Integrated Volatile Compounds and Transcriptional Gene Analysis Elucidate the Deterioration Mechanism of Embryo Rice During Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Embryo Rice Storage Test
2.3. Determination of Basic Indexes
2.3.1. Physicochemical Index
2.3.2. Fatty Acid Value, Lipase Activity, and MDA Determination
2.3.3. Catalase, DPPH, and FRAP
2.3.4. Texture
2.3.5. Taste Value
2.3.6. Color
2.4. Determination of Volatile Compounds
2.4.1. E-Nose
2.4.2. HS-SPME-GC-MS
2.4.3. Evaluation of Major Volatile Compounds
2.5. Transcriptome Analysis
2.5.1. RNA Extraction, Library Construction, and Sequencing
2.5.2. Differential Expression Analysis and Functional Enrichment
2.6. Data Analysis
3. Results and Discussion
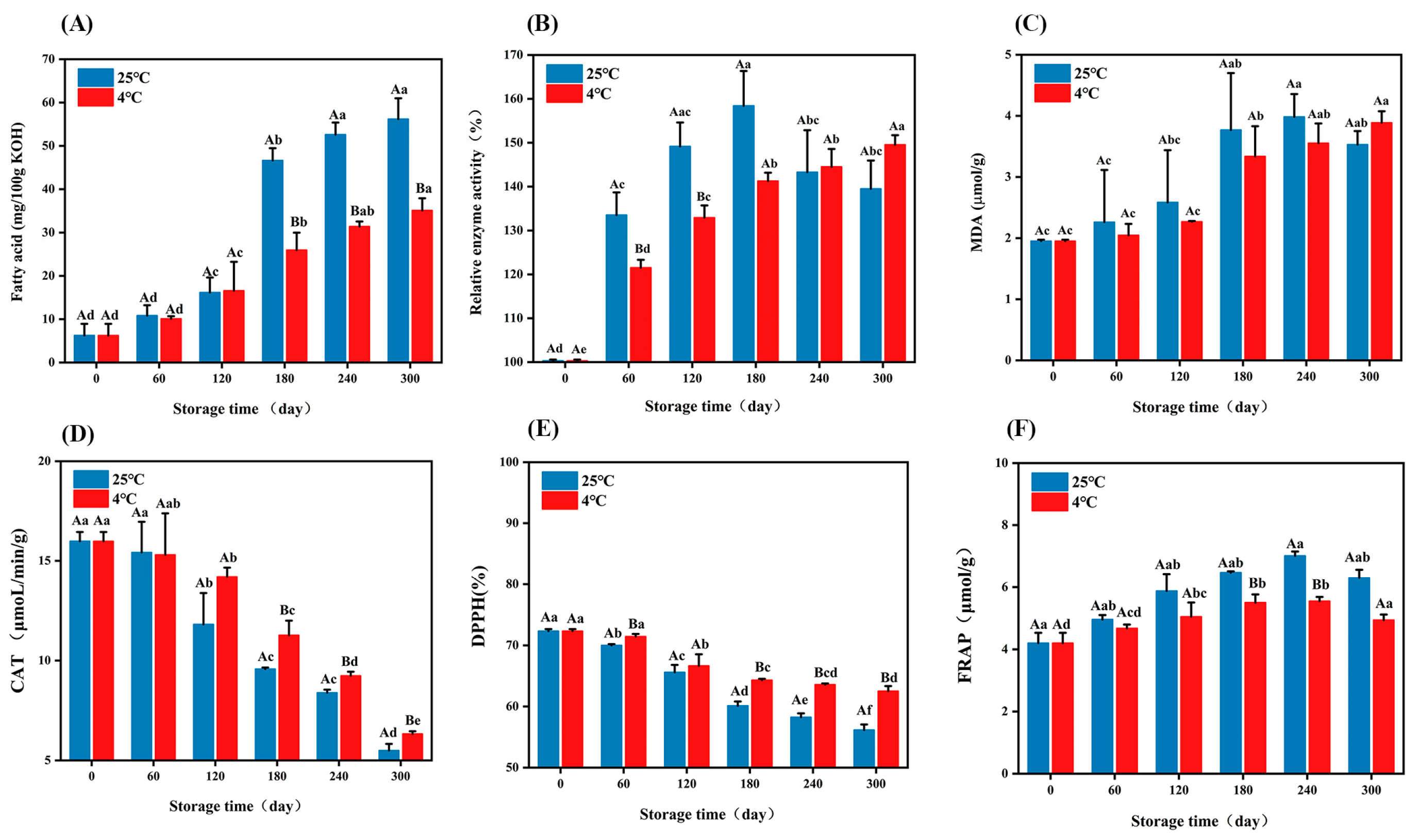
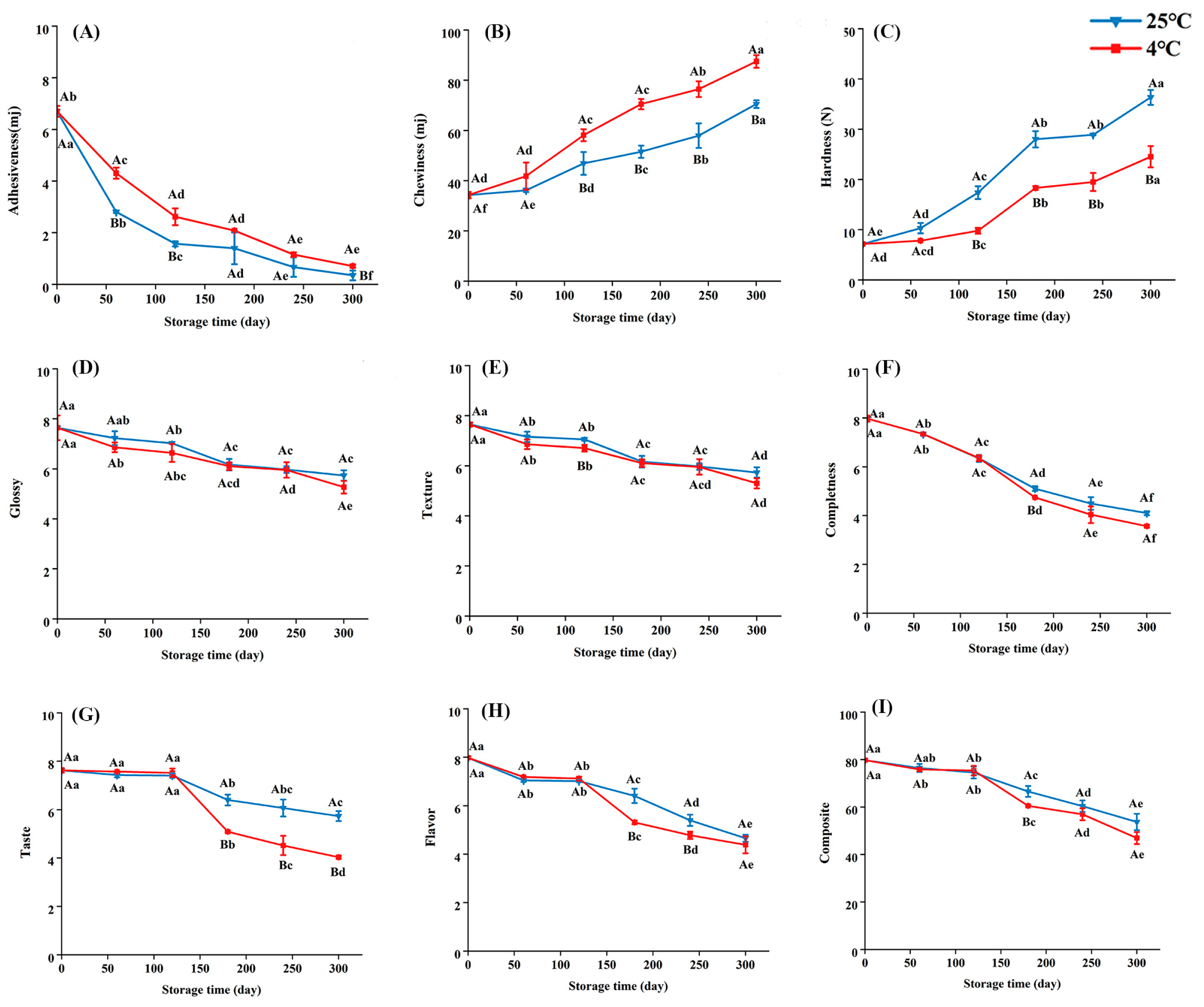
3.1. Changes in Basic Indexes During Embryo Rice Storage
3.1.1. Changes in Oxidation Index During Embryo Rice Storage
3.1.2. Changes in Sensory Quality of Embryo Rice During Storage
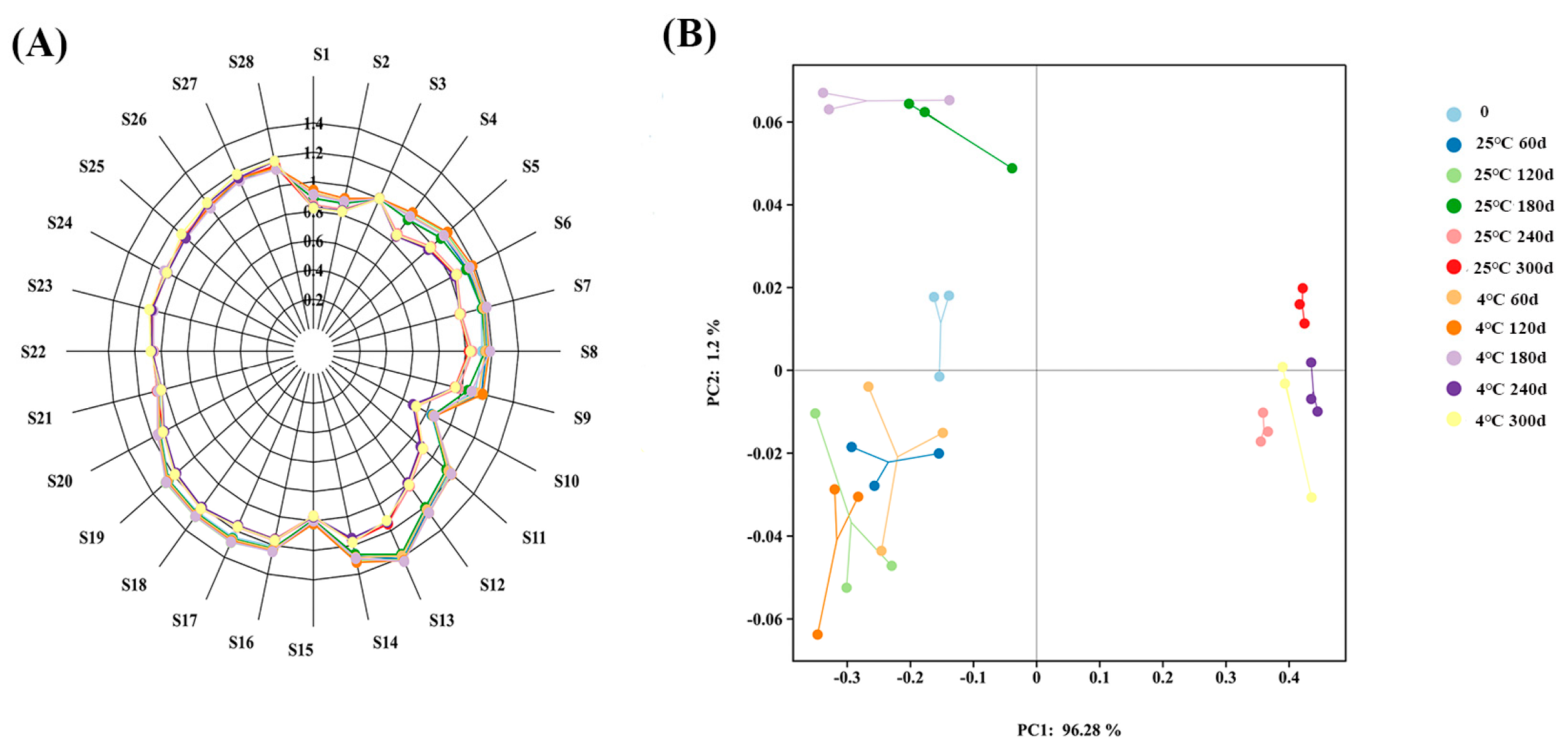
3.2. Analysis of Volatile Compounds of Embryo Rice During Storage
3.2.1. Analysis of Odor of Embryo Rice During Storage
3.2.2. Analysis of HS-SPME-GC-MS of Embryo Rice During Storage
3.2.3. Analysis of ROAV of Embryo Rice During Storage
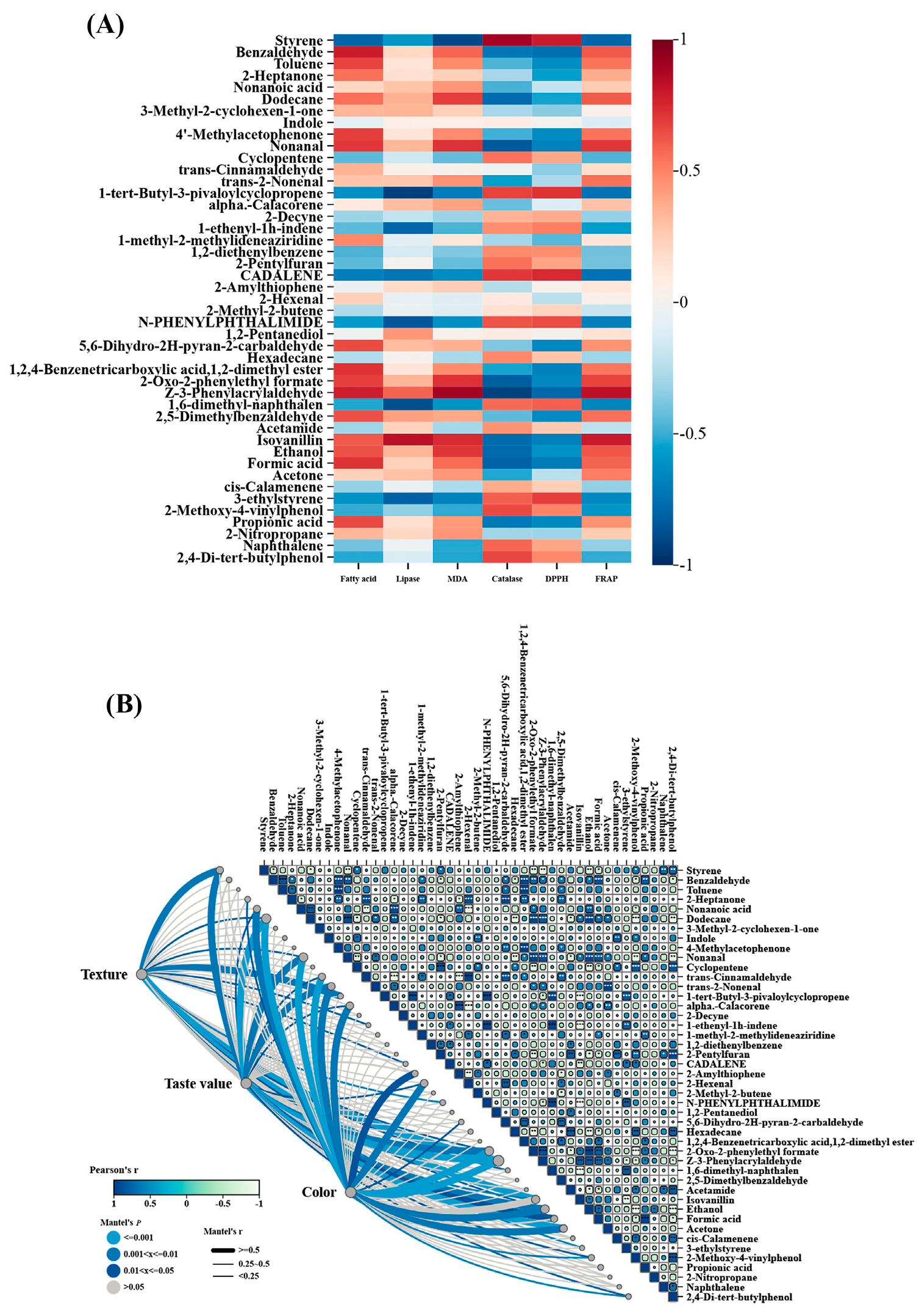
3.2.4. Correlation Analysis Between Volatile Compounds and Basic Indexes
3.3. Analysis of RNA-Seq of Embryo Rice During Storage
3.3.1. Identification of DEGs Under Different Storage Conditions of Embryo Rice
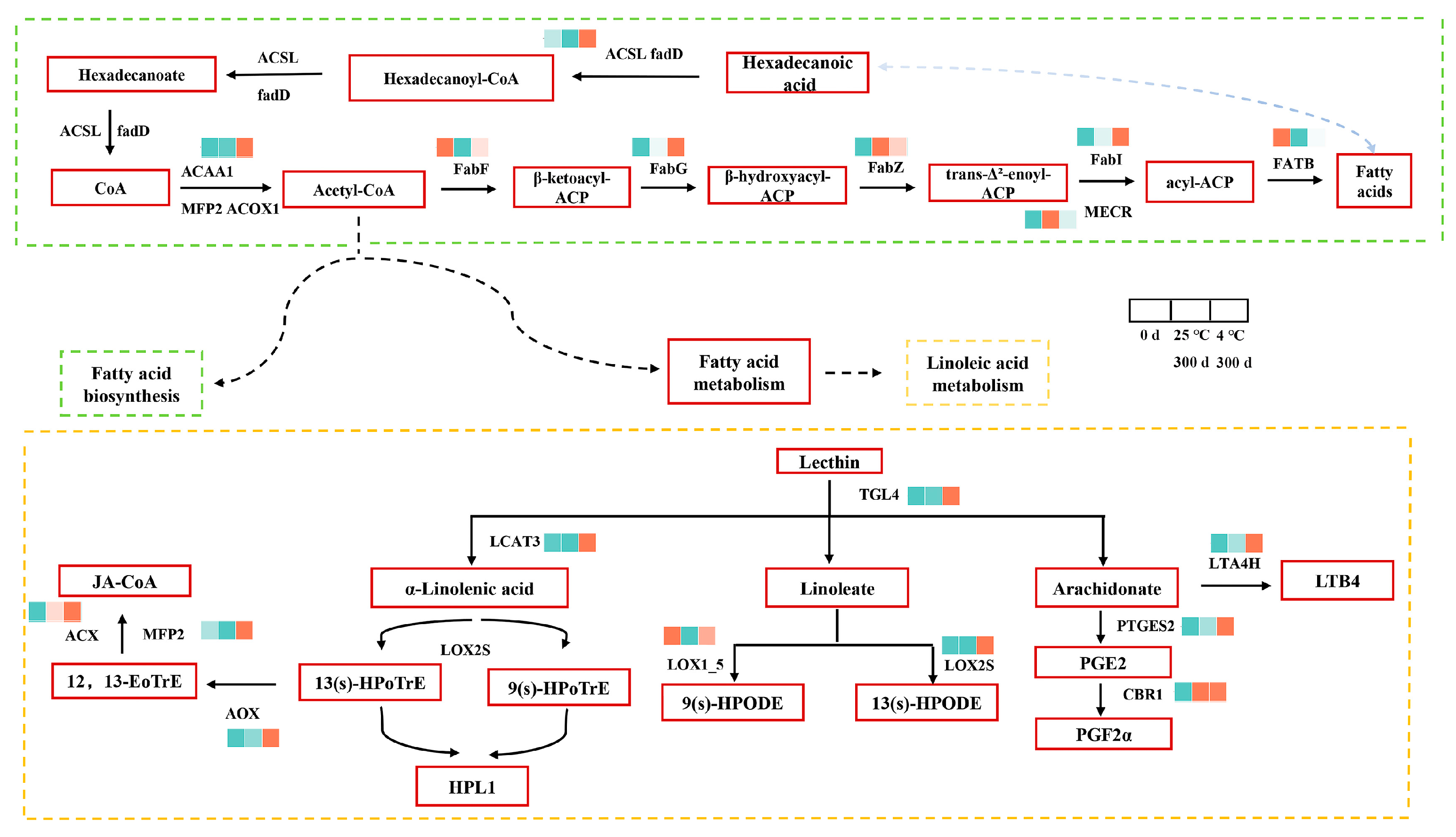
3.3.2. Changes in Gene Expression Related to Lipid Metabolism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDA | malondialdehyde |
| CAT | Catalase |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| FRAP | Ferric Reducing Antioxidant Power |
| E-Nose | Electronic Nose |
| HS-SPME-GC-MS | Headspace–Solid-Phase Micro-Extraction–Gas Chromatography–Mass Spectrometry |
References
- Liu, X.F.; Li, X.; Meng, Q.N.; Liu, C.; Zhang, N. Study on Optimization of Preparation Technology and Quality Characteristics of Selenium-enriched Rice with Retained Germ. Shipin Gongye Keji 2023, 44, 174–184. [Google Scholar]
- Xia, Q.L.; Kang, S.M.; Jin, Z.; Xu, J.J.; Cao, L.L.; Pang, M. Difference analysis of key aroma compounds between Thai fragrant rice and common rice based on odor activity values. Shipin Anquan Zhiliang Jiance Xuebao 2023, 14, 62–70. [Google Scholar]
- Hong, Y.; Shao, Z.H.; Cao, L.; Song, Y.; Tao, P.; Liu, C.; Gong, X.; Zhu, J.Q. Comprehensive Analysis of the Impact of Protein Oxidation on Edible Quality of Germinated Rice during Storage and Its Correlation. Henong Xuebao 2024, 38, 1617–1626. [Google Scholar]
- Hong, Y.; Shao, Z.H.; Cao, L.; Song, Y.; Tao, P.; Liu, C.; Gong, X.; Pan, L.S.; Hong, C. Lipid oxidation and changes of volatile substances of germ rice during storage. Shipin Yu Fajiao Gongye 2024, 50, 248–255. [Google Scholar]
- Zhu, T.Y.; Chen, F.X. Study on storage stability of rice with remained germ. Cereals Oils 2023, 36, 90–93+98. [Google Scholar]
- Zhou, X.Q.; Zhu, F.Q.; Zhang, Y.R.; Peng, C. Analysis of the Storage Property, Physiological, Biochemical Indicators Parameters and the Pasting Characteristics of Rice in Different Storage Time. Zhongguo Liangyou Xuebao 2020, 35, 108–114+124. [Google Scholar]
- Gao, M.H. Study on Quality of Japonica Rice with Excellent Food Taste in Northeast Chin; Shenyang Agricultural University: Shenyang, China, 2022. [Google Scholar]
- Tian, J.y.; Ji, G.m.; Zhang, J.f.; Luo, D.q.; Zhang, F.; Li, L.j.; Jiang, M.j.; Zhu, D.w.; Li, M. Variety Screening and Characterization Analysis of Storage Stability of Eating Quality of Rice. Foods 2024, 13, 4140. [Google Scholar] [CrossRef]
- Sun, Y.X.; Du, F.; Huang, Y.Q.; Miao, J.J.; Lai, K.Q. Effects of heat treatments, storage and reheating on volatile compounds in pork and screening for characteristic volatile compounds. Meat Sci. 2025, 222, 109740. [Google Scholar] [CrossRef]
- Wang, L.Q.; Wang, L.D.; Zhou, Y.; Chen, K.X.; Zheng, X.Z.; Lu, S.W. Recent Development in Key Storage Technologies for Improvement of Lipid Stability of Rice with Remained Germ. Shipin Kexue 2022, 43, 337–344. [Google Scholar]
- Ma, J.J.; Qiao, Z.Y.; Huang, G.L.; Sun, L.X.; Sui, S.Y.; Wang, S.N. Analysis of Flavor Characteristics and Qualities of Suxiangjing Rice. Zhongguo Liangyou Xuebao 2022, 37, 166–174. [Google Scholar]
- Zhang, G.C.; Wang, X.Q.; Wang, Y.Q.; Hua, D.; Lv, H.N.; Liu, J.R.; Zhou, D.Y.; He, Y.T.; Lu, B.X.; Liu, H.; et al. Combined transcriptome and metabolome analyses reveal the mechanism of abundant bioactive compounds and high antioxidant activity in germinated weedy rice. J. Food Sci. 2024, 89, 5517–5530. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.J.; Xiong, F.; Niu, X.Q.; Gong, S.F.; Sun, X.W.; Xiao, Y.; Yang, Y.D.; Chen, F.S. Molecular mechanism of quality changes in solid endosperm of tender coconut during room temperature storage based on transcriptome and metabolome. Food Chem. 2024, 436, 137615. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.D.; Zhao, W.T.; Wang, P.; Zhao, S.; Wang, D.; Zhao, X.Y. Transcriptome analysis integrated with changes in cell wall polysaccharides of different fresh-cut chili pepper cultivars during storage reveals the softening mechanism. Food Chem. 2024, 452, 139445. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.J.; Fu, Z.X.; Dou, P.P.; Yu, C.; Dong, X.Y.; Hong, H.; Luo, Y.k.; Tan, Y.Q. Decoding nattokinase efficacy: From digestion and absorption to lipid pathway modulation in high-fat diet-induced atherosclerosis. Food Biosci. 2025, 66, 106203. [Google Scholar] [CrossRef]
- Wang, X.L.; Chao, H.; Ma, W.Y.; Li, Y.Z.; Yang, H.; Chen, W.; Li, L. Preservation mechanism of cold plasma pretreatment on the antioxidant activity and quality of prune during storage. Food Res. Int. 2025, 206, 116081. [Google Scholar] [CrossRef]
- Tang, R.F.; Zhou, Y.J.; Chen, Z.S.Z.; Zeng, J.; Huang, H.; Jiang, Y.M.; Qu, H.X.; Zhu, H. Involvement of miRNA-mediated anthocyanin and energy metabolism in the storability of litchi fruit. Postharvest Biol. Technol. 2020, 165, 111200. [Google Scholar] [CrossRef]
- Yuan, F.; Yan, J.; Yan, X.X.; Liu, H.B.; Pan, S.Y. Comparative transcriptome analysis of genes involved in volatile compound synthesis in blueberries (Vaccinium virgatum) during postharvest storage. Postharvest Biol. Technol. 2020, 170, 111327. [Google Scholar] [CrossRef]
- Li, Y.T.; Liu, S.Y.; Kuang, H.Y.; Zhang, J.Y.; Wang, B.; Wang, S.J. Transcriptomic and Physiological Analysis Reveals the Possible Mechanism of Inhibiting Strawberry Aroma Changes by Ultrasound after Harvest. Foods 2024, 13, 2231. [Google Scholar] [CrossRef]
- GB 5009.3-2016; National Standards for Food Safety. Determination of Moisture in Food. Chinese Standard: Beijing, China, 2016.
- GB 5009.5-2016; National Standards for Food Safety. Determination of Protein in Food. Chinese Standard: Beijing, China, 2016.
- GB/T 20569-2006; State Grain Administration. Rules for Determining Rice Storage Quality. Chinese Standard: Beijing, China, 2006.
- GB/T 5523-2008; National Grain and Oil Standardization Technical Committee. Determination of Lipase Activity of Grain and Oil. Chinese Standard: Beijing, China, 2008.
- Choi, S.; Seo, H.S.; Lee, K.R.; Lee, S.; Lee, J.; Lee, J. Effect of milling and long-term storage on volatiles of black rice (Oryza sativa L.) determined by headspace solid-phase microextraction with gas chromatography–mass spectrometry. Food Chem. 2018, 276, 572–582. [Google Scholar] [CrossRef]
- Liu, D.Y.; Zhou, G.H.; Xu, X.L. “ROAV” Method: A New Method for Determining Key Odor Compounds of Rugao Ham. Shipin Kexue 2008, 29, 370–374. [Google Scholar]
- Shao, Z.H.; Gong, X.; Song, Y.; Tao, S.; Hong, Y.; Liu, C.; Zhu, J.Q.; Wang, M.C.; Cao, L.; Ye, J.X.; et al. Effects of different treatments on storage stability and flavor characteristics of wheat germ. Shipin Gongye Keji 2025, 46, 1–13. [Google Scholar] [CrossRef]
- Li, Z.T.; Yan, C.C.; Wang, F.; Wang, R.H.; Hua, Z.T. Quality Changes of Different Soft Brown Rice during Storage. Shipin Yanjiu Yu Kaifa 2024, 45, 21–29. [Google Scholar]
- Xu, P.C.; XU, R.; Dai, Z.H.; Jin, G.Q.; Wang, T.; Feng, W.; Zhang, H.; Zhou, X.; Wang, R. Effects of packaging methods on physicochemical properties and eating qualities of rice with remained germ. Zhongguo Liangyou Xuebao 2022, 37, 1–7. [Google Scholar]
- Wang, C.L.; Wang, Z.J.; Lin, Z.S.; Chen, F.R.; Li, B. Quality changes of rice during storage. Liangshi Yu Siliao Gongye 2014, 5–9+14. [Google Scholar]
- Li, J.J.; Li, T.Y.; Wang, P.C.; Wu, X.Y. Effects of different storage temperatures on microbial quantity and quality of high quality indica rice. Grain Oil Storage Technol. Newsl. 2025, 41, 47–51. [Google Scholar]
- Shen, L.Z.; Sheng, Y.H.; Lu, Q.F.; Li, J.; Lv, Y.T.; Li, J.J.; Zeng, R.; Cai, C.G.; Cai, H.Y. Progress in Functional Research and Food Development of Tartary Buckwheat (Fagopyrum tataricum). Shipin Yanjiu Yu Kaifa 2021, 42, 192–199. [Google Scholar]
- Yusof, F.A.M.; Azman, E.M.; Adzahan, N.M.; Yusof, N.L. The influence of exogenous melatonin treatment on quality, nutritional profile, and antioxidant system of fresh-cut carambola during cold storage. J. Agric. Food Res. 2025, 19, 101704. [Google Scholar]
- Zhao, W.J.; Zhang, Q.L.; Xue, J.Y.; Ruan, J.F. Effect of Storage Time on Main Components and Antioxidant Activity of Gongmei White Tea. Shipin Gongye 2023, 44, 17–20. [Google Scholar]
- Chen, X.; Zhang, X.X.; Wang, B.Y.; Chen, P.R.; Xu, Y.; Du, X.F. Investigation of water migration and its impacts on eating qualities of black rice during cooking process. J. Cereal Sci. 2019, 89, 102810. [Google Scholar] [CrossRef]
- Bi, W.Y.; Dong, Z.; Zhang, L.L.; Shi, T.Y. Study on Effects of Low and Quasi-Low and Normal Temperature Storage on Texture and Pasting of Properties “Yong You 15”. Zhongguo Liangyou Xuebao 2022, 37, 137–142. [Google Scholar]
- Ma, Z.Q.; Zhai, X.T.; Zhang, N.; Tan, B. Effects of Germination, Fermentation and Extrusion on the Nutritional, Cooking and Sensory Properties of Brown Rice Products: A Comparative Study. Foods 2023, 12, 1542. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.J.; Qiao, Y.J.; Wang, X.; Wang, C.F.; Liu, C.X. Study on the storage quality and shelf life of rice with different moisture content. Cereals Oils 2022, 35, 104–108+113. [Google Scholar]
- Yang, Z.T.; Xu, R.D.; Chang, Y.Y.; Yang, H.Y.; Guo, Y.E.; Fu, L.H. Quality Characteristics and Flavor Components of Quinoa Rice during Storage at Room Temperature. Shipin Gongye Keji 2025, 1–15. [Google Scholar] [CrossRef]
- Yang, W.Z.; Wang, C.L.; Yang, Q.; Yang, S.Y.; Gong, K.K.; Jiang, G.P.; Liu, B. Effect of Temperature on Storage Quality of Vacuum Packed Garlic. J. Refrig. Technol. 2024, 47, 35–41. [Google Scholar]
- Wang, Y.Y.; Ma, Y.H.; Duan, J.L.; Wang, B.; Ma, T.Z.; Jiang, Y.M.; Zhang, B. Discrimination and characterization of the volatile organic compounds in red and black raspberry wines fermented with different commercial Saccharomyces cerevisiae: An integrated analysis using E-nose, GC-MS, GC-IMS, and multivariate statistical models. Food Chem. 2025, 478, 143678. [Google Scholar] [CrossRef]
- Zhou, Y.W.; Chen, Y.W.; Dong, J.F.; Wang, Q.; Yan, F.L.; Tan, J.J.; Xu, Y.C.; Zhu, G.F.; Fan, Y.p.; Ye, Y.J. Postharvest fragrance dynamics of different scented cut lilies: Insights from HS–SPME–GC–MS and electronic nose. Postharvest Biol. Technol. 2025, 222, 113378. [Google Scholar] [CrossRef]
- Chen, Q.C.; Zhu, Y.; Liu, Y.F.; Liu, Y.; Dong, C.W.; Lin, Z.; Teng, J. Black tea aroma formation during the fermentation period. Food Chem. 2022, 374, 131640. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Zhou, Y.B.; Wen, H.T. Analysis of Differences in Volatile Components of Rucheng Baimao (Camellia pubescens) Black Tea in Different Seasons. Foods 2025, 14, 763. [Google Scholar] [CrossRef]
- Mi, Y.J.; Wang, Z.H.; Guan, L.N.; Zhang, M.; Li, S.X.; Ye, G.D.; Ren, X.; Liang, S. Analysis of volatile compounds in rice porridge of different japonica rice varieties in Northeast China. J. Cereal Sci. 2023, 113, 103749. [Google Scholar] [CrossRef]
- Hu, X.Q.; Lu, L.; Guo, Z.L.; Zhu, Z.W. Volatile compounds, affecting factors and evaluation methods for rice aroma: A review. Trends Food Sci. Technol. 2020, 97, 136–146. [Google Scholar] [CrossRef]
- Lu, L.; Hu, Z.Q.; Fang, C.Y.; Hu, X.Q. Characteristic Flavor Compounds and Functional Components of Fragrant Rice with Different Flavor Types. Foods 2023, 12, 2185. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Tian, A.L.; Zhao, K.; Zhang, R.; Lei, Z.X.; Qin, X.H.; Wu, X.Q.; Liu, Y.J.; Liu, P.J.; Yang, S.Q.; et al. Effect of cooking methods on volatile compounds and texture properties in rice porridge. LWT-Food Sci. Technol. 2023, 184, 115111. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, Y.B.; Xu, Q.J.; Zhou, X.X.; Ding, Y.T.; Wang, W.J. Effect of storage positions on the Volatile Flavor Compounds (VFCs) of paddy rice through Gas Chromatography-Ion Mobility Spectroscopy (GC-IMS) analysis. Shipin Gongye Keji 2023, 44, 375–382. [Google Scholar]
- Salinas Moreno, Y.; Martínez Ortiz, M.Á.; Padilla Camberos, E.; Ramírez Díaz, J.L.; Ledesma Miramontes, A.; Alemán de la Torre, I.; Santillán Fernández, A. Effect of Solvent and Grain Color on the Biological Activities of Maize Grain. Foods 2025, 14, 1163. [Google Scholar] [CrossRef]
- Kang, W.C.; Lin, H.; Man, Z.X. Analysis for Volatile Gases of Rice with Different Storage Periods Based on GC-MS and Multivariate Analysis Method. Zhongguo Liangyou Xuebao 2018, 33, 94–101. [Google Scholar]
- Zhang, Y.X.; Jiu, C.S.; He, K.R.; Wang, H.B.; Liu, Y.X.; Ning, M.; Wu, R.N.; Zhang, S.L.; Wu, J.R. HS-SPME-GC-MS Combined with ROAV to Analyze the Effect of Different Packaging Materials on the Quality of Milk Powder. Shipin Gongye Keji 2025, 1–16. [Google Scholar] [CrossRef]
- Yue, L.; Zulipiya, M.M.T.; Wang, J.M.; Mao, H.Y.; Yu, M.; Reyilamu, H.L.L. Effect of oil-processing technology on the characteristic aroma components of turnip seed oil by HS-GC-IMS combined with ROAV analysis. Zhongguo Youzhi 2024, 1–12. [Google Scholar] [CrossRef]
- Zhao, K.Y.; Zhu, X.Q.; Yuan, S.Z.; Xu, X.B.; Shi, J.Y.; Zuo, J.H.; Yue, X.Z.; Su, T.B.; Wang, Q. Transcriptomic, metabolomic, and physiological analysis of two varieties of Chinese cabbage (Brassica rapa L. ssp. pekinensis) that differ in their storability. Postharvest Biol. Technol. 2024, 210, 112750. [Google Scholar] [CrossRef]
- Liu, X.; Wang, W.F.; Li, Z.; Xu, L.; Lan, D.M.; Wang, Y.H. Lipidomics analysis unveils the dynamic alterations of lipid degradation in rice bran during storage. Food Res. Int. 2024, 184, 114243. [Google Scholar] [CrossRef]
- Kumar, B.R.; Kshitija, S.; Ranjeet, K.; Sumandeep, K.; Kirti, C. A Holistic View of the Genetic Factors Involved in Triggering Hydrolytic and Oxidative Rancidity of Rice Bran Lipids. Food Rev. Int. 2023, 39, 441–466. [Google Scholar]
- Barros Santos, M.C.; Barouh, N.; Lullien-Pellerin, V.; Micard, V.; Villeneuve, P.; Zhou, B.; Oger, C.; Vigor, C.; Dur, T.; Ferreira, M.S.; et al. Rice Bran Lipidome Identifies Novel Phospholipids, Glycolipids, and Oxylipins with Roles in Lipid Metabolism of Hypercholesterolemic Children. Mol. Nutr. Food Res. 2022, 67, e2200111. [Google Scholar]
- Koch, E.; Wiebel, M.; Löwen, A.; Willenberg, I.; Schebb, N.H. Characterization of the Oxylipin Pattern and Other Fatty Acid Oxidation Products in Freshly Pressed and Stored Plant Oils. J. Agric. Food Chem. 2022, 70, 12935–12945. [Google Scholar]
- Hoang, A.T.; Tabatabaei, M.; Aghbashlo, M.; Carlucci, A.P.; Ölçer, A.I.; Le, A.T.; Ghassemi, A. Rice bran oil-based biodiesel as a promising renewable fuel alternative to petrodiesel: A review. Renew. Sustain. Energy Rev. 2021, 135, 110204. [Google Scholar] [CrossRef]


| 0 d | 60 d | 120 d | 180 d | 240 d | 300 d | |
|---|---|---|---|---|---|---|
| 25 °C | ||||||
| L* | 66.52 ± 1.92 Aa | 65.57 ± 2.03 Aa | 65.49 ± 1.20 Aa | 63.43 ± 1.41 Aab | 58.75 ± 6.39 Ab | 58.63 ± 3.66 Ab |
| a* | −0.11 ± 0.05 Aa | −0.34 ± 0.09 Aa | −0.36 ± 0.05 Ab | −0.39 ± 0.04 Ab | −0.39 ± 0.05 Ab | −0.55 ± 0.03 Ac |
| b* | 10.55 ± 1.37 Ad | 12.02 ± 0.82 Ac | 12.62 ± 0.92 Abc | 13.77 ± 0.29 Aab | 13.94 ± 0.36 Aa | 14.61 ± 0.25 Aa |
| ΔE | - | 2.90 ± 1.54 Aa | 1.82 ± 1.45 Aab | 2.75 ± 1.28 Aa | 0.32 ± 0.19 Ab | 3.45 ± 2.80 Aa |
| 4 °C | ||||||
| L* | 66.52 ± 1.92 Aa | 64.95 ± 2.03 Aa | 64.85 ± 1.34 Aa | 64.06 ± 0.90 Aa | 57.07 ± 3.78 Ab | 50.63 ± 6.16 Bc |
| a* | −0.11 ± 0.05 Ab | −0.16 ± 0.03 Bb | −0.28 ± 0.06 Bc | −0.29 ± 0.02 Bc | −0.36 ± 0.04 Ad | −0.37 ± 0.02 Bd |
| b* | 10.55 ± 1.37 Ab | 11.71 ± 0.80 Aab | 12.36 ± 0.90 Aa | 12.75 ± 0.79 Ba | 12.96 ± 0.29 Ba | 12.87 ± 0.71 Ba |
| ΔE | - | 3.02 ± 1.13 Abc | 2.55 ± 0.79 Ac | 1.81 ± 0.87 Ac | 7.04 ± 3.91 Bab | 7.50 ± 5.18 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Su, T.; Ma, L.; Mu, X.; Wang, H.; Xu, L.; Wang, L.; Wang, B.; Yao, D.; Wang, C. Integrated Volatile Compounds and Transcriptional Gene Analysis Elucidate the Deterioration Mechanism of Embryo Rice During Storage. Foods 2025, 14, 1482. https://doi.org/10.3390/foods14091482
Yang X, Su T, Ma L, Mu X, Wang H, Xu L, Wang L, Wang B, Yao D, Wang C. Integrated Volatile Compounds and Transcriptional Gene Analysis Elucidate the Deterioration Mechanism of Embryo Rice During Storage. Foods. 2025; 14(9):1482. https://doi.org/10.3390/foods14091482
Chicago/Turabian StyleYang, Xiyuan, Tingting Su, Lixue Ma, Xindi Mu, Hui Wang, Lei Xu, Lidong Wang, Baijun Wang, Di Yao, and Changyuan Wang. 2025. "Integrated Volatile Compounds and Transcriptional Gene Analysis Elucidate the Deterioration Mechanism of Embryo Rice During Storage" Foods 14, no. 9: 1482. https://doi.org/10.3390/foods14091482
APA StyleYang, X., Su, T., Ma, L., Mu, X., Wang, H., Xu, L., Wang, L., Wang, B., Yao, D., & Wang, C. (2025). Integrated Volatile Compounds and Transcriptional Gene Analysis Elucidate the Deterioration Mechanism of Embryo Rice During Storage. Foods, 14(9), 1482. https://doi.org/10.3390/foods14091482





