Quality Attributes, Structural Characteristics, and Functional Properties of Brewer’s Spent Grain Protein Concentrates as Affected by Alkaline and Pulsed Electric Field-Assisted Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material Preparation
2.2. Alkaline Extraction
2.3. Pulsed Electric Field-Assisted Extraction
2.4. Proximate Analysis
2.5. Protein Quality Determination
2.5.1. In Vitro Gastrointestinal Digestion
2.5.2. Amino Acid Profile Determination
2.5.3. Amino Acid Score Determination
2.5.4. Protein Digestibility-Corrected Amino Acid Score Determination
2.6. Determination of Protein Secondary Structure Changes
2.7. Functional Properties Determinations
2.7.1. Solubility
2.7.2. Foaming Properties
2.7.3. Emulsifying Properties
2.7.4. Oil-Holding Capacity
2.8. Statistical Analysis
3. Results and Discussion
3.1. Effect of PEF on Protein Extraction
3.1.1. Model Fitting and Statistical Analysis
3.1.2. Relationship Between Studied Parameters and Protein Purity
3.1.3. Model Validation
3.2. Effect of PEF on Protein Quality
3.2.1. Chemical Composition of Protein Concentrates
3.2.2. Amino Acid Profile of ALK-Treated and PEF-Treated Protein Concentrates
3.2.3. In Vitro Digestibility of ALK-Treated and PEF-Treated Protein Concentrates
3.2.4. Amino Acid Score and PDCAAS of ALK-Treated and PEF-Treated Protein Concentrates
3.2.5. Effect of PEF on Secondary Structure of Protein Concentrates
3.3. Effect of PEF on Functional Properties of Protein Concentrates
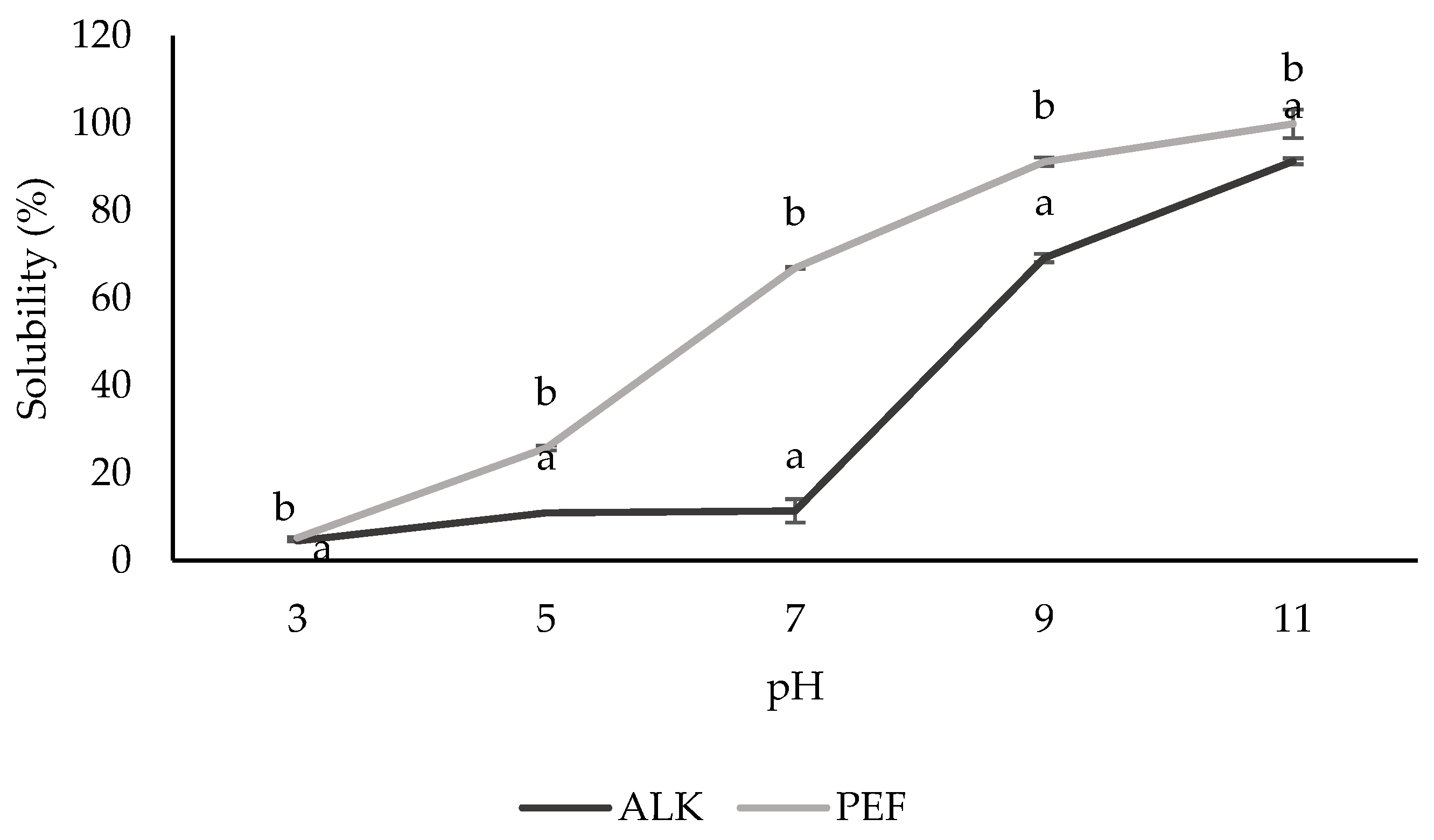
3.3.1. Solubility
3.3.2. Foaming Properties
3.3.3. Emulsifying Properties
3.3.4. Oil-Holding Capacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galanakis, C.M. The future of food. Foods 2024, 13, 506. [Google Scholar] [CrossRef] [PubMed]
- Ruben, R.; Cavatassi, R.; Lipper, L.; Smaling, E.; Winters, P. Towards food systems transformation—five paradigm shifts for healthy, inclusive and sustainable food systems. Food Secur. 2021, 13, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Aschemann-Witzel, J.; Gantriis, R.F.; Fraga, P.; Perez-Cueto, F.J. Plant-based food and protein trend from a business perspective: Markets, consumers, and the challenges and opportunities in the future. Crit. Rev. Food Sci. Nutr. 2021, 61, 3119–3128. [Google Scholar] [CrossRef]
- Mylan, J.; Andrews, J.; Maye, D. The big business of sustainable food production and consumption: Exploring the transition to alternative proteins. Proc. Natl. Acad. Sci. USA 2023, 120, e2207782120. [Google Scholar] [CrossRef]
- Jaeger, A.; Zannini, E.; Sahin, A.W.; Arendt, E.K. Barley protein properties, extraction and applications, with a focus on brewers’ spent grain protein. Foods 2021, 10, 1389. [Google Scholar] [CrossRef]
- Meneses, N.G.; Martins, S.; Teixeira, J.A.; Mussatto, S.I. Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Sep. Purif. Technol. 2013, 108, 152–158. [Google Scholar] [CrossRef]
- Pasquet, P.-L.; Villain-Gambier, M.; Trébouet, D. By-Product valorization as a means for the brewing industry to move toward a circular bioeconomy. Sustainability 2024, 16, 3472. [Google Scholar] [CrossRef]
- Olivares-Galván, S.; Marina, M.; García, M. Extraction of valuable compounds from brewing residues: Malt rootlets, spent hops, and spent yeast. Trends Food Sci. Technol. 2022, 127, 181–197. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Potkule, J.; Verma, R.; Punia, S.; Mahapatra, A.; Belwal, T.; Dahuja, A.; Joshi, S.; Berwal, M.K. Advances in the plant protein extraction: Mechanism and recommendations. Food Hydrocoll. 2021, 115, 106595. [Google Scholar] [CrossRef]
- Chan, C.-H.; Yeoh, H.K.; Yusoff, R.; Ngoh, G.C. A first-principles model for plant cell rupture in microwave-assisted extraction of bioactive compounds. J. Food Eng. 2016, 188, 98–107. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, L.; Ju, H.; Bao, Z.; Zeng, X.-a.; Lin, S. Research advances and application of pulsed electric field on proteins and peptides in food. Food Res. Int. 2021, 139, 109914. [Google Scholar] [CrossRef]
- Das, R.S.; Tiwari, B.K.; Chemat, F.; Garcia-Vaquero, M. Impact of ultrasound processing on alternative protein systems: Protein extraction, nutritional effects and associated challenges. Ultrason. Sonochem. 2022, 91, 106234. [Google Scholar]
- Pojić, M.; Mišan, A.; Tiwari, B. Eco-innovative technologies for extraction of proteins for human consumption from renewable protein sources of plant origin. Trends Food Sci. Technol. 2018, 75, 93–104. [Google Scholar] [CrossRef]
- Kumari, B.; Tiwari, B.K.; Hossain, M.B.; Brunton, N.P.; Rai, D.K. Recent advances on application of ultrasound and pulsed electric field technologies in the extraction of bioactives from agro-industrial by-products. Food Bioprocess Technol. 2018, 11, 223–241. [Google Scholar] [CrossRef]
- Ghoshal, G. Comprehensive review on pulsed electric field in food preservation: Gaps in current studies for potential future research. Heliyon 2023, 9, e17532. [Google Scholar] [CrossRef]
- Tylewicz, U.; Tappi, S.; Mannozzi, C.; Romani, S.; Dellarosa, N.; Laghi, L.; Ragni, L.; Rocculi, P.; Rosa, M.D. Effect of pulsed electric field (PEF) pre-treatment coupled with osmotic dehydration on physico-chemical characteristics of organic strawberries. J. Food Eng. 2017, 213, 2–9. [Google Scholar] [CrossRef]
- Thongkong, S.; Klangpetch, W.; Unban, K.; Tangjaidee, P.; Phimolsiripol, Y.; Rachtanapun, P.; Jantanasakulwong, K.; Schönlechner, R.; Thipchai, P.; Phongthai, S. Impacts of electroextraction using the pulsed electric field on properties of rice bran protein. Foods 2023, 12, 835. [Google Scholar] [CrossRef]
- Vanga, S.K.; Wang, J.; Jayaram, S.; Raghavan, V. Effects of pulsed electric fields and ultrasound processing on proteins and enzymes: A review. Processes 2021, 9, 722. [Google Scholar] [CrossRef]
- Na Lamphun, J.; Phangkham, K.; Pruakpromma, J.; Prom-ngam, P.; Tongdonyod, S.; Klangpetch, W. Development of non-alcoholic beer from malted barley added with Kum Jao Morchor 107 rice bran. In International Conference on Food and Applied Bioscience; Kantary Hills Hotel: Chiang Mai, Thailand, 2024. [Google Scholar]
- Siriwat, W.; Ungwiwatkul, S.; Unban, K.; Laokuldilok, T.; Klunklin, W.; Tangjaidee, P.; Potikanond, S.; Kaur, L.; Phongthai, S. Extraction, enzymatic modification, and anti-cancer potential of an alternative plant-based protein from Wolffia globosa. Foods 2023, 12, 3815. [Google Scholar] [CrossRef]
- Qu, K.-C.; Li, H.-Q.; Tang, K.-K.; Wang, Z.-Y.; Fan, R.-F. Selenium mitigates cadmium-induced adverse effects on trace elements and amino acids profiles in chicken pectoral muscles. Biol. Trace Elem. Res. 2020, 193, 234–240. [Google Scholar] [CrossRef]
- Zhou, Y.; He, Q.; Zhou, D. Optimization extraction of protein from mussel by high-intensity pulsed electric fields. J. Food Process. Preserv. 2017, 41, e12962. [Google Scholar] [CrossRef]
- Malik, M.A.; Sheikh, M.A.; Mir, N.A. A review on pulsed electric field modification of proteins: Effect on the functional and structural properties. Food Biosci. 2024, 61, 104636. [Google Scholar] [CrossRef]
- Mustafa, G.; Arshad, M.U.; Saeed, F.; Afzaal, M.; Niaz, B.; Hussain, M.; Raza, M.A.; Nayik, G.A.; Obaid, S.A.; Ansari, M.J. Comparative study of raw and fermented oat bran: Nutritional composition with special reference to their structural and antioxidant profile. Fermentation 2022, 8, 509. [Google Scholar] [CrossRef]
- Pop, A.; Păucean, A.; Socaci, S.A.; Alexa, E.; Man, S.M.; Mureșan, V.; Chiş, M.S.; Salanță, L.; Popescu, I.; Berbecea, A. Quality characteristics and volatile profile of macarons modified with walnut oilcake by-product. Molecules 2020, 25, 2214. [Google Scholar] [CrossRef] [PubMed]
- Day, L.; Cakebread, J.A.; Loveday, S.M. Food proteins from animals and plants: Differences in the nutritional and functional properties. Trends Food Sci. Technol. 2022, 119, 428–442. [Google Scholar] [CrossRef]
- Silva, E.S.; Roohinejad, S.; Koubaa, M.; Barba, F.J.; Jambrak, A.R.; Vukušić, T.; Santos, M.D.; Queirós, R.P.; Saraiva, J.A. Effect of pulsed electric fields on food constituents. In Springer eBooks; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–19. [Google Scholar]
- Roobab, U.; Zeng, X.-A.; Ahmed, W.; Madni, G.M.; Manzoor, M.F.; Aadil, R.M. Effect of pulsed electric field on the chicken meat quality and taste-related amino acid stability: Flavor simulation. Foods 2023, 12, 710. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Oey, I.; Bremer, P.; Silcock, P.; Carne, A. Proteolytic pattern, protein breakdown and peptide production of ovomucin-depleted egg white processed with heat or pulsed electric fields at different pH. Food Res. Int. 2018, 108, 465–474. [Google Scholar] [CrossRef]
- Siddeeg, A.; Zeng, X.A.; Rahaman, A.; Manzoor, M.F.; Ahmed, Z.; Ammar, A.F. Effect of pulsed electric field pretreatment of date palm fruits on free amino acids, bioactive components, and physicochemical characteristics of the alcoholic beverage. J. Food Sci. 2019, 84, 3156–3162. [Google Scholar] [CrossRef]
- Shen, Y.; Abeynayake, R.; Sun, X.; Ran, T.; Li, J.; Chen, L.; Yang, W. Feed nutritional value of brewers’ spent grain residue resulting from protease aided protein removal. J. Anim. Sci. Biotechnol. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Ren, Y.; Li, Q.; Zhang, C.; Qian, J.-Y. Implications for structural and digestive properties of broad bean protein modified by pulsed electric field. J. Food Eng. 2025, 391, 112444. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Mason, S.L.; Jayawardena, S.R.; Mungure, T.; Bekhit, A.E.-D.A. Cooking does not impair the impact of pulsed electric field on the protein digestion of venison (Cervus elaphus) during in vitro gastrointestinal digestion. Int. J. Food Sci. Technol. 2021, 56, 3026–3033. [Google Scholar] [CrossRef]
- Bhat, Z.; Morton, J.D.; Mason, S.L.; Bekhit, A.E.A. Pulsed electric field improved protein digestion of beef during in-vitro gastrointestinal simulation. LWT—Food Sci. Technol. 2019, 102, 45–51. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Matter, M.A.; Rady, M.R. Assessment of some barley germplasms based on RAPD analysis and anti-nutritional factors. J. Crop Sci. Biotechnol. 2010, 13, 61–68. [Google Scholar] [CrossRef]
- Wu, D.; Wu, W.; Zhang, N.; Soladoye, O.P.; Aluko, R.E.; Zhang, Y.; Fu, Y. Tailoring soy protein/corn zein mixture by limited enzymatic hydrolysis to improve digestibility and functionality. Food Chem. X 2024, 23, 101550. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, G. Advantages and limitations of the protein digestibility-corrected amino acid score (PDCAAS) as a method for evaluating protein quality in human diets. Br. J. Nutr. 2012, 108 (Suppl. 2), S333–S336. [Google Scholar] [CrossRef]
- Kudełka, W.; Kowalska, M.; Popis, M. Quality of soybean products in terms of essential amino acids composition. Molecules 2021, 26, 5071. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.-J.; Jiang, W.; Qian, J.-Y. Effect of pulsed electric field on functional and structural properties of canola protein by pretreating seeds to elevate oil yield. LWT—Food Sci. Technol. 2017, 84, 73–81. [Google Scholar] [CrossRef]
- Zhang, Q.; Shao, D.; Xu, P.; Jiang, Z. Effects of an electric field on the conformational transition of the protein: Pulsed and oscillating electric fields with different frequencies. Polymers 2021, 14, 123. [Google Scholar] [CrossRef]
- Podtelezhnikov, A.A.; Wild, D.L. Reconstruction and stability of secondary structure elements in the context of protein structure prediction. Biophys. J. 2009, 96, 4399–4408. [Google Scholar] [CrossRef]
- Pelegrine, D.H.G.; Gasparetto, C.A. Whey proteins solubility as function of temperature and pH. LWT—Food Sci. Technol. 2005, 38, 77–80. [Google Scholar] [CrossRef]
- Gulzar, S.; Martín-Belloso, O.; Soliva-Fortuny, R. Tailoring the techno-functional properties of fava bean protein isolates: A comparative evaluation of Ultrasonication and pulsed electric field treatments. Foods 2024, 13, 376. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, X.; Mo, H. Effects of pulsed electric fields on physicochemical properties of soybean protein isolates. LWT—Food Sci. Technol. 2007, 40, 1167–1175. [Google Scholar] [CrossRef]
- Li, J.; Yang, X.; Swallah, M.S.; Fu, H.; Ji, L.; Meng, X.; Yu, H.; Lyu, B. Soy protein isolate: An overview on foaming properties and air–liquid interface. Int. J. Food Sci. Technol. 2022, 57, 188–200. [Google Scholar] [CrossRef]
- Teng, Y.; Wang, Y.; Xu, X.; Wang, R.; Chen, B.; Wang, L.; Zhan, F.; Han, Z.; Li, Y.; Zhu, X. Enhancement of chickpea protein functionalities through higher-intensity pulsed electric field: Insights into protein aggregations and structural changes. Food Hydrocoll. 2025, 164, 111227. [Google Scholar] [CrossRef]
- Lai, X.-J.; Chen, J.-Q.; Nie, J.; Guo, P.-F.; Faisal Manzoor, M.; Huang, Y.-Y.; Li, J.; Lin, S.-Y.; Zeng, X.-A.; Wang, R. Enhancement of extraction efficiency and functional properties of chickpea protein isolate using pulsed electric field combined with ultrasound treatment. Ultrason. Sonochem. 2024, 111, 107089. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Zheng, X.; Wen, W.; Wang, X. Characteristics of quinoa protein isolate treated by pulsed electric field. Foods 2024, 13, 148. [Google Scholar] [CrossRef]
- Mahalaxmi, S.; Himashree, P.; Malini, B.; Sunil, C. Effect of microwave treatment on the structural and functional properties of proteins in lentil flour. Food Chem. Adv. 2022, 1, 100147. [Google Scholar] [CrossRef]
- Zhao, Q.; Xie, T.; Hong, X.; Zhou, Y.; Fan, L.; Liu, Y.; Li, J. Modification of functional properties of perilla protein isolate by high-intensity ultrasonic treatment and the stability of o/w emulsion. Food Chem. 2022, 368, 130848. [Google Scholar] [CrossRef]
- Guo, L.; Wang, X.; Ren, Y.; Zhang, X.; Li, Q.; Zhang, C.; Qian, J.-Y. Outcomes of structure, function and flavor of pea protein isolate treated by AC, DC and pulsed electric fields. Food Res. Int. 2024, 176, 113817. [Google Scholar] [CrossRef]
- McClements, D.; Großmann, L. Proposed methods for testing and comparing the emulsifying properties of proteins from animal, plant, and alternative sources. Colloids Interfaces 2022, 6, 19. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, L.; Liu, Y.; Huang, S.; Li, J. Effects of proteins on emulsion stability: The role of proteins at the oil–water interface. Food Chem. 2022, 397, 133726. [Google Scholar] [CrossRef] [PubMed]
- Melchior, S.; Calligaris, S.; Bisson, G.; Manzocco, L. Understanding the impact of moderate-intensity pulsed electric fields (MIPEF) on structural and functional characteristics of pea, rice and gluten concentrates. Food Bioprocess Technol. 2020, 13, 2145–2155. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Y.-H.; Zhao, X.-D.; Zhang, L.; Li, Q.; Wu, C.; Ding, X.; Qian, J.-Y. Assessment of impact of pulsed electric field on functional, rheological and structural properties of vital wheat gluten. LWT—Food Sci. Technol. 2021, 147, 111536. [Google Scholar] [CrossRef]

| No. | Experimental Design | Responses | |||
|---|---|---|---|---|---|
| Number of Pulse (Pulse) | Field Strength (kV/cm) | Frequency (Hz) | Protein Purity (%) | Protein Recovery (%) | |
| 1 | 5000 | 8 | 9 | 85.28 | 15.58 |
| 2 | 9000 | 8 | 9 | 78.83 | 13.40 |
| 3 | 5000 | 10 | 9 | 91.60 | 21.64 |
| 4 | 9000 | 10 | 9 | 75.89 | 9.21 |
| 5 | 5000 | 9 | 8 | 90.87 | 25.48 |
| 6 | 9000 | 9 | 8 | 75.62 | 16.14 |
| 7 | 5000 | 9 | 10 | 89.96 | 8.75 |
| 8 | 9000 | 9 | 10 | 77.61 | 12.47 |
| 9 | 7000 | 8 | 8 | 77.48 | 9.32 |
| 10 | 7000 | 10 | 8 | 81.14 | 7.63 |
| 11 | 7000 | 8 | 10 | 80.80 | 15.86 |
| 12 | 7000 | 10 | 10 | 81.87 | 3.54 |
| 13 | 7000 | 9 | 9 | 77.60 | 13.14 |
| 14 | 7000 | 9 | 9 | 78.33 | 11.00 |
| 15 | 7000 | 9 | 9 | 76.98 | 11.68 |
| Predicted value | 5385.84 | 9.80 | 10.21 | 90.49 | 22.70 |
| Experimental value | 5386 | 10 | 10 | 89.26 | 23.72 |
| ALK extraction (pH 10, stirring 90 min at 50 °C) | 73.00 | 12.96 | |||
| Responses | Source | Sum of Square | DF | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|---|
| Protein purity | Model | 420.17 | 9 | 46.69 | 39.28 | 0.0004 |
| X1 | 309.51 | 1 | 309.51 | 260.4 | <0.0001 | |
| X2 | 8.22 | 1 | 8.22 | 6.92 | 0.0465 | |
| X3 | 3.29 | 1 | 3.29 | 2.77 | 0.1571 | |
| X12 | 66 | 1 | 66 | 55.53 | 0.0007 | |
| X22 | 3.96 | 1 | 3.96 | 3.33 | 0.1276 | |
| X32 | 10.06 | 1 | 10.06 | 8.46 | 0.0334 | |
| X1 × 2 | 21.44 | 1 | 21.44 | 18.04 | 0.0081 | |
| X1 × 3 | 2.1 | 1 | 2.1 | 1.77 | 0.241 | |
| X2 × 3 | 1.68 | 1 | 1.68 | 1.41 | 0.2882 | |
| Residual | 5.94 | 5 | 1.19 | |||
| Lack of Fit | 0.2214 | |||||
| R2 | 0.9861 | |||||
| Adj R2 | 0.9609 | |||||
| Protein recovery | Model | 316.28 | 9 | 35.14 | 1.61 | 0.3122 |
| X1 | 51.24 | 1 | 51.24 | 2.35 | 0.1862 | |
| X2 | 18.41 | 1 | 18.41 | 0.84 | 0.4007 | |
| X3 | 40.32 | 1 | 40.32 | 1.85 | 0.2324 | |
| X12 | 85.71 | 1 | 85.71 | 3.92 | 0.1045 | |
| X22 | 12.03 | 1 | 12.03 | 0.55 | 0.4915 | |
| X32 | 4.07 | 1 | 4.07 | 0.19 | 0.6841 | |
| X1 × 2 | 23.23 | 1 | 26.23 | 1.20 | 0.3231 | |
| X1 × 3 | 42.64 | 1 | 42.64 | 1.95 | 0.2122 | |
| X2 × 3 | 28.19 | 1 | 28.19 | 1.29 | 0.3074 | |
| Residual | 109.22 | 5 | 21.84 | |||
| Lack of Fit | 0.0327 | |||||
| R2 | 0.7433 | |||||
| Adj R2 | 0.2813 |
| ALK | PEF | |
|---|---|---|
| Chemical composition (%) | ||
| Moisture | 4.41 ± 0.02 a | 3.72 ± 0.03 b |
| Protein | 73.00 ± 0.46 b | 89.26 ± 0.93 a |
| Fat | 1.90 ± 0.02 a | 1.10 ± 0.04 b |
| Ash | 2.31 ± 0.05 a | 1.02 ± 0.01 b |
| Fiber | 2.34 ± 0.55 a | 1.06 ± 0.06 b |
| Carbohydrate | 16.04 ± 0.88 a | 3.84 ± 0.12 b |
| Portion of secondary structures (%) | ||
| α-helices | 22.95 ± 0.13 a | 22.18 ± 0.20 b |
| β-sheets | 22.15 ± 0.18 b | 23.01 ± 0.13 a |
| β-turns | 21.14 ± 0.51 a | 20.25 ± 0.23 b |
| Random coils | 33.76 ± 0.25 ns | 34.56 ± 0.29 ns |
| Amino Acids | Amount of Essential Amino Acid (mg/100 g Sample) | Amino Acid Score ** | ||
|---|---|---|---|---|
| ALK | PEF | ALK | PEF | |
| Essential amino acids | ||||
| Histidine | 1556.05 ± 0.90 | 2159.31 ± 10.59 | 1.42 ± 0.00 | 1.97 ± 0.01 |
| Isoleucine | 1150.96 ± 17.49 | 1456.10 ± 350.66 | 0.53 ± 0.01 * | 0.66 ± 0.16 * |
| Leucine | 1595.54 ± 14.70 | 2103.90 ± 2.44 | 0.37 ± 0.00 * | 0.49 ± 0.00 * |
| Lysine | 2046.99 ± 9.32 | 2697.46 ± 11.79 | 0.63 ± 0.00 * | 0.82 ± 0.00 * |
| Methionine | 991.32 ± 2.42 | 1293.33 ± 41.83 | 0.62 ± 0.00 * | 0.81 ± 0.03 * |
| Phenylalanine | 2896.92 ± 4.79 | 3108.10 ± 29.11 | 1.05 ± 0.00 | 1.31 ± 0.00 |
| Threonine | 2198.47 ± 17.95 | 3099.73 ± 4.43 | 1.32 ± 0.01 | 1.85 ± 0.00 |
| Valine | 2856.95 ± 6.11 | 3844.27 ± 15.25 | 1.01 ± 0.00 | 1.35 ± 0.01 |
| Total | 15,293.20 ± 71.14 | 20,288.28 ± 447.24 | - | - |
| In vitro digestibility (%) | - | - | 49.84 ± 0.10 b | 54.96 ± 0.48 a |
| PDCAAS (%) | - | - | 18.44 ± 0.11 b (Leucine) | 24.42 ± 0.02 a (Leucine) |
| Protein Properties | ALK | PEF |
|---|---|---|
| HB/HL balance | 0.63 ± 0.00 b | 0.74 ± 0.01 a |
| Amphipathic amino acids (mg/100 g) | 3127.10 ± 17.12 b | 4219.55 ± 73.30 a |
| Foaming properties | ||
| Foamability (%) | 7.84 ± 0.51 b | 30.86 ± 0.24 a |
| Foam stability (%) | 68.89 ± 1.92 b | 79.37 ± 5.02 a |
| Emulsifying properties | ||
| Emulsifying ability (%) | 96.69 ± 1.41 b | 99.58 ± 0.72 a |
| Emulsion stability (%) | 90.98 ± 2.26 ns | 91.20 ± 2.25 ns |
| OHC (g/g sample) | 1.88 ± 0.09 ns | 1.87 ± 0.06 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paksin, P.; Tangjaidee, P.; Klangpetch, W.; Unban, K.; Khumsap, T.; Klunklin, W.; Yawootti, A.; Jantanasakulwong, K.; Rachtanapun, P.; Phongthai, S. Quality Attributes, Structural Characteristics, and Functional Properties of Brewer’s Spent Grain Protein Concentrates as Affected by Alkaline and Pulsed Electric Field-Assisted Extraction. Foods 2025, 14, 1515. https://doi.org/10.3390/foods14091515
Paksin P, Tangjaidee P, Klangpetch W, Unban K, Khumsap T, Klunklin W, Yawootti A, Jantanasakulwong K, Rachtanapun P, Phongthai S. Quality Attributes, Structural Characteristics, and Functional Properties of Brewer’s Spent Grain Protein Concentrates as Affected by Alkaline and Pulsed Electric Field-Assisted Extraction. Foods. 2025; 14(9):1515. https://doi.org/10.3390/foods14091515
Chicago/Turabian StylePaksin, Parichat, Pipat Tangjaidee, Wannaporn Klangpetch, Kridsada Unban, Tabkrich Khumsap, Warinporn Klunklin, Artit Yawootti, Kittisak Jantanasakulwong, Pornchai Rachtanapun, and Suphat Phongthai. 2025. "Quality Attributes, Structural Characteristics, and Functional Properties of Brewer’s Spent Grain Protein Concentrates as Affected by Alkaline and Pulsed Electric Field-Assisted Extraction" Foods 14, no. 9: 1515. https://doi.org/10.3390/foods14091515
APA StylePaksin, P., Tangjaidee, P., Klangpetch, W., Unban, K., Khumsap, T., Klunklin, W., Yawootti, A., Jantanasakulwong, K., Rachtanapun, P., & Phongthai, S. (2025). Quality Attributes, Structural Characteristics, and Functional Properties of Brewer’s Spent Grain Protein Concentrates as Affected by Alkaline and Pulsed Electric Field-Assisted Extraction. Foods, 14(9), 1515. https://doi.org/10.3390/foods14091515









