Microwave-Assisted Valorization of Tomato Pomace for Pectin Recovery: Improving Yields and Environmental Footprint
Abstract
:1. Introduction
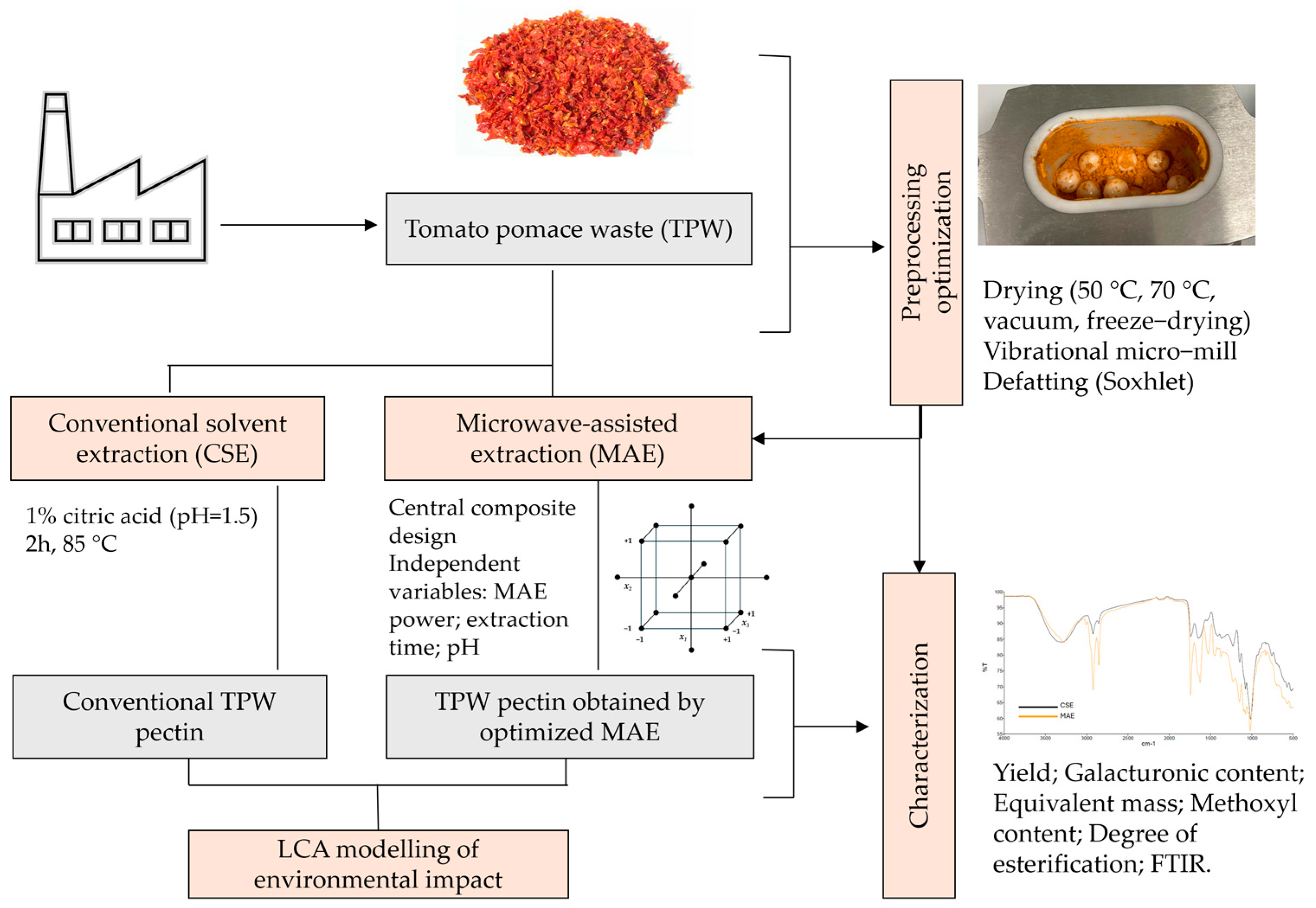
2. Materials and Methods
2.1. Raw Material and Organization of Experiments
2.2. Experimental Design
2.3. Pectin Extraction
2.3.1. Conventional Solvent Extraction of Pectin
2.3.2. Microwave-Assisted Extraction of Pectin
2.4. Pectin Characterization
2.4.1. Pectin Yield
2.4.2. Chemical Characterization of Pectin
2.4.3. Fourier Transform Infrared Spectroscopy
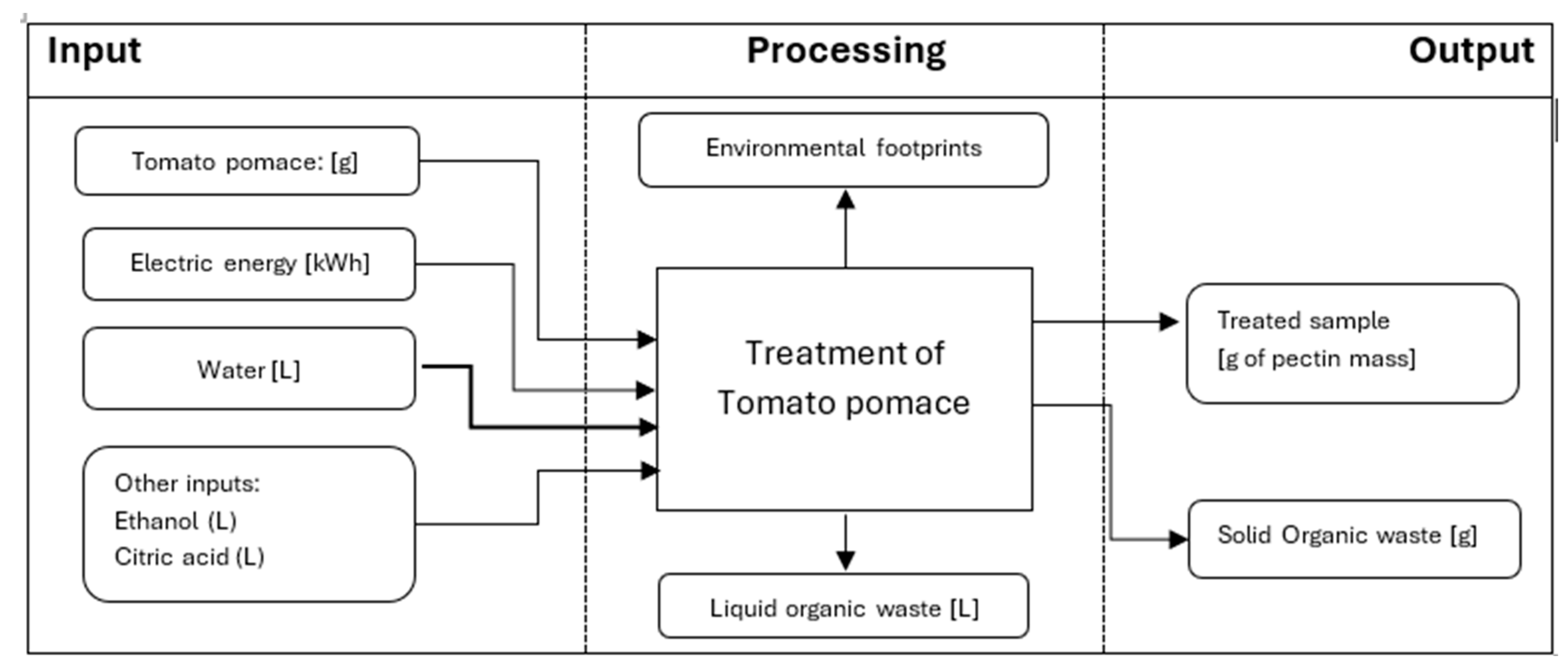
2.5. Modeling of Environmental Impact Using LCA
2.6. Data Analysis
3. Results and Discussion
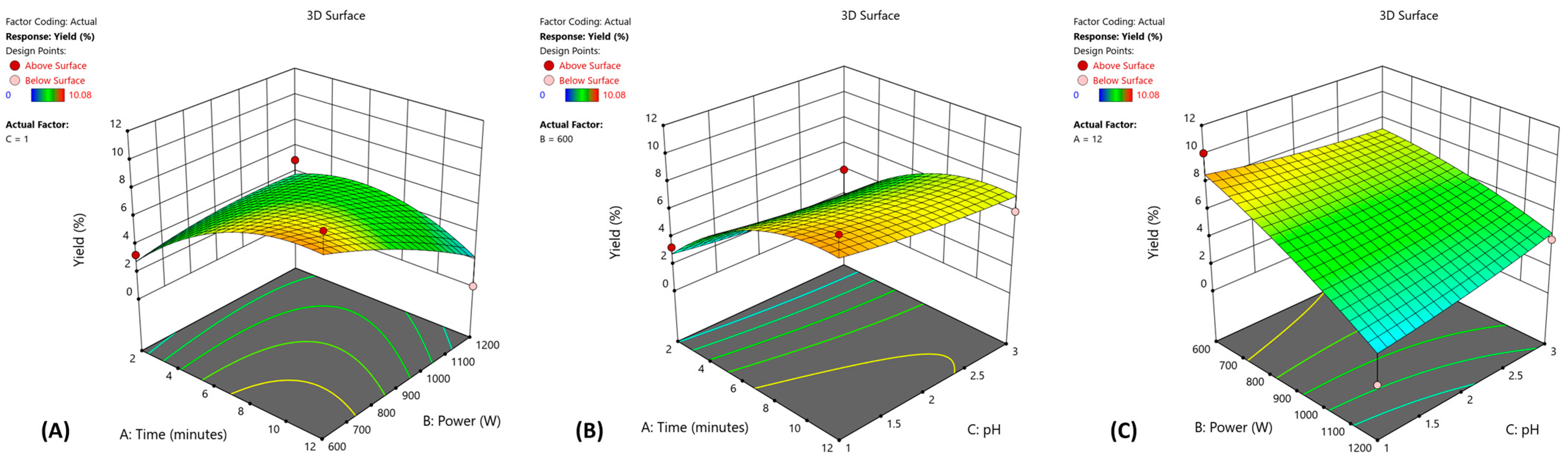
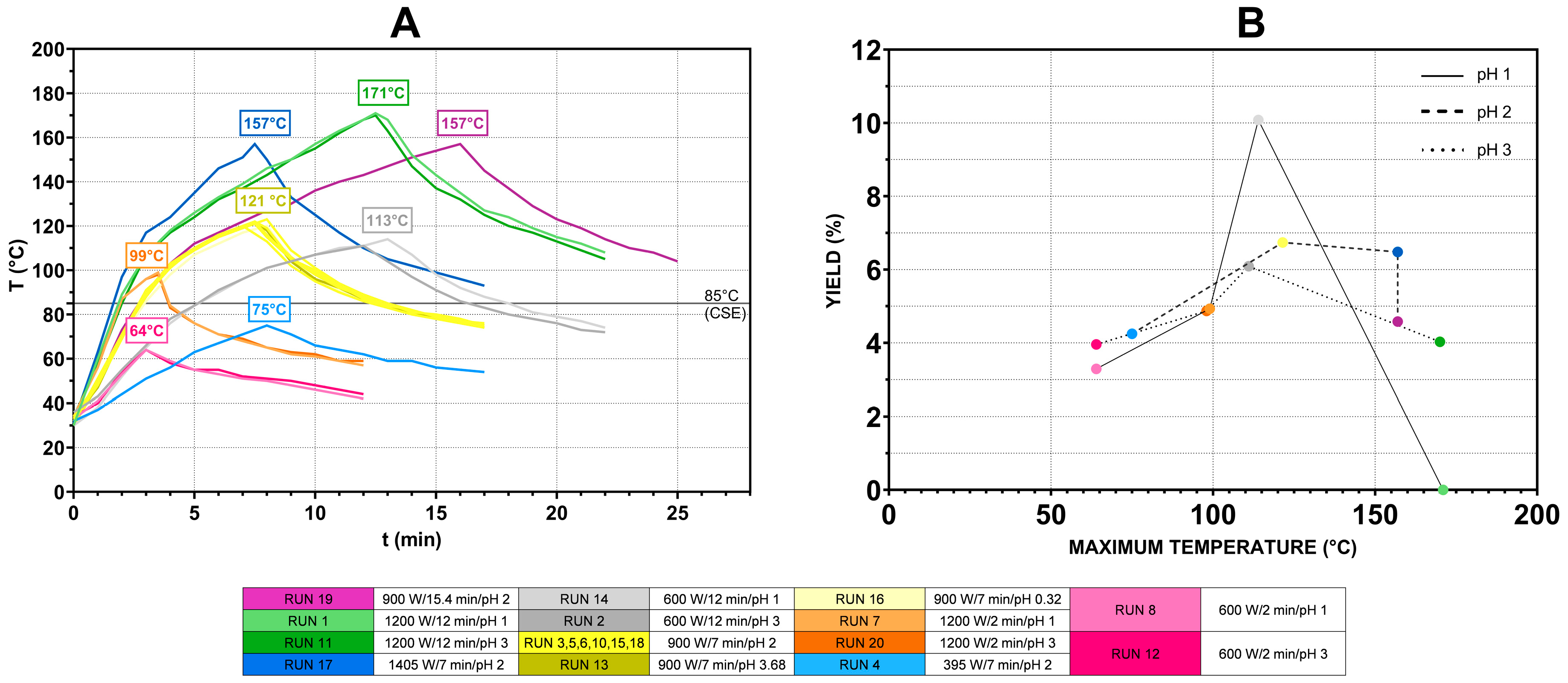
3.1. Optimization of Pectin Extraction
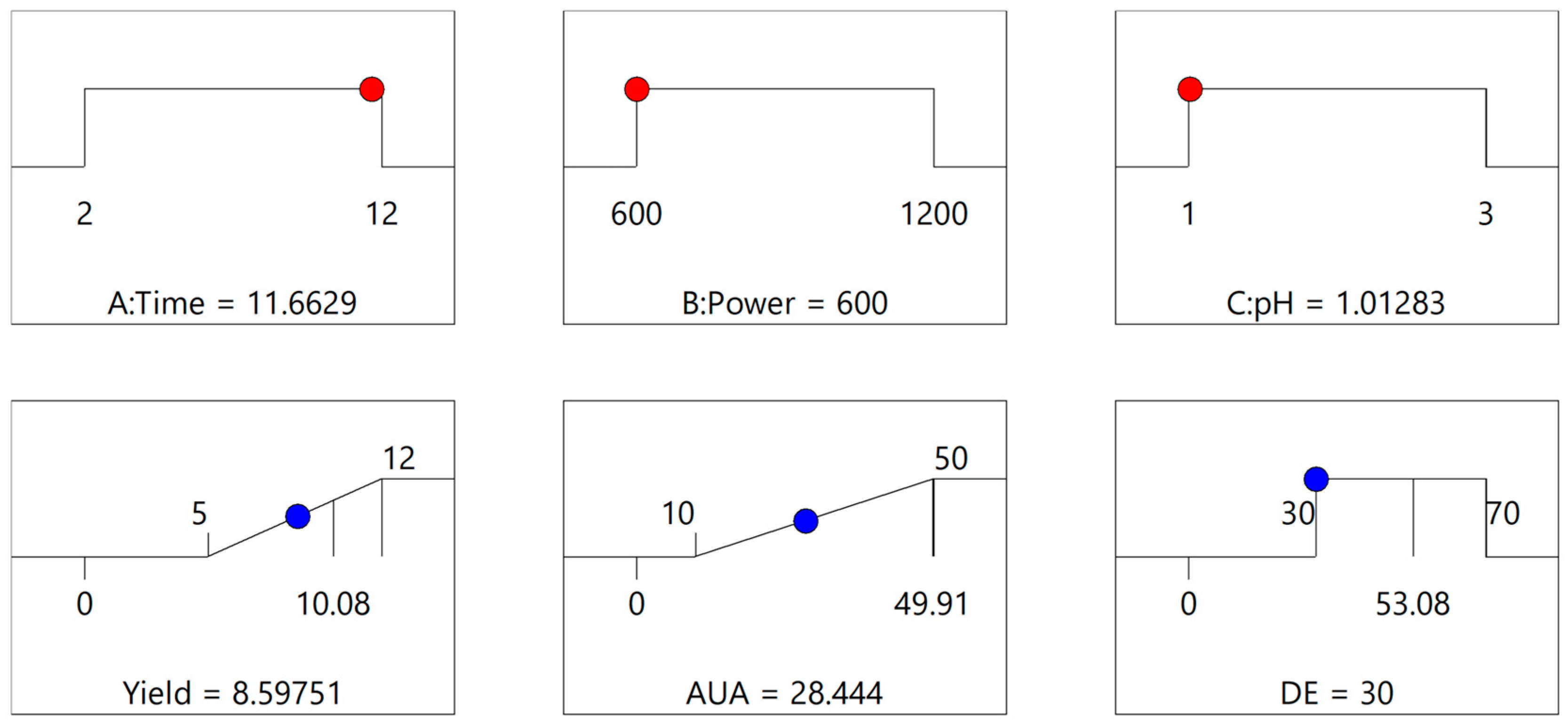
3.1.1. Fitting the Model
| Independent Variables | Experimental Values | |||||
|---|---|---|---|---|---|---|
| Variable 1: Time (min) | Variable 2: Power (W) | Variable 3: pH | Yield (%) | AUA (%) | DE (%) | |
| 1 | 12 | 1200 | 1 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 2 | 12 | 600 | 3 | 6.09 ± 0.09 | 24.56 ± 1.68 | 53.08 ± 2.25 |
| 3 | 7 | 900 | 2 | 7.09 ± 0.08 | 25.73 ± 1.55 | 45.72 ± 1.50 |
| 4 | 7 | 395 | 2 | 4.25 ± 0.04 | 39.49 ± 2.85 | 34.62 ± 1.37 |
| 5 | 7 | 900 | 2 | 7.04 ± 0.46 | 28.95 ± 1.12 | 39.67 ± 1.39 |
| 6 | 7 | 900 | 2 | 5.83 ± 0.12 | 20.87 ± 0.26 | 31.46 ± 0.96 |
| 7 | 2 | 1200 | 1 | 4.93 ± 0.06 | 32.69 ± 1.24 | 25.26 ± 0.75 |
| 8 | 2 | 600 | 1 | 3.29 ± 0.12 | 41.08 ± 9.23 | 31.64 ± 1.49 |
| 9 | 0 | 900 | 2 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 10 | 7 | 900 | 2 | 6.83 ± 0.37 | 25.90 ± 0.49 | 36.06 ± 0.87 |
| 11 | 12 | 1200 | 3 | 4.03 ± 0.15 | 26.56 ± 1.21 | 48.52 ± 2.50 |
| 12 | 2 | 600 | 3 | 3.96 ± 0.19 | 39.63 ± 1.34 | 45.48 ± 0.22 |
| 13 | 7 | 900 | 3.68 | 8.27 ± 0.43 | 28.98 ± 1.26 | 47.49 ± 1.11 |
| 14 | 12 | 600 | 1 | 10.08 ± 0.20 | 38.02 ± 1.00 | 35.13 ± 0.62 |
| 15 | 7 | 900 | 2 | 6.64 ± 0.35 | 29.70 ± 2.01 | 42.63 ± 0.61 |
| 16 | 7 | 900 | 0.32 | 6.22 ± 0.49 | 49.92 ± 4.85 | 14.37 ± 0.24 |
| 17 | 7 | 1405 | 2 | 6.48 ± 0.19 | 35.10 ± 1.29 | 44.97 ± 1.12 |
| 18 | 7 | 900 | 2 | 7.04 ± 0.34 | 25.54 ± 1.61 | 46.14 ± 1.38 |
| 19 | 15.41 | 900 | 2 | 4.58 ± 0.03 | 30.93 ± 0.22 | 48.92 ± 1.96 |
| 20 | 2 | 1200 | 3 | 4.87 ± 0.13 | 25.26 ± 0.55 | 51.56 ± 0.52 |
3.1.2. Multiple Response Optimization and Response Surface Analysis
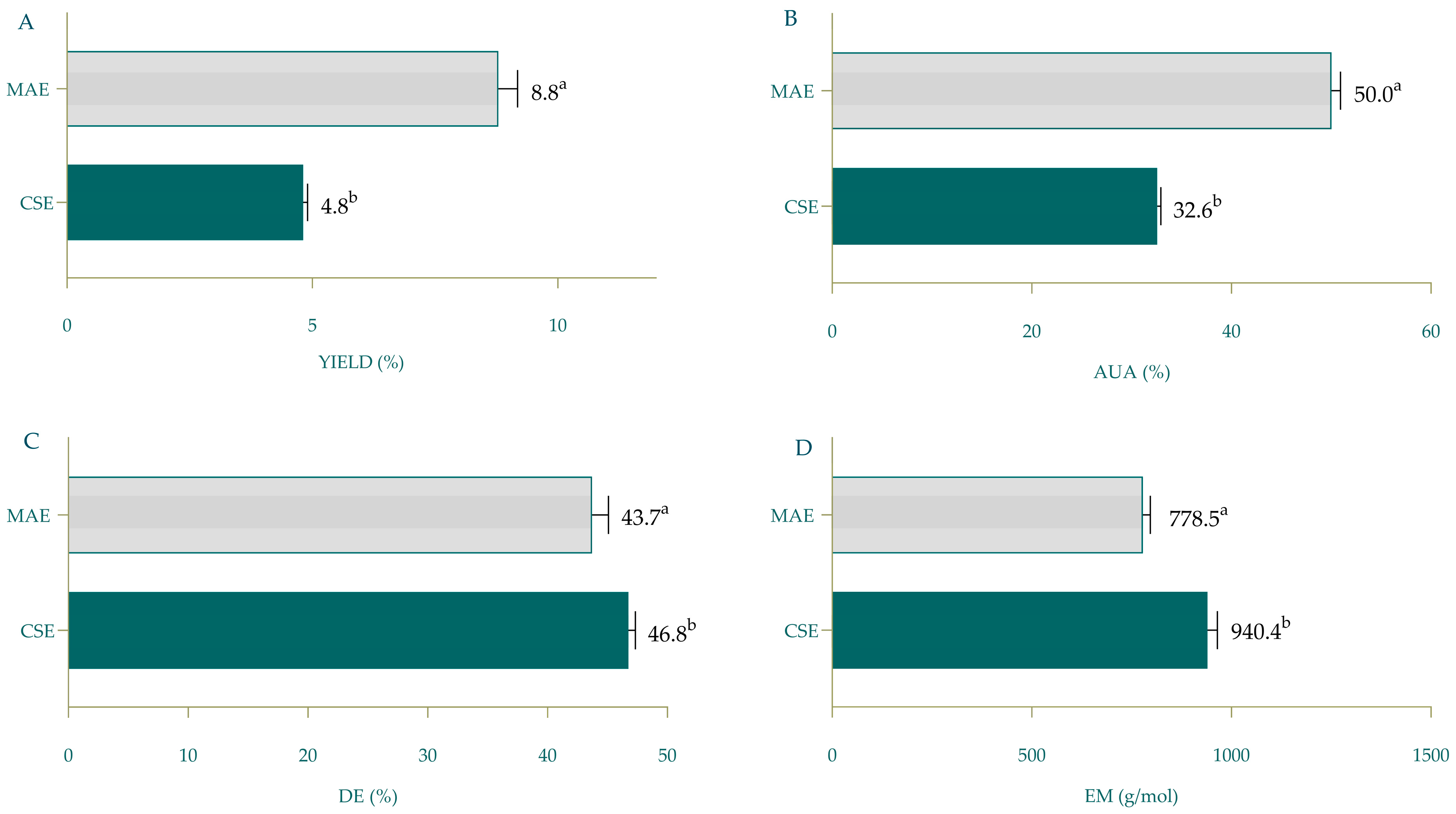
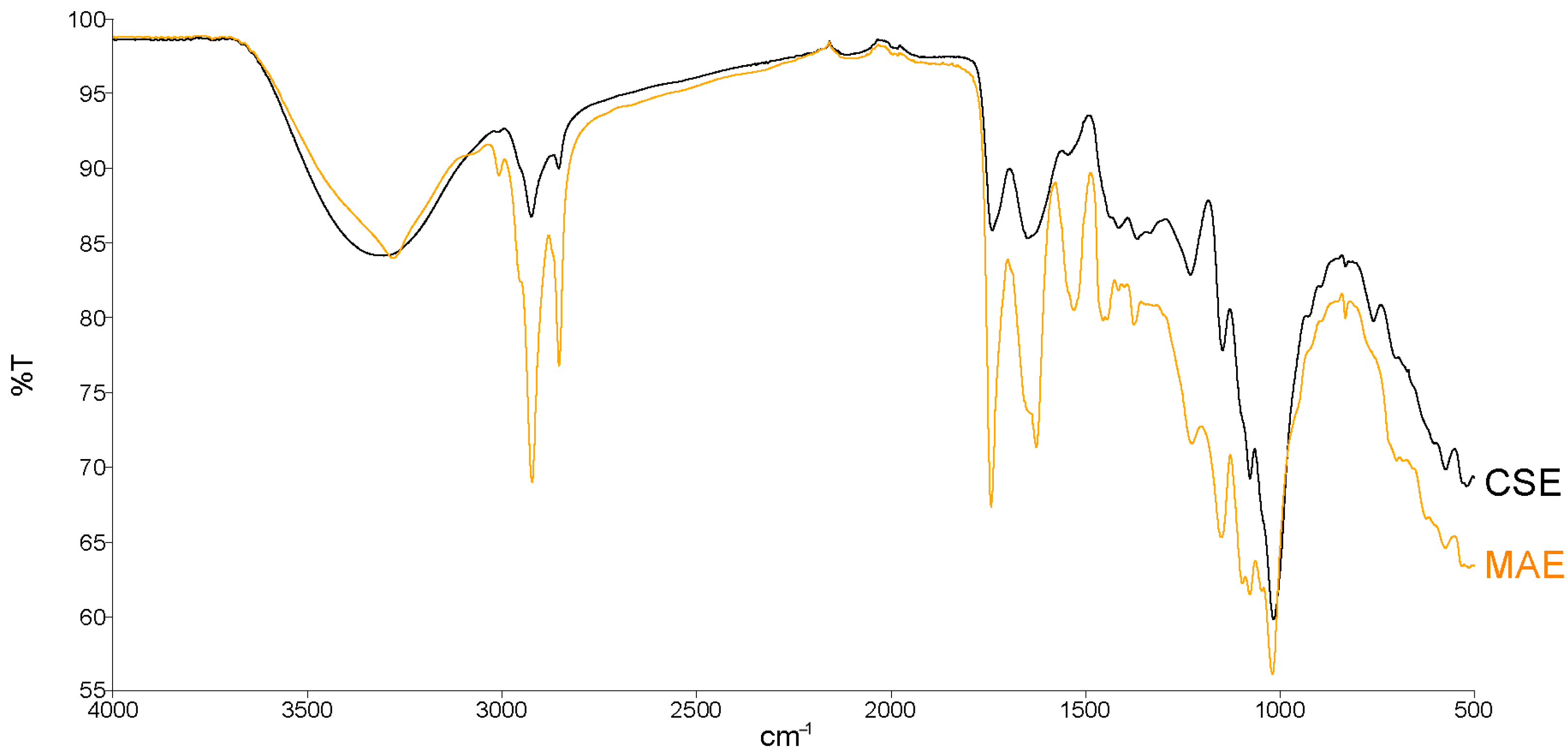
3.2. Comparison of TPW Pectin Obtained by CSE and MAE
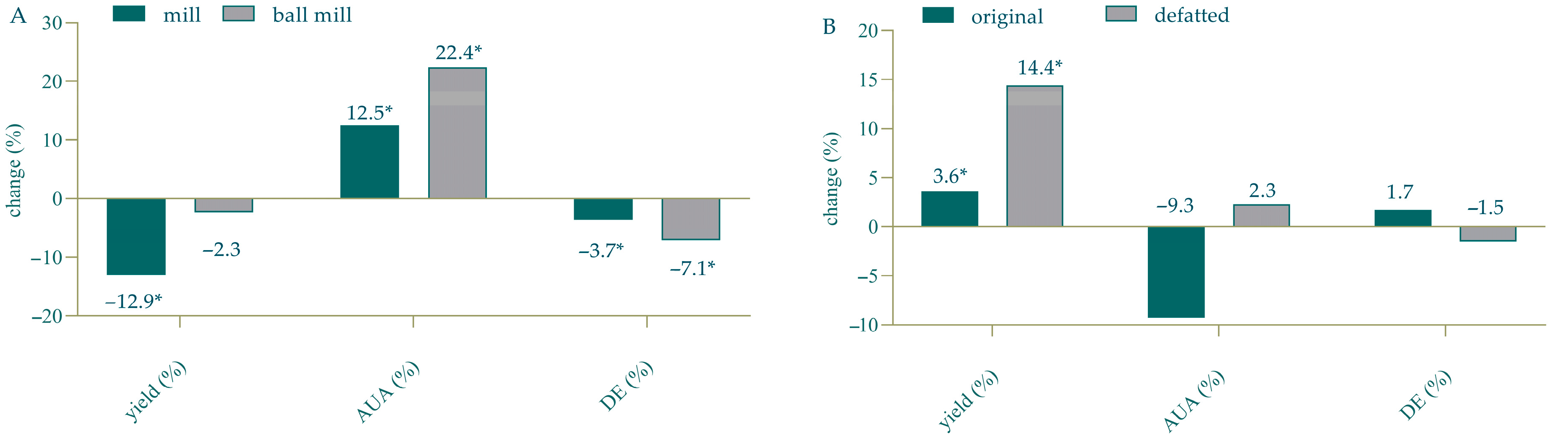
3.3. Impact of TPW Pretreatment on the Yield and Characteristics of Pectin Obtained via Optimized MAE
3.4. Environmental Impact of Tomato Pomace Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| TPW | Tomato pomace waste |
| MAE | Microwave-assisted extraction |
| CSE | Conventional solvent extraction |
| LCA | Life-cycle assessment |
| AUA | Anhydrouronic acid content |
| DE | Degree of esterification |
| DM | Degree of methylation |
| EM | Equivalent mass |
| FTIR | Fourier-transform infrared |
References
- DrugBank. Pectin. Available online: https://go.drugbank.com/drugs/DB11158 (accessed on 17 March 2025).
- Zdunek, A.; Pieczywek, P.M.; Cybulska, J. The Primary, Secondary, and Structures of Higher Levels of Pectin Polysaccharides. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1101–1117. [Google Scholar] [CrossRef] [PubMed]
- IndustryARC. Pectin Market Size, Share, Industry Trend & Forecast 2030. Available online: https://www.industryarc.com/Report/15977/pectin-market.html (accessed on 17 March 2025).
- Global Market Insights. Pectin Market Size & Share, Industry Analysis Report 2024–2032. Available online: https://www.gminsights.com/industry-analysis/pectin-market (accessed on 29 August 2024).
- Mellinas, C.; Ramos, M.; Jiménez, A.; Garrigós, M.C. Recent Trends in the Use of Pectin from Agro-Waste Residues as a Natural-Based Biopolymer for Food Packaging Applications. Materials 2020, 13, 673. [Google Scholar] [CrossRef] [PubMed]
- Nadar, C.G.; Arora, A.; Shastri, Y. Sustainability Challenges and Opportunities in Pectin Extraction from Fruit Waste. ACS Eng. Au 2022, 2, 61–74. [Google Scholar] [CrossRef]
- Bhatt, T.; Patel, K. Carotenoids: Potent to Prevent Diseases Review. Nat. Products Bioprospect. 2020, 10, 109–117. [Google Scholar] [CrossRef]
- Radić, K.; Galić, E.; Vinković, T.; Golub, N.; Vitali Čepo, D. Tomato Waste as a Sustainable Source of Antioxidants and Pectins: Processing, Pretreatment and Extraction Challenges. Sustainability 2024, 16, 9158. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, F.; Liu, X.; Luo, J.; Wu, J.; Wang, Z. Pectin from Black Tomato Pomace: Characterization, Interaction with Gallotannin, and Emulsifying Stability Properties. Starch—Stärke 2019, 71, 1800172. [Google Scholar] [CrossRef]
- Grassino, A.N.; Brnčić, M.; Vikić-Topić, D.; Roca, S.; Dent, M.; Brnčić, S.R. Ultrasound Assisted Extraction and Characterization of Pectin from Tomato Waste. Food Chem. 2016, 198, 93–100. [Google Scholar] [CrossRef]
- Almeida, P.V.; Gando-Ferreira, L.M.; Quina, M.J. Tomato Residue Management from a Biorefinery Perspective and towards a Circular Economy. Foods 2024, 13, 1873. [Google Scholar] [CrossRef]
- Bagade, S.B.; Patil, M. Recent Advances in Microwave Assisted Extraction of Bioactive Compounds from Complex Herbal Samples: A Review. Crit. Rev. Anal. Chem. 2021, 51, 138–149. [Google Scholar] [CrossRef]
- Petigny, L.; Périno, S.; Minuti, M.; Visinoni, F.; Wajsman, J.; Chemat, F. Simultaneous Microwave Extraction and Separation of Volatile and Non-Volatile Organic Compounds of Boldo Leaves. From Lab to Industrial Scale. Int. J. Mol. Sci. 2014, 15, 7183–7198. [Google Scholar] [CrossRef]
- Périno, S.; Pierson, J.T.; Ruiz, K.; Cravotto, G.; Chemat, F. Laboratory to Pilot Scale: Microwave Extraction for Polyphenols Lettuce. Food Chem. 2016, 204, 108–114. [Google Scholar] [CrossRef]
- Lasunon, P.; Phonkerd, N.; Tettawong, P.; Sengkhamparn, N. Effect of Microwave—Assisted Extraction on Bioactive Compounds from Industrial Tomato Waste and Its Antioxidant Activity. Food Res. 2021, 5, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Sengar, A.S.; Rawson, A.; Pare, A.; Kumar, K.S. Valorization of Tomato Processing Waste: Characterization and Process Optimization of Pectin Extraction. Int. J. Chem. Stud. 2019, 7, 3790–3794. [Google Scholar]
- Van Audenhove, J.; Bernaerts, T.; De Smet, V.; Delbaere, S.; Van Loey, A.M.; Hendrickx, M.E. The Structure and Composition of Extracted Pectin and Residual Cell Wall Material from Processing Tomato: The Role of a Stepwise Approach versus High-Pressure Homogenization-Facilitated Acid Extraction. Foods 2021, 10, 1064. [Google Scholar] [CrossRef] [PubMed]
- Golub, N.; Galić, E.; Radić, K.; Jagodić, A.-M.; Predović, N.; Katelan, K.; Tesla, L.; Pedisić, S.; Vinković, T.; Vitali Čepo, D. Phyto-Assisted Synthesis of Nanoselenium–Surface Modification and Stabilization by Polyphenols and Pectins Derived from Agricultural Wastes. Foods 2023, 12, 1117. [Google Scholar] [CrossRef]
- Casas-Orozco, D.; Villa, A.L.; Bustamante, F.; González, L.M. Process Development and Simulation of Pectin Extraction from Orange Peels. Food Bioprod. Process. 2015, 96, 86–98. [Google Scholar] [CrossRef]
- Ranganna, S. Hand Book of Analysis and Quality Control for Fruit and Vegetable Products, 2nd ed.; Tata-McGraw-Hill: New Delhi, India, 1995. [Google Scholar]
- Liew, S.Q.; Chin, N.L.; Yusof, Y.A. Extraction and Characterization of Pectin from Passion Fruit Peels. Agric. Agric. Sci. Procedia 2014, 2, 231–236. [Google Scholar] [CrossRef]
- Khamsucharit, P.; Laohaphatanalert, K.; Gavinlertvatana, P.; Sriroth, K.; Sangseethong, K. Characterization of Pectin Extracted from Banana Peels of Different Varieties. Food Sci. Biotechnol. 2018, 27, 623–629. [Google Scholar] [CrossRef]
- ISO 14040:2006; Environmental Management—Life Cycle Assessment—Principles and Framework. International Organization for Standardization (ISO): Geneva, Switzerland, 2006.
- Djekic, I.; Pojić, M.; Tonda, A.; Putnik, P.; Kovačević, D.B.; Režek-Jambrak, A.; Tomasevic, I. Scientific Challenges in Performing Life-Cycle Assessment in the Food Supply Chain. Foods 2019, 8, 301. [Google Scholar] [CrossRef]
- Gaffey, J.; Matinez, A.A.; Andrade, T.A.; Ambye-Jensen, M.; Bishop, G.; Collins, M.N.; Styles, D. Assessing the Environmental Footprint of Alternative Green Biorefinery Protein Extraction Techniques from Grasses and Legumes. Sci. Total Environ. 2024, 949, 175035. [Google Scholar] [CrossRef]
- de Moura, F.A.; Macagnan, F.T.; de Oliveira Petkowicz, C.L.; da Silva, L.P. Partially Hydrolyzed Pectin Extracted from Passion Fruit Peel: Molar Mass and Physicochemical Properties. Bioact. Carbohydrates Diet. Fibre 2020, 21, 100206. [Google Scholar] [CrossRef]
- Riyamol; Gada Chengaiyan, J.; Rana, S.S.; Ahmad, F.; Haque, S.; Capanoglu, E. Recent Advances in the Extraction of Pectin from Various Sources and Industrial Applications. ACS Omega 2023, 8, 46309–46324. [Google Scholar] [CrossRef]
- Rodulfo, R.S.; Castillo-Israel, K.A.T.; Gaban, P.J.V.; Ilano, M.C.R.; Benedicto, J.B.; Badua, M.A.A.; Rivadeneira, J.P. Downstream Processing of Crude Ultrasound-Extracted Pectin From Saba Banana (Musa Acuminata x Balbisiana (BBB Group) “Saba”) Peel. Int. J. Food Sci. 2024, 2024, 9892858. [Google Scholar] [CrossRef] [PubMed]
- Alancay, M.M.; Lobo, M.O.; Quinzio, C.M.; Iturriaga, L.B. Extraction and Physicochemical Characterization of Pectin from Tomato Processing Waste. J. Food Meas. Charact. 2017, 11, 2119–2130. [Google Scholar] [CrossRef]
- Spinei, M.; Oroian, M. Microwave-Assisted Extraction of Pectin from Grape Pomace. Sci. Rep. 2022, 12, 12722. [Google Scholar] [CrossRef] [PubMed]
- Thu Dao, T.A.; Webb, H.K.; Malherbe, F. Optimization of Pectin Extraction from Fruit Peels by Response Surface Method: Conventional versus Microwave-Assisted Heating. Food Hydrocoll. 2021, 113, 106475. [Google Scholar] [CrossRef]
- Swamy, G.J.; Muthukumarappan, K. Optimization of Continuous and Intermittent Microwave Extraction of Pectin from Banana Peels. Food Chem. 2017, 220, 108–114. [Google Scholar] [CrossRef]
- Rahmani, Z.; Khodaiyan, F.; Kazemi, M.; Sharifan, A. Optimization of Microwave-Assisted Extraction and Structural Characterization of Pectin from Sweet Lemon Peel. Int. J. Biol. Macromol. 2020, 147, 1107–1115. [Google Scholar] [CrossRef]
- Sarah, M.; Hasibuan, I.M.; Misran, E.; Maulina, S. Optimization of Microwave-Assisted Pectin Extraction from Cocoa Pod Husk. Molecules 2022, 27, 6544. [Google Scholar] [CrossRef]
- Chandel, V.; Biswas, D.; Roy, S.; Vaidya, D.; Verma, A.; Gupta, A. Current Advancements in Pectin: Extraction, Properties and Multifunctional Applications. Foods 2022, 11, 2683. [Google Scholar] [CrossRef]
- Belkheiri, A.; Forouhar, A.; Ursu, A.V.; Dubessay, P.; Pierre, G.; Delattre, C.; Djelveh, G.; Abdelkafi, S.; Hamdami, N.; Michaud, P. Extraction, Characterization, and Applications of Pectins from Plant by-Products. Appl. Sci. 2021, 11, 6596. [Google Scholar] [CrossRef]
- Majid, M.; Fallah-Joshaqani, S.; Dalvi-Isfahan, M.; Hamdami, R. Comparison of Conventional and Microwave-Assisted Extraction of Pectin from Watermelon Rind. J. Polym. Environ. 2023, 31, 3363–3371. [Google Scholar] [CrossRef]
- Yu, Y.; Lu, P.; Yang, Y.; Ji, H.; Zhou, H.; Chen, S.; Qiu, Y.; Chen, H. Differences in Physicochemical Properties of Pectin Extracted from Pomelo Peel with Different Extraction Techniques. Sci. Rep. 2024, 14, 9182. [Google Scholar] [CrossRef] [PubMed]
- Bagherian, H.; Zokaee Ashtiani, F.; Fouladitajar, A.; Mohtashamy, M. Comparisons between Conventional, Microwave- and Ultrasound-Assisted Methods for Extraction of Pectin from Grapefruit. Chem. Eng. Process. Process Intensif. 2011, 50, 1237–1243. [Google Scholar] [CrossRef]
- Košťálová, Z.; Aguedo, M.; Hromádková, Z. Microwave-Assisted Extraction of Pectin from Unutilized Pumpkin Biomass. Chem. Eng. Process. Process Intensif. 2016, 102, 9–15. [Google Scholar] [CrossRef]
- Galbe, M.; Wallberg, O. Pretreatment for Biorefineries: A Review of Common Methods for Efficient Utilisation of Lignocellulosic Materials. Biotechnol. Biofuels 2019, 12, 294. [Google Scholar] [CrossRef]
- Peral, C. Biomass Pretreatment Strategies (Technologies, Environmental Performance, Economic Considerations, Industrial Implementation). In Biotransformation of Agricultural Waste and By-Products; Poltronieri, P., D’Urso, O.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 125–160. [Google Scholar]
- De Laet, E.; Bernaerts, T.; Dewettinck, K.; Hendrickx, M.E.; Van Loey, A.M. The Effect of Different Particle Size Reduction Techniques on the Biomass Microstructure and the Influence on the Pectin Extraction Yield and Structure; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Ramirez Cabrera, P.A.; Lozano Pérez, A.S.; Lozada Castro, J.J.; Sierra Vargas, F.E.; Guerrero Fajardo, C.A. Revolutionizing Biomass Processing: The Design and Functionality of an Innovative Extruder for Sugarcane Bagasse Milling Pretreatment. Designs 2024, 8, 85. [Google Scholar] [CrossRef]
- Djurle, S.; Andersson, A.A.M.; Andersson, R. Milling and Extrusion of Six Barley Varieties, Effects on Dietary Fibre and Starch Content and Composition. J. Cereal Sci. 2016, 72, 146–152. [Google Scholar] [CrossRef]
- Yi, J.; Li, X.; He, J.; Duan, X. Drying Efficiency and Product Quality of Biomass Drying: A Review. Dry. Technol. 2020, 38, 2039–2054. [Google Scholar] [CrossRef]
- Siriwattananon, L.; Maneerate, J. Effect of Drying Methods on Dietary Fiber Content in Dried Fruit and Vegetable from Non-Toxic Agricultural Field. Int. J. Geomate 2016, 11, 2896–2900. [Google Scholar] [CrossRef]
- Nutrizio, M.; Dukić, J.; Sabljak, I.; Samardžija, A.; Biondić Fučkar, V.; Djekić, I.; Režek Jambrak, A. Upcycling of Food By-Products and Waste: Nonthermal Green Extractions and Life Cycle Assessment Approach. Sustainability 2024, 16, 9143. [Google Scholar] [CrossRef]
- Djekic, I.; Tomasević, I. Analysis and Comparison of Environmental Impacts of Nonthermal Food Technologies. In Nonthermal Processing in Agri-Food-Bio Sciences; Režek Jambrak, A., Ed.; Springer: Cham, Switzerland, 2022; pp. 671–685. [Google Scholar]
- Yadav, R.D.; Dhamole, P.B. Techno Economic and Life Cycle Assessment of Lycopene Production from Tomato Peels Using Different Extraction Methods. Biomass Convers. Biorefinery 2024, 14, 25495–25511. [Google Scholar] [CrossRef]
| Independent Variables | Levels | |||||
|---|---|---|---|---|---|---|
| Uncoded | Coded | −α | −1 | 0 | +1 | +α |
| Microwave power (W) | X1 | 395 | 600 | 900 | 1200 | 1405 |
| Extraction time (min) | X2 | 0 | 2 | 7 | 12 | 15.4 |
| pH | X3 | 0.32 | 1 | 2 | 3 | 3.68 |
| Analysis | Predicted Mean | SE Mean | 95% CI Low | 95% CI High | Observed Mean |
|---|---|---|---|---|---|
| Yield | 8.59 ± 1.75 | 1.37 | 5.53 | 11.66 | 8.79 ± 0.39 |
| AUA | 28.44 ± 12.04 | 2.69 | 22.8 | 34.08 | 50.03 ± 0.91 |
| DE | 30.00 ± 12.32 | 6.28 | 16.67 | 43.32 | 43.71 ± 1.37 |
| Conventional Drying | Vacuum Drying | Freeze Drying | ||
|---|---|---|---|---|
| 70 °C | 50 °C | |||
| Yield (%) | 13.9 ± 0.21 a | 15.4 ± 0.07 a | 14.8 ± 0.00 a | 15.2 ± 0.90 a |
| EM (g/mol) | 782.9 ± 28.1 a | 706.2 ± 53.4 b | 805.9 ± 3.36 a | 799.0 ± 9.07 a |
| MC (%) | 4.43 ± 0.30 ab | 3.49 ± 0.46 a | 4.10 ± 0.49 ab | 4.77 ± 0.79 b |
| AUA (%) | 47.6 ± 2.25 a | 44.9 ± 4.31 a | 45.1 ± 2.79 a | 49.1 ± 4.21 a |
| DE (%) | 41.2 ± 0.95 a | 38.5 ± 0.71 b | 41.4 ± 1.29 a | 38.3 ± 2.04 b |
| CSE | MAE | Index | CSE | MAE | Index | ||
|---|---|---|---|---|---|---|---|
| FU1–One Treatment | FU2–Pectin [g] | ||||||
| Acidification | mol H+ eq | 0.29 | 0.13 | 2.14 | 0.17 | 0.09 | 1.81 |
| Climate change | kg CO2e | 38.97 | 18.15 | 2.15 | 23.20 | 12.78 | 1.81 |
| Freshwater eutrophication | kg P eq | 2.69 × 10−3 | 1.16 × 10−3 | 2.33 | 1.60 × 10−3 | 8.14 × 10−4 | 1.97 |
| Human toxicity-cancerogenic | CTUh | 5.81 × 10−8 | 2.62 × 10−8 | 2.22 | 3.46 × 10−8 | 1.84 × 10−8 | 1.88 |
| Human toxicity-non cancerogenic | CTUh | 1.28 × 10−6 | 5.91 × 10−7 | 2.17 | 7.63 × 10−7 | 4.16 × 10−7 | 1.83 |
| Ionizing radiation | kBq U-235 | 3.00 | 1.40 | 2.14 | 1.79 | 0.99 | 1.81 |
| Ozone depletion | kg CFC11 eq | 6.55 × 10−8 | 2.81 × 10−8 | 2.33 | 3.90 × 10−8 | 1.98 × 10−8 | 1.97 |
| Use of fossil resource | MJ | 587.96 | 272.66 | 2.16 | 349.97 | 192.01 | 1.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golub, N.; Galić, E.; Radić, K.; Smigic, N.; Djekić, I.; Pedisić, S.; Čepo, D.V. Microwave-Assisted Valorization of Tomato Pomace for Pectin Recovery: Improving Yields and Environmental Footprint. Foods 2025, 14, 1516. https://doi.org/10.3390/foods14091516
Golub N, Galić E, Radić K, Smigic N, Djekić I, Pedisić S, Čepo DV. Microwave-Assisted Valorization of Tomato Pomace for Pectin Recovery: Improving Yields and Environmental Footprint. Foods. 2025; 14(9):1516. https://doi.org/10.3390/foods14091516
Chicago/Turabian StyleGolub, Nikolina, Emerik Galić, Kristina Radić, Nada Smigic, Ilija Djekić, Sandra Pedisić, and Dubravka Vitali Čepo. 2025. "Microwave-Assisted Valorization of Tomato Pomace for Pectin Recovery: Improving Yields and Environmental Footprint" Foods 14, no. 9: 1516. https://doi.org/10.3390/foods14091516
APA StyleGolub, N., Galić, E., Radić, K., Smigic, N., Djekić, I., Pedisić, S., & Čepo, D. V. (2025). Microwave-Assisted Valorization of Tomato Pomace for Pectin Recovery: Improving Yields and Environmental Footprint. Foods, 14(9), 1516. https://doi.org/10.3390/foods14091516









