Expression of Genes Associated with Carotenoid Accumulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Swofford, C.A.; Sinskey, A.J. Modular engineering for microbial production of carotenoids. Metab. Eng. Commun. 2020, 10, e00118. [Google Scholar] [CrossRef] [PubMed]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid metabolism in plants. Mol. Plant 2015, 8, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Rao, S.; Zhou, X.; Li, L. Plant carotenoids: Recent advances and future perspectives. Mol. Hortic. 2022, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef]
- Ram, S.; Mitra, M.; Shah, F.; Tirkey, S.R.; Mishra, S. Bacteria as an alternate biofactory for carotenoid production: A review of its applications, opportunities and challenges. J. Funct. Foods 2020, 67, 103867. [Google Scholar] [CrossRef]
- Yao, Y.; Goh, H.M.; Kim, J.E. The roles of carotenoid consumption and bioavailability in cardiovascular health. Antioxidants 2021, 10, 1978. [Google Scholar] [CrossRef]
- Sandmann, G. Carotenoids and their biosynthesis in fungi. Molecules 2022, 27, 1431. [Google Scholar] [CrossRef]
- Ye, Z.W.; Jiang, J.G.; Wu, G.H. Biosynthesis and regulation of carotenoids in Dunaliella: Progresses and prospects. Biotechnol. Adv. 2008, 26, 352–360. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, S.L.; Chen, H.Y.; Zou, Y.; Zheng, Q.W.; Guo, L.Q.; Wu, G.H.; Lu, J.; Lin, J.F.; Ye, Z.W. Enhancement of carotenoid production and its regulation in edible mushroom Cordyceps militaris by abiotic stresses. Enzyme Microb. Technol. 2021, 148, 109808. [Google Scholar] [CrossRef]
- Zhao, D.; Li, C. Effects of TiO2 and H2O2 treatments on the biosynthesis of carotenoids and lipids in oleaginous red yeast Rhodotorula glutinis ZHK. LWT-Food Sci. Technol. 2023, 180, 114733. [Google Scholar] [CrossRef]
- Fischer, B.B.; Krieger-Liszkay, A.; Eggen, R.I. Oxidative stress induced by the photosensitizers neutral red (type I) or rose bengal (type II) in the light causes different molecular responses in Chlamydomonas reinhardtii. Plant Sci. 2005, 168, 747–759. [Google Scholar] [CrossRef]
- Schroeder, W.A.; Johnson, E.A. Singlet oxygen and peroxyl radicals regulate carotenoid biosynthesis in Phaffia rhodozyma. J. Biol. Chem. 1995, 270, 18374–18379. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Wang, Y.C.; Chen, X.; Gao, N.; Wu, Y.; Zhang, H.F. Protoplast fusion between Blakeslea trispora 14,271 (+) and 14,272 (−) enhanced the yield of lycopene and β-carotene. World J. Microbiol. Biotechnol. 2021, 37, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mantzouridou, F.T.; Naziri, E. Scale translation from shaken to diffused bubble aerated systems for lycopene production by Blakeslea trispora under stimulated conditions. Appl. Microbiol. Biotechnol. 2017, 101, 1845–1856. [Google Scholar] [CrossRef]
- Luo, W.; Wang, Y.; Yang, P.; Qu, Y.; Yu, X. Multilevel regulation of carotenoid synthesis by light and active oxygen in Blakeslea trispora. J. Agric. Food Chem. 2021, 69, 10974–10988. [Google Scholar] [CrossRef]
- Giani, M.; Martínez-Espinosa, R.M. Carotenoids as a protection mechanism against oxidative stress in Haloferax mediterranei. Antioxidants 2020, 9, 1060. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.Y.; Cho, E.S.; Kim, S.; Kim, K.; Seo, M.J. Optimization of bacterioruberin production from Halorubrum ruber and assessment of its antioxidant potential. Microb. Cell Factories 2024, 23, 2. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, S.; Liu, H.; Shen, H.; Lin, X.; Yang, F.; Zhou, Y.J.; Jin, G.; Ye, M.; Zou, H.; et al. A multi-omic map of the lipid-producing yeast Rhodosporidium toruloides. Nat. Commun. 2012, 3, 1112. [Google Scholar] [CrossRef]
- Deeba, F.; Kumar, K.K.; Rajacharya, G.H.; Gaur, N.A. Metabolomic profiling revealed diversion of cytidinediphosphate-diacylglycerol and glycerol pathway towards denovo triacylglycerol synthesis in Rhodosporidium toruloides. J. Fungi 2021, 7, 967. [Google Scholar] [CrossRef]
- Xie, Z.T.; Mi, B.Q.; Lu, Y.J.; Chen, M.T.; Ye, Z.W. Research progress on carotenoid production by Rhodosporidium toruloides. Appl. Microbiol. Biotechnol. 2024, 108, 7. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, W.Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. Res. 2015, 35, 600–604. [Google Scholar] [CrossRef]
- Niekerk, L.A.; Gokul, A.; Basson, G.; Badiwe, M.; Nkomo, M.; Klein, A.; Keyster, M. Heavy metal stress and mitogen activated kinase transcription factors in plants: Exploring heavy metal-ROS influences on plant signalling pathways. Plant Cell Environ. 2024, 47, 2793–2810. [Google Scholar] [CrossRef]
- Kumar, K.; Raina, S.K.; Sultan, S.M. Arabidopsis MAPK signaling pathways and their cross talks in abiotic stress response. J. Plant Biochem. Biotechnol. 2020, 29, 700–714. [Google Scholar] [CrossRef]
- Lim, F.-H. Molecular cloning of Ganoderma boninense Hog1-type mitogen-activated protein kinase (MAPK) cDNA and transcriptional response to salinity stress. J. Oil Palm Res. 2018, 30, 380–389. [Google Scholar] [CrossRef]
- Wang, L.; Chen, R.; Weng, Q.; Lin, S.; Wang, H.; Li, L.; Fuchs, B.B.; Tan, X.; Mylonakis, E. SPT20 regulates the Hog1-MAPK pathway and is involved in Candida albicans response to hyperosmotic stress. Front. Microbiol. 2020, 11, 213. [Google Scholar] [CrossRef]
- Prodromou, C.; Aran-Guiu, X.; Oberoi, J.; Perna, L.; Chapple, J.P.; van der Spuy, J. HSP70-HSP90 chaperone networking in protein-misfolding disease. In The Networking of Chaperones by Co-Chaperones; Edkins, A.L., Blatch, G.L., Eds.; Springer: Cham, Switzerland, 2023; pp. 389–425. [Google Scholar] [CrossRef]
- Roy, A.; Tamuli, R. Heat shock proteins and the calcineurin-crz1 signaling regulate stress responses in fungi. Arch. Microbiol. 2022, 204, 240. [Google Scholar] [CrossRef]
- Ohama, N.; Kusakabe, K.; Mizoi, J.; Zhao, H.M.; Kidokoro, S.; Koizumi, S.; Takahashi, F.; Ishida, T.; Yanagisawa, S.; Shinozaki, K.; et al. The transcriptional cascade in the heat stress response of Arabidopsis is strictly regulated at the level of transcription factor expression. Plant Cell 2016, 28, 181–201. [Google Scholar] [CrossRef]
- Wang, H.B.; Luo, J.; Huang, X.Y.; Lu, M.B.; Yu, L.J. Oxidative stress response of Blakeslea trispora induced by H2O2 during β-carotene biosynthesis. J. Ind. Microbiol. Biotechnol. 2014, 41, 555–561. [Google Scholar] [CrossRef]
- Mantzouridou, F.T.; Sferopoulou, E.; Thanou, P. Uncovering the hidden potential of phytoene production by the fungus Blakeslea trispora. Foods 2024, 13, 2882. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, T.; Liu, Y.F.; Wang, X.Y.; Zhang, L.; Ku, T.; Quek, S.Y. Qualitative and quantitative assessment of DNA quality of frozen beef based on DNA yield, gel electrophoresis and PCR amplification and their correlations to beef quality. Food Chem. 2018, 260, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Gong, Z.; Li, N.; Zhao, Y.; Zhang, H.; Yang, X.; Liu, Y.; Rao, Z.; Yu, X. A negative regulator of carotenogenesis in Blakeslea trispora. Appl. Environ. Microbiol. 2020, 86, e02462-02419. [Google Scholar] [CrossRef]
- Zhao, F.; Maren, N.A.; Kosentka, P.Z.; Liao, Y.Y.; Lu, H.; Duduit, J.R.; Huang, D.; Ashrafi, H.; Zhao, T.; Huerta, A.I.; et al. An optimized protocol for stepwise optimization of real-time RT-PCR analysis. Hortic. Res. 2021, 8, 179. [Google Scholar] [CrossRef]
- Steinberg, G.; Peñalva, M.A.; Riquelme, M.; Wösten, H.A.; Harris, S.D. Cell biology of hyphal growth. Microbiol. Spectr. 2017, 5, 1128. [Google Scholar] [CrossRef]
- Ge, X.; Li, R.; Zhang, X.; Zhao, J.; Zhang, Y.; Xin, Q. Transcriptome sequencing and global analysis of blue light-responsive genes provide clues for high carotenoid yields in Blakeslea trispora. Int. Microbiol. 2022, 25, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, K.K. Photoprotection revisited: Genetic and molecular approaches. Annu. Rev. Plant Biol. 1999, 50, 333–359. [Google Scholar] [CrossRef]
- Pagels, F.; Salvaterra, D.; Amaro, H.M.; Guedes, A.C. Pigments from microalgae. In Handbook of Microalgae-Based Processes and Products: Fundamentals and Advances in Energy, Food, Feed, Fertilizer, and Bioactive Compounds; Jacob-Lopes, E., Maroneze, M.M., Queiroz, M.I., Zepka, L.Q., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 465–492. [Google Scholar] [CrossRef]
- Ashokkumar, V.; Flora, G.; Sevanan, M.; Sripriya, R.; Chen, W.H.; Park, J.H.; Rajesh Banu, J.; Kumar, G. Technological advances in the production of carotenoids and their applications- A critical review. Bioresour. Technol. 2023, 367, 128215. [Google Scholar] [CrossRef] [PubMed]
- Krieger-Liszkay, A.; Fufezan, C.; Trebst, A. Singlet oxygen production in photosystem II and related protection mechanism. Photosynth. Res. 2008, 98, 551–564. [Google Scholar] [CrossRef]
- Wang, L.; Yang, T.; Pan, Y.; Shi, L.; Jin, Y.; Huang, X. The metabolism of reactive oxygen species and their effects on lipid biosynthesis of microalgae. Int. J. Mol. Sci. 2023, 24, 11041. [Google Scholar] [CrossRef]
- Liang, M.H.; Zhu, J.; Jiang, J.G. Carotenoids biosynthesis and cleavage related genes from bacteria to plants. Crit. Rev. Food Sci. Nutr. 2018, 58, 2314–2333. [Google Scholar] [CrossRef]
- Liu, C.; Hu, B.; Cheng, Y.L.; Guo, Y.H.; Yao, W.R.; Qian, H. Carotenoids from fungi and microalgae: A review on their recent production, extraction, and developments. Bioresour. Technol. 2021, 337, 125398. [Google Scholar] [CrossRef]
- Sun, T.; Li, L. Toward the ‘golden’ era: The status in uncovering the regulatory control of carotenoid accumulation in plants. Plant Sci. 2020, 290, 110331. [Google Scholar] [CrossRef] [PubMed]
- Turra, D.; Segorbe, D.; Di Pietro, A. Protein kinases in plant-pathogenic fungi: Conserved regulators of infection. Annu. Rev. Phytopathol. 2014, 52, 267–288. [Google Scholar] [CrossRef] [PubMed]
- Manna, M.; Rengasamy, B.; Sinha, A.K. Revisiting the role of MAPK signalling pathway in plants and its manipulation for crop improvement. Plant Cell Environ. 2023, 46, 2277–2295. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, H. ROS regulation in Dunaliella salina by fulvic acid: Induction of enzymes related to the ascorbate-glutathione pathway and antioxidant metabolites. J. Appl. Phycol. 2024, 36, 3231–3241. [Google Scholar] [CrossRef]
- Lamers, P.P.; van de Laak, C.C.; Kaasenbrood, P.S.; Lorier, J.; Janssen, M.; De Vos, R.C.; Bino, R.J.; Wijffels, R.H. Carotenoid and fatty acid metabolism in light-stressed Dunaliella salina. Biotechnol. Bioeng. 2010, 106, 638–648. [Google Scholar] [CrossRef]
- Cheng, D.; Liu, H.; Zhang, H.; Soon, T.K.; Ye, T.; Li, S.; Ma, H.; Zheng, H. Differential expressions of HSP70 gene between golden and brown noble scallops Chlamys nobilis under heat stress and bacterial challenge. Fish Shellfish Immunol. 2019, 94, 924–933. [Google Scholar] [CrossRef]
- Haak, D.C.; Fukao, T.; Grene, R.; Hua, Z.; Ivanov, R.; Perrella, G.; Li, S. Multilevel regulation of abiotic stress responses in plants. Front. Plant Sci. 2017, 8, 1564. [Google Scholar] [CrossRef]
- Mo, S.; Qian, Y.; Zhang, W.; Qian, L.; Wang, Y.; Cailin, G.; Ding, H. Mitogen-activated protein kinase action in plant response to high-temperature stress: A mini review. Protoplasma 2021, 258, 477–482. [Google Scholar] [CrossRef]
- Kou, Y.P.; Liu, M.J.; Sun, P.P.; Dong, Z.Q.; Liu, J. High light boosts salinity stress-induced biosynthesis of astaxanthin and lipids in the green alga Chromochloris zofingiensis. Algal Res. 2020, 50, 101976. [Google Scholar] [CrossRef]
- Andrasi, N.; Rigo, G.; Zsigmond, L.; Perez-Salamo, I.; Papdi, C.; Klement, E.; Pettko-Szandtner, A.; Baba, A.I.; Ayaydin, F.; Dasari, R.; et al. The mitogen-activated protein kinase 4-phosphorylated heat shock factor A4A regulates responses to combined salt and heat stresses. J. Exp. Bot. 2019, 70, 4903–4918. [Google Scholar] [CrossRef]
- Åkerfelt, M.; Morimoto, R.I.; Sistonen, L. Heat shock factors: Integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 2010, 11, 545–555. [Google Scholar] [CrossRef]
- Carmona-Gutierrez, D.; Bauer, M.A.; Zimmermann, A.; Aguilera, A.; Austriaco, N.; Ayscough, K.; Balzan, R.; Bar-Nun, S.; Barrientos, A.; Belenky, P. Guidelines and recommendations on yeast cell death nomenclature. Microb. Cell 2018, 5, 4–31. [Google Scholar] [CrossRef]
- Choi, Y.E.; Rhee, J.K.; Kim, H.S.; Ahn, J.W.; Hwang, H.; Yang, J.W. Chemical genetics approach reveals importance of cAMP and MAP kinase signaling to lipid and carotenoid biosynthesis in microalgae. J. Microbiol. Biotechnol. 2015, 25, 637–647. [Google Scholar] [CrossRef]
| Gene | Description | GenBank Accession Number | Primer Sequence (Forward/Reverse) |
|---|---|---|---|
| Tef1 | elongation factor 1-alpha | AF157235 | AACTCGGTAAGGGTTCCTTCAAG CGGGAGCATCAATAACGGTAAC |
| CarB | phytoene dehydrogenase | AY176663 | CGCTTGCACTTGTTTGGTAAGATC CAACCATGTTGAAACCACCACG |
| CarG | geranylgeranyl pyrophosphate synthase | JQ289995 | CCCAAGGACGATTTGATCGTC CATGTTGGGCTTGTTCAACTTGG |
| CarRA | bifunctional lycopene cyclase/phytoene synthase | AY17666 | CCCAACACTTCTTGGAAGCAAAC GCCACAGGTCGACGCAAGA |
| HMGR | 3-hydroxy-3-methylglutaryl-coenzyme A reductase | KAI8378970 | GATCTCCCGTGACTCTCGTAC CCTTCCGTAGTTGCCATGGG |
| FUS3 | mitogen-activated protein kinase | JAIWNE010000030 BD560DRAFT_397017 | CGCTGGTATCGTGCACCTG CATCCATTGTAGGTGTGCCGAG |
| HOG1 | mitogen-activated protein kinase | JAIWNE010000026 BD560DRAFT_454271 | GTGTCTACCAGATACTATCGTGC GCAATCAAATCATCGGATGGTG |
| HSP20 | heat shock protein 20 | JAIWNE010000004 BD560DRAFT_383523 | CCAGCCACGGATATGATTGAG GTTTTTCATTCGCATCCTTAGGC |
| HSP70 | heat shock protein 70 | JAIWNE010000001 BD560DRAFT_380704 | CTATGGCACTGTGATTGGTATCG CAGTACGTGTAGGGTTAGCAGAG |
| HSP100 | heat shock protein 100 | JAIWNE010000035 BD560DRAFT_398661 | CCAACTTTTGCTTCAAGTCACGG GAACGTAAACGCTTCCAACTCG |
| MPK1 | mitogen-activated protein kinase | JAIWNE010000044 BD560DRAFT_401363 | CTTCACCTGAGGGTAATGCTGG CCTTTGAAGAGAGGTCGGCC |
| DanJ | DanJ domain protein | JAIWNE010000001 BD560DRAFT_380444 | GCCTACGAAATTCTATCTGATCC GTAGGTCTGTCCATCATGAAAGGC |
| ACC | Acetyl-CoA carboxylase | JAIWNE010000022 BD560DRAFT_446170 | CCCCAGACTTCAAGTTGAGCATC GTAGGACGACGATGGCTTGTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, J.; Chen, Y.; Lin, S.; Shao, Y.; Zou, Y.; Zheng, Q.; Guo, L.; Lin, J.; Chen, M.; Ye, Z. Molecular Regulation of Carotenoid Accumulation Enhanced by Oxidative Stress in the Food Industrial Strain Blakeslea trispora. Foods 2025, 14, 1452. https://doi.org/10.3390/foods14091452
Deng J, Chen Y, Lin S, Shao Y, Zou Y, Zheng Q, Guo L, Lin J, Chen M, Ye Z. Molecular Regulation of Carotenoid Accumulation Enhanced by Oxidative Stress in the Food Industrial Strain Blakeslea trispora. Foods. 2025; 14(9):1452. https://doi.org/10.3390/foods14091452
Chicago/Turabian StyleDeng, Jiawei, Yuyang Chen, Siting Lin, Yilu Shao, Yuan Zou, Qianwang Zheng, Liqiong Guo, Junfang Lin, Moutong Chen, and Zhiwei Ye. 2025. "Molecular Regulation of Carotenoid Accumulation Enhanced by Oxidative Stress in the Food Industrial Strain Blakeslea trispora" Foods 14, no. 9: 1452. https://doi.org/10.3390/foods14091452
APA StyleDeng, J., Chen, Y., Lin, S., Shao, Y., Zou, Y., Zheng, Q., Guo, L., Lin, J., Chen, M., & Ye, Z. (2025). Molecular Regulation of Carotenoid Accumulation Enhanced by Oxidative Stress in the Food Industrial Strain Blakeslea trispora. Foods, 14(9), 1452. https://doi.org/10.3390/foods14091452






