Absorption Patterns of Fucoidan Oligosaccharides from Kjellmaniella crassifolia in the Caco-2 Monolayer Cell Model and Their Pharmacokinetics in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Instruments
2.3. Preparation of Fucoidan Oligosaccharides
2.4. Chemical Constituents of F, SPF1, and SPF2
2.5. Caco-2 Cytotoxicity Assay
2.6. Establishment of Caco-2 Cell Monolayer Model
2.7. Transport and Uptake Experiments
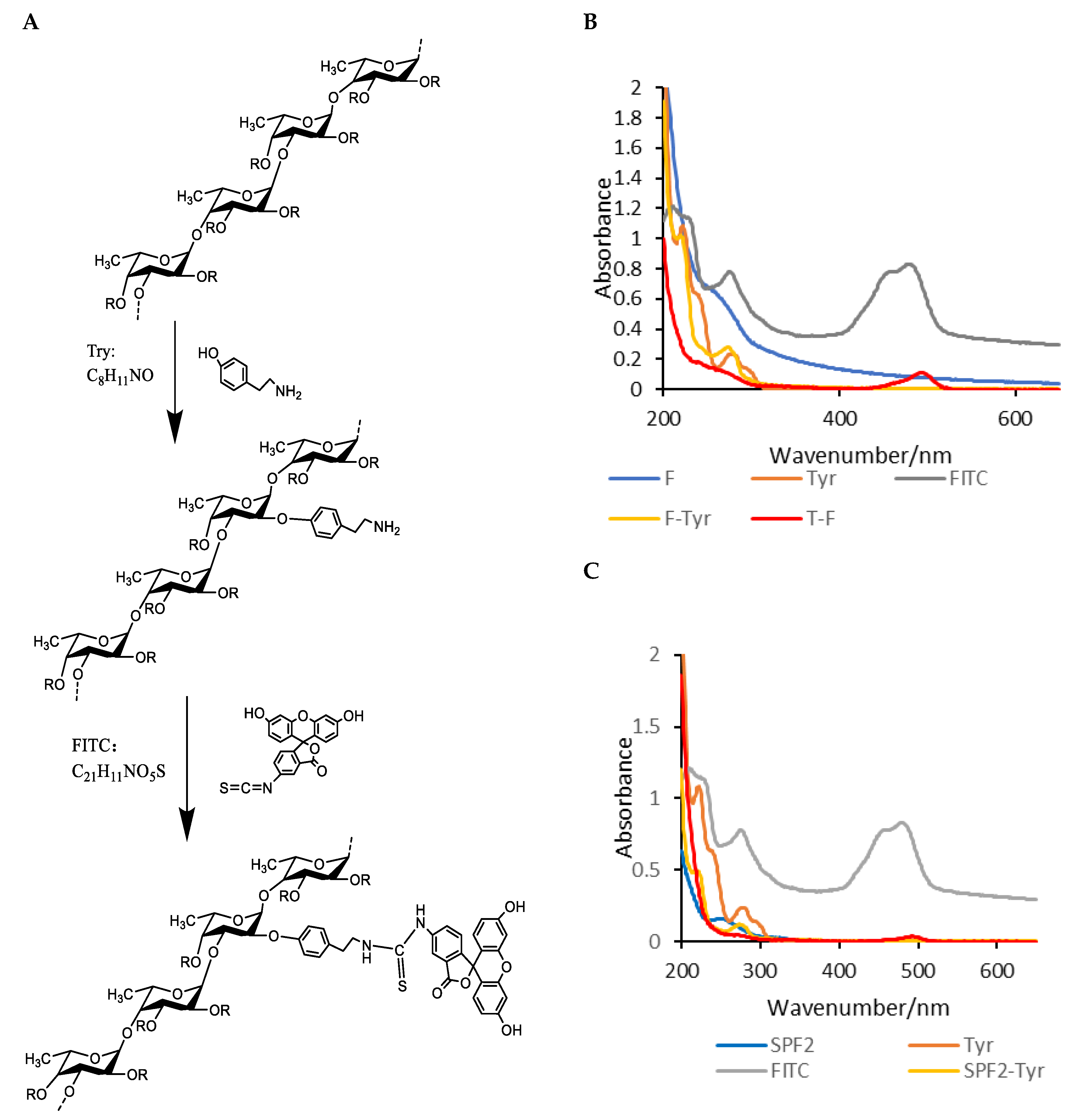
2.8. Fluorescent Labeling of F and SPF2
2.9. T-F and T-SPF2 Quantitative Analysis
2.10. Metabolic Kinetics Study in Mice
2.11. Statistical Analysis
3. Results
3.1. Preparation of SPF1 and SPF2
3.2. Molecular Characterization of F, SPF1, and SPF2
3.2.1. Chemical Composition of F, SPF1, and SPF2
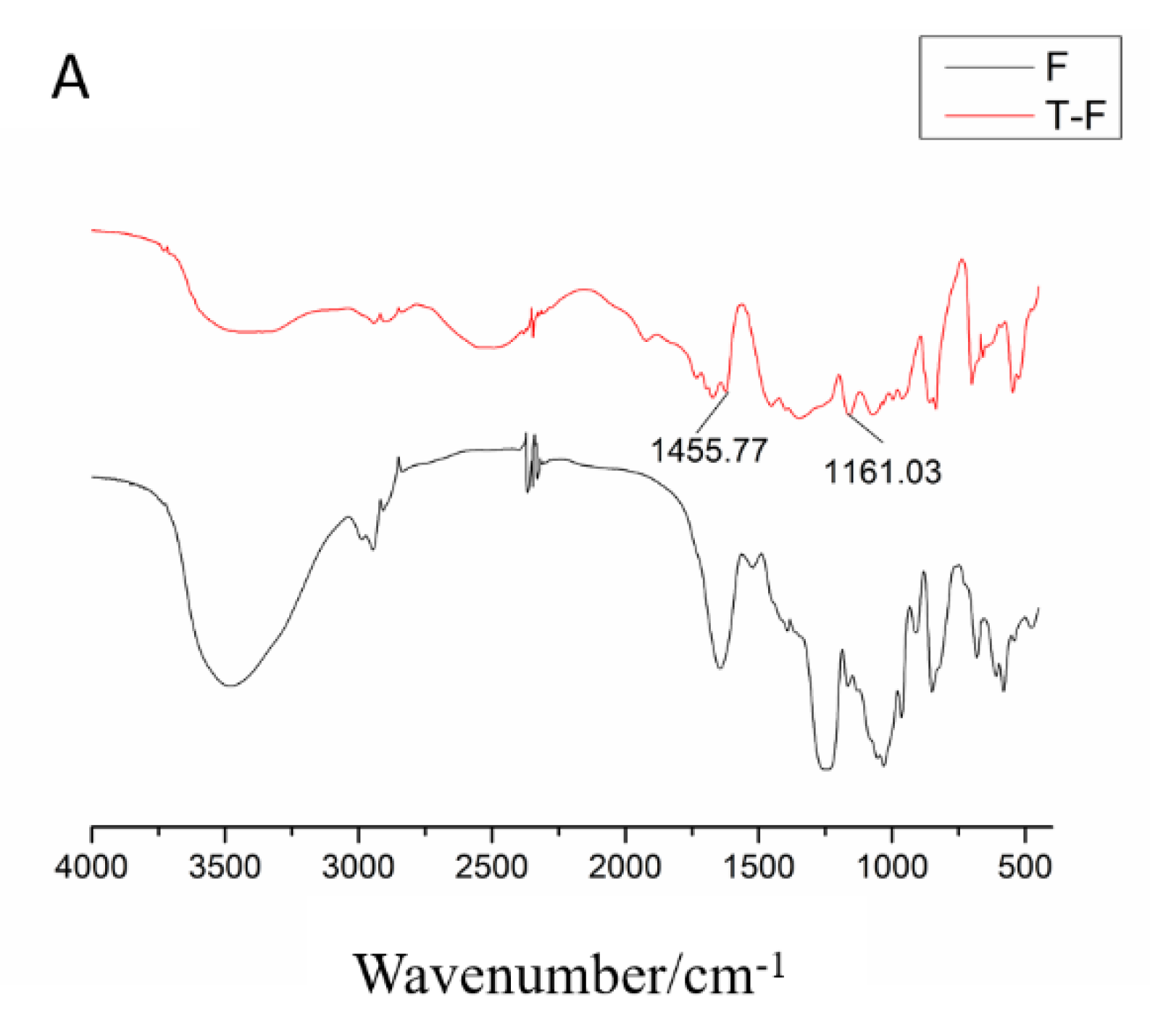
3.2.2. Infrared Spectroscopy Analysis
3.3. Cell Viability Assay
3.4. Establishment of the Cellular Intestinal Model
3.5. Transport and Uptake Experiments
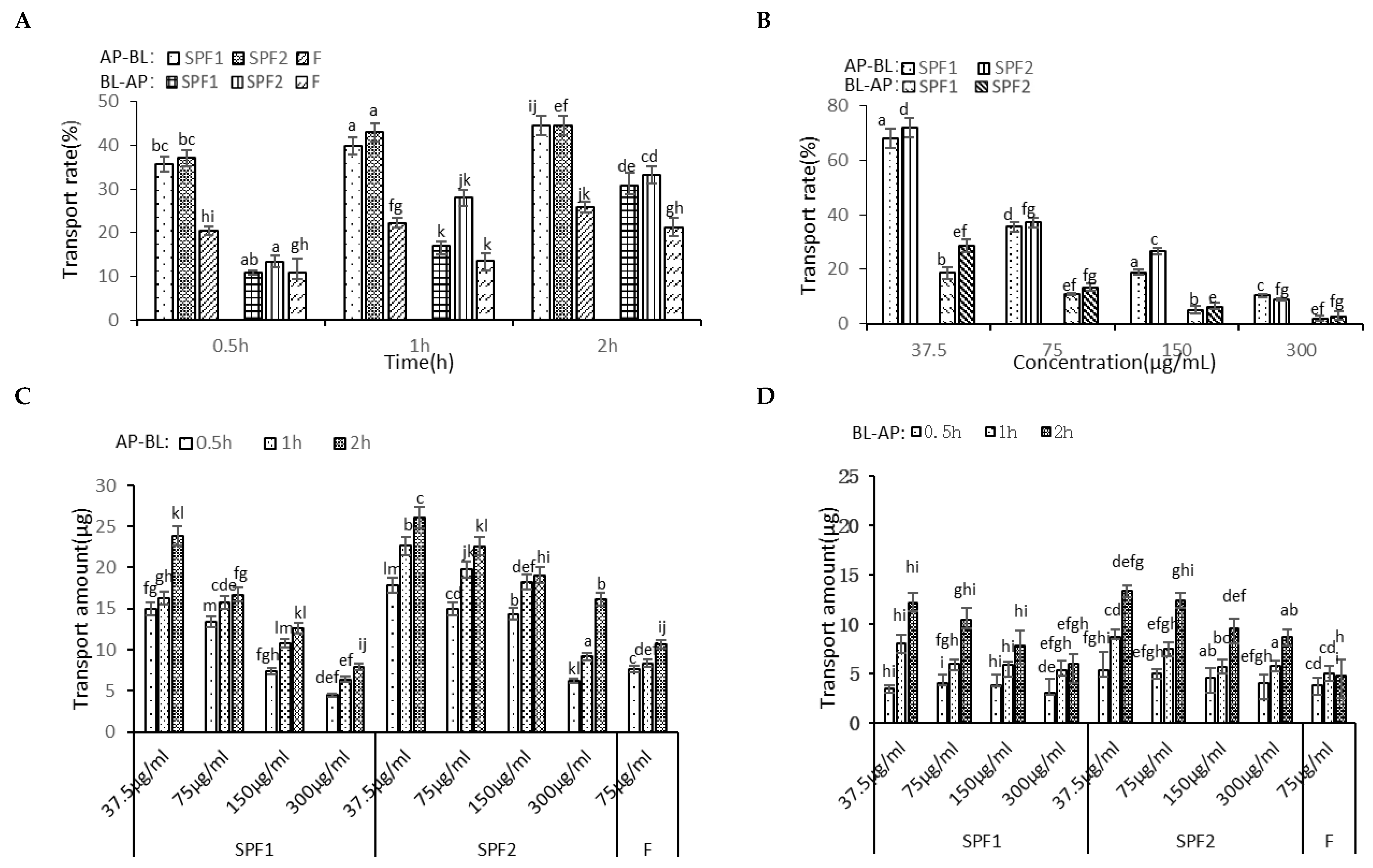
3.5.1. Transport Experiment
3.5.2. Uptake Experiments
3.6. Fluorescent Labeling of F and SPF2
3.6.1. Degree of Substitution
3.6.2. UV-Vis Spectrum
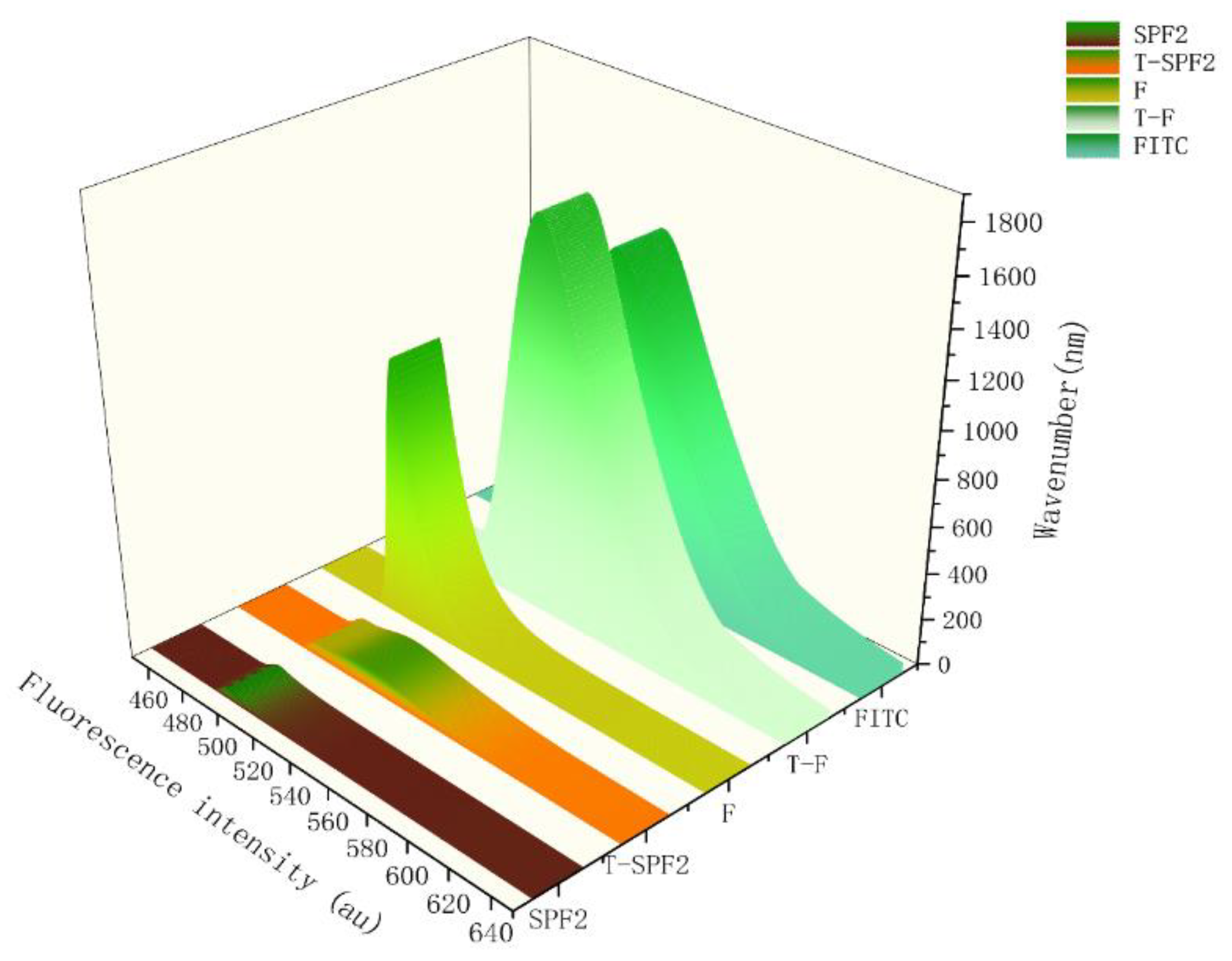
3.6.3. Fluorescence Spectrum
3.6.4. Fourier-Transform Infrared Spectroscopy
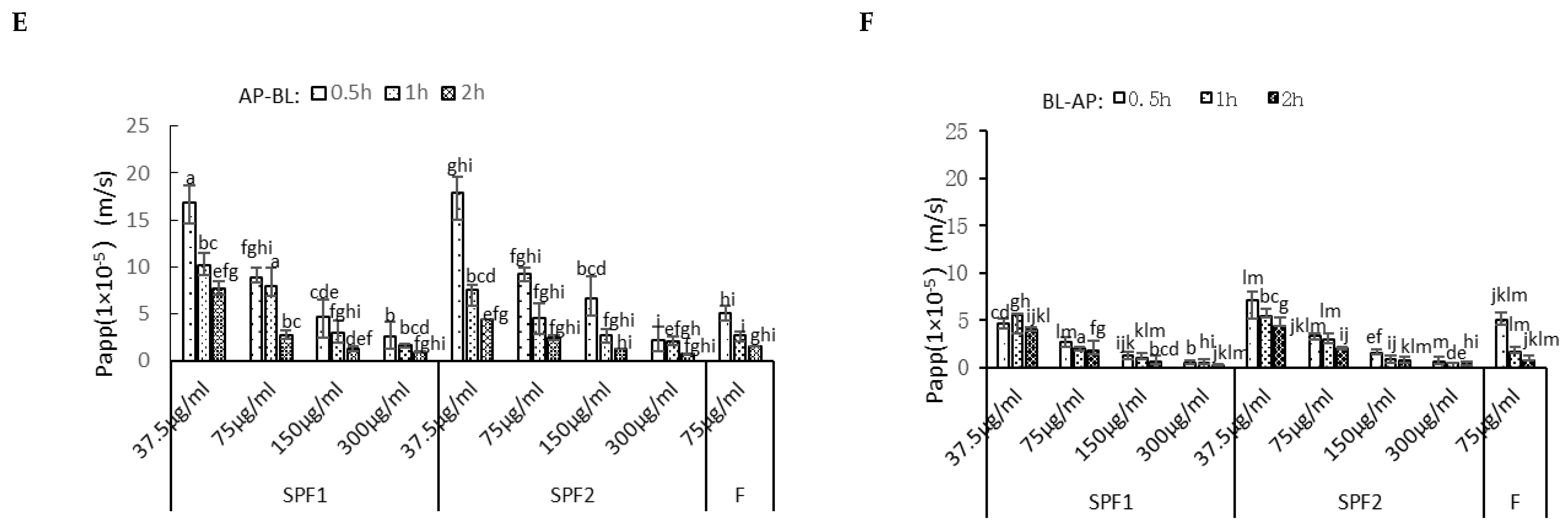
3.7. Quantitative Analysis of T-F and T-SPF2
3.7.1. Standard Curve Plotting
3.7.2. Recovery, Precision, and Stability
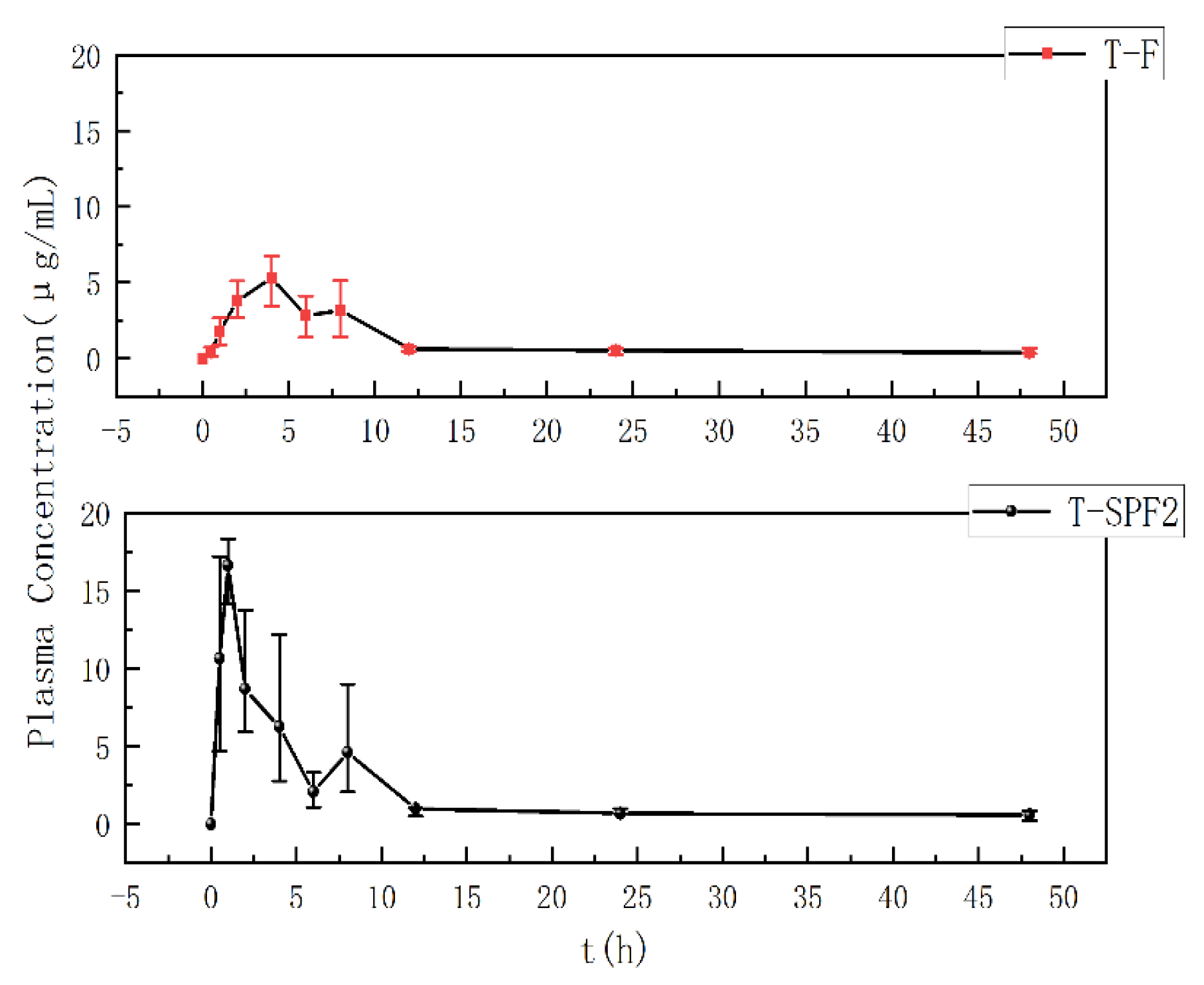
3.8. Metabolic Kinetics Study in Mice
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, X.; Meilan, X.; Jing, L.; Yiqing, M.; Yutong, W.; Huaqi, Z.; Hui, L. Fucoidan Improves D-Galactose-Induced Cognitive Dysfunction by Promoting Mitochondrial Biogenesis and Maintaining Gut Microbiome Homeostasis. Nutrients 2024, 16, 1512. [Google Scholar] [CrossRef] [PubMed]
- Citkowska, A.; Szekalska, M.; Winnicka, K. Possibilities of fucoidan utilization in the development of pharmaceutical dosage forms. Mar. Drugs 2019, 17, 458. [Google Scholar] [CrossRef] [PubMed]
- Min, W.; Suresh, V.; Saiyi, Z.; Kit-Leong, C. Fucoidan-Derived Functional Oligosaccharides: Recent Developments, Preparation, and Potential Applications. Foods 2023, 12, 878. [Google Scholar] [CrossRef]
- Catarina, O.; Nuno, M.N.; Rui, L.R.; Albino, M.; Tiago, H.S. A review on fucoidan antitumor strategies: From a biological active agent to a structural component of fucoidan-based systems. Carbohydr. Polym. 2020, 239, 116131. [Google Scholar]
- Zhang, Z.; Till, S.; Jiang, C.; Knappe, S.; Reutterer, S.; Scheiflinger, F.; Szabo, C.M.; Dockal, M. Structure-activity relationship of the pro- and anticoagulant effects of Fucus vesiculosus fucoidan. Thromb. Haemost. 2014, 111, 429–437. [Google Scholar] [CrossRef]
- Noelia, F.; Maria, D.T.; Maria, J.G.; Herminia, D. Recovery of Bioactive and Gelling Extracts from Edible Brown Seaweed Laminaria ochroleuca by Non-Isothermal Autohydrolysis. Food Chem. 2018, 277, 353–361. [Google Scholar]
- Yoo, H.J.; You, D.-J.; Lee, K.-W. Characterization and Immunomodulatory Effects of High Molecular Weight Fucoidan Fraction from the Sporophyll of Undaria pinnatifida in Cyclophosphamide-Induced Immunosuppressed Mice. Mar. Drugs 2019, 17, 447. [Google Scholar] [CrossRef]
- Elisaveta, A.; Paolina, L.; Alexandra, B.; Plamen, K.; Mariana, N.; Ilia, I.; Lyudmil, P.; Bogdan, T.; Florin, O.; Cedric, D.; et al. Immunomodulatory and Anti-Inflammatory Effects of Fucoidan: A Review. Polymers 2020, 12, 2338. [Google Scholar] [CrossRef]
- Xie, X.-T.; Cheong, K.-L. Recent advances in marine algae oligosaccharides: Structure, analysis, and potential prebiotic activities. Crit. Rev. Food Sci. Nutr. 2022, 62, 7703–7717. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.J.; Ahn, G. Step Gradient Alcohol Precipitation for the Purification of Low Molecular Weight Fucoidan from Sargassum siliquastrum and Its UVB Protective Effects. Int. J. Biol. Macromol. 2020, 163, 26–35. [Google Scholar]
- Nagamine, T.; Nakazato, K.; Tomioka, S.; Iha, M.; Nakajima, K. Intestinal absorption of fucoidan extracted from the brown seaweed Cladosiphon okamuranus. Mar. Drugs 2014, 13, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Kizuku, K.; Makoto, T.; Masahiko, I.; Takeaki, N. Absorption Study of Mozuku Fucoidan in Japanese Volunteers. Mar. Drugs 2018, 16, 254. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Fengjun, G.; Jing, H.; Lijuan, Z.; Changhu, X.; Zhaohui, Z.; Bafang, L. Antithrombotic activity of oral administered low molecular weight fucoidan from Laminaria japonica. Thromb. Res. 2016, 144, 46–52. [Google Scholar]
- Zhang, E.; Fulong, C.; Lixu, X.; Hao, L.; Shuliang, S.; Aiguo, J. Use of Fluorescein Isothiocyanate Isomer I to Study the Mechanism of Intestinal Absorption of Fucoidan Sulfate in Vivo and in Vitro. Biopharm. Drug Dispos. 2018, 39, 298–307. [Google Scholar] [CrossRef]
- Baozhen, L.; Zhuo, W.; Jianping, C.; Xuehua, C.; Jiarui, L.; Yinghua, L.; Rui, L.; Xiaofei, L.; Bingbing, S.; Kit-Leong, C.; et al. Physicochemical Characterization and Antitumor Activity of Fucoidan and Its Degraded Products from Sargassum hemiphyllum (turner) C. Agardh. Molecules 2023, 28, 2610. [Google Scholar] [CrossRef]
- Shengjun, C.; Huan, L.; Xianqing, Y.; Laihao, L.; Bo, Q.; Xiao, H.; Haixia, M.; Chunsheng, L.; Chuang, P. Degradation of sulphated polysaccharides from Grateloupia livida and antioxidant activity of the degraded components. Int. J. Biol. Macromol. 2020, 156, 660–668. [Google Scholar]
- Jing, Y.; Qianqian, L.; Jun, W.; Xiaotong, Y.; Shiping, Y.; Wei, Z.; Yang, L.; Wei, T.; Shaoping, N.; Amira, H.; et al. Fucoidan Extracted from Sporophyll of Undaria pinnatifida Grown in Weihai, China–Chemical Composition and Comparison of Antioxidant Activity of Different Molecular Weight Fractions. Front. Nutr. 2021, 8, 636930. [Google Scholar]
- Hui, Z.; Lin, S.; Xiaolin, C.; Pengcheng, L. An Exploration of Seaweed Polysaccharides Stimulating Denitrifying Bacteria for Safer Nitrate Removal. Molecules 2021, 26, 3390. [Google Scholar] [CrossRef]
- Qiang, L.; Shuxin, J.; Weiwei, S.; Xiaohui, Q.; Weiguo, S.; Jiaojiao, M.; Jie, Y. Structure characterization, antioxidant and immunoregulatory properties of a novel fucoidan from the sea cucumber Stichopus chloronotus. Carbohydr. Polym. 2020, 231, 115767. [Google Scholar]
- Zayed, A.; El-Aasr, M.; Ibrahim, A.R.S. Fucoidan characterization: Determination of purity and physicochemical and chemical properties. Mar. Drugs 2020, 18, 571. [Google Scholar] [CrossRef]
- Fei, L.; Yunlu, W.; Jing, Z.; Guoyong, Y.; Linlin, H.; Quanhong, L. Transport mechanism and subcellular localization of a polysaccharide from Cucurbia moschata across Caco-2 cells model. Int. J. Biol. Macromol. 2021, 182, 1003–1014. [Google Scholar]
- Qingfang, X.; Weijie, Z.; Qian, L.; Jie, Z.; Weiwei, F.; Ting, Z.; Guanghua, M.; Yao, C.; Xiangyang, W.; Liuqing, Y.; et al. Investigation of the uptake and transport of polysaccharide from Se-enriched Grifola frondosa in Caco-2 cells model. Int. J. Biol. Macromol. 2020, 158, 1330–1341. [Google Scholar]
- Ziming, Z.; Xianglin, P.; Jingya, X.; Zhijing, W.; Yu, Z.; Kaiping, W. Advances in Tracking of Polysaccharides in Vivo: Labeling Strategies, Potential Factors and Applications Based on Pharmacokinetic Characteristics. Int. J. Biol. Macromol. 2020, 163, 1403–1420. [Google Scholar]
- Liu, Y.; Xiao, M.; Zhao, J.; Zhang, X.; Hu, X.; Goff, H.D.; Guo, Q. Fluorescent labeling affected the structural/conformational properties of arabinoxylans. Carbohydr. Polym. 2021, 265, 118064. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef]
- Seng, J.L.; Wan, M.W.A.; Sonja, S.; Thomas, R.; Stefan, B. Structural elucidation of fucoidan from Cladosiphon okamuranus (Okinawa mozuku). Food Chem. 2019, 272, 222–226. [Google Scholar]
- Yuxi, W.; Luying, G.; Hengsheng, Z.; Chao, A.; Xiaozhou, H.; Mingfu, W.; Yuyu, Z.; Chao, Z. Opportunities and Challenges of Algal Fucoidan for Diabetes Management. Trends Food Sci. Technol. 2021, 111, 628–641. [Google Scholar]
- Panjaitan, R.S.; Natalia, L. Ekstraksi Polisakarida Sulfat Dari Sargassum Polycystum Dengan Metode Microwave Assisted Extraction Dan Uji Toksisitasnya. J. Pascapanen Bioteknol. Kelaut. Perikanan 2021, 16, 23–32. [Google Scholar] [CrossRef]
- Marcella, D.; Antonella, S.; Clara, D.F.; Jianbo, X.; Laura, C.; Domenico, T. In vitro intestinal transport and anti-inflammatory properties of ideain across Caco-2 transwell model. Fitoterapia 2020, 146, 104723. [Google Scholar]
- Qiong, Y.; Maochen, X.; Ke, W.; Qiang, W.; Jiarui, Z.; Yuan, W.; Kai, J.; Shuliang, S. Application of Fucoidan in Caco-2 Model Establishment. Pharmaceuticals 2022, 15, 418. [Google Scholar] [CrossRef]
- Zichao, W.; Huiru, Z.; Yingbin, S.; Xiaoxiao, Z.; Xueqin, W.; Jiaqi, W.; Kun, F.; Xiaobei, Z. Characterization of a novel polysaccharide from Ganoderma lucidum and its absorption mechanism in Caco-2 cells and mice model. Int. J. Biol. Macromol. 2018, 118, 320–326. [Google Scholar]
- Ying, W.; Jiazhuo, L.; Zhiwei, Z.; Ronghui, Z.; Qun, S.; Peng, W. Halo-fluorescein for Photodynamic Bacteria Inactivation in Extremely Acidic Conditions. Nat. Commun. 2021, 12, 526. [Google Scholar]
- Lim, J.-M.; Yoo, H.J.; Lee, K.-W. High Molecular Weight Fucoidan Restores Intestinal Integrity by Regulating Inflammation and Tight Junction Loss Induced by Methylglyoxal-Derived Hydroimidazolone-1. Mar. Drugs 2022, 20, 580. [Google Scholar] [CrossRef]
- Kaiping, W.; Fang, C.; Xianglin, P.; Tao, Z.; Xiqiu, L.; Ziming, Z.; Li, L.; Yu, Z. Investigation of the transport and absorption of Angelica sinensis polysaccharide through gastrointestinal tract both in vitro and in vivo. Drug Deliv. 2017, 24, 1360–1371. [Google Scholar]
- Naoya, M.; Asami, O.; Yoshiyuki, A.; Takuya, F.; Kiyohiko, S. Bottom-Up Physiologically Based Oral Absorption Modeling of Free Weak Base Drugs. Pharmaceutics 2020, 12, 844. [Google Scholar]
- Xu, B.; Zhang, E.; Bo, H.; Hao, L.; Shuliang, S.; Aiguo, J. Study on Absorption Mechanism and Tissue Distribution of Fucoidan. Molecules 2020, 25, 1087. [Google Scholar] [CrossRef]
- Chengcan, L.; Kejia, F.; Kangna, C.; Jiali, W.; Jing, Z.; Di, Z.; Ning, L.; Yang, L.; Xijing, C.; Yongjie, Z. Permeability and transport mechanism of trihexyphenidyl hydrochloride in Caco-2 cell monolayer model with a validated UPLC-MS/MS method. J. Pharm. Biomed. Anal. 2020, 178, 112924. [Google Scholar]
- Walters, H.C.; Craddock, A.L.; Fusegawa, H.; Willingham, M.C.; Dawson, P.A. Expression, transport properties, and chromosomal location of organic anion transporter subtype 3. Am. J. Physiol.-Gastrointest. Liver Physiol. 2000, 279, G1188–G1200. [Google Scholar] [CrossRef]
- Jundi, L.; Po-Yun, T.; Woo, K.K.; Todd, J.A. Assay Considerations for Fluorescein Isothiocyanate-Dextran (Fitc-D): An Indicator of Intestinal Permeability in Broiler Chickens. Poult. Sci. 2021, 100, 101202. [Google Scholar]
- Pengli, L.; Chunxia, L.; Yiting, X.; Yang, Z.; Hongbing, L.; Xia, Z.; Guangli, Y.; Huashi, G. Preparation, characterization and pharmacokinetics of fluorescence labeled propylene glycol alginate sodium sulfate. J. Ocean. Univ. China 2014, 13, 683–690. [Google Scholar]
- Paul, C.; Cathal, O.; Pamela, J.M.; Emeir, M.M.; Philip, J.A. Risks and benefits of consuming edible seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar]
- Jiaojiao, T.; Yimin, S.; Jing, W.; Ning, W.; Yang, Y.; Quanbin, Z.l. Pharmacokinetics of Fucoidan and Low Molecular Weight Fucoidan from Saccharina japonica after Oral Administration to Mice. J. Oceanol. Limnol. 2023, 41, 1900–1909. [Google Scholar]
- Zhan, E.; Fulong, C.; Tongde, Z.; Yuchao, C.; Hao, L.; Shuliang, S.; Aiguo, J. Determination of Fucoidan in Rat Plasma by HPLC and Its Application in Pharmacokinetics. Pak. J. Pharm. Sci. 2020, 33, 1–9. [Google Scholar] [PubMed]
- Shan, L.; Junhui, L.; Guizhu, M.; Lufeng, Y.; Yaqin, H.; Xingqian, Y.; Ding, T.; Robert, J.L.; Shiguo, C. Effect of the Sulfation Pattern of Sea Cucumber-Derived Fucoidan Oligosaccharides on Modulating Metabolic Syndromes and Gut Microbiota Dysbiosis Caused by HFD in Mice. J. Funct. Foods 2019, 55, 193–210. [Google Scholar]
- Zizhe, A.; Zhaohui, Z.; Xiaomei, Z.; Huicheng, Y.; Haiyan, L.; Mengyang, L.; Ying, S.; Xue, Z.; Hongwei, Z. Oligosaccharide Mapping Analysis by HILIC-ESI-HCD-MS/MS for Structural Elucidation of Fucoidan from Sea Cucumber Holothuria Floridana. Carbohydr. Polym. 2021, 275, 118694. [Google Scholar]
- Keemink, J.; Bergström, C.A.S. Caco-2 cell conditions enabling studies of drug absorption from digestible lipid-based formulations. Pharm. Res. 2018, 35, 74. [Google Scholar] [CrossRef]
- Hidalgo, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 1989, 96, 736–749. [Google Scholar] [CrossRef]
- Wilson, G.; Hassan, I.F.; Dix, C.J.; Williamson, I.; Artursson, P. Transport and permeability properties of human Caco-2 cells: An in vitro model of the intestinal epithelial cell barrier. J. Control. Release 1990, 11, 25–40. [Google Scholar] [CrossRef]
- Benwei, Z.; Fang, N.; Qiang, X.; Zhong, Y. Marine Oligosaccharides Originated From Seaweeds: Source, Preparation, Structure, Physiological Activity And Applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 60–74. [Google Scholar]
- Yu, W.; Xu, B.; Bo, H.; Maochen, X.; Qi, C.; Aiguo, J.; Shuliang, S. Transport mechanisms of polymannuronic acid and polyguluronic acid across Caco-2 cell monolayers. Pharmaceutics 2020, 12, 167. [Google Scholar] [CrossRef]
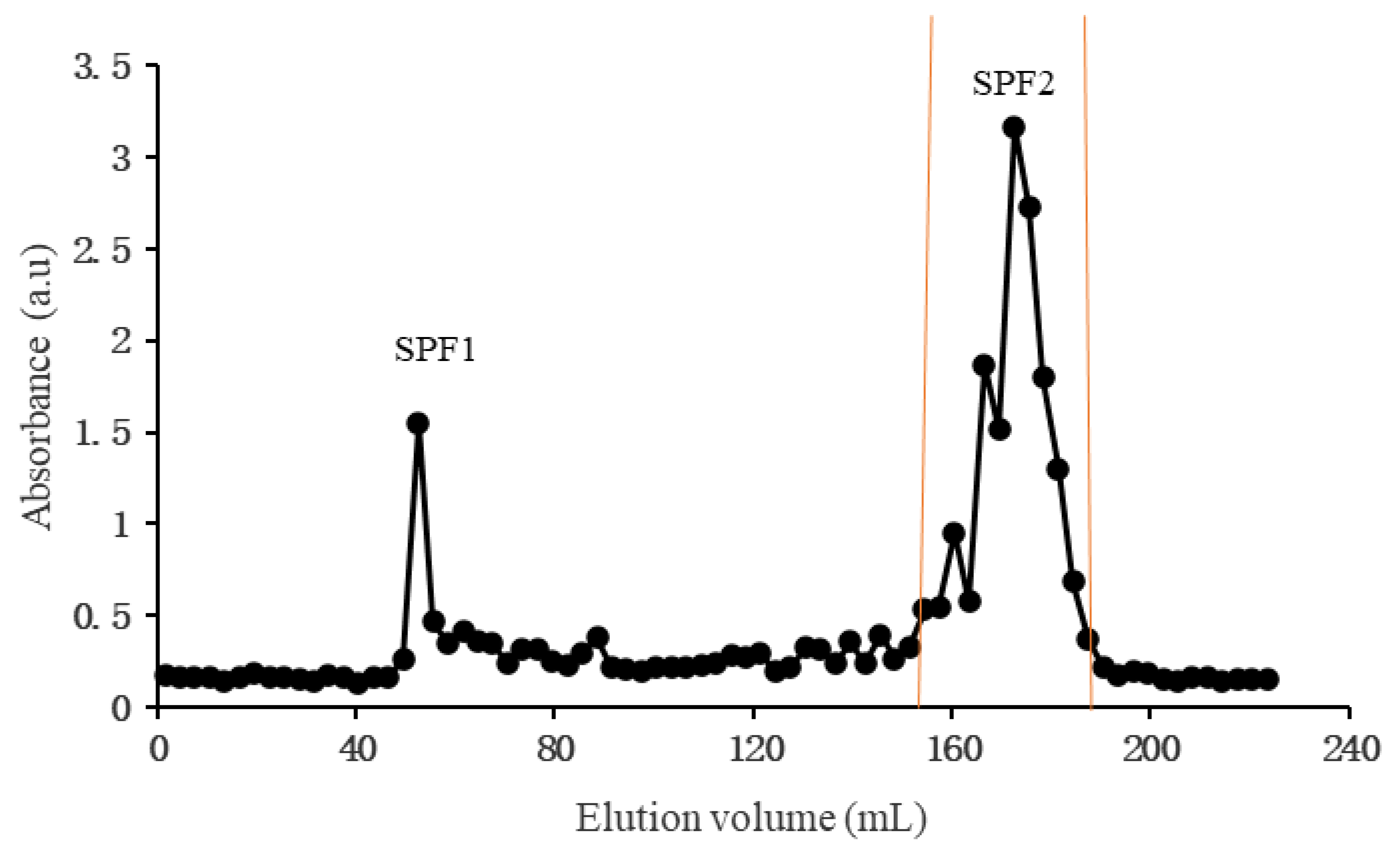
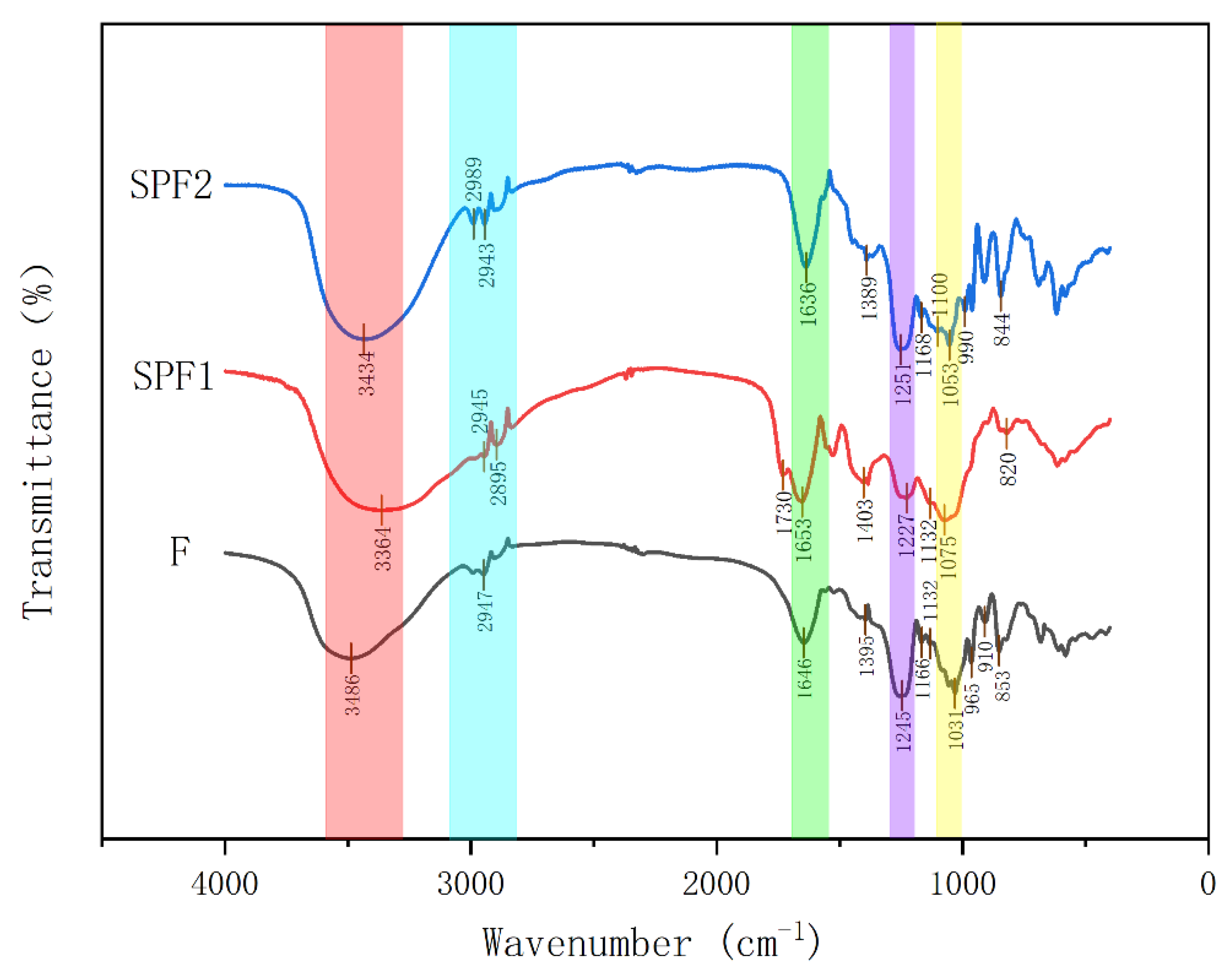
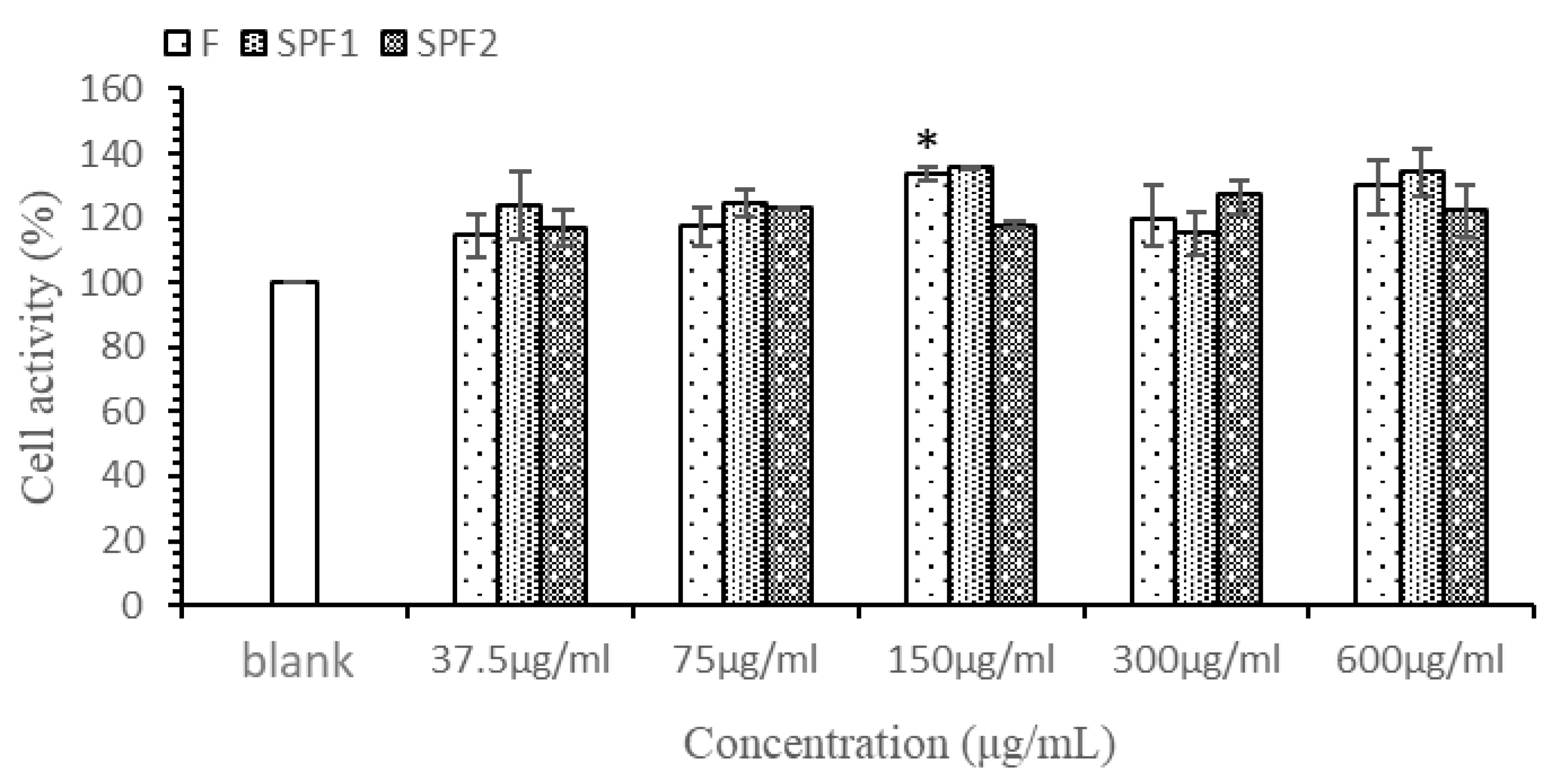
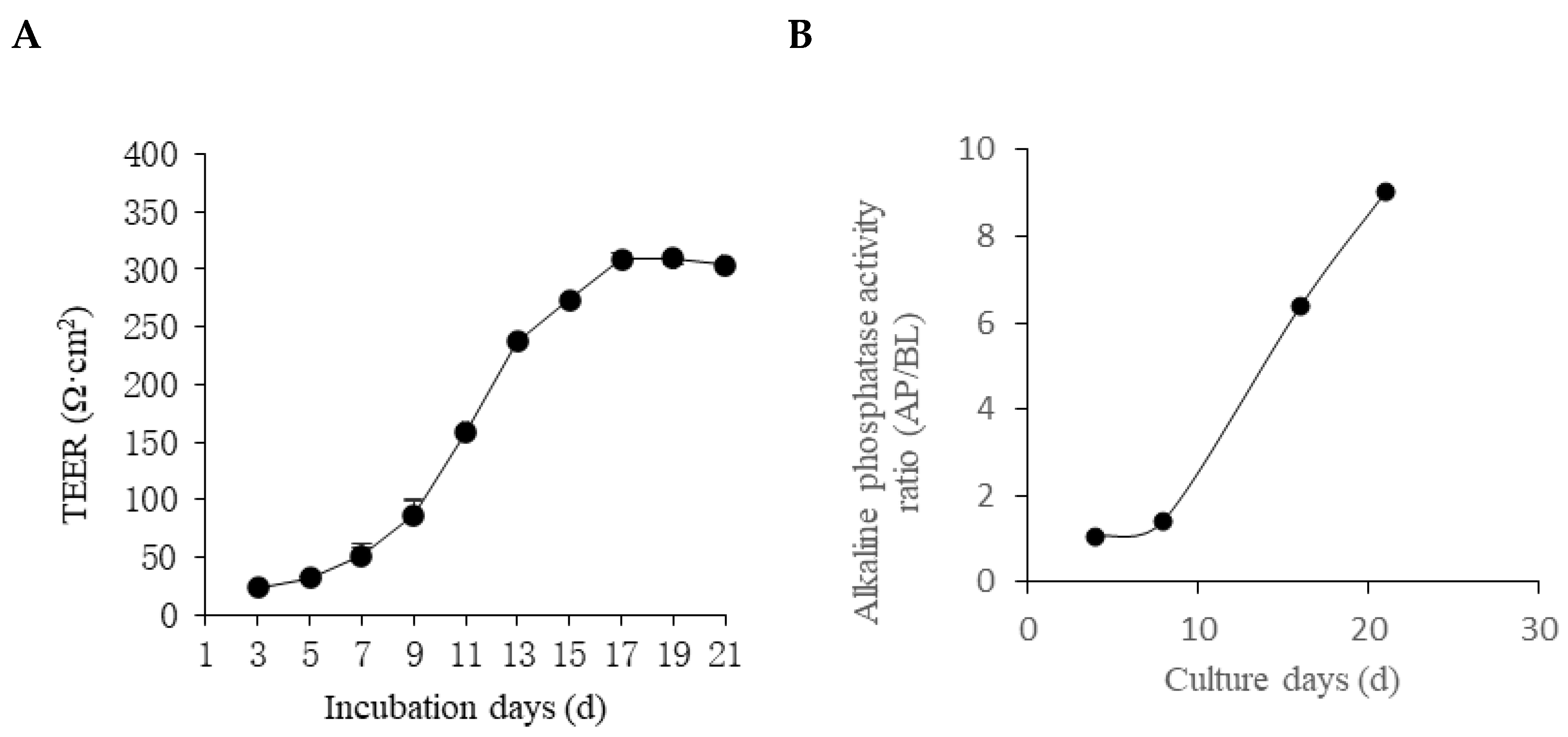


| Sample | F | SPF1 | SPF2 |
|---|---|---|---|
| Total sugar (%) | 51.90 ± 1.21 c | 68.67 ± 2.90 a | 59.70 ± 0.97 b |
| Sulfate group (%) | 25.58 ± 1.02 a | 15.33 ± 0.41 c | 16.89 ± 0.50 b |
| Aldonic acid (%) | - | 2.15 ± 0.35 | - |
| Range of molecular weight (Da) | - | 1.63 × 104~2.14 × 105 | 244.22~1545.36 |
| Composition of monosaccharides (%) | |||
| Fucose | 83.97 | 12.76 | 97.94 |
| Xylose | 0.59 | 5.00 | 0.28 |
| Rhamnose | 0.57 | 5.36 | 0.25 |
| Mannose | 6.47 | 38.87 | 0.19 |
| Galactose | 2.76 | 8.67 | 0.81 |
| Glucuronic acid | 3.37 | 25.59 | 0.17 |
| Galacturonic acid | - | 0.07 | - |
| Glucose | 2.27 | 3.69 | 0.35 |
| Linear Equation | R2 | Linear Range | |
|---|---|---|---|
| T-F | y = 30.005x + 0.9579 | 0.9991 | 1~25 μg/mL |
| T-SPF2 | y = 27.37x + 4.0118 | 0.9994 | 1~25 μg/mL |
| Sample | Concentration (μg/mL) | Recovery | Precision | Stability | |||
|---|---|---|---|---|---|---|---|
| RSD (%) | RSD (%) Intra-day | RSD (%) Inter-day | RSD (%) Room Temperature for 24 h | RSD (%) Refrigerate for 24 h | RSD (%) Re-Dissolve Three Times | ||
| T-F | 1 | 10.76 | 10.2 | 3.75 | 5.56 | 6.49 | 7.63 |
| 5 | 10.52 | 4.14 | 5.96 | 4.04 | 6.02 | 6.24 | |
| 25 | 5.53 | 5.12 | 1.74 | 4.66 | 3.00 | 3.90 | |
| T-SPF2 | 1 | 10.83 | 10.83 | 1.38 | 9.21 | 9.00 | 8.24 |
| 5 | 6.17 | 6.17 | 2.32 | 6.10 | 9.45 | 7.33 | |
| 25 | 4.34 | 4.34 | 1.98 | 2.74 | 1.71 | 4.26 | |
| Sample | Cmax | Tmax | T1/2 | AUC0–t | AUC0–∞ | MRT0–∞ | CL/F |
|---|---|---|---|---|---|---|---|
| (mg/L) | (h) | (h) | (mg/L h) | (mg/L h) | (h) | (L/h/kg) | |
| T-F | 5.28 | 4.00 | 51.12 | 43.79 | 73.03 | 51.03 | 3.10 |
| T-SPF2 | 16.63 | 1.00 | 12.11 | 274.02 | 375.95 | 17.95 | 0.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Xia, Q.; Li, L.; Shi, Y.; Gao, Y.; Ma, Y.; Liu, S.; He, Y.; Wang, Q.; Ren, D. Absorption Patterns of Fucoidan Oligosaccharides from Kjellmaniella crassifolia in the Caco-2 Monolayer Cell Model and Their Pharmacokinetics in Mice. Foods 2025, 14, 1486. https://doi.org/10.3390/foods14091486
Xu Z, Xia Q, Li L, Shi Y, Gao Y, Ma Y, Liu S, He Y, Wang Q, Ren D. Absorption Patterns of Fucoidan Oligosaccharides from Kjellmaniella crassifolia in the Caco-2 Monolayer Cell Model and Their Pharmacokinetics in Mice. Foods. 2025; 14(9):1486. https://doi.org/10.3390/foods14091486
Chicago/Turabian StyleXu, Zhiying, Qing Xia, Liu Li, Yuxin Shi, Yuan Gao, Yichao Ma, Shu Liu, Yunhai He, Qiukuan Wang, and Dandan Ren. 2025. "Absorption Patterns of Fucoidan Oligosaccharides from Kjellmaniella crassifolia in the Caco-2 Monolayer Cell Model and Their Pharmacokinetics in Mice" Foods 14, no. 9: 1486. https://doi.org/10.3390/foods14091486
APA StyleXu, Z., Xia, Q., Li, L., Shi, Y., Gao, Y., Ma, Y., Liu, S., He, Y., Wang, Q., & Ren, D. (2025). Absorption Patterns of Fucoidan Oligosaccharides from Kjellmaniella crassifolia in the Caco-2 Monolayer Cell Model and Their Pharmacokinetics in Mice. Foods, 14(9), 1486. https://doi.org/10.3390/foods14091486






