Comparative Proteome and Weighted Gene Co-Expression Network Analyses Uncover the Mechanism of Wheat Grain Protein Accumulation in Response to Nitrogen Fertilizer Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Grain Flour Quality Analysis
2.3. Flour Proteome Analysis
2.4. WGCNA
2.5. Gene Expression Analysis Using Quantitative Real-Time PCR (qRT-PCR)
2.6. Data Analysis
3. Results
3.1. Grain Protein Content and Quality Parameters Under Different Nitrogen Treatments
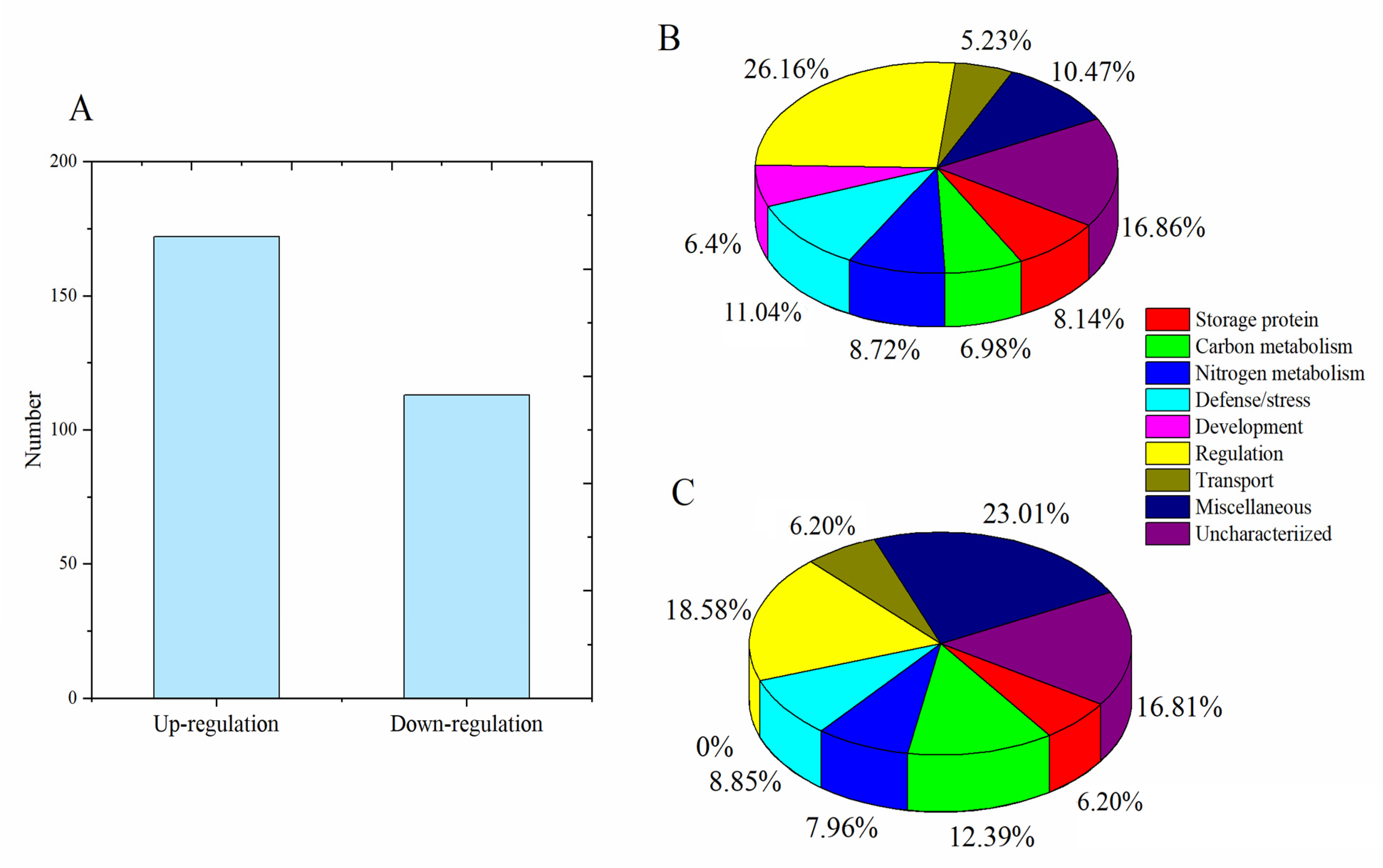
3.2. Grain Protein Components Identified by Proteomic Analysis
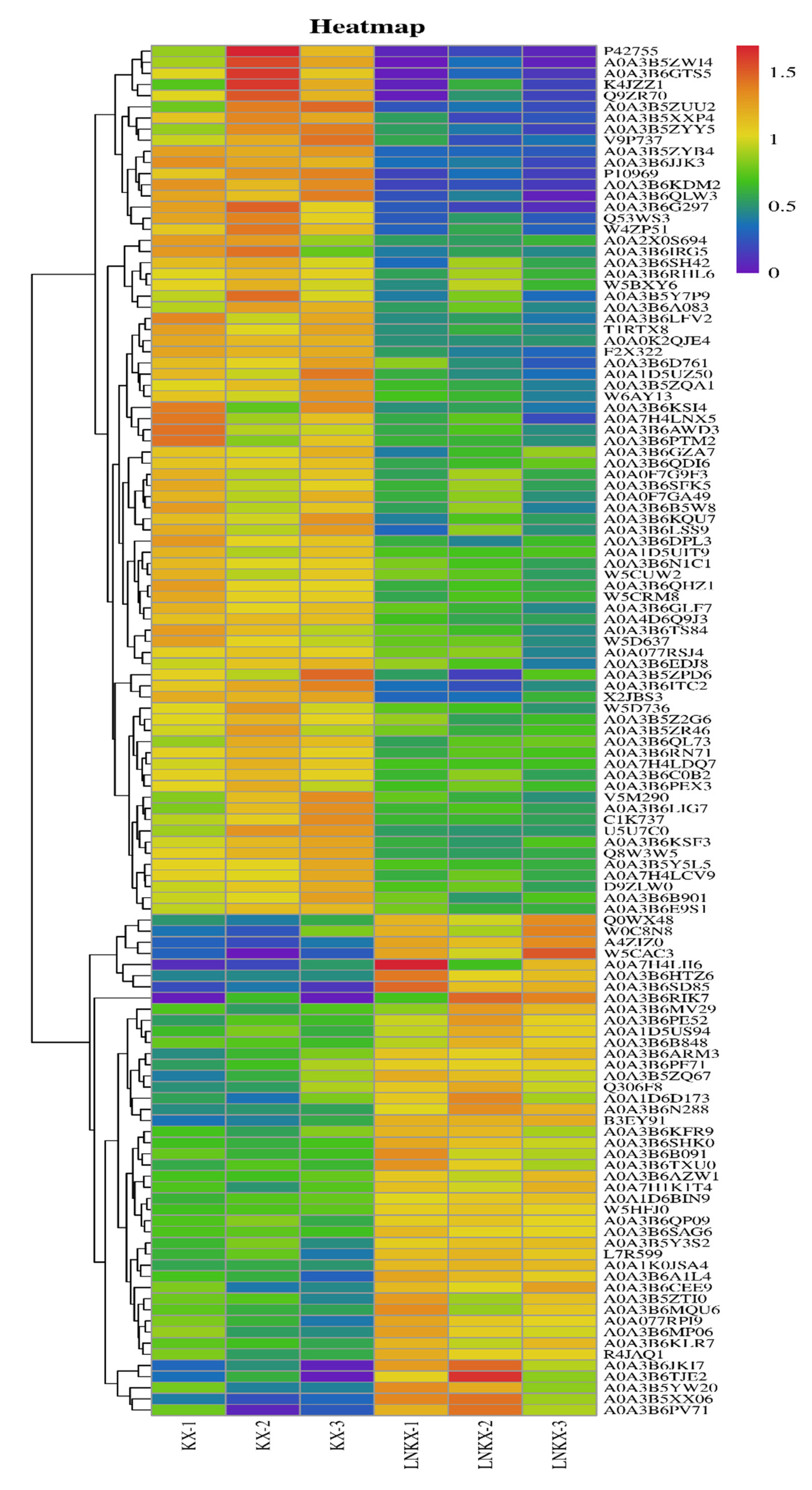
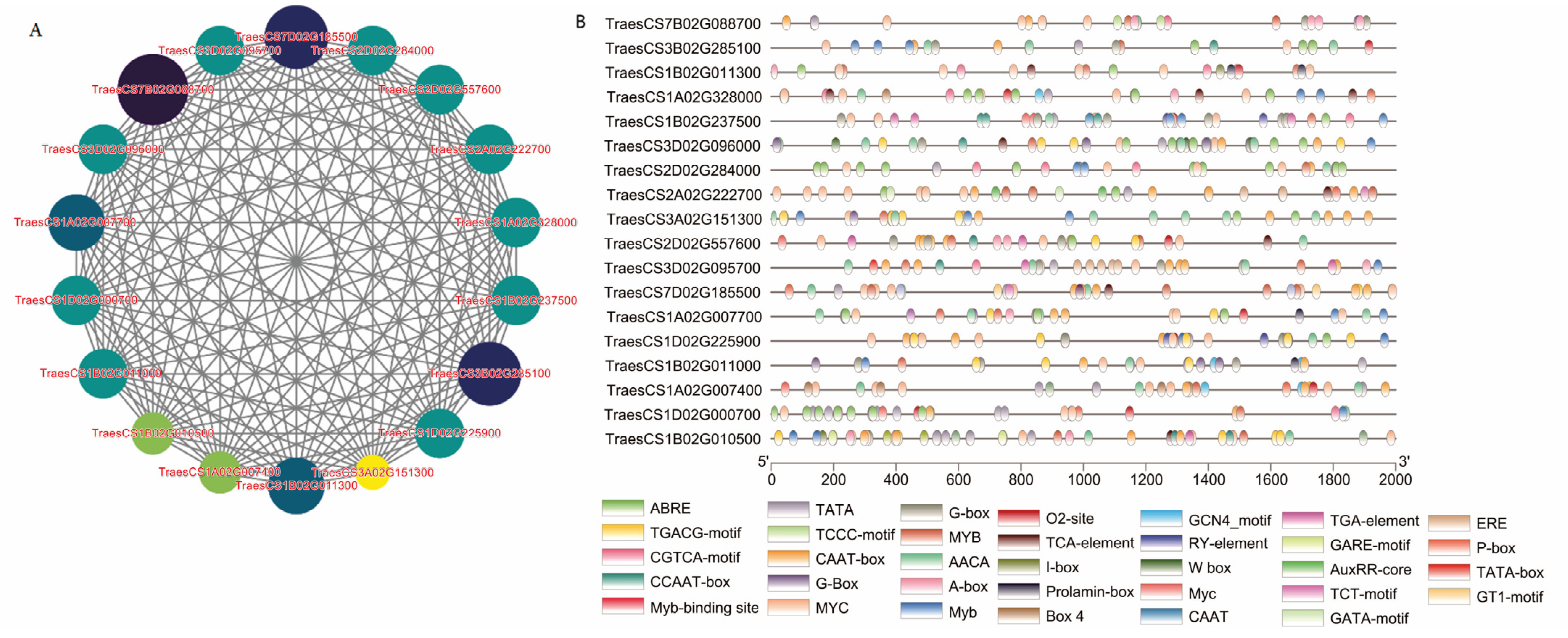
3.3. WGCNA and Screening of Hub Genes
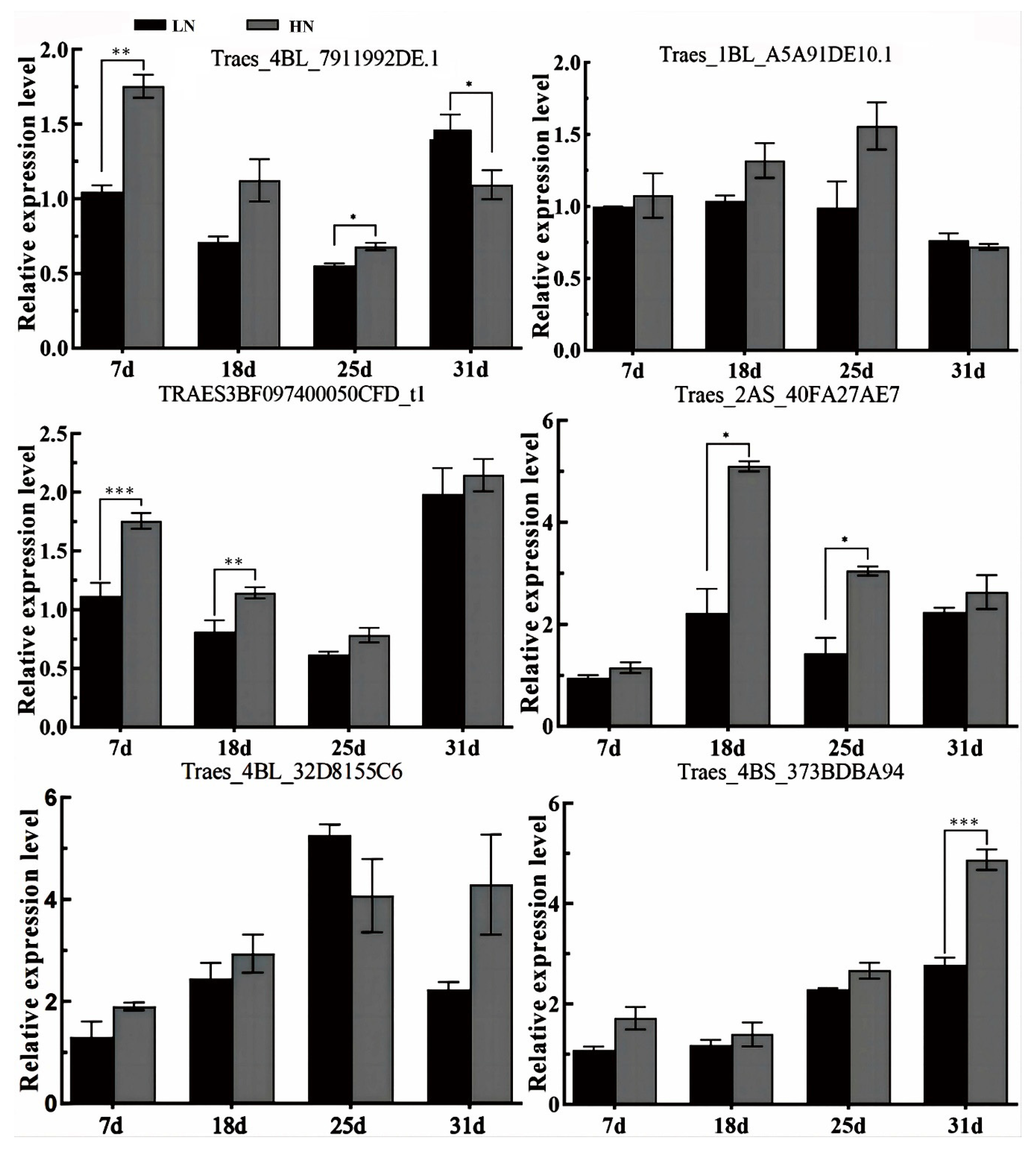
3.4. Gene Expression Verification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaur, A.; Shevkani, K.; Katyal, M.; Singh, N.; Ahlawat, A.K.; Singh, A.M. Physicochemical and rheological properties of starch and flour from different durum wheat varieties and their relationships with noodle quality. J. Food Sci. Technol. 2016, 4, 2127–2138. [Google Scholar] [CrossRef] [PubMed]
- UthayakUmaran, S.; Wrigley, C. Durum Wheat: Grain-quality characteristics and management of quality requirements. In Cereal Grains; Woodhead Publishing: Cambridge, UK, 2017; pp. 135–151. [Google Scholar]
- Shewry, P.R.; Tatham, A.S. The characteristics, structures and evolutionary relationships of prolamins. In Seed Proteins; Springer Netherlands: Dordrecht, The Netherlands, 1999; Volume 2, pp. 11–33. [Google Scholar]
- Caballero, P.A.; Gómez, M.; Rosell, C.M. Bread quality and dough rheology of enzyme-supplemented wheat flour. Eur. Food Res. Technol. 2007, 224, 525–534. [Google Scholar] [CrossRef]
- Wang, X.L.; Appels, R.; Zhang, X.K.; Bekes, F.; Torok, K.; Tomoskozi, S.; Diepeveen, D.; Ma, W.J.; Islam, S. Protein-transitions in and out of the dough matrix in wheat flour mixing. Food Chem. 2017, 217, 542–551. [Google Scholar] [CrossRef]
- Zhang, L.L.; Guan, E.Q.; Yang, Y.L.; Liu, Y.X.; Zhang, T.J.; Bian, K. Impact of wheat globulin addition on dough rheological properties and quality of cooked noodles. Food Chem. 2021, 362, 130170. [Google Scholar] [CrossRef] [PubMed]
- Zilic, S.; Barac, M.; Pesic, M.; Hadzi-Taskovic-Sukalovic, V.; Dodiga, D.; Mladenovic-Drinic, S.; Jankovic, M. Genetic variability of albumin-globulin content, and lipoxygenase, peroxidase activities among bread and durum wheat genotypes. Genetika 2011, 43, 503–516. [Google Scholar] [CrossRef]
- Altuna, L.; Ribotta, P.D.; Tadini, C.C. Effect of a combination of enzymes on dough rheology and physical and sensory properties of bread enriched with resistant starch. LWT—Food Sci Technol. 2015, 64, 867–873. [Google Scholar] [CrossRef]
- Bonet, A.; Rosell, C.M.; Caballero, P.A.; Gómez, M.; Pérez-Munuera, I.; Lluch, M.A. Glucose oxidase effect on dough rheology and bread quality: A study from macroscopic to molecular level. Food Chem. 2006, 99, 408–415. [Google Scholar] [CrossRef]
- Johansson, E.; Prieto-Linde, M.L.; Jonsson, J.O. Effects of wheat cultivar and nitrogen application on storage protein composition and breadmaking quality. Cereal Chem. 2001, 78, 19–25. [Google Scholar] [CrossRef]
- Zhen, S.; Deng, X.; Xu, X.; Liu, N.; Zhu, D.; Wang, Z.; Yan, Y. Effect of high-nitrogen fertilizer on gliadin and glutenin subproteomes during kernel development in wheat (Triticum aestivum L.). Crop J. 2020, 18, 38–52. [Google Scholar] [CrossRef]
- Shen, Y.X.; Han, X.J.; Feng, H.X.; Han, Z.D.; Mao, W.; Ma, D.Y.; Jin, J.M.; Li, S.J.; Ma, G.; Zhang, Y.F.; et al. Wheat GSPs and processing quality are affected by irrigation and nitrogen through nitrogen remobilization. Foods 2023, 12, 4407. [Google Scholar] [CrossRef]
- Yang, T.; Zhou, Q.; Wang, Q.; Wang, X.X.; Cai, J.; Huang, M.; Jiang, D. Effects of Nitrogen Fertilizer on Quality Characteristics of Wheat with the Absence of Different Individual High-Molecular-Weight Glutenin Subunits (HMW-GSs). Int. J. Mol. Sci. 2022, 23, 2178. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.Q.; Jiang, J.L.; Wang, L.L.; Huang, M.; Zhou, Q.; Cai, J.; Wang, X.; Dai, T.B.; Jiang, D. Reducing Nitrogen Rate and Increasing Plant Density Accomplished High Yields with Satisfied Grain Quality of Soft Wheat via Modifying the Free Amino Acid Supply and Storage Protein Gene Expression. J. Agric. Food Chem. 2022, 70, 2146–2159. [Google Scholar] [CrossRef] [PubMed]
- Butterbach-bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. B 2013, 368, 1621. [Google Scholar] [CrossRef] [PubMed]
- Langenkämper, G.; Zörb, C. Modern aspects of wheat grain proteins. J. Appl. Bot. Food Qual. 2019, 92, 240–245. [Google Scholar]
- Hu, J.X.; Yu, M.; Chang, Y.A.; Tang, H.L.; Wang, W.X.; Du, L.P.; Wang, K.; Yan, Y.M.; Ye, X.G. Functional analysis of TaPDI genes on storage protein accumulation by CRISPR/Cas9 edited wheat mutants. Int. J. Bio. Macromol. 2022, 196, 131–143. [Google Scholar] [CrossRef]
- Li, J.H.; Xie, L.N.; Tian, X.L.; Liu, S.Y.; Xu, D.A.; Jin, H.; Song, J.; Dong, Y.; Zhao, D.H.; Li, G.Y.; et al. TaNAC100 acts as an integrator of seed protein and starch synthesis exerting pleiotropic effects on agronomic traits in wheat. Plant J. 2021, 108, 829–840. [Google Scholar] [CrossRef]
- Uauy, C.; Distelfeld, A.; Fahima, T.; Blechl, A.; Dubcovsky, J. A NAC gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science 2006, 314, 1298–1301. [Google Scholar] [CrossRef]
- Hou, G.; Du, C.; Gao, H.; Liu, S.; Sun, W.; Lu, H.; Kang, J.; Xie, Y.; Ma, D.; Wang, C. Identification of microRNAs in developing wheat grain that are potentially involved in regulating grain characteristics and the response to nitrogen levels. BMC Plant Biol. 2020, 20, 87. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiong, S.; Wei, Y.; Meng, X.; Wang, X.; Ma, X. The role of glutamine synthetase isozymes in enhancing nitrogen use efficiency of N-efficient winter wheat. Sci. Rep. 2017, 7, 1000. [Google Scholar] [CrossRef]
- Zhang, M.W.; Ma, D.Y.; Ma, G.; Wang, C.Y.; Xie, X.D.; Kang, G.Z. Responses of glutamine synthetase activity and gene expression to nitrogen levels in winter wheat cultivars with different grain protein content. J. Cereal Sci. 2017, 74, 187–193. [Google Scholar] [CrossRef]
- Feng, J.; Jia, Y.; Xu, B.; Bi, X.; Ge, Z.; Ma, G.; Xie, Y.; Wang, C.; Ma, D. Quantitative proteomic analysis for characterization of protein components related to dough quality and celiac disease in wheat flour, dough, and heat-treated dough. Food Chem. 2024, 461, 140624. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Xue, X.; Jin, X.; Niu, B.; Chen, Z.; Ji, X.; Shi, M.; He, Y. Effect of annealing using plasma-activated water on the structure and properties of wheat flour. Front. Nutr. 2022, 9, 951588. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewski, J.R.; Zougman, A.; Nagarajuna, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Zhang, B.; Horvath, S. A general framework for weighted gene co-expression network analysis. Stat. Appl. Genet. Mol. Biol. 2005, 4, 17. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinf. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.Q.; Li, Y.J.; Zhao, Y.Y.; Shi, H.Z. Physiological, transcriptome and co-expression network analysis of chlorophyll-deficient mutants in flue-cured tobacco. BMC Plant Biol. 2023, 23, 153. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction net-works. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Chin, C.H.; Chen, S.H.; Wu, H.H.; Ho, C.W.; Ko, M.T.; Lin, C.Y. CytoHubba: Identif ying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8 (Suppl. S4), S11. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using realtime quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhou, Z.F.; Zhang, Z.W.; Jia, L.H.; Qiu, H.X.; Guan, H.Y.; Liu, C.C.; Qin, M.M.; Wang, Y.H.; Li, W.X.; Yao, W.; et al. Genetic basis of gluten aggregation properties in wheat (Triticum aestivum L.) dissected by QTL mapping of GlutoPeak parameters. Front. Plant Sci. 2020, 11, 28. [Google Scholar] [CrossRef]
- Gaines, C.S. Report of the AACC committee on soft wheat flour. Method 56–11. Solvent retention capacity profile. Cereal Foods World 2000, 45, 303–306. [Google Scholar]
- Song, F.Y.; Li, Y.; Huang, J.; Lu, W.J.; Guo, Z.Y.; Deng, W. Integrated transcriptomic and proteomic analyses reveal the impact of drought and heat stress combination on Morus alba. Environ. Exp. Bot. 2024, 228, 105988. [Google Scholar] [CrossRef]
- Ma, G.; Liu, W.X.; Li, S.S.; Zhang, P.P.; Wang, C.Y.; Lu, H.F.; Wang, L.F.; Xie, Y.X.; Ma, D.Y.; Kang, G.Z. Determining the optimal N input to improve grain yield and quality in winter wheat with reduced apparent N loss in the North China Plain. Front. Plant Sci. 2019, 10, 181. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, L.; Napoli, M.; Mancini, M.; Masella, P.; Cappelli, A.; Parenti, A.; Orlandini, S. Wheat grain composition, dough rheology and bread quality as affected by nitrogen and sulfur fertilization and seeding density. Agronomy 2022, 10, 233. [Google Scholar] [CrossRef]
- Liu, T.; Li, M.; Qi, X.K.; Wang, R.L. Effect of glow discharge cold plasma treatment on improvement of wheat processing quality. Food Sci. 2023, 44, 87–93. [Google Scholar]
- Mejia, C.D.; Mauer, L.J.; Hamaker, B.R. Similarities and differences in secondary structure of viscoelastic polymers of maize α-zein and wheat gluten proteins. J. Cereal Sci. 2007, 45, 353–359. [Google Scholar] [CrossRef]
- Bosmali, I.; Kotsiou, K.; Matsakidou, A.; Irakli, M.; Madesis, P.; Biliaderis, C.G. Fortification of wheat bread with an alternative source of bean proteins using raw and roasted Phaseolus coccineus flours: Impact on physicochemical, nutritional and quality attributes. Food Hydrocoll. 2025, 158, 110527. [Google Scholar] [CrossRef]
- Liu, L.; Shi, Z.J.; Wang, X.L.; Ren, T.; Ma, Z.; Li, X.P.; Xu, B.; Hu, X.Z. Interpreting the correlation between repeated sheeting process and wheat noodle qualities: From water molecules movement perspective. LWT-Food Sci. Technol. 2021, 151, 112219. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Z.C.; Wang, Z.H.; Duan, Y.Q.; Guo, H.M.; Liang, Y.; Zhang, X.; Zhang, Y.J.; Wang, J.S. Effect of high-molecular-weight glutenin subunits silencing on dough aggregation characteristics. Food Chem. 2024, 443, 138371. [Google Scholar] [CrossRef]
- Revanappa, S.B.; Salimath, P.V.; Rao, P. Effect of peroxidase on textural quality of dough and arabinoxylan characteristics isolated from whole wheat flour dough. Int. J. Food Prop. 2014, 17, 2131–2141. [Google Scholar] [CrossRef]
- Wang, Y.J.; Wang, D.M.; Tao, Z.Q.; Yang, Y.S.; Gao, Z.X.; Zhao, G.C.; Chang, X.H. Impact of nitrogen deficiency on wheat (Triticum aestivum L.) grain during the medium filling stage: Transcriptomic and metabolomic comparisons. Front. Plant Sci. 2021, 12, 674433. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Wang, R.L.; Li, J.N.; Zhou, Q.Y.; Cui, C. Regulatory mechanism analysis of signal transduction genes during rapeseed (Brassica napus L.) germination under aluminum stress using WGCNA combination with QTL. Front. Plant Sci. 2025, 16, 1546572. [Google Scholar] [CrossRef]
- Shang, P.P.; Bi, L.; Li, W.W.; Zhou, X.L.; Feng, Y.L.; Wu, J.H.; Zeng, B. Exploration of key genes and pathways in response to submergence stress in red clover (Trifolium pratense L.) by WGCNA. BMC Plant Biol. 2025, 25, 4. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; He, D.; Cai, T.; Wang, C.; Wang, G.; Meng, F.; Han, Z.; Li, N.; Wang, Z. Effecting of spraying ABA on glutenin fraction content and GMP size distribution in wheat grain. Sci. Agric. Sin. 2010, 43, 2595–2602. [Google Scholar]
- Fauteux, F.; Stromvik, M.V. Seed storage protein gene promoters contain conserved DNA motifs in Brassicaceae, Fabaceae and Poaceae. BMC Plant Biol. 2009, 9, 126. [Google Scholar] [CrossRef]
- Bernard, V.; Brunaud, V.; Lecharny, A. TC-motifs at the TATA-box expected position in plant genes: A novel class of motifs involved in the transcription regulation. BMC Genom. 2010, 11, 166. [Google Scholar] [CrossRef]
- Li, J.H.; Wang, K.; Li, G.Y.; Li, Y.L.; Zhang, Y.; Liu, Z.Y.; Ye, X.G.; Xia, X.C.; He, Z.H.; Cao, S.H. Dissecting conserved cis-regulatory modules of Glu-1 promoters which confer the highly active endosperm-specific expression via stable wheat transformation. Crop J. 2019, 7, 8–18. [Google Scholar] [CrossRef]

| Traits | HN | LN | |
|---|---|---|---|
| Protein and gluten content | Protein content (%) | 14.58 ± 0.47 a | 13.01 ± 0.28 b |
| Wet gluten content (%) | 31.00 ± 1.56 a | 27.18 ± 1.04 b | |
| Gluten index | 0.97 ± 0.01 a | 0.95 ± 0.03 a | |
| Rheological properties | Water absorption rate (%) | 68.00 ± 1.84 a | 66.60 ± 1.31 a |
| Development time (min) | 25.00 ± 0.01 a | 9.80 ± 5.54 b | |
| Stability time (min) | 23.00 ± 0.17 a | 17.57 ± 1.44 b | |
| Energy area (cm2) | 183.00 ± 17.00 a | 176.7 ± 31.66 a | |
| Extension resistance (EU) | 629.00 ± 3.00 a | 562.7 ± 27.15 b | |
| Extensibility (mm) | 155.50 ± 1.50 a | 162.33 ± 7.76 a | |
| Protein secondary structure | β-sheets (%) | 46. 81 ± 0.004 a | 44.55 ± 0.013 b |
| α-helix (%) | 12.29 ± 0.007 a | 12.82 ± 0.002 a | |
| β-turns (%) | 29.46 ± 0.009 a | 27.29 ± 0.011 b | |
| Irregular curl (%) | 11.44 ± 0.011 b | 15.34 ± 0.005 a | |
| Solvent retention capacity | Water (%) | 93.54 ± 0.72 a | 89.98 ± 3.01 a |
| Sucrose (%) | 138.54 ± 3.17 a | 132.84 ± 1.27 b | |
| Sodium carbonate (%) | 120.03 ± 0.13 a | 109.11 ± 1.68 b | |
| Lactic acid (%) | 122.48 ± 0.94 a | 116.50 ± 6.45 a | |
| Bread-making quality | Volume (cm3) | 707.50 ± 6.36 a | 690.00 ± 1.41 a |
| Score | 76.90 ± 0.26 a | 71.20 ± 0.06 b | |
| Core Gene ID | Homologous Gene ID in A. thaliana | Gene Function |
|---|---|---|
| TraesCS7B02G088700 | AT1G74720 | Encodes a putative transmembrane protein carrying four C(2) domains; involved in organ development. |
| TraesCS3B02G285100 | AT3G02480 | LEA protein that is upregulated by ABA, NaCl, and light deprivation. |
| TraesCS1B02G011300 | AT1G77140 | A peripheral membrane protein that associates with microsomal membranes, likely to function in the transport of proteins to the vacuole. |
| TraesCS1A02G328000 | AT5G22470 | PARP3 is one of three canonical PARPs in Arabidopsis. |
| TraesCS1B02G237500 | AT3G51810 | Encodes an ABA-inducible protein that accumulates during seed maturation. |
| TraesCS3D02G096000 | AT1G66180 | Encodes a putative aspartyl protease. |
| TraesCS2D02G284000 | AT5G50590 | Encodes a putative hydroxysteroid dehydrogenase |
| TraesCS2A02G222700 | AT2G34740 | protein phosphatase 2C family protein. |
| TraesCS3A02G151300 | AT1G64110 | Target promoter of the male germline-specific transcription factor DUO1. |
| TraesCS2D02G557600 | AT2G26530 | Pheromone receptor-like protein involved in the early elicitor signaling events. |
| TraesCS3D02G095700 | AT1G74490 | Protein kinase superfamily protein. |
| TraesCS7D02G185500 | AT4G10250 | Columbia endomembrane-localized small heat shock protein. |
| TraesCS1A02G007700 | AT4G31580 | Encodes a Serine/arginine-rich (SR) protein RSZp22. |
| TraesCS1D02G225900 | AT1G11960 | Calcium channel that is phosphorylated by BIK1 in the presence of PAMPS and required for stomatal immunity. |
| TraesCS1B02G011000 | AT1G07560 | Leucine-rich repeat protein kinase family protein. |
| TraesCS1A02G007400 | AT2G01150 | Encodes a RING-H2 finger protein. |
| TraesCS1D02G000700 | AT2G32160 | Methyltransferase gene. |
| TraesCS1B02G010500 | AT2G01150 | Encodes a RING-H2 finger protein. |
| Gene ID | Transcription Factor ID | Transcription Factor Family |
|---|---|---|
| TraesCS1B02G237500 | Traes_4BL_7911992DE.1 | Dof |
| Traes_1BL_A5A91DE10.1 | Trihelix | |
| TraesCS1B02G011000 | TRAES3BF097400050CFD_t1 | ARF |
| Traes_2AS_40FA27AE7 | MYB | |
| TraesCS1A02G007400 | Traes_4BL_32D8155C6 | G2-like |
| Traes_4BS_373BDBA94 | NAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, B.; Jia, Y.; Feng, J.; Yang, Y.; Ma, G.; Zhang, Y.; Xie, Y.; Ma, D. Comparative Proteome and Weighted Gene Co-Expression Network Analyses Uncover the Mechanism of Wheat Grain Protein Accumulation in Response to Nitrogen Fertilizer Application. Foods 2025, 14, 1481. https://doi.org/10.3390/foods14091481
Xu B, Jia Y, Feng J, Yang Y, Ma G, Zhang Y, Xie Y, Ma D. Comparative Proteome and Weighted Gene Co-Expression Network Analyses Uncover the Mechanism of Wheat Grain Protein Accumulation in Response to Nitrogen Fertilizer Application. Foods. 2025; 14(9):1481. https://doi.org/10.3390/foods14091481
Chicago/Turabian StyleXu, Beiming, Yuku Jia, Jianchao Feng, Yang Yang, Geng Ma, Yanfei Zhang, Yingxin Xie, and Dongyun Ma. 2025. "Comparative Proteome and Weighted Gene Co-Expression Network Analyses Uncover the Mechanism of Wheat Grain Protein Accumulation in Response to Nitrogen Fertilizer Application" Foods 14, no. 9: 1481. https://doi.org/10.3390/foods14091481
APA StyleXu, B., Jia, Y., Feng, J., Yang, Y., Ma, G., Zhang, Y., Xie, Y., & Ma, D. (2025). Comparative Proteome and Weighted Gene Co-Expression Network Analyses Uncover the Mechanism of Wheat Grain Protein Accumulation in Response to Nitrogen Fertilizer Application. Foods, 14(9), 1481. https://doi.org/10.3390/foods14091481





