The Anti-Inflammatory Effects and Molecular Mechanism of Citri Reticulatae Pericarpium Essential Oil: A Combined GC-MS and Network Pharmacology Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Essential Oils
2.2. Cell Culture
2.3. LPS-Induced Inflammatory Response in RAW 264.7 Cells
2.4. Cell Viability Assay (CCK-8)
2.5. Measurement of Inflammatory Factors
2.6. Real-Time Quantitative PCR (RT-qPCR) Analyses
2.7. Gas Chromatography-Mass Spectrometry (GCMS) Analysis
2.8. Network Pharmacology Analysis
2.9. Molecular Docking
2.10. Molecular Dynamics (MD) Simulation
2.11. Statistical Analysis
3. Results and Discussion
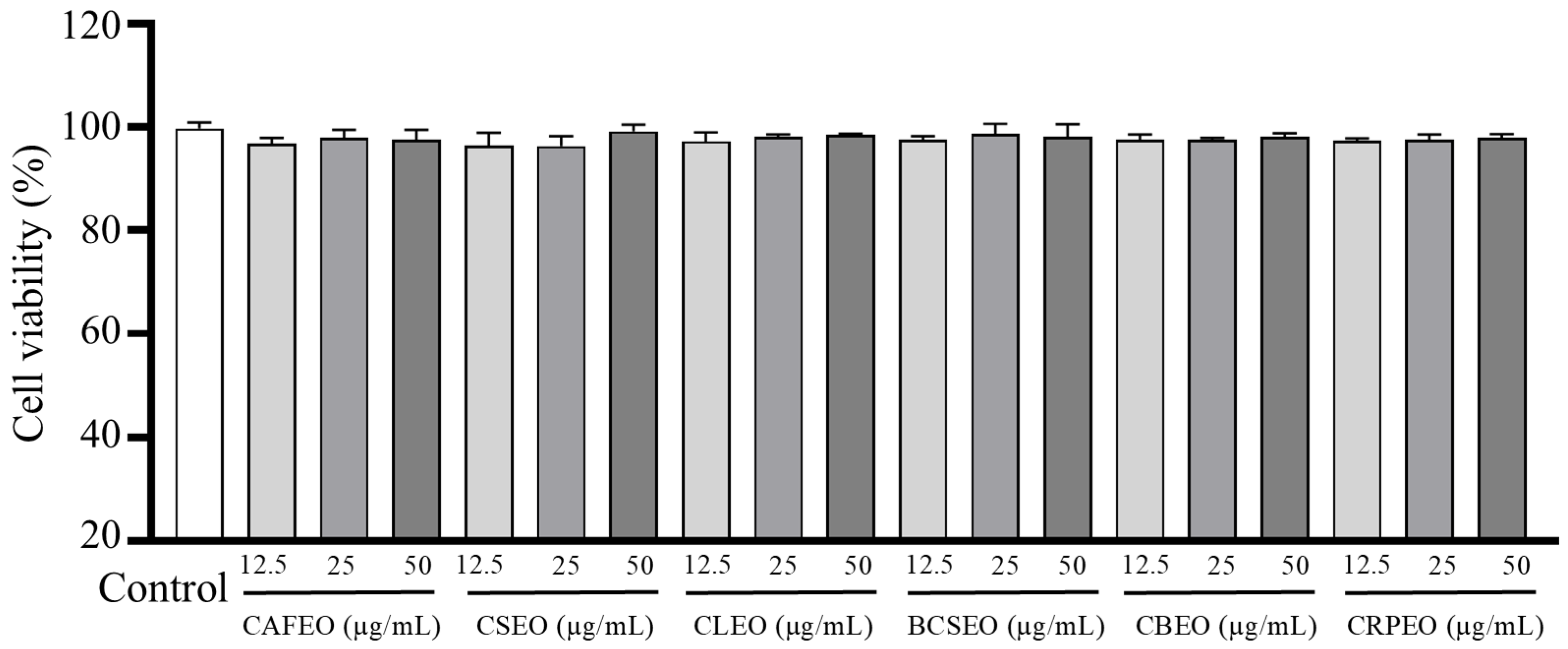
3.1. Cytotoxicity
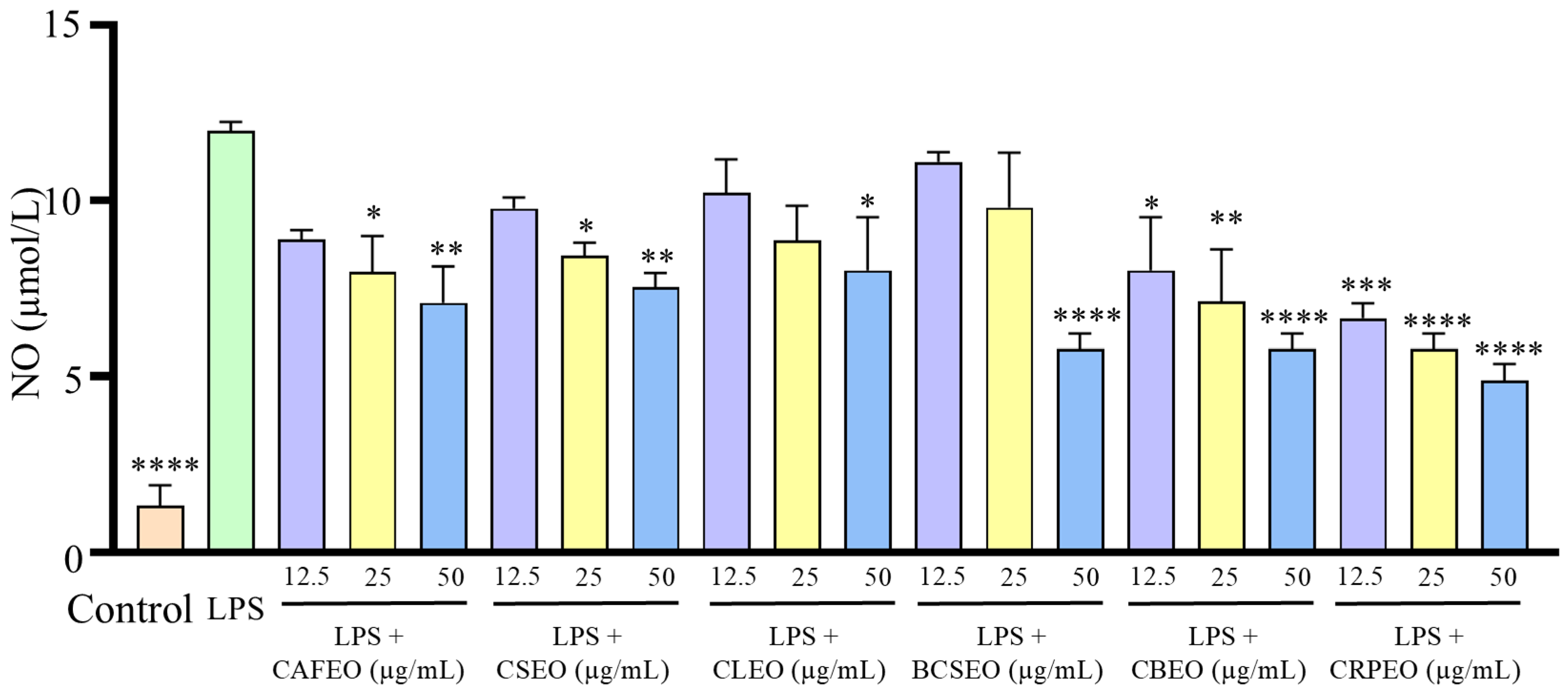
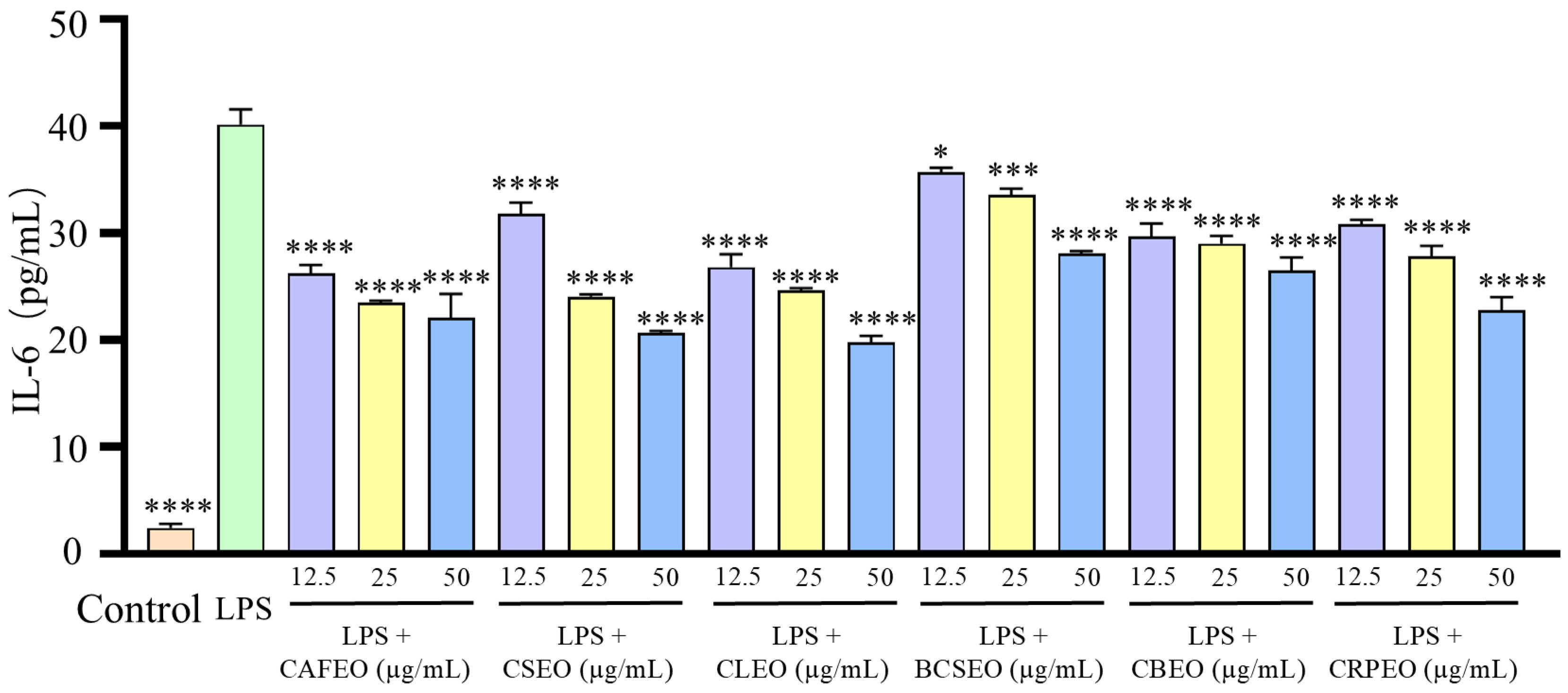
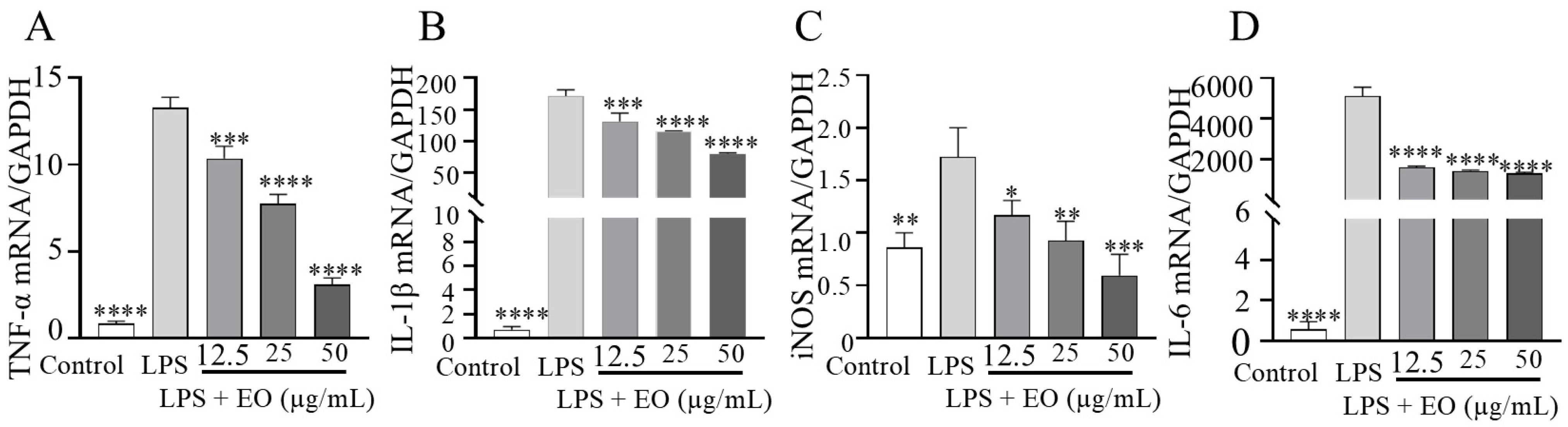
3.2. LPS-Induced Inflammation in RAW 264.7 Cells
3.3. Chemical Components of Rutaceae Essential Oils
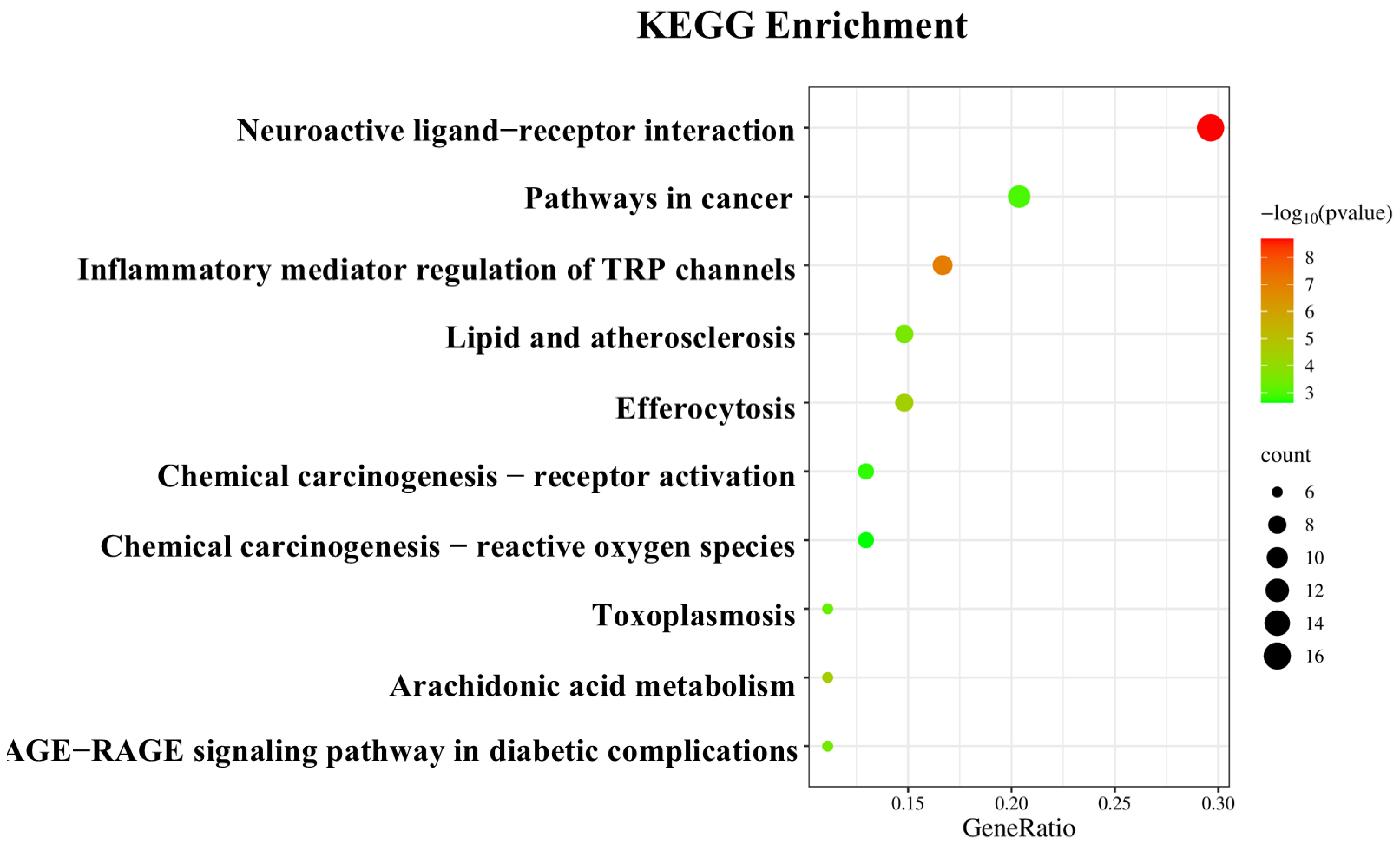
3.4. “Component–Target Pathway” of the Anti-Inflammatory Effects of Essential Oils
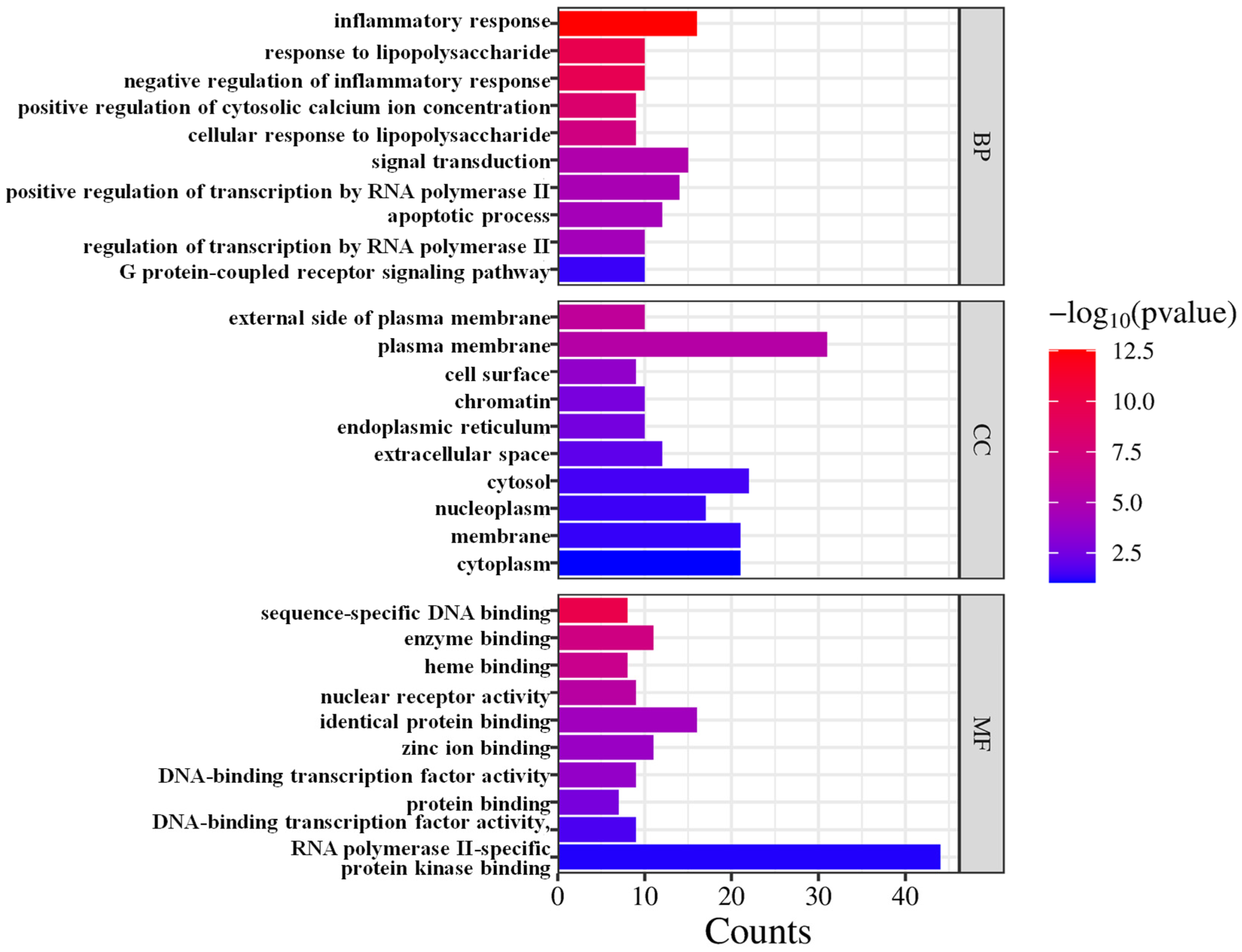
3.5. GO Enrichment Analysis
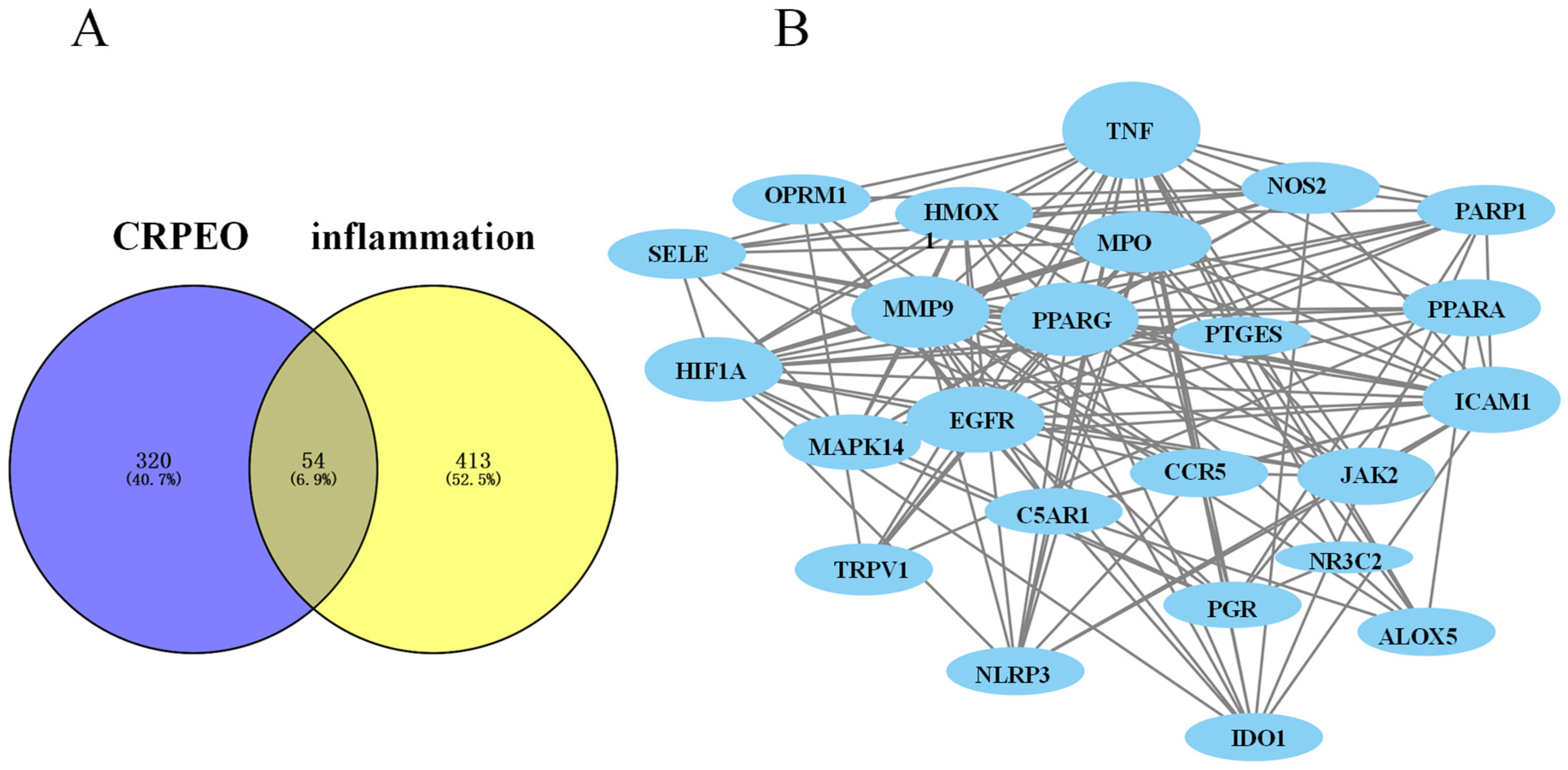
3.6. Protein–Protein Interaction (PPI) Network Analysis
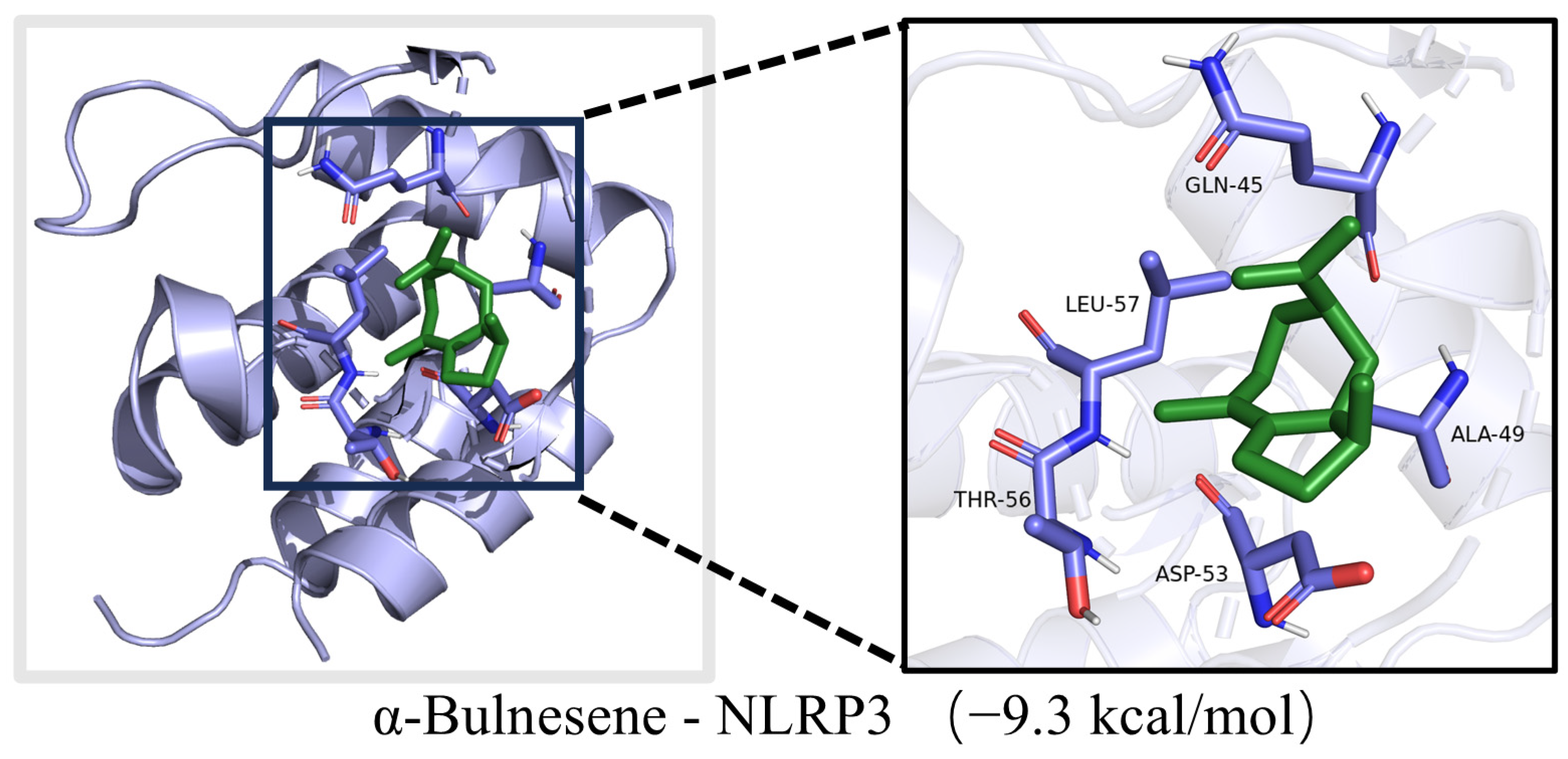
3.7. Molecular Docking Analysis
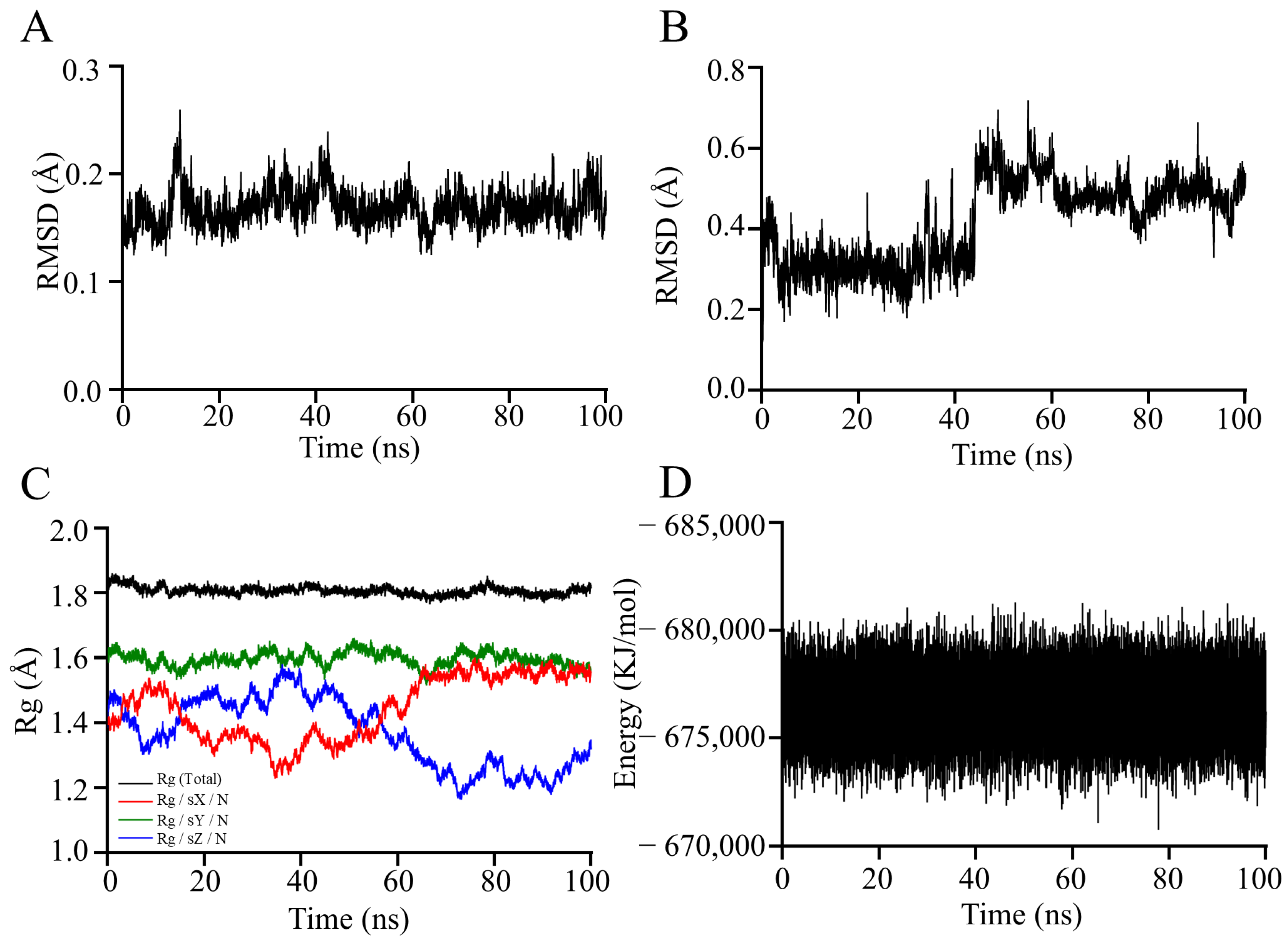
3.8. Molecular Dynamics Simulation Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GC-MS | gas chromatography-mass spectrometry |
| TNF-α | tumor necrosis factor (TNF)-α |
| IL-6 | interleukin (IL)-6 |
| NO | nitric oxide |
| iNOS | inducible nitric oxide synthase |
| NLRP3 | NLR Family Pyrin Domain-Containing 3 |
| HaCaT | human epidermal keratinocytes |
| DMEM | Dulbecco’s modified Eagle medium |
| FBS | fetal bovine serum |
| LPS | lipopolysaccharide |
| CCK-8 | Cell Counting Kit-8 |
| ELISA | enzyme-linked immunosorbent assay |
| RT-qPCR | real-time quantitative PCR |
| STP | SwissTargetPrediction |
| PPI | protein–protein interaction |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| 3D | three-dimensional |
| PDB | Protein Data Bank |
| SD | standard deviation |
| EOs | essential oils |
| CAFEO | Citrus aurantium flower essential oil |
| CSEO | Citrus sinensis essential oil |
| CLEO | Citrus limon essential oil |
| BCSEO | Brazilian Citrus sinensis essential oil |
| CBEO | Citrus bergamia essential oil |
| CRPEO | Citri Reticulatae Pericarpium essential oil |
| CB2 | cannabinoid receptor type 2 |
| COX-2 | cyclooxygenase-2 |
Appendix A
| No. | Compound | Content (%) | RI | RT (min) | |
|---|---|---|---|---|---|
| Measured | Documented | ||||
| 1 | Pseudolimonene | 3.09 | 1002 | 1004 | 3.878 |
| 2 | 4-Thujanol | 1.20 | 1065 | 1070 | 4.05 |
| 3 | D-Limonene | 57.77 | 1031 | 1028 | 4.275 |
| 4 | γ-Terpinene | 11.29 | 1057 | 1059 | 4.55 |
| 5 | Terpinolene | 5.50 | 1086 | 1089 | 4.76 |
| 6 | Fenchol | 0.38 | 1123 | 1121 | 5.083 |
| 7 | 1-Terpineol | 0.61 | 1136 | 1140 | 5.206 |
| 8 | β-Terpineol | 0.50 | 1151 | 1153 | 5.358 |
| 9 | Terpinen-4-ol | 0.43 | 1176 | 1180 | 5.75 |
| 10 | α-Terpineol | 3.93 | 1186 | 1190 | 5.965 |
| 11 | α-Terpinyl acetate | 0.73 | 1330 | 1328 | 5.999 |
| 12 | Citral | 0.51 | 1245 | 1242 | 6.803 |
| 13 | Neryl Acetate | 0.70 | 1360 | 1362 | 8.531 |
| 14 | α-Bergamotene | 1.67 | 1435 | 1433 | 9.707 |
| 15 | α-Himachalene | 2.78 | 1452 | 1448 | 11.157 |
| 16 | δ-Tocopherol | 0.54 | 2951 | 2953 | 35.945 |
| 17 | Farnesol | 5.44 | 1709 | 1711 | 36.587 |
| 18 | γ-Tocopherol | 0.84 | 3051 | 3055 | 37.346 |
| 19 | α-Tocopherol | 0.37 | 3110 | 3112 | 38.345 |
| Total (%) | 98.28 | ||||
| No. | Compound | Content (%) | RI | RT (min) | |
|---|---|---|---|---|---|
| Measured | Documented | ||||
| 1 | 3-Carene | 0.5 | 1007 | 1009 | 3.976 |
| 2 | D-Limonene | 35.06 | 1031 | 1028 | 4.163 |
| 3 | Isopulegol | 0.18 | 1151 | 1146 | 4.55 |
| 4 | Dihydrocarveol | 0.4 | 1187 | 1190 | 4.314 |
| 5 | Linalool | 4.04 | 1100 | 1101 | 4.799 |
| 6 | p-Mentha-2,8-dienol | 11.61 | 1081 | 1078 | 5.216 |
| 7 | 5-Caranol | 0.53 | 1281 | 1286 | 5.323 |
| 8 | Chrysanthenol | 0.9 | 1162 | 1164 | 5.377 |
| 9 | Carveol | 12.44 | 1204 | 1200 | 5.745 |
| 10 | Pseudocarveol | 0.77 | 1191 | 1186 | 5.877 |
| 11 | Thujyl Alcohol | 5.55 | 1148 | 1146 | 5.617 |
| 12 | Isopiperitenol | 1.72 | 1754 | 1752 | 5.745 |
| 13 | Perillyl Alcohol | 0.35 | 1291 | 1294 | 6.097 |
| 14 | D-Carvone | 3.22 | 1241 | 1245 | 6.666 |
| 15 | Citral | 1.71 | 1245 | 1242 | 6.857 |
| 16 | Perillal | 0.79 | 1274 | 1276 | 7.101 |
| 17 | Cyclohexanol | 1.18 | 851 | 849 | 7.273 |
| 18 | α-Copaene | 0.56 | 1374 | 1372 | 8.728 |
| 19 | β-Copaene | 1.13 | 1421 | 1424 | 8.919 |
| 20 | Valencene | 2.1 | 1474 | 1479 | 10.941 |
| 21 | δ-Cadinene | 0.73 | 1483 | 1486 | 11.372 |
| 22 | Spathulenol | 0.42 | 1576 | 1578 | 8.326 |
| 23 | Sesquibenihiol | 0.84 | 1786 | 1788 | 8.458 |
| 24 | α-terpinene | 0.19 | 1014 | 1016 | 19.963 |
| 25 | Elaidic Acid Methyl Ester | 0.72 | 2081 | 2084 | 22.823 |
| 26 | Tangeretin | 2.6 | 3196 | 3198 | 38.781 |
| 27 | Stigmasterol | 0.54 | 3174 | 3170 | 39.893 |
| Total (%) | 90.78 | ||||
| No. | Compound | Content (%) | RI | RT (min) | |
|---|---|---|---|---|---|
| Measured | Documented | ||||
| 1 | Pseudolimonene | 0.48 | 1002 | 1004 | 3.952 |
| 2 | Terpilene | 3.1 | 1019 | 1016 | 4.04 |
| 3 | D-Limonene | 21.74 | 1031 | 1028 | 4.099 |
| 4 | γ-Terpinene | 3.28 | 1057 | 1059 | 4.182 |
| 5 | α-Fenchene | 30.19 | 943 | 947 | 4.261 |
| 6 | Isoterpinene | 5.39 | 1057 | 1083 | 4.437 |
| 7 | Linalyl Formate | 15.46 | 1216 | 1219 | 4.53 |
| 8 | Fenchol | 1.86 | 1123 | 1121 | 4.711 |
| 9 | Chrysanthenol | 1.68 | 1162 | 1164 | 4.902 |
| 10 | α-Terpineol | 3.07 | 1186 | 1190 | 5.23 |
| 11 | Verbenol | 0.57 | 1152 | 1145 | 5.402 |
| 12 | Citral | 3 | 1245 | 1242 | 5.617 |
| 13 | Neryl Acetate | 1.02 | 1360 | 1362 | 6.024 |
| 14 | Myrac Aldehyde | 0.94 | 1506 | 1509 | 6.778 |
| Total (%) | 91.78 | ||||
| No. | Compound | Content (%) | RI | RT (min) | |
|---|---|---|---|---|---|
| Measured | Documented | ||||
| 1 | 3-Carene | 0.78 | 1007 | 1009 | 3.976 |
| 2 | D-Limonene | 34.6 | 1031 | 1028 | 4.192 |
| 3 | Linalool | 7.07 | 1100 | 1101 | 4.809 |
| 4 | trans-Mentha-2,8-dien-1-ol | 1.29 | 1126 | 1123 | 5.078 |
| 5 | trans-p-Mentha-2,8-dienol | 0.56 | 1075 | 1078 | 5.186 |
| 6 | p-Mentha-2,8-dien-1-ol | 1.47 | 1137 | 1134 | 5.23 |
| 7 | Neoisopulegol | 0.78 | 1141 | 1144 | 5.304 |
| 8 | α-Terpineol | 6.97 | 1186 | 1190 | 5.999 |
| 9 | Carveol | 4.55 | 1204 | 1200 | 6.034 |
| 10 | (+)-3-Carene | 0.5 | 991 | 995 | 6.504 |
| 11 | Linalyl acetate | 0.77 | 1249 | 1251 | 6.558 |
| 12 | Carvone | 1.07 | 1247 | 1243 | 6.602 |
| 13 | Citral | 2.21 | 1245 | 1242 | 6.856 |
| 14 | Perillal | 0.84 | 1274 | 1276 | 7.082 |
| 15 | Estragole | 0.48 | 1196 | 1200 | 7.165 |
| 16 | p-Mentha-1(7),8(10)-dien-9-ol | 0.85 | 1281 | 1287 | 7.243 |
| 17 | Isoanethole | 0.64 | 1195 | 1199 | 7.395 |
| 18 | α-Copaene | 0.98 | 1374 | 1372 | 8.722 |
| 19 | Cyclododecanol | 1.64 | 1574 | 1575 | 9.173 |
| 20 | β-Guaiene | 0.62 | 1486 | 1490 | 10.921 |
| 21 | 2,5-Di-Tert-Butylphenol | 1.51 | 1509 | 1514 | 10.991 |
| 22 | δ-Cadinene | 1.85 | 1483 | 1486 | 11.382 |
| 23 | γ-himachalene | 0.78 | 1486 | 1482 | 12.621 |
| 24 | β-Costol | 0.45 | 1774 | 1778 | 12.753 |
| 25 | β-Sinensal | 1.55 | 1690 | 1694 | 14.908 |
| 26 | α-Sinensal | 1.2 | 1746 | 1748 | 16.04 |
| 27 | Docosahexaenoic acid methyl ester | 0.43 | 2410 | 2413 | 17.21 |
| 28 | Hexadecanoic acid, methyl ester | 0.94 | 1925 | 1927 | 19.522 |
| 29 | Elaidic Acid Methyl Ester | 3.52 | 2081 | 2084 | 22.852 |
| 30 | Methyl Lineoleate | 0.42 | 2088 | 2092 | 24.4 |
| 31 | Squalene Oxide | 0.48 | 2695 | 2693 | 34.206 |
| 32 | Tangeretin | 5.79 | 3196 | 3198 | 38.825 |
| 33 | Scutellarein Tetramethyl Ether | 2.26 | 3183 | 3186 | 38.879 |
| 34 | Stigmasterol | 0.92 | 3174 | 3170 | 39.893 |
| Total (%) | 90.77 | ||||
| No. | Compound | Content (%) | RI | RT (min) | |
|---|---|---|---|---|---|
| Measured | Documented | ||||
| 1 | Isomenthone | 10.62 | 1174 | 1170 | 5.485 |
| 2 | α-Terpineol | 1.3 | 1186 | 1190 | 5.642 |
| 3 | Citronellol | 29.56 | 1241 | 1237 | 5.955 |
| 4 | Nerol | 9.02 | 1258 | 1254 | 6.455 |
| 5 | 3,7-Dimethyloct-6-enyl ethyl carbonate | 5.99 | 1517 | 1514 | 6.744 |
| 6 | Myrtanol | 0.41 | 1253 | 1257 | 6.93 |
| 7 | Geranyl Ethyl Carbonate | 0.99 | 1549 | 1553 | 7.038 |
| 8 | Guaia-6,9-Diene | 11.52 | 1443 | 1447 | 7.268 |
| 9 | 3-Carene | 0.35 | 1007 | 1009 | 8.056 |
| 10 | α-Copaene | 0.92 | 1374 | 1372 | 8.169 |
| 11 | β-Bourbonene | 2.83 | 1381 | 1384 | 8.556 |
| 12 | α-Guaiene | 1.05 | 1440 | 1442 | 8.909 |
| 13 | γ-himachalene | 3.85 | 1486 | 1482 | 9.58 |
| 14 | α-Muurolene | 1.77 | 1468 | 1469 | 9.966 |
| 15 | β-copaene | 0.41 | 1427 | 1424 | 10.03 |
| 16 | Ledene | 2.58 | 1497 | 1495 | 10.099 |
| 17 | γ-Cadinene | 0.45 | 1517 | 1513 | 10.26 |
| 18 | T-Cadinol | 3.65 | 1645 | 1640 | 10.334 |
| 19 | Calamenene | 1.47 | 1518 | 1522 | 10.495 |
| 20 | Neryl Isobutyrate | 1.85 | 1514 | 1511 | 10.633 |
| 21 | Sesquibenihiol | 0.78 | 1786 | 1788 | 10.721 |
| 22 | Dehydrosaussurea Lactone | 0.36 | 1836 | 1838 | 11.005 |
| 23 | Geranyl tiglate | 2.3 | 1699 | 1703 | 11.313 |
| Total (%) | 94.03 | ||||
References
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and Cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease Across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, A.; Zulkipli, M.A.; Mohd Hatta, F.H.; Jahidin, A.H.; Abdul Nasir, N.A.; Hazizul Hasan, M. Therapeutic Potentials of Iridoids Derived from Rubiaceae against in Vitro and in Vivo Inflammation: A Scoping Review. Saudi Pharm. J. 2024, 32, 101876–101894. [Google Scholar] [CrossRef] [PubMed]
- De Amorim, M.S.; Verdan, M.H.; Oliveira, C.S.; Santos, A.D.C. Essential Oils of Neotropical Myrtaceae Species from 2011 until 2023: An Update. Chem. Biodivers. 2025, 22, e202401503. [Google Scholar] [CrossRef]
- Pham, T.V.; Hoang, T.X.; Do, B.H.; Nguyen, K.T.; Nguyen, N.H.; Tran, G. Chemical Compositions, Molecular Docking, Anti-Inflammatory, and Anti-Cancer Effects of the Leaf Essential Oils Isolated from Three Species of the Rutaceae Family in Vietnam. Chem. Biodivers. 2025, 22, e202401466. [Google Scholar] [CrossRef]
- Santos, A.R.; De Paula, V.F.; Barbosa, L.C.A. Conchocarpus J. C. Mikan (Rutaceae): Chemical Constituents and Biological Activities. Chem. Biodivers. 2025, 22, e202402119. [Google Scholar] [CrossRef]
- Sharma, M.S.; Sharma, D.S.; Singh, M.S.; Pathak, M.S. A Review: Citrus Plants-a Gift of Nature. World J. Pharm. Pharm. Sci. 2020, 9, 551–560. [Google Scholar]
- Nahar, L.; El-Seedi, H.R.; Khalifa, S.A.M.; Mohammadhosseini, M.; Sarker, S.D. Ruta Essential Oils: Composition and Bioactivities. Molecules 2021, 26, 4766. [Google Scholar] [CrossRef]
- Boudjema, K.; Mouhouche, M.; Guerdouba, A.; Hali, L. Composition, physicochemical analysis, antimicrobial and anti-inflammatory activities of the essential oils obtained from Ruta chalepensis L. growing wild in northern Algeria. J. Chem. Soc. Pak. 2018, 40, 1054–1062. [Google Scholar]
- Anandakumar, P.; Kamaraj, S.; Vanitha, M.K. D-limonene: A Multifunctional Compound with Potent Therapeutic Effects. Food Biochem. 2021, 45, e13566. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, L.; Wang, S.; Gao, Y.; Jin, F. Molecular Mechanism of the Anti-Inflammatory Effects of Plant Essential Oils: A Systematic Review. J. Ethnopharmacol. 2023, 301, 115829–115852. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Li, Z.; Sun, Y.; Zhang, Y.; Wang, S.; Zhang, Q.; Cai, T.; Xiang, W.; Zeng, C.; Tang, J. D-Limonene: Promising and Sustainable Natural Bioactive Compound. Appl. Sci. 2024, 14, 4605. [Google Scholar] [CrossRef]
- Sajid, A.; Sarfraz, R.; Hanif, M.A.; Shahid, M. Evaluation of Chemical Composition and Biological Activities of Citrus Pseudolimon and Citrus Grandis Peel Essential Oils. J.Chem.Soc.Pak. 2016, 38, 266–273. [Google Scholar]
- Aziz, Z.A.A.; Ahmad, A.; Setapar, S.H.M.; Karakucuk, A.; Azim, M.M.; Lokhat, D.; Rafatullah, M.; Ganash, M.; Kamal, M.A.; Ashraf, G.M. Essential Oils: Extraction Techniques, Pharmaceutical and Therapeutic Potential—A Review. Curr. Drug Metab. 2018, 19, 1100–1110. [Google Scholar] [CrossRef]
- Xia, N.; Wang, J.; Jia, Y.; Duan, J.; Wang, X.; Li, J.; Zhou, P.; Xie, Y.; Shi, H.; Zhao, C.; et al. Optimization of the Process of Extracting Essential Oil of Rosemary by Hydro Distillation with Different Auxiliary Methods. LWT 2025, 215, 117266–117278. [Google Scholar] [CrossRef]
- Xiao, S.; Liu, S.; Yu, H.; Xie, Y.; Guo, Y.; Fan, J.; Yao, W. A Study on the Mechanism of the Sedative-Hypnotic Effect of Cin-namomum camphora chvar. Borneol Essential Oil Based on Network Pharmacology. J. Oleo Sci. 2022, 71, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Narke, D.; Kurade, M.; Frey, K.M.; Rajalingam, S.; Siddiquee, A.; Mustafa, S.J.; Ledent, C.; Ponnoth, D.S. Limo-nene-Induced Activation of A2A Adenosine Receptors Reduces Airway Inflammation and Reactivity in a Mouse Model of Asthma. Purinergic Signal. 2020, 16, 415–426. [Google Scholar] [CrossRef]
- Raka, R.N.; Zhiqian, D.; Yue, Y.; Luchang, Q.; Suyeon, P.; Junsong, X.; Hua, W. Pingyin Rose Essential Oil Alleviates LPS-Induced Inflammation in RAW 264.7 Cells via the NF-κB Pathway: An Integrated in Vitro and Network Pharmacology Analysis. BMC Complement. Med. Ther. 2022, 22, 272–288. [Google Scholar] [CrossRef]
- Tian, M.; Wang, Q.; Jia, X.; Tian, Y.; Hong, Y.; Zhou, Y. Chemical Constituents, Antibacterial and Anti-Inflammatory Properties of Pyrus calleryana Dcne. Essential Oil. Ind. Crop. Prod. 2023, 204, 117353–117364. [Google Scholar] [CrossRef]
- Li, H.; Wang, R.; Chen, Y.; Zhao, M.; Lan, S.; Zhao, C.; Li, X.; Li, W. Integrated Network Pharmacology and Pharmacological Investigations to Discover the Active Compounds of Toona Sinensis Pericarps against Diabetic Nephropathy. J. Ethnopharmacol. 2024, 333, 118441–118453. [Google Scholar] [CrossRef]
- Mohanty, S.; Ray, A.; Sahoo, C.; Sahoo, A.; Jena, S.; Panda, P.C.; Nayak, S. Volatile Profiling Coupled with Multivariate Analysis, Antiproliferative and Anti-Inflammatory Activities of Rhizome Essential Oil of Four Hedychium Species from India. J. Ethnopharmacol. 2023, 317, 116835–116852. [Google Scholar] [CrossRef]
- Santos, P.L.; Matos, J.P.S.C.F.; Picot, L.; Almeida, J.R.G.S.; Quintans, J.S.S.; Quintans-Júnior, L.J. Citronellol, a Monoterpene Alcohol with Promising Pharmacological Activities-A Systematic Review. Food Chem. Toxicol. 2019, 123, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Ammar, A.H.; Bouajila, J.; Lebrihi, A.; Mathieu, F.; Romdhane, M.; Zagrouba, F. Chemical composition and in vitro antimicrobial and antioxidant activities of Citrus aurantium L. flowers essential oil (Neroli oil). Pak. J. Biol. Sci. 2012, 15, 1034–1040. [Google Scholar] [CrossRef]
- El Hachlafi, N.; Elbouzidi, A.; Batbat, A.; Taibi, M.; Jeddi, M.; Addi, M.; Naceiri Mrabti, H.; Fikri-Benbrahim, K. Chemical Composition and Assessment of the Anti-Inflammatory, Antioxidant, Cytotoxic and Skin Enzyme Inhibitory Activities of Citrus sinensis (L.) Osbeck Essential Oil and Its Major Compound Limonene. Pharmaceuticals 2024, 17, 1652. [Google Scholar] [CrossRef] [PubMed]
- Bouzenna, H.; Hfaiedh, N.; Giroux-Metges, M.A.; Elfeki, A.; Talarmin, H. Protective effects of essential oil of Citrus limon against aspirin-induced toxicity in IEC-6 cells. Appl. Physiol. Nutr. Metab. 2017, 42, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Camberos, E.; Sanchez-Hernandez, I.M.; Torres-Gonzalez, O.R.; Gallegos-Ortiz, M.D.R.; Méndez-Mona, A.L.; Baez-Moratilla, P.; Flores-Fernandez, J.M. Natural essential oil mix of sweet orange peel, cumin, and allspice elicits anti-inflammatory activity and pharmacological safety similar to non-steroidal anti-inflammatory drugs. Saudi. J. Biol. Sci. 2022, 29, 3830–3837. [Google Scholar] [CrossRef]
- Yu, X.; Sun, S.; Guo, Y.; Liu, Y.; Yang, D.; Li, G.; Lü, S. Citri Reticulatae Pericarpium (Chenpi): Botany, Ethnopharmacology, Phytochemistry, and Pharmacology of a Frequently Used Traditional Chinese Medicine. J. Ethnopharmacol. 2018, 220, 265–282. [Google Scholar] [CrossRef]
- Rasheed, H.A.; Rehman, A.; Chen, X.; Aziz, T.; Al-Asmari, F.; Alhomrani, M.; Alamri, A.S.; Cui, H.; Lin, L. Unveiling the Anti-Listerial Effect of Citrus Bergamia Essential Oil: Mechanism of Membrane Disruption and Anti-Hemolytic Activity. Food Bioscienc. 2024, 61, 104742–104752. [Google Scholar] [CrossRef]
- Ahn, C.; Lee, J.; Park, M.; Kim, J.; Yang, J.; Yoo, Y.; Jeung, E. Cytostatic Effects of Plant Essential Oils on Human Skin and Lung Cells. Exp. Ther. Med. 2020, 19, 2008–2018. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, L.; Liang, Y.; Li, H.; Yuan, D.; Gao, H.; Zeng, M. Comparative Analysis of Essential Oil Components in Pericarpium Citri Reticulatae Viride and Pericarpium Citri Reticulatae by GC-MS Combined with Chemometric Resolution Method. J Pharm. Biomed. Anal. 2008, 46, 66–74. [Google Scholar] [CrossRef]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.-Z.; Xie, X.-Q.; Altmann, K.-H.; Karsak, M.; Zimmer, A. Beta-Caryophyllene is a Dietary Cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef] [PubMed]
- Silva-Filho, S.E.; Wiirzler, L.A.M.; Cavalcante, H.A.O.; Uchida, N.S.; de Souza Silva-Comar, F.M.; Cardia, G.F.E.; da Silva, E.L.; Aguiar, R.P.; Bersani-Amado, C.A.; Cuman, R.K.N. Effect of Patchouli (Pogostemon cablin) Essential Oil on in Vitro and in Vivo Leukocytes Behavior in Acute Inflammatory Response. Biomed. Pharmacother. 2016, 84, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Gu, Y.; Wang, C.; Zhang, J.; Zhang, J.; Wang, G.; Wang, F. A Systematic Review of the Anti-Inflammatory and Immunomodulatory Properties of 16 Essential Oils of Herbs. Evid. Based Complement. Altern. Med. 2020, 2020, 8878927–8878941. [Google Scholar] [CrossRef]
- Lee, S.-E.; Park, S.; Jang, G.Y.; Lee, J.; Moon, M.; Ji, Y.-J.; Jung, J.W.; Nam, Y.; Shin, S.J.; Lee, Y.; et al. Extract of Aster koraiensis Nakai Leaf Ameliorates Memory Dysfunction via Anti-Inflammatory Action. Int. J. Mol. Sci. 2023, 24, 5765. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-Related Inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Li, B.; Yang, X.; Zhang, P.; Guo, J.; Rong, K.; Wang, X.; Cao, X.; Zhou, T.; Zhao, J. Engeletin Alleviates the Inflammation and Apoptosis in Intervertebral Disc Degeneration via Inhibiting the NF-κB and MAPK Pathways. J. Inflamm. Res. 2022, 15, 5767–5783. [Google Scholar] [CrossRef] [PubMed]
- She, H.; He, Y.; Zhao, Y.; Mao, Z. Release the Autophage Brake on Inflammation: The MAPK14/P38α-ULK1 Pedal. Autophagy 2018, 14, 1097–1098. [Google Scholar] [CrossRef]
- Bougaki, M.; Searles, R.J.; Kida, K.; Yu, J.; Buys, E.S.; Ichinose, F. Nos3 Protects Against Systemic Inflammation and Myocardial Dysfunction in Murine Polymicroboal Sepsis. Shock 2010, 34, 281–290. [Google Scholar] [CrossRef]
- Rayego-Mateos, S.; Rodrigues-Diez, R.; Morgado-Pascual, J.L.; Valentijn, F.; Valdivielso, J.M.; Goldschmeding, R.; Ruiz-Ortega, M. Role of Epidermal Growth Factor Receptor (EGFR) and Its Ligands in Kidney Inflammation and Damage. Mediat. Inflamm. 2018, 2018, 8739473–8739495. [Google Scholar] [CrossRef]
- Ahmad, S.B.; Rehman, M.U.; Fatima, B.; Ahmad, B.; Hussain, I.; Ahmad, S.P.; Farooq, A.; Muzamil, S.; Razzaq, R.; Rashid, S.M.; et al. Antifibrotic Effects of D-Limonene (5(1-Methyl-4-[1-Methylethenyl]) Cyclohexane) in CCl(4) Induced Liver Toxicity in Wistar Rats. Environ. Toxicol. 2018, 33, 361–369. [Google Scholar] [CrossRef]
- Ke, Q.; Zhu, J.; Su, D.; Pan, F.; Meng, Q.; Kou, X. Preparation, Physicochemical Characterization, and Computational Studies of Aldehyde Aroma Compounds/Cyclodextrin Inclusion Complexes. Ind. Crop. Prod. 2024, 211, 118245–118262. [Google Scholar] [CrossRef]
- Lobanov, M.I.; Bogatyreva, N.S.; Galzitskaia, O.V. [Radius of gyration is indicator of compactness of protein structure]. Mol. Biol. 2008, 42, 701–706. [Google Scholar] [CrossRef]
- Deng, C.; Cao, C.; Zhang, Y.; Hu, J.; Gong, Y.; Zheng, M.; Zhou, Y. Formation and Stabilization Mechanism of β-Cyclodextrin Inclusion Complex with C10 Aroma Molecules. Food Hydrocoll. 2022, 123, 107013–107025. [Google Scholar] [CrossRef]
- He, Z.-X.; Gao, G.; Qiao, H.; Dong, G.; Dan, Z.; Li, Y.; Qi, Y.; Zhang, Q.; Yuan, S.; Liu, H.-M.; et al. Discovery of 1,2,4-Triazole-3-Thione Derivatives as Potent and Selective DCN1 Inhibitors for Pathological Cardiac Fibrosis and Remodeling. J. Med. Chem. 2024, 67, 18699–18723. [Google Scholar] [CrossRef] [PubMed]





| Gene | Primer Sequences |
|---|---|
| TNF-α | F: 5′ TTCTGTCTACTGAACTTC3′ R: 5′ CCATAGAACTGATGAGAG3′ |
| IL-6 | F: 5′ GCCAGAGTCCTTCAGAGAGA3′ R: 5′ TGGTCCTTAGCCACTCCTTC3′ |
| IL-1β | F: 5′ CAATGGACAGAATATCAAC3′ R: 5′ ACAGGACAGGTATAGATT3′ |
| iNOS | F: 5′ TACGGAAGTCAGAAGATG3′ R: 5′ TAATGGAGGAGTAGTATTGG3′ |
| GAPDH | F: 5′ AGTGGCAAAGTGGAGATT3′ R: 5′ GTGGAGTCATACTGGAACA3′ |
| No. | Compound | Content (%) | RI | RT (min) | |
|---|---|---|---|---|---|
| Measured | Documented | ||||
| 1 | α-Pinene | 2.68 | 933 | 931 | 7.397 |
| 2 | β-Pinene | 0.34 | 976 | 975 | 8.852 |
| 3 | β-Myrcene | 0.81 | 991 | 988 | 9.127 |
| 4 | 2-Nonen-1-ol, (E)- | 0.23 | 1144 | 1149 | 9.484 |
| 5 | o-Cymene | 1.3 | 1023 | 1018 | 10.427 |
| 6 | D-Limonene | 76.51 | 1031 | 1028 | 10.622 |
| 7 | Sabinen | 0.32 | 965 | 969 | 10.686 |
| 8 | γ-Terpinene | 1.20 | 1054 | 1056 | 11.601 |
| 9 | Terpilene | 0.16 | 1019 | 1016 | 11.845 |
| 10 | Terpinolen | 0.56 | 1082 | 1083 | 12.586 |
| 11 | Linalool | 2.11 | 1100 | 1101 | 13.036 |
| 12 | Limonene oxide | 0.22 | 1140 | 1138 | 14.3 |
| 13 | Limonene oxide, trans- | 0.25 | 1137 | 1139 | 14.439 |
| 14 | Perillol | 0.34 | 1291 | 1294 | 14.645 |
| 15 | Dihydroterpineol | 0.62 | 1145 | 1142 | 14.904 |
| 16 | 4-Terpineol | 0.14 | 1176 | 1180 | 16.03 |
| 17 | Terpinyl formate | 0.28 | 1181 | 1183 | 16.536 |
| 18 | β-Patchoulene | 0.38 | 1384 | 1381 | 23.089 |
| 19 | Caryophyllene | 0.29 | 1411 | 1415 | 24.177 |
| 20 | α-Guaiene | 0.61 | 1440 | 1442 | 24.588 |
| 21 | Sativene | 0.45 | 1394 | 1390 | 25.155 |
| 22 | α-Bulnesene | 0.34 | 1396 | 1394 | 26.666 |
| 23 | Patchouli alcohol | 0.22 | 1661 | 1663 | 31.702 |
| Total (%) | 90.36 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, J.; Cui, J.; Yang, H.; Cao, J.; Xiao, S.; Cheng, G. The Anti-Inflammatory Effects and Molecular Mechanism of Citri Reticulatae Pericarpium Essential Oil: A Combined GC-MS and Network Pharmacology Study. Foods 2025, 14, 1455. https://doi.org/10.3390/foods14091455
Pu J, Cui J, Yang H, Cao J, Xiao S, Cheng G. The Anti-Inflammatory Effects and Molecular Mechanism of Citri Reticulatae Pericarpium Essential Oil: A Combined GC-MS and Network Pharmacology Study. Foods. 2025; 14(9):1455. https://doi.org/10.3390/foods14091455
Chicago/Turabian StylePu, Junmei, Jiabao Cui, Hui Yang, Jianxin Cao, Shanshan Xiao, and Guiguang Cheng. 2025. "The Anti-Inflammatory Effects and Molecular Mechanism of Citri Reticulatae Pericarpium Essential Oil: A Combined GC-MS and Network Pharmacology Study" Foods 14, no. 9: 1455. https://doi.org/10.3390/foods14091455
APA StylePu, J., Cui, J., Yang, H., Cao, J., Xiao, S., & Cheng, G. (2025). The Anti-Inflammatory Effects and Molecular Mechanism of Citri Reticulatae Pericarpium Essential Oil: A Combined GC-MS and Network Pharmacology Study. Foods, 14(9), 1455. https://doi.org/10.3390/foods14091455







