Development of an Integrated Multifunctional Column for Rapid Pretreatment and Determination of Trichothecenes in Cereals and Feeds with HPLC-MS/MS
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Standards
2.3. Preparation of Solutions
2.4. Instruments and Equipment
2.5. Hybrid Sorbents Optimization
2.6. Sample Collection
2.7. Sample Preparation
2.8. LC-MS/MS Analysis
2.9. Data Analysis
2.10. Method Validation
3. Results and Discussion
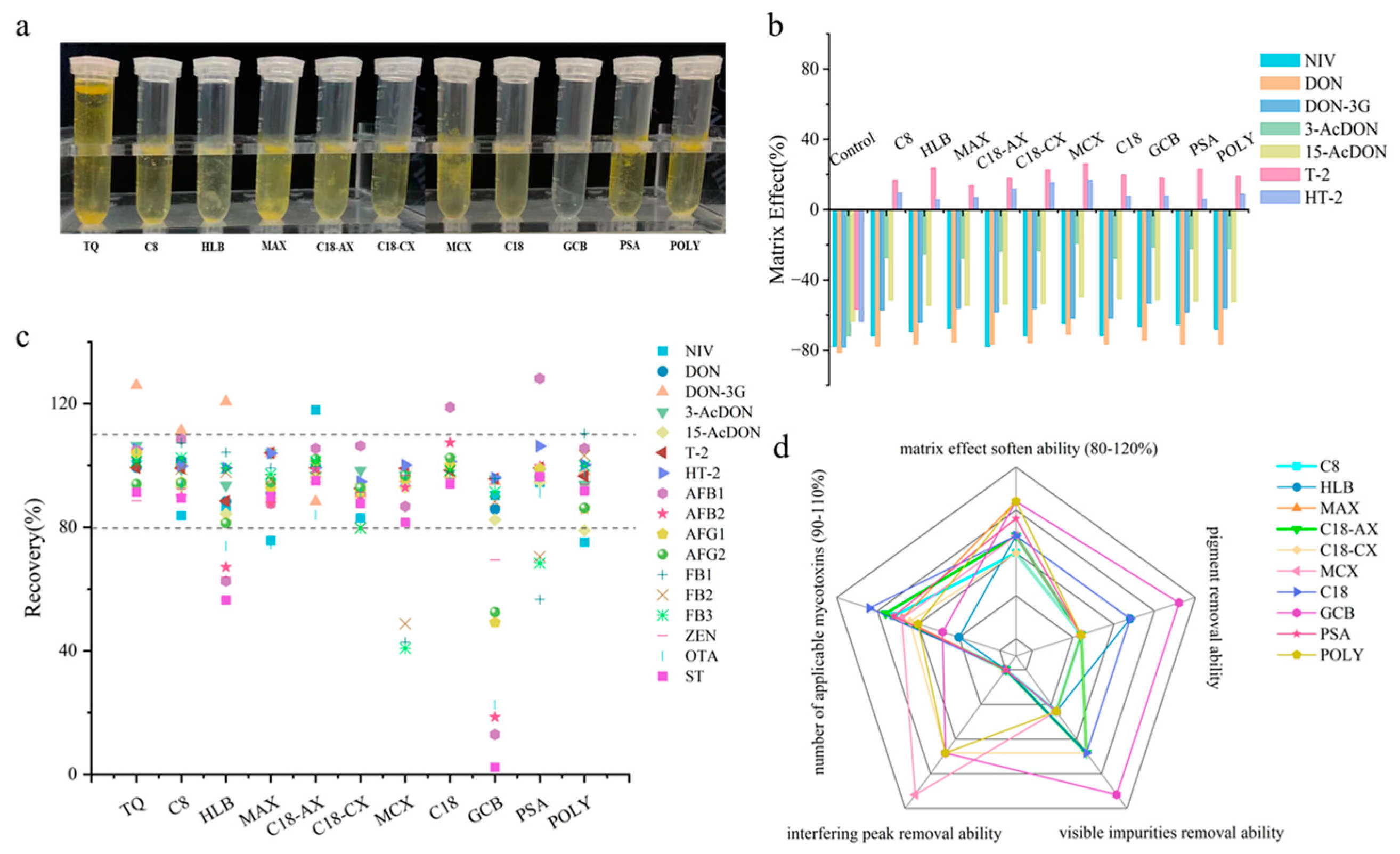
3.1. Selection, Optimization, and Combination of Sorbents
3.1.1. Construction of a Database for the Purification Capacity of Individual Sorbent
3.1.2. Optimization of Hybrid Sorbents
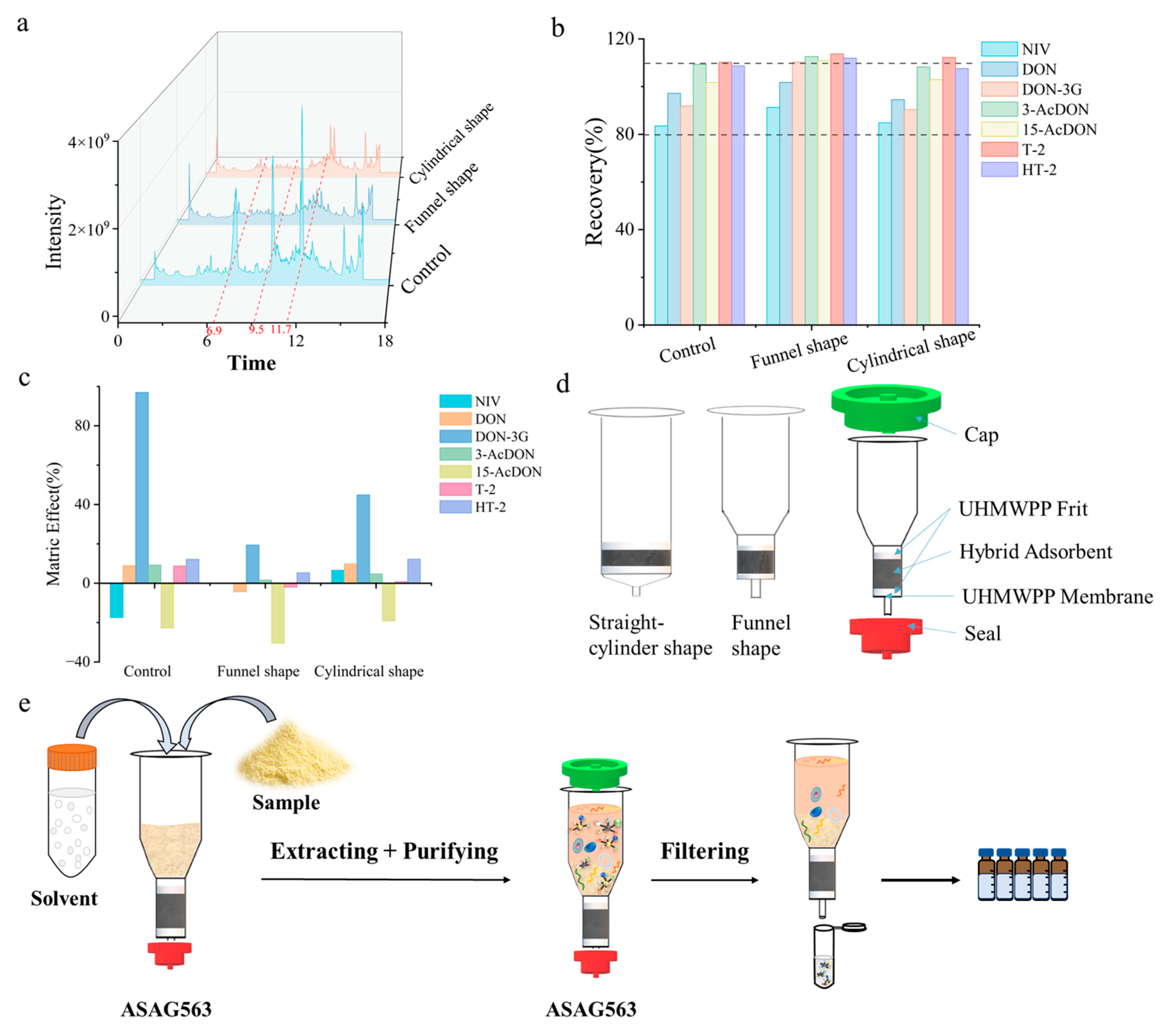
3.2. Design, Construction, and Optimization of Multifunctional Pretreatment Column
3.2.1. Selection of Sieve Plate and Filter Membrane
3.2.2. Design of an Integrated Multifunctional Pretreatment Column
3.3. Optimization of Extraction and Elution Solvents
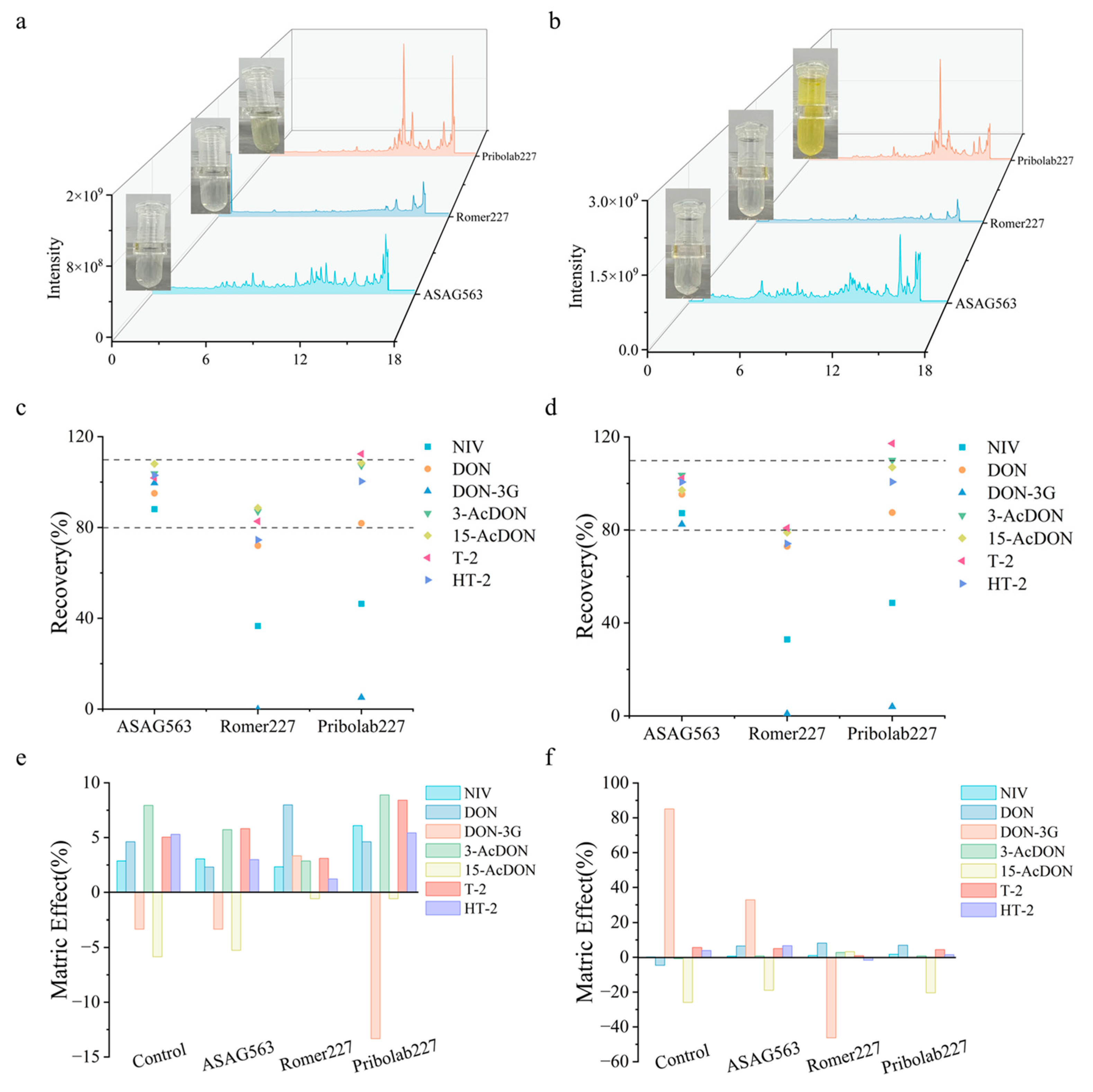
3.4. Analytical Performance of ASAG563
3.5. Method Validation
3.6. Comparison with Commercial MFC
3.7. Application of ASAG563 in Maize for Feedstuff Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nomura, M.; Shidara, K.; Yasuda, I.; Aoyama, K.; Takahashi, A.; Ishibashi, T. Development of a simultaneous quantification method for ten trichothecenes including deoxynivalenol-3-glucoside in feed. Mycotoxin Res. 2020, 36, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Mahato, D.K.; Pandhi, S.; Kamle, M.; Gupta, A.; Sharma, B.; Panda, B.K.; Srivastava, S.; Kumar, M.; Selvakumar, R.; Pandey, A.K.; et al. Trichothecenes in food and feed: Occurrence, impact on human health and their detection and management strategies. Toxicon 2022, 208, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Takushima, R.; Tanaka, A.; Okada, A.; Matsui, K.; Maeda, K.; Aikawa, S.; Kimura, M.; Takahashi-Ando, N. Reduced Toxicity of Trichothecenes, Isotrichodermol, and Deoxynivalenol, by Transgenic Expression of the Tri101 3-O-Acetyltransferase Gene in Cultured Mammalian FM3A Cells. Toxins 2019, 11, 654. [Google Scholar] [CrossRef]
- Berthiller, F.; Schuhmacher, R.; Buttinger, G.; Krska, R. Rapid simultaneous determination of major type A- and B-trichothecenes as well as zearalenone in maize by high performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2005, 1062, 209–216. [Google Scholar] [CrossRef]
- Gab-Allah, M.A.; Choi, K.; Kim, B. Accurate determination of type B trichothecenes and conjugated deoxynivalenol in grains by isotope dilution–liquid chromatography tandem mass spectrometry. Food Control 2021, 121, 107557. [Google Scholar] [CrossRef]
- Gosetti, F.; Mazzucco, E.; Zampieri, D.; Gennaro, M.C. Signal suppression/enhancement in high-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 3929–3937. [Google Scholar] [CrossRef]
- Ji, C.; Sun, X.; Fang, Y.; Li, P. Determination of Aflatoxin B1 in Grains by Aptamer Affinity Column Enrichment and Purification Coupled with High Performance Liquid Chromatography Detection. Foods 2024, 13, 640. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.; Li, X.; Zhou, S.; Ma, J.; Zhao, X.; Zhang, Q.; Yin, X. Determination of multi-mycotoxins in vegetable oil via liquid chromatography-high resolution mass spectrometry assisted by a complementary liquid–liquid extraction. Food Chem. X 2023, 20, 100887. [Google Scholar] [CrossRef]
- Raposo, F.; Barceló, D. Challenges and strategies of matrix effects using chromatography-mass spectrometry: An overview from research versus regulatory viewpoints. TrAC Trends Anal. Chem. 2021, 134, 116068. [Google Scholar] [CrossRef]
- Ertekin, Ö.; Kaymak, T.; Pirinçci, Ş.Ş.; Akçael, E.; Öztürk, S. Aflatoxin-specific Monoclonal Antibody Selection for Immunoaffinity Column Development. BioTechniques 2019, 66, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, A.; Mao, X.; Sun, M.; Yang, S.; Li, J.; You, Y.; Wu, Y.; Jiang, G. Multiple antibodied based immunoaffinity columns preparation for the simultaneous analysis of deoxynivalenol and T-2 toxin in cereals by liquid chromatography tandem mass spectrometry. Food Chem. 2021, 337, 127802. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Dai, H.; Wan, X.; Shan, D.; Zhang, Q.; Li, J.; Xu, Q.; Wang, C. Simultaneous determination of multiple mycotoxins in corn and wheat by high efficiency extraction and purification based on polydopamine and ionic liquid bifunctional nanofiber mat. Anal. Chim. Acta 2023, 1267, 341361. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Manzanares, N.; García-Campaña, A.M.; Gámiz-Gracia, L. Multiclass mycotoxin analysis in Silybum marianum by ultra high performance liquid chromatography–tandem mass spectrometry using a procedure based on QuEChERS and dispersive liquid–liquid microextraction. J. Chromatogr. A 2013, 1282, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Lahane, V.; Singh, S.; Mishra, S.; Yadav, A.K. Development and validation of multiresidue analysis method for biomonitoring of pesticides and metabolites in human blood and urine by LC-QToF-MS. Talanta 2025, 282, 126968. [Google Scholar] [CrossRef]
- Lin, S.; Qiu, W.; Hua, Y.; Yang, Y. Rapid determination of caine-based anesthetics and their metabolite residues in fish using a modified QuEChERS method coupled with UPLC-MS/MS. Food Chem. X 2024, 24, 102032. [Google Scholar] [CrossRef]
- Su, Y.; Lu, J.; Guo, X.; Liu, J.; Fu, H.; Yu, L. Method development, residue level analysis, and dietary risk assessment of six morpholine pesticide residues in plant-derived foods based on modified QuEChERS–liquid chromatography–tandem mass spectrometry. J. Food Compos. Anal. 2024, 132, 106303. [Google Scholar] [CrossRef]
- Turner, N.W.; Subrahmanyam, S.; Piletsky, S.A. Analytical methods for determination of mycotoxins: A review. Anal. Chim. Acta 2009, 632, 168–180. [Google Scholar] [CrossRef]
- Drabińska, N.; Marcinkowska, M.A.; Wieczorek, M.N.; Jeleń, H.H. Application of Sorbent-Based Extraction Techniques in Food Analysis. Molecules 2023, 28, 7985. [Google Scholar] [CrossRef]
- Rochat, B.; Waridel, P.; Barblan, J.; Sottas, P.-E.; Quadroni, M. Robust and sensitive peptidomics workflow for plasma based on specific extraction, lipid removal, capillary LC setup and multinozzle ESI emitter. Talanta 2021, 223, 121617. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; El-Nouby, M.A.M.; Kimani, P.K.; Lim, L.W.; Rabea, E.I. A review of the modern principles and applications of solid-phase extraction techniques in chromatographic analysis. Anal. Sci. 2022, 38, 1457–1487. [Google Scholar] [CrossRef]
- Hashemi, B.; Zohrabi, P.; Shamsipur, M. Recent developments and applications of different sorbents for SPE and SPME from biological samples. Talanta 2018, 187, 337–347. [Google Scholar] [CrossRef]
- Rahmani, Z.; Shafiei-Alavijeh, M.; Kazemi, A.; Rashidi, A.M. Synthesis of MIL-101@nanoporous graphene composites as hydrophobic adsorbents for oil removal. J. Taiwan Inst. Chem. Eng. 2018, 91, 597–608. [Google Scholar] [CrossRef]
- Guo, D.; Huang, Q.; Zhao, R.; Guo, W.; Fan, K.; Han, Z.; Zhao, Z.; Nie, D. MIL-101(Cr)@Fe3O4 nanocomposites as magnetic solid-phase extraction adsorbent for the determination of multiple mycotoxins in agricultural products by ultra-high-performance liquid chromatography tandem mass spectrometry. Food Control 2023, 146, 109540. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; Peñalver-Soler, R.; Campillo, N.; Viñas, P. Dispersive Solid-Phase Extraction using Magnetic Carbon Nanotube Composite for the Determination of Emergent Mycotoxins in Urine Samples. Toxins 2020, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- EU Commission. Commission Implementing Regulation (EU) 2023/2782 laying down the methods of sampling and analysis for the control of the levels of mycotoxins in food and repealing Regulation (EC) No 401/2006. Off. J. Eur. Union 2023, 44, 36. [Google Scholar]
- SANTE/11312/2021. Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed. EU 2021, 20–49. Available online: https://eurl-pesticides.eu/docs/public/tmplt_article.asp?CntID=727 (accessed on 1 January 2024).
- Li, S.; Liu, N.; Cai, D.; Liu, C.; Ye, J.; Li, B.; Wu, Y.; Li, L.; Wang, S.; van der Fels-Klerx, H.J. A predictive model on deoxynivalenol in harvested wheat in China: Revealing the impact of the environment and agronomic practicing. Food Chem. 2023, 405, 134727. [Google Scholar] [CrossRef]
- Wu, Y.; Ye, J.; Zhang, B.; Wang, S.; Li, L.; Li, R.; Li, S.; Cui, H.; Xie, G.; Wang, S.; et al. A Fast Analytical Approach for Determination of 16 Kinds of Mycotoxins in Vegetable Oils Using Stable Isotope Dilution and Ultra High Performance Liquid Chromatography-Tandem Mass Spectrometry. Chin. J. Anal. Chem. 2018, 46, 975–984. [Google Scholar] [CrossRef]
- EU Commission. Commission regulation (EU) No 519/2014 amending Regulation (EC) No 401/2006 as regards methods of sampling of large lots, spices and food supplements, performance criteria for T-2, HT-2 toxin and citrinin and screening methods of analysis. Off. J. Eur. Union 2014, 147, 35–38. [Google Scholar]
- Sapozhnikova, Y.; Nuñez, A. Non-targeted analysis with liquid chromatography—High resolution mass spectrometry for the identification of food packaging migrants. J. Chromatogr. A 2022, 1676, 463215. [Google Scholar] [CrossRef]
- Hennion, M.-C. Graphitized carbons for solid-phase extraction. J. Chromatogr. A 2000, 885, 73–95. [Google Scholar] [CrossRef]
- Fontanals, N.; Borrull, F.; Marcé, R.M. Overview of mixed-mode ion-exchange materials in the extraction of organic compounds. Anal. Chim. Acta 2020, 1117, 89–107. [Google Scholar] [CrossRef] [PubMed]
- Basko, A.; Pochivalov, K. Current State-of-the-Art in Membrane Formation from Ultra-High Molecular Weight Polyethylene. Membranes 2022, 12, 1137. [Google Scholar] [CrossRef]
- Taylor, N.; Ma, W.J.; Kristopeit, A.; Wang, S.-c.; Zydney, A.L. Retention characteristics of sterile filters—Effect of pore size and structure. J. Membr. Sci. 2021, 635, 119436. [Google Scholar] [CrossRef]
- Carson, A.R.; Smith, E.N.; Matsui, H.; Brækkan, S.K.; Jepsen, K.; Hansen, J.B.; Frazer, K.A. Effective filtering strategies to improve data quality from population-based whole exome sequencing studies. BMC Bioinform. 2014, 15, 15. [Google Scholar] [CrossRef]
- Seah, M.Q.; Chua, S.F.; Ang, W.L.; Lau, W.J.; Mansourizadeh, A.; Thamaraiselvan, C. Advancements in polymeric membranes for challenging water filtration environments: A comprehensive review. J. Environ. Chem. Eng. 2024, 12, 112628. [Google Scholar] [CrossRef]
- Chen, F.; Ji, Z.; Qi, Q. Effect of pore size and layers on filtration performance of coalescing filters with different wettabilities. Sep. Purif. Technol. 2018, 201, 71–78. [Google Scholar] [CrossRef]
- Puts, G.J.; Crouse, P.; Ameduri, B.M. Polytetrafluoroethylene: Synthesis and Characterization of the Original Extreme Polymer. Chem. Rev. 2019, 119, 1763–1805. [Google Scholar] [CrossRef]
- Shim, E.; Jang, J.-P.; Moon, J.-J.; Kim, Y. Improvement of Polytetrafluoroethylene Membrane High-Efficiency Particulate Air Filter Performance with Melt-Blown Media. Polymers 2021, 13, 4067. [Google Scholar] [CrossRef]
- Scientific Working Group for Forensic Toxicology. Scientific Working Group for Forensic Toxicology (SWGTOX) Standard Practices for Method Validation in Forensic Toxicology. J. Anal. Toxicol. 2013, 37, 452–474. [Google Scholar] [CrossRef]
- Song, L.; Han, Y.; Yang, J.; Qin, Y.; Zeng, W.; Xu, S.; Pan, C. Rapid single-step cleanup method for analyzing 47 pesticide residues in pepper, chili peppers and its sauce product by high performance liquid and gas chromatography-tandem mass spectrometry. Food Chem. 2019, 279, 237–245. [Google Scholar] [CrossRef]
- Wu, Y.; Ye, J.; Wang, S.; Han, X.; Li, L.; Zhu, L.; Li, S.; Liu, H.; Wang, Y.; Shao, Y.; et al. An accurate and efficient method for simultaneous determination of 16 trichothecenes in maize and wheat by UHPLC-Q-Orbitrap-HRMS combined one-step pretreatment with a novel isotope multipoint calibration strategy. Food Control 2023, 149, 109708. [Google Scholar] [CrossRef]
- GB13078-2017; Hygienical Standard for Feed. China’s Administration for Quality Supervision, Inspection and Quarantine (AQSIQ): Beijing, China, 2017.



| Background of Column | Recovery of Column | ||
|---|---|---|---|
| C (μg/kg) | Recovery (%) | RSD (%) | |
| NIV | <LOD | 97.4 | 3.5 |
| DON | <LOD | 99.4 | 2.4 |
| DON-3G | <LOD | 88.4 | 3.8 |
| 3-AcDON | <LOD | 99.7 | 3.0 |
| 15-AcDON | <LOD | 100.8 | 2.5 |
| T-2 | <LOD | 95.8 | 2.8 |
| HT-2 | <LOD | 100.0 | 3.4 |
| Intra-Day | Inter-Day | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low Concentration Level | Medium Concentration Level | High Concentration Level | Medium Concentration Level | |||||||||||
| Matrix | TCTs | ME (%) | C (μg/kg) | REC (%) | RSDr (%) | C (μg/kg) | REC (%) | RSDr (%) | C (μg/kg) | REC (%) | RSDr (%) | C (μg/kg) | REC (%) | RSDR (%) |
| Maize for feedstuff | NIV | 103.0 | 800 | 92.2 | 0.3 | 1600 | 92.4 | 2.9 | 3200 | 92.4 | 2.9 | 1600 | 92.9 | 1.2 |
| DON | 102.3 | 600 | 103.9 | 0.6 | 1200 | 100.0 | 1.4 | 2400 | 100.2 | 1.5 | 1200 | 99.1 | 2.0 | |
| DON-3G | 96.7 | 100 | 94.4 | 5.1 | 200 | 99.6 | 2.0 | 400 | 117.8 | 5.9 | 200 | 96.8 | 2.6 | |
| 3-AcDON | 105.7 | 160 | 109.8 | 2.4 | 320 | 106.3 | 2.0 | 640 | 105.7 | 1.3 | 320 | 104.8 | 1.2 | |
| 15-AcDON | 94.7 | 80 | 105.8 | 1.2 | 160 | 103.4 | 0.3 | 320 | 104.8 | 1.4 | 160 | 104.6 | 3.5 | |
| T-2 | 105.8 | 8 | 113.8 | 2.5 | 16 | 103.6 | 1.3 | 32 | 100.2 | 6.8 | 16 | 105.0 | 1.9 | |
| HT-2 | 103.0 | 40 | 108.8 | 5.6 | 80 | 104.9 | 0.1 | 160 | 96.1 | 2.8 | 80 | 105.8 | 0.9 | |
| DDGS | NIV | 100.7 | 1600 | 90.2 | 0.2 | 3200 | 84.6 | 3.1 | 6400 | 80.8 | 1.3 | 3200 | 85.1 | 3.4 |
| DON | 106.5 | 1200 | 102.4 | 1.2 | 2400 | 93.6 | 1.1 | 4800 | 85.4 | 0.8 | 2400 | 91.6 | 2.2 | |
| DON-3G | 132.8 | 200 | 94.3 | 3.0 | 400 | 86.0 | 3.1 | 800 | 78.9 | 4.8 | 400 | 84.3 | 1.7 | |
| 3-AcDON | 100.8 | 320 | 110.2 | 0.8 | 640 | 104.5 | 0.8 | 1280 | 96.8 | 0.6 | 640 | 105.5 | 2.0 | |
| 15-AcDON | 81.0 | 160 | 107.1 | 4.1 | 320 | 97.9 | 1.8 | 640 | 93.3 | 1.0 | 320 | 96.5 | 2.8 | |
| T-2 | 105.0 | 16 | 111.5 | 3.1 | 32 | 112.0 | 1.4 | 64 | 101.7 | 0.4 | 32 | 110.6 | 1.4 | |
| HT-2 | 106.6 | 80 | 100.5 | 3.7 | 160 | 109.2 | 0.5 | 320 | 102.1 | 2.6 | 160 | 109.0 | 3.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Wu, Y.; Liu, T.; Ye, J.; Li, L.; Guan, X.; Wang, S. Development of an Integrated Multifunctional Column for Rapid Pretreatment and Determination of Trichothecenes in Cereals and Feeds with HPLC-MS/MS. Foods 2025, 14, 1466. https://doi.org/10.3390/foods14091466
Liu S, Wu Y, Liu T, Ye J, Li L, Guan X, Wang S. Development of an Integrated Multifunctional Column for Rapid Pretreatment and Determination of Trichothecenes in Cereals and Feeds with HPLC-MS/MS. Foods. 2025; 14(9):1466. https://doi.org/10.3390/foods14091466
Chicago/Turabian StyleLiu, Sisi, Yu Wu, Tongtong Liu, Jin Ye, Li Li, Xiao Guan, and Songxue Wang. 2025. "Development of an Integrated Multifunctional Column for Rapid Pretreatment and Determination of Trichothecenes in Cereals and Feeds with HPLC-MS/MS" Foods 14, no. 9: 1466. https://doi.org/10.3390/foods14091466
APA StyleLiu, S., Wu, Y., Liu, T., Ye, J., Li, L., Guan, X., & Wang, S. (2025). Development of an Integrated Multifunctional Column for Rapid Pretreatment and Determination of Trichothecenes in Cereals and Feeds with HPLC-MS/MS. Foods, 14(9), 1466. https://doi.org/10.3390/foods14091466






