Addressing Quality, Safety, and Sustainability Challenges in Artisanal Pico Cheese Production: Proteolysis Indexes, Staphylococci, and Whey Valorization
Abstract
1. Introduction
2. Materials and Methods
2.1. Assessing the Effect of Salt Levels and Maturation Time on Physicochemical Characteristics, Maturation Indexes, and Microbiological Profile of Model Cheeses
2.1.1. Experimental Cheese Manufacture
2.1.2. Physicochemical Analyses
2.1.3. Proteolysis Parameters
2.1.4. Microbiological Analyses
2.2. Characterization, Virulence Factors, and Antibiotic Resistance of Coagulase-Positive Isolates Obtained from the Experimental Cheeses
2.3. Potential of Autochthonous LAB Cultures for the Control of S. aureus Populations
2.3.1. Effect of an Autochthonous LAB on S. aureus Strains (ATCC 9144 or ATCC 25923) in Pasteurized Whey
2.3.2. Effect of LAB Addition on CoPS Numbers in Raw Milk Cheeses
2.3.3. Assessing the Effect of LAB Addition Level on CoPS Numbers in Pasteurized- and Raw-Milk Cheeses by a Challenge Test
2.4. Screening of Whey Cheese as a Vehicle for Lactococcal Strains
2.4.1. Growth of Lactococci in Whey
2.4.2. Fate of Autochthonous Lactococci in requeijão
2.5. Statistical Analyses
3. Results
3.1. Effects of Maturation Time and Salt Addition Levels on Physicochemical Parameters, Proteolysis Indexes, and Microbial Populations in Experimental Cheeses
3.2. Characterization, Virulence Factors and Antibiotic Resistance of Coagulase-Positive Isolates Obtained from the Experimental Cheeses
3.3. Screening of Whey Cheese as a Vehicle for Lactococcal Strains
3.3.1. Effect of Autochthonous LAB on the Growth of S. aureus Strains in a Whey Model
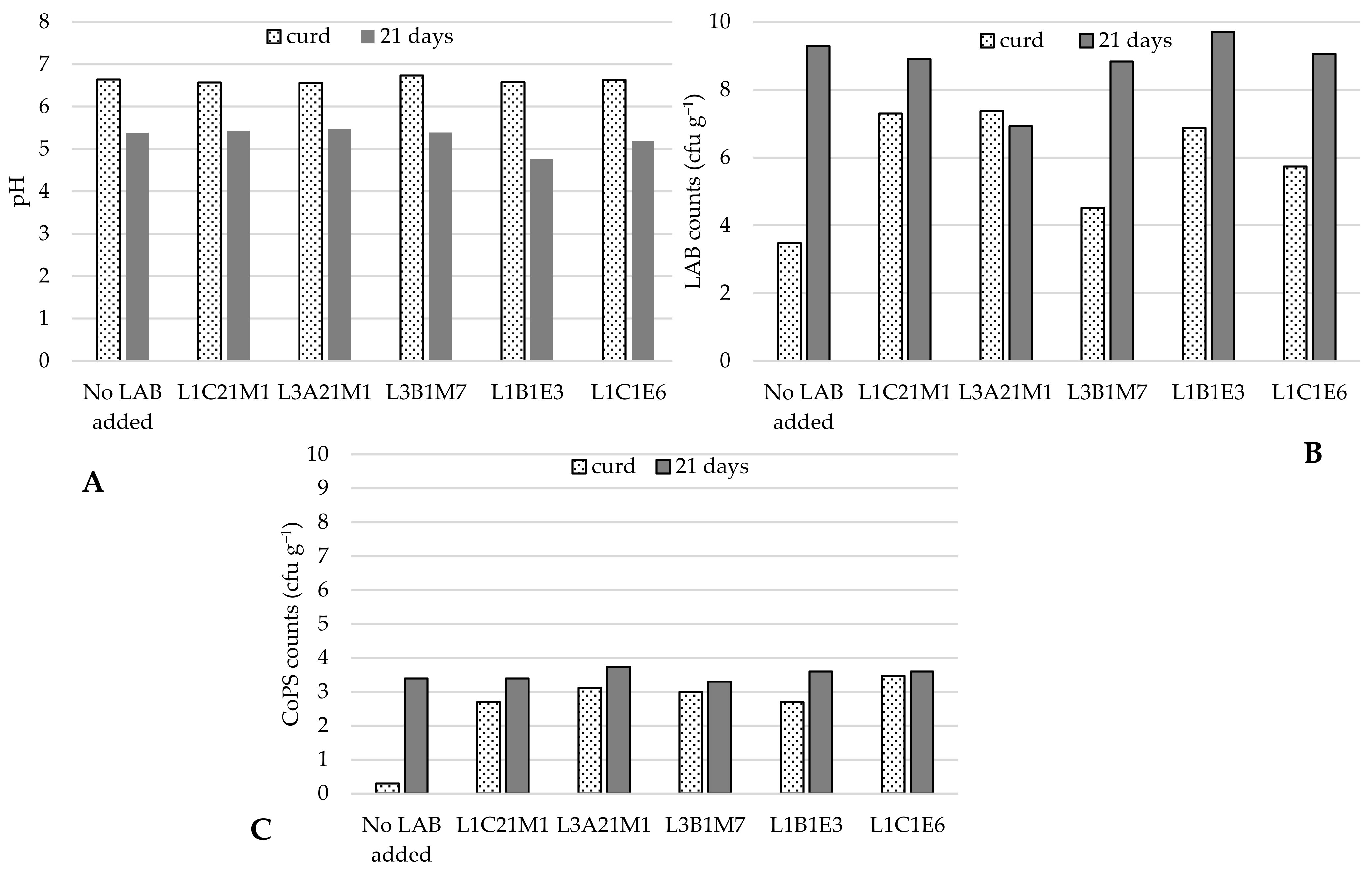
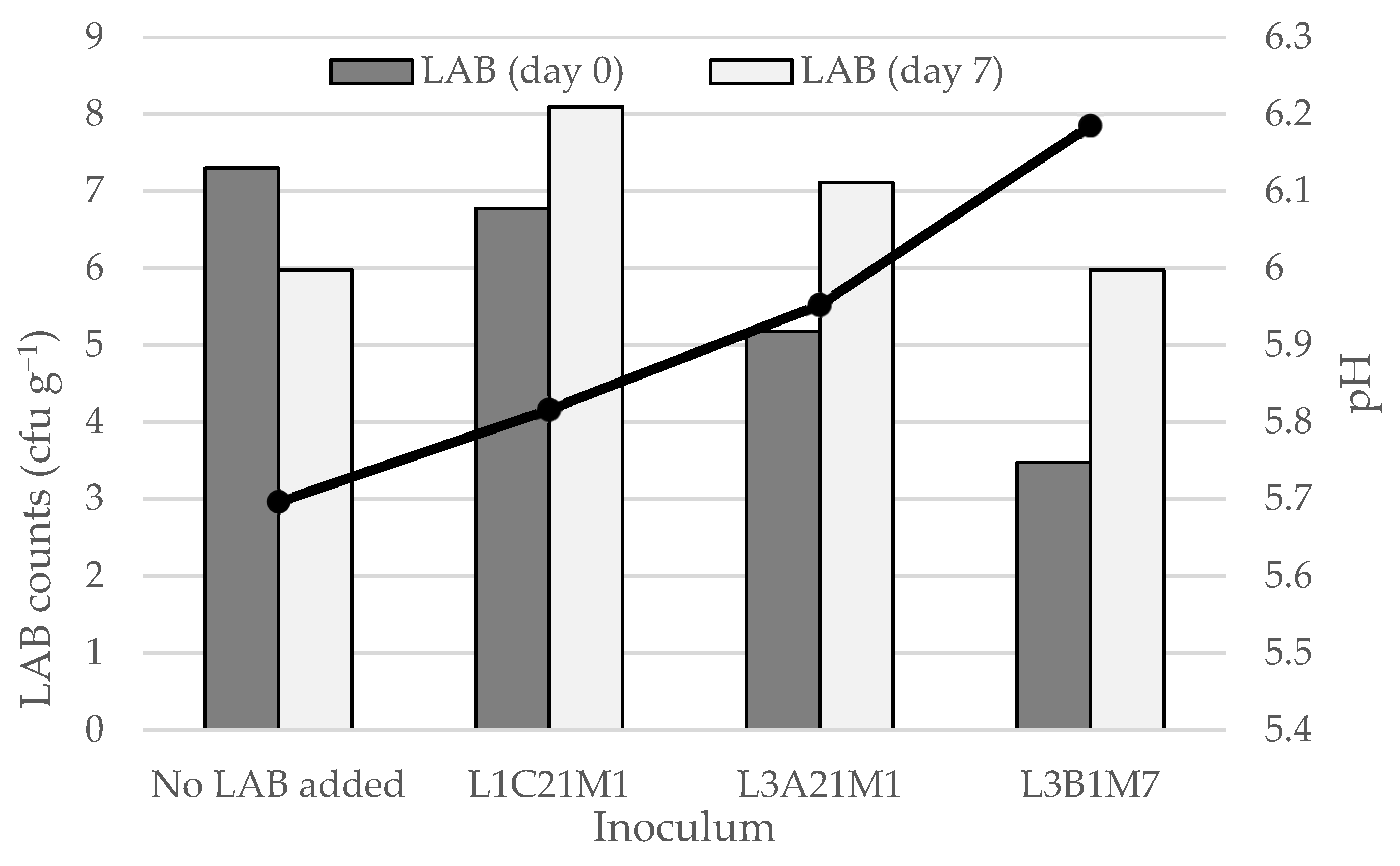
3.3.2. Effect of Adding Autochthonous LAB to Raw-Milk Model Cheeses
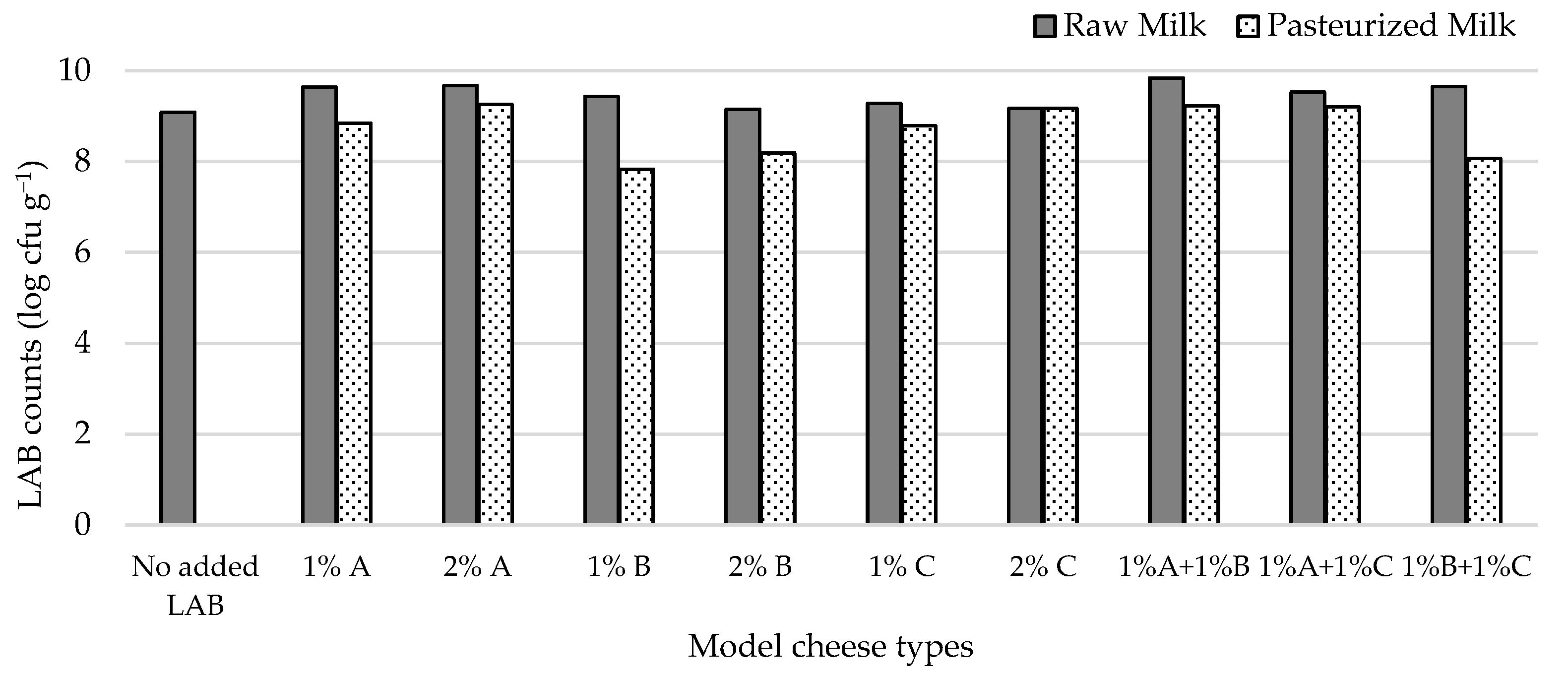
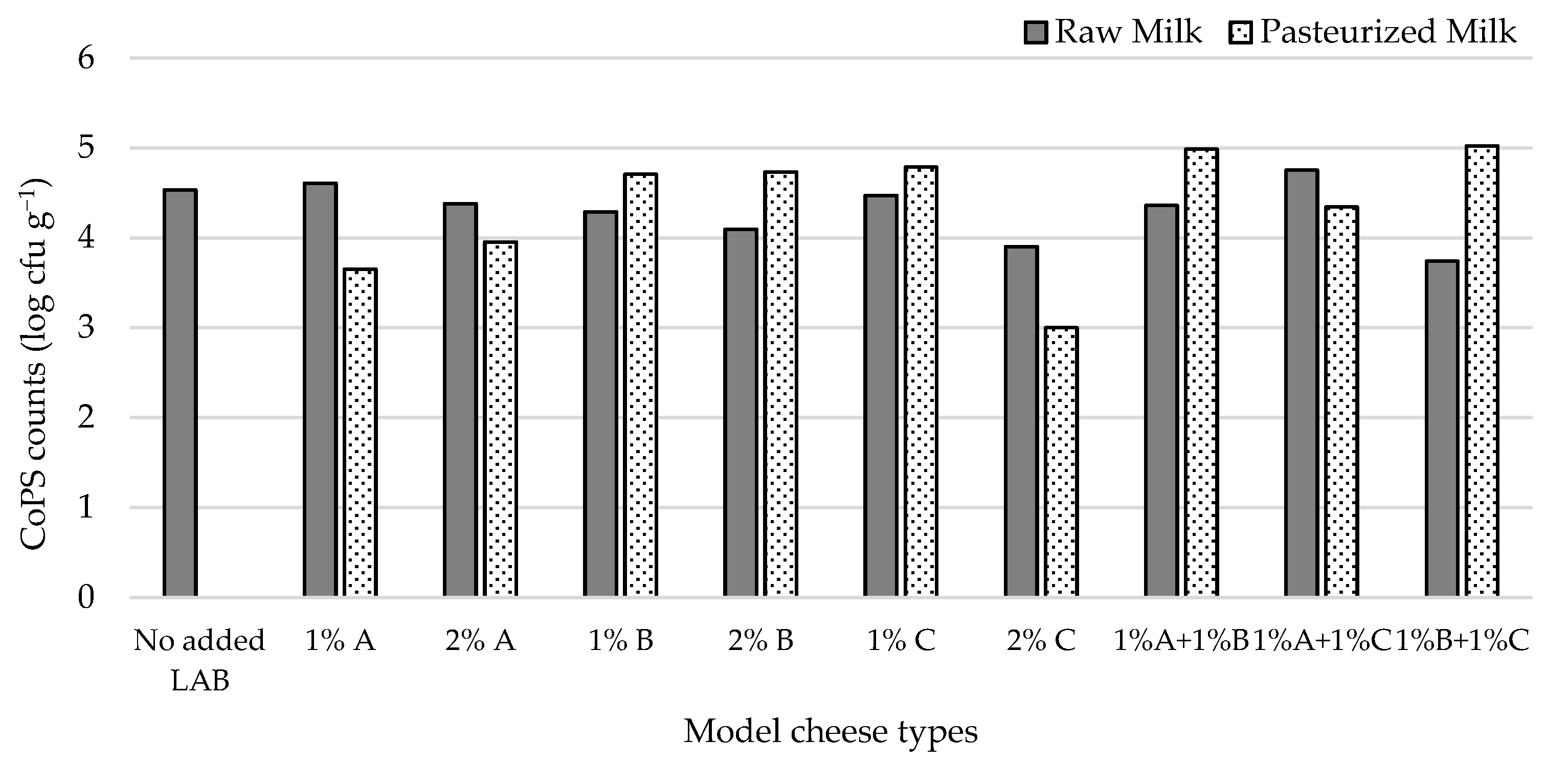
3.3.3. Effect of Adding Autochthonous Lactococci on CoPS Levels in Pasteurized and Raw Milk Cheese Models by a Challenge Test
3.4. Screening of Whey Cheese as a Vehicle for Lactococcal Strains with Probiotic Potential
3.4.1. Fate of Autochthonous Lactococci in Whey
3.4.2. Fate of Autochthonous Lactococci in Whey Cheese
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FDA. Food Code; U.S. Food and Drug Administration: Washington, DC, USA, 2013; Available online: http://www.fda.gov/Food/GuidanceRegulation/RetailFoodProtection/FoodCode/ucm374275.htm (accessed on 12 July 2024).
- Trmčić, A.; Ralyea, R.; Meunier-Goddik, L.; Donnelly, C.; Glass, K.; D’Amico, D.; Meredith, E.; Kehler, M.; Tranchina, N.; McCue, C.; et al. Consensus categorization of cheese based on water activity and pH—A rational approach to systemizing cheese diversity. J. Dairy Sci. 2017, 100, 841–847. [Google Scholar] [CrossRef]
- Câmara, S.P.A.; Dapkevicius, A.; Rosa, H.J.D.; Silva, C.C.G.; Malcata, F.X.; Enes Dapkevicius, M.L.N. Physicochemical, biochemical, microbiological and safety aspects of Pico cheese: Assessment throughout maturation and on the final product. Int. J. Dairy Technol. 2017, 70, 542–555. [Google Scholar] [CrossRef]
- Paula, A.C.L.; Medeiros, J.D.; Fernades, G.R.; da Silva, V.L.; Diniz, C.G. Microbiome of industrialized Minas Frescal Cheese reveals high prevalence of putative bacteria: A concern in the One Health context. LWT 2020, 139, 110791. [Google Scholar] [CrossRef]
- Câmara, S.P.A.; Dapkevicius, A.; Silva, C.C.G.; Malcata, F.X.; Enes Dapkevicius, M.L.N. Artisanal Pico cheese as reservoir of Enterococcus species possessing virulence and antibiotic resistance properties: Implications for food safety. Food Biotechnol. 2020, 34, 25–41. [Google Scholar] [CrossRef]
- Becker, K. Methicillin-Resistant Staphylococci and Macrococci at the Interface of Human and Animal Health. Toxins 2021, 13, 61. [Google Scholar] [CrossRef] [PubMed]
- Emiliano, J.V.S.; Fusieger, A.; Camargo, A.C.; Rodrigues, F.F.C.; Nero, L.A.; Perone, I.T.; Carvalho, Â.F. Staphylococcus aureus in dairy industry: Enterotoxin production, biofilm formation and use of lactic acid bacteria for its biocontrol. Foodborne Pathog. Dis. 2024, 21, 601–616. [Google Scholar] [CrossRef]
- Verkade, E.; Kluytmans, J. Livestock-associated Staphylococcus aureus CC398: Animal reservoirs and human infections. Infect. Genet. Evol. 2014, 21, 523–530. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Lin, Y.-T.; Wan, T.-W.; Wang, D.-Y.; Lin, H.-Y.; Lin, C.-Y.; Chen, Y.-C.; Teng, L.-J. Distribution of antibiotic resistance genes among Staphylocoocus species isolated from ready-to-eat foods. J. Food Drug Anal. 2019, 27, 841–848. [Google Scholar] [CrossRef]
- Chajęcka-Wierzchowska, W.; Zadernowska, A.; Nalepa, B.; Sierpińska, M.; Łaniewska-Trokenheim, Ł. Retail ready-to-eat food as a potential vehicle for Staphylococcus spp. harboring antibiotic resistance genes. J. Food Prot. 2014, 77, 993–998. [Google Scholar] [CrossRef]
- Perin, L.M.; Savo-Sardaro, M.L.; Nero, L.A.; Neviani, E.; Gatti, M. Bacterial ecology of artisanal Minas cheeses assessed by culture-dependent and -independent methods. Food Microbiol. 2017, 65, 160–169. [Google Scholar] [CrossRef]
- O’Brien, M.; Hunt, K.; McSweeney, S.; Jordan, K. Occurrence of foodborne pathogens in Irish farmhouse cheese. Food Microbiol. 2009, 26, 910–914. [Google Scholar] [CrossRef]
- Estepar, J.; Sánchez, M.M.; Alonso, L.; Mayo, B. Biochemical and microbiological characterization of artisanal “Peñamellera” cheese: Analysis of its indigenous lactic acid bacteria. Int. Dairy J. 1999, 9, 737–746. [Google Scholar] [CrossRef]
- Johler, S.; Macori, G.; Bellio, A.; Acutis, P.L.; Gallina, S.; Decastelli, L. Characterization of Staphylococcus aureus isolated along the raw milk cheese production process in artisan dairies in Italy. J. Dairy Sci. 2018, 101, 2915–2929. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, C.; Câmara, S.; Dapkevicius, M.d.L.; Vinuesa, P.; da Silva, C.C.; Malcata, F.X.; Rego, O.A. Characterization of the bacterial biodiversity in Pico cheese (an artisanal Azorean food). Int. J. Food Microbiol. 2015, 192, 86–94. [Google Scholar] [CrossRef]
- Zareie, Z.; Moayedi, A.; Garavand, F.; Tabar-Heydar, K.; Khomeiri, M.; Maghsoudlou, Y. Probiotic Properties, Safety Assessment, and Aroma-Generating Attributes of Some Lactic Acid Bacteria Isolated from Iranian Traditional Cheese. Fermentation 2023, 9, 338. [Google Scholar] [CrossRef]
- Margalho, L.; Jorge, G.P.; Noleto, D.A.P.; Silva, C.E.; Abreu, J.S.; Piran, M.V.F.; Brocchi, M.; Sat’Ana, A.S. Biopreservation and probiotic potential of a large set of lactic acid bacteria isolated from Brazilian artisanal cheeses: From screening to in product approach. Microbiol. Res. 2021, 242, 126622. [Google Scholar] [CrossRef] [PubMed]
- Câmara, S.P.; Dapkevicius, A.; Riquelmem, C.; Elias, R.B.; Silva, C.C.G.; Malcata, F.X.; Dapkevicius, M.L.N.E. Potential of lactic acid bacteria from Pico cheese for starter culture development. Food Sci. Technol. Int. 2019, 25, 303–317. [Google Scholar] [CrossRef]
- Cabezas, L.; Sánchez, I.; Poveda, J.M.; Seseña, S.; Palop, M.L.L. Comparison of microflora, chemical and sensory characteristics of artisanal Manchego cheeses from two dairies. Food Control 2007, 18, 11–17. [Google Scholar] [CrossRef]
- Franco, I.; Prieto, B.; Urdiales, R.; Fresno, J.M.; Carballo, J. Study of the biochemical changes during ripening of Ahumado de Áliva cheese: A Spanish traditional variety. Food Chem. 2001, 74, 463–469. [Google Scholar] [CrossRef]
- Cuesta, P.; Férnandez-García, E.; González de Llano, D.; Montilla, A.; Rodríguez, A. Evolution of the microbiological and biochemical characteristics of Afuega’l Pitu cheese during ripening. J. Dairy Sci. 1996, 79, 1693–1698. [Google Scholar] [CrossRef]
- Guinee, T.P. Salting and the role of salt in cheese. Int. J. Dairy Technol. 2004, 57, 99–109. [Google Scholar] [CrossRef]
- Zandona, E.; Blažić, M.; Jambrak, A.J. Whey utilization: Sustainable uses and environmental approach. Food Technol. Biotechnol. 2021, 59, 147–161. [Google Scholar] [CrossRef]
- Domingos-Lopes, M.F.P.; Stanton, C.; Ross, R.P.; Silva, C.C.G. Histamine and cholesterol lowering abilities of lactic acid bacteria isolated from artisanal Pico cheese. J. Appl. Microbiol. 2020, 129, 1428–1440. [Google Scholar] [CrossRef]
- Ribeiro, S.C.; O’Connor, P.M.; Ross, R.P.; Stanton, C.; Silva, C.C.G. An anti-listerial Lactococcus lactis strain isolated from Azorean Pico cheese produces lacticin 481. Int. Dairy J. 2016, 63, 18–28. [Google Scholar] [CrossRef]
- Câmara, S.P.A.; Maduro-Dias, C.; Rocha, L.; Dapkevicius, A.; Rosa, H.J.D.; Borba, A.E.; Silveira, M.G.; Malcata, F.X.; Dapkevicius, M.L.E. Assessment of autochthonous lactic acid bacteria as starter cultures for improved manufacture of Pico cheese using a cheese model. Int. Dairy J. 2022, 128, 105294. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemistry, 20th ed.; AOAC International: Rockville, MD, USA, 2016. [Google Scholar]
- Macedo, A.; Malcata, F.X. Secondary proteolysis in Serra cheese during ripening and throughout the cheese-making season. Z. Lebens Unter Forsch. A 1997, 204, 173–179. [Google Scholar] [CrossRef]
- Brito, M.A.V.P.; Campos, G.M.D.M.; Brito, J.R.F. Esquema simplificado para identificação de estafilococos coagulase-positivos isolados de mastite bovina. Ciência Rural 2002, 32, 79–82. [Google Scholar] [CrossRef]
- Pereira, C.I.; Graça, J.A.; Ogando, N.S.; Gomes, A.M.; Malcata, F.X. Bacterial dynamics in model cheese systems, aiming at safety and quality of Portuguese-style traditional ewe’s cheeses. J. Food Prot. 2009, 72, 2243–2251. [Google Scholar] [CrossRef]
- O’Toole, G.A. Microtiter dish biofilm formation assay. J. Vis. Exp. 2011, 30, 2437. [Google Scholar] [CrossRef]
- EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 14.0. The European Committee on Antimicrobial Susceptibility Testing. 2024. Available online: http://www.eucast.org (accessed on 4 November 2024).
- CLSI. Performance Standards for Antimicrobial Testing, 34th ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2024. [Google Scholar]
- USDA. Combined Database for Predictive Microbiology (ComBase). A Web Resource for Quantitative and Predictive Food Microbiology. DMFit software. Available online: https://combasebrowser.errc.ars.usda.gov (accessed on 3 February 2025).
- Ribeiro, S.C.; Coelho, M.C.; Todorov, S.D.; Franco, B.D.; Dapkevicius, M.L.; Silva, C.C. Technological properties of bacteriocin-producing lactic acid bacteria isolated from Pico cheese an artisanal cow’s milk cheese. J. Appl. Microbiol. 2014, 116, 573–585. [Google Scholar] [CrossRef] [PubMed]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards); Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Hilbert, F.; et al. Statement on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 15: Suitability of taxonomic units notified to EFSA until September 2021. EFSA J. 2022, 20, 7045. [Google Scholar] [CrossRef]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L.H. Fundamentals of Cheese Science; Aspen Publishers: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Li, N.; Richoux, R.; Boutinaud, M.; Martin, P.; Gagnaire, V. Role of somatic cells on dairy processes and products: A review. Dairy Sci. Technol. 2014, 94, 517–538. [Google Scholar] [CrossRef]
- Sionek, B.; Szydłowska, A.; Trząskowska, M.; Kołożyn-Krajewska, D. The Impact of Physicochemical Conditions on Lactic Acid Bacteria Survival in Food Products. Fermentation 2024, 10, 298. [Google Scholar] [CrossRef]
- Medved’ová, A.; Koňuchová, M.; Kvočiková, K.; Hatalová, I.; Valík, L. Effect of Lactic Acid Bacteria Addition on the Microbiological Safety of Pasta-Filata Types of Cheeses. Front. Microbiol. 2020, 11, 612528. [Google Scholar] [CrossRef]
- Settanni, L.; Moschetti, G. Non-starter lactic acid bacteria used to improve cheese quality and provide health benefits. Food Microbiol. 2010, 27, 691–697. [Google Scholar] [CrossRef]
- Bertelloni, F.; Cagnoli, G.; Ebani, V.V. Virulence and Antimicrobial Resistance in Canine Staphylococcus spp. Isolates. Microorganisms 2021, 9, 515. [Google Scholar] [CrossRef]
- Haas, B.; Bonifait, L.; Vaillancourt, K.; Charette, S.J.; Gottschalk, M.; Grenier, D. Characterization of DNase activity and gene in Streptococcus suis and evidence for a role as virulence factor. BMC Res. Notes 2014, 7, 424. [Google Scholar] [CrossRef]
- Ribeiro, S.C.; Stanton, C.; Yang, B.; Ross, P.R.; Silva, C.C.G. Conjugated linoleic acid production and probiotic assessment of Lactobacillus plantarum isolated from Pico cheese. LWT 2018, 90, 403–411. [Google Scholar] [CrossRef]
- Lister, J.L.; Horswill, A.R. Staphylococcus aureus biofilms: Recent developments in biofilm dispersal. Front. Cell Infect. Microbiol. 2017, 4, 178. [Google Scholar] [CrossRef]
- Miao, J.; Liang, Y.; Chen, L.; Wang, W.; Wang, J.; Li, B.; Li, L.; Chen, D.; Xu, Z. Formation and development of Staphylococcus biofilm: With focus on food safety. J. Food Saf. 2017, 37, e12358. [Google Scholar] [CrossRef]
- Cai, H.; Kou, X.; Ji, H.; Wang, X.; Wang, H.; Zhang, Y.; Lu, S.; Dong, J.; Wang, Q.; Jing, Z.; et al. Prevalence and characteristics of Staphylococcus aureus isolated from Kazak cheese in Xinjiang, China. Food Control 2021, 123, 107759. [Google Scholar] [CrossRef]
- Lowy, F.D. Antimicrobial resistance: The example of Staphylococcus aureus. J. Clin. Investig. 2003, 111, 1265–1273. [Google Scholar] [CrossRef]
- Wörmann, M.E.; Pech, J.; Reich, F.; Tenhagen, B.-A.; Wichmann-Schauer, H.; Lienen, T. Growth of methicillin-resistant Staphylococcus aureus during raw milk soft cheese-production and the inhibitory effect of starter cultures. Food Microbiol. 2024, 119, 104451. [Google Scholar] [CrossRef]
- Silva, C.C.; Domingos-Lopes, M.F.; Magalhães, V.A.; Freitas, D.A.; Coelho, M.C.; Rosa, H.J.; Dapkevicius, M.L. Short communication: Latin-style fresh cheese enhances lactic acid bacteria survival but not Listeria monocytogenes resistance under in vitro simulated gastrointestinal conditions. J. Dairy Sci. 2015, 98, 4377–4383. [Google Scholar] [CrossRef]
- Domingos-Lopes, M.F.; Stanton, C.; Ross, P.R.; Dapkevicius, M.L.E.; Silva, C.C.G. Genetic diversity, safety and technological characterization of lactic acid bacteria isolated from artisanal Pico cheese. Food Microbiol. 2017, 63, 178–190. [Google Scholar] [CrossRef]
- Cieza, M.Y.R.; Bonsaglia, E.C.R.; Rall, V.L.M.; dos Santos, M.V.; Silva, N.C.C. Staphylococcal enterotoxins: Description and importance in food. Pathogens 2024, 13, 676. [Google Scholar] [CrossRef]
- Bertazzoni, E.; Donelli, G.; Midtvedt, T.; Nicoli, J.; Sanz, Y. Probiotics and clinical effects: Is the number what counts? J. Chemother. 2013, 25, 193–212. [Google Scholar] [CrossRef]
| Antibiotic Target | Classification | Antibiotic(s) | Disk Charge (µg) 1 |
|---|---|---|---|
| Cell wall | Penicillins | Penicillin G | 1 unit |
| Cephalosporins | Cefoxitin 2 | 30 | |
| Cefoperazone 3 | 30 | ||
| Ceftiofur 3 | 30 | ||
| Ceftaroline 4 | 30 | ||
| Protein synthesis | Aminoglycosides | Gentamycin | 10 |
| Kanamycin | 30 | ||
| Tobramycin | 30 | ||
| Tetracyclines | Tetracycline | 30 | |
| Minocycline | 30 | ||
| Phenicols | Chloramphenicol | 30 | |
| Fusidanes | Fusidic acid | 50 | |
| Macrolides | Erythromycin | 15 | |
| Ansamycins | Rifampicin | 5 | |
| Oxazolidinones | Linezolid | 10 | |
| Streptogramins | Quinupristin-dalfopristin | 15 | |
| Monocarboxylic acids | Mupirocin | 200 | |
| Folate synthesis | Diaminopyrimidines | Trimethoprim | 5 |
| Sulfonamide-diaminopyrimidine | Sulfamethoxazole-trimethoprim | 25 | |
| DNA | Fluoroquinolones | Moxifloxacin | 5 |
| Norfloxacin | 10 | ||
| Cell wall—DNA | Penicillin-aminocoumarin | Penicillin-novobiocin | 40 |
| Parameters | Maturation Time (d) | Added Salt (g 100−1 Cheese) | |||
|---|---|---|---|---|---|
| Moisture (g 100 g−1of cheese) | 0.2 a | 0.5 a | 0.7 a | 0.9 a | |
| 20 a | 42.0 ± 1.8 | 41.3 ± 2.1 | 42.5 ± 1.4 | 42.5 ± 1.5 | |
| 40 b | 31.5 ± 0.9 | 33.7 ± 2.1 | 32.2 ± 1.6 | 32.4 ± 3.1 | |
| 60 c | 29.5 ± 0.1 | 28.2 ± 1.9 | 28.2 ± 1.3 | 28.0 ± 1.9 | |
| Ash (g 100 g−1TS) | 0.2 a | 0.5 a | 0.7 ab | 0.9 b | |
| 20 a | 5.1 ± 04 | 5.2 ± 0.4 | 5.6 ± 0.3 | 6.2 ± 0.3 | |
| 40 a | 5.9 ± 1.6 | 5.2 ± 0.0 | 5.8 ± 0.3 | 6.2 ± 0.3 | |
| 60 a | 5.2 ± 0.1 | 5.5 ± 0.2 | 5.4 ± 0.3 | 6.0 ± 0.0 | |
| Protein (g 100 g−1TS) | 0.2 a | 0.5 a | 0.7 a | 0.9 a | |
| 20 a | 39.8 ± 0.3 | 39.4 ± 2.0 | 36.4 ± 5.7 | 38.6 ± 1.9 | |
| 40 a | 39.7 ± 0.1 | 39.4 ± 0.8 | 39.5 ± 0.3 | 38.3 ± 1.1 | |
| 60 a | 39.4 ± 0.3 | 38.9 ± 1.3 | 37.1 ± 4.2 | 38.8 ± 1.8 | |
| Fat (g 100 g−1 TS) | 0.2 a | 0.5 a | 0.7 a | 0.9 a | |
| 20 a | 48.5 ± 0.2 | 48.2 ± 0.7 | 47.5 ± 1.1 | 48.4 ± 1.4 | |
| 40 b | 48.6 ± 2.4 | 51.3 ± 1.6 | 50.6 ± 2.0 | 50.8 ± 1.5 | |
| 60 b | 50.5 ± 0.8 | 52.0 ± 1.7 | 51.9 ± 0.3 | 49.9 ± 1.3 | |
| NaCl (g 100 g−1TS) | 0.2 a | 0.5 b | 0.7 c | 0.9 d | |
| 20 a | 0.4 ± 0.0 | 0.7 ± 0.0 | 1.0 ± 0.1 | 1.6 ± 0.1 | |
| 40 b | 0.5 ± 0.0 | 0.8 ± 0.0 | 1.2 ± 0.1 | 1.6 ± 0.1 | |
| 60 c | 0.5 ± 0.0 | 0.9 ± 0.1 | 1.4 ± 0.1 | 1.6 ± 0.1 | |
| WSN (g 100 g−1TN) | 0.2 a | 0.5 a | 0.7 a | 0.9 a | |
| 20 a | 11.2 ± 2.7 | 11.8 ± 5.1 | 17.5 ± 7.0 | 13.9 ± 4.4 | |
| 40 b | 10.5 ± 2.7 | 8.3 ± 0.6 | 9.1 ± 1.5 | 9.3 ± 1.2 | |
| 60 b | 7.9 ± 0.9 | 7.9 ± 0.5 | 8.7 ± 2.1 | 8.4 ± 1.3 | |
| 12% TCAN (g 100 g−1TN) | 0.2 a | 0.5 a | 0.7 a | 0.9 a | |
| 20 a | 8.4 ± 1.7 | 6.5 ± 3.5 | 10.2 ± 3.7 | 9.3 ± 3.1 | |
| 40 b | 6.3 ± 1.0 | 4.7 ± 1.1 | 5.1 ± 1.4 | 5.9 ± 1.8 | |
| 60 b | 5.4 ± 0.3 | 3.7 ± 0.7 | 5.8 ± 1.0 | 3.5 ± 0.5 | |
| 5% PTAN (g 100 g−1TN) | 0.2 a | 0.5 a | 0.7 a | 0.9 a | |
| 20 a | 0.4 ± 0.0 | 0.7 ± 0.0 | 1.0 ± 0.1 | 1.6 ± 0.1 | |
| 40 a | 0.5 ± 0.0 | 0.8 ± 0.0 | 1.2 ± 0.1 | 1.6 ± 0.1 | |
| 60 a | 0.5 ± 0.0 | 0.9 ± 0.1 | 1.4 ± 0.1 | 1.6 ± 0.1 | |
| Parameters | Manufacture Stage | Added Salt (g 100−1 Cheese) | |||
|---|---|---|---|---|---|
| pH | 0.2 a | 0.5 ab | 0.7 a | 0.9 b | |
| Raw milk a | 6.86 ± 0.05 | 6.86 ± 0.01 | 6.73 ± 0.01 | 6.82 ± 0.01 | |
| Curd a | 6.77 ± 0.05 | 6.72 ± 0.02 | 6.74 ± 0.02 | 6.74 ± 0.02 | |
| Cheese, 20 days b | 5.39 ± 0.25 | 5.51 ± 0.11 | 5.67 ± 0.02 | 5.67 ± 0.16 | |
| Cheese, 40 days c | 5.18 ± 0.23 | 5.41 ± 0.08 | 5.15 ± 0.14 | 5.55 ± 0.10 | |
| Cheese, 60 days d | 5.08 ± 0.12 | 5.20 ± 0.08 | 5.09 ± 0.14 | 5.41 ± 0.11 | |
| Titratable acidity | 0.2 ab | 0.5 a | 0.7 b | 0.9 ab | |
| Cheese, 20 days a | 1.18 ± 0.42 | 1.05 ± 0.04 | 0.86 ± 0.12 | 0.77 ± 0.14 | |
| Cheese, 40 days b | 1.31 ± 0.06 | 1.07 ± 0.06 | 1.97 ± 0.30 | 1.74 ± 0.30 | |
| Cheese, 60 days b | 1.76 ± 0.00 | 1.30 ± 0.11 | 2.27 ± 0.42 | 1.84 ± 0.31 | |
| aw | 0.2 a | 0.5 b | 0.7 c | 0.9 a | |
| Cheese, 20 days a | 0.63 ± 0.02 | 0.80 ± 0.01 | 0.74 ± 0.01 | 0.64 ± 0.01 | |
| Cheese, 40 days b | 0.58 ± 0.00 | 0.62 ± 0.00 | 0.62 ± 0.02 | 0.59 ± 0.01 | |
| Cheese, 60 days b | 0.63 ± 0.00 | 0.58 ± 0.00 | 0.59 ± 0.00 | 0.62 ± 0.00 | |
| Salt-in-moisture | 0.2 a | 0.5 b | 0.7 c | 0.9 d | |
| Cheese, 20 days a | 1.00 ± 0.04 | 1.69 ± 0.08 | 3.36 ± 0.42 | 2.10 ± 0.93 | |
| Cheese, 40 days b | 1.46 ± 0.09 | 2.42 ± 0.18 | 4.94 ± 0.67 | 3.22 ± 1.47 | |
| Cheese, 60 days c | 1.76 ± 0.00 | 3.14 ± 0.51 | 5.63 ± 0.24 | 3.66 ± 1.51 | |
| TAM counts (log cfu g−1) | 0.2 a | 0.5 a | 0.7 a | 0.9 a | |
| Raw milk a | 3.52 ± 0.28 | 4.12 ± 0.42 | 3.41 ± 0.17 | 3.47 ± 0.15 | |
| Curd b | 3.67 ± 0.49 | 4.15 ± 0.27 | 3.82 ± 0.11 | 3.62 ± 0.28 | |
| Cheese, 20 days c | 8.91 ± 00.09 | 8.81 ± 0.16 | 8.92 ± 0.19 | 8.89 ± 0.07 | |
| Cheese, 40 days c | 8.61 ± 0.43 | 8.58 ± 0.39 | 8.79 ± 0.16 | 8.78 ± 0.14 | |
| Cheese, 60 days d | 8.11 ± 0.41 | 7.75 ± 0.16 | 8.40 ± 0.39 | 8.24 ± 0.32 | |
| LAB counts (log cfu g−1) | 0.2 a | 0.5 a | 0.7 a | 0.9 a | |
| Raw milk a | 2.33 ± 0.85 | 2.8 ± 0.16 | 2.26 ± 0.24 | 2.42 ± 0.05 | |
| Curd b | 1.32 ± 2.28 | 3.06 ± 0.10 | 0.9 ± 1.56 | 0.00 ± 0.00 | |
| Cheese, 20 days c | 8.83 ± 0.11 | 8.57 ± 0.38 | 8.63 ± 0.15 | 8.73 ± 0.17 | |
| Cheese, 40 days c | 8.85 ± 0.13 | 8.41 ± 0.38 | 8.79 ± 0.16 | 8.66 ± 0.08 | |
| Cheese, 60 days c | 8.77±0.10 | 8.19 ± 0.04 | 8.74 ± 0.19 | 8.12 ± 0.11 | |
| CoPS counts (log cfu g−1) | 0.2 a | 0.5 a | 0.7 b | 0.9 ab | |
| Raw milk a | 2.30 ± 0.30 | 2.18 ± 0.44 | 2.81 ± 0.26 | 2.65 ± 0.22 | |
| Curd a | 1.00 ± 1.73 | 2.28 ± 1.98 | 2.96 ± 0.24 | 2.06 ± 1.79 | |
| Cheese, 20 days b | 5.37 ± 0.22 | 5.21 ± 0.61 | 6.13 ± 0.46 | 5.71 ± 0.15 | |
| Cheese, 40 days b | 4.48 ± 0.59 | 4.99 ± 0.90 | 6.25 ± 0.17 | 5.60 ± 0.03 | |
| Cheese, 60 days b | 4.33 ± 0.22 | 4.54 ± 0.73 | 6.09 ± 0.21 | 5.50 ± 0.14 | |
| Isolate | DNAse | Biofilm Production (OD570) | Antibiotic Resistance/Sensitivity | ||
|---|---|---|---|---|---|
| 24 h | 48 h | Penicillin | Cefoxitin | ||
| A201 | − | 0.18 ± 0.07 | 0.20 ± 0.06 | R | S |
| A202 | − | 0.16 ± 0.04 | 0.17 ± 0.07 | R | S |
| A203 | − | 0.19 ± 0.04 | 0.20 ± 0.08 | S | R |
| A204 | + | 0.53 ± 0.10 | 0.70 ± 0.14 | S | S |
| A604 | + | 0.37 ± 0.16 | 1.17 ± 0.68 | S | R |
| C201 | + | 0.18 ± 0.03 | 2.29 ± 0.90 | S | R |
| C202 | + | 0.28 ± 0.11 | 1.84 ± 1.10 | S | R |
| C203 | + | 0.17 ± 0.07 | 0.46 ± 0.30 | S | R |
| C204 | + | 0.13 ± 0.08 | 1.23 ± 1.34 | S | S |
| C603 | + | 0.15 ± 0.02 | 0.64 ± 0.33 | S | R |
| C604 | + | 0.14 ± 0.05 | 1.22 ± 1.48 | S | R |
| Inocula | N0 | λ | µ | A | R2 | SE of Fit |
|---|---|---|---|---|---|---|
| L1C21M1 | 6.219 ± 0.065 | 13.908 ± 5.162 | 0.033 ± 0.005 | 7.579 ± 0.050 | 0.982 | 0.0866 |
| SA9144 | 7.079 ± 0.087 | no lag | 0.012 ± 0.003 | 8.283 ± 0.077 | 0.941 | 0.1340 |
| L1C21M1+SA9144 | 7.013 ± 0.048 | no lag | 0.002 ± 0.001 | 7.565 ± 0.082 | 0.857 | 0.0948 |
| SA25923 | 6.844 ± 0.012 | 29.437 ± 4.965 | 0.008 ± 0.000 | 8.092 ± 0.013 | 0.999 | 0.0186 |
| L1C21M1+SA25923 | 6.757 ± 0.025 | no lag | 0.002 ± 0.000 | 7.222 ± 0.034 | 0.948 | 0.0479 |
| Lactococcal Strains | Cell Number Variation (Log Cycles) | DMFit Outputs | |||
|---|---|---|---|---|---|
| Lag Phase Length (h) | Maximum Specific Rate (h−1) | SE of Fit | R2 | ||
| L1C21M1 | +1.460 | 14.077 ± 5.486 | 0.030 ± 0.005 | 0.081 | 0.985 |
| L3A21M1 | −0.619 | -- | −0.002 ± 0.000 | 0.051 | 0.982 |
| L3B1M7 | +3.050 | 0 | 0.027 ± 0.005 | 0.051 | 0.964 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Câmara, S.P.A.; Maduro Dias, C.; Nunes, H.P.B.; Martin, R.; Pimentel, F.; Gomes, J.V.; Silveira, M.d.G.A.; Rosa, H.J.D.; Dapkevicius, A.; Borba, A.E.S.; et al. Addressing Quality, Safety, and Sustainability Challenges in Artisanal Pico Cheese Production: Proteolysis Indexes, Staphylococci, and Whey Valorization. Foods 2025, 14, 1487. https://doi.org/10.3390/foods14091487
Câmara SPA, Maduro Dias C, Nunes HPB, Martin R, Pimentel F, Gomes JV, Silveira MdGA, Rosa HJD, Dapkevicius A, Borba AES, et al. Addressing Quality, Safety, and Sustainability Challenges in Artisanal Pico Cheese Production: Proteolysis Indexes, Staphylococci, and Whey Valorization. Foods. 2025; 14(9):1487. https://doi.org/10.3390/foods14091487
Chicago/Turabian StyleCâmara, Sandra P. A., Cristiana Maduro Dias, Hélder P. B. Nunes, Raphael Martin, Francisca Pimentel, Júlia V. Gomes, Maria da Graça A. Silveira, Henrique J. D. Rosa, Airidas Dapkevicius, Alfredo E. S. Borba, and et al. 2025. "Addressing Quality, Safety, and Sustainability Challenges in Artisanal Pico Cheese Production: Proteolysis Indexes, Staphylococci, and Whey Valorization" Foods 14, no. 9: 1487. https://doi.org/10.3390/foods14091487
APA StyleCâmara, S. P. A., Maduro Dias, C., Nunes, H. P. B., Martin, R., Pimentel, F., Gomes, J. V., Silveira, M. d. G. A., Rosa, H. J. D., Dapkevicius, A., Borba, A. E. S., & Dapkevicius, M. d. L. N. E. (2025). Addressing Quality, Safety, and Sustainability Challenges in Artisanal Pico Cheese Production: Proteolysis Indexes, Staphylococci, and Whey Valorization. Foods, 14(9), 1487. https://doi.org/10.3390/foods14091487







