Exploring the Complexities of Seafood: From Benefits to Contaminants
Abstract
:1. Introduction
2. Global Demand, Production, and Consumption of Seafood
3. Benefits of Seafood Consumption
3.1. Health Benefits
3.2. Nutritional Benefits
3.2.1. Protein
3.2.2. Omega-3 Fatty Acids
3.2.3. Health Benefits of Omega-3 Fatty Acids
3.2.4. Vitamin D and Fish
3.2.5. Choline
3.2.6. Zinc
3.2.7. Iodine
3.2.8. Selenium
4. Harms of Seafood Consumption
4.1. Physical Contaminants
4.1.1. Micro- and Nanoplastic Contamination of Fish and Shellfish
The Effect of Microplastics on Fish and Shellfish
The Effect of Microplastics on the Human Body
Microplastics as Transmitters of Heavy Metals and Microbial Pathogens
4.2. Chemical Contaminants
4.2.1. Heavy Metals in Fish and Shellfish
Mercury
Lead
Cadmium
4.2.2. Per- and Polyfluoroalkyl Substances
Adverse Health Effects of Per- and Polyfluoroalkyl Substances
Seafoods as a Source of PFAS Compounds
4.2.3. Biogen Amines in Seafoods
Effects of Biogen Amines on Human Health
4.2.4. Allergens in Seafoods
| Phylum | Class | Common Name |
|---|---|---|
| Shellfish Mollusca | Gastropoda Bivalvia | Abalone Mussels, scallops, clams, and oysters |
| Arthropoda | Cephalopoda Crustacea | Squids, cuttlefish, octopus, lobster, crayfish, shrimp, prawn, and crab |
| Fish Chordata | Bony fish-Osteichthyes | Tuna, hake, cod, herring, salmon, sole, pilchard, anchovy, yellowfin, trout, and swordfish |
Effects of Allergens on Human Health
4.3. Biological Contaminants
4.3.1. Foodborne Pathogens in Seafood and Their Implications on Human Health
Viruses as Pathogens in Seafood
Bacteria as Pathogens in Seafood and Their Antimicrobial Resistance
Parasites as Pathogens in Seafood
Harmful Algal Blooms and Their Biotoxins as Pathogens in Seafood
| Pathogen | Source | Country | Reference | |
|---|---|---|---|---|
| Genus | Genotypes/Species/Strains/Serovars | |||
| Viruses | ||||
| Norovirus (NoV) | HuNoV Group II (GII) | Oysters, clams, shrimps, and finfish | India | [298] |
| Norovirus GI and GII | Sea urchins | Portugal | [299] | |
| Norovirus GI and GII | Oysters | USA | [300] | |
| Hepatitis A (HAV) | N.D. | Fish and shrimps | Iran | [301] |
| N.D. | Oysters | USA | [300] | |
| Genotype IA | Mussels and clams | Italy | [302] | |
| Hepatitis E (HEV) | G3 HEV | Mussels and clams | Italy | [303] |
| G3 HEV | Mussels and oysters | Scotland | [304] | |
| 4 Sub-genotypes (4b/4d) | A. granosa, S. subcrenata, and R. philippinarum | China | [305] | |
| Adenovirus (AdV) | N.D. | Finfish, bivalve mollusks, crustaceans, and cephalopods | India | [306] |
| A species HAdVs serotype 12, F species HAdVs serotype 41, and C species PAdVs serotype 5 | Oysters | Taiwan | [307] | |
| HAdV | Mussels | Spain, Greece, and Finland | [308] | |
| Sapovirus (SaV) | N.D. | Clams, cockles, and oysters | Morocco | [309] |
| N.D. | Shrimp, oysters, Atlantic salmon, and Arctic surf clams | China | [310] | |
| GI.1, GI.2, GI.3, GII.4, and GV.1 | Mussels | Spain | [311] | |
| Rotavirus (RV) | N.D. | Mussels and clams | Italy | [302] |
| N.D. | Mussels and clams | India | [312] | |
| G1, G2, G3, and G9 | Oysters | Thailand | [313] | |
| Aichivirus (AiV) | AiV-1 | Oysters and clams | Vietnam | [314] |
| AiV-1, genotype B | Mussels | South Africa | [315] | |
| AiV-1, genotype B | Mussels, oysters, and clams | Italy | [316] | |
| Bacteria | ||||
| Vibrio | V. parahaemolyticus | Oysters | USA | [317] |
| V. parahaemolyticus, V. vulnificus, V. alginolyticus, and V. mimicus | Oysters, clams, mussels, fish, and shrimp | Mexico | [172] | |
| V. parahaemolyticus, V. vulnificus, and V. cholerae | Mussels, clams, oysters, fish, cephalopods, and crustaceans | Italy | [318] | |
| Shewanella | S. algae | Oysters, clams, and abalone | Taiwan | [319] |
| S. algae | Shrimps | India | [320] | |
| S. abalonesis, S. algae, S. baltica, S. hafniensis, S. marisflavi, and S. putrefaciens | Oysters | USA | [321] | |
| Pseudomonas | P. aeruginosa | Fish (fresh, smoked, salted dried, and frozen) | Iran | [322] |
| P. fluorescens, P. fragi, P. lundensis, P. marginalis, P. syringae, P. taetrolens, P. chlororaphis, P. tolaasii, and P. viridilivida | Salmon, plaice, and northern cod fillets | Italy | [191] | |
| P. brenneri, P. defensor, P. haemolytica, P. lactis, P. lundensis, P. lurida, P. mandelii, P. meridiana, P. migulae, P. proteolytica, P. simiae, and P. weihenstephanensis, | Rainbow, Black Sea, brook, and brown trout | Turkey | [323] | |
| Clostridium | C. botulinum | Canned tuna, sardine, and mackerel | Egypt | [201] |
| C. perfringes | Oysters, mud snails, scallops, clams, loach, and monopteros albus | China | [181] | |
| C. difficile | Oysters | USA | [324] | |
| Escherichia coli | E. coli | Shrimp, catfish, and tilapia | USA | [174] |
| β-lactamase and carbapenemase-producing E. coli | Clams, oysters, razor clams, cockles, and mussels | Portugal | [325] | |
| Shiga-toxin-producing E. coli (STEC) | Shrimps, crabs, and oysters | Egypt | [220] | |
| Salmonella | S. Enterica subsp. enterica Typhimurium and monophasic variant I 1;4, [5], and 12:i:- | Oysters, mussels, and clams | Canada | [326] |
| S. Typhimurium, S. Enteritidis, S. Branderup, S. Infantis, S. Virchow, S. Agona, and S. Derby | Clams, mussels, oysters, and scallops | Poland | [225] | |
| S. Typhimurium | Shrimp, catfish, and tilapia | USA | [174] | |
| Shigella | Shigella spp. | Red mullet, red sea bream, and emperor fish | Jordan | [237] |
| Shigella spp. | Salmon, shrimp, and tilapia | USA | [251] | |
| S. dysenteriae and S. flexneri | Tilapia, common carp, and catfish | Ethiopia | [327] | |
| Listeria | L. monocytogenes | Smoked seafood, seafood salads, and fresh crab meat or sushi | USA | [328] |
| L. monocytogenes | Bogue, horse mackerel, hake, chub mackerel, European anchovy, and European pilchard | Greece | [242] | |
| Listeria spp. and L. monocytogenes | Fish products, sushi, and other ready-to-eat foods | Poland | [248] | |
| Staphylococcus | S. aureus | Raw processed fish and other ready-to-eat foods | Bangladesh | [253] |
| S. aureus, S. xylosus, S. lugdunensis, S. hominis, S. haemolyticus, S. lentus, S. sciuri, and S. capitis | Dried, smoked, and braised fish | Burkina Faso | [329] | |
| S. aureus | Freshwater fish, saltwater fish, and shrimp | China | [254] | |
| Campylobacter | C. jejuni | Shrimp, catfish, and tilapia | USA | [174] |
| C. lari and C. jejuni | Mussels | Croatia | [259] | |
| C. lari, C. jejuni, C. lari, and C. peloridis | Oysters, mussels, and common cockle | France | [264] | |
| Algae blooms | ||||
| Alexandrium | A. Pacificum and A. minutum | Shellfish and water | Italy | [330] |
| A. catenella, A. foedum, A. insuetum, A. lee, A. margalefi, A. minutum, A. pseudogonyaulax, and A. tamarense | Clam and water | Tunisia | [331] | |
| A. tamarense | Seawater | Scotland | [332] | |
| Dinophysis | D. acuminata, D. norvegica, D. fortii, D. ovum, and D. caudata | Water | USA | [333] |
| D. cf. acuminata | Water | Greece | [334] | |
| D. acuminata, D. norvegica, and D. acuta | Water | Norway | [335] | |
| Gymnodium | Gymnodinum spp. | Seawater | Morocco | [336] |
| G. catenatum | Water | Portugal | [337] | |
| Karlodinium | K. australe | Water | Malaysia | [338] |
| K. veneficum | Water | China | [339] | |
| K. veneficum | Water | China | [340] | |
| Prorocentrum | P. minimum | Water | Mexico | [341] |
| P. lima complex, P. caipirignum, P. cf. concavum, P. cf. emarginatum, P. cf. fukuyoi, and/or P. cf. rhathymum | Water | Japan | [342] | |
| P. lima complex, P. rhathymum, P. borbonicum, P. levis, P. rhathymum, and P. emarginatum | Water | Greece | [343] | |
| Pathogen | Contaminated Seafood | Year | Facts | Symptoms | Country/Continent | Reference |
|---|---|---|---|---|---|---|
| Listeria monocytogenes ST173 | Fish products | 2012–2024 | Multi-country (7); prolonged outbreak; 73 cases; 14 deaths | N.R. | Europe | [344] |
| Listeria monocytogenes ST1607 | Smoked salmon products | 2021–2024 | Multi-country (3); prolonged outbreak; 20 cases; 5 deaths | N.R. | Europe | [345] |
| Listeria monocytogenes ST155 | Ready-to-eat fish products | 2016–2023 | Multi-country (5); prolonged outbreak; 64 cases; 10 deaths | N.R. | Europe | [346] |
| Norovirus | Raw oysters | 2022 | Multistate (8); 211 illnesses | Fever, nausea, diarrhea, vomiting, abdominal cramps, chills, and headache | USA | [347] |
| Norovirus | Raw oysters | 2022 | Multistate (13); 192 illnesses | Diarrhea, vomiting, nausea, fever, headache, body ache, and stomach pain | USA | [347] |
| Salmonella Litchfield | Fresh fish | 2022 | Multistate (4); 39 illnesses; 15 hospitalizations; 0 deaths | N.R. | USA | [348] |
| Vibrio parahaemolyticus ST417 and ST50 | Raw oysters | 2021–2022 | 184 confirmed cases; 27 hospitalizations; 0 deaths | Diarrhea and abdominal pain | Australia | [349] |
| Salmonella Thompson | Seafood | 2021 | Multistate (15); 115 illnesses; 20 hospitalizations; 0 deaths | N.R. | USA | [350] |
| Salmonella Weltevreden | Frozen cooked shrimp | 2021 | Multistate (4); 9 illnesses; 3 hospitalizations; 0 deaths | N.R. | USA | [351] |
| N.I. | Mixed spicy seafood salad | 2020 | 368 cases; 0 deaths | Abdominal pain, watery diarrhea, nausea, vomiting, fever, and bloody diarrhea | Thailand | [352] |
| Hepatitis A | Clams, snapping shrimps, cockle, corbicula, and oysters | 2020 | 191 cases | N.R. | China | [353] |
| Vibrio parahaemolyticus, Shigella flexneri, Shiga toxin-producing E. coli non-O157, Vibrio albensis, Campylobacter lari, and norovirus genogroup 1. | Raw oysters | 2019 | Multistate (5); 16 illnesses/cases; 2 hospitalizations; 0 deaths | Diarrhea (that may be watery or bloody), stomach cramps or pain, nausea, vomiting, and fever | USA | [354] |
| Salmonella Newport | Frozen raw tuna | 2019 | Multistate (8); 15 illnesses/cases; 2 hospitalizations; 0 deaths | Diarrhea, fever, and stomach cramps | USA | [355] |
| Vibrio mimicus ctx-negative | Steamed blue crab, snow crab, shrimp, and oysters | 2019 | One state (Florida); 6 cases; 4 hospitalizations; 1 intensive care | Vomiting, headache, and nausea | USA | [356] |
| Listeria monocytogenes ST1247 (clonal complex 8) | Cold-smoked fish products | 2014–2019 | Multi-country (5); prolonged outbreak; 22 cases; 5 deaths | N.R. | Europe | [357] |
| Vibrio parahaemolyticus | Fresh crab meat | 2018 | 8 jurisdictions; 26 illnesses/cases; 9 hospitalizations; 0 deaths | N.R. | USA | [358] |
| Listeria monocytogenes ST8 | Salmon products | 2015–2018 | Multi-country (3); prolonged outbreak; 12 cases; 4 deaths | N.R. | Europe | [359] |
| Hepatitis A virus | Frozen scallops | 2016 | 1 state (Hawaii), 74 hospitalizations | Yellow eyes or skin, abdominal pain, pale stools, and dark urine | USA | [360] |
| Vibrio cholerae | Raw seafood | 2016 | 3 cases | Watery diarrhea, anorexia, vomiting, yellowish diarrhea, abdominal pain, myalgia, and acute renal failure | South Korea | [361] |
| Salmonella Paratyphi B variant L(+) tartrate(+) and Weltevreden | Frozen raw tuna | 2015 | Multistate (11); 65 illnesses/cases; 11 hospitalizations; 0 deaths | N.R. | USA | [362] |
| N.I. (suspected shellfish poisoning) | Clams | 2015 | 20 cases (January); 199 cases (April) | Dizziness, vomiting, nausea, headache, abdominal cramps, diarrhea, fever, and perioral and distal paresthesia | India | [363] |
5. Future Perspectives: Strategies to Control the Risks Associated with Seafood Consumption
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lubchenco, J.; Haugan, P.M. The Blue Compendium; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar] [CrossRef]
- Parsaeimehr, A.; Ozbay, G. Utilizing HRPzyme, a cost-effective Vibrio parahaemolyticus detection method. LWT 2023, 189, 115461. [Google Scholar] [CrossRef]
- Phillips, J.A. Dietary Guidelines for Americans, 2020–2025. Workplace Health Saf. 2021, 69, 395. [Google Scholar] [CrossRef] [PubMed]
- Djedjibegovic, J.; Marjanovic, A.; Tahirovic, D.; Caklovica, K.; Turalic, A.; Lugusic, A.; Omeragic, E.; Sober, M.; Caklovica, F. Heavy metals in commercial fish and seafood products and risk assessment in adult population in Bosnia and Herzegovina. Sci. Rep. 2020, 10, 13238. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Casagrande, N.; Verones, F.; Sobral, P.; Martinho, G. Physical properties of microplastics affecting the aquatic biota: A review. Environ. Adv. 2024, 17, 100566. [Google Scholar] [CrossRef]
- La Bella, G.; Basanisi, M.G.; Nobili, G.; Terio, V.; Suffredini, E.; La Salandra, G. First Report of Hepatitis E Virus in Shellfish in Southeast Italy. Appl. Sci. 2021, 11, 43. [Google Scholar] [CrossRef]
- Roy, P.K.; Roy, A.; Jeon, E.B.; DeWitt, C.A.M.; Park, J.W.; Park, S.Y. Comprehensive analysis of predominant pathogenic bacteria and viruses in seafood products. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13410. [Google Scholar] [CrossRef] [PubMed]
- Manjusha, L.; Kumar, H.S.; Nayak, B.B. Emerging Pathogens of Public Health Significance Associated with Seafood. In Advances in Fish Processing Technologies; Apple Academic Press: Palm Bay, FL, USA, 2023; pp. 377–394. [Google Scholar]
- Tlusty, M.F.; Tyedmers, P.; Bailey, M.; Ziegler, F.; Henriksson, P.J.G.; Béné, C.; Bush, S.; Newton, R.; Asche, F.; Little, D.C.; et al. Reframing the sustainable seafood narrative. Glob. Environ. Chang. 2019, 59, 101991. [Google Scholar] [CrossRef]
- Guillen, J.; Natale, F.; Carvalho, N.; Casey, J.; Hofherr, J.; Druon, J.N.; Fiore, G.; Gibin, M.; Zanzi, A.; Martinsohn, J.T. Global seafood consumption footprint. Ambio 2019, 48, 111–122. [Google Scholar] [CrossRef]
- Tran, N.; Chu, L.; Chan, C.Y.; Genschick, S.; Phillips, M.J.; Kefi, A.S. Fish supply and demand for food security in Sub-Saharan Africa: An analysis of the Zambian fish sector. Mar. Policy 2019, 99, 343–350. [Google Scholar] [CrossRef]
- Issifu, I.; Deffor, E.W.; Deyshappriya, N.P.R.; Dahmouni, I.; Sumaila, U.R. Drivers of Seafood Consumption at Different Geographical Scales. J. Sustain. Res. 2022, 4, e220012. [Google Scholar] [CrossRef]
- Naylor, R.L.; Kishore, A.; Sumaila, U.R.; Issifu, I.; Hunter, B.P.; Belton, B.; Bush, S.R.; Cao, L.; Gelcich, S.; Gephart, J.A.; et al. Blue food demand across geographic and temporal scales. Nat. Commun. 2021, 12, 5413. [Google Scholar] [CrossRef]
- Anderson Asche, F.; Garlock, T. Globalization and commoditization: The transformation of the seafood market. J. Commod. Mark. 2018, 12, 2–8. [Google Scholar] [CrossRef]
- Froehlich, H.E.; Couture, J.; Falconer, L.; Krause, G.; Morris, J.A.; Perez, M.; Stentiford, G.D.; Vehviläinen, H.; Halpern, B.S. Mind the gap between ICES nations’ future seafood consumption and aquaculture production. ICES J. Mar. Sci. 2021, 78, 468–477. [Google Scholar] [CrossRef]
- FAO. Brief to The State of World Fisheries and Aquaculture 2024; Blue Transformation in Action: Rome, Italy, 2024. [Google Scholar]
- Adeleke, B.; Robertson-Andersson, D.; Moodley, G.; Taylor, S. Aquaculture in Africa: A Comparative Review of Egypt, Nigeria, and Uganda Vis-À-Vis South Africa. Rev. Fish. Sci. Aquac. 2020, 29, 167–197. [Google Scholar] [CrossRef]
- Malcorps, W.; Newton, R.W.; Maiolo, S.; Eltholth, M.; Zhu, C.; Zhang, W.; Li, S.; Tlusty, M.; Little, D.C. Global seafood trade: Insights in sustainability messaging and claims of the major producing and consuming regions. Sustainability 2021, 13, 11720. [Google Scholar] [CrossRef]
- Lu, J.; Lin, Y.; Wu, J.; Zhang, C. Continental-scale spatial distribution, sources, and health risks of heavy metals in seafood: Challenge for the water-food-energy nexus sustainability in coastal regions? Environ. Sci. Pollut. Res. 2021, 28, 63815–63828. [Google Scholar] [CrossRef]
- Shamshak, G.L.; Anderson, J.L.; Asche, F.; Garlock, T.; Love, D.C. U.S. seafood consumption. J. World Aquac. Soc. 2019, 50, 715–727. [Google Scholar] [CrossRef]
- Dey, M.M.; Surathkal, P.; Chen, O.L.; Engle, C.R. Market trends for seafood products in the USA: Implication for Southern aquaculture products. Aquac. Econ. Manag. 2017, 21, 25–43. [Google Scholar] [CrossRef]
- Rene Blickem, E.; Bell, J.W.; Oliveira, A.C.M.; Mona Baumgartel, D.; DeBeer, J. An Analysis of Seafood Recalls in the United States, 2002 Through 2022. J. Food Prot. 2023, 86, 100090. [Google Scholar] [CrossRef]
- Love, D.C.; Asche, F.; Young, R.; Nussbaumer, E.M.; Anderson, J.L.; Botta, R.; Conrad, Z.; Froehlich, H.E.; Garlock, T.M.; Gephart, J.A.; et al. An Overview of Retail Sales of Seafood in the USA, 2017–2019. Rev. Fish. Sci. Aquac. 2022, 30, 259–270. [Google Scholar] [CrossRef]
- Golden, C.D.; Koehn, J.Z.; Shepon, A.; Passarelli, S.; Free, C.M.; Viana, D.F.; Matthey, H.; Eurich, J.G.; Gephart, J.A.; Fluet-Chouinard, E.; et al. Aquatic foods to nourish nations. Nature 2021, 598, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Seafood Health Facts. Seafood Nutrition Overview. Available online: https://www.seafoodhealthfacts.org/nutrition/seafood-nutrition-overview/ (accessed on 30 March 2025).
- U.S. Department of Agriculture, Agricultural Research Service BHNRC. Food Data Central. Available online: https://fdc.nal.usda.gov/ (accessed on 30 March 2025).
- Linus Pauling Institute at Oregon State University. Essential Fatty Acids. Last Reviewed 2019. Available online: https://lpi.oregonstate.edu/mic/other-nutrients/essential-fatty-acids#metabolism-bioavailability. (accessed on 30 October 2023).
- NIH Office of Dietary Supplements. Omega-3 Fatty Acids for Health Professionals. Updated 2023. Available online: https://ods.od.nih.gov/factsheets/Omega3FattyAcids-HealthProfessional/ (accessed on 25 October 2023).
- Burdge, G. Alpha-linolenic acid metabolism in men and women: Nutritional and biological implications. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Adarme-Vega, T.C.; Lim, D.K.; Timmins, M.; Vernen, F.; Li, Y.; Schenk, P.M. Microalgal biofactories: A promising approach towards sustainable omega-3 fatty acid production. Microb. Cell Fact. 2012, 11, 96. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cardoso, C.; Afonso, C.; Bandarra, N.M. Dietary DHA and health: Cognitive function ageing. Nutr. Res. Rev. 2016, 29, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Herrick, K.A.; Storandt, R.J.; Afful, J.; Pfeiffer, C.M.; Schleicher, R.L.; Gahche, J.J.; Potischman, N. Vitamin D status in the United States, 2011–2014. Am. J. Clin. Nutr. 2019, 110, 150–157. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cui, A.; Xiao, P.; Ma, Y.; Fan, Z.; Zhou, F.; Zheng, J.; Zhang, L. Prevalence, trend, and predictor analyses of vitamin D deficiency in the US population, 2001-2018. Front. Nutr. 2022, 9, 965376. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- NIHOffice of Dietary Supplements Vitamin, D. Updated 2023. Available online: https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/ (accessed on 31 October 2023).
- Schmid, A.; Walther, B. Natural vitamin D content in animal products. Adv. Nutr. 2013, 4, 453–462. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, Z.; Chen, T.C.; Zhang, A.; Persons, K.S.; Kohn, N.; Berkowitz, R.; Martinello, S.; Holick, M.F. An evaluation of the vitamin D3 content in fish: Is the vitamin D content adequate to satisfy the dietary requirement for vitamin D? J. Steroid Biochem. Mol. Biol. 2007, 103, 642–644. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jakobsen, J.; Smith, C.; Bysted, A.; Cashman, K.D. Vitamin D in Wild and Farmed Atlantic Salmon (Salmo Salar)-What Do We Know? Nutrients 2019, 11, 982. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academies Press: Washington, DC, USA, 1998. [Google Scholar] [PubMed]
- Wiedeman, A.M.; Barr, S.I.; Green, T.J.; Xu, Z.; Innis, S.M.; Kitts, D.D. Dietary Choline Intake: Current State of Knowledge Across the Life Cycle. Nutrients 2018, 10, 1513. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wallace, T.C.; Blusztajn, J.K.; Caudill, M.A.; Klatt, K.C.; Natker, E.; Zeisel, S.H.; Zelman, K.M. Choline: The Underconsumed and Underappreciated Essential Nutrient. Nutr. Today 2018, 53, 240–253. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- NIH Office of Dietary Supplements. Choline. Updated 2022. Available online: https://ods.od.nih.gov/factsheets/Choline-HealthProfessional/ (accessed on 31 October 2023).
- NIH Office of Dietary Supplements. Zinc. Updated 2022. Available online: https://ods.od.nih.gov/factsheets/zinc-healthprofessional/ (accessed on 1 November 2023).
- Maxfield, L.; Shukla, S.; Crane, J.S. Zinc Deficiency. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK493231/ (accessed on 28 June 2023).
- Choudhry, H.; Nasrullah, M. Iodine consumption and cognitive performance: Confirmation of adequate consumption. Food Sci. Nutr. 2018, 6, 1341–1351, Erratum in Food Sci. Nutr. 2021, 9, 1256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- NIH Office of Dietary Supplements. Iodine. Updated 2023. Available online: https://ods.od.nih.gov/factsheets/Iodine-HealthProfessional/ (accessed on 2 November 2023).
- NIH National Institute for Environmental Health Sciences. Endocrine Disruptors. Available online: https://www.niehs.nih.gov/health/topics/agents/endocrine#:~:text=Endocrine%20disruptors%20are%20found%20in,diet%2C%20skin%2C%20and%20water (accessed on 2 April 2024).
- Wang, M.; Wang, X.; Liu, J.; Wang, Z.; Jin, T.; Zhu, G.; Chen, X. The Association Between Cadmium Exposure and Osteoporosis: A Longitudinal Study and Predictive Model in a Chinese Female Population. Front. Public Health 2021, 9, 762475. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; Institute of Medicine: Washington, DC, USA, 2000. [Google Scholar]
- Fernández-Bautista, T.; Gómez-Gómez, B.; Gracia-Lor, E.; Pérez-Corona, T.; Madrid, Y. Selenium Health Benefit Values and Hg and Se speciation studies for elucidating the quality and safety of highly consumed fish species and fish-derived products. Food Chem. 2024, 435, 137544. [Google Scholar] [CrossRef]
- Kosnett, M.; Chan, L.; Faustman, E.; Foldy, S.; Miller, G.; Takaro, T.; Ahmed, S.; Allen, P.; Boehme, J.; Gabriel, M.; et al. A Review of Human Health Impacts of Selenium in Aquatic Systems. A report submitted to the International Joint Commission by the Health Professionals Advisory Board. 2020. Available online: https://ijc.org/sites/default/files/2020-09/HPAB_SeleniumHealthReview_2020.pdf (accessed on 15 April 2025).
- Burger, J.; Gochfeld, M.; Jeitner, C.; Donio, M.; Pittfield, T. Selenium: Mercury molar ratios in freshwater fish from Tennessee: Individual, species, and geographical variations have implications for management. Ecohealth 2012, 9, 171–182. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oxford Languages Dictionary. Available online: https://www.oxfordlearnersdictionaries.com/definition/english/microplastic (accessed on 15 April 2025).
- National Oceanic and Atmospheric Administration. What Are Microplastics? Available online: https://oceanservice.noaa.gov/facts/microplastics.html (accessed on 15 April 2024).
- Gigault, J.; Halle, A.T.; Baudrimont, M.; Pascal, P.Y.; Gauffre, F.; Phi, T.L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, O.A.; Rahman, M.S. An ecotoxicological approach to microplastics on terrestrial and aquatic organisms: A systematic review in assessment, monitoring and biological impact. Environ. Toxicol. Pharmacol. 2021, 84, 103615. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhao, F.; Tong, L.; Wang, S.; Zhou, D. Contamination, bioaccumulation mechanism, detection, and control of human norovirus in bivalve shellfish: A review. Crit. Rev. Food Sci. Nutr. 2021, 62, 8972–8985. [Google Scholar] [CrossRef]
- Watson-Wright, W.M.; Wells, P.G.; Duce, R.A.; Gilardi, K.V.; Girvan, A.S.T.; Huber, M.E.; Kershaw, P.J.; Linders, J.B.H.J.; Luit, R.J.; Vivian, C.M.G.; et al. The UN Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection (GESAMP)—An ocean science-policy interface standing the test of time. Mar. Pollut. Bull. 2024, 199, 115917. [Google Scholar] [CrossRef]
- Guzzetti, E.; Sureda, A.; Tejada, S.; Faggio, C. Microplastic in marine organism: Environmental and toxicological effects. Environ. Toxicol. Pharmacol. 2018, 64, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef] [PubMed]
- Andra, S.S.; Makris, K.C. Thyroid disrupting chemicals in plastic additives and thyroid health. J. Environ. Sci. Health Part C 2012, 30, 107–151. [Google Scholar] [CrossRef] [PubMed]
- Stevens, S.; McPartland, M.; Bartosova, Z.; Skåland, H.S.; Völker, J.; Wagner, M. Plastic Food Packaging from Five Countries Contains Endocrine- and Metabolism-Disrupting Chemicals. Environ. Sci. Technol. 2024, 58, 4859–4871. [Google Scholar] [CrossRef] [PubMed]
- Dan, D.; Qu, K. Environmental Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Ciro, M.; Rongioletti, A.; Baiocco, F.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Mišľanová, C.; Valachovičová, M.; Slezáková, Z. An Overview of the Possible Exposure of Infants to Microplastics. Life 2024, 14, 371. [Google Scholar] [CrossRef] [PubMed]
- Domenech, J.; Annangi, B.; Marcos, R.; Hernández, A.; Catalán, J. Insights into the potential carcinogenicity of micro- and nano-plastics. Mutat. Res. Rev. Mutat. Res. 2023, 791, 108453. [Google Scholar] [CrossRef] [PubMed]
- Garrido Gamarro, E.; Costanzo, V. Microplastics in Food Commodities—A Food Safety Review on Human Exposure Through Dietary Sources; Food Safety and Quality Series No. 18; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Ullah, S.; Ahmad, S.; Guo, X.; Ullah, S.; Ullah, S.; Nabi, G.; Wanghe, K. A review of the endocrine disrupting effects of micro and nano plastic and their associated chemicals in mammals. Front. Endocrinol. 2023, 13, 1084236. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism, and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- David, G.; Isangedighi, I.I. Heavy Metals Contamination in Fish: Effects on Human Health. J. Aquat. Sci. Mar. Biol. 2019, 2, 7–12. [Google Scholar] [CrossRef]
- Has-Schön, E.; IBogut, I. Strelec. Heavy metal profile in five fish species included in human diet, domiciled in the end flow of River Neretva (Croatia). Arch. Environ. Contam. Toxicol. 2006, 50, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Kuras, R.; Janasik, B.; Stanislawska, M.; Kozlowska, L.; Wasowicz, W. Assessment of Mercury Intake from Fish Meals Based on Intervention Research in the Polish Subpopulation. Biol. Trace Elem. Res. 2017, 179, 23–31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Winiarska-Mieczan, A.; Florek, M.; Kwiecień, M.; Kwiatkowska, K.; Krusiński, R. Cadmium and Lead Content in Chosen Commercial Fishery Products Consumed in Poland and Risk Estimations on Fish Consumption. Biol. Trace Elem. Res. 2018, 182, 373–380. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kimáková, T.; Kuzmová, L.; Nevolná, Z.; Bencko, V. Fish, and fish products as risk factors of mercury exposure. Ann. Agric. Environ. Med. 2018, 25, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Castro-González, M.I.; Méndez-Armenta, M. Heavy metals: Implications associated to fish consumption. Environ. Toxicol. Pharmacol. 2008, 26, 263–271. [Google Scholar] [CrossRef] [PubMed]
- EPAc. Mercury Compounds. Available online: https://www.epa.gov/system/files/documents/2021-12/mercury-compounds_12-3-2021_final.pdf (accessed on 11 October 2023).
- Bradley, M.A.; Barst, B.D.; Basu, N. A review of mercury bioavailability in humans and fish. Int. J. Environ. Res. Public Health 2017, 14, 169. [Google Scholar] [CrossRef] [PubMed]
- EPAd. Technical Factsheet on Lead. Available online: https://archive.epa.gov/water/archive/web/pdf/archived-technical-fact-sheet-on-lead.pdf#:~:text=Lead%20does%20not%20appear%20to%20bioconcentrate%20significantly%20in,portions%20do%20not%20pose%20a%20human%20health%20danger (accessed on 11 October 2023).
- Pastorelli, A.A.; Baldini, M.; Stacchini, P.; GBaldini, S.; Morelli, E.; Sagratella, S.; Zaza, S.; Ciardullo, S. Human exposure to lead, cadmium and mercury through fish and seafood product consumption in Italy: A pilot evaluation. Food Addit. Contam. Part A 2012, 29, 1913–1921. [Google Scholar] [CrossRef]
- Garofalo, L.; Sala, M.; Focardi, C.; Pasqualetti, P.; Delfino, D.; D’Onofrio, F.; Droghei, B.; Pasquali, F.; Nicolini, V.; Galli, F.S.; et al. Monitoring of Cadmium, Lead, and Mercury Levels in Seafood Products: A Ten-Year Analysis. Foods 2025, 14, 451. [Google Scholar] [CrossRef]
- Barchiesi, F.; Branciari, R.; Latini, M.; Roila, R.; Lediani, G.; Filippini, G.; Scortichini, G.; Piersanti, A.; Rocchegiani, E.; Ranucci, D. Heavy Metals Contamination in Shellfish: Benefit-Risk Evaluation in Central Italy. Foods 2020, 9, 1720. [Google Scholar] [CrossRef]
- WHO Lead. Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/Home/Chemical/3511 (accessed on 11 October 2023).
- Centers for Disease Control Prevention (CDC). Surveillance for Foodborne Disease Outbreaks United States, 2016: Annual Report; U.S. Department of Health and Human Services, CDC: Atlanta, GA, USA, 2018. [Google Scholar]
- FDA Cadmium in Food and Foodwares. 2024. Available online: https://www.fda.gov/food/environmental-contaminants-food/cadmium-food-and-foodwares (accessed on 30 March 2025).
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Scientific Opinion on the risks for animal and public health related to the presence of T-2 and HT-2 toxin in food and feed. EFSA J. 2011, 9, 2481. [CrossRef]
- British Columbia Centre for Disease Control. Cadmium in BC Shellfish. 2013. Available online: http://www.bccdc.ca/resource-gallery/Documents/Educational%20Materials/EH/FPS/Fish/CadmiuminBCShellfishNov13.pdf (accessed on 30 March 2025).
- He, Y.; Fang, H.; Pan, X.; Zhu, B.; Chen, J.; Wang, J.; Zhang, R.; Chen, L.; Qi, X.; Zhang, H. Cadmium Exposure in Aquatic Products and Health Risk Classification Assessment in Residents of Zhejiang, China. Foods 2023, 12, 3094. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Palermo, T.; Meliker, J. Seafood intake and blood cadmium in a cohort of adult avid seafood consumers. Int. J. Hyg. Environ. Health 2015, 218, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.G.; De-la-Torre, G.E. Per-and polyfluoroalkyl substances (PFASs) in consumable species and food products. J. Food Sci. Technol. 2023, 60, 2319–2336. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority) Panel on Contaminants in the Food Chain; Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.C. Risk to human health related to the presence of perfluoroalkyl substances in food. EFSA J. 2020, 18, e06223. [Google Scholar]
- Barbo, N.; Stoiber, T.; Naidenko, O.V.; Andrews, D.Q. Locally caught freshwater fish across the United States are likely a significant source of exposure to PFOS and other perfluorinated compounds. Environ. Res. 2023, 220, 115165. [Google Scholar] [CrossRef]
- EPA National Rivers and Streams Assessment: The Third Collaborative Survey. 2023. Available online: https://riverstreamassessment.epa.gov/webreport/#per-and-polyfluoroalkyl-substances-pfas (accessed on 10 February 2024).
- National Academies of Sciences, Engineering, and Medicine (NASEM). Exposure to Contaminants Associated with Consumption of Seafood. In The Role of Seafood Consumption in Child Growth and Development; National Academies Press US: Washington, DC, USA, 2024. [Google Scholar]
- Hamade, A. Fish consumption benefits and PFAS risks: Epidemiology and public health recommendations. Toxicol. Rep. 2024, 13, 101736. [Google Scholar] [CrossRef]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A Review of the Pathways of Human Exposure to Poly- and Perfluoroalkyl Substances (PFASs) and Present Understanding of Health Effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.Y.; Raymond, M.; Blackowicz, M.; Liu, Y.; Thompson, B.A.; Anderson, H.A.; Turyk, M. Perfluoroalkyl substances and fish consumption. Environ. Res. 2017, 154, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Chemicals, L.C.P. Action Plan. 2020. Available online: https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/long-chain-perfluorinated-chemicals-pfcs-action-plan (accessed on 30 March 2025).
- McCarthy, C.; Kappleman, W.; DiGuiseppi, W. Ecological considerations of per-and polyfluoroalkyl substances (PFAS). Curr. Pollut. Rep. 2017, 3, 289–301. [Google Scholar] [CrossRef]
- Bedi, M.; Sapozhnikova, Y.; Taylor, R.B.; Ng, C. Per-and polyfluoroalkyl substances (PFAS) measured in seafood from a cross-section of retail stores in the United States. J. Hazard. Mater. 2023, 459, 132062. [Google Scholar] [CrossRef]
- Brendel, S.; Fetter, É.; Staude, C.; Vierke, L.; Biegel-Engler, A. Short chain perfluoroalkyl acids: Environmental concerns and a regulatory strategy under REACH. Environ. Sci. Eur. 2018, 30, 9. [Google Scholar] [CrossRef] [PubMed]
- De Silva, A.O.; Spencer, C.; Scott, B.F.; Backus, S.; Muir, D.C. Detection of a cyclic perfluorinated acid, perfluoroethylcyclohexane sulfonate, in the Great Lakes of North America. Environ. Sci. Technol. 2011, 45, 8060–8066. [Google Scholar] [CrossRef]
- De Silva, A.O.; Armitage, J.M.; Bruton, T.A.; Dassuncao, C.; Heiger-Bernays, W.; Hu, X.C.; Kärrman, A.; Kelly, B.; Ng, C.; Robuck, A.; et al. PFAS exposure pathways for humans and wildlife: A synthesis of current knowledge and key gaps in understanding. Environ. Toxicol. Chem. 2021, 40, 631–657. [Google Scholar] [CrossRef]
- Dassuncao, C.; Hu, X.C.; Nielsen, F.; Weihe, P.; Grandjean, P.; Sunderland, E.M. Shifting global exposures to poly-and perfluoroalkyl substances (PFASs) evident in longitudinal birth cohorts from a seafood-consuming population. Environ. Sci. Technol. 2018, 52, 3738–3747. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; et al. Risk to human health related to the presence of perfluorooctane sulfonic acid and perfluorooctanoic acid in food. EFSA J. 2018, 16, e05194. [Google Scholar]
- Young, W.; Wiggins, S.; Limm, W.; Fisher, C.M.; DeJager, L.; Genualdi, S. Analysis of per-and poly (fluoroalkyl) substances (PFASs) in highly consumed seafood products from US markets. J. Agric. Food Chem. 2022, 70, 13545–13553. [Google Scholar] [CrossRef] [PubMed]
- Ruffle, B.; Vedagiri, U.; Bogdan, D.; Maier, M.; Schwach, C.; Murphy-Hagan, C. Perfluoroalkyl Substances in US market basket fish and shellfish. Environ. Res. 2020, 190, 109932. [Google Scholar] [CrossRef] [PubMed]
- Fair, P.A.; Wolf, B.; White, N.D.; Arnott, S.A.; Kannan, K.; Karthikraj, R.; Vena, J.E. Perfluoroalkyl substances (PFASs) in edible fish species from Charleston Harbor and tributaries, South Carolina, United States: Exposure and risk assessment. Environ. Res. 2019, 171, 266–277. [Google Scholar] [CrossRef]
- Biji, K.B.; Ravishankar, C.N.; Venkateswarlu, R.; Mohan, C.O.; and Gopal, T.S. Biogenic amines in seafood: A review. J. Food Sci. technology 2016, 53, 2210–2218. [Google Scholar] [CrossRef]
- Oktariani, A.F.; Ramona, Y.; Sudaryatma, P.E.; Dewi, I.A.M.M.; Shetty, K. Role of marine bacterial contaminants in histamine formation in seafood products: A review. Microorganisms 2022, 10, 1197. [Google Scholar] [CrossRef]
- Lin, C.S.; Kung, H.F.; Lin, C.M.; Tsai, H.C.; Tsai, Y.H. Histamine production by Raoultella ornithinolytica in mahi-mahi meat at various storage temperatures. J. Food Drug Anal. 2016, 24, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Enache, E.; Kataoka, A.I.; Black, D.G.; Weddig, L.; Hayman, M.; Bjornsdottir-Butler, K. Heat resistance of histamine-producing bacteria in irradiated tuna loins. J. Food protection 2013, 76, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M.; Ferrer, S.; Pardo, I. Which lactic acid bacteria are responsible for histamine production in wine? J. Appl. Microbiol. 2005, 99, 580–586. [Google Scholar] [CrossRef]
- Møller, C.D.A.; Ücok, E.F.; Rattray, F.P. Histamine forming behaviour of bacterial isolates from aged cheese. Food Res. Int. 2020, 128, 108719. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.; del Rio, B.; Ladero, V.; Redruello, B.; Fernández, M.; Martin, M.C.; Alvarez, M.A. Isolation and typification of histamine-producing Lactobacillus vaginalis strains from cheese. Int. J. Food Microbiol. 2015, 215, 117–123. [Google Scholar] [CrossRef]
- Ohshima, C.; Sato, F.; Takahashi, H.; Kuda, T.; Kimura, B. Development of the genus and species determination method for histamine producing bacteria isolated from fishery product with high-resolution melting analysis. Food Hyg. Saf. Sci. (Shokuhin Eiseigaku Zasshi) 2019, 60, 168–175. [Google Scholar] [CrossRef]
- Kanki, M.; Yoda, T.; Tsukamoto, T.; Baba, E. Histidine decarboxylases and their role in accumulation of histamine in tuna and dried saury. Appl. Environ. Microbiol. 2007, 73, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Lunestad, B.T.; Levsen, A.; Rosnes, J.T. Tracing pathogens in fish production chains. In Tracing Pathogens in the Food Chain; Woodhead Publishing: Cambridge, UK, 2011; pp. 433–464. [Google Scholar]
- Taylor, S.L.; Scanlan, R.A. (Eds.) Food Toxicology: A Perspective on the Relative Risks; Marcel Dekker Inc.: New York, NY, USA, 1989; pp. xiii+–466. [Google Scholar]
- Nuñez, M.; del Olmo, A.; Calzada, J. Biogenic Amines. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 416–423. [Google Scholar]
- Traylor, J.; Mathew, D. Histamine Toxicity. [Updated 2023 Jun 26]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://pubmed.ncbi.nlm.nih.gov/29763046/ (accessed on 15 April 2025).
- Bjornsdottir-Butler, K.; Bencsath, F.A.; McCarthy, S.; Benner, R.A., Jr. Heat resistance of histidine decarboxylase from gram-negative histamine-producing bacteria in seafood. J. Food Prot. 2017, 80, 1273–1279. [Google Scholar] [CrossRef]
- Tantasuttikul, A.; Mahakarnchanakul, W. Growth parameters and sanitizer resistance of Raoultella ornithinolytica and Raoultella terrigena isolated from seafood processing plant. Cogent Food Agric. 2019, 5, 1569830. [Google Scholar] [CrossRef]
- Hu, J.W.; Cao, M.J.; Guo, S.C.; Zhang, L.J.; Su, W.J.; Liu, G.M. Identification and inhibition of histamine-forming bacteria in blue scad (Decapterus maruadsi) and chub mackerel (Scomber japonicus). J. Food Prot. 2015, 78, 383–389. [Google Scholar] [CrossRef]
- Dityanawarman, A.; Puspita, I.D.; Ratnawati, S.E.; Ekantari, N.; Tamplin, M. Growth rate and histamine production of Klebsiella sp. CK02 Isolated from skipjack tuna compared with Morganella morganii ATCC 25830 at various incubation temperatures. Squalen. Bull. Mar. Fish. Postharvest Biotechnol. 2020, 15, 1–9. [Google Scholar] [CrossRef]
- Landete, J.M.; De Las Rivas, B.; Marcobal, A.; Munoz, R. Updated molecular knowledge about histamine biosynthesis by bacteria. Crit. Rev. Food Sci. Nutr. 2008, 48, 697–714. [Google Scholar] [CrossRef]
- García-Tapia, G.; Barba-Quintero, G.; Gallegos-Infante, J.A.; Aguilar, R.P.; Ruíz-Cortés, J.A.; Ramírez, J.A. Influence of physical damage and freezing on histamine concentration and microbiological quality of yellowfin tuna during processing. Food Sci. Technol. 2013, 33, 463–467. [Google Scholar] [CrossRef]
- Björnsdóttir-Butler, K.; Bolton, G.E.; Jaykus, L.A.; McClellan-Green, P.D.; Green, D.P. Development of molecular-based methods for determination of high histamine producing bacteria in fish. Int. J. Food Microbiol. 2010, 139, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Debeer, J.; Bell, J.W.; Nolte, F.; Arcieri, J.; Correa, G. Histamine limits by country: A survey and review. J. Food Prot. 2021, 84, 1610–1628. [Google Scholar] [CrossRef] [PubMed]
- Schink, M.; Konturek, P.C.; Tietz, E.; Dieterich, W.; Pinzer, T.C.; Wirtz, S.; Neurath, M.F.; Zopf, Y. Microbial patterns in patients with histamine intolerance. J. Physiol. Pharmacol. 2018, 69, 579–593. [Google Scholar]
- Emborg, J. Morganella Psychrotolerans-Identification, Histamine Formation Importance for Histamine Fish Poisoning. Ph.D. Thesis, Danish Institute for Fisheries Research, Technical University of Denmark, Kongens Lyngby, Denmark, December 2007. [Google Scholar]
- Mommert, S.; Hüer, M.; Schaper-Gerhardt, K.; Gutzmer, R.; Werfel, T. Histamine up-regulates oncostatin M expression in human M1 macrophages. Br. J. Pharmacol. 2020, 177, 600–613. [Google Scholar] [CrossRef]
- Saito, H. Mast cell research. In History of Allergy; Karger Publishers: Basel, Switzerland, 2014; Volume 100, pp. 165–171. [Google Scholar]
- Dwidar, M.; Yokobayashi, Y. Development of a histamine aptasensor for food safety monitoring. Sci. Rep. 2019, 9, 16659. [Google Scholar] [CrossRef]
- Barcik, W.; Pugin, B.; Brescó, M.S.; Westermann, P.; Rinaldi, A.; Groeger, D.; Van Elst, D.; Sokolowska, M.; Krawczyk, K.; Frei, R.; et al. Bacterial secretion of histamine within the gut influences immune responses within the lung. Allergy 2019, 74, 899–909. [Google Scholar] [CrossRef]
- Khan, F.; Orson, F.; Ogawa, Y.; Parker, C.; and Davis, C.M. Adult seafood allergy in the Texas Medical Center: A 13-year experience. Allergy Rhinol. 2011, 2, ar-2011. [Google Scholar] [CrossRef]
- Lopata, A.L.; Lehrer, S.B. New insights into seafood allergy. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Villacis, J.; Rice, T.R.; Bucci, L.R.; El-Dahr, J.M.; Wild, L.; Demerell, D.; Soteres, D.; Lehrer, S.B. Do shrimp-allergic individuals tolerate shrimp-derived glucosamine? Clin. Exp. Allergy 2006, 36, 1457–1461. [Google Scholar] [CrossRef]
- James, J.M.; Crespo, J.F. Allergic reactions to foods by inhalation. Curr. Allergy Asthma Rep. 2007, 7, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Lopata, A.L.; Jeebhay, M.F. Seafood allergy in south Africa-studies in the domestic and occupational setting. Allergy Clin. Immunol. Int. 2001, 13, 204–210. [Google Scholar] [CrossRef]
- Ben-Shoshan, M.; Harrington, D.W.; Soller, L.; Fragapane, J.; Joseph, L.; St Pierre, Y.; Godefroy, S.B.; Elliot, S.J.; Clarke, A.E. A population-based study on peanut, tree nut, fish, shellfish, and sesame allergy prevalence in Canada. J. Allergy Clin. Immunol. 2010, 125, 1327–1335. [Google Scholar] [CrossRef]
- Ross, M.P.; Ferguson, M.; Street, D.; Klontz, K.; Schroeder, T.; Luccioli, S. Analysis of food-allergic and anaphylactic events in the National Electronic Injury Surveillance System. J. Allergy Clin. Immunol. 2008, 121, 166–171. [Google Scholar] [CrossRef]
- Cianferoni, A.; Novembre, E.; Mugnaini, L.; Lombardi, E.; Bernardini, R.; Pucci, N.; Vierucci, A. Clinical features of acute anaphylaxis in patients admitted to a university hospital: An 11-year retrospective review (1985–1996). Ann. Allergy Asthma Immunol. 2001, 87, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.J.; Thalayasingam, M.; Lee, B.W. Food allergy in Asia: How does it compare? Asia Pac. Allergy 2013, 3, 3–14. [Google Scholar] [CrossRef]
- Turner, P.; Ng, I.; Kemp, A.; Campbell, D. Seafood allergy in children: A descriptive study. Ann. Allergy Asthma Immunol. 2011, 106, 494–501. [Google Scholar] [CrossRef]
- Zhang, Y.; Matsuo, H.; Morita, E. Cross-reactivity among shrimp, crab, and scallops in a patient with a seafood allergy. J. Dermatol. 2006, 33, 174–177. [Google Scholar] [CrossRef]
- Maulitz, R.M.; Pratt, D.S.; and Schocket, A.L. Exercise-induced anaphylactic reaction to shellfish. J. Allergy Clin. Immunol. 1979, 63, 433–434. [Google Scholar] [CrossRef] [PubMed]
- Prester, L. Seafood allergy, toxicity, and intolerance: A review. J. Am. Coll. Nutr. 2016, 35, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Rona, R.J.; Keil, T.; Summers, C.; Gislason, D.; Zuidmeer, L.; Sodergren, E.; Sigurdardottir, S.T.; Lindner, T.; Goldhahn, K.; Dahlstrom, J.; et al. The prevalence of food allergy: A meta-analysis. J. Allergy Clin. Immunol. 2007, 120, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, A.; Cruz, L.; and Oliveira, J.F. Shrimp allergy: Clinical characteristics and food challenge outcome in a centre in the north of Portugal. Clin. Transl. Allergy 2013, 3, 1. [Google Scholar] [CrossRef]
- Sharp, M.F.; Lopata, A.L. Fish allergy: In review. Clin. Rev. Allergy Immunol. 2014, 46, 258–271. [Google Scholar] [CrossRef]
- Dumen, E.; Ekici, G.; Ergin, S.; Bayrakal, G.M. Presence of Foodborne Pathogens in Seafood and Risk Ranking for Pathogens. Foodborne Pathog. Dis. 2020, 17, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, S. Seafood-borne parasitic diseases: A “one-health” approach is needed. Fishes 2019, 4, 9. [Google Scholar] [CrossRef]
- Iwamoto, M.; Ayers, T.; Mahon, B.E.; Swerdlow, D.L. Epidemiology of seafood-associated infections in the United States. Clin. Microbiol. Rev. 2010, 23, 399–411. [Google Scholar] [CrossRef]
- Barrett, K.A.; Nakao, J.H.; Taylor, E.V.; Eggers, C.; Gould, L.H. Fish-Associated Foodborne Disease Outbreaks: United States, 1998-2015. Foodborne Pathog. Dis. 2017, 14, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Khora, S.S. Risk from viral pathogens in seafood. In Diet, Microbiome and Health; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Holst, M.; Beth, W.; Carolyn, C.; Jeffrey, T.; Irving, D.; David, N. Contributing Factors of Foodborne Illness Outbreaks—National Outbreak Reporting System, United States, 2014–2022. MMWR. Surveill. Summ. 2025, 74, 1–12. [Google Scholar] [CrossRef]
- Rowan, N.J. Current decontamination challenges and potentially complementary solutions to safeguard the vulnerable seafood industry from recalcitrant human norovirus in live shellfish: Quo Vadis? Sci. Total Environ. 2023, 874, 162380. [Google Scholar] [CrossRef]
- Sun, Y.; Liang, M.; Zhao, F.; Su, L. Research Progress on Biological Accumulation, Detection and Inactivation Technologies of Norovirus in Oysters. Foods 2023, 12, 3891. [Google Scholar] [CrossRef] [PubMed]
- Fehrenbach, G.W.; Murphy, E.; Pogue, R.; Carter, F.; Clifford, E.; Major, I. Comprehensive analysis and assessment of exposure to enteric viruses and bacteria in shellfish. Mar. Environ. Res. 2024, 196, 106404. [Google Scholar] [CrossRef] [PubMed]
- Gokoglu, N. Shellfish Processing and Preservation; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- McLeod, C.; Polo, D.; Le Saux, J.C.; Le Guyader, F.S. Depuration and Relaying: A Review on Potential Removal of Norovirus from Oysters. Compr. Rev. Food Sci. Food Saf. 2017, 16, 692–706. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, G.; Guercio, A.; Gucciardi, F.; Di Bella, S.; La Rosa, G.; Suffredini, E.; Randazzo, W.; Purpari, G. Occurrence of Human Enteric Viruses in Shellfish along the Production and Distribution Chain in Sicily, Italy. Foods 2021, 10, 1384. [Google Scholar] [CrossRef] [PubMed]
- CDC Norovirus Outbreaks. 2025. Available online: https://www.cdc.gov/norovirus/outbreak-basics/ (accessed on 30 March 2025).
- Hunt, K.; Doré, B.; Keaveney, S.; Rupnik, A.; Butler, F. A quantitative exposure assessment model for norovirus in oysters harvested from a classified production area. Microb. Risk Anal. 2023, 23, 100247. [Google Scholar] [CrossRef]
- Yu, Z.; Xu, Z.; Chen, J.; Chen, L.; Liao, N.; Zhang, R.; Cheng, D. Quantitative Risk Assessment of Five Foodborne Viruses in Shellfish Based on Multiplex qPCR. Foods 2023, 12, 3462. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, V.; Elois, M.; Pereira, B.; Marília, S.; Juliano, M.; Lindner, D.D. Bioaccumulation Dynamic by Crassostrea gigas Oysters of Viruses That Are Proposed as Surrogates for Enteric Virus Contamination in Environmental Samples. Food Environ. Virol. 2023, 15, 1–7. [Google Scholar] [CrossRef]
- Di Cola, G.; Fantilli, A.C.; Pisano, M.B.; Ré, V.E. Foodborne transmission of hepatitis A and hepatitis E viruses: A literature review. Int. J. Food Microbiol. 2021, 338, 108986. [Google Scholar] [CrossRef]
- Krahulcová, M.; Cverenkárová, K.; Koreneková, J.; Oravcová, A.; Koščová, J.; Bírošová, L. Occurrence of Antibiotic-Resistant Bacteria in Fish and Seafood from Slovak Market. Foods 2023, 12, 3912. [Google Scholar] [CrossRef]
- Kumar, S.; Lekshmi, M.; Parvathi, A.; Nayak, B.B.; Varela, M.F. Antibiotic resistance in seafood-borne pathogens. In Foodborne Pathogens and Antibiotic Resistance; El-Samragy, Y., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2016; pp. 397–415. [Google Scholar] [CrossRef]
- Parlapani, F.F. Microbial diversity of seafood. Curr. Opin. Food Sci. 2021, 37, 45–51. [Google Scholar] [CrossRef]
- Chatreman, N.; Seecharran, D.; Ansari, A.A. Prevalence and distribution of pathogenic bacteria found in fish and fishery products: A review. J. Fish. Life Sci. 2020, 5, 53–65. [Google Scholar]
- Álvarez-Contreras, A.K.; Quiñones-Ramírez, E.I.; Vázquez-Salinas, C. Prevalence, detection of virulence genes and antimicrobial susceptibility of pathogen Vibrio species isolated from different types of seafood samples at “La Nueva Viga” market in Mexico City. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2021, 114, 1417–1429. [Google Scholar] [CrossRef]
- Afreen, M.; Ucak, I. Food-borne pathogens in seafood. Eurasian J. Agric. Res. 2021, 5, 44–58. [Google Scholar]
- Elbashir, S.; Jahncke, M.; DePaola, A.; Bowers, J.; Schwarz, J.; Punchihewage-Don, A.J.; Min, B.; Rippen, T.; Parveen, S. Prevalence and Abundance of Bacterial Pathogens of Concern in Shrimp, Catfish and Tilapia Obtained at Retail Stores in Maryland, USA. Pathogens 2023, 12, 187. [Google Scholar] [CrossRef]
- Diner, R.E.; Zimmer-Faust, A.; Cooksey, E.; Allard, S.; Kodera, S.M.; Kunselman, E.; Garodia, Y.; Allen, A.E.; Griffith, J.; Gilbert, J.A. Host and Water Microbiota are Differentially Linked to Potential Human Pathogen Accumulation in Oysters. BioRxiv 2022. [Google Scholar] [CrossRef] [PubMed]
- CDC. About Vibrio Infection. 2024. Available online: https://www.cdc.gov/vibrio/about/?CDC_AAref_Val=https://www.cdc.gov/vibrio/faq.html (accessed on 30 March 2025).
- Abioye, O.E.; Nontongana, N.; Osunla, C.A.; Okoh, A.I. Antibiotic resistance and virulence genes profiling of Vibrio cholerae and Vibrio mimicus isolates from some seafood collected at the aquatic environment and wet markets in Eastern Cape Province, South Africa. PLoS ONE 2023, 18, e0290356. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.T.T.; Hoang, T.T.H.; Fleischmann, S.; Pham, H.N.; Lai, T.L.H.; Cam, T.T.H.; Truong, L.O.; Le, V.P.; Alter, T. Quantification and Antimicrobial Resistance of Vibrio parahaemolyticus in Retail Seafood in Hanoi, Vietnam. J. Food Prot. 2022, 85, 786–791. [Google Scholar] [CrossRef]
- Yu Huang, Z.; Xiao, Y.; Wang, D. Shewanella infection in humans: Epidemiology, clinical features and pathogenicity. Virulence 2022, 13, 1515–1532. [Google Scholar] [CrossRef]
- Jung-Schroers, V.; Jung, A.; Ryll, M.; Bauer, J.; Teitge, F.; Steinhagen, D. Methods for identification and differentiation of different Shewanella spp. isolates for diagnostic use. J. Fish Dis. 2018, 41, 689–714. [Google Scholar] [CrossRef]
- Li, Y.; Qi, R.; Yang, H.; Zhang, X.; Wu, Y.; Huang, B.; Zhao, Q.; Gu, Y. Analysis of clinical characteristics of infections caused by shewanella species. Indian J. Med. Microbiol. 2024, 49, 100574. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.N.N.; Nasir, N.M.; Sahrani, F.K.; Ahmad, A.; Sairi, F. Characterization of putative pathogenic Shewanella algae isolated from ballast water. Vet. World 2021, 14, 678–688. [Google Scholar] [CrossRef]
- Kalathuru, S.; Singla, A.; Kumar, A.; Swami, A. Shewanella algae, an Emerging Pathogen, Causing Skin and Soft Tissue Infections. Infect. Dis. Clin. Pract. 2023, 31, e1286. [Google Scholar] [CrossRef]
- Ainoda, Y.; Tanaka, E.; Wajima, T.; Nakaminami, H.; Hirota, Y.; Matsushita, T.; Hirai, Y. A case of Shewanella algae-induced bacteremia in Japan: Case report and literature review. J. Infect. Chemother. 2022, 28, 1430–1432. [Google Scholar] [CrossRef]
- Zhang, W.; Lv, X.; Liu, Z.; Ni, L. The spoilage and adhesion inhibitory effects of Bacillus subtilis against Shewanella and Pseudomonas in large yellow croaker (Pseudosciaena crocea). Food Sci. Technol. 2022, 42, e02721. [Google Scholar] [CrossRef]
- Zan, Z.; Han, Z.; Hou, Y.; Zhang, Y.; Sun, J. Development of a loop-mediated isothermal amplification method for detection of Shewanella algae in fish. Aquac. Rep. 2022, 26, 101292. [Google Scholar] [CrossRef]
- Paździor, E.; Pękala-Safińska, A.; Wasyl, D. Genotypic diversity among Shewanella spp. collected from freshwater fish. J. Fish Dis. 2019, 42, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, W.F.; Giambiagi-deMarval, M.; Laport, M.S. Shewanella harboring antimicrobial and copper resistance genes in sea urchins (Paracentrotus lividus) from the Crozon peninsula (Brittany, France). Infect. Genet. Evol. 2020, 85, 104437. [Google Scholar] [CrossRef]
- Marathe, N.P.; Salvà-Serra, F.; Nimje, P.S.; Moore, E.R.B. Novel Plasmid Carrying Mobile Colistin Resistance Gene mcr-4.3 and Mercury Resistance Genes in Shewanella baltica: Insights into Mobilization of mcr-4.3 in Shewanella Species. Microbiol. Spectr. 2022, 10, e0203722. [Google Scholar] [CrossRef]
- Kang, C.H.; So, J.S. Antibiotic and heavy metal resistance in Shewanella putrefaciens strains isolated from shellfishes collected from West Sea, Korea. Mar. Pollut. Bull. 2016, 112, 111–116. [Google Scholar] [CrossRef]
- Ben Mhenni, N.; Alberghini, G.; Giaccone, V.; Truant, A.; Catellani, P. Prevalence and Antibiotic Resistance Phenotypes of Pseudomonas spp. in Fresh Fish Fillets. Foods 2023, 12, 950. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.A.; El-Tahlawy, A.S.; Abdelmoneim, H.M.; Abdallah, K.M.E.; El Bayomi, R.M. Pseudomonas aeruginosa in Fish and Fish Products: A Review on the Incidence, Public Health Significance, Virulence Factors, Antimicrobial Resistance, and Biofilm Formation. J. Adv. Vet. Res. 2023, 13, 1464–1468. [Google Scholar]
- Abril, A.G.; Calo-Mata, P.; Böhme, K.; Villa, T.G.; Barros-Velázquez, J.; Sánchez-Pérez, Á.; Pazos, M.; Carrera, M. Shotgun proteomic analyses of Pseudomonas species isolated from fish products. Food Chem. 2024, 450, 139342. [Google Scholar] [CrossRef] [PubMed]
- Dehkordi, S.M.H.; Anvar, S.A.; Rahimi, E.; Ahari, H.; Ataee, M. Molecular investigation of prevalence, phenotypic and genotypic diversity, antibiotic resistance, frequency of virulence genes and genome sequencing in Pseudomonas aeruginosa strains isolated from lobster. Int. J. Food Microbiol. 2022, 382, 109901. [Google Scholar] [CrossRef] [PubMed]
- Li Gu, N.; Huang, T.Y.; Zhong, F.; Peng, G. Pseudomonas aeruginosa: A typical biofilm forming pathogen and an emerging but underestimated pathogen in food processing. Front. Microbiol. 2023, 13, 1114199. [Google Scholar] [CrossRef]
- Maravić, A.; Šamanić, I.; Šprung, M.; Fredotović, Ž.; Ilić, N.; Dragičević, J.; Puizina, J. Broad-spectrum resistance of Pseudomonas aeruginosa from shellfish: Infrequent acquisition of novel resistance mechanisms. Environ. Monit. Assess. 2018, 190, 81. [Google Scholar] [CrossRef]
- CDC. About Pseudomonas aeruginosa. 2024. Available online: https://www.cdc.gov/pseudomonas-aeruginosa/about/index.html (accessed on 30 March 2025).
- Thomassen, G.M.B.; Reiche, T.; Tennfjord, C.E.; Mehli, L. Antibiotic Resistance Properties among Pseudomonas spp. Associated with Salmon Processing Environments. Microorganisms 2022, 10, 1420. [Google Scholar] [CrossRef]
- Dehkordi, S.M.H.; Anvar, S.A.; Rahimi, E.; Ahari, H.; Ataee, M. Prevalence, phenotypic and genotypic diversity, antibiotic resistance, and frequency of virulence genes in Pseudomonas aeruginosa isolated from shrimps. Aquac. Int. 2022, 30, 131–156. [Google Scholar] [CrossRef]
- Elbashir, S.; Parveen, S.; Schwarz, J.; Rippen, T.; Jahncke, M.; DePaola, A. Seafood pathogens and information on antimicrobial resistance: A review. Food Microbiol. 2018, 70, 85–93. [Google Scholar] [CrossRef]
- Hamad, G.; Hafez, E.E.; Sobhy, S.E.; Mehany, T.; Elfayoumy, R.A.; Elghazaly, E.M.; Eskander, M.; Tawfik, R.G.; Hussein, S.M.; Pereira, L. Detection of Clostridium botulinum in Some Egyptian Fish Products, Its Control In Vitro Using Citrus Leaves Extracts, and Applicability of Citrus limon Leaf Extract in Tuna. Foods 2023, 12, 1466. [Google Scholar] [CrossRef]
- Ali, A.; Parisi, A.; Conversano, M.C.; Iannacci, A.; D’Emilio, F.; Mercurio, V.; Normanno, G. Food-borne bacteria associated with seafoods: A brief review. J. Food Qual. Hazards Control 2020, 7, 4–10. [Google Scholar] [CrossRef]
- Hafeez, M.; Ahmad, I.; Qureshi, S.; Kashoo, Z.; Farooq, S.; Asmi, O.; Shah, F.; Razak, N. Clostridium perfringens from fresh water fish of Kashmir Himalaya and their aquatic environment: Toxinotyping and phylogenetic analysis. Anaerobe 2022, 77, 102619. [Google Scholar] [CrossRef] [PubMed]
- Bendary, M.M.; Abd El-Hamid, M.I.; El-Tarabili, R.M.; Hefny, A.A.; Algendy, R.M.; Elzohairy, N.A.; Ghoneim, M.M.; Al-Sanea, M.M.; Nahari, M.H.; Moustafa, W.H. Clostridium perfringens Associated with Foodborne Infections of Animal Origins: Insights into Prevalence, Antimicrobial Resistance, Toxin Genes Profiles, and Toxinotypes. Biology 2022, 11, 551. [Google Scholar] [CrossRef]
- CDC. About C. perfringens Food Poisoning. 2024. Available online: https://www.cdc.gov/clostridium-perfringens/about/index.html (accessed on 30 March 2025).
- Agnoletti, F.; Arcangeli, G.; Barbanti, F.; Barco, L.; Brunetta, R.; Cocchi, M.; Conedera, G.; D’Este, L.; Drigo, I.; Spigaglia, P.; et al. Survey, characterization and antimicrobial susceptibility of Clostridium difficile from marine bivalve shellfish of North Adriatic Sea. Int. J. Food Microbiol. 2019, 298, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Yadav, J.P.; Das, S.C.; Dhaka, P.; Vijay, D.; Kumar, M.; Mukhopadhyay, A.K.; Chowdhury, G.; Chauhan, P.; Singh, R.; Dhama, K.; et al. Molecular characterization and antimicrobial resistance profile of Clostridium perfringens type A isolates from humans, animals, fish and their environment. Anaerobe 2017, 47, 120–124. [Google Scholar] [CrossRef]
- Mudadu, A.G.; Spanu, C.; Pantoja, J.C.F.; Dos Santos, M.C.; De Oliveira, C.D.; Salza, S.; Piras, G.; Uda, M.T.; Virgilio, S.; Giagnoni, L.; et al. Association between Escherichia coli and Salmonella spp. food safety criteria in live bivalve molluscs from wholesale and retail markets. Food Control 2022, 137, 108942. [Google Scholar] [CrossRef]
- Bai, Z.; Xu, X.; Wang, C.; Wang, T.; Sun, C.; Liu, S.; Li, D. A comprehensive review of detection methods for Escherichia coli O157:H7. TrAC Trends Anal. Chem. 2022, 152, 116646. [Google Scholar] [CrossRef]
- Kavinesan, K.; Sugumar, G.; Chrisolite, B.; Muthiahsethupathy, A.; Sudarshan, S.; Parthiban, F.; Mansoor, M. Phenotypic and genotypic characterization of pathogenic Escherichia coli identified in resistance mapping of β-lactam drug-resistant isolates from seafood along Tuticorin coast. Environ. Sci. Pollut. Res. 2023, 30, 68111–68128. [Google Scholar] [CrossRef]
- Kim Lee, M.S.; Kim, J.H. Recent Updates on Outbreaks of Shiga Toxin-Producing Escherichia coli and Its Potential Reservoirs. Front. Cell Infect. Microbiol. 2020, 10, 273. [Google Scholar] [CrossRef]
- Ramos, S.; Silva, V.; de Lurdes Enes Dapkevicius, M.; Caniça, M.; Tejedor-Junco, M.T.; Igrejas, G.; Poeta, P. Escherichia coli as commensal and pathogenic bacteria among food-producing animals: Health implications of extended spectrum β-lactamase (ESBL) production. Animals 2020, 10, 2239. [Google Scholar] [CrossRef]
- Prakasan, S.; Lekshmi, M.; Ammini, P.; Balange, A.K.; Nayak, B.B.; Kumar, S.H. Occurrence, pathogroup distribution and virulence genotypes of Escherichia coli from fresh seafood. Food Control 2022, 133, 108669. [Google Scholar] [CrossRef]
- Marshall, K.E.; Hexemer, A.; Seelman, S.L.; Fatica, M.K.; Blessington, T.; Hajmeer, M.; Kisselburgh, H.; Atkinson, R.; Hill, K.; Sharma, D.; et al. Lessons Learned from a Decade of Investigations of Shiga Toxin-Producing Escherichia coli Outbreaks Linked to Leafy Greens, United States and Canada. Emerg. Infect. Dis. 2020, 26, 2319–2328. [Google Scholar] [CrossRef] [PubMed]
- ECDC. European Centre for Disease Prevention and Control. STEC infection. In Annual Epidemiological Report for 2020; ECDC: Stockholm, Sweden, 2022; Available online: https://www.ecdc.europa.eu/en/publications-data/stec-infection-annual-epidemiological-report-2021 (accessed on 30 March 2025).
- Rubini, S.; Galletti, G.; D’Incau, M.; Govoni, G.; Boschetti, L.; Berardelli, C.; Barbieri, S.; Merialdi, G.; Formaglio, A.; Guidi, E.; et al. Occurrence of Salmonella enterica subsp. enterica in bivalve molluscs and associations with Escherichia coli in molluscs and faecal coliforms in seawater. Food Control 2018, 84, 429–435. [Google Scholar] [CrossRef]
- Odumosu, B.T.; Obeten, H.I.; Bamidele, T.A. Incidence of Multidrug-Resistant Escherichia coli Harbouring blaTEM and tetA Genes Isolated from Seafoods in Lagos Nigeria. Curr. Microbiol. 2021, 78, 2414–2419. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, F.; Pezzi, A.; Galletti, G.; Tamba, M.; Merialdi, G.; Piva, S.; Serraino, A.; Rubini, S. Antimicrobial resistance patterns in Salmonella enterica subsp. enterica and Escherichia coli isolated from bivalve molluscs and marine environment. Food Control 2021, 121, 107590. [Google Scholar] [CrossRef]
- Ryu, S.H.; Park, S.G.; Choi, S.M.; Hwang, Y.O.; Ham, H.J.; Kim, S.U.; Lee, Y.K.; Kim, M.S.; Park, G.Y.; Kim, K.S.; et al. Antimicrobial resistance and resistance genes in Escherichia coli strains isolated from commercial fish and seafood. Int. J. Food Microbiol. 2012, 152, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Al Qabili, D.M.A.; Aboueisha, A.K.M.; Ibrahim, G.A.; Youssef, A.I.; El-Mahallawy, H.S. Virulence and antimicrobial-resistance of shiga toxin-producing E. coli (STEC) Isolated from edible shellfish and its public health significance. Arch. Microbiol. 2022, 204, 510. [Google Scholar] [CrossRef]
- Tamber, S.; Montgomery, A.; Eloranta, K.; Buenaventura, E. Enumeration and survival of salmonella enterica in live oyster shellstock harvested from Canadian waters. J. Food Prot. 2020, 83, 6–12. [Google Scholar] [CrossRef]
- Miao, S.; Liu, L.; Fu, Z. Prevalence of Salmonella in Chinese Food Commodities: A Meta-Analysis. J. Food Prot. 2022, 85, 859–870. [Google Scholar] [CrossRef]
- Ferrari, R.G.; Rosario, D.K.A.; Cunha-Neto, A.; Mano, S.B.; Figueiredo, E.E.S.; Conte-Juniora, C.A. Worldwide epidemiology of Salmonella serovars in animal-based foods: A meta-analysis. Appl. Environ. Microbiol. 2019, 85, e00591-19. [Google Scholar] [CrossRef]
- Salam, F.; Lekshmi, M.; Prabhakar, P.; Kumar, S.H.; Nayak, B.B. Physiological characteristics and virulence gene composition of selected serovars of seafood-borne Salmonella enterica. Vet. World 2023, 16, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Lopatek, M.; Wieczorek, K.; Osek, J. Prevalence and Antimicrobial Resistance of Bacterial Foodborne Pathogens Isolated from Raw Bivalve Molluscs Subjected to Consumption in Poland during a Ten-Year Period. Foods 2022, 11, 3521. [Google Scholar] [CrossRef]
- Beshiru, A.; Igbinosa, I.H.; Igbinosa, E.O. Prevalence of antimicrobial resistance and virulence gene elements of Salmonella serovars from ready-to-eat (RTE) shrimps. Front. Microbiol. 2019, 10, 1613. [Google Scholar] [CrossRef] [PubMed]
- Odeyemi, O.A.; Amin, M.; Dewi, F.R.; Kasan, N.A.; Onyeaka, H.; Stratev, D.; Odeyemi, O.A. Prevalence of Antibiotic-Resistant Seafood-Borne Pathogens in Retail Seafood Sold in Malaysia: A Systematic Review and Meta-Analysis. Antibiotics 2023, 12, 829. [Google Scholar] [CrossRef] [PubMed]
- Bai Li, X.; Liu, X.; Xing, Z.; Su, R.; Wang, Y.; Xia, X.; Shi, C. Antibacterial Effect of Eugenol on Shigella flexneri and Its Mechanism. Foods 2022, 11, 2565. [Google Scholar] [CrossRef] [PubMed]
- Alejandro, D.J.C.-S.; Luis, D.E.-C.; Raquel, G.-B.; Alejandra, L.S.M.-A. Fish, Tilapia, and Shigellosis: A review. Afr. J. Agric. Res. 2021, 17, 498–512. [Google Scholar] [CrossRef]
- Nisa, I.; Qasim, M.; Yasin, N.; Ullah, R.; Ali, A. Shigella flexneri: An emerging pathogen. Folia Microbiol. 2020, 65, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Shen, Q.; Wu, J.; Dai, Z.; Wang, Y. Naked-eyes detection of Shigella flexneri in food samples based on a novel gold nanoparticle-based colorimetric aptasensor. Food Control 2019, 98, 333–341. [Google Scholar] [CrossRef]
- Halimeh, F.B.; Rafei, R.; Osman, M.; Kassem, I.I.; Diene, S.M.; Dabboussi, F.; Rolain, J.M.; Hamze, M. Historical, current, and emerging tools for identification and serotyping of Shigella. Braz. J. Microbiol. 2021, 52, 2043–2055. [Google Scholar] [CrossRef]
- CDC. Shigellosis CDC Yellow Book 2024. Available online: https://wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/shigellosis (accessed on 30 March 2025).
- CDC. About Shigella Infection. 2024. Available online: https://www.cdc.gov/shigella/about/index.html (accessed on 30 March 2025).
- Garcia-Williams, A.G.; Logan, N.; Marsh, Z.A. Chapter 12—Shigella. In Foodborne Infections and Intoxications, 5th ed.; Morris, J.G., Vugia, D.J., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 221–236. [Google Scholar] [CrossRef]
- Luo, W.; Dai, W.; Zhang, X.; Zheng, L.; Zhao, J.; Xie, X.; Xu, Y. Effects of Shigella flexneri exposure on development of Xenopus Tropicals embryo and its immune response. J. Hazard. Mater. 2022, 427, 128153. [Google Scholar] [CrossRef]
- Obaidat, M.M.; Salman, A.E.B. Antimicrobial resistance percentages of salmonella and Shigella in seafood imported to Jordan: Higher percentages and more diverse profiles in Shigella. J. Food Prot. 2017, 80, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Marijani, E. Prevalence and Antimicrobial Resistance of Bacteria Isolated from Marine and Freshwater Fish in Tanzania. Int. J. Microbiol. 2022, 2022, 4652326. [Google Scholar] [CrossRef] [PubMed]
- Lasagabaster, A.; Jiménez, E.; Lehnherr, T.; Miranda-Cadena, K.; Lehnherr, H. Bacteriophage biocontrol to fight Listeria outbreaks in seafood. Food Chem. Toxicol. 2020, 145, 11168. [Google Scholar] [CrossRef] [PubMed]
- Mohan, V.; Cruz, C.D.; van Vliet, A.H.M.; Pitman, A.R.; Visnovsky, S.B.; Rivas, L.; Gilpin, B.; Fletcher, G.C. Genomic diversity of Listeria monocytogenes isolates from seafood, horticulture and factory environments in New Zealand. Int. J. Food Microbiol. 2021, 347, 109166. [Google Scholar] [CrossRef]
- Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernández Escámez, P.S.; Girones, R.; Herman, L.; Koutsoumanis, K.; Nørrung, B.; et al. Listeria monocytogenes contamination of ready-to-eat foods and the risk for human health in the EU. EFSA J. 2018, 16, e05134. [Google Scholar] [CrossRef] [PubMed]
- Peratikos, P.; Tsitsos, A.; Damianos, A.; Kyritsi, M.A.; Hadjichristodoulou, C.; Soultos, N.; Economou, V. Listeria monocytogenes from Marine Fish and the Seafood Market Environment in Northern Greece: Prevalence, Molecular Characterization, and Antibiotic Resistance. Appl. Sci. 2024, 14, 2725. [Google Scholar] [CrossRef]
- CDC. About Listeria Infection. 2024. Available online: https://www.cdc.gov/listeria/about/index.html (accessed on 30 March 2025).
- Selvaganapathi, R.; Jeyasekaran, G.; Shakila, R.J.; Sukumar, D.; Kumar, M.P.; Sivaraman, B. Occurrence of Listeria monocytogenes on the seafood contact surfaces of Tuticorin Coast of India. J. Food Sci. Technol. 2018, 55, 2808–2812. [Google Scholar] [CrossRef] [PubMed]
- Palma, F.; Brauge, T.; Radomski, N.; Mallet, L.; Felten, A.; Mistou, M.Y.; Brisabois, A.; Guillier, L.; Midelet-Bourdin, G. Dynamics of mobile genetic elements of Listeria monocytogenes persisting in ready-to-eat seafood processing plants in France. BMC Genom. 2020, 21, 130. [Google Scholar] [CrossRef]
- Wiktorczyk-Kapischke, N.; Wałecka-Zacharska, E.; Skowron, K.; Kijewska, A.; Bernaciak, Z.; Bauza-Kaszewska, J.; Kraszewska, Z.; Gospodarek-Komkowska, E. Comparison of Selected Phenotypic Features of Persistent and Sporadic Strains of Listeria monocytogenes Sampled from Fish Processing Plants. Foods 2022, 11, 1492. [Google Scholar] [CrossRef]
- Ryser, E.T. Chapter 11—Listeria. In Foodborne Infections and Intoxications, 5th ed.; Morris, J.G., Vugia, D.J., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 201–220. [Google Scholar] [CrossRef]
- Szymczak, B.; Szymczak, M.; Trafiałek, J. Prevalence of Listeria species and L. monocytogenes in ready-to-eat foods in the West Pomeranian region of Poland: Correlations between the contamination level, serogroups, ingredients, and producers. Food Microbiol. 2020, 91, 103532. [Google Scholar] [CrossRef]
- Wieczorek, K.; Osek, J. Prevalence, genetic diversity and antimicrobial resistance of Listeria monocytogenes isolated from fresh and smoked fish in Poland. Food Microbiol. 2017, 64, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Abdollahzadeh, E.; Ojagh, S.M.; Hosseini, H.; Ghaemi, E.A.; Irajian, G.; Naghizadeh Heidarlo, M. Antimicrobial resistance of Listeria monocytogenes isolated from seafood and humans in Iran. Microb. Pathog. 2016, 100, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Jiang, L.; Yang, Q.; Han, F.; Chen, S.; Pu, S.; Vance, A.; Ge, B. Prevalence and antimicrobial susceptibility of major foodborne pathogens in imported seafood. J. Food Prot. 2011, 74, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Naas, H.T.; Edarhoby, R.A.; Garbaj, A.M.; Azwai, S.M.; Abolghait, S.K.; Gammoudi, F.T.; Moawad, A.A.; Barbieri, I.; Eldaghayes, I.M. Occurrence, characterization, and antibiogram of Staphylococcus aureus in meat, meat products, and some seafood from Libyan retail markets. Vet. World 2019, 12, 925–931. [Google Scholar] [CrossRef]
- Islam, M.A.; Parveen, S.; Rahman, M.; Huq, M.; Nabi, A.; Khan, Z.U.M.; Ahmed, N.; Wagenaar, J.A. Occurrence and characterization of methicillin resistant Staphylococcus aureus in processed raw foods and ready-to-eat foods in an urban setting of a developing country. Front. Microbiol. 2019, 10, 503. [Google Scholar] [CrossRef] [PubMed]
- Rong, D.; Wu, Q.; Xu, M.; Zhang, J.; Yu, S. Prevalence, virulence genes, antimicrobial susceptibility, and genetic diversity of Staphylococcus aureus from retail aquatic products in China. Front. Microbiol. 2017, 8, 714. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Huang, J.; Wu, S.; Zhang, F.; Li, Y.; Rong, D.; Zhao, M.; Ye, Q.; Gu, Q.; Zhang, Y.; et al. Occurrence, Antibiotic Susceptibility, Biofilm Formation and Molecular Characterization of Staphylococcus aureus Isolated from Raw Shrimp in China. Foods 2023, 12, 2651. [Google Scholar] [CrossRef]
- Matuszewska, M.; Dabrowska, A.; Murray, G.G.R.; Kett, S.M.; Vick, A.J.A.; Banister, S.C.; Pantoja Munoz, L.; Cunningham, P.; Welch, J.J.; Holmes, M.A.; et al. Absence of Staphylococcus aureus in Wild Populations of Fish Supports a Spillover Hypothesis. Microbiol. Spectr. 2023, 11, e04858-22. [Google Scholar] [CrossRef]
- Othman, B.R.; Kuan, C.H.; Mohammed, A.S.; Cheah, Y.K.; Tan, C.W.; New, C.Y.; Thung, T.Y.; San Chang, W.; Loo, Y.Y.; Nakaguchi, Y.; et al. Occurrence of methicillin-resistant Staphylococcus aureus in raw shellfish at retail markets in Malaysia and antibacterial efficacies of black seed (Nigella sativa) oil against MRSA. Food Control 2018, 90, 324–331. [Google Scholar] [CrossRef]
- Carvalho, J.S.; Neto, A.F.L.; Melo, I.M.; Varjão, L.M.; Andrade, C.A.D.N.; Xavier, D.E.; Leal, N.C.; De Castro Almeida, R.C. Occurrence of methicillin-resistant staphylococcus aureus in ready-to-eat raw fish from Japanese Cuisine Restaurants in Salvador, Brazil. J. Food Prot. 2020, 83, 991–995. [Google Scholar] [CrossRef]
- Jurinović, L.; Ječmenica, B.; Džafić, N.; Brlek Gorski, D.; Šimpraga, B.; Krstulović, F.; Amšel Zelenika, T.; Humski, A. First Data on Campylobacter spp. Presence in Shellfish in Croatia. Pathogens 2022, 11, 943. [Google Scholar] [CrossRef]
- Habib, I.; Mohamed, M.Y.I.; Khan, M. Current state of Salmonella, campylobacter and listeria in the food chain across the arab countries: A descriptive review. Foods 2021, 10, 2369. [Google Scholar] [CrossRef] [PubMed]
- CDC. About Campylobacter Infection. 2024. Available online: https://www.cdc.gov/campylobacter/about/index.html (accessed on 30 March 2025).
- Sher, A.A.; Ashraf, M.A.; Mustafa, B.E.; Raza, M.M. Epidemiological trends of foodborne Campylobacter outbreaks in the United States of America, 1998–2016. Food Microbiol. 2021, 97, 10375. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Sanchéz, A.D.J. Food, Fish and Campylobacteriosis. Int. J. Food Stud. 2020, 9, 394–406. [Google Scholar] [CrossRef]
- Rincé, A.; Balière, C.; Hervio-Heath, D.; Cozien, J.; Lozach, S.; Parnaudeau, S.; Le Guyader, F.S.; Le Hello, S.; Giard, J.C.; Sauvageot, N.; et al. Occurrence of bacterial pathogens and human noroviruses in shellfish- Harvesting areas and their catchments in France. Front. Microbiol. 2018, 9, 2443. [Google Scholar] [CrossRef] [PubMed]
- CDC. CDC’s 2019 Antibiotic Resitance Thread Report: Drug-Resistant Campylobacter; Department of Health and Human Services Center for Disease Control and Prevention: Atlanta, Georgia, 2019; pp. 1–2. [Google Scholar]
- Lozano-León, A.; Rodríguez-Souto, R.R.; González-Escalona, N.; Llovo-Taboada, J.; Iglesias-Canle, J.; Álvarez-Castro, A.; Garrido-Maestu, A. Detection, molecular characterization, and antimicrobial susceptibility, of Campylobacter spp. isolated from shellfish. Microb. Risk Anal. 2021, 18, 100176. [Google Scholar] [CrossRef]
- Soonthornchaikul, N.; Garelick, H. Antimicrobial resistance of Campylobacter species isolated from edible bivalve molluscs purchased from bangkok markets, Thailand. Foodborne Pathog. Dis. 2009, 6, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Deardorff, T.L.; Overstreet, R.M. Seafood-transmitted zoonoses in the United States: The fishes, the dishes, and the worms. In Microbiology of Marine Food Products; Springer: Boston, MA, USA, 1991; pp. 211–265. [Google Scholar]
- Yang, R.; Reid, A.; Lymbery, A.; Ryan, U. Identification of zoonotic Giardia genotypes in fish. Int. J. Parasitol. 2010, 40, 779–785. [Google Scholar] [CrossRef]
- Chai, J.Y.; Murrell, K.D.; Lymbery, A.J. Fish-borne parasitic zoonoses: Status and issues. Int. J. Parasitol. 2005, 35, 1233–1254. [Google Scholar] [CrossRef]
- McCarthy, J.; Moore, T.A. Emerging helminth zoonoses. Int. J. Parasitol. 2000, 30, 1351–1359. [Google Scholar] [CrossRef]
- Sumner, J.; Antananawat, S.; Kiermeier, A.; McLeod, C.; Shamsi, S. Raw fish consumption in Australia: How safe is it? Food Aust. 2015, 67, 24–26. [Google Scholar]
- Habibi, F.; Shamsi, S. Preliminary report of occurrence of Corynosoma spp.(Acanthocephala: Polymorphidae) in Southern Caspian sprat (Clupeonella grimmi). Parasitol. Res. 2018, 117, 3327–3331. [Google Scholar] [CrossRef]
- Fujita, T.; Waga, E.; Kitaoka, K.; Imagawa, T.; Komatsu, Y.; Takanashi, K.; Anbo, F.; Anbo, T.; Katuki, S.; Ichihara, S.; et al. Human infection by acanthocephalan parasites belonging to the genus Corynosoma found from small bowel endoscopy. Parasitol. Int. 2016, 65, 491–493. [Google Scholar] [CrossRef]
- Cong, W.; Elsheikha, H.M. Biology, epidemiology, clinical features, diagnosis, and treatment of selected fish-borne parasitic zoonoses. Yale J. Biol. Med. 2021, 94, 297–309. [Google Scholar] [PubMed]
- Craig, N. Fish tapeworm and sushi. Can. Fam. Physician 2012, 58, 654–658. [Google Scholar] [PubMed]
- Chai, J.Y.; Jung, B.K. General overview of the current status of human foodborne trematodiasis. Parasitology 2022, 149, 1262–1285. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, S.; Suthar, J.; Zhu, X.; Barton, D.P. Infection levels of Gnathostomatidae (Nematoda) larvae in commercial fishes in north-eastern Australian waters and related food safety concerns. Int. J. Food Microbiol. 2023, 403, 110340. [Google Scholar] [CrossRef]
- Hughes, A.J.; Biggs, B.A. Parasitic worms of the central nervous system: An Australian perspective. Intern. Med. J. 2002, 32, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Boreham, R.E.; Hendrick, S.; O’donoghue, P.J.; Stenzel, D.J. Incidental finding of Myxobolus spores (Protozoa: Myxozoa) in stool samples from patients with gastrointestinal symptoms. J. Clin. Microbiol. 1998, 36, 3728–3730. [Google Scholar] [CrossRef]
- Sha, J.; Xiong, H.; Li, C.; Lu, Z.; Zhang, J.; Zhong, H.; Zhang, W.; Yan, B. Chemosphere Harmful algal blooms and their eco-environmental indication. Chemosphere 2021, 274, 129912. [Google Scholar] [CrossRef]
- Dorantes-Aranda, J. Harmful Algae Impacting Aquatic Organisms: Recent Field and Laboratory Observations. Toxins 2023, 15, 339. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Fensin, E.; Gobler, C.J.; Hoeglund, A.E.; Hubbard, K.A.; Kulis, D.M.; Landsberg, J.H.; Lefebvre, K.A.; Provoost, P.; Richlen, M.L.; et al. Marine harmful algal blooms (HABs) in the United States: History, current status and future trends. Harmful Algae 2021, 102, 101975. [Google Scholar] [CrossRef]
- Karlson, B.; Andersen, P.; Arneborg, L.; Cembella, A.; Eikrem, W.; John, U.; Joy, J.; Klemm, K.; Kobos, J.; Lehtinen, S.; et al. Harmful algal blooms and their effects in coastal seas of Northern Europe. Harmful Algae 2021, 102, 101989. [Google Scholar] [CrossRef] [PubMed]
- Holland, D.S.; Leonard, J. Is a delay a disaster? economic impacts of the delay of the California dungeness crab fishery due to a harmful algal bloom. Harmful Algae 2020, 98, 101904. [Google Scholar] [CrossRef]
- CDC. Summary Report—One Health Harmful Algal Bloom System (OHHABS), United States. 2021. Available online: https://www.cdc.gov/ohhabs/data/summary-report-united-states-2021.html?CDC_AAref_Val=https://www.cdc.gov/habs/data/2021-ohhabs-data-summary.html (accessed on 30 March 2025).
- Neves, R.A.F.; Nascimento, S.M.; Santos, L.N. Harmful algal blooms and shellfish in the marine environment: An overview of the main molluscan responses, toxin dynamics, and risks for human health. Environ. Sci. Pollut. Res. Int. 2021, 28, 55846–55868. [Google Scholar] [CrossRef] [PubMed]
- Grattan, L.M.; Holobaugh, S.; Morris, J.G. Harmful algal blooms and public health. Harmful Algae 2016, 57, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Rolton, A.; Rhodes, L.; Hutson, K.S.; Biessy, L.; Bui, T.; Mackenzie, L.; Symonds, J.E.; Smith, K.F. Effects of Harmful Algal Blooms on Fish and Shellfish Species: A Case Study of New Zealand in a Changing Environment. Toxins 2022, 14, 341. [Google Scholar] [CrossRef] [PubMed]
- Zastepa, A.; Westrick, J.A.; Liang, A.; Birbeck, J.A.; Furr, E.; Watson, L.C.; Stockdill, J.L.; Ramakrishna, B.S.; Crevecoeur, S. Broad screening of toxic and bioactive metabolites in cyanobacterial and harmful algal blooms in Lake of the Woods (Canada and USA), 2016. J. Great Lakes Res. 2023, 49, 134–146. [Google Scholar] [CrossRef]
- Joseph, M.; Henderson, S.B.; Clermont, H.; Saha, N.; Mcintyre, L. Heliyon The health risks of marine biotoxins associated with high seafood consumption: Looking beyond the single dose, single outcome paradigm with a view towards addressing the needs of coastal Indigenous populations in British Columbia. Heliyon 2024, 10, e27146. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, Y.; Wu, L.; Gao, X.; Huang, H.; Han, Y.; Zhu, T. Advances in Biosensors for the Rapid Detection of Marine Biotoxins: Current Status and Future Perspectives. Biosensors 2024, 14, 203. [Google Scholar] [CrossRef]
- Young, N.; Sharpe, R.A.; Barciela, R.; Nichols, G.; Davidson, K.; Berdalet, E.; Fleming, L.E. Marine harmful algal blooms and human health: A systematic scoping review. Harmful Algae 2020, 98, 101901. [Google Scholar] [CrossRef]
- Nicolas, J.; Hoogenboom, R.L.A.P.; Hendriksen, P.J.M.; Bodero, M.; Bovee, T.F.H.; Rietjens, I.M.C.M.; Gerssen, A. Marine biotoxins and associated outbreaks following seafood consumption: Prevention and surveillance in the 21st century. Glob. Food Secur. 2017, 15, 11–21. [Google Scholar] [CrossRef]
- Sinno-tellier, S.; Abadie, E.; Guillotin, S.; Nicolas, M.; Delcourt, N.; Bosse, A. Human shell fish poisoning: Implementation of a national surveillance program in France. Front. Mar. Sci. 2023, 9, 1089585. [Google Scholar] [CrossRef]
- Valenti, W.C.; Barros, H.P.; Moraes-Valenti, P.; Bueno, G.W.; Cavalli, R.O. Aquaculture in Brazil: Past, present and future. Aquac. Rep. 2021, 19, 100611. [Google Scholar] [CrossRef]
- O’Mahony, M. Eu regulatory risk management of marine biotoxins in the marine bivalve mollusc food-chain. Toxins 2018, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Das, O.; Lekshmi, M.; Kumar, S.; Nayak, B.B. Incidence of norovirus in tropical seafood harbouring fecal indicator bacteria. Mar. Pollut. Bull. 2020, 150, 110777. [Google Scholar] [CrossRef]
- Santos-Ferreira, N.; Mesquita, J.R.; Rivadulla, E.; Inácio, Â.S.; Nascimento, M.S.J.; Romalde, J.; Martins da Costa, P. Norovirus contamination of sea urchins (Paracentrotus lividus): Potential food risk for consumers. Food Control 2020, 111, 107041. [Google Scholar] [CrossRef]
- Woods, J.W.; Burkhardt, W. Occurrence of norovirus and hepatitis a virus in U.S. Oysters. Food Environ. Virol. 2010, 2, 176–182. [Google Scholar] [CrossRef]
- Amroabadi, M.A.; Rahimi, E.; Shakerian, A.; Momtaz, H. Incidence of hepatitis A and hepatitis E viruses and norovirus and rotavirus in fish and shrimp samples caught from the Persian Gulf. Arq. Bras. Med. Vet. Zootecnia 2021, 73, 169–178. [Google Scholar] [CrossRef]
- Fusco, G.; Anastasio, A.; Kingsley, D.H.; Amoroso, M.G.; Pepe, T.; Fratamico, P.M.; Cioffi, B.; Rossi, R.; La Rosa, G.; Boccia, F. Detection of hepatitis a virus and other enteric viruses in shellfish collected in the gulf of Naples, Italy. Int. J. Environ. Res. Public Health 2019, 16, 2588. [Google Scholar] [CrossRef]
- La Rosa, G.; Proroga, Y.T.R.; De Medici, D.; Capuano, F.; Iaconelli, M.; Della Libera, S.; Suffredini, E. First Detection of Hepatitis E Virus in Shellfish and in Seawater from Production Areas in Southern Italy. Food Environ. Virol. 2018, 10, 127–131. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, Z.; Crossan, C.; Craft, J.; Scobie, L. First Report of the Presence of Hepatitis E Virus in Scottish-Harvested Shellfish Purchased at Retail Level. Food Environ. Virol. 2018, 10, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Li, D.; Zha, E.; Zhou, T.; Wang, S.; Yue, X. Surveillance of hepatitis e virus contamination in shellfish in China. Int. J. Environ. Res. Public Health 2015, 12, 2026–2036. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Lekshmi, M.; Das, O.; Kumar, S.; Nayak, B.B. Occurrence of Human Enteric Adenoviruses in Fresh Tropical Seafood from Retail Markets and Landing Centers. J. Food Sci. 2019, 84, 2256–2260. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, V.; Chen, J.S.; Hsu, G.J.; Chen, H.P.; Chao, H.C.; Huang, S.W.; Tsai, I.-S.; Hsu, B.M. Surveillance of Adenovirus and Norovirus Contaminants in the Water and Shellfish of Major Oyster Breeding Farms and Fishing Ports in Taiwan. Pathogens 2022, 11, 316. [Google Scholar] [CrossRef]
- Diez-Valcarce, M.; Kokkinos, P.; Söderberg, K.; Bouwknegt, M.; Willems, K.; de Roda-Husman, A.M.; von Bonsdorff, C.H.; Bellou, M.; Hernández, M.; Maunula, L.; et al. Occurrence of Human Enteric Viruses in Commercial Mussels at Retail Level in Three European Countries. Food Environ. Virol. 2012, 4, 73–80. [Google Scholar] [CrossRef]
- Benabbes, L.; Ollivier, J.; Schaeffer, J.; Parnaudeau, S.; Rhaissi, H.; Nourlil, J.; Le Guyader, F.S. Norovirus and Other Human Enteric Viruses in Moroccan Shellfish. Food Environ. Virol. 2013, 5, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Wang, W.; Wang, Q.; He, F.; Hao, J.; Pang, B. Surveillance of enteric pathogens in imported seafood and environmental surfaces in five seafood markets before the outbreak of COVID-19. Biosaf. Health 2021, 3, 183–186. [Google Scholar] [CrossRef]
- Varela, M.F.; Hooper, A.S.; Rivadulla, E.; Romalde, J.L. Human Sapovirus in Mussels from Ría do Burgo, A Coruña (Spain). Food Environ. Virol. 2016, 8, 187–193. [Google Scholar] [CrossRef]
- Mohan, V.; Rawat, S.; Lokesh, K.M.; Mohan, H.V.; Avinash Reddy, D.; Kumar, A.; Bhilegaonkar, K.N. Prevalence of Rotavirus in shellfish from Southern Kerala. Vet. World 2014, 7, 821–824. [Google Scholar] [CrossRef]
- Kittigul, L.; Panjangampatthana, A.; Rupprom, K.; Pombubpa, K. Genetic diversity of rotavirus strains circulating in environmental water and bivalve shellfish in Thailand. Int. J. Environ. Res. Public Health 2014, 11, 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- Suffredini, E.; Le, Q.H.; Di Pasquale, S.; Pham, T.D.; Vicenza, T.; Losardo, M.; To, K.A.; De Medici, D. Occurrence and molecular characterization of enteric viruses in bivalve shellfish marketed in Vietnam. Food Control 2020, 108, 106828. [Google Scholar] [CrossRef]
- Onosi, O.; Upfold, N.S.; Jukes, M.D.; Luke, G.A.; Knox, C. The First Molecular Detection of Aichi Virus 1 in Raw Sewage and Mussels Collected in South Africa. Food Environ. Virol. 2019, 11, 96–100. [Google Scholar] [CrossRef]
- Terio, V.; Bottaro, M.; Di Pinto, A.; Fusco, G.; Barresi, T.; Tantillo, G.; Martella, V. Occurrence of Aichi virus in retail shellfish in Italy. Food Microbiol. 2018, 74, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Almuhaideb, E.; Chintapenta, L.K.; Abbott, A.; Parveen, S.; Ozbay, G. Assessment of Vibrio parahaemolyticus levels in oysters (Crassostrea virginica) and seawater in Delaware Bay in relation to environmental conditions and the prevalence of molecular markers to identify pathogenic Vibrio parahaemolyticus strains. PLoS ONE 2020, 15, e0242229. [Google Scholar] [CrossRef] [PubMed]
- Castello, A.; Alio, V.; Sciortino, S.; Oliveri, G.; Cardamone, C.; Butera, G.; Costa, A. Occurrence and Molecular Characterization of Potentially Pathogenic Vibrio spp. in Seafood Collected in Sicily. Microorganisms 2023, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.Y.; Liu, P.Y.; Lee, Y.H.; Wu, Z.Y.; Huang, C.C.; Cheng, C.C.; Tung, K.C. The pathogenicity of Shewanella algae and ability to tolerate a wide range of temperatures and salinities. Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 6976897. [Google Scholar] [CrossRef]
- Dutta, C.; Manna, S.K.; Sengupta, C. The Occurrence of Emerging Human Pathogen Shewanella algae in Shrimp Seafood. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 2677–2685. [Google Scholar] [CrossRef]
- Richards, G.P.; Watson, M.A.; Crane, E.J.; Burt, I.G.; Bushek, D. Shewanella and Photobacterium spp. in oysters and seawater from the Delaware bay. Appl. Environ. Microbiol. 2008, 74, 3323–3327. [Google Scholar] [CrossRef]
- Shahrokhi, G.R.; Rahimi, E.; Shakerian, A. The Prevalence Rate, Pattern of Antibiotic Resistance, and Frequency of Virulence Factors of Pseudomonas aeruginosa Strains Isolated from Fish in Iran. J. Food Qual. 2022, 2022, 8990912. [Google Scholar] [CrossRef]
- Duman, M.; Mulet, M.; Altun, S.; Saticioglu, I.B.; Ozdemir, B.; Ajmi, N.; Lalucat, J.; García-Valdés, E. The diversity of Pseudomonas species isolated from fish farms in Turkey. Aquaculture 2021, 535, 736369. [Google Scholar] [CrossRef]
- Montazeri, N.; Liu, D.; Janes, M.E. Occurrence of Toxigenic Clostridium difficile in Louisiana Oysters (Crassostrea virginica) and Environmental Waters. Food Nutr. Sci. 2015, 6, 1065–1070. [Google Scholar] [CrossRef]
- Freire, S.; Grilo, T.; Rodrigues, B.; Oliveira, R.; Esteves, C.; Marques, A.; Poirel, L.; Aires-de-Sousa, M. ESBL- and Carbapenemase-Producing Escherichia coli and Klebsiella pneumoniae among Bivalves from Portuguese Shellfish Production Areas. Microorganisms 2023, 11, 415. [Google Scholar] [CrossRef] [PubMed]
- Hodges, L.M.; Cooper, A.; Koziol, A.; Carrillo, C.D. Characterization of MLST-99 Salmonella Typhimurium and the monophasic variant I:4,[5],12:i:-isolated from Canadian Atlantic coast shellfish. Microbiology 2024, 170, 001456. [Google Scholar] [CrossRef] [PubMed]
- Dissasa, G.; Lemma, B.; Mamo, H. Isolation and identification of major bacteria from three Ethiopian rift valley lakes live and processed fish, and water samples: Implications in sanitary system of fish products. BMC Vet. Res. 2022, 18, 439. [Google Scholar] [CrossRef]
- Luchansky, J.B.; Chen, Y.; Porto-Fett, A.C.S.; Pouillot, R.; Shoyer, B.A.; Johnson-Derycke, R.; Eblen, D.R.; Hoelzer, K.; Shaw, W.K.; Van Doren, J.M.; et al. Survey for listeria monocytogenes in and on ready-to-eat foods from retail establishments in the United States (2010 through 2013): Assessing potential changes of pathogen prevalence and levels in a decade. J. Food Prot. 2017, 80, 903–921. [Google Scholar] [CrossRef]
- Ouédraogo, A.; Ouédraogo, G.A.; Ouédraogo, H.S.; Tchoumbougnang, F.; Zongo, C.; Savadogo, A. Microbial Quality and Molecular Identification of Enterotoxigenic Staphylococcus Strains Isolated from Dried, Smoked, and Braised Fish Sold in Ouagadougou Markets. Adv. Microbiol. 2024, 1, 59–76. [Google Scholar] [CrossRef]
- Bazzoni, A.M.; Cangini, M.; Mudadu, A.G.; Lorenzoni, G.; Arras, I.; Sanna, G.; Pino, F.; Milandri, A.; Virgilio, S. Recent findings of paralytic shellfish toxins linked to the genus Alexandrium Halim in Mediterranean mollusc production areas. Toxicon 2020, 174, 48–56. [Google Scholar] [CrossRef]
- Armi, Z.; Milandri, A.; Turki, S.; Hajjem, B. Alexandrium catenella and Alexandrium tamarense in the North Lake of Tunis: Bloom characteristics and the occurrence of paralytic shellfish Toxin. Afr. J. Aquat. Sci. 2011, 36, 47–56. [Google Scholar] [CrossRef]
- Touzet, N.; Davidson, K.; Pete, R.; Flanagan, K.; McCoy, G.R.; Amzil, Z.; Maher, M.; Chapelle, A.; Raine, R. Co-occurrence of the West European (Gr.III) and North American (Gr.I) ribotypes of Alexandrium tamarense (Dinophyceae) in Shetland, Scotland. Protist 2010, 161, 370–384. [Google Scholar] [CrossRef]
- Ayache, N.; Bill, B.D.; Brosnahan, M.L.; Campbell, L.; Deeds, J.R.; Fiorendino, J.M.; Gobler, C.J.; Handy, S.M.; Harrington, N.; Kulis, D.M.; et al. A survey of Dinophysis spp.; their potential to cause diarrhetic shellfish poisoning in coastal waters of the United States. J. Phycol. 2023, 59, 658–680. [Google Scholar] [CrossRef] [PubMed]
- Koukaras, K.; Nikolaidis, G. Dinophysis blooms in Greek coastal waters (Thermaikos Gulf, NW Aegean Sea). J. Plankton Res. 2004, 26, 445–457. [Google Scholar] [CrossRef]
- Naustvoll, L.J.; Gustad, E.; Dahl, E. Monitoring of Dinophysis species and diarrhetic shellfish toxins in Flødevigen Bay, Norway: Inter-annual variability over a 25-year time-series. Food Addit. Contam.-Part A 2012, 29, 1605–1615. [Google Scholar] [CrossRef] [PubMed]
- Abouabdellah, R.; Taleb, H.; Bennouna, A.; Erler, K.; Chafik, A.; Moukrim, A. Paralytic shellfish poisoning toxin profile of mussels Perna perna from southern Atlantic coasts of Morocco. Toxicon 2008, 51, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Botelho, M.J.; Vale, C.; Ferreira, J.G. Profiles of paralytic shellfish toxins in bivalves of low and elevated toxicities following exposure to Gymnodinium catenatum blooms in Portuguese estuarine and coastal waters. Chemosphere 2015, 138, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.C.; Leaw, C.P.; Tan, T.H.; Kon, N.F.; Yek, L.H.; Hii, K.S.; Teng, S.T.; Razali, R.M.; Usup, G.; Iwataki, M.; et al. A bloom of Karlodinium australe (Gymnodiniales, Dinophyceae) associated with mass mortality of cage-cultured fishes in West Johor Strait, Malaysia. Harmful Algae 2014, 40, 51–62. [Google Scholar] [CrossRef]
- Zhou, C.; Place, A.R.; Yan, X.; Xu, J.; Luo, Q.; William, E.; Jiang, Y. Interactions between Karlodinium veneficum and Prorocentrum donghaiense from the East China Sea. Harmful Algae 2015, 49, 50–57. [Google Scholar] [CrossRef]
- Wang, X.; Liu, C.; Zhang, Q.-C.; Chen, J.-F.; Wang, J.-X.; Zhao, Q.-Y.; Yan, T.; Yu, R.-C. A dinoflagellate bloom caused by multiple species of Kareniaceae in the coastal waters of Fujian in June 2022 and its adverse impacts on Brachionus plicatilis and Artemia salina. Mar. Pollut. Bull. 2023, 196, 115685. [Google Scholar] [CrossRef]
- Sierra-Beltrán, A.P.; Cortés-Altamirano, R.; Cortés-Lara, M.C. Occurrences of Prorocentrum minimum (Pavillard) in México. Harmful Algae 2005, 4, 507–517. [Google Scholar] [CrossRef]
- Nishimura, T.; Uchida, H.; Noguchi, R.; Oikawa, H.; Suzuki, T.; Funaki, H.; Ihara, C.; Hagino, K.; Arimitsu, S.; Tanii, Y.; et al. Abundance of the benthic dinoflagellate Prorocentrum and the diversity, distribution, and diarrhetic shellfish toxin production of Prorocentrum lima complex and P. caipirignum in Japan. Harmful Algae 2020, 96, 101687. [Google Scholar] [CrossRef]
- Aligizaki, K.; Nikolaidis, G.; Katikou, P.; Baxevanis, A.D.; Abatzopoulos, T.J. Potentially toxic epiphytic Prorocentrum (Dinophyceae) species in Greek coastal waters. Harmful Algae 2009, 8, 299–311. [Google Scholar] [CrossRef]
- ECDC. Prolonged Multi-Country Outbreak of Listeria monocytogenes ST173 Linked to Consumption of Fish Products. 2024. Available online: https://www.ecdc.europa.eu/en/publications-data/prolonged-multi-country-outbreak-listeria-monocytogenes-st173-linked-consumption (accessed on 30 March 2025).
- ECDC. Rapid Outbreak Assessment: Prolonged Multi-Country Outbreak of Listeria Monocytogenes ST1607 Linked to Smoked Salmon Products. 2024. Available online: https://www.ecdc.europa.eu/en/publications-data/rapid-outbreak-assessment-prolonged-multi-country-outbreak-listeria-monocytogenes (accessed on 30 March 2025).
- ECDC. Rapid Outbreak Assessment: Prolonged Multi Country Cluster of Listeria Monocytogenes ST155 Infections. 2023. Available online: https://www.ecdc.europa.eu/en/publications-data/prolonged-multi-country-cluster-listeria-monocytogenes-st155-infections (accessed on 30 March 2025).
- CDC. Norovirus. 2024. Available online: https://www.cdc.gov/norovirus/outbreak-basics/previous-outbreaks.html (accessed on 30 March 2025).
- FDA. Outbreak Investigation of Salmonella: Seafood (October 2022). 2022. Available online: https://www.fda.gov/food/outbreaks-foodborne-illness/outbreak-investigation-salmonella-seafood-october-2022 (accessed on 30 March 2025).
- Fearnley, E.; Leong, L.E.; Centofanti, A.; Dowsett, P.; Combs, B.G.; Draper, A.D.; Hocking, H.; Howden, B.; Horan, K.; Wilmot, M.; et al. Vibrio parahaemolyticus foodborne illness associated with oysters, Australia, 2021–2022. Emerg. Infect. Dis. 2024, 30, 2271. [Google Scholar] [CrossRef] [PubMed]
- FDA. Outbreak Investigation of Salmonella Thompson: Seafood (October 2021). 2021. Available online: https://www.fda.gov/food/outbreaks-foodborne-illness/outbreak-investigation-salmonella-thompson-seafood-october-2021 (accessed on 30 March 2025).
- FDA. Outbreak Investigation of Salmonella Weltevreden: Frozen Pre-Cooked Shrimp (April 2021). 2021. Available online: https://www.fda.gov/food/outbreaks-foodborne-illness/outbreak-investigation-salmonella-weltevreden-frozen-pre-cooked-shrimp-april-2021 (accessed on 30 March 2025).
- Janekrongtham, C.; Dejburum, P.; Sujinpram, S.; Rattanathumsakul, T.; Swaddiwudhipong, W. Outbreak of seafood-related food poisoning from undetectable Vibrio parahaemolyticus-like pathogen, Chiang Mai Province, Thailand, December 2020. Trop. Med. Int. Health 2022, 27, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, Y.; Fang, X.; Wang, Y.; Han, Y.; Liu, Y.; Cao, J.; Zhou, W.; Zheng, H.; Yao, W. An Epidemic of Hepatitis A—Liaoning Province, 2020. China CDC Wkly. 2020, 2, 570–574. [Google Scholar] [CrossRef] [PubMed]
- CDC. Multistate Outbreak of Gastrointestinal Illnesses Linked to Oysters Imported from Mexico. 2019. Available online: https://archive.cdc.gov/#/details?url=https://www.cdc.gov/vibrio/investigations/rawoysters-05-19/index.html (accessed on 30 March 2025).
- CDC. 2019 Salmonella Infections Linked to Frozen Raw Tuna. 2019. Available online: https://archive.cdc.gov/#/details?url=https://www.cdc.gov/salmonella/newport-04-19/index.html (accessed on 30 March 2025).
- Alam, M.T.; Stern, S.R.; Frison, D.; Taylor, K.; Tagliamonte, M.S.; Nazmus, S.S.; Paisie, T.; Hilliard, N.B.; Jones, R.G.; Iovine, N.M.; et al. Seafood-Associated Outbreak of ctx-Negative Vibrio mimicus Causing Cholera-Like Illness, Florida, USA. Emerg. Infect. Dis. 2023, 29, 2141–2144. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Multi-Country Outbreak of Listeria Monocytogenes Clonal Complex 8 Infections Linked to Consumption of Cold-Smoked Fish Products. 2019. Available online: https://www.ecdc.europa.eu/en/publications-data/multi-country-outbreak-listeria-monocytogenes-fish-products (accessed on 30 March 2025).
- CDC. Vibrio parahaemolyticus Infections Linked to Fresh Crab Meat Imported from Venezuela (Final Update). 2018. Available online: https://archive.cdc.gov/#/details?url=https://www.cdc.gov/vibrio/investigations/vibriop-07-18/index.html (accessed on 30 March 2025).
- ECDC. Multi-Country Outbreak of Listeria monocytogenes Sequence Type 8 Infections Linked to Consumption of Salmon Products. 2018. Available online: https://www.ecdc.europa.eu/en/publications-data/multi-country-outbreak-listeria-monocytogenes-sequence-type-8-infections-linked (accessed on 30 March 2025).
- HDOH. Hepatitis A Outbreak 2016. 2016. Available online: https://health.hawaii.gov/docd/hepatitis-a-outbreak-2016/ (accessed on 30 March 2025).
- Kim, J.H.; Lee, J.; Hong, S.; Lee, S.; Na, H.Y.; Jeong, Y.-I.; Choi, E.J.; Kim, J.; Kawk, H.S.; Cho, E. Cholera outbreak due to raw seafood consumption in South Korea, 2016. Am. J. Trop. Med. Hyg. 2018, 99, 168–170. [Google Scholar] [CrossRef]
- CDC. 2015 Salmonella Outbreak Linked to Frozen Raw Tuna. 2015. Available online: https://archive.cdc.gov/#/details?url=https://www.cdc.gov/salmonella/paratyphi-b-05-15/index.html (accessed on 30 March 2025).
- Velayudhan, A.; Nayak, J.; Murhekar, M.V.; Dikid, T.; Sodha, S.V. Shellfish poisoning outbreaks in Cuddalore District, Tamil Nadu, India. Indian J. Public Health 2021, 65, S29–S33. [Google Scholar] [CrossRef]
- Al-Busaidi, M.A.; Jukes, D.J.; Bose, S. Seafood safety and quality: An analysis of the supply chain in the Sultanate of Oman. Food Control 2016, 59, 651–662. [Google Scholar] [CrossRef]
- Desdouits, M.; Reynaud, Y.; Philippe, C.; Guyader, F.S.L. A Comprehensive Review for the Surveillance of Human Pathogenic Microorganisms in Shellfish. Microorganisms 2023, 11, 2218. [Google Scholar] [CrossRef]
- CDC. Foodborne Diseases Active Surveillance Network. Available online: https://www.cdc.gov/foodnet/about/index.html (accessed on 30 March 2025).
- ECDC The European Surveillance System (TESSy). Available online: https://www.ecdc.europa.eu/en/publications-data/european-surveillance-system-tessy (accessed on 30 March 2025).
- Wu, Y.; Chen, J. Food safety monitoring and surveillance in China: Past, present and future. Food Control 2018, 90, 429–439. [Google Scholar] [CrossRef]
- Taiwo, O.R.; Onyeaka, H.; Oladipo, E.K.; Oloke, J.K.; Chukwugozie, D.C. Advancements in Predictive Microbiology: Integrating New Technologies for Efficient Food Safety Models. Int. J. Microbiol. 2024, 2024, 6612162. [Google Scholar] [CrossRef]
- Ferone, M.; Gowen, A.; Fanning, S.; Scannell, A.G. Microbial detection and identification methods: Bench top assays to omics approaches. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3106–3129. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Cai, Q.; Miao, Q.; Song, Z.; Fang, Y.; Hu, B. High-throughput metagenomics for identification of pathogens in the clinical settings. Small Methods 2021, 5, 2000792. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.H.; Kim, M.I. Nanomaterial-mediated paper-based biosensors for colorimetric pathogen detection. TrAC Trends Anal. Chem. 2020, 132, 116038. [Google Scholar] [CrossRef] [PubMed]
- Soroka, M.; Wasowicz, B.; Rymaszewska, A. Loop-mediated isothermal amplification (LAMP): The better sibling of PCR? Cells 2021, 10, 1931. [Google Scholar] [CrossRef] [PubMed]
- Suminda, G.G.D.; Bhandari, S.; Won, Y.; Goutam, U.; Pulicherla, K.K.; Son, Y.O.; Ghosh, M. High-throughput sequencing technologies in the detection of livestock pathogens, diagnosis, and zoonotic surveillance. Comput. Struct. Biotechnol. J. 2022, 20, 5378–5392. [Google Scholar] [CrossRef] [PubMed]
- Panwar, S.; Duggirala, K.S.; Yadav, P.; Debnath, N.; Yadav, A.K.; Kumar, A. Advanced diagnostic methods for identification of bacterial foodborne pathogens: Contemporary and upcoming challenges. Crit. Rev. Biotechnol. 2023, 43, 982–1000. [Google Scholar] [CrossRef]
- Mu, W.; Kleter, G.A.; Bouzembrak, Y.; Dupouy, E.; Frewer, L.J.; Radwan Al Natour, F.N.; Marvin, H.J. Making food systems more resilient to food safety risks by including artificial intelligence, big data, and internet of things into food safety early warning and emerging risk identification tools. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13296. [Google Scholar] [CrossRef]
- Naseem, S.; Rizwan, M. The role of artificial intelligence in advancing food safety: A strategic path to zero contamination. Food Control 2025, 6, 111292. [Google Scholar] [CrossRef]
- Leal, M.C.; Pimentel, T.; Ricardo, F.; Rosa, R.; Calado, R. Seafood traceability: Current needs, available tools, and biotechnological challenges for origin certification. Trends Biotechnol. 2015, 33, 331–336. [Google Scholar] [CrossRef]
- Hoque, M.Z.; Akhter, N.; Chowdhury, M.S.R. Consumers’ Preferences for the Traceability Information of Seafood Safety. Foods 2022, 11, 1675. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.; Mitchell, M.; Dean, M.; Elliott, C.; Campbell, K. The seafood supply chain from a fraudulent perspective. Food Secur. 2018, 10, 939–963. [Google Scholar] [CrossRef]
- Lewis, S.G.; Boyle, M. The Expanding Role of Traceability in Seafood: Tools and Key Initiatives. J. Food Sci. 2017, 82, A13–A21. [Google Scholar] [CrossRef]
- Rejeb, A.; Keogh, J.G.; Zailani, S.; Treiblmaier, H.; Rejeb, K. Blockchain Technology in the Food Industry: A Review of Potentials, Challenges and Future Research Directions. Logistics 2020, 4, 27. [Google Scholar] [CrossRef]
- Stentiford, G.D.; Bateman, I.J.; Hinchliffe, S.J.; Bass, D. 1.; Hartnell, R.; Santos, E.M.; Devlin, M.J.; Feist, S.W.; Taylor, N.G.; Verner-Jeffreys, D.W.; et al. Sustainable aquaculture through the One Health lens. Nat. Food 2020, 1, 468–474. [Google Scholar] [CrossRef]
- Muniesa, A.; Furones, D.; Rodgers, C.; Basurco, B. An assessment of health management and biosecurity procedures in marine fish farming in Spain. Aquac. Rep. 2022, 25, 101199. [Google Scholar] [CrossRef]
- Subasinghe, R.; Alday-Sanz, V.; Bondad-Reantaso, M.G.; Jie, H.; Shinn, A.P.; Sorgeloos, P. Biosecurity: Reducing the burden of disease. J. World Aquac. Soc. 2023, 54, 397–426. [Google Scholar] [CrossRef]
- Okocha, R.C.; Olatoye, I.O.; Adedeji, O.B. Food safety impacts of antimicrobial use and their residues in aquaculture. Public Health Rev. 2018, 39, 21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, H.; He, S.; Feng, Q.; Xia, S.; Zhou, Q.; Wu, Z.; Zhang, Y. Navigating the depths of seafood authentication: Technologies, regulations, and future prospects. Meas. Food 2024, 14, 100165. [Google Scholar] [CrossRef]
- Paolacci, S.; Mendes, R.; Klapper, R.; Velasco, A.; Ramilo-Fernandez, G.; Munoz-Colmenero, M.; Potts, T.; Martins, S.; Avignon, S.; Maguire, J.; et al. Labels on seafood products in different European countries and their compliance to EU legislation. Mar. Policy 2021, 134, 104810. [Google Scholar] [CrossRef]
- Hicks, D.T. Seafood Safety and Quality: The Consumer’s Role. Foods 2016, 5, 71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
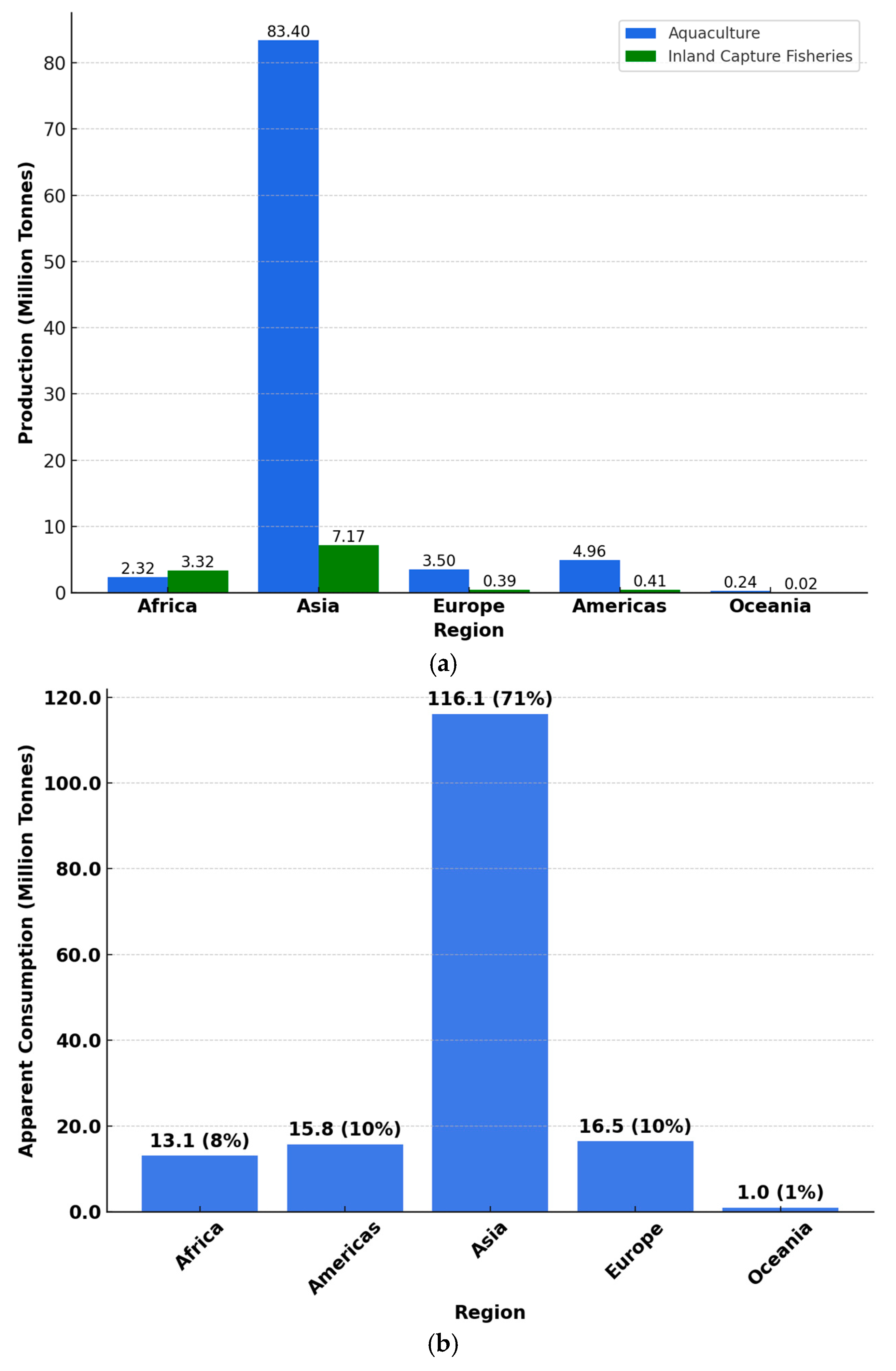

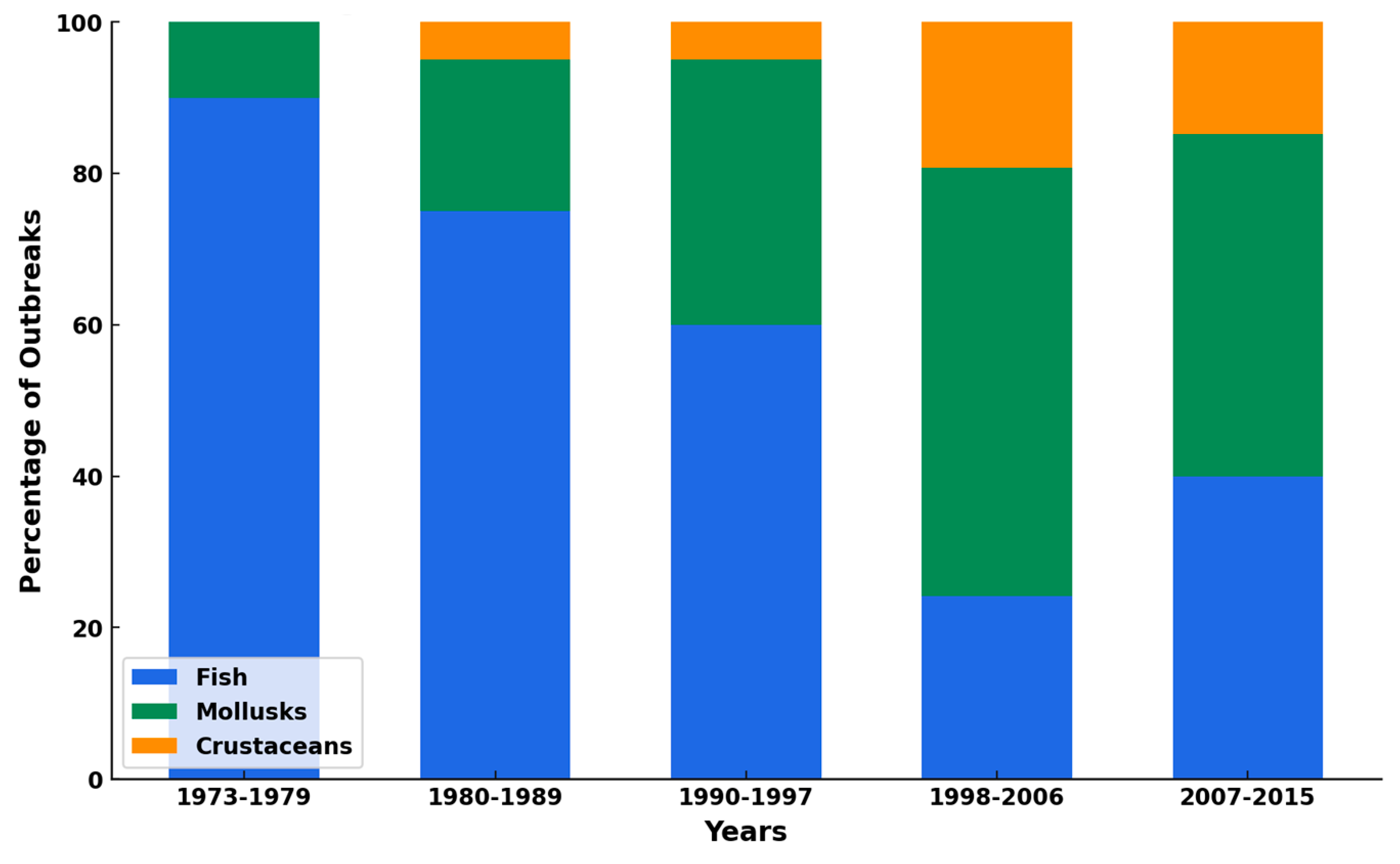

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taylor, B.; Ofori, K.F.; Parsaeimehr, A.; Akdemir Evrendilek, G.; Attarwala, T.; Ozbay, G. Exploring the Complexities of Seafood: From Benefits to Contaminants. Foods 2025, 14, 1461. https://doi.org/10.3390/foods14091461
Taylor B, Ofori KF, Parsaeimehr A, Akdemir Evrendilek G, Attarwala T, Ozbay G. Exploring the Complexities of Seafood: From Benefits to Contaminants. Foods. 2025; 14(9):1461. https://doi.org/10.3390/foods14091461
Chicago/Turabian StyleTaylor, Bettina, Kelvin Fynn Ofori, Ali Parsaeimehr, Gulsun Akdemir Evrendilek, Tahera Attarwala, and Gulnihal Ozbay. 2025. "Exploring the Complexities of Seafood: From Benefits to Contaminants" Foods 14, no. 9: 1461. https://doi.org/10.3390/foods14091461
APA StyleTaylor, B., Ofori, K. F., Parsaeimehr, A., Akdemir Evrendilek, G., Attarwala, T., & Ozbay, G. (2025). Exploring the Complexities of Seafood: From Benefits to Contaminants. Foods, 14(9), 1461. https://doi.org/10.3390/foods14091461









