Effect of a Gelatin-Based Film Including Gelidium sp. Algal Flour on Antimicrobial Properties Against Spoilage Bacteria and Quality Enhancement of Refrigerated Trachurus trachurus
Abstract
1. Introduction
2. Materials and Methods
2.1. Initial Algal Flour and Biopolymer Film Preparation
2.2. Raw Fish, Packaging, and Sampling
2.3. Determination of Chemical Indices Related to Quality Loss
2.4. Statistical Analysis
3. Results
3.1. Bacterial Evolution in Refrigerated Fish
3.2. Evolution of Chemical Parameters Related to Microbial Activity in Refrigerated Fish
3.3. Determination of Lipid Oxidation in Refrigerated Fish
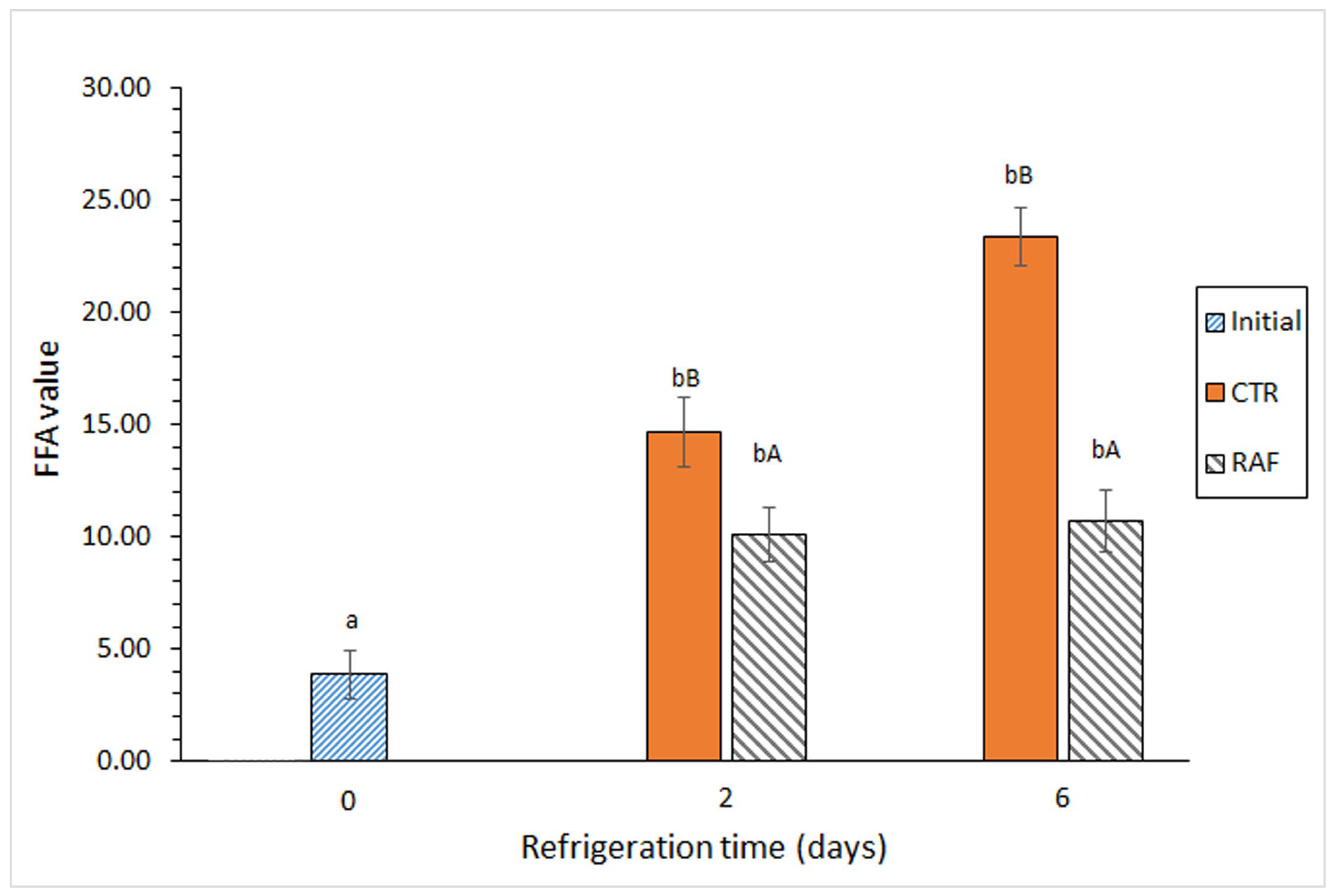
3.4. Determination of Lipid Hydrolysis in Refrigerated Fish
4. Discussion
4.1. Evolution of Microbial Development in Refrigerated Fish
4.2. Evolution of Lipid Oxidation in Refrigerated Fish
4.3. Evolution of Lipid Hydrolysis in Refrigerated Fish
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olafsdóttir, G.; Martinsdóttir, E.; Oehlenschläger, J.; Dalgaard, P.; Jensen, B.; Undeland, I.; Mackie, I.; Henehan, G.; Nielsen, J.; Nilsen, H. Methods to evaluate fish freshness in research and industry. Trends Food Sci. Technol. 1997, 8, 258–265. [Google Scholar] [CrossRef]
- Aubourg, S.P. Damage detection in horse mackerel (Trachurus trachurus) during chilled storage. J. Am. Oil Chem. Soc. 2001, 78, 857–862. [Google Scholar] [CrossRef]
- Özoğul, Y. Methods for freshness quality and deterioration. In Handbook of Seafood and Seafood Products Analysis; Nollet, L., Toldrá, F., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, Fl, USA, 2010; pp. 189–214. [Google Scholar]
- Mei, J.; Ma, X.; Xie, J. Review on natural preservatives for extending fish shelf life. Foods 2019, 8, 490. [Google Scholar] [CrossRef] [PubMed]
- Gökoğlu, N. Novel natural food preservatives and applications in seafood preservation: A review. J. Sci. Food Agric. 2019, 99, 2068–2077. [Google Scholar] [CrossRef]
- Amaral, R.A.; Pinto, C.A.; Lima, V.; Tavares, J.; Martins, A.P.; Fidalgo, L.G.; Silva, A.M.; Gil, M.M.; Teixeora, P.; Barbosa, J.; et al. Chemical-based methodologies to extend the shelf life of fresh fish—A Review. Foods 2021, 10, 2300. [Google Scholar] [CrossRef]
- Aubourg, S.P. Employment of flake ice systems including natural preservative compounds for the quality enhancement of chilled seafood. A review. Antioxidants 2021, 10, 1499. [Google Scholar] [CrossRef]
- López-Rubio, A.; Gavara, R.; Lagarón, J. Bioactive packaging: Turning foods into healthier foods through biomaterials. Trends Food Sci. Technol. 2006, 17, 567–575. [Google Scholar] [CrossRef]
- Umaraw, P.; Munekata, P.; Verma, A.; Barba, F.J.; Singh, V.P.; Kumar, P.; Lorenzo, J.M. Edible films/coating with tailored properties for active packaging of meat, fish and derived products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar] [CrossRef]
- Giménez, B.; López de Lacey, A.; Pérez-Santín, E.; López-Caballero, M.E.; Montero, P. Release of active compounds from agar and agar gelatin films with green tea extract. Food Hydrocoll. 2013, 30, 264–271. [Google Scholar] [CrossRef]
- Martucci, J.; Gende, L.; Neira, L.; Ruseckaite, R. Oregano and lavender essential oils as antioxidant and antimicrobial additives of biogenic gelatin films. Ind. Crops Prod. 2015, 71, 205–213. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Jafari, S.M.; Salejhabadi, A.; Nafchi, A.M.; Kumar, S.U.; Khalil, H.P.S. Biodegradable green packaging with antimicrobial functions based on the bioactive compounds from tropical plants and their byproducts. Trend Food Sci. Technol. 2020, 100, 262–277. [Google Scholar] [CrossRef]
- Rustad, T.; Storro, I.; Slizyte, R. Possibilities for the utilization of marine byproducts. Int. J. Food Sci. Technol. 2011, 46, 2001–2014. [Google Scholar] [CrossRef]
- Özyurt, G.; Özkütük, A.S.; Boğa, M.; Durmuş, M.; Boğa, E.K. Biotransformation of seafood processing wastes fermented with natural lactic acid bacteria; the quality of fermented products and their use in animal feeding. Turk. J. Fish Aquat. Sci. 2017, 17, 543–555. [Google Scholar]
- Rubio-Rodríguez, N.; Beltrán, S.; Jaime, I.; de Diego, S.M.; Sanz, M.T.; Carballido, J.R. Production of omega-3 polyunsaturated fatty acid concentrates: A review. Innov. Food Sci. Emerg. Technol. 2010, 11, 1–12. [Google Scholar] [CrossRef]
- Olsen, R.L.; Toppe, J.; Karunasagar, I. Challenges and realistic opportunities in the use of byproducts from processing of fish and shellfish. Trends Food Sci. Technol. 2014, 36, 144–152. [Google Scholar] [CrossRef]
- Hu, X.; Tao, N.; Wang, X.; Xiao, J.; Wang, M. Marine-derived bioactive compounds with anti-obesity effect: A Review. J. Funct. Foods 2016, 21, 372–387. [Google Scholar] [CrossRef]
- Atef, M.; Ojagh, M. Health benefits and food applications of bioactive compounds from fish byproducts: A review. J. Funct. Foods 2017, 35, 673–681. [Google Scholar] [CrossRef]
- Kohli, K.; Prajapati, R.; Shah, R.; Das, M.; Sharma, B.K. Food waste: Environmental impact and possible solutions. Sustain. Food Technol. 2024, 2, 70–80. [Google Scholar] [CrossRef]
- Gupta, D.; Lall, A.; Kumar, S.; Patil, T.D.; Gaikwad, K.K. Plant-based edible films and coatings for food-packaging applications: Recent advances, applications, and trends. Sustain. Food Technol. 2024, 2, 1428–1455. [Google Scholar] [CrossRef]
- Hussain, S.; Akhter, R.; Maktedar, S.S. Advancements in sustainable food packaging: From eco-friendly materials to innovative technologies. Sustain. Food Technol. 2024, 2, 1297–1364. [Google Scholar] [CrossRef]
- Metha, C.; Pawar, S.; Suvarna, V. Recent advancements in alginate-based films for active food packaging applications. Sustain. Food Technol. 2024, 2, 1246–1265. [Google Scholar] [CrossRef]
- Sandsdalen, E.; Haug, T.; Stensvag, K.; Styrvold, O. The antibacterial effect of a polyhydroxylated fucophlorethol from the marine brown alga, Fucus vesiculosus. World J. Microb. Biotechnol. 2003, 19, 777–782. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci. Technol. 2011, 22, 315–326. [Google Scholar] [CrossRef]
- Echave, J.; Otero, P.; Garcia-Oliveira, P.; Munekata, P.E.S.; Pateiro, M.; Lorenzo, J.M.; Simal-Gandara, J.; Prieto, M.A. Seaweed-derived proteins and peptides: Promising marine bioactives. Antioxidants 2022, 11, 176. [Google Scholar] [CrossRef]
- MacArtain, P.; Gill, C.I.R.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional value of edible seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef]
- Jiang, J.L.; Zhang, W.Z.; Ni, W.X.; Shao, J.W. Insight on structure-property relationships of carrageenan from marine red algal: A review. Carb. Polym. 2021, 257, 117642. [Google Scholar] [CrossRef]
- Agarwal, P.; Kayala, P.; Chandrasekaran, N.; Mukherjee, A.; Shah, S.; Thomas, J. Antioxidant and antibacterial activity of Gelidium pusillum (Stackhouse) against Aeromonas caviae and its applications in aquaculture. Aquac. Int. 2021, 29, 845–858. [Google Scholar] [CrossRef]
- Wang, F.; Kong, L.M.; Xie, Y.Y.; Wang, C.; Wang, X.L.; Wang, Y.B.; Fu, L.L.; Zhou, T. Purification, structural characterization, and biological activities of degraded polysaccharides from Porphyra yezoensis. J. Food Biochem. 2021, 45, e13661. [Google Scholar] [CrossRef]
- Seedevi, P.; Moovendhan, M.; Viramani, S.; Shanmugam, A. Bioactive potential and structural characterization of sulfated polysaccharide from seaweed (Gracilaria corticata). Carb. Polym. 2017, 155, 516–524. [Google Scholar] [CrossRef]
- Arulkumar, A.; Rosemary, T.; Paramasivam, S.; Rajendran, R.B. Phytochemical composition, in vitro antioxidant, antibacterial potential and GC–MS analysis of red seaweeds (Gracilaria corticata and Gracilaria edulis) from Palk Bay, India. Biocatal. Agricult. Biotechnol. 2018, 15, 63–71. [Google Scholar] [CrossRef]
- Pei, R.; Zhai, H.; Qi, B.; Hao, S.; Huang, H.; Yang, X. Isolation, purification and monosaccharide composition analysis of polysaccharide from Gelidium amansii. Food Ferment. Indust. 2020, 7, 57–62. [Google Scholar]
- Mostafavi, F.S.; Zaeim, D. Agar-based edible films for food packaging applications—A review. Int. J. Biol. Macrom. 2020, 159, 1165–1176. [Google Scholar] [CrossRef]
- Yu, G.; Zhang, Q.; Wang, Y.; Yang, Q.; Yu, H.; Li, H.; Chen, J.; Fu, L. Sulfated polysaccharides from red seaweed Gelidium amansii: Structural characteristics, antioxidant and anti-glycation properties, and development of bioactive films. Food Hydrocoll. 2021, 119, 106820. [Google Scholar] [CrossRef]
- Commission Regulation (EU). No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food Text with EEA relevance. OJEU 2011, L12, 1–89. [Google Scholar]
- Gran Vision Research. Seaweed Packaging Market Size, Share & Trends Analysis Report by Source (Plant, Animal), by Packaging Process, by Application (Food, Beverages, Pharmaceuticals), by Region, and Segment Forecasts, 2024–2030. Available online: https://www.grandviewresearch.com/industry-analysis/seaweed-packaging-market-report (accessed on 4 April 2025).
- Ortiz-Viedma, J.; Aguilera, J.M.; Flores, M.; Lemus-Mondaca, R.; Larrazabal, M.J.; Miranda, J.M.; Aubourg, S.P. Protective effect of red algae (Rhodophyta) extracts on essential dietary components of heat-treated salmon. Antioxidants 2021, 10, 1108. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.M.; Trigo, M.; Barros-Velázquez, J.; Aubourg, S.P. Antimicrobial activity of red alga flour (Gelidium sp.) and its effect on quality retention of Scomber scombrus during refrigerated storage. Foods 2022, 11, 904. [Google Scholar] [CrossRef]
- López, L.; Gómez, A.; Trigo, M.; Miranda, J.M.; Barros-Velázquez, J.; Aubourg, S.P. Preservative effect of a gelatin-based film including a Gelidium sp. flour extract on refrigerated Atlantic mackerel. App. Sci. 2024, 14, 8817. [Google Scholar] [CrossRef]
- AOAC. Official Methods for Analysis of the Association of Analytical Chemistry, 15th ed.; Association of Official Chemists, Inc.: Arlington, VA, USA, 1990; pp. 931–937. [Google Scholar]
- Stejskal, N.; Miranda, J.M.; Martucci, J.F.; Ruseckaite, R.A.; Barros-Velázquez, J.; Aubourg, S.P. Quality enhancement of refrigerated hake muscle by active packaging with a protein concentrate from Spirulina platensis. Food Bioprocess Technol. 2020, 13, 1110–1118. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Lesieur, S.; Labarre, D.; Jayakrishnan, A. Periodate oxidation of sodium alginate in water and in ethanol–water mixture: A comparative study. Carb. Polym. 2005, 340, 1425–1429. [Google Scholar] [CrossRef]
- Tozawa, H.; Erokibara, K.; Amano, K. Proposed modification of Dyer’s method for trimethylamine determination in codfish. In Fish Inspection and Quality Control; Kreuzer, R., Ed.; Fishing News Books Ltd.: London, UK, 1971; pp. 187–190. [Google Scholar]
- Bligh, E.; Dyer, W. A rapid method of total extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Lowry, R.; Tinsley, I. Rapid colorimetric determination of free fatty acids. J. Am. Oil Chem. Soc. 1976, 53, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.; McKay, J. The estimation of peroxides in fats and oils by the ferric thiocyanate method. J. Am. Oil Chem. Soc. 1949, 26, 360–363. [Google Scholar] [CrossRef]
- Vyncke, W. Direct determination of the thiobarbituric acid value in trichloroacetic acid extracts of fish as a measure of oxidative rancidity. Fette Seifen Anstrichm. 1970, 72, 1084–1087. [Google Scholar] [CrossRef]
- Aubourg, S.P.; Medina, I.; Pérez-Martín, R. A comparison between conventional and fluorescence detection methods of cooking-induced damage to tuna fish lipids. Z. Lebensm. Unters. Forsch. 1995, 200, 252–255. [Google Scholar] [CrossRef]
- Perez, M.J.; Falqué, E.; Dominguez, H. Antimicrobial action of compounds from marine seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef]
- Chauhan, K.; Rao, A. Clean-label alternatives for food preservation: An emerging trend. Heliyon 2024, 10, e35815. [Google Scholar] [CrossRef]
- Bai, H.; Wang, Z.; Zhang, H.; Liu, R. Study on synergistic effect of polyphenols and an Auricularia auricula polysaccharides combination on antioxidant activity. Sci. Technol. Food Ind. 2013, 22, 124–127. [Google Scholar]
- Yin, J.; Zhao, D.; Song, J.; Gao, R.; Wang, X.; Rao, H.; Gao, X.; Hao, J. Synergistic antioxidant activity of Lycium barbarum Polysaccharide and chlorogenic acid and its effect on inflammatory response of NR8383 cells. Foods 2024, 13, 3696. [Google Scholar] [CrossRef]
- Zuorro, A.; Lavecchia, R.; Contreras-Ropero, J.E.; Martínez, J.B.G.; Barajas-Ferreira, C.; Barajas-Solano, A.F. Natural Antimicrobial Agents from Algae: Current Advances and Future Directions. Int. J. Mol. Sci. 2024, 25, 11826. [Google Scholar] [CrossRef]
- Akter, A.; Alam Sobuj, M.K.; Islam, M.; Chakroborty, K.; Tasnim, N.; Ayon, M.H.; Hossain, F.; Rafiquzzaqman, S.M. Seaweed polysaccharides: Sources, structure and biomedical applications with special emphasis on antiviral potentials. Fut. Foods 2024, 10, 100440. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Antibacterial derivatives of marine algae: An overview of pharmacological mechanisms and applications. Mar. Drugs 2016, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- El-Baroty, G.S.; El-Baz, F.K.; Abd-Elmoein, A.; El-Baky, H.H.A.; Ali, M.M.; Ibrahim, A.E. Evaluation of glycolipids of some Egyptian marine algae as a source of bioactive substances. Electr. J. Environm. Agric. Food Chem. 2011, 10, 2114–2128. [Google Scholar]
- Zeid, A.H.A.; Aboutabl, E.A.; Sleem, A.A.; El-Rafie, H.M. Water soluble polysaccharides extracted from Pterocladia capillacea and Dictyopteris membranacea and their biological activities. Carb. Polym. 2014, 113, 62–66. [Google Scholar] [CrossRef]
- Cui, M.; Zhou, R.; Wang, Y.; Zhang, M.; Liu, K.; Ma, C. Beneficial effects of sulfated polysaccharides from the red seaweed Gelidium pacificum Okamura on mice with antibiotic-associated diarrhea. Food Funct. 2020, 11, 4625–4637. [Google Scholar] [CrossRef]
- Lim, G.O.; Hong, Y.H.; Song, K.B. Incorporating grapefruit seed extract into Gelidium corneum-whey protein isolate blend packaging film increases the shelf life of fish paste. J. Food Sci. Nutr. 2008, 13, 370–374. [Google Scholar] [CrossRef]
- Wu, X.F.; Zhang, M.; Adhikari, B.; Sun, J.C. Recent developments in novel freezing and thawing technologies applied to foods. Crit. Rev. Food Sci. Nutr. 2017, 57, 3620–3631. [Google Scholar] [CrossRef]
- Sikorski, Z.E.; Kolakowski, E. Endogenous enzyme activity and seafood quality: Influence of chilling, freezing, and other environmental factors. In Seafood Enzymes. Utilization and Influence on Postharvest Seafood Quality; Haard, N.F., Simpson, B.K., Eds.; Marcel Dekker: New York, NY, USA, 2000; pp. 451–487. [Google Scholar]
- Howell, N.K. Interaction of proteins with small molecules. In Ingredient Interactions—Effects on Food Quality; Gaonkar, A., Ed.; Marcel Dekker: New York, NY, USA, 1995; pp. 269–289. [Google Scholar]
- Pokorný, J. Browning from lipid-protein interactions. Prog. Food Nutr. Sci. 1981, 5, 421–428. [Google Scholar]
- Jassbi, A.R.; Mohabati, M.; Eslami, S.; Sohrabipour, J.; Miri, R. Biological activity and chemical constituents of red and brown algae from the Persian Gulf. Iranian J. Pharm. Res. 2013, 12, 339–348. [Google Scholar]
- Widowati, I.; Lubac, D.; Puspita, M.; Bourgougnon, N. Antibacterial and antioxidant properties of the red alga Gracilaria verrucosa from the North coast of Java, Semarang, Indonesia. Int. J. Latest Res. Sci. Technol. 2014, 3, 179–185. [Google Scholar]
- Reboleira, J.; Ganhão, R.; Mendes, S.; Adão, P.; Andrade, M.; Vilarinho, F.; Sanches-Silva, A.; Sousa, D.; Mateus, A.; Bernardino, S. Optimization of extraction conditions for Gracilaria gracilis extracts and their antioxidative stability as part of microfiber food coating additives. Molecules 2020, 25, 4060. [Google Scholar] [CrossRef]
- Kim, S.; Back, S.; Song, K. Physical and antioxidant properties of alginate films prepared from Sargassum fulvellum with black chokeberry extract. Food Pack. Shelf Life 2018, 18, 157–163. [Google Scholar] [CrossRef]
- Arulkumar, K.; Raja, R.; Sameer Kumar, V.B.; Joseph, A.; Shilpa, T.; Carvalho, I.S. Antioxidant and cytotoxic activities of sulfated polysaccharides from five different edible seaweeds. J. Food Meas. Charact. 2021, 15, 567–576. [Google Scholar] [CrossRef]
- Ji, C.; Pan, C.; Huang, H.; Tao, F.; Lin, S.; Chen, S.; Qi, B.; Hu, X.; Yang, X. Effects of origin and harvest period on characterization, structure and antioxidant activity of polysaccharides derived from Porphyra haitanensis. Int. J. Food Sci. Technol. 2022, 57, 123–136. [Google Scholar] [CrossRef]
- Pan, C.; Ma, J.; Tao, F.; Ji, C.; Zhao, Y.; Chen, S.; Yang, X. Novel insight into the antioxidant proteins derived from laver (Porphyra haitanensis) by proteomics analysis and protein based bioinformatics. Food Biosci. 2021, 42, 101134. [Google Scholar] [CrossRef]
- Barbosa, R.G.; Trigo, M.; Zhang, B.; Aubourg, S.P. Effect of alga flour extract on lipid damage evolution in heated fish muscle system. Antioxidants 2022, 11, 807. [Google Scholar] [CrossRef]
- Whittle, K.; Hardy, R.; Hobbs, G. Chilled fish and fishery products. In Chilled Foods. The State of the Art; Gormley, T., Ed.; Elsevier Applied Science: New York, NY, USA, 1990; pp. 87–116. [Google Scholar]
- Ghali, A.; Dave, D.; Budge, S.; Brooks, M. Fish spoilage mechanisms and preservation: Review. Am. J. Appl. Sci. 2010, 7, 859–877. [Google Scholar] [CrossRef]
- Sista, R.; Erickson, M.; Shewfelt, R. Quality deterioration in frozen foods associated with hydrolytic enzyme activities. In Quality in Frozen Food; Erickson, M., Hung, Y.-C., Eds.; Chapman and Hall: New York, NY, USA, 1997; pp. 101–110. [Google Scholar]
- Babakhani, A.; Farvin, K.; Jacobsen, C. Antioxidative effect of seaweed extracts in chilled storage of minced Atlantic mackerel (Scomber scombrus): Effect on lipid and protein oxidation. Food Bioprocess Technol. 2016, 9, 352–364. [Google Scholar] [CrossRef]
- Bilican, I. Preparation and properties of novel mucilage composite fils reinforced with polydimethylsiloxane. Macromol. Mater. Eng. 2024, 309, 2300317. [Google Scholar] [CrossRef]
- Koc-Bilican, B. Linden-based mucilage biodegradable films: A green perspective of functional and sustainable food packaging. Int. J. Biolol. Macromol. 2024, 261, 129805. [Google Scholar] [CrossRef]
- El Hassani, N.E.A.; Baraket, A.; Alem, C. Recent advances in natural food preservatives: A sustainable solution for food safety and shelf life extension. J. Food Meas. Charact. 2015, 19, 293–315. [Google Scholar] [CrossRef]
- Li, W.; Liu, B.; Li, Y.; Xue, P.; Lu, Y.; Song, S.; Li, Y.; Szeto, I.M.Y.; Ren, F.; Guo, H. The application of lactoferrin in infant formula: The past, present and future. Crit. Rev. Food Sci. Nutr. 2024, 64, 5748. [Google Scholar] [CrossRef] [PubMed]
- Sugrue, I.; Ross, R.P.; Hill, C. Bacteriocin diversity, function, discovery and application as antimicrobials. Nat. Rev. Microbiol. 2024, 22, 256. [Google Scholar] [CrossRef]

| Microbial Parameter | Packaging Condition | Refrigeration Time (Days) | ||
|---|---|---|---|---|
| 0 | 2 | 6 | ||
| Enterobacteriaceae | CTR | 1.00 ± 0.00 a | 1.00 ± 0.00 aA | 2.00 ± 0.23 bA |
| RAF | 1.00 ± 0.00 a | 1.07 ± 0.12 aA | 1.69 ± 0.82 aA | |
| Psychrotrophic bacteria | CTR | 3.91 ± 0.47 a | 4.68 ± 0.33 aB | 6.35 ± 0.39 bA |
| RAF | 3.91 ± 0.47 a | 3.22 ± 0.16 aA | 6.13 ± 0.27 bA | |
| Proteolytics | CTR | 2.57 ± 0.56 a | 3.33 ± 0.39 aB | 5.95 ± 0.41 bA |
| RAF | 2.57 ± 0.56 a | 2.84 ± 0.06 aA | 5.43 ± 0.16 bA | |
| Lipolytics | CTR | 2.00 ± 0.00 a | 2.20 ± 0.35 aA | 3.90 ± 0.56 bA |
| RAF | 2.00 ± 0.00 a | 2.10 ± 0.17 aA | 3.65 ± 0.67 bA | |
| Chemical Index | Packaging Condition | Refrigeration Time (Days) | ||
|---|---|---|---|---|
| 0 | 2 | 6 | ||
| pH | CTR | 6.11 ± 0.04 a | 6.34 ± 0.04 bA | 6.53 ± 0.12 cB |
| RAF | 6.11 ± 0.04 a | 6.26 ± 0.08 bA | 6.29 ± 0.02 bA | |
| TMA (mg TMA-N·kg−1 muscle) | CTR | 0.4 ± 0.1 a | 2.6 ± 0.8 bA | 16.4 ± 0.5 cB |
| RAF | 0.4 ± 0.1 a | 2.4 ± 0.9 bA | 10.5 ± 1.5 cA | |
| Lipid Oxidation Index *** | Packaging Condition | Refrigeration Time (Days) | ||
|---|---|---|---|---|
| 0 | 2 | 6 | ||
| PV (meq active oxygen·kg−1 lipids) | CTR | 3.54 ± 1.74 a | 6.70 ± 0.25 bA | 12.12 ± 2.35 cA |
| RAF | 3.54 ± 1.74 a | 10.03 ± 0.84 bB | 16.7 ± 1.43 cB | |
| TBA-i (mg malondialdehy-de·kg−1 muscle) | CTR | 0.36 ± 0.03 a | 1.32 ± 0.40 bA | 3.26 ± 0.56 cA |
| RAF | 0.36 ± 0.03 a | 1.31 ± 0.23 bA | 5.15 ± 1.38 cA | |
| FR | CTR | 0.54 ± 0.11 a | 1.51 ± 0.83 bA | 1.63 ± 0.16 bB |
| RAF | 0.54 ± 0.11 a | 1.54 ± 0.37 bA | 1.23 ± 0.17 bA | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez, A.; López, L.; Miranda, J.M.; Trigo, M.; Barros-Velázquez, J.; Aubourg, S.P. Effect of a Gelatin-Based Film Including Gelidium sp. Algal Flour on Antimicrobial Properties Against Spoilage Bacteria and Quality Enhancement of Refrigerated Trachurus trachurus. Foods 2025, 14, 1465. https://doi.org/10.3390/foods14091465
Gómez A, López L, Miranda JM, Trigo M, Barros-Velázquez J, Aubourg SP. Effect of a Gelatin-Based Film Including Gelidium sp. Algal Flour on Antimicrobial Properties Against Spoilage Bacteria and Quality Enhancement of Refrigerated Trachurus trachurus. Foods. 2025; 14(9):1465. https://doi.org/10.3390/foods14091465
Chicago/Turabian StyleGómez, Antonio, Lucía López, José M. Miranda, Marcos Trigo, Jorge Barros-Velázquez, and Santiago P. Aubourg. 2025. "Effect of a Gelatin-Based Film Including Gelidium sp. Algal Flour on Antimicrobial Properties Against Spoilage Bacteria and Quality Enhancement of Refrigerated Trachurus trachurus" Foods 14, no. 9: 1465. https://doi.org/10.3390/foods14091465
APA StyleGómez, A., López, L., Miranda, J. M., Trigo, M., Barros-Velázquez, J., & Aubourg, S. P. (2025). Effect of a Gelatin-Based Film Including Gelidium sp. Algal Flour on Antimicrobial Properties Against Spoilage Bacteria and Quality Enhancement of Refrigerated Trachurus trachurus. Foods, 14(9), 1465. https://doi.org/10.3390/foods14091465









