Protective Effect of Lactic Acid Bacteria Isolated from Ripened Foods Against Listeria monocytogenes in Plant-Based Fermented Dry-Cured Sausages
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbial Cultures
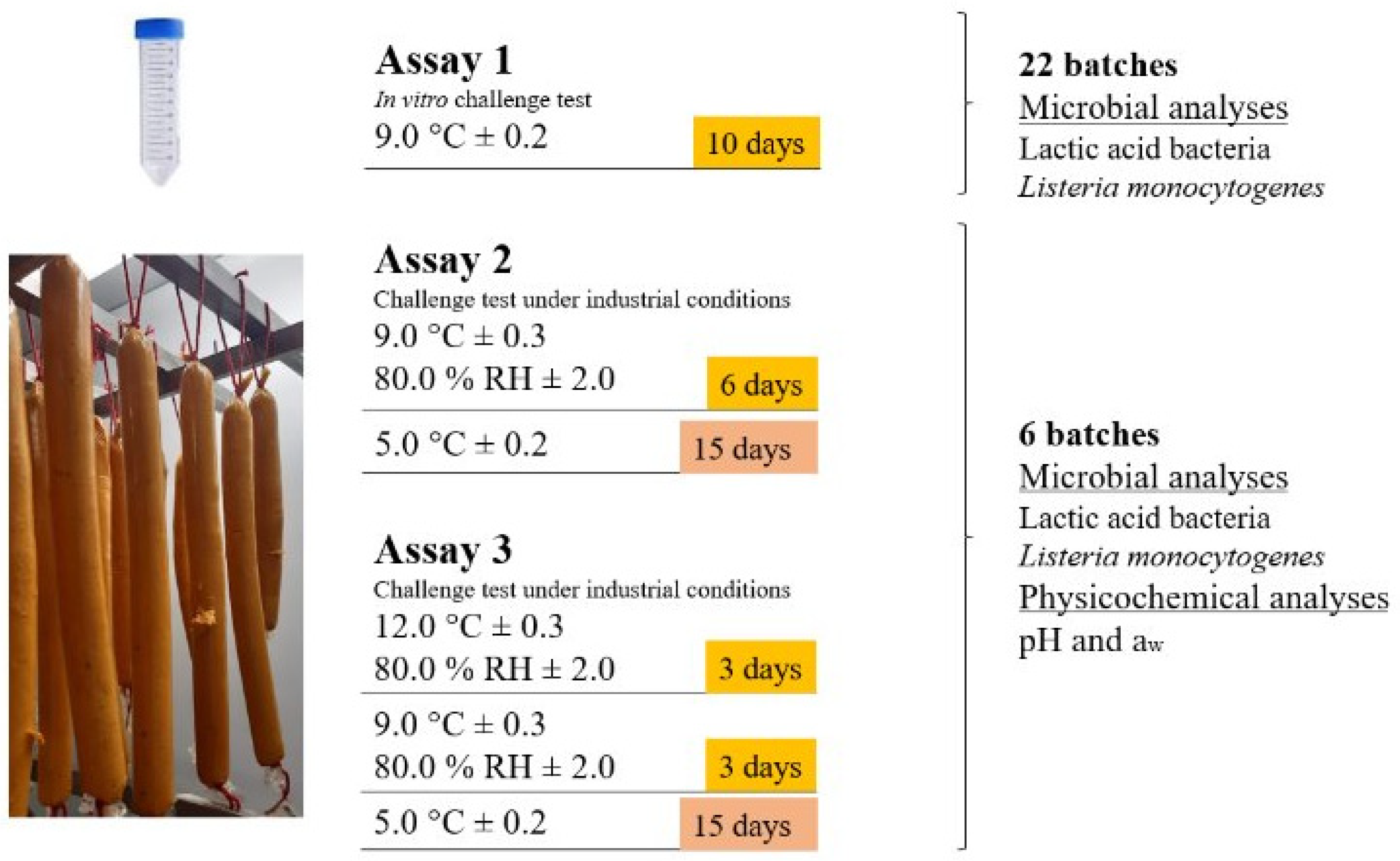
2.2. Preparation of the Plant-Based Analog and Experimental Design
2.3. Challenge Tests
2.3.1. In Vitro Assay
2.3.2. Assays Under Industrial Conditions
2.4. Physicochemical Analyses
2.5. Sensory Evaluation
2.6. Data Analysis
3. Results and Discussion
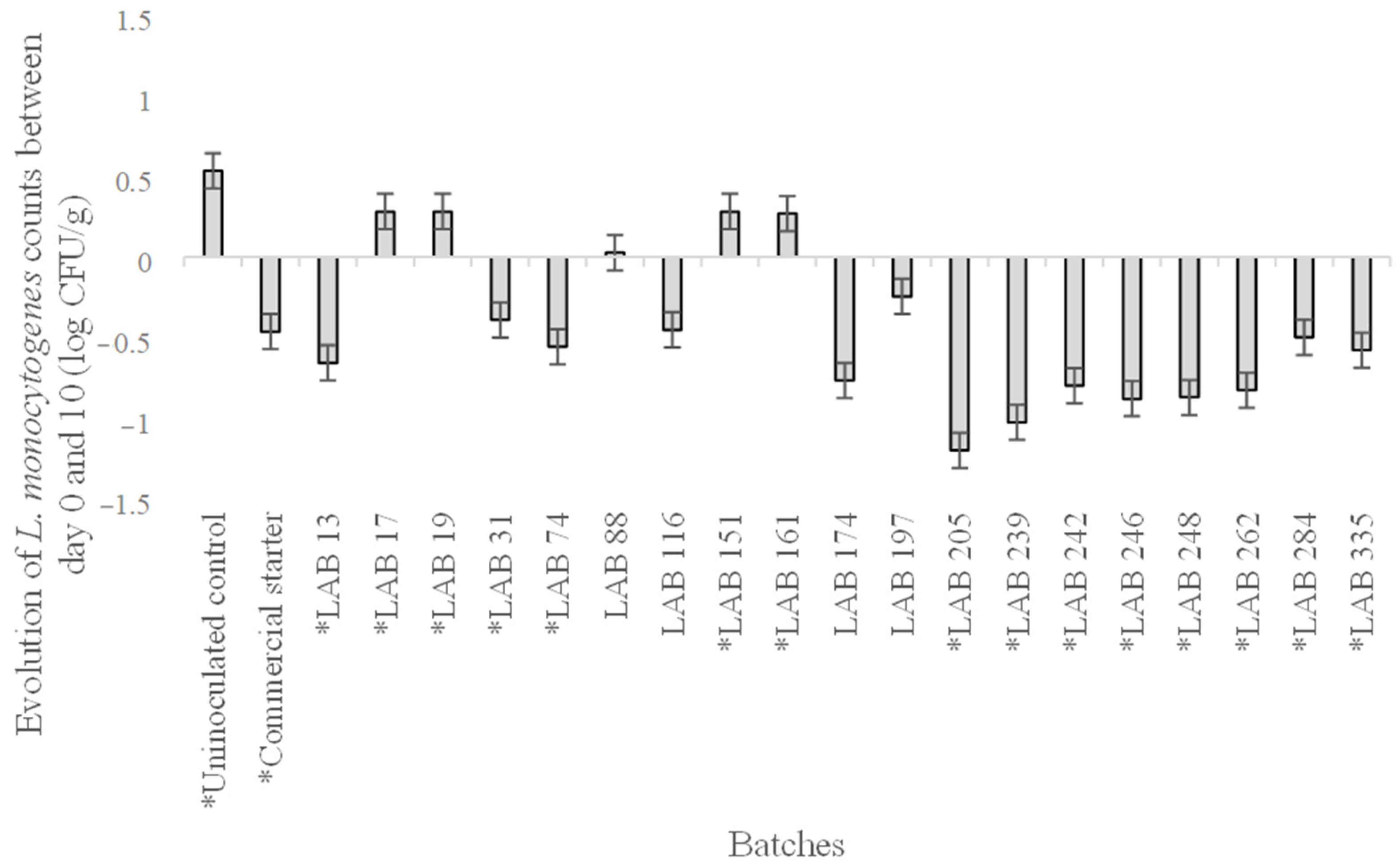
3.1. In Vitro Assay
3.2. Assay Under Industrial Conditions
3.2.1. Physicochemical Results
3.2.2. Microbial Results
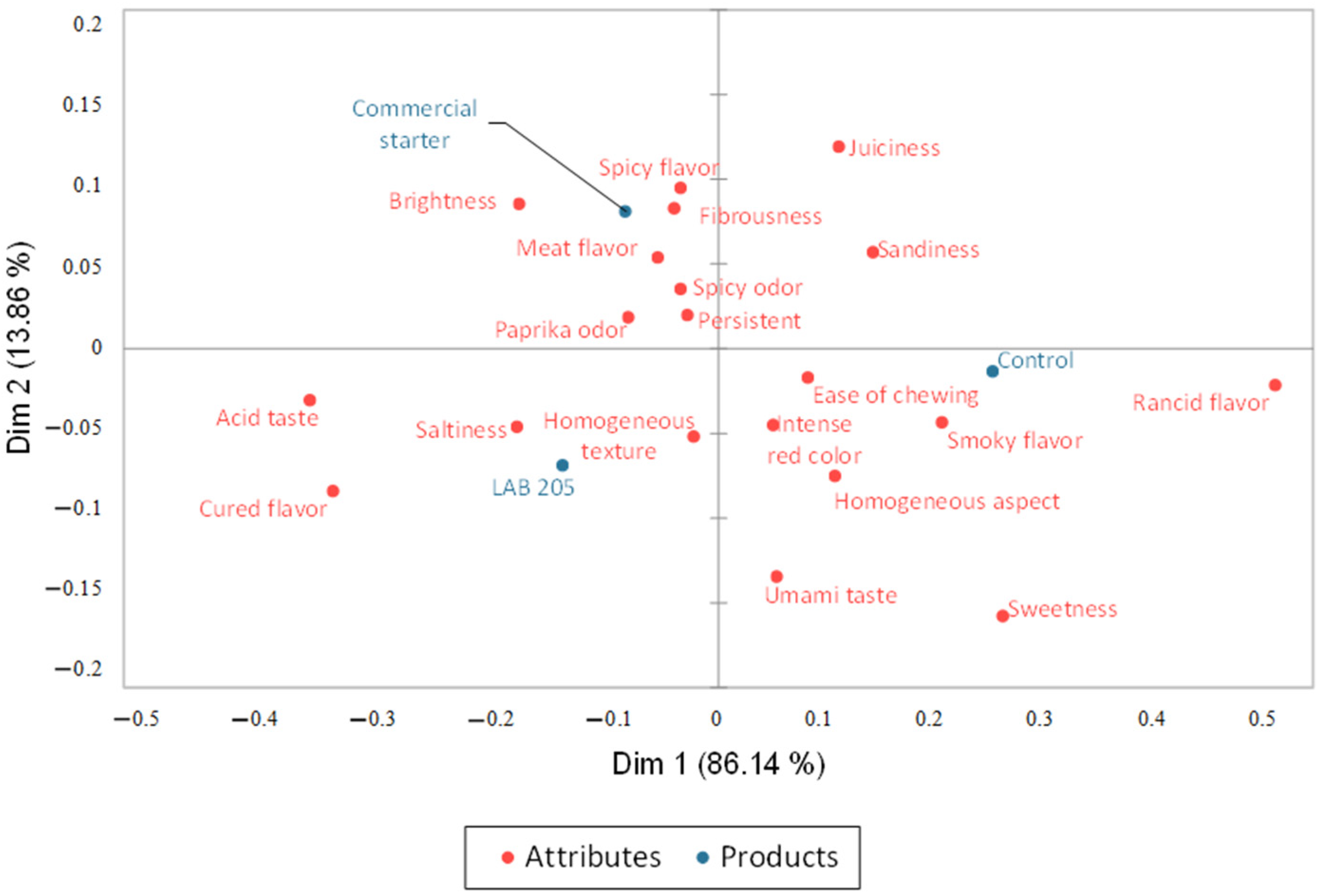
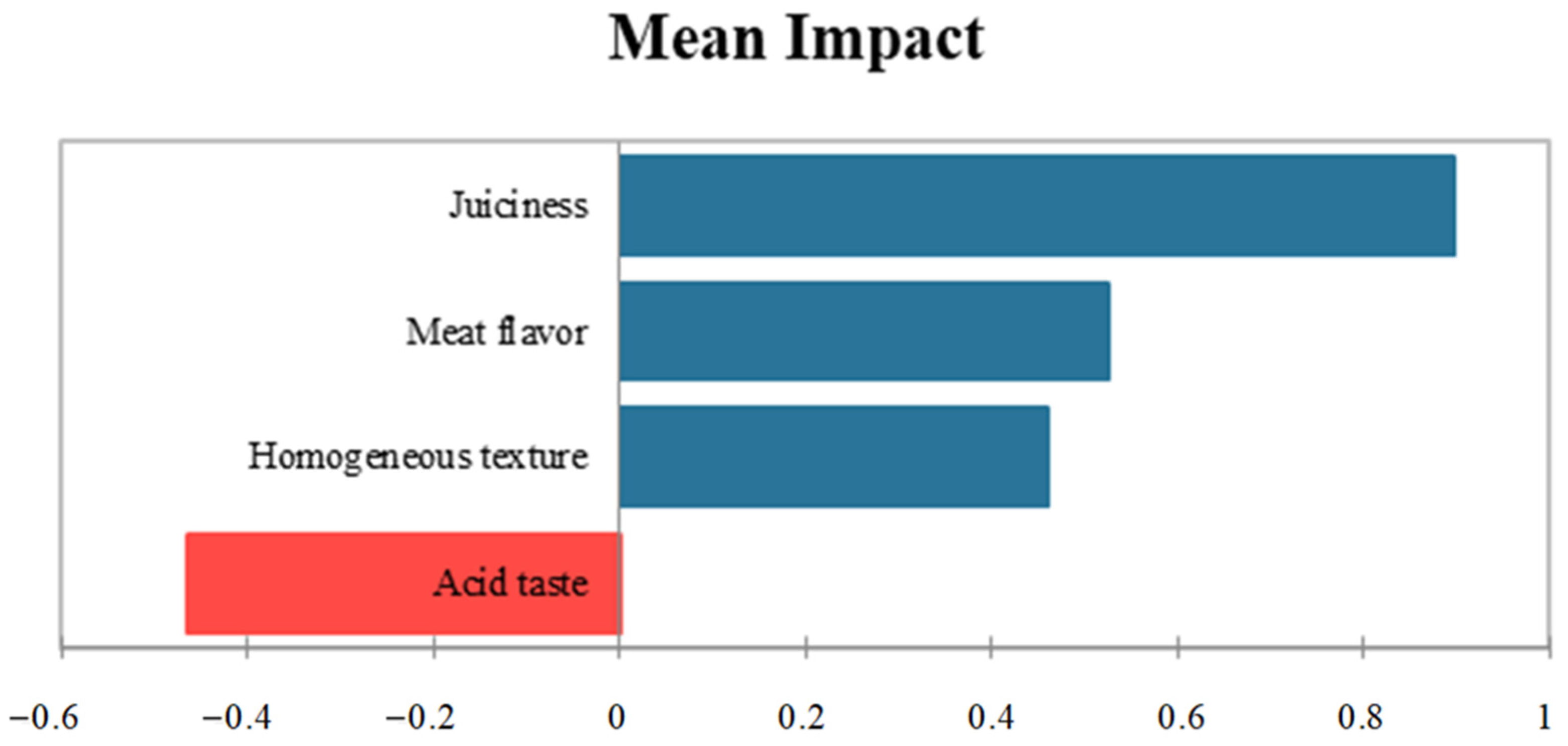
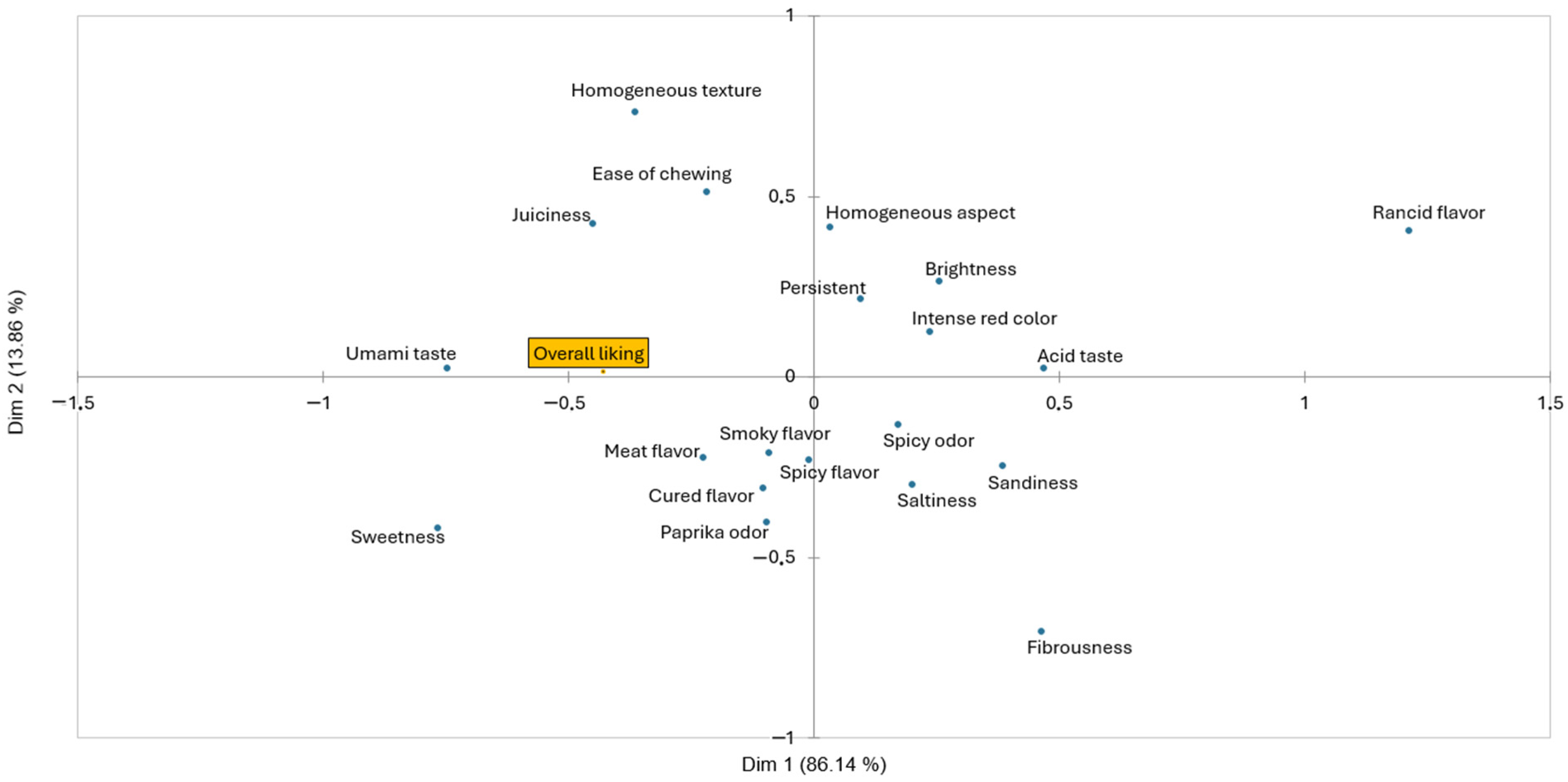
3.3. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, K.; Zang, M.; Wang, S.; Zhang, Z.; Li, D.; Li, X. Development of Meat Analogs: Focus on the Current Status and Challenges of Regulatory Legislation. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1006–1029. [Google Scholar] [CrossRef] [PubMed]
- Wild, F.; Czerny, M.; Janssen, A.; Kole, A.; Zunabovic, M.; Domig, K. The Evolution of a Plant-Based Alternative to Meat: From Niche Markets to Widely Accepted Meat Alternatives. Agro. Food Ind. Hi Tech 2014, 21, 45–49. [Google Scholar]
- Muhialdin, B.J.; Ubbink, J. Effects of PH and Aging on the Texture and Physicochemical Properties of Extruded Pea Protein Isolate. Food Hydrocoll. 2023, 140, 108639. [Google Scholar] [CrossRef]
- Yadav, P.; Ahlawat, S.; Gauri Jairath, G.; Monika Rani, M.; Bishnoi, S. Studies on Physico-Chemical Properties and Shelf Life of Developed Chicken Meat Analogue Rolls. Haryana Vet. 2015, 54, 25–28. [Google Scholar]
- Silberbauer, A.; Schmid, M. Packaging Concepts for Ready-to-Eat Food: Recent Progress. J. Packag. Technol. Res. 2017, 1, 113–126. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2023 Zoonoses Report. EFSA J. 2024, 22, e9106. [Google Scholar] [CrossRef]
- Letchumanan, V.; Wong, P.C.; Goh, B.H.; Ming, L.C.; Pus-Parajah, P.; Wong, S.H.; Mutalib, N.S.A.; Lee, L.H. A Review on the Characteristics, Taxanomy and Prevalence of Listeria monocytogenes. Prog. Microbes Mol. Biol. 2018, 1, 1–8. [Google Scholar] [CrossRef]
- Townsend, A.; Strawn, L.K.; Chapman, B.J.; Dunn, L.L. A Systematic Review of Listeria Species and Listeria monocytogenes Prevalence, Persistence, and Diversity throughout the Fresh Produce Supply Chain. Foods 2021, 10, 1427. [Google Scholar] [CrossRef]
- Leclercq, A.; Tourdjman, M.; Mattheus, W.; Friesema, I.; Van Sorge, N.M.; Halbedel, S.; Wilking, H.; Lecuit, M. Outbreak of Listeriosis Associated with Consumption of Vegan Cheese. N. Engl. J. Med. 2024, 390, 1439–1440. [Google Scholar] [CrossRef]
- Archer, D.L. The Evolution of FDA’s Policy on Listeria monocytogenes in Ready-to-Eat Foods in the United States. Curr. Opin. Food Sci. 2018, 20, 64–68. [Google Scholar] [CrossRef]
- Imran, M.; Liyan, Z. Production of Plant-Based Meat: Functionality, Limitations and Future Prospects. Eur. Food Res. Technol. 2023, 249, 2189–2213. [Google Scholar] [CrossRef]
- Kyriakopoulou, K.; Keppler, J.K.; van der Goot, A.J. Functionality of Ingredients and Additives in Plant-Based Meat Analogues. Foods 2021, 10, 600. [Google Scholar] [CrossRef] [PubMed]
- Filho, G.; Vessoni Penna, T.C.; Schaffner, D.W. Microbiological Quality of Vegetable Proteins during the Preparation of a Meat Analog. Ital. J. Food Sci. 2005, 17, 269–283. [Google Scholar]
- Szűcs, V.; Szabó, E.; Guerrero, L.; Tarcea, M.; Bánáti, D. Modelling of Avoidance of Food Additives: A Cross Country Study. Int. J. Food Sci. Nutr. 2019, 70, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Wu, L.; Chen, X.; Huang, Z.; Hu, W. Effects of Food-Additive-Information on Consumers’ Willingness to Accept Food with Additives. Int. J. Environ. Res. Public Health 2018, 15, 2394. [Google Scholar] [CrossRef]
- Roland, W.S.U.; Pouvreau, L.; Curran, J.; Van De Velde, F.; De Kok, P.M.T. Flavor Aspects of Pulse Ingredients. Cereal Chem. 2017, 94, 58–65. [Google Scholar] [CrossRef]
- Ahmad, M.; Qureshi, S.; Akbar, M.H.; Siddiqui, S.A.; Gani, A.; Mushtaq, M.; Hassan, I.; Dhull, S.B. Plant-Based Meat Alternatives: Compositional Analysis, Current Development and Challenges. Appl. Food Res. 2022, 2, 100154. [Google Scholar] [CrossRef]
- Delgado, J.; Álvarez, M.; Cebrián, E.; Martín, I.; Roncero, E.; Rodríguez, M. Biocontrol of Pathogen Microorganisms in Ripened Foods of Animal Origin. Microorganisms 2023, 11, 1578. [Google Scholar] [CrossRef]
- Fischer, S.W.; Titgemeyer, F. Protective Cultures in Food Products: From Science to Market. Foods 2023, 12, 1541. [Google Scholar] [CrossRef]
- Allende, A.; Alvarez-Ordóñez, A.; Bortolaia, V.; Bover-Cid, S.; De Cesare, A.; Dohmen, W.; Guillier, L.; Jacxsens, L.; Nauta, M.; Mughini-Gras, L.; et al. Update of the List of Qualified Presumption of Safety (QPS) Recommended Microbiological Agents Intentionally Added to Food or Feed as Notified to EFSA 21: Suitability of Taxonomic Units Notified to EFSA until September 2024. EFSA J. 2025, 23, e9169. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Y.; Nychas, G.J.E.; Luo, X.; Zhu, L.; Mao, Y.; Dong, P.; Zhang, Y. Utilizing Lactic Acid Bacteria and Their Metabolites for Controlling Listeria monocytogenes in Meat Products: Applications, Limitations, and Future Perspectives. Trends Food Sci. Technol. 2024, 152, 104699. [Google Scholar] [CrossRef]
- Huang, L.; Hwang, C.A.; Liu, Y.; Renye, J.; Jia, Z. Growth Competition between Lactic Acid Bacteria and Listeria monocytogenes during Simultaneous Fermentation and Drying of Meat Sausages—A Mathematical Modeling. Food Res. Int. 2022, 158, 111553. [Google Scholar] [CrossRef] [PubMed]
- Molina, G.E.S.; Ras, G.; da Silva, D.F.; Duedahl-Olesen, L.; Hansen, E.B.; Bang-Berthelsen, C.H. Metabolic Insights of Lactic Acid Bacteria in Reducing Off-Flavors and Antinutrients in Plant-Based Fermented Dairy Alternatives. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70134. [Google Scholar] [CrossRef] [PubMed]
- Erem, E.; Kilic-Akyilmaz, M. The Role of Fermentation with Lactic Acid Bacteria in Quality and Health Effects of Plant-Based Dairy Analogues. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13402. [Google Scholar] [CrossRef]
- Martín, I.; Rodríguez, A.; Alía, A.; Martínez, R.; Córdoba, J.J. Selection and Characterization of Lactic Acid Bacteria with Activity against Listeria monocytogenes from Traditional RTE Ripened Foods. LWT 2022, 163, 113579. [Google Scholar] [CrossRef]
- Martín-Miguélez, J.M.; Souza Olegario, L.; González-Mohino, A.; Ventanas, S.; Delgado, J. Physicochemical, Sensory, and Safety Evaluation of Dry-Cured Fermented Sausages and Its Plant-Based Meat Analog. LWT 2024, 208, 116704. [Google Scholar] [CrossRef]
- Gürel, A.E.; Ağbulut, Ü.; Ergün, A.; Ceylan, İ. Environmental and Economic Assessment of a Low Energy Consumption Household Refrigerator. Eng. Sci. Technol. Int. J. 2020, 23, 365–372. [Google Scholar] [CrossRef]
- Arienzo, A.; Murgia, L.; Fraudentali, I.; Gallo, V.; Angelini, R.; Antonini, G. Microbiological Quality of Ready-to-Eat Leafy Green Salads during Shelf-Life and Home-Refrigeration. Foods 2020, 9, 1421. [Google Scholar] [CrossRef]
- AOAC Association of Official Analytical Chemists. Official Methods of Analysis of the AOAC International; AOAC Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Borges, A.F.; Cózar, A.; Patarata, L.; Gama, L.T.; Alfaia, C.M.; Fernandes, M.J.; Fernandes, M.H.; Pérez, H.V.; Fraqueza, M.J. Effect of High Hydrostatic Pressure Challenge on Biogenic Amines, Microbiota, and Sensory Profile in Traditional Poultry- and Pork-Based Semidried Fermented Sausage. J. Food Sci. 2020, 85, 1256–1264. [Google Scholar] [CrossRef]
- Dučić, M.; Barcenilla, C.; Cobo-Díaz, J.F.; López, M.; Álvarez-Ordóñez, A.; Prieto, M. High Pressure Processing at the Early Stages of Ripening Enhances the Safety and Quality of Dry Fermented Sausages Elaborated with or without Starter Culture. Food Res. Int. 2023, 163, 112162. [Google Scholar] [CrossRef]
- Herz, E.; Kinne, T.; Terjung, N.; Gibis, M.; Weiss, J. Influence of Extrudate to SPI-Gel-Binder Ratios and Transglutaminase Crosslinking on Texture of a Plant-Based Salami Analogue. Future Foods 2023, 7, 100235. [Google Scholar] [CrossRef]
- Martín, I.; García, C.; Rodríguez, A.; Córdoba, J.J. Effect of a Selected Protective Culture of Lactilactobacillus Sakei on the Evolution of Volatile Compounds and on the Final Sensorial Characteristics of Traditional Dry-Cured Fermented “Salchichón”. Biology 2023, 12, 88. [Google Scholar] [CrossRef] [PubMed]
- Bonacina, M.; da Silva, G.S.; Mitterer-Daltoé, M.L. Physicochemical Quality and Consumer Discrimination of Industrial and Traditional Fermented Sausages. Ciência Rural 2020, 50, e20200143. [Google Scholar] [CrossRef]
- Ventanas, S.; González-Mohino, A.; Olegario, L.S.; Estévez, M. Newbie Consumers Try Pizzas in Which Bacon Is Replaced by Tenebrio molitor L. Larvae: Not as Healthy as Expected and Not as Terrible as They Thought. Int. J. Gastron. Food Sci. 2022, 29, 100553. [Google Scholar] [CrossRef]
- González-Mohino, A.; Ventanas, S.; Estévez, M.; Olegario, L.S. Sensory Characterization of Iberian Dry-Cured Loins by Using Check-All-That-Apply (CATA) Analysis and Multiple-Intake Temporal Dominance of Sensations (TDS). Foods 2021, 10, 1983. [Google Scholar] [CrossRef]
- Straadt, I.K.; Aaslyng, M.D.; Bertram, H.C. Sensory and Consumer Evaluation of Pork Loins from Crossbreeds between Danish Landrace, Yorkshire, Duroc, Iberian and Mangalitza. Meat Sci. 2013, 95, 27–35. [Google Scholar] [CrossRef]
- Gelinski, J.M.L.N.; Baratto, C.M.; Casagrande, M.; de Oliveira, T.P.; Megiolaro, F.; de Martini Soares, F.A.S.; de Souza, E.M.B.; Vicente, V.A.; Fonseca, G.G. Control of Pathogens in Fresh Pork Sausage by Inclusion of Lactobacillus Sakei BAS0117. Can. J. Microbiol. 2019, 65, 831–841. [Google Scholar] [CrossRef]
- Hu, Y.; Tian, Y.; Zhu, J.; Wen, R.; Chen, Q.; Kong, B. Technological Characterization and Flavor-Producing Potential of Lactic Acid Bacteria Isolated from Traditional Dry Fermented Sausages in Northeast China. Food Microbiol. 2022, 106, 104059. [Google Scholar] [CrossRef]
- Siddi, G.; Piras, F.; Meloni, M.P.; Casti, D.; Spanu, C.; Pala, C.; Mocci, A.M.; Piga, C.; Salvo, R.D.; Santis, E.D.; et al. Evaluation of Vacuum Packaging for Extending the Shelf Life of Sardinian Fermented Sausage. Ital. J. Food Saf. 2023, 12, 10819. [Google Scholar] [CrossRef]
- Leroy, F.; Verluyten, J.; De Vuyst, L. Functional Meat Starter Cultures for Improved Sausage Fermentation. Int. J. Food Microbiol. 2006, 106, 270–285. [Google Scholar] [CrossRef]
- Fernández, M.; Hospital, X.F.; Caballero, N.; Jiménez, B.; Sánchez-Martín, V.; Morales, P.; Haza, A.I.; Hierro, E. Potential of Selected Bacteriocinogenic Lactic Acid Bacteria to Control Listeria monocytogenes in Nitrite-Reduced Fermented Sausages. Food Control 2023, 150, 109724. [Google Scholar] [CrossRef]
- Panebianco, F.; Giarratana, F.; Caridi, A.; Sidari, R.; De Bruno, A.; Giuffrida, A. Lactic Acid Bacteria Isolated from Traditional Italian Dairy Products: Activity against Listeria monocytogenes and Modelling of Microbial Competition in Soft Cheese. LWT 2021, 137, 110446. [Google Scholar] [CrossRef]
- Siddi, G.; Piras, F.; Spanu, V.; Meloni, M.P.; Sanna, R.; Carta, N.; Errico, M.; Cuccu, M.; De Santis, E.P.L.; Scarano, C. Selection of Commercial Protective Cultures to Be Added in Sardinian Fermented Sausage to Control Listeria monocytogenes. Ital. J. Food Saf. 2022, 11, 10368. [Google Scholar] [CrossRef]
- Cenci-Goga, B.T.; Ranucci, D.; Miraglia, D.; Cioffi, A. Use of Starter Cultures of Dairy Origin in the Production of Salame Nostrano, an Italian Dry-Cured Sausage. Meat Sci. 2008, 78, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Martín, I.; Alía, A.; Rodríguez, A.; Gómez, F.; Córdoba, J.J. Growth and Expression of Virulence Genes of Listeria monocytogenes during the Processing of Dry-Cured Fermented “Salchichón” Manufactured with a Selected Lactilactobacillus sakei. Biology 2021, 10, 1258. [Google Scholar] [CrossRef]
- Martín, I.; Barbosa, J.; Pereira, S.I.A.; Rodríguez, A.; Córdoba, J.J.; Teixeira, P. Study of Lactic Acid Bacteria Isolated from Traditional Ripened Foods and Partial Characterization of Their Bacteriocins. LWT 2023, 173, 114300. [Google Scholar] [CrossRef]
- Martín, I.; Rodríguez, A.; Córdoba, J.J. Application of Selected Lactic-Acid Bacteria to Control Listeria monocytogenes in Soft-Ripened “Torta Del Casar” Cheese. LWT 2022, 168, 113873. [Google Scholar] [CrossRef]
- Martín, I.; Rodríguez, A.; Sánchez-Montero, L.; Padilla, P.; Córdoba, J.J. Effect of the Dry-Cured Fermented Sausage “Salchichón” Processing with a Selected Lactobacillus sakei in Listeria monocytogenes and Microbial Population. Foods 2021, 10, 856. [Google Scholar] [CrossRef]
- Martín-Miguélez, J.M.; Robledo, J.; Martín, I.; Castaño, C.; Delgado, J.; Córdoba, J.J. Biocontrol of L. monocytogenes with Selected Autochthonous Lactic Acid Bacteria in Raw Milk Soft-Ripened Cheese under Different Water Activity Conditions. Foods 2024, 13, 172. [Google Scholar] [CrossRef]
- Jeong, C.H.; Lee, S.H.; Yoon, Y.; Choi, H.Y.; Kim, H.Y. Identification of Optimal Fermentation Temperature for Dry-Fermented Sausage Using Strains Isolated from Korean Fermented Foods. Foods 2022, 12, 137. [Google Scholar] [CrossRef]
- Seleshe, S.; Kang, S.N. Effect of Different Pediococcus pentosaceus and Lactobacillus plantarum Strains on Quality characteristics of Dry Fermented Sausage after Completion of Ripening Period. Food Sci. Anim. Resour. 2021, 41, 636. [Google Scholar] [CrossRef] [PubMed]
- European Commission Commission Regulation (EU) 2020/205 of 14 February 2020 Amending Regulation (EC) No 2073/2005 as Regards Salmonella in Reptile Meat (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/eli/reg/2020/205/oj/eng (accessed on 6 August 2024).
- Hadjicharalambous, C.; Grispoldi, L.; Goga, B.C. Quantitative Risk Assessment of Listeria monocytogenes in a Traditional RTE Product. EFSA J. 2019, 17, e170906. [Google Scholar] [CrossRef] [PubMed]
- Ameer, A.; Seleshe, S.; Kang, S.N. Effect of Modified Atmosphere Packaging Varying in CO2 and N2 Composition on Quality Characteristics of Dry Fermented Sausage during Refrigeration Storage. Food Sci. Anim. Resour. 2022, 42, 639. [Google Scholar] [CrossRef]
- Gonzalez-Fandos, E.; de Castro, M.V.; Martinez-Laorden, A.; Perez-Arnedo, I. Behavior of Listeria monocytogenes and Other Microorganisms in Sliced Riojano Chorizo (Spanish Dry-Cured Sausage) during Storage under Modified Atmospheres. Microorganisms 2021, 9, 1384. [Google Scholar] [CrossRef]
- Mudadu, A.G.; Piras, G.; Melillo, R.; Salza, S.; Cau, S.; Virgilio, S.; Meloni, D.; Mele, P. Survival of Naturally Contaminating Listeria monocytogenes in Commercial Mediterranean-Style Dry Fermented Sausages during Storage. J. Food Prot. 2022, 85, 1576–1583. [Google Scholar] [CrossRef]
- Porto-Fett, A.C.S.; Espuña, E.; Shane, L.E.; Shoyer, B.A.; Mcgeary, L.; Vinyard, B.T.; Stahler, L.J.; Osoria, M.; Luchansky, J.B. Viability of Shiga Toxin–Producing Escherichia coli, Salmonella spp., and Listeria monocytogenes during Preparation and Storage of Fuet, a Traditional Dry-Cured Spanish Pork Sausage. J. Food Prot. 2022, 85, 879–889. [Google Scholar] [CrossRef]
- Meloni, D.; Galluzzo, P.; Mureddu, A.; Piras, F.; Griffiths, M.; Mazzette, R. Listeria monocytogenes in RTE Foods Marketed in Italy: Prevalence and Automated EcoRI Ribotyping of the Isolates. Int. J. Food Microbiol. 2009, 129, 166–173. [Google Scholar] [CrossRef]
- Meloni, D.; Consolati, S.G.; Mazza, R.; Mureddu, A.; Fois, F.; Piras, F.; Mazzette, R. Presence and Molecular Characterization of the Major Serovars of Listeria monocytogenes in Ten Sardinian Fermented Sausage Processing Plants. Meat Sci. 2014, 97, 443–450. [Google Scholar] [CrossRef]
- Spanu, C.; Jordan, K. Listeria monocytogenes Environmental Sampling Program in Ready-to-Eat Processing Facilities: A Practical Approach. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2843–2861. [Google Scholar] [CrossRef]
- Jeong, C.H.; Lee, S.-H.; Kim, H.-Y. Proteolysis Analysis and Sensory Evaluation of Fermented Sausages Strains Isolated from Korean Fermented Foods. Food Sci. Anim. Resour. 2023, 43, 877. [Google Scholar] [CrossRef]
- ISO 11290-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria Monocytogenes and of Listeria spp.—Part 1: Detection Method. International Organization for Standardization: Geneva, Switzerland, 2017.
- Xia, L.; Qian, M.; Cheng, F.; Wang, Y.; Han, J.; Xu, Y.; Zhang, K.; Tian, J.; Jin, Y. The Effect of Lactic Acid Bacteria on Lipid Metabolism and Flavor of Fermented Sausages. Food Biosci. 2023, 56, 103172. [Google Scholar] [CrossRef]
- Li, X.; Li, J. The Flavor of Plant-Based Meat Analogues. Cereal Foods World 2020, 65. [Google Scholar] [CrossRef]
- Yu, D.; Feng, M.-q.; Sun, J. Influence of Mixed Starters on the Degradation of Proteins and the Formation of Peptides with Antioxidant Activities in Dry Fermented Sausages. Food Control 2021, 123, 107743. [Google Scholar] [CrossRef]
- Sugahara, H.; Kato, S.; Nagayama, K.; Sashihara, K.; Nagatomi, Y. Heterofermentative Lactic Acid Bacteria Such as Limosilactobacillus as a Strong Inhibitor of Aldehyde Compounds in Plant-Based Milk Alternatives. Front. Sustain. Food Syst. 2022, 6, 965986. [Google Scholar] [CrossRef]
- Paglarini, C.d.S.; Vidal, V.A.S.; dos Santos, M.; Coimbra, L.O.; Esmerino, E.A.; Cruz, A.G.; Pollonio, M.A.R. Using Dynamic Sensory Techniques to Determine Drivers of Liking in Sodium and Fat-Reduced Bologna Sausage Containing Functional Emulsion Gels. Food Res. Int. 2020, 132, 109066. [Google Scholar] [CrossRef]
| Codification | LAB Species |
|---|---|
| Commercial starter | Latilactobacillus curvatus |
| 13, 74, 239, 242, 248, 262, 335 | Lacticaseibacillus paracasei |
| 17, 31, 88, 116, 246 | Lacticaseibacillus casei |
| 19, 174, 197, 205 | Latilactobacillus sakei |
| 151 | Lactococcus garvieae |
| 161 | Enterococcus durans |
| 284 | Lactiplantibacillus plantarum |
| Attributes | ||
|---|---|---|
| Acid taste | Smoky flavor | Juiciness |
| Sandiness | Meat flavor | Paprika odor |
| Brightness | Cured flavor | Spicy odor |
| Sweet taste | Rancid flavor | Persistent |
| Ease of chewing | Homogeneous aspect | Saltiness |
| Fibrousness | Homogeneous texture | Umami taste |
| Spicy flavor | Intense red color |
| Batches | Initial Day (log CFU/g) | Final Day (log CFU/g) |
|---|---|---|
| Uninoculated control | 4.98 ± 0.02 * | 7.89 ± 0.82 * |
| Listeria monocytogenes | 4.98 ± 0.02 * | 7.89 ± 0.82 * |
| L. monocytogenes and commercial starter | 8.78 ± 0.03 * | 9.02 ± 0.09 * |
| L. monocytogenes and LAB 13 | 8.75 ± 0.06 | 8.85 ± 0.21 |
| L. monocytogenes and LAB 17 | 8.51 ± 0.01 * | 9.06 ± 0.06 * |
| L. monocytogenes and LAB 19 | 8.53 ± 0.02 * | 8.98 ± 0.07 * |
| L. monocytogenes and LAB 31 | 8.37 ± 0.04 * | 8.49 ± 0.06 * |
| L. monocytogenes and LAB 74 | 8.82 ± 0.10 | 8.75 ± 0.10 |
| L. monocytogenes and LAB 88 | 8.18 ± 0.10 * | 8.57 ± 0.19 * |
| L. monocytogenes and LAB 116 | 9.23 ± 0.12 * | 8.76 ± 0.10 * |
| L. monocytogenes and LAB 151 | 8.55 ± 0.01 | 8.40 ± 0.38 |
| L. monocytogenes and LAB 161 | 9.05 ± 0.05 * | 8.59 ± 0.07 * |
| L. monocytogenes and LAB 174 | 6.78 ± 0.08 * | 8.70 ± 0.12 * |
| L. monocytogenes and LAB 197 | 7.33 ± 0.09 * | 8.69 ± 0.10 * |
| L. monocytogenes and LAB 205 | 6.92 ± 0.07 * | 8.61 ± 0.13 * |
| L. monocytogenes and LAB 239 | 9.09 ± 0.02 * | 8.33 ± 0.04 * |
| L. monocytogenes and LAB 242 | 9.03 ± 0.01 * | 8.27 ± 0.14 * |
| L. monocytogenes and LAB 246 | 9.25 ± 0.03 * | 8.15 ± 0.15 * |
| L. monocytogenes and LAB 248 | 9.36 ± 0.07 * | 8.31 ± 0.14 * |
| L. monocytogenes and LAB 262 | 9.24 ± 0.04 * | 8.43 ± 0.23 * |
| L. monocytogenes and LAB 284 | 8.85 ± 0.02 * | 8.61 ± 0.09 * |
| L. monocytogenes and LAB 335 | 9.07 ± 0.03 * | 8.61 ± 0.14 * |
| First assay under industrial conditions (9 °C) | |||
| Batches | Day 0 | Day 6 | Day 6 + 15 |
| pH | |||
| Uninoculated batch | 5.60 ± 0.01 * | 5.64 ± 0.12 4* | 5.57 ± 0.03 5* |
| Commercial starter | 5.60 ± 0.01 b* | 4.97 ± 0.04 a2 | 4.97 ± 0.06 a4* |
| LAB 31 | 5.60 ± 0.01 c* | 5.31 ± 0.04 b3* | 4.34 ± 0.07 a1 |
| LAB 74 | 5.60 ± 0.01 c* | 4.90 ± 0.01 b2* | 4.12 ± 0.08 a12* |
| LAB 116 | 5.60 ± 0.01 c* | 4.90 ± 0.11 b2* | 4.55 ± 0.14 a23 |
| LAB 205 | 5.60 ± 0.01 b* | 4.64 ± 0.03 a1* | 4.72 ± 0.09 a34 |
| aw | |||
| Uninoculated batch | 0.966 ± 0.001 b | 0.960 ± 0.001 ab | 0.957 ± 0.001 a |
| Commercial starter | 0.966 ± 0.001 b | 0.959 ± 0.001 ab | 0.954 ± 0.002 a |
| LAB 31 | 0.966 ± 0.001 b | 0.959 ± 0.001 ab | 0.954 ± 0.002 a |
| LAB 74 | 0.966 ± 0.001 b | 0.958 ± 0.001 ab | 0.956 ± 0.001 a |
| LAB 116 | 0.966 ± 0.001 b | 0.958 ± 0.001 ab | 0.956 ± 0.002 a |
| LAB 205 | 0.966 ± 0.001 b | 0.958 ± 0.001 ab | 0.954 ± 0.002 a |
| Second assay under industrial conditions (12 + 9 °C) | |||
| Batches | Day 0 | Day 6 | Day 6 + 15 |
| pH | |||
| Uninoculated batch | 6.00 ± 0.01 * | 6.11 ± 0.12 3* | 5.68 ± 0.03 3* |
| Commercial starter | 6.00 ± 0.01 c* | 4.93 ± 0.04 b12 | 4.69 ± 0.06 a23* |
| LAB 31 | 6.00 ± 0.01 c* | 5.11 ±0.04 b123* | 4.47 ± 0.07 a1 |
| LAB 74 | 6.00 ± 0.01 b* | 5.26 ± 0.01 ab23* | 4.47 ± 0.08 a12* |
| LAB 116 | 6.00 ± 0.01 c* | 5.19 ± 0.11 b23* | 4.56 ± 0.14 a12 |
| LAB 205 | 6.00 ± 0.01 b* | 4.87 ± 0.03 a1* | 4.60 ± 0.09 a123 |
| aw | |||
| Uninoculated batch | 0.966 ± 0.001 b | 0.960 ± 0.001 ab | 0.957 ± 0.001 a |
| Commercial starter | 0.966 ± 0.001 b | 0.959 ± 0.001 ab | 0.954 ± 0.002 a |
| LAB 31 | 0.966 ± 0.001 b | 0.959 ± 0.001 ab | 0.954 ± 0.002 a |
| LAB 74 | 0.966 ± 0.001 b | 0.958 ± 0.001 ab | 0.956 ± 0.001 a |
| LAB 116 | 0.966 ± 0.001 b | 0.958 ± 0.001 ab | 0.956 ± 0.002 a |
| LAB 205 | 0.966 ± 0.001 b | 0.958 ± 0.001 ab | 0.954 ± 0.002 a |
| Batches | Day 0 (log CFU/g) | Day 6 (log CFU/g) | Day 6 + 15 (log CFU/g) |
|---|---|---|---|
| First assay under industrial conditions (9 °C) | |||
| Uninoculated control | 4.87 ± 0.03 a1* | 9.59 ± 0.12 c2* | 8.78 ± 0.04 b12* |
| L. monocytogenes | 5.52 ± 0.03 a2* | 9.33 ± 0.29 c12* | 8.54 ± 0.09 b1* |
| L. monocytogenes and commercial starter | 6.59 ± 0.26 a3 | 9.28 ± 0.10 b12* | 8.88 ± 0.15 b12 |
| L. monocytogenes and LAB 31 | 6.61 ± 0.03 a3* | 9.44 ± 0.18 c12* | 8.99 ± 0.15 b12 |
| L. monocytogenes and LAB 74 | 6.44 ± 0.07 a3* | 9.07 ± 0.19 b1* | 9.17 ± 0.15 b2* |
| L. monocytogenes and LAB 116 | 6.49 ± 0.08 a3* | 9.31 ± 0.08 b12* | 9.10 ± 0.31 b2 |
| L. monocytogenes and LAB 205 | 6.43 ± 0.09 a3* | 9.02 ± 0.04 b1* | 8.71 ± 0.29 b12 |
| Second assay under industrial conditions (12 + 9 °C) | |||
| Uninoculated control | 5.44 ± 0.03 a1* | 7.53 ± 0.03 b1* | 7.54 ± 0.24 b12* |
| L. monocytogenes | 5.44 ± 0.03 a1* | 7.87 ± 0.14 b1* | 6.91 ± 0.72 ab1* |
| L. monocytogenes and commercial starter | 6.44 ± 0.26 a123 | 9.06 ± 0.06 b3* | 9.07 ± 0.05 b3 |
| L. monocytogenes and LAB 31 | 6.80 ± 0.03 a3* | 8.62 ± 0.09 b2* | 8.78 ± 0.09 b23 |
| L. monocytogenes and LAB 74 | 6.86 ± 0.07 a3* | 8.56 ± 0.22 b123* | 8.87 ± 0.04 b23* |
| L. monocytogenes and LAB 116 | 7.14 ± 0.08 a3* | 8.51 ± 0.09 b2* | 8.82 ± 0.11 c23 |
| L. monocytogenes and LAB 205 | 6.05 ± 0.09 a2* | 8.87 ± 0.04 b23* | 8.82 ± 0.12 b23 |
| Batches | Day 0 (log CFU/g) | Day 6 (log CFU/g) | Day 6 + 15 (log CFU/g) |
|---|---|---|---|
| First assay under industrial conditions (9 °C) | |||
| L. monocytogenes | 3.74 ± 0.06 a1* | 5.17 ± 0.10 b2* | 5.60 ± 0.16 b3* |
| L. monocytogenes and commercial starter | 4.15 ± 0.02 b2* | 5.46 ± 0.13 c2* | 2.59 ± 0.26 a1* |
| L. monocytogenes and LAB 31 | 4.36 ± 0.03 b3* | 5.34 ± 0.33 c2* | 3.58 ± 0.56 a123* |
| L. monocytogenes and LAB 74 | 4.53 ± 0.04 b4* | 5.59 ± 0.21 c2* | 3.02 ± 0.22 a1* |
| L. monocytogenes and LAB 116 | 4.31 ± 0.09 b23* | 5.47 ± 0.23 c2* | 4.00 ± 0.15 a2* |
| L. monocytogenes and LAB 205 | 4.15 ± 0.10 b2* | 2.98 ± 0.05 a1* | 2.18 ± 0.30 a1* |
| Second assay under industrial conditions (12 + 9 °C) | |||
| L. monocytogenes | 5.33 ± 0.06 a* | 5.92 ± 0.33 ab2* | 6.15 ± 0.02 b2* |
| L. monocytogenes and commercial starter | 5.35 ± 0.02 a* | 6.21 ± 0.06 b2* | 4.62 ± 0.24 a1* |
| L. monocytogenes and LAB 31 | 5.22 ± 0.03 a* | 6.39 ± 0.03 b2* | 5.09 ± 0.11 a1* |
| L. monocytogenes and LAB 74 | 5.32 ± 0.04 b* | 6.34 ± 0.09 c2* | 4.90 ± 0.01 a1* |
| L. monocytogenes and LAB 116 | 5.32 ± 0.09 b* | 4.55 ± 0.17 a1* | 4.54 ± 0.26 a1* |
| L. monocytogenes and LAB 205 | 5.26 ± 0.10 b* | 6.16 ± 0.12 c2* | 4.44 ± 0.18 a1* |
| Attributes | Control | Commercial Starter | LAB 205 |
|---|---|---|---|
| Acid taste | 0.167 a | 0.412 b | 0.480 b |
| Brightness | 0.088 | 0.157 | 0.137 |
| Cured flavor | 0.078 a | 0.167 ab | 0.216 b |
| Ease of chewing | 0.833 | 0.775 | 0.784 |
| Fibrousness | 0.088 | 0.118 | 0.098 |
| Homogeneous aspect | 0.363 | 0.294 | 0.343 |
| Homogeneous texture | 0.343 | 0.363 | 0.412 |
| Intense red color | 0.324 | 0.304 | 0.333 |
| Juiciness | 0.373 | 0.402 | 0.284 |
| Meat flavor | 0.216 | 0.284 | 0.255 |
| Paprika odor | 0.373 | 0.490 | 0.480 |
| Persistent | 0.206 | 0.245 | 0.235 |
| Rancid flavor | 0.265 b | 0.127 ab | 0.108 a |
| Saltiness | 0.245 a | 0.363 ab | 0.422 b |
| Sandiness | 0.343 | 0.324 | 0.265 |
| Smoky flavor | 0.324 | 0.235 | 0.245 |
| Spicy flavor | 0.343 | 0.461 | 0.373 |
| Spicy odor | 0.333 | 0.412 | 0.382 |
| Sweet taste | 0.127 | 0.069 | 0.098 |
| Umami taste | 0.098 | 0.078 | 0.108 |
| Acceptability | 2.76 ± 1.17 | 3.04 ± 1.07 | 3.06 ± 1.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Miguélez, J.M.; Castaño, C.; Delgado, J.; Olegario, L.S.; González-Mohino, A. Protective Effect of Lactic Acid Bacteria Isolated from Ripened Foods Against Listeria monocytogenes in Plant-Based Fermented Dry-Cured Sausages. Foods 2025, 14, 1491. https://doi.org/10.3390/foods14091491
Martín-Miguélez JM, Castaño C, Delgado J, Olegario LS, González-Mohino A. Protective Effect of Lactic Acid Bacteria Isolated from Ripened Foods Against Listeria monocytogenes in Plant-Based Fermented Dry-Cured Sausages. Foods. 2025; 14(9):1491. https://doi.org/10.3390/foods14091491
Chicago/Turabian StyleMartín-Miguélez, José M., Cristina Castaño, Josué Delgado, Lary Souza Olegario, and Alberto González-Mohino. 2025. "Protective Effect of Lactic Acid Bacteria Isolated from Ripened Foods Against Listeria monocytogenes in Plant-Based Fermented Dry-Cured Sausages" Foods 14, no. 9: 1491. https://doi.org/10.3390/foods14091491
APA StyleMartín-Miguélez, J. M., Castaño, C., Delgado, J., Olegario, L. S., & González-Mohino, A. (2025). Protective Effect of Lactic Acid Bacteria Isolated from Ripened Foods Against Listeria monocytogenes in Plant-Based Fermented Dry-Cured Sausages. Foods, 14(9), 1491. https://doi.org/10.3390/foods14091491








