Multifaceted Biological Activities of Culinary Herb and Spice Extracts: In Vitro and In Silico Simulation Insights into Inflammation-Related Targets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Samples
2.3. HS-SPME Procedure to Extract Volatile Organic Metabolites
2.4. GC-MS Conditions
2.5. Molecular Modeling
2.5.1. Preparation of Target Proteins
2.5.2. Preparation of Small-Molecule Inhibitors
2.5.3. Molecular Docking Simulations
2.6. In Vitro Assessment of Total Phenolic Content, Total Flavonoids Content, and Antioxidant and Anti-Inflammatory Activities of Culinary Herb and Spice Extracts
2.6.1. Total Phenolic Content
2.6.2. Total Flavonoid Content
2.6.3. Total Anthocyanin Content
2.6.4. 2,2-Diphenyl-1-Picrylhydrazyl Scavenging Assay (DPPH)
2.6.5. 2,2′-Azinobis-(3-Ethylbenzothiazoline-6-Sulfonic Acid) Scavenging Assay (ABTS)
2.6.6. Oxygen Radical Absorbance Capacity (ORAC)
2.6.7. Anti-Inflammatory Activity
2.7. Statistical Analysis
3. Results and Discussion
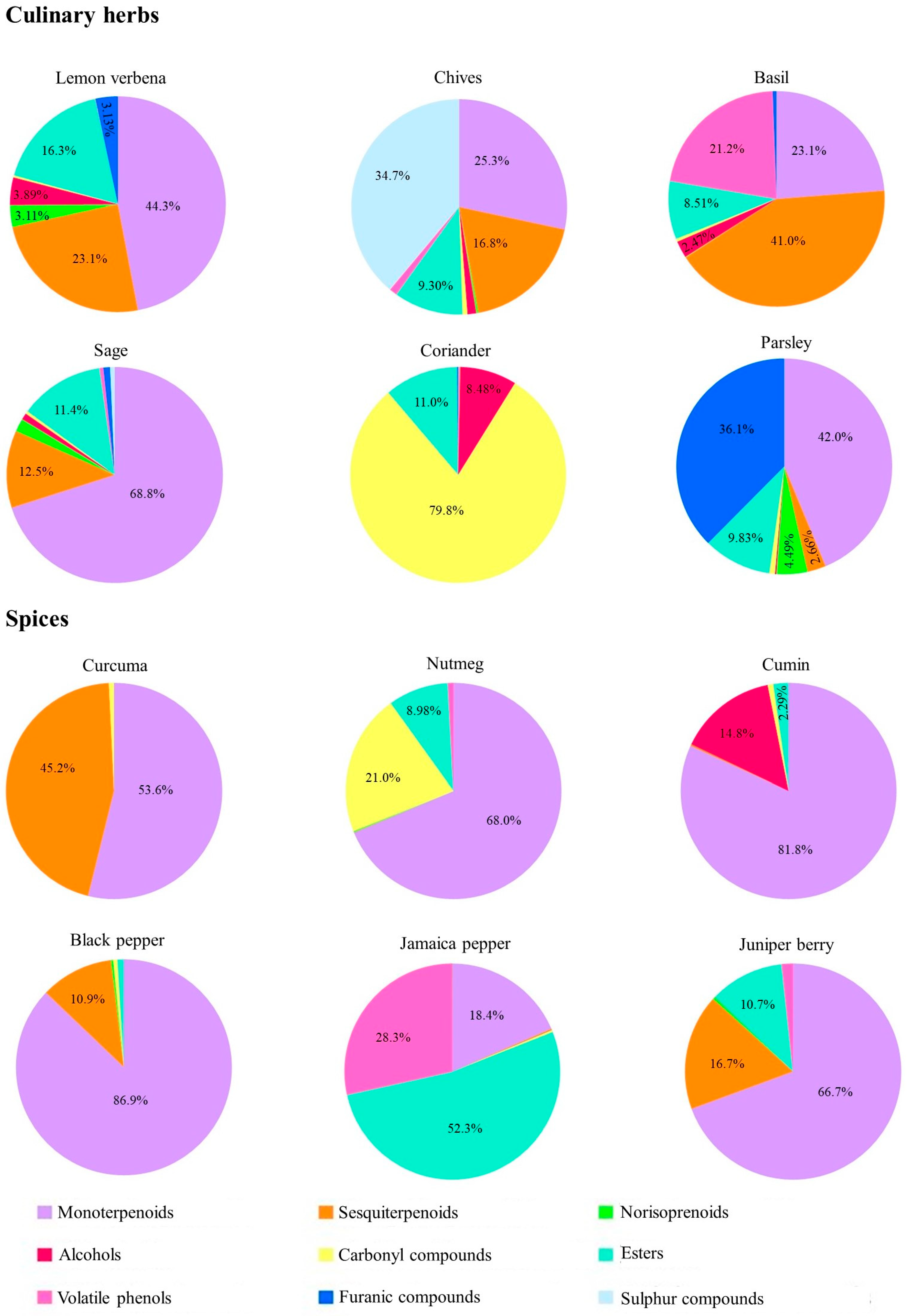
3.1. Volatilomic Fingerprint of Culinary Herbs and Spices
3.1.1. Culinary Herbs
3.1.2. Spices
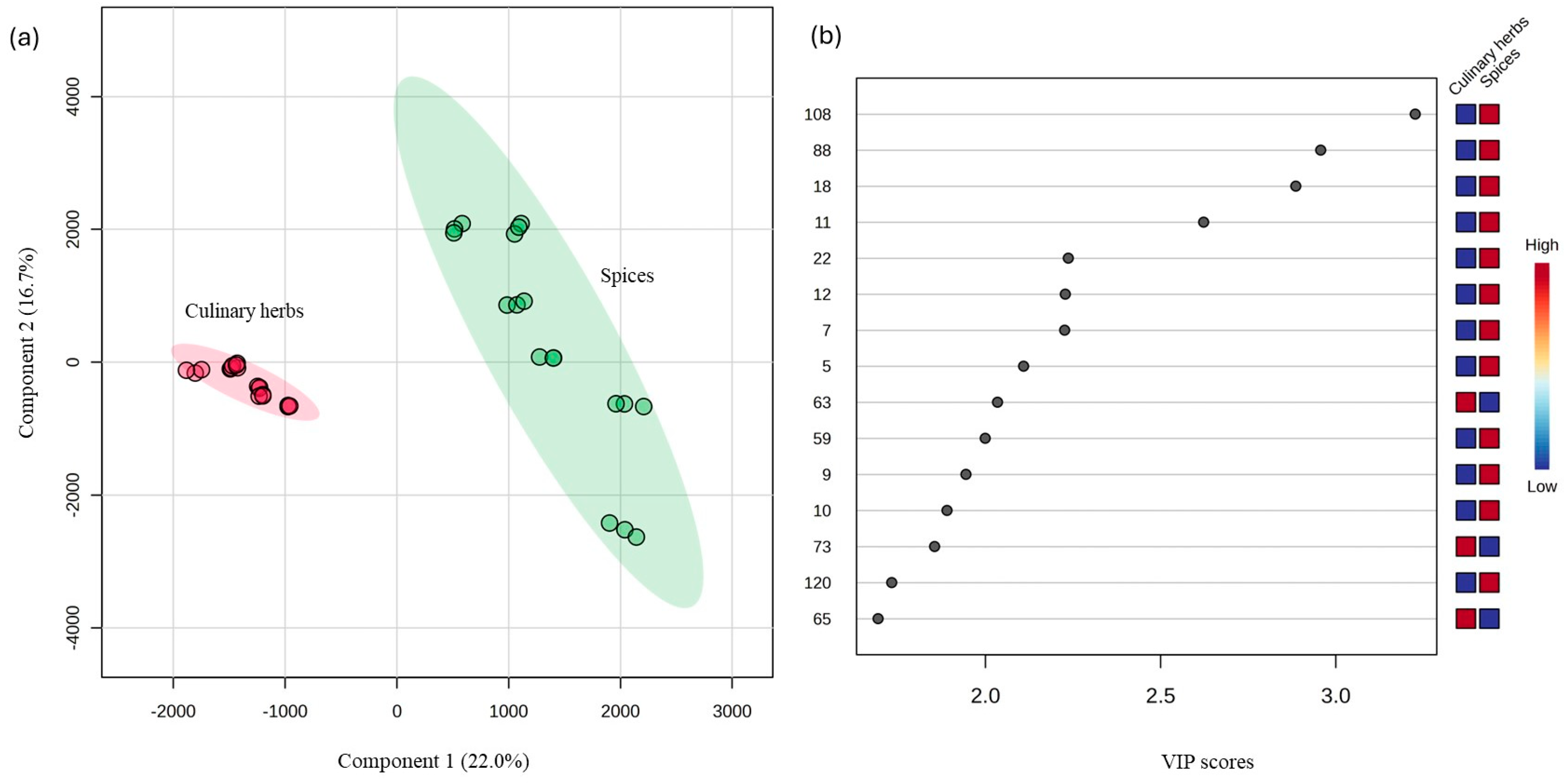
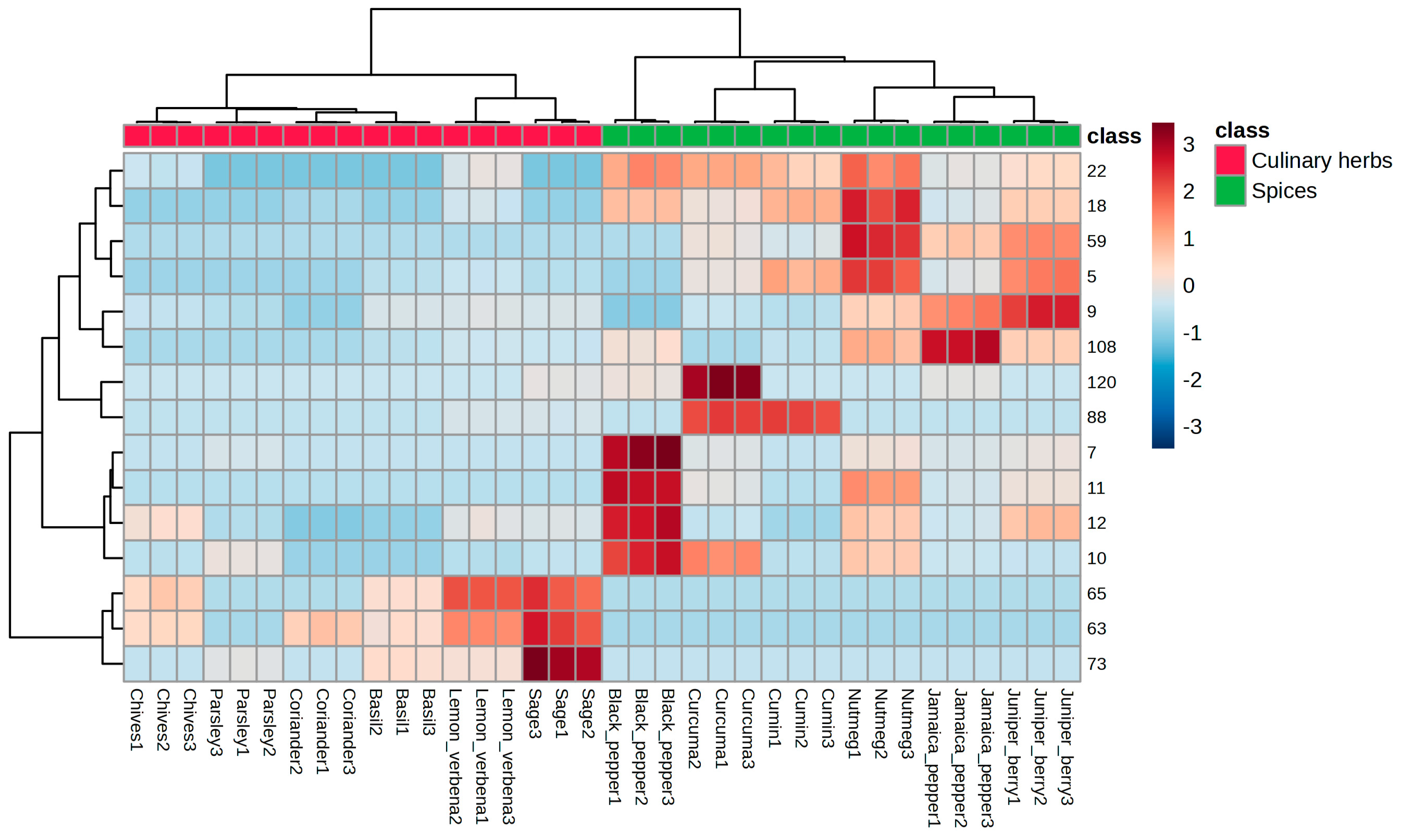
3.2. Statistical Analysis
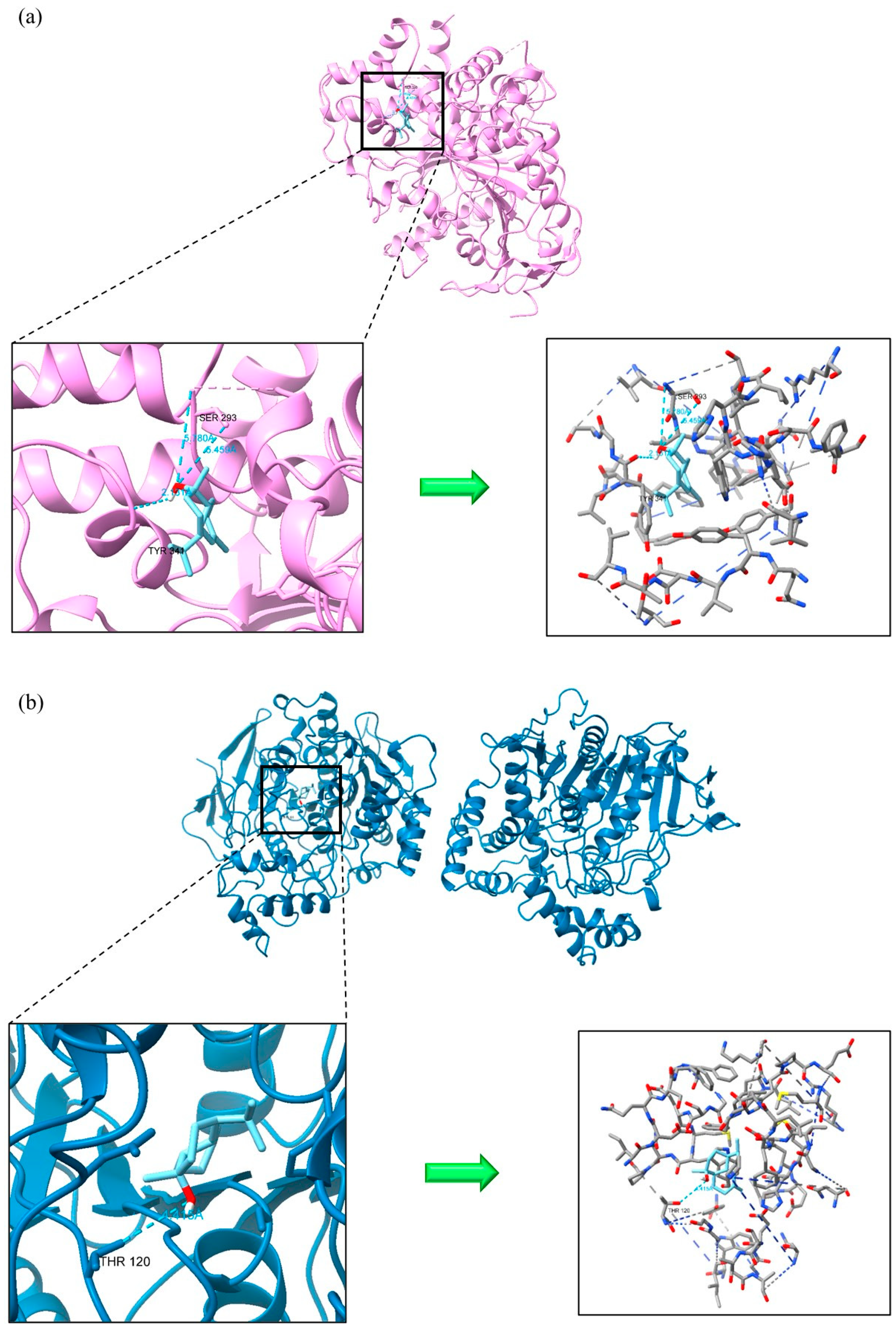
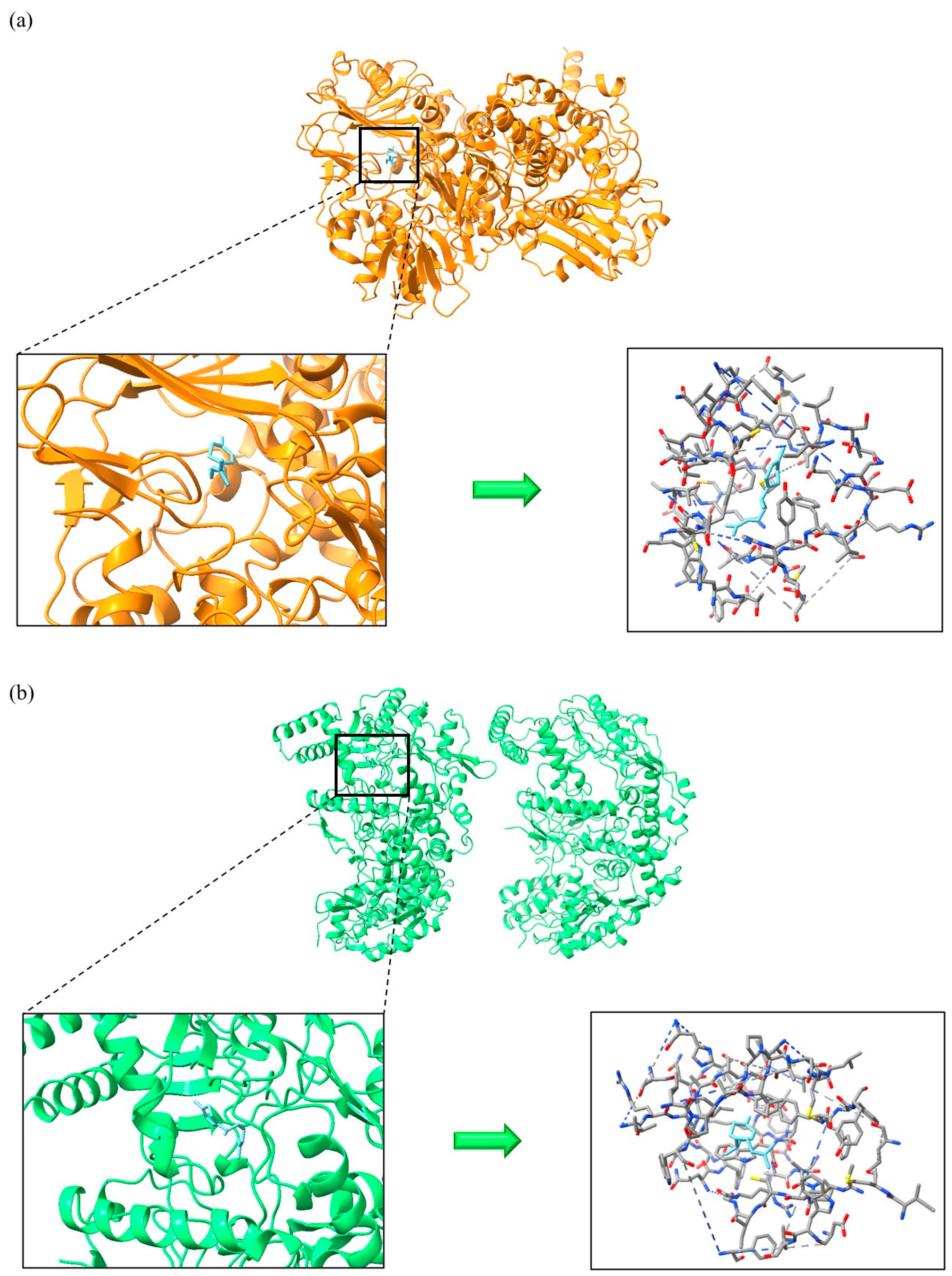
3.3. Molecular Docking Simulations
3.4. Assessment of the Total Phenolics and Flavonoids and Antioxidant and Anti-Inflammatory Activities of Culinary Herbs and Spices
3.4.1. Total Phenolic, Flavonoid, and Anthocyanin Content
3.4.2. Antioxidant Activity
3.4.3. Anti-Inflammatory Activity
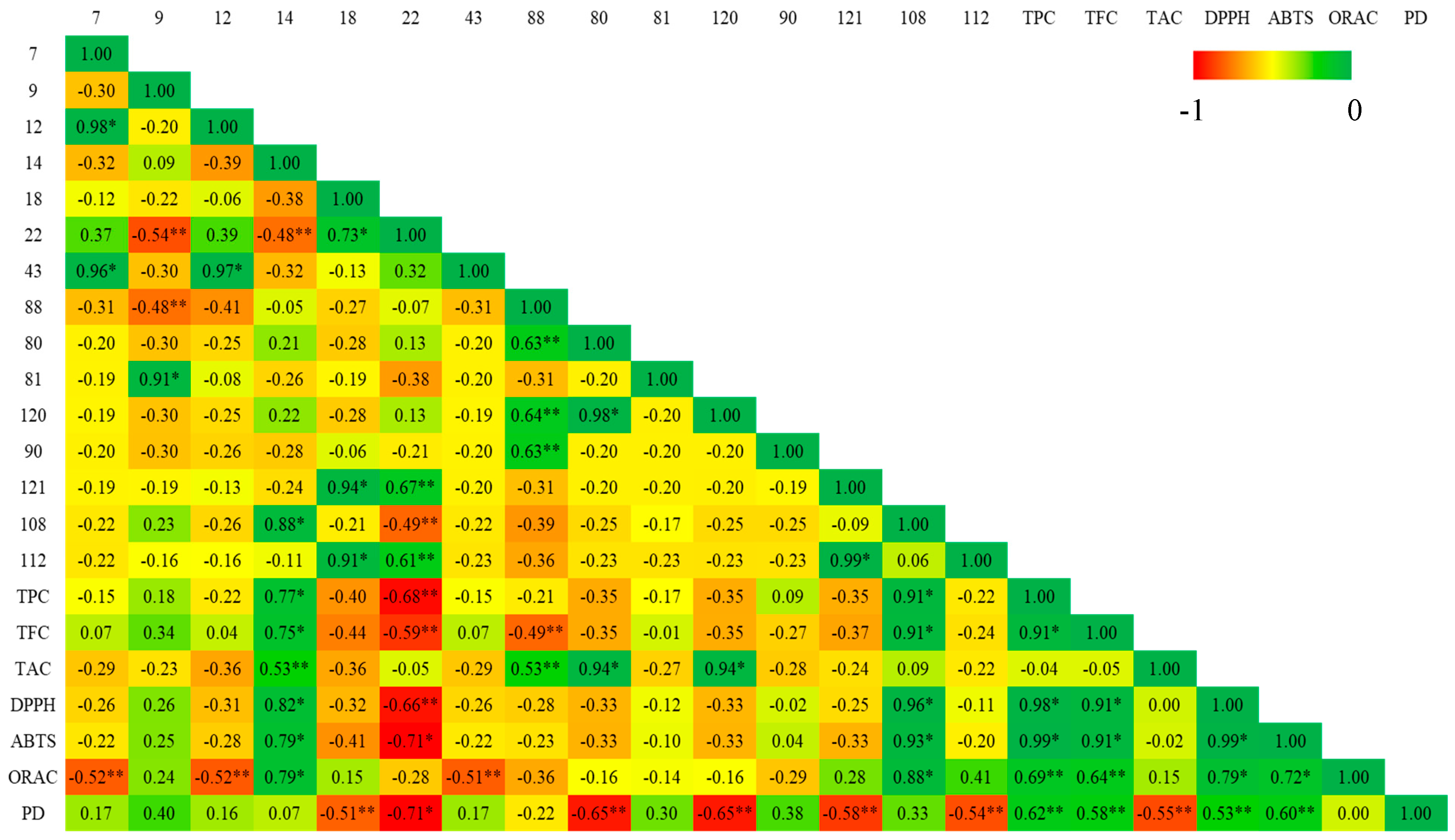
3.5. Pearson Correlation Between Main Volatile Organic Metabolites and Biological Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-LOX | 5-lipoxygenase |
| ABTS | 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) |
| AChE | acetylcholinesterase |
| ADT | AutoDockTools |
| Aβ | amyloid-beta |
| BChE | butyrylcholinesterase |
| C3GE | cyanidin-3-glucoside equivalents |
| COX-2 | cyclooxygenase-2 |
| CPO | cold-pressed oil |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| DW | dry weight |
| FDA | Food and Drug Administration |
| GAE | gallic acid equivalent |
| GC-MS | gas chromatography–mass spectrometry |
| HCA | hierarchical cluster analysis |
| HS-SPME | headspace solid-phase microextraction |
| iNOS | inducible nitric oxide synthase |
| KI | Kovat index |
| MAO-B | monoamine oxidase B |
| ORAC | oxygen radical absorbance capacity |
| PBS | phosphate-buffered saline |
| PDB | Protein Data Bank |
| PDBQT | Protein Data Bank, partial charge, and atom type |
| PLS-DA | partial least squares-discriminant analysis |
| QE | quercetin equivalent |
| ROS | reactive oxygen species |
| RT | retention time |
| TAC | total anthocyanin content |
| TE | Trolox equivalents |
| TFC | total flavonoid content |
| TPC | total phenolic content |
| VIP | variable importance in projection |
| VOMs | volatile organic metabolites |
| ΔG | Gibbs free energy |
References
- Athanasiadis, V.; Chatzimitakos, T.; Makrygiannis, I.; Kalompatsios, D.; Bozinou, E.; Lalas, S.I. Antioxidant-Rich Extracts from Lemon Verbena (Aloysia citrodora L.) Leaves through Response Surface Methodology. Oxygen 2024, 4, 1–19. [Google Scholar] [CrossRef]
- Bukvicki, D.; Gottardi, D.; Prasad, S.; Novakovic, M.; Marin, P.D.; Tyagi, A.K. The Healing Effects of Spices in Chronic Diseases. Curr. Med. Chem. 2020, 27, 4401–4420. [Google Scholar] [CrossRef] [PubMed]
- Şener, G.; Karakadıoglu, G.; Ozbeyli, D.; Ede, S.; Yanardag, R.; Sacan, O.; Aykac, A. Petroselinum Crispum Extract Ameliorates Scopolamine-Induced Cognitive Dysfunction: Role on Apoptosis, Inflammation and Oxidative Stress. Food Sci. Hum. Wellness 2022, 11, 1290–1298. [Google Scholar] [CrossRef]
- Singh, V.; Kaur, K.; Kaur, S.; Shri, R.; Singh, T.G.; Singh, M. Trimethoxyflavones from Ocimum basilicum L. Leaves Improve Long Term Memory in Mice by Modulating Multiple Pathways. J. Ethnopharmacol. 2022, 295, 115438. [Google Scholar] [CrossRef]
- Bajer, T.; Ligor, M.; Ligor, T.; Buszewski, B. Design of the Extraction Process for Terpenes and Other Volatiles from Allspice by Solid-Phase Microextraction and Hydrodistillation. J. Sep. Sci. 2016, 39, 769–775. [Google Scholar] [CrossRef]
- Dai, X.; Jia, C.; Lu, J.; Yu, Z. The Dynamics of Bioactive Compounds and Their Contributions to the Antioxidant Activity of Postharvest Chive (Allium schoenoprasum L.). Food Res. Int. 2023, 174, 113600. [Google Scholar] [CrossRef]
- Mahmoud, E.; Starowicz, M.; Ciska, E.; Topolska, J.; Farouk, A. Determination of Volatiles, Antioxidant Activity, and Polyphenol Content in the Postharvest Waste of Ocimum basilicum L. Food Chem. 2022, 375, 131692. [Google Scholar] [CrossRef]
- Milenković, A.; Stanojević, J.; Stanojević, L. Comparative Analysis of Chemical Composition and Antioxidant Activity of Essential Oil and Hydrolate from Black Pepper Fruit (Piper nigrum L.). Maced. J. Chem. Chem. Eng. 2022, 41, 209–220. [Google Scholar] [CrossRef]
- Muchtaridi; Subarnas, A.; Apriyantono, A.; Mustarichie, R. Identification of Compounds in the Essential Oil of Nutmeg Seeds (Myristica fragrans Houtt.) That Inhibit Locomotor Activity in Mice. Int. J. Mol. Sci. 2010, 11, 4771–4781. [Google Scholar] [CrossRef]
- Pachura, N.; Zimmer, A.; Grzywna, K.; Figiel, A.; Szumny, A.; Łyczko, J. Chemical Investigation on Salvia officinalis L. Affected by Multiple Drying Techniques—The Comprehensive Analytical Approach (HS-SPME, GC–MS, LC-MS/MS, GC-O and NMR). Food Chem. 2022, 397, 133802. [Google Scholar] [CrossRef]
- Qiang, Y.; Si, R.; Tan, S.; Wei, H.; Huang, B.; Wu, M.; Shi, M.; Fang, L.; Fu, J.; Zeng, S. Spatial Variation of Volatile Organic Compounds and Antioxidant Activity of Turmeric (Curcuma longa L.) Essential Oils Harvested from Four Provinces of China. Curr. Res. Food Sci. 2021, 4, 882–890. [Google Scholar] [CrossRef]
- Rashid, H.M.; Mahmod, A.I.; Afifi, F.U.; Talib, W.H. Antioxidant and Antiproliferation Activities of Lemon Verbena (Aloysia citrodora): An In Vitro and In Vivo Study. Plants 2022, 11, 785. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Lyu, J.; Wei, L.; Xie, B.; Wei, J.; Zhang, G.; Li, J.; Gao, C.; Xiao, X.; Yu, J. Chemometric Approaches for the Optimization of Headspace-Solid Phase Microextraction to Analyze Volatile Compounds in Coriander (Coriandrum sativum L.). LWT 2022, 167, 113842. [Google Scholar] [CrossRef]
- Drinić, Z.; Pljevljakušić, D.; Janković, T.; Zdunić, G.; Bigović, D.; Šavikin, K. Hydro-Distillation and Microwave-Assisted Distillation of Sideritis Raeseri: Comparison of the Composition of the Essential Oil, Hydrolat and Residual Water Extract. Sustain. Chem. Pharm. 2021, 24, 100538. [Google Scholar] [CrossRef]
- Palmieri, S.; Pellegrini, M.; Ricci, A.; Compagnone, D.; Lo Sterzo, C. Chemical Composition and Antioxidant Activity of Thyme, Hemp and Coriander Extracts: A Comparison Study of Maceration, Soxhlet, UAE and RSLDE Techniques. Foods 2020, 9, 1221. [Google Scholar] [CrossRef]
- Luca, S.V.; Kittl, T.; Minceva, M. Supercritical CO2 Extraction of Spices: A Systematic Study with Focus on Terpenes and Piperamides from Black Pepper (Piper nigrum L.). Food Chem. 2023, 406, 135090. [Google Scholar] [CrossRef]
- Kim, N.; Lee, D. Headspace Solid-Phase Microextraction for Characterization of Fragrances of Lemon Verbena (Aloysia triphylla) by Gas Chromatography-Mass Spectrometry. J. Sep. Sci. 2004, 27, 96–100. [Google Scholar] [CrossRef]
- Baky, M.H.; Elkenawy, N.M.; El-Nashar, H.A.S.; Abib, B.; Farag, M.A. Comparison of Autoclaving and γ-Radiation Impact on Four Spices Aroma Profiles and Microbial Load Using HS-SPME GC–MS and Chemometric Tools. Sci. Rep. 2024, 14, 5752. [Google Scholar] [CrossRef]
- Farag, M.A.; Dokalahy, E.U.; Eissa, T.F.; Kamal, I.M.; Zayed, A. Chemometrics-Based Aroma Discrimination of 14 Egyptian Mango Fruits of Different Cultivars and Origins, and Their Response to -1 SPME Coupled to GC–MS. ACS Omega 2022, 7, 2377–2390. [Google Scholar] [CrossRef]
- Mostafa, N.M.; Mostafa, A.M.; Ashour, M.L.; Elhady, S.S. Neuroprotective Effects of Black Pepper Cold-Pressed Oil on Scopolamine-Induced Oxidative Stress and Memory Impairment in Rats. Antioxidants 2021, 10, 1993. [Google Scholar] [CrossRef]
- Izcara, S.; Perestrelo, R.; Morante-Zarcero, S.; Sierra, I.; Câmara, J.S. Volatilomic Fingerprinting from Edible Flowers. Unravelling Some Impact Compounds behind Its Attractiveness. Food Biosci. 2022, 50, 102188. [Google Scholar] [CrossRef]
- El-Sayed, A.M. The Pherobase: Database of Insect Pheromones and Semiochemicals. Available online: http://www.pherobase.com (accessed on 15 July 2024).
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure Visualization for Researchers, Educators, and Developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Sanner, M.F. Python: A Programming Language for Software Integration and Development. J. Mol. Graph Model. 1999, 17, 57–61. [Google Scholar] [PubMed]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Abreu, T.; Jasmins, G.; Bettencourt, C.; Teixeira, J.; Câmara, J.S.; Perestrelo, R. Tracing the Volatilomic Fingerprint of Grape Pomace as a Powerful Approach for Its Valorization. Curr. Res. Food Sci. 2023, 7, 100608. [Google Scholar] [CrossRef]
- Ribeiro, L.F.; Ribani, R.H.; Francisco, T.M.G.; Soares, A.A.; Pontarolo, R.; Haminiuk, C.W.I. Profile of Bioactive Compounds from Grape Pomace (Vitis vinifera and Vitis labrusca) by Spectrophotometric, Chromatographic and Spectral Analyses. J. Chromatogr. B 2015, 1007, 72–80. [Google Scholar] [CrossRef]
- Zulueta, A.; Esteve, M.J.; Frígola, A. ORAC and TEAC Assays Comparison to Measure the Antioxidant Capacity of Food Products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
- Gunathilake, K.; Ranaweera, K.; Rupasinghe, H. In Vitro Anti-Inflammatory Properties of Selected Green Leafy Vegetables. Biomedicines 2018, 6, 107. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; De Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the Gap between Raw Spectra and Functional Insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
- Hanif, M.; Xie, B.; Wei, S.; Li, J.; Gao, C.; Wang, R.; Ali, S.; Xiao, X.; Yu, J.; Al-Hashimi, A.; et al. Characterization of the Volatile Profile from Six Different Varieties of Chinese Chives by HS-SPME/GC–MS Coupled with E. NOSE. J. King Saud Univ.-Sci. 2022, 34, 101971. [Google Scholar] [CrossRef]
- Du, P.; Yuan, H.; Chen, Y.; Zhou, H.; Zhang, Y.; Huang, M.; Jiangfang, Y.; Su, R.; Chen, Q.; Lai, J.; et al. Identification of Key Aromatic Compounds in Basil (Ocimum L.) Using Sensory Evaluation, Metabolomics and Volatilomics Analysis. Metabolites 2023, 13, 85. [Google Scholar] [CrossRef] [PubMed]
- Peitzika, S.-C.; Pontiki, E. A Review on Recent Approaches on Molecular Docking Studies of Novel Compounds Targeting Acetylcholinesterase in Alzheimer Disease. Molecules 2023, 28, 1084. [Google Scholar] [CrossRef]
- Iova, O.-M.; Marin, G.-E.; Lazar, I.; Stanescu, I.; Dogaru, G.; Nicula, C.A.; Bulboacă, A.E. Nitric Oxide/Nitric Oxide Synthase System in the Pathogenesis of Neurodegenerative Disorders—An Overview. Antioxidants 2023, 12, 753. [Google Scholar] [CrossRef]
- Jalil, S.; Basri, R.; Aziz, M.; Shafiq, Z.; Ejaz, S.A.; Hameed, A.; Iqbal, J. Pristine 2-Chloroquinoline-Based-Thiosemicarbazones as Multitarget Agents against Alzheimer’s Disease: In Vitro and in Silico Studies of Monoamine Oxidase (MAO) and Cholinesterase (ChE) Inhibitors. J. Mol. Struct. 2024, 1306, 137841. [Google Scholar] [CrossRef]
- Joshi, Y.B.; Praticò, D. The 5-Lipoxygenase Pathway: Oxidative and Inflammatory Contributions to the Alzheimer’s Disease Phenotype. Front. Cell. Neurosci. 2015, 8, 436. [Google Scholar] [CrossRef]
- Chu, J.; Praticò, D. The 5-Lipoxygenase as Modulator of Alzheimer’s γ-Secretase and Therapeutic Target. Brain Res. Bull. 2016, 126, 207–212. [Google Scholar] [CrossRef]
- Javed, M.A.; Bibi, S.; Jan, M.S.; Ikram, M.; Zaidi, A.; Farooq, U.; Sadiq, A.; Rashid, U. Diclofenac Derivatives as Concomitant Inhibitors of Cholinesterase, Monoamine Oxidase, Cyclooxygenase-2 and 5-Lipoxygenase for the Treatment of Alzheimer’s Disease: Synthesis, Pharmacology, Toxicity and Docking Studies. RSC Adv. 2022, 12, 22503–22517. [Google Scholar] [CrossRef]
- Moussa, N.; Dayoub, N. Exploring the Role of COX-2 in Alzheimer’s Disease: Potential Therapeutic Implications of COX-2 Inhibitors. Saudi Pharm. J. 2023, 31, 101729. [Google Scholar] [CrossRef]
- Gligorić, E.; Igić, R.; Teofilović, B.; Grujić-Letić, N. Phytochemical Screening of Ultrasonic Extracts of Salix Species and Molecular Docking Study of Salix-Derived Bioactive Compounds Targeting Pro-Inflammatory Cytokines. Int. J. Mol. Sci. 2023, 24, 11848. [Google Scholar] [CrossRef] [PubMed]
- Minhas, R.; Bansal, Y.; Bansal, G. Inducible Nitric Oxide Synthase Inhibitors: A Comprehensive Update. Med. Res. Rev. 2020, 40, 823–855. [Google Scholar] [CrossRef]
- Lu, S.; Tang, L.; Zhou, L.; Lai, Y.; Liu, L.; Duan, Y. Study on the Multitarget Mechanism and Active Compounds of Essential Oil from Artemisia Argyi Treating Pressure Injuries Based on Network Pharmacology. Evid.-Based Complement. Altern. Med. 2022, 2022, 1019289. [Google Scholar] [CrossRef] [PubMed]
- Iorio, R.; Celenza, G.; Petricca, S. Multi-Target Effects of ß-Caryophyllene and Carnosic Acid at the Crossroads of Mitochondrial Dysfunction and Neurodegeneration: From Oxidative Stress to Microglia-Mediated Neuroinflammation. Antioxidants 2022, 11, 1199. [Google Scholar] [CrossRef] [PubMed]
- Omari, Z.; Kazunori, S.; Sabti, M.; Bejaoui, M.; Hafidi, A.; Gadhi, C.; Isoda, H. Dietary Administration of Cumin-Derived Cuminaldehyde Induce Neuroprotective and Learning and Memory Enhancement Effects to Aging Mice. Aging 2021, 13, 1671–1685. [Google Scholar] [CrossRef]
- Francik, S.; Francik, R.; Sadowska, U.; Bystrowska, B.; Zawiślak, A.; Knapczyk, A.; Nzeyimana, A. Identification of Phenolic Compounds and Determination of Antioxidant Activity in Extracts and Infusions of Salvia Leaves. Materials 2020, 13, 5811. [Google Scholar] [CrossRef]
- Muzolf-Panek, M.; Stuper-Szablewska, K. Comprehensive Study on the Antioxidant Capacity and Phenolic Profiles of Black Seed and Other Spices and Herbs: Effect of Solvent and Time of Extraction. J. Food Meas. Charact. 2021, 15, 4561–4574. [Google Scholar] [CrossRef]
- Alcântara, M.A.; de Lima Brito Polari, I.; de Albuquerque Meireles, B.R.L.; de Lima, A.E.A.; da Silva Junior, J.C.; de Andrade Vieira, É.; dos Santos, N.A.; de Magalhães Cordeiro, A.M.T. Effect of the Solvent Composition on the Profile of Phenolic Compounds Extracted from Chia Seeds. Food Chem. 2019, 275, 489–496. [Google Scholar] [CrossRef]
- Ferreira, F.S.; de Oliveira, V.S.; Chávez, D.W.H.; Chaves, D.S.; Riger, C.J.; Sawaya, A.C.H.F.; Guizellini, G.M.; Sampaio, G.R.; Torres, E.A.F.D.S.; Saldanha, T. Bioactive Compounds of Parsley (Petroselinum crispum), Chives (Allium schoenoprasum L) and Their Mixture (Brazilian cheiro-verde) as Promising Antioxidant and Anti-Cholesterol Oxidation Agents in a Food System. Food Res. Int. 2022, 151, 110864. [Google Scholar] [CrossRef]
- Kiani, H.S.; Ali, B. Optimized Extraction of Polyphenols, LC-MS/MS, and GC-MS Identification of Metabolites from the Selected Medicinal Herbs, Their Antioxidant and Anti-Diabetic Potential. Preprints 2023, 11, 2721. [Google Scholar]
- Tashtoush, S.H.; Ereifej, K.I.; Feng, H.; Rababah, T.M.; Al-U’datt, M.H.; Gammoh, S.; Al-Rabadi, G.J. Temperature and Acidified Solvent Effect on Total Anthocyanins and RP-HPLC Phenolic Acids Determination in Selected Spices. Food Nutr. Sci. 2016, 07, 20–29. [Google Scholar] [CrossRef]
- Kozłowska, M.; Ścibisz, I.; Przybył, J.; Ziarno, M.; Żbikowska, A.; Majewska, E. Phenolic Contents and Antioxidant Activity of Extracts of Selected Fresh and Dried Herbal Materials. Pol. J. Food Nutr. Sci. 2021, 71, 269–278. [Google Scholar] [CrossRef]
- Trifan, A.; Zengin, G.; Korona-Glowniak, I.; Skalicka-Woźniak, K.; Luca, S.V. Essential Oils and Sustainability: In Vitro Bioactivity Screening of Myristica fragrans Houtt. Post-Distillation By-Products. Plants 2023, 12, 1741. [Google Scholar] [CrossRef]
- Bailey-Shaw, Y.A.; Williams, L.A.D.; Green, C.E.; Rodney, S.; Smith, A.M. In-Vitro Evaluation of the Anti-Inflammatory Potential of Selected Jamaican Plant Extracts Using the Bovine Serum Albumin Protein Denaturation Assay. Int. J. Pharm. Sci. Rev. Res. 2017, 47, 145–153. [Google Scholar]
- Oliveira, S.D.D.S.; De Oliveira E Silva, A.M.; Blank, A.F.; Nogueira, P.C.D.L.; Nizio, D.A.D.C.; Almeida-Pereira, C.S.; Pereira, R.O.; Menezes-Sá, T.S.A.; Santana, M.H.D.S.; Arrigoni-Blank, M.D.F. Radical Scavenging Activity of the Essential Oils from Croton grewioides Baill Accessions and the Major Compounds Eugenol, Methyl Eugenol and Methyl Chavicol. J. Essent. Oil Res. 2021, 33, 94–103. [Google Scholar] [CrossRef]
- Kim, T.; Song, B.; Cho, K.S.; Lee, I.-S. Therapeutic Potential of Volatile Terpenes and Terpenoids from Forests for Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 2187. [Google Scholar] [CrossRef]
- Kudva, A.K.; Manoj, M.N.; Swamy, B.M.; Ramadoss, C.S. Complexation of Amphotericin B and Curcumin with Serum Albumins: Solubility and Effect on Erythrocyte Membrane Damage. J. Exp. Pharmacol. 2011, 2011, 1–6. [Google Scholar] [CrossRef]
- Behjati Hosseini, S.; Asadzadeh-Lotfabad, M.; Erfani, M.; Babayan-Mashhadi, F.; Mokaberi, P.; Amiri-Tehranizadeh, Z.; Saberi, M.R.; Chamani, J. A Novel Vision into the Binding Behavior of Curcumin with Human Serum Albumin-Holo Transferrin Complex: Molecular Dynamic Simulation and Multi-Spectroscopic Perspectives. J. Biomol. Struct. Dyn. 2022, 40, 11154–11172. [Google Scholar] [CrossRef]


| Samples | TPC mgGAE/g | TFC mgQE/g | TAC mgC3GE/g | DPPH mgTE/g | ABTS mgTE/g | ORAC µMTE/g | Egg Albumin Denaturation (%) |
|---|---|---|---|---|---|---|---|
| Lemon verbena | 2.50 ± 0.01 a | 2.28 ± 0.02 a | n.d. | 1.97 ± 0.05 a | 5.85 ± 0.48 a | 1.70 ± 0.09 a | 3.65 ± 0.17 a |
| Chives | 4.65 ± 0.12 b | 3.35 ± 0.20 a,b | n.d. | 6.78 ± 1.05 b | 20.7 ± 1.40 b | 63.9 ± 2.40 b | 32.2 ± 1.58 b |
| Basil | 6.06 ± 0.02 b,c | 2.02 ± 0.01 a,b | 0.70 ± 0.01 a | 4.79 ± 0.44 b | 7.78 ± 0.75 a,c | 48.1 ± 0.82 c | 37.9 ± 0.57 b |
| Sage | 6.73 ± 0.13 c | 3.49 ± 0.23 a,b | 2.06 ± 0.06 b | 11.8 ± 0.82 c | 15.9 ± 1.93 b,c | 87.1 ± 6.90 d | 47.3 ± 1.10 c |
| Coriander | 5.39 ± 0.36 b,c | 1.16 ± 0.05 a,c | n.d. | 5.72 ± 0.25 b | 34.8 ± 1.32 d | 46.7 ± 0.63 c | 10.3 ± 0.35 d |
| Parsley | 5.36 ± 0.24 b,c | 1.10 ± 0.01 a,c | n.d. | 5.70 ± 0.79 b | 25.4 ± 2.61 b,c | 94.4 ± 9.60 d,e | 48.9 ± 1.94 c |
| Curcuma | 6.16 ± 0.21 b,c | 0.99 ± 0.01 a,c | 23.9 ± 0.22 c | 6.14 ± 0.19 b | 35.4 ± 2.61 d | 62.7 ± 0.76 b | 45.5 ± 2.07 c |
| Nutmeg | 6.33 ± 0.10 c | 0.62 ± 0.03 c | 1.18 ± 0.06 d | 11.2 ± 0.08 c | 35.2 ± 4.77 d | 82.5 ± 0.37 d | 47.6 ± 4.54 c |
| Cumin | 26.4 ± 0.25 d | 3.31 ± 0.02 a,b | 0.31 ± 0.03 e | 25.5 ± 0.52 d | 79.7 ± 1.61 e | 56.5 ± 1.15 b,c | 77.0 ± 0.39 e |
| Black pepper | 15.3 ± 0.24 e | 13.1 ± 1.24 d | n.d. | 10.5 ± 1.12 c | 48.9 ± 2.79 f | 46.3 ± 0.60 c | 70.6 ± 0.35 f |
| Jamaica pepper | 65.4 ± 1.38 f | 36.9 ± 0.64 e | 8.45 ± 0.16 f | 87.3 ± 0.12 e | 187 ± 4.87 g | 107 ± 2.84 e | 76.6 ± 0.23 e |
| Juniper berry | 14.6 ± 0.50 e | 10.6 ± 0.64 d | 0.61 ± 0.02 a | 19.3 ± 1.10 f | 62.6 ± 3.66 h | 63.3 ± 2.94 b | 74.5 ± 0.37 e,f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hontman, N.; Gonçalves, J.; Câmara, J.S.; Perestrelo, R. Multifaceted Biological Activities of Culinary Herb and Spice Extracts: In Vitro and In Silico Simulation Insights into Inflammation-Related Targets. Foods 2025, 14, 1456. https://doi.org/10.3390/foods14091456
Hontman N, Gonçalves J, Câmara JS, Perestrelo R. Multifaceted Biological Activities of Culinary Herb and Spice Extracts: In Vitro and In Silico Simulation Insights into Inflammation-Related Targets. Foods. 2025; 14(9):1456. https://doi.org/10.3390/foods14091456
Chicago/Turabian StyleHontman, Nance, Jéssica Gonçalves, José S. Câmara, and Rosa Perestrelo. 2025. "Multifaceted Biological Activities of Culinary Herb and Spice Extracts: In Vitro and In Silico Simulation Insights into Inflammation-Related Targets" Foods 14, no. 9: 1456. https://doi.org/10.3390/foods14091456
APA StyleHontman, N., Gonçalves, J., Câmara, J. S., & Perestrelo, R. (2025). Multifaceted Biological Activities of Culinary Herb and Spice Extracts: In Vitro and In Silico Simulation Insights into Inflammation-Related Targets. Foods, 14(9), 1456. https://doi.org/10.3390/foods14091456







