Sensory Properties of Fermented Blends of Sunflower Press Cake and Whey
Abstract
:1. Introduction
2. Materials and General Methods
2.1. Materials
2.2. General Methods
2.2.1. Strain Management
2.2.2. Fermentation Experiments
2.2.3. Assessment of Microbial Safety
2.2.4. Chemical and Physical Analysis of Fermented Blends
3. Experiment 1: Initial Sensory Screening
3.1. Experimental Details
3.1.1. Fermentation of Sunflower Press Cake/Whey Blends
3.1.2. Sample Assessment by Free Choice Profiling
3.1.3. Distinguishability of Fermented Blends
3.1.4. Quantitative Descriptive Analysis
3.2. Results
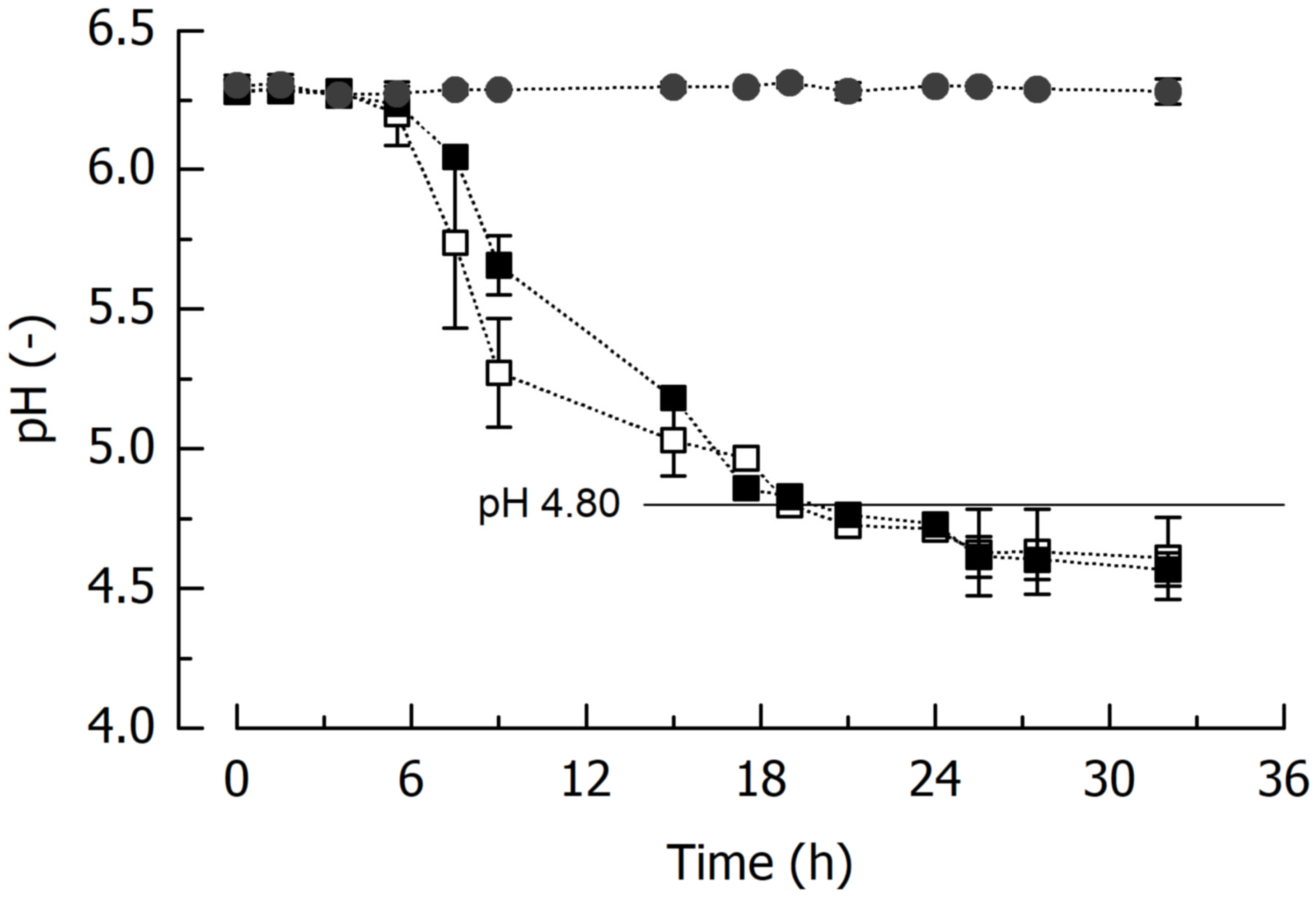
3.2.1. Fermentation Characteristics
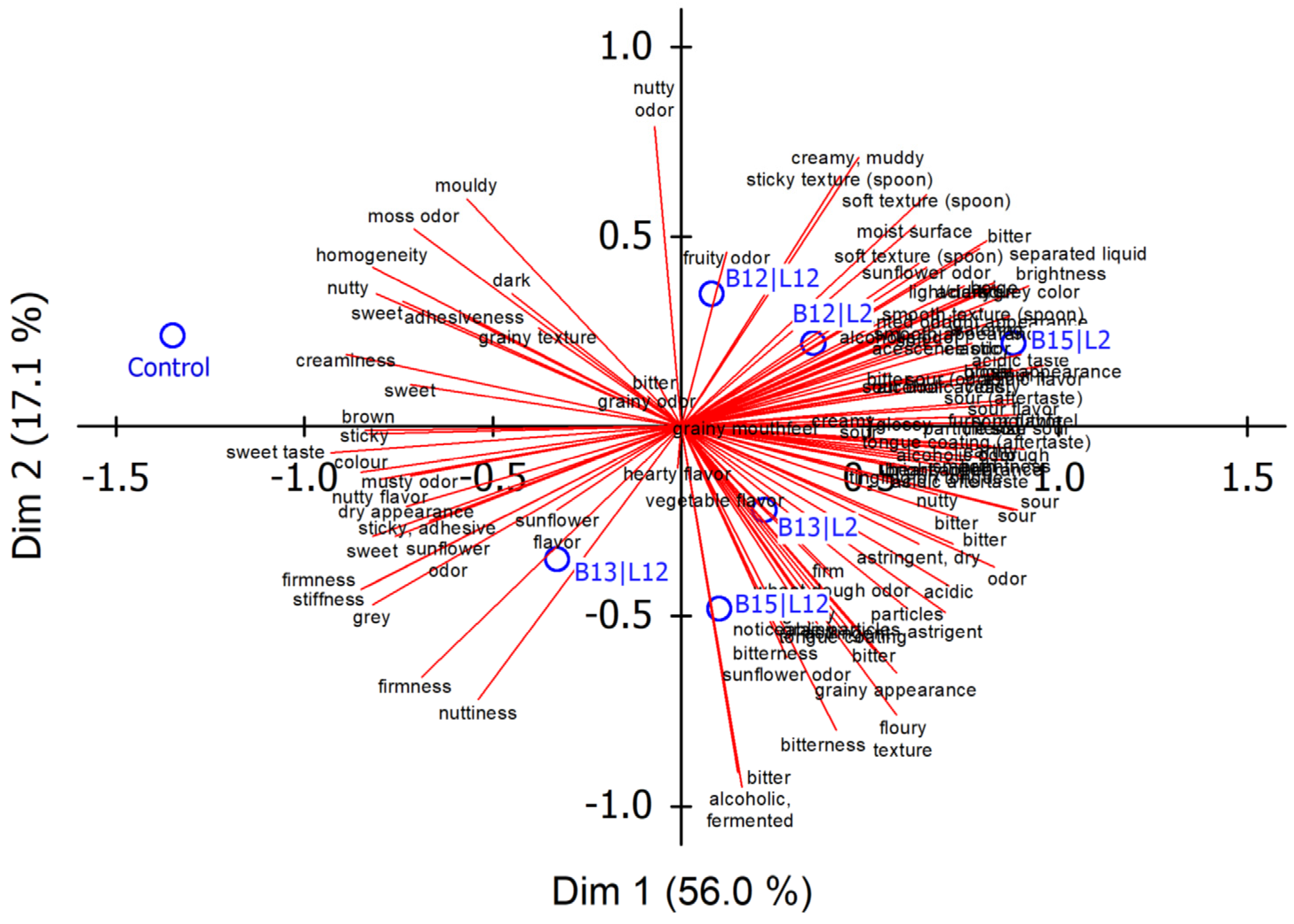
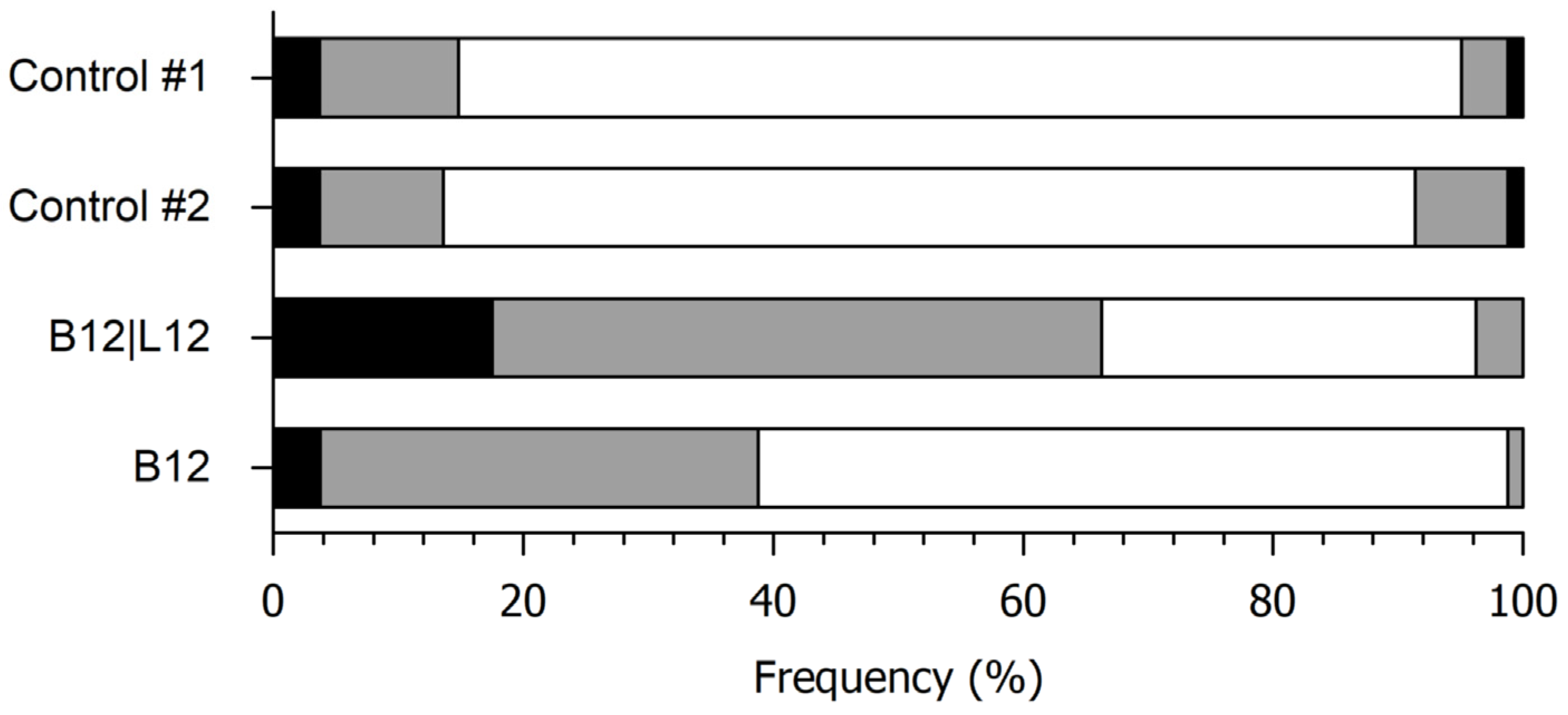
3.2.2. Sensory Discrimination of the Fermented Blends
3.2.3. Fermented Blend Viscosity
3.3. Conclusions and Implications for Subsequent Testing
- Time-based fermentation turned out to be unfeasible because of the different acidification activity of the involved LAB, and for the subsequent experiments, it was decided to ferment until pH 4.80.
- L. citreum (B13) acidified the slowest, to a pH significantly higher than for the other LAB. This might be a risk concerning product hygiene and safety, so this strain was not considered further.
- The samples fermented with mixtures of LAB and K. marxianus (L2) were more intense with respect to the ‘fermented, yeasty’ characteristic and described as more unpleasant by the subjects. Therefore, L2 was no longer considered.
- Furthermore, we decided to include a preliminary washing step for reducing bitterness induced by phenolic compounds present in the native press cake.
- The blank, unfermented sample appeared as not appropriate for being compared with fermented blends. After performing some minor experiments, it was decided to include a blend acidified to pH 4.80 by lactic acid, the main metabolite of the LAB, as a more appropriate control sample.
4. Experiment 2: Selecting Blends for the Final Sensory Evaluation
4.1. Experimental Details
4.1.1. Preparation and Fermentation of Sunflower Press Cake/Whey Blends
4.1.2. Sensory Evaluation
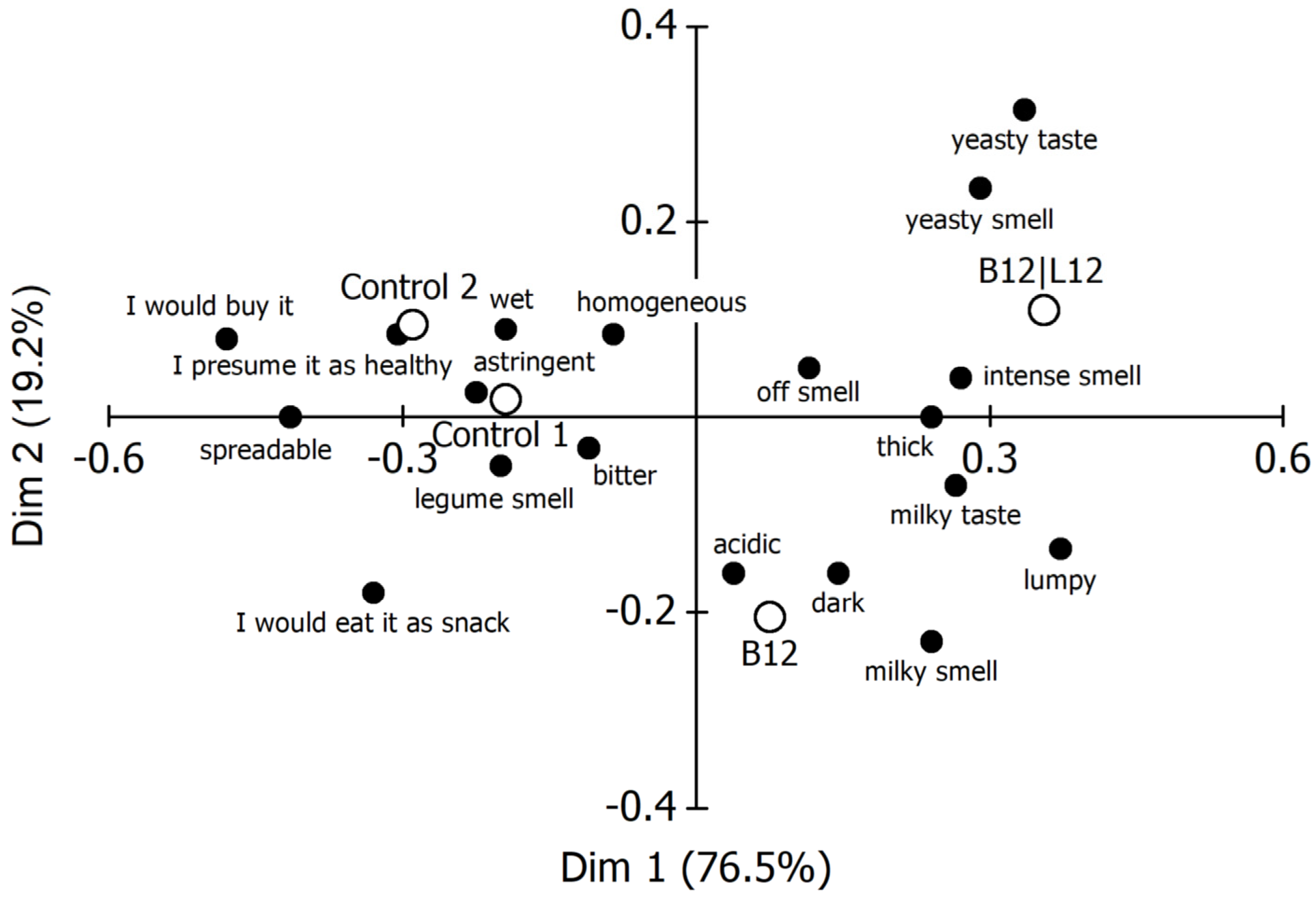
4.2. Results
4.3. Conclusions and Implications for Subsequent Testing
- Because of the explicit result of the initial discrimination test, including the verbal descriptions of the significant difference, it was decided to use blends of washed sunflower press cake and whey for the final consumer study.
- To allow including a sample for evaluating scoring consistency without introducing too much stress to the panel, we decided to eliminate also the B15|L12 consortium (P. pentosaceus + S. cerevisiae), mainly because of the ‘fermented, yeasty’ impression observed in QDA. In addition, its incubation time of approx. 70 h to reach pH 4.80 was considered as being too long.
- Based on a small questionnaire given to the subjects in the QDA test (details not given here), it was decided to include ‘Spreadability’ as the most important characteristic when using spread as a descriptor for a subsequent Check-all-that-apply test.
5. Experiment 3: Consumer Tests
5.1. Experimental Details
5.1.1. Preparation of Blends
5.1.2. Sensory Evaluation
5.2. Results
5.2.1. Chemical Analyses
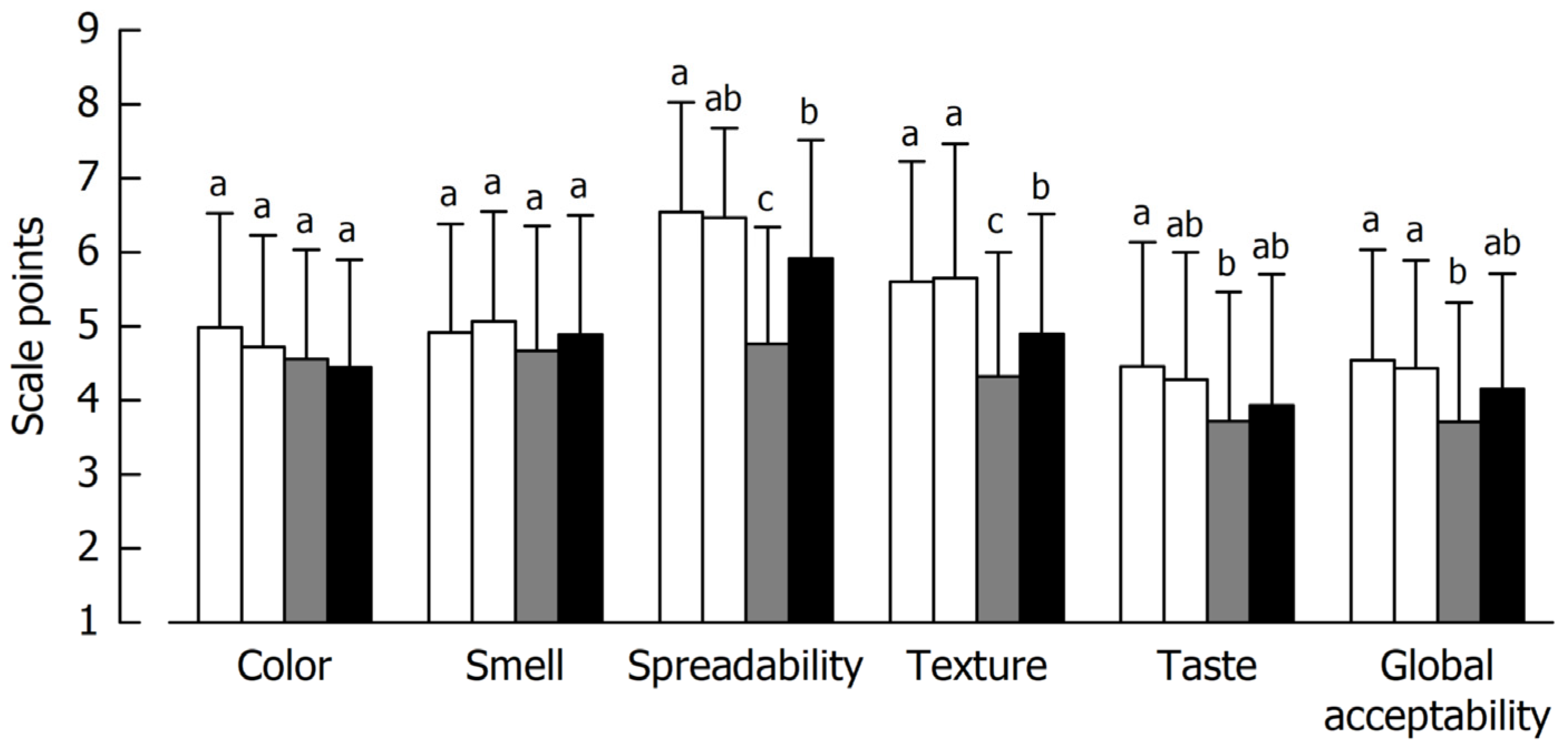
5.2.2. Hedonic Classification
5.2.3. Check-All-That-Apply Classification
5.2.4. Just-About-Right Scoring of Spreadability
6. Limitations
7. General Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cano, M.E.; García-Martin, A.; Comendador Morales, P.; Wojtusik, M.; Santos, V.E.; Kovensky, J.; Ladero, M. Production of oligosaccharides from agrofood wastes. Fermentation 2020, 6, 31. [Google Scholar] [CrossRef]
- Eyinade, G.A.; Mushunje, A.; Gbolahan Yusuf, S.F. The willingness to consume organic food: A review. Food Agric. Immunol. 2021, 32, 78–104. [Google Scholar] [CrossRef]
- Dal Gobbo, A.; Forno, F.; Magnani, N. Making “good food”more practicable? The reconfiguration of alternative food provisioning in the online world. Sust. Prod. Consumpt. 2022, 29, 862–871. [Google Scholar]
- Rohm, H.; Brennan, C.; Turner, C.; Günther, E.; Campbell, G.; Hernando, I.; Struck, S.; Kontogiorgos, V. Adding value to fruit processing waste: Innovative ways to incorporate fibers from berry pomace in baked and extruded cereal-based foods—A SUSFOOD Project. Foods 2015, 4, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Rohm, H.; Oostindjer, M.; Aschemann-Witzel, J.; Symmank, C.; Almli, V.L.; de Hooge, I.E.; Normann, A.; Karantininis, K. Consumers in a sustainable food supply chain (COSUS): Understanding consumer behavior to encourage food waste reduction. Foods 2017, 6, 104. [Google Scholar] [CrossRef]
- Russo, G.L.; Langellotti, A.L.; Blasco, T.; Oliviero, M.; Sacchi, R.; Masi, P. Production of Omega-3 oil by Aurantiochytrium mangrovei using spent osmotic solution from candied fruit industry as sole organic carbon source. Processes 2021, 9, 1834. [Google Scholar] [CrossRef]
- Viti, C.; Bellabarba, V.; Daghio, M.; Mengoni, A.; Mele, M.; Buccioni, A.; Pacini, G.C.; Bekki, A.; Azim, K.; Hafidi, H.; et al. Alfalfa for a sustainable ovine farming system: Proposed research for a new feeding strategy based on alfalfa and ecological leftovers in drought conditions. Sustainability 2021, 13, 3880. [Google Scholar] [CrossRef]
- Raak, N.; Struck, S.; Jaros, D.; Hernando, I.; Gülseren, I.; Michalska-Ciechanowska, A.; Foschino, R.; Corredig, M.; Rohm, H. Blending side streams. A potential solution to reach a resource efficient, circular, zero-waste food system. Future Foods 2022, 6, 100207. [Google Scholar] [CrossRef]
- Hagman, L. Sustainable side-stream management in Swedish food processing companies using external actors and biogas solutions. Front. Food Sci. Technol. 2023, 3, 1073663. [Google Scholar] [CrossRef]
- Arrutia, F.; Binner, E.; Williams, P.; Waldron, K.W. Oilseeds beyond oil: Press cakes and meals supplying global protein requirements. Trends Food Sci. Technol. 2020, 100, 88–102. [Google Scholar] [CrossRef]
- Baümler, E.R.; Carrin, M.E.; Carelli, A.A. Extraction of sunflower oil using ethanol as solvent. J. Food Eng. 2016, 178, 190–197. [Google Scholar] [CrossRef]
- Ahlström, C.; Thuvander, J.; Rayner, M.; Matos, M.; Gutiérrez, G.; Östbring, K. The effect of precipitation pH on protein recovery yield and emulsifying properties in the extraction of protein from cold-pressed rapeseed press cake. Molecules 2022, 27, 2957. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.W.; Miklavčič Višnjevec, A.; Krienke, D.; Preskett, D.; Schwarzkopf, M.; Charlton, A. Pilot scale extraction of protein from cold and hot-pressed rapeseed cake: Preliminary studies on the effect of upstream mechanical processing. Food Bioprod. Proc. 2022, 133, 132–139. [Google Scholar] [CrossRef]
- Parodi, E.; La Nasa, J.; Ribechini, E.; Petri, A.; Piccolo, O. Extraction of proteins and residual oil from flax (Linum usitatissimum), camelina (Camelina sativa), and sunflower (Helianthus annuus) oilseed press cakes. Biomass Conv. Bioref. 2023, 13, 1915–1926. [Google Scholar] [CrossRef]
- Sert, D.; Rohm, H.; Struck, S. Ultrasound-assisted extraction of protein from pumpkin seed press cake: Impact on protein yield and techno-functionality. Foods 2022, 11, 4029. [Google Scholar] [CrossRef] [PubMed]
- Cabral Ribeiro, P.P.; Xavier Junior, F.H.; Gleyse do Nascimento Silva, C.; Bezerra da Silva, T.M.; Mota Corrêa, B.B.; Oliveira de Veras, B.; Tribuzy de Magalhães Cordeiro, A.M.; de Andrade Vieira, E.; Canindé de Sousa Júnior, F.; Florentino da Silva Chaves Damasceno, K.S.; et al. Faveleira (Cnidoscolus quercifolius Pohl) seed press cake flour: Production, characterization and application for use in cookies. J. Sci. Food Agric. 2023, 103, 627–636. [Google Scholar] [CrossRef]
- Čolić, L.M.; Jukić, M.; Nakov, G.; Lukinac, J.; Antunović, M. Sustainable utilization of hemp press cake flour in ice cream production: Physicochemical, rheological, textural, and sensorial properties. Sustainability 2024, 16, 8354. [Google Scholar] [CrossRef]
- Eurostat. Agriculture, Forestry and Fishery Statistics; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar]
- Nicolás, P.; Luján Ferreira, M.; Lassalle, V. A review of magnetic separation of whey proteins and potential application to whey proteins recovery, isolation and utilization. J. Food Eng. 2019, 246, 7–15. [Google Scholar] [CrossRef]
- Ostertag, F.; Schmidt, C.M.; Berensmeier, S.; Hinrichs, J. Development and validation of an RP-HPLC DAD method for the simultaneous quantification of minor and major whey proteins. Food Chem. 2021, 342, 128176. [Google Scholar] [CrossRef]
- Pires, A.F.; Marnotes, N.G.; Rubio, O.D.; Garcia, A.C.; Pereira, C.D. Dairy byproducts: A review on the valorization of whey and second cheese whey. Foods 2021, 10, 1067. [Google Scholar] [CrossRef]
- Lappa, I.; Papadaki, A.; Kachrimanidou, V.; Terpou, A.; Koulougliotis, D.; Eriotou, E.; Kopsahelis, N. Cheese whey processing: Integrated biorefinery concepts and emerging food applications. Foods 2019, 8, 347. [Google Scholar] [CrossRef]
- Paraman, I.; Sharif, M.K.; Supriyadi, S.; Rizvi, S.S.H. Agro-food industry by-products in to value-added extruded foods. Food Bioprod. Proc. 2015, 96, 78–85. [Google Scholar] [CrossRef]
- Morejón Caraballo, S.; Trültzsch, S.; Rohm, H.; Struck, S. Dry fractionation of sunflower press cake as tool to improve its technofunctional properties. LWT—Food Sci. Technol. 2024, 214, 117148. [Google Scholar] [CrossRef]
- Raak, N.; Corredig, M. Towards creating sustainable foods from side streams: Heat-induced structure formation in blends of sunflower seed press cakes and cheese whey under moderate shear. Food Hydrocoll. 2023, 144, 108932. [Google Scholar] [CrossRef]
- Mangieri, N.; Rosciano, G.; Porcellato, D.; Winther, A.R.; De Noni, I.; Fracassetti, D.; Foschino, R.; Vigentini, I. Sustainability of food side streams: A case study of fermented blends made with sour whey and sunflower press cake powder using the back-slopping technique. Front. Sustain. Food Syst. 2023, 7, 1166002. [Google Scholar] [CrossRef]
- Raak, N.; Mangieri, N.; Foschino, R.; Corredig, M. Shaping future foods through fermentation of side streams: Microbial, chemical, and physical characteristics of fermented blends from sunflower seed press cake and cheese whey. Foods 2023, 12, 4099. [Google Scholar] [CrossRef]
- Michalska-Ciechanowska, A.; Masztalerz, K.; Mangieri, N.; Foschino, R.; Lech, K.; Wojdyło, A.; Nowicka, P.; Brzezowska, J. Unlocking the potential of oil by-products: Investigating the influence of co-culture fermentation on phenolic acid degradation in side stream blends from oil and dairy industry. Future Foods 2024, 9, 100324. [Google Scholar] [CrossRef]
- Michalska-Ciechanowska, A.; Brzezowska, J.; Lech, K.; Masztalerz, K.; Korzeniowska, M.; Zambrowicz, A.; Szoltysik, M. Exploiting the potential of powdered blends of recovered sunflower seed cake phenolics and whey—Development of sustainable food additives. Foods 2024, 13, 1433. [Google Scholar] [CrossRef]
- Günes, Z.S.; Raak, N.; Corredig, M.; Gülseren, I. The influence of pectinase treatment on the characteristics of pre-treated and lyophilized sunflower meal-whey blends. Int. J. Food Sci. Technol. 2024, 59, 4562–4571. [Google Scholar] [CrossRef]
- ISO 15214; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Mesophilic Lactic Acid Bacteria—Colony-Count Technique at 30 °C. International Organization for Standardization: Geneva, Switzerland, 1998.
- ISO 21871; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Determination of Low Numbers of Presumptive Bacillus Cereus—Most Probable Number Technique and Detection Method. International Organization for Standardization: Geneva, Switzerland, 2006.
- ISO 6888-1; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus Aureus and Other Species). Part 1: Method using Baird-Parker agar medium. International Organization for Standardization: Geneva, Switzerland, 2021.
- ISO 16649-1; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia coli, Part 1: Colony-Count Technique at 44 Degrees C Using Membranes and 5-Bromo-4-Chloro-3-Indolyl Beta-D-Glucuronide. International Organization for Standardization: Geneva, Switzerland, 2018.
- ISO 21527-1; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds. Part 1: Colony Count Technique in Products with Water Activity Greater than 0.95. International Organization for Standardization: Geneva, Switzerland, 2008.
- ISO Standard 11290-1; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria Monocytogenes and of Listeria spp. Part 1: Detection Method. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 6579-1; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella Part 1: Detection of Salmonella spp. International Organization for Standardization: Geneva, Switzerland, 2017.
- Biggs, D.A.; Szijarto, L. Method for routine determination of lactose in milk. J. Dairy Sci. 1963, 46, 1196–1200. [Google Scholar] [CrossRef]
- Jaros, D.; Thamke, I.; Raddatz, H.; Rohm, H. Single-cultivar cloudy juice made from table apples: An attempt to identify the driving force for sensory preference. Eur. Food Res. Technol. 2009, 229, 51–61. [Google Scholar] [CrossRef]
- ISO Standard 8586; Sensory analysis—Selection and Training of Sensory Assessors. International Organization for Standardization: Geneva, Switzerland, 2023.
- Deliza, R.; MacFie, H.; Hedderley, D. The consumer sensory perception of passion-fruit juice using free-choice profiling. J. Sensory Stud. 2005, 20, 17–27. [Google Scholar] [CrossRef]
- Lachnit, M.; Busch-Stockfisch, M.; Kunert, J.; Krahl, T. Suitability of free choice profiling for assessment of orange-based carbonated soft-drinks. Food Qual. Pref. 2003, 14, 257–263. [Google Scholar] [CrossRef]
- ISO 4120; Sensory Analysis—Methodology—Triangle Test. International Organization for Standardization: Geneva, Switzerland, 2021.
- Powers, J.J. Current practices and applications of descriptive methods. In Sensory Analysis of Foods, 2nd ed.; Piggott, J.R., Ed.; Elsevier Applied Science: London, UK, 1988; pp. 187–265. [Google Scholar]
- Good Kitzberger, S.C.; dos Santos Scholz, M.B.; Gonçalves Dias da Silva, J.B.; de Toledo Benassi, M.; Protasio Pereira, L.F. Free choice profiling sensory analysis to discriminate coffees. AIMS Agricult. Food 2016, 1, 455–469. [Google Scholar] [CrossRef]
- Thamke, I.; Dürrschmid, K.; Rohm, H. Sensory description of dark chocolate by consumers. LWT—Food Sci. Technol. 2009, 42, 534–539. [Google Scholar] [CrossRef]
- O’Mahony, M. Sensory Evaluation of Food; Marcel Dekker: New York, NY, USA, 1986. [Google Scholar]
- Surber, G.; Schäfer, C.; Wefers, D.; Rohm, H.; Jaros, D. Exopolysaccharides from Lactococcus lactis affect manufacture, texture and sensory properties of concentrated acid milk gel suspensions (fresh cheese). Int. Dairy J. 2021, 112, 104854. [Google Scholar] [CrossRef]
- Mendo, S.; Da Costa, I.; Cattaneo, S.; Masotti, F.; Stuknytė, M.; De Noni, I.; Foschino, R. Fermented blend from sunflower seed press-cake and bovine sweet whey: Protein breakdown during in vitro gastrointestinal digestion. Food Chem. X 2024, 23, 101745. [Google Scholar] [CrossRef]
- ISO 5534; Cheese and Processed Cheese—Determination of the Total Solids Content (Reference Method). International Organization for Standardization: Geneva, Switzerland, 2004.
- Lawless, H.; Heymann, H. Sensory Evaluation of Food: Principles and Practices. Springer: New York, NY, USA, 2010. [Google Scholar]
- Rothman, L.; Parker, M.J. Just-About-Right (JAR) Scales: Design, Usage, Benefits and Risks; ASTM International: West Consohocken, PA, USA, 2011. [Google Scholar]
- Xiong, R.; Meullenet, J.F. A PLS dummy variable approach to assess the impact of jar attributes on liking. Food Qual. Pref. 2006, 17, 188–198. [Google Scholar] [CrossRef]
- Zajsek, K.; Gorsek, A. Effect of natural starter culture activity on ethanol content in fermented dairy products. Int. J. Dairy Technol. 2010, 23, 113–118. [Google Scholar] [CrossRef]
- Bower, J.A.; Boyd, R. Effect of health concern and consumption patterns on measures of sweetness by hedonic and just-about-right scales. J. Sensory Stud. 2003, 18, 235–248. [Google Scholar] [CrossRef]
- Epler, S.; Chambers, E., IV; Kemp, K.E. Hedonic scales are a better predictor than just-about-right scales of optimal sweetness in lemonade. J. Sensory Stud. 1998, 13, 191–197. [Google Scholar] [CrossRef]



| Code | Species | Origin |
|---|---|---|
| B12 | Lactococcus lactis | Commercial milk kefir |
| B13 | Leuconostoc citreum | Homemade sugary kefir |
| B15 | Pediococcus pentosaceus | Blend of sunflower press cake and reconstituted whey |
| L2 | Kluyveromyces lactis | Household milk kefir |
| L12 | Saccharomyces cerevisiae | Homemade kvass |
| Strain Association | B12|L2 | B12|L12 | B13|L2 | B13|L12 | B15|L2 | B15|L12 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Batch | #1 | #2 | #1 | #2 | #1 | #2 | #1 | #2 | #1 | #2 | #1 | #2 |
| pH after fermentation | 4.57 | 4.54 | 4.66 | 4.59 | 4.81 | 5.20 | 5.23 | 5.29 | 4.45 | 4.47 | 4.72 | 5.02 |
| LAB (log CFU/g) at 0 h | 6.60 | 5.91 | 6.66 | 6.00 | 6.74 | 6.10 | 6.73 | 6.10 | 6.06 | 6.06 | 6.16 | 6.09 |
| LAB (log CFU/g) after fermentation | 9.31 | 9.67 | 9.38 | 9.44 | 9.50 | 9.63 | 9.50 | 9.50 | 9.35 | 9.56 | 9.37 | 9.08 |
| Yeasts (log CFU/g) at 0 h | 4.96 | 5.06 | 5.34 | 5.08 | 5.06 | 5.14 | 5.12 | 5.09 | 5.00 | 5.06 | 5.16 | 5.19 |
| Yeasts (log CFU/g) after fermentation | 6.65 | 7.15 | 7.39 | 7.10 | 6.24 | 6.48 | 6.89 | 6.89 | 7.27 | 7.30 | 7.49 | 7.00 |
| B. cereus (log CFU/g) at 0 h | <2.00 | <2.00 | <2.00 | <2.00 | <2.00 | <2.00 | <2.00 | <2.00 | <2.00 | <2.00 | <2.00 | <2.00 |
| B. cereus (log CFU/g) after fermentation | <2.00 | <2.00 | <2.00 | <2.00 | <2.00 | <2.00 | <2.00 | <2.00 | <2.00 | <2.00 | <2.00 | >2.00 * |
| Title 1 | B12|L12 | B12 | Control (Chemical Acidification) |
|---|---|---|---|
| Sugars (g/100 g) Galactose Glucose Lactose Raffinose Sucrose | |||
| n.d. | n.d. | 0.11 ± 0.02 | |
| n.d. 3.36 ± 0.09 n.d. n.d. | n.d. 3.37 ± 0.01 0.10 ± 0.05 0.18 ± 0.03 | 0.06 ± 0.02 4.53 ± 0.01 0.07 ± 0.04 0.14 ± 0.06 | |
| Organic acids and alcohol (g/100 g) Acetic acid Lactic acid Ethanol | 0.02 ± 0.00 1.54 ± 0.12 | 0.02 ± 0.00 1.52 ± 0.07 | n.d. 1.55 ± 0.03 |
| 0.12 ± 0.03 | n.d. | n.d. | |
| Viable counts (log CFU/g) Lactic acid bacteria Yeasts | |||
| 9.26 | 8.61 | <2 | |
| 7.06 | <2 | <2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rohm, H.; Morejón Caraballo, S.; Salvador, A.; Mendo, S.; Llorca, E.; Cattaneo, S.; De Noni, I.; Struck, S.; Foschino, R.; Hernando, I. Sensory Properties of Fermented Blends of Sunflower Press Cake and Whey. Foods 2025, 14, 1489. https://doi.org/10.3390/foods14091489
Rohm H, Morejón Caraballo S, Salvador A, Mendo S, Llorca E, Cattaneo S, De Noni I, Struck S, Foschino R, Hernando I. Sensory Properties of Fermented Blends of Sunflower Press Cake and Whey. Foods. 2025; 14(9):1489. https://doi.org/10.3390/foods14091489
Chicago/Turabian StyleRohm, Harald, Sophie Morejón Caraballo, Ana Salvador, Sofia Mendo, Empar Llorca, Stefano Cattaneo, Ivano De Noni, Susanne Struck, Roberto Foschino, and Isabel Hernando. 2025. "Sensory Properties of Fermented Blends of Sunflower Press Cake and Whey" Foods 14, no. 9: 1489. https://doi.org/10.3390/foods14091489
APA StyleRohm, H., Morejón Caraballo, S., Salvador, A., Mendo, S., Llorca, E., Cattaneo, S., De Noni, I., Struck, S., Foschino, R., & Hernando, I. (2025). Sensory Properties of Fermented Blends of Sunflower Press Cake and Whey. Foods, 14(9), 1489. https://doi.org/10.3390/foods14091489











