Where Do Milk Microbes Originate? Traceability of Microbial Community Structure in Raw Milk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. DNA Preparation, PCR Amplification and Sequencing
2.3. Sequence Analysis and Statistics
2.4. Nucleotide Sequence Availability
3. Results
3.1. Quality Safety Index Analysis of Raw Milk Samples
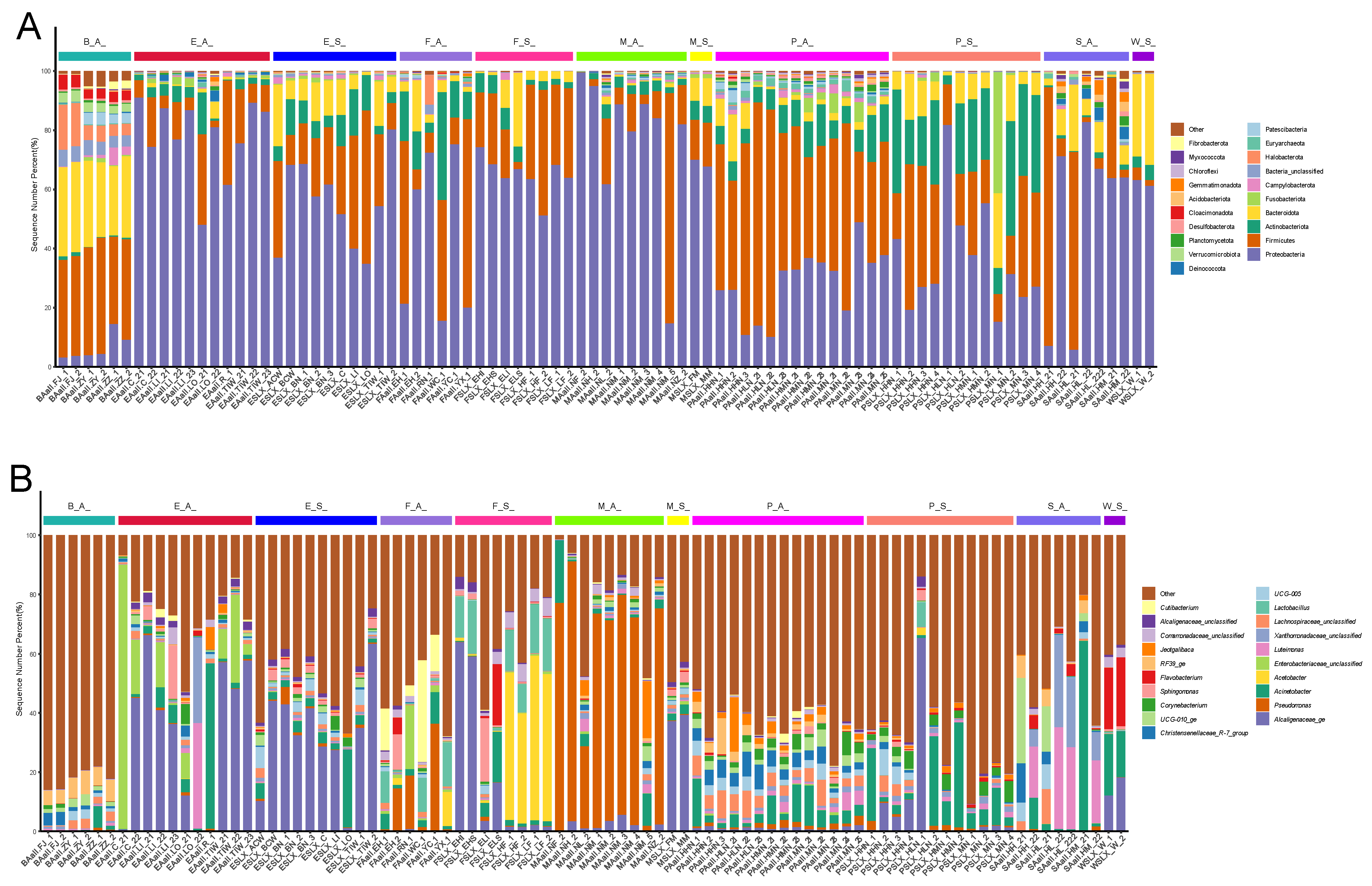
3.2. Variations in Microbial Abundance and Composition Across Sample Types
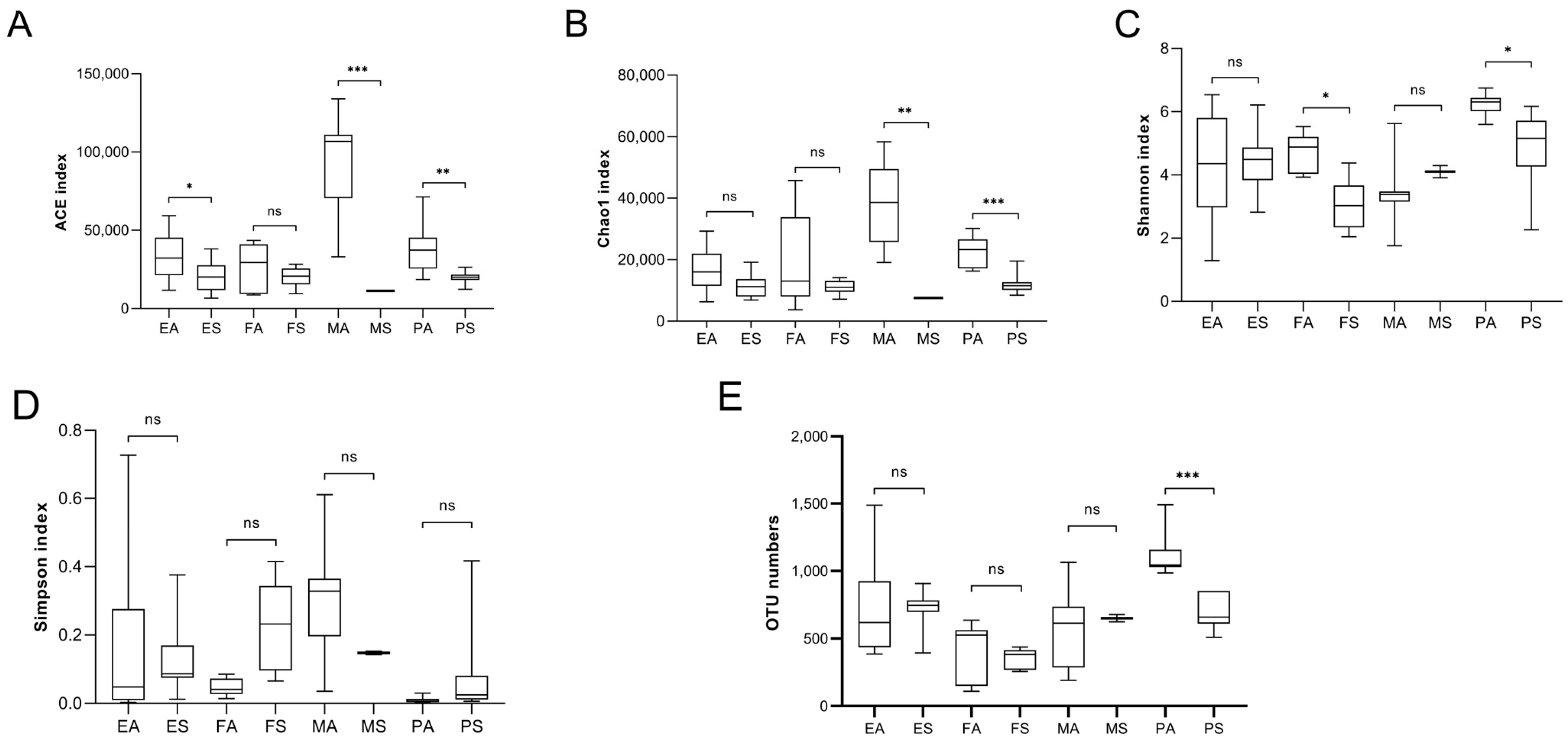
3.3. OTU-Based Analysis
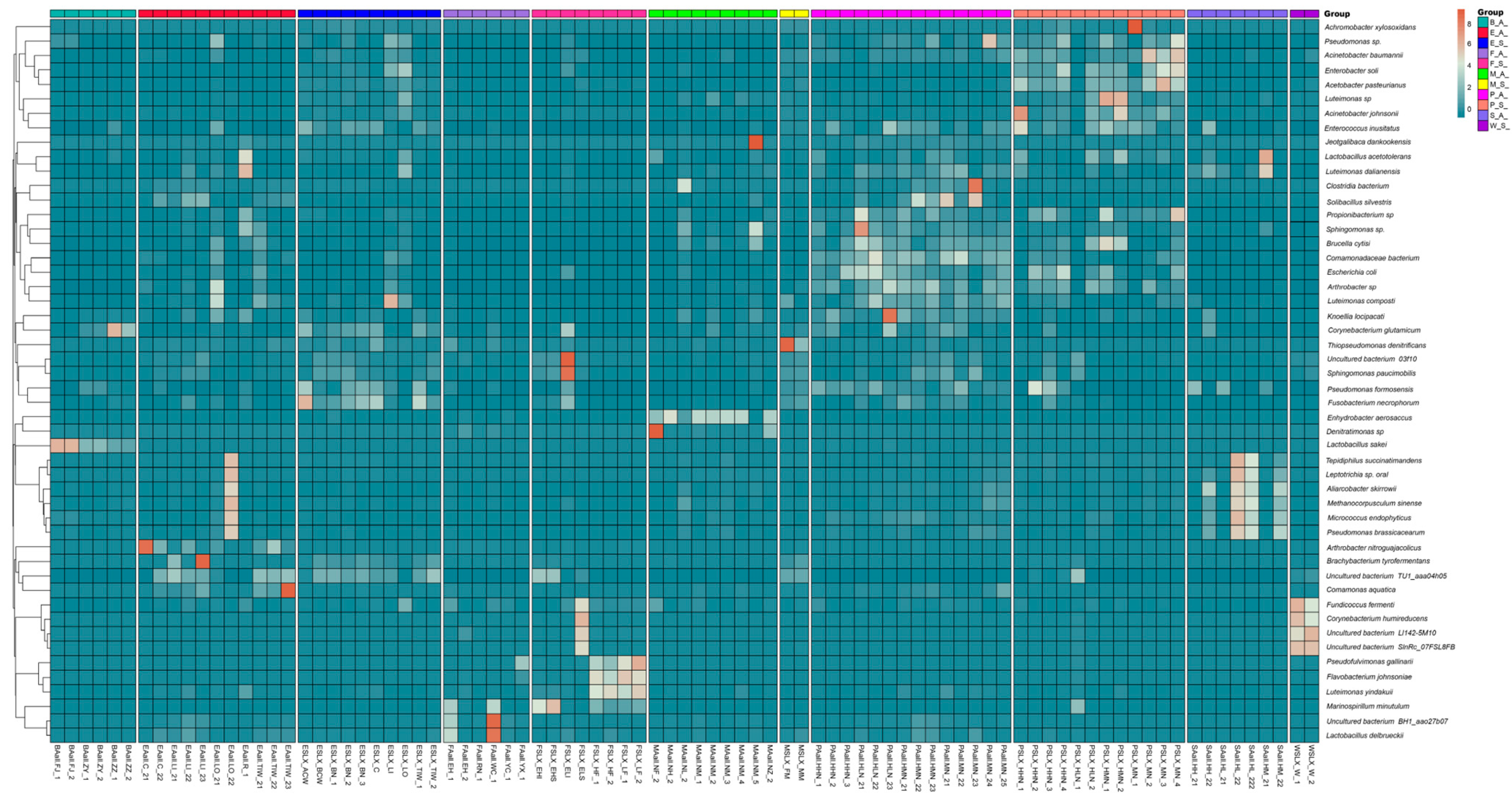
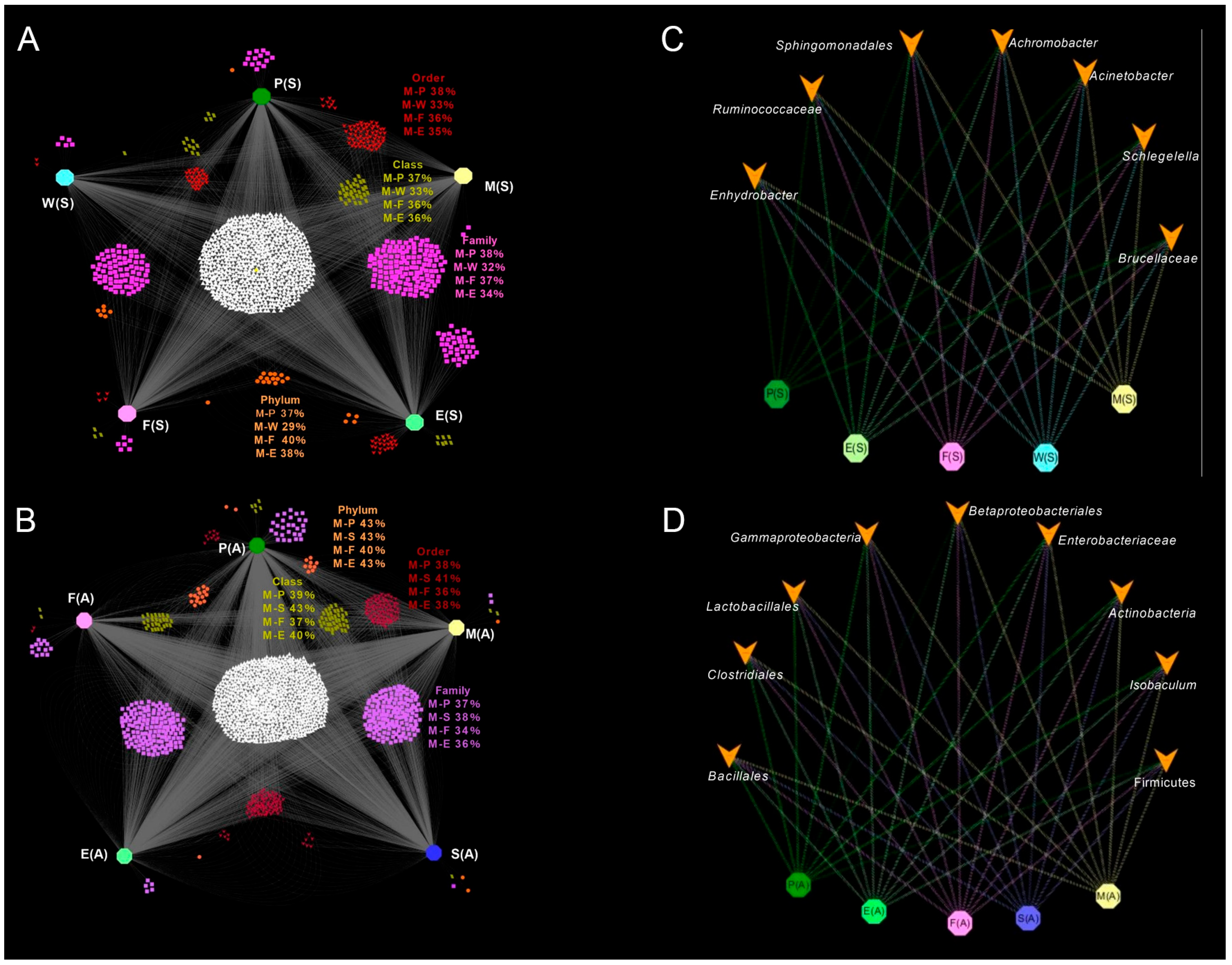
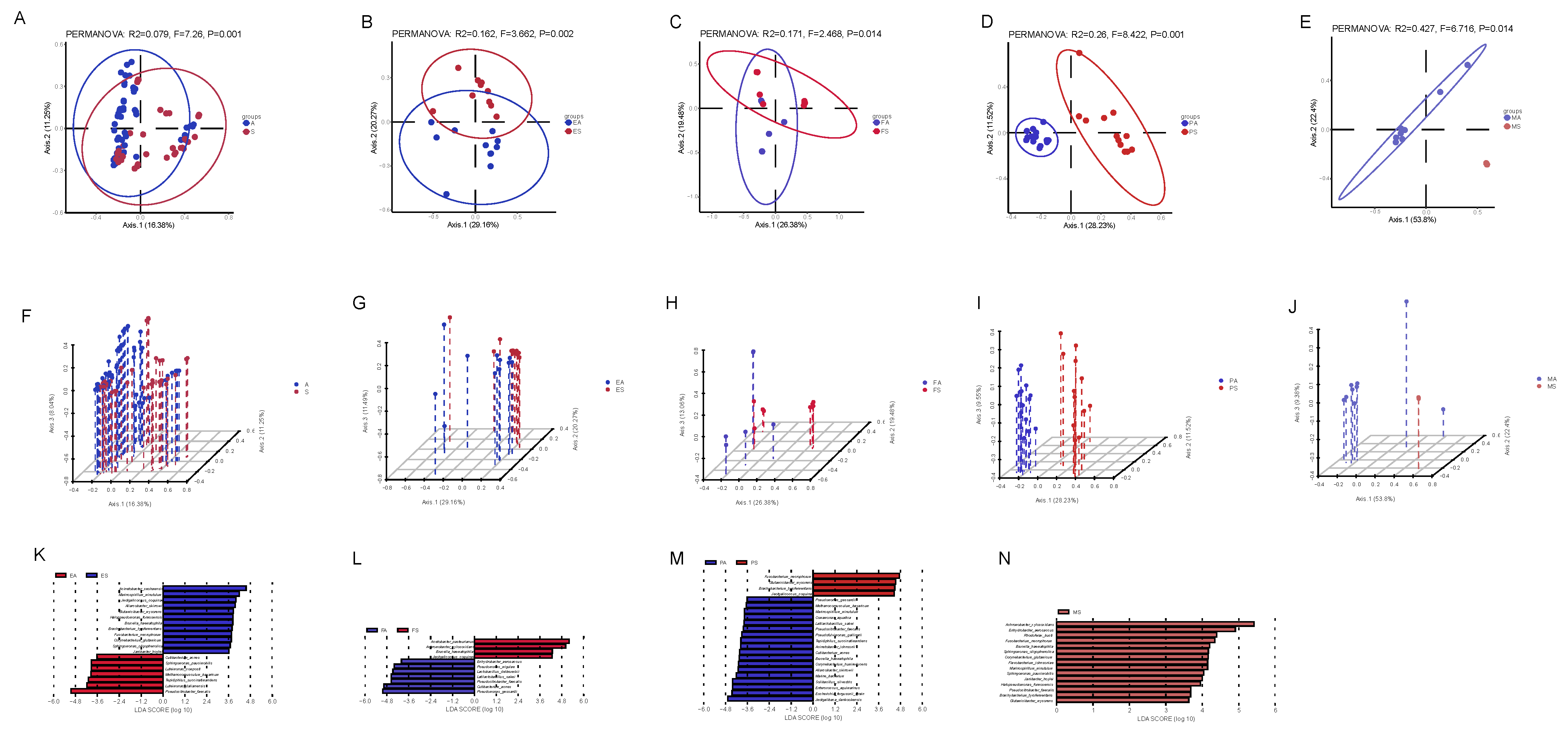
3.4. Relationship Between Raw Milk and Other Types of Sample Microbial Communities
3.5. Seasonal Differences in Microbial Communities of Raw Milk and Other Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fardellone, P.; Sejourne, A.; Blain, H.; Cortet, B.; Thomas, T.; Committee, G.S. Osteoporosis: Is milk a kindness or a curse? Jt. Bone Spine 2017, 84, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Gaucheron, F. Milk and Dairy Products: A Unique Micronutrient Combination. J. Am. Coll. Nutr. 2011, 30, 400s–409s. [Google Scholar] [CrossRef] [PubMed]
- Antunes, I.C.; Bexiga, R.; Pinto, C.; Roseiro, L.C.; Quaresma, M.A.G. Cow’s Milk in Human Nutrition and the Emergence of Plant-Based Milk Alternatives. Foods 2022, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Liu, Y.; Yang, S.; Liu, Y.; Shi, H.; Yue, X.; Wu, R.; Wu, J. High-throughput sequencing provides new insights into the roles and implications of core microbiota present in pasteurized milk. Food Res. Int. 2020, 137, 109586. [Google Scholar] [CrossRef]
- Li, Y.; Weng, P.; Wu, Z.; Liu, Y. Extending the Shelf Life of Raw Milk and Pasteurized Milk with Plantaricin FB-2. Foods 2023, 12, 608. [Google Scholar] [CrossRef]
- Endara, P.; Wiedmann, M.; Adalja, A. Consumer willingness to pay for shelf life of high-temperature, short-time-pasteurized fluid milk: Implications for smart labeling and food waste reduction. J. Dairy Sci. 2023, 106, 5940–5957. [Google Scholar] [CrossRef]
- Li, N.; Wang, Y.; You, C.; Ren, J.; Chen, W.; Zheng, H.; Liu, Z. Variation in Raw Milk Microbiota Throughout 12 Months and the Impact of Weather Conditions. Sci. Rep. 2018, 8, 2371. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, Y.; Jay-Russell, M.; Lemay, D.G.; Mills, D.A. Reservoirs of antimicrobial resistance genes in retail raw milk. Microbiome 2020, 8, 99. [Google Scholar] [CrossRef]
- Ranvir, S.; Sharma, R.; Gandhi, K.; Mann, B. Assessment of physico-chemical changes in UHT milk during storage at different temperatures. J. Dairy Res. 2020, 87, 243–247. [Google Scholar] [CrossRef]
- Porcellato, D.; Aspholm, M.; Skeie, S.B.; Monshaugen, M.; Brendehaug, J.; Mellegård, H. Microbial diversity of consumption milk during processing and storage. Int. J. Food Microbiol. 2018, 266, 21–30. [Google Scholar] [CrossRef]
- Zhuang, J.; Hou, Y.; Wang, Y.; Gao, Y.; Chen, Y.; Qi, J.; Li, P.; Bian, Y.; Ju, N. Relationship between microorganisms and milk metabolites during quality changes in refrigerated raw milk: A metagenomic and metabolomic exploration. Int. J. Food Microbiol. 2024, 425, 110891. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Wu, S.; Du, Q.; Min, L. The Investigation of Changes in Bacterial Community of Pasteurized Milk during Cold Storage. Foods 2024, 13, 451. [Google Scholar] [CrossRef] [PubMed]
- Verdier-Metz, I.; Gagne, G.; Bornes, S.; Monsallier, F.; Veisseire, P.; Delbès-Paus, C.; Montel, M.-C. Cow Teat Skin, a Potential Source of Diverse Microbial Populations for Cheese Production. Appl. Environ. Microbiol. 2012, 78, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Doyle Conor, J.; Gleeson, D.; O’Toole Paul, W.; Cotter Paul, D. Impacts of Seasonal Housing and Teat Preparation on Raw Milk Microbiota: A High-Throughput Sequencing Study. Appl. Environ. Microbiol. 2016, 83, e02694-16. [Google Scholar] [CrossRef]
- O’Connell, A.; McParland, S.; Ruegg, P.L.; O’Brien, B.; Gleeson, D. Seasonal trends in milk quality in Ireland between 2007 and 2011. J. Dairy Sci. 2015, 98, 3778–3790. [Google Scholar] [CrossRef]
- Sevi, A.; Albenzio, M.; Muscio, A.; Casamassima, D.; Centoducati, P. Effects of litter management on airborne particulates in sheep houses and on the yield and quality of ewe milk. Livest. Prod. Sci. 2003, 81, 1–9. [Google Scholar] [CrossRef]
- Vacheyrou, M.; Normand, A.C.; Guyot, P.; Cassagne, C.; Piarroux, R.; Bouton, Y. Cultivable microbial communities in raw cow milk and potential transfers from stables of sixteen French farms. Int. J. Food Microbiol. 2011, 146, 253–262. [Google Scholar] [CrossRef]
- Amagliani, G.; Petruzzelli, A.; Omiccioli, E.; Tonucci, F.; Magnani, M.; Brandi, G. Microbiological Surveillance of a Bovine Raw Milk Farm Through Multiplex Real-Time PCR. Foodborne Pathog. Dis. 2012, 9, 406–411. [Google Scholar] [CrossRef]
- Van Kessel, J.A.S.; Karns, J.S.; Lombard, J.E.; Kopral, C.A. Prevalence of Virulence and Factors in Bulk Tank Milk and In-Line Filters from US Dairies. J. Food Prot. 2011, 74, 759–768. [Google Scholar] [CrossRef]
- Oultram, J.W.H.; Ganda, E.K.; Boulding, S.C.; Bicalho, R.C.; Oikonomou, G. A Metataxonomic Approach Could Be Considered for Cattle Clinical Mastitis Diagnostics. Front. Vet. Sci. 2017, 4, 36. [Google Scholar] [CrossRef]
- Makovec, J.A.; Ruegg, P.L. Results of milk samples submitted for microbiological examination in Wisconsin from 1994 to 2001. J. Dairy Sci. 2003, 86, 3466–3472. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, G.; Teixeira, A.G.V.; Foditsch, C.; Bicalho, M.L.; Machado, V.S.; Bicalho, R.C. Fecal Microbial Diversity in Pre-Weaned Dairy Calves as Described by Pyrosequencing of Metagenomic 16S rDNA. Associations of Faecalibacterium Species with Health and Growth. PLoS ONE 2013, 8, e63157. [Google Scholar] [CrossRef] [PubMed]
- Falentin, H.; Rault, L.; Nicolas, A.; Bouchard, D.S.; Lassalas, J.; Lamberton, P.; Aubry, J.-M.; Marnet, P.-G.; Le Loir, Y.; Even, S. Bovine Teat Microbiome Analysis Revealed Reduced Alpha Diversity and Significant Changes in Taxonomic Profiles in Quarters with a History of Mastitis. Front. Microbiol. 2016, 7, 480. [Google Scholar] [CrossRef]
- Kable Mary, E.; Srisengfa, Y.; Laird, M.; Zaragoza, J.; McLeod, J.; Heidenreich, J.; Marco Maria, L. The Core and Seasonal Microbiota of Raw Bovine Milk in Tanker Trucks and the Impact of Transfer to a Milk Processing Facility. mBio 2016, 7, e00836-16. [Google Scholar] [CrossRef]
- Oikonomou, G.; Machado, V.S.; Santisteban, C.; Schukken, Y.H.; Bicalho, R.C. Microbial Diversity of Bovine Mastitic Milk as Described by Pyrosequencing of Metagenomic 16s rDNA. PLoS ONE 2012, 7, e47671. [Google Scholar] [CrossRef]
- Oikonomou, G.; Bicalho, M.L.; Meira, E.; Rossi, R.E.; Foditsch, C.; Machado, V.S.; Teixeira, A.G.V.; Santisteban, C.; Schukken, Y.H.; Bicalho, R.C. Microbiota of Cow’s Milk; Distinguishing Healthy, Sub-Clinically and Clinically Diseased Quarters. PLoS ONE 2014, 9, e85904. [Google Scholar] [CrossRef]
- Metzger, S.A.; Hernandez, L.L.; Suen, G.; Ruegg, P.L. Understanding the Milk Microbiota. Vet. Clin. N. Am. Food Anim. Pract. 2018, 34, 427–438. [Google Scholar] [CrossRef]
- Brunk Clifford, F.; Eis, N. Quantitative Measure of Small-Subunit rRNA Gene Sequences of the Kingdom Korarchaeota. Appl. Environ. Microbiol. 1998, 64, 5064–5066. [Google Scholar] [CrossRef]
- Reysenbach, A.L.; Giver, L.J.; Wickham, G.S.; Pace, N.R. Differential amplification of rRNA genes by polymerase chain reaction. Appl. Environ. Microbiol. 1992, 58, 3417–3418. [Google Scholar] [CrossRef]
- Schloss Patrick, D.; Westcott Sarah, L.; Ryabin, T.; Hall Justine, R.; Hartmann, M.; Hollister Emily, B.; Lesniewski Ryan, A.; Oakley Brian, B.; Parks Donovan, H.; Robinson Courtney, J.; et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Pruesse, E.; Quast, C.; Knittel, K.; Fuchs, B.M.; Ludwig, W.; Peplies, J.; Glöckner, F.O. SILVA: A comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007, 35, 7188–7196. [Google Scholar] [CrossRef] [PubMed]
- Bailey, V.L.; Fansler, S.J.; Stegen, J.C.; McCue, L.A. Linking microbial community structure to β-glucosidic function in soil aggregates. ISME J. 2013, 7, 2044–2053. [Google Scholar] [CrossRef] [PubMed]
- Falardeau, J.; Keeney, K.; Trmcic, A.; Kitts, D.; Wang, S.Y. Farm-to-fork profiling of bacterial communities associated with an artisan cheese production facility. Food Microbiol. 2019, 83, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Regasa, S.; Mengistu, S.; Abraha, A. Milk Safety Assessment, Isolation, and Antimicrobial Susceptibility Profile of Staphylococcus aureus in Selected Dairy Farms of Mukaturi and Sululta Town, Oromia Region, Ethiopia. Vet. Med. Int. 2019, 2019, 3063185. [Google Scholar] [CrossRef]
- Orwa, J.D.; Matofari, J.W.; Muliro, P.S. Handling practices and microbial contamination sources of raw milk in rural and peri urban small holder farms in Nakuru County, Kenya. Int. J. Livest. Prod. 2017, 8, 5–11. [Google Scholar] [CrossRef]
- Perkins, N.R.; Kelton, D.F.; Leslie, K.E.; Hand, K.J.; MacNaughton, G.; Berke, O. An analysis of the relationship between wash water quality and bulk tank milk quality on Ontario dairy farms. J. Dairy Sci. 2007, 90, 330. [Google Scholar] [CrossRef]
- Jayarao, B.M.; Pillai, S.R.; Sawant, A.A.; Wolfgang, D.R.; Hegde, N.V. Guidelines for monitoring bulk tank milk somatic cell and bacterial counts. J. Dairy Sci. 2004, 87, 3561–3573. [Google Scholar] [CrossRef]
- Quigley, L.; O’Sullivan, O.; Stanton, C.; Beresford, T.P.; Ross, R.P.; Fitzgerald, G.F.; Cotter, P.D. The complex microbiota of raw milk. FEMS Microbiol. Rev. 2013, 37, 664–698. [Google Scholar] [CrossRef]
- Gran, H.M.; Mutukumira, A.N.; Wetlesen, A.; Narvhus, J.A. Smallholder dairy processing in Zimbabwe: The production of fermented milk products with particular emphasis on sanitation and microbiological quality. Food Control 2002, 13, 161–168. [Google Scholar] [CrossRef]
- Bava, L.; Zucali, M.; Sandrucci, A.; Brasca, M.; Vanoni, L.; Zanini, L.; Tamburini, A. Effect of cleaning procedure and hygienic condition of milking equipment on bacterial count of bulk tank milk. J. Dairy Res. 2011, 78, 211–219. [Google Scholar] [CrossRef]
- Amoureux, L.; Bador, J.; Verrier, T.; Mjahed, H.; De Curraize, C.; Neuwirth, C. Achromobacter xylosoxidans is the predominant Achromobacter species isolated from diverse non-respiratory samples. Epidemiol. Infect. 2016, 144, 3527–3530. [Google Scholar] [CrossRef] [PubMed]
- Neuwirth, C.; Freby, C.; Ogier-Desserrey, A.; Perez-Martin, S.; Houzel, A.; Péchinot, A.; Duez, J.-M.; Huet, F.; Siebor, E. VEB-1 in Achromobacter xylosoxidans from Cystic Fibrosis Patient, France. Emerg. Infect. Dis. J. 2006, 12, 1737. [Google Scholar] [CrossRef] [PubMed]
- Thaker, H.; Brahmbhatt, M.; Nayak, J. Isolation and identification of Staphylococcus aureus from milk and milk products and their drug resistance patterns in Anand, Gujarat. Vet. World 2013, 5, 10–13. [Google Scholar] [CrossRef]
- Burns, E.M.; Ahmed, H.; Isedeh, P.N.; Kohli, I.; Van der Pol, W.; Shaheen, A.; Muzaffar, A.F.; Al-Sadek, C.; Foy, T.M.; Abdelgawwad, M.S.; et al. Ultraviolet radiation, both UVA and UVB, influences the composition of the skin microbiome. Exp. Dermatol. 2019, 28, 136–141. [Google Scholar] [CrossRef]
- Angel, R.; Soares, M.I.M.; Ungar, E.D.; Gillor, O. Biogeography of soil archaea and bacteria along a steep precipitation gradient. ISME J. 2010, 4, 553–563. [Google Scholar] [CrossRef]
- Kraal, L.; Abubucker, S.; Kota, K.; Fischbach, M.A.; Mitreva, M. The Prevalence of Species and Strains in the Human Microbiome: A Resource for Experimental Efforts. PLoS ONE 2014, 9, e97279. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Richa; Kumar, A.; Tyagi, M.B.; Sinha, R.P. Molecular Mechanisms of Ultraviolet Radiation-Induced DNA Damage and Repair. J. Nucleic Acids 2010, 2010, 592980. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Coelho, M.C.; Malcata, F.X.; Silva, C.C.G. Lactic Acid Bacteria in Raw-Milk Cheeses: From Starter Cultures to Probiotic Functions. Foods 2022, 11, 2276. [Google Scholar] [CrossRef]
- Widyastuti, Y.; Febrisiantosa, A.; Tidona, F. Health-Promoting Properties of Lactobacilli in Fermented Dairy Products. Front. Microbiol. 2021, 12, 673890. [Google Scholar] [CrossRef]
- Qin, J.J.; Li, R.Q.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Quigley, L.; McCarthy, R.; O’Sullivan, O.; Beresford, T.P.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C.; Cotter, P.D. The microbial content of raw and pasteurized cow milk as determined by molecular approaches. J. Dairy Sci. 2013, 96, 4928–4937. [Google Scholar] [CrossRef] [PubMed]
- Driessche, E.V.; Houf, K.; Vangroenweghe, F.; Zutter, L.D.; Hoof, J.V. Prevalence, enumeration and strain variation of Arcobacter species in the faeces of healthy cattle in Belgium. Vet. Microbiol. 2005, 105, 149–154. [Google Scholar] [CrossRef]
- Yesilmen, S.; Vural, A.; Erkan, M.E.; Yildirim, I.H. Prevalence and antimicrobial susceptibility of Arcobacter species in cow milk, water buffalo milk and fresh village cheese. Int. J. Food Microbiol. 2014, 188, 11–14. [Google Scholar] [CrossRef]
- De Smet, S.; De Zutter, L.; Houf, K. Small ruminants as carriers of the emerging foodborne pathogen Arcobacter on small and medium farms. Small Rumin. Res. 2011, 97, 124–129. [Google Scholar] [CrossRef]
- Botta, C.; Buzzanca, D.; Chiarini, E.; Chiesa, F.; Rubiola, S.; Ferrocino, I.; Fontanella, E.; Rantsiou, K.; Houf, K.; Alessandria, V. Microbial contamination pathways in a poultry abattoir provided clues on the distribution and persistence of Arcobacter spp. Appl. Environ. Microbiol. 2024, 90, e00296-24. [Google Scholar] [CrossRef]
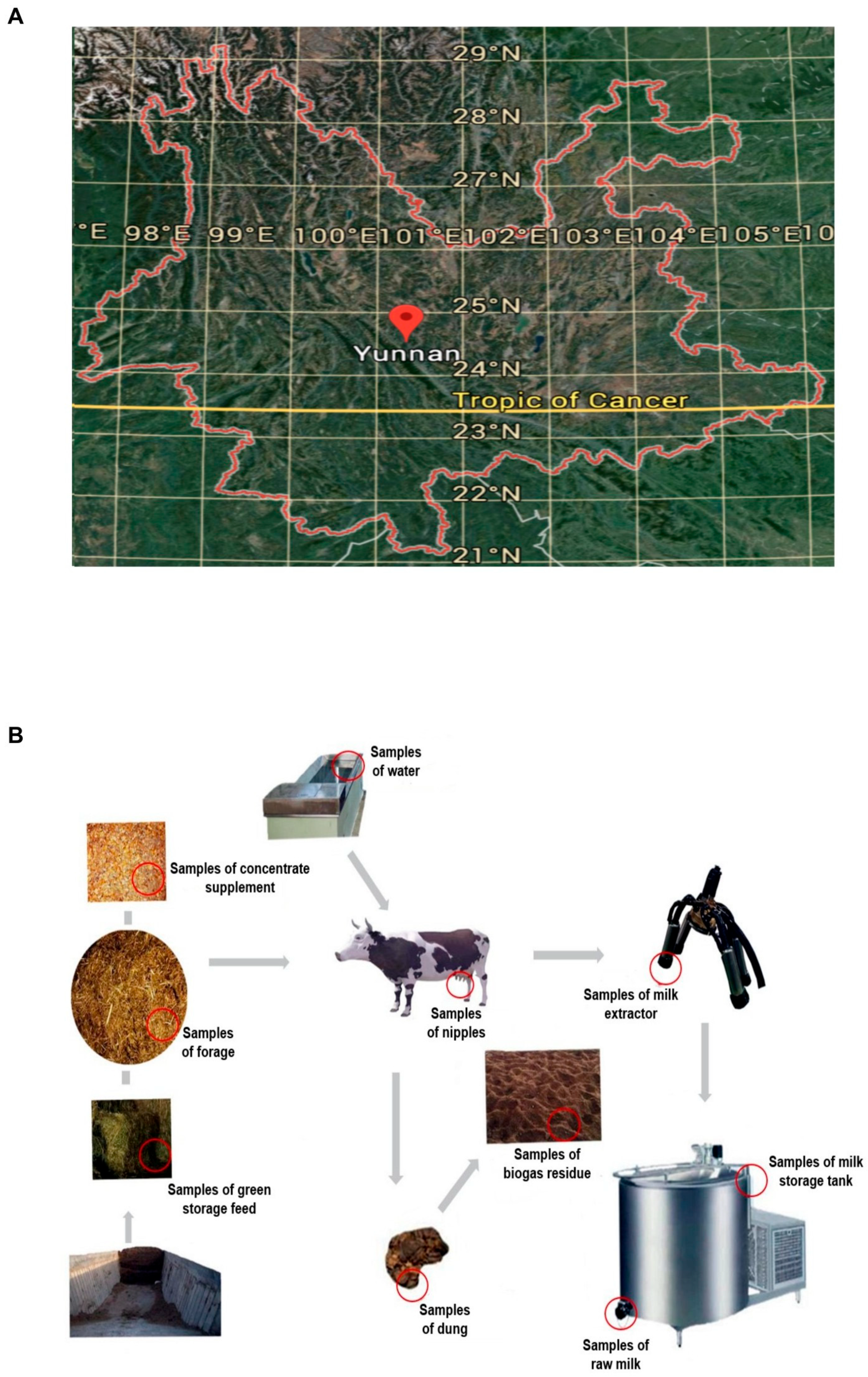

| Season | Sample Type | Abbreviation | Number of Samples | Description |
|---|---|---|---|---|
| Spring | Environment | E(S) | 10 | Inner cover wipes of the milking extractor and milk storage tank |
| Teat surfaces | P(S) | 12 | Swabs from high-, medium-, and low-yielding cows (daily milk ≥ 35 kg, 18–35 kg) | |
| Raw milk | M(S) | 2 | Collected during milking | |
| Forage | F(S) | 8 | Corn silage and concentrated feed (cornmeal and soybean meal) | |
| Drinking water | W(S) | 2 | Purified reservoir water | |
| Autumn | Environment | E(A) | 11 | Inner cover wipes of the milking extractor and milk storage tank |
| Teat surfaces | P(A) | 14 | Swabs from high-, medium-, and low-yielding cows | |
| Raw milk | M(A) | 9 | Collected during milking | |
| Forage | F(A) | 5 | Alfalfa hay and silage | |
| Animal feces | S(A) | 6 | Feces from high-, medium-, and low-yielding cows | |
| Biogas residue | B(A) | 5 | Dried residues from biogas production (used as bedding material) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Zhang, Y.; Liu, C.; Li, X. Where Do Milk Microbes Originate? Traceability of Microbial Community Structure in Raw Milk. Foods 2025, 14, 1490. https://doi.org/10.3390/foods14091490
Li S, Zhang Y, Liu C, Li X. Where Do Milk Microbes Originate? Traceability of Microbial Community Structure in Raw Milk. Foods. 2025; 14(9):1490. https://doi.org/10.3390/foods14091490
Chicago/Turabian StyleLi, Shuqi, Yuwang Zhang, Chenjian Liu, and Xiaoran Li. 2025. "Where Do Milk Microbes Originate? Traceability of Microbial Community Structure in Raw Milk" Foods 14, no. 9: 1490. https://doi.org/10.3390/foods14091490
APA StyleLi, S., Zhang, Y., Liu, C., & Li, X. (2025). Where Do Milk Microbes Originate? Traceability of Microbial Community Structure in Raw Milk. Foods, 14(9), 1490. https://doi.org/10.3390/foods14091490







