Impact of Pumpkin Seed, Brown Rice, Yellow Pea, and Hemp Seed Proteins on the Physicochemical, Technological, and Sensory Properties of Green Lentil Cookies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ingredients Used for Preparation of Samples
2.2. Preparation of Cookie Samples
2.3. Reagents and Standards Used for Chemical Determinations
2.4. Determination of Baking Loss and Geometry
2.5. Color Measurement
2.6. Determination of Antioxidant Properties
2.7. Determination of Crude Protein Content
2.8. pH Measurement
2.9. Texture Profile Analysis
2.10. Sensory Tests
2.11. Data Analysis
3. Results and Discussion
3.1. Physicochemical Properties of Vegan Lentil Cookies
3.1.1. Color
3.1.2. Total Polyphenol and Flavonoid Content, Antioxidant Capacity, and Crude Protein Content
3.1.3. Baking Loss and Geometry
3.2. Texture Properties of Vegan Lentil Cookies
3.3. Sensory Properties of Vegan Lentil Cookies
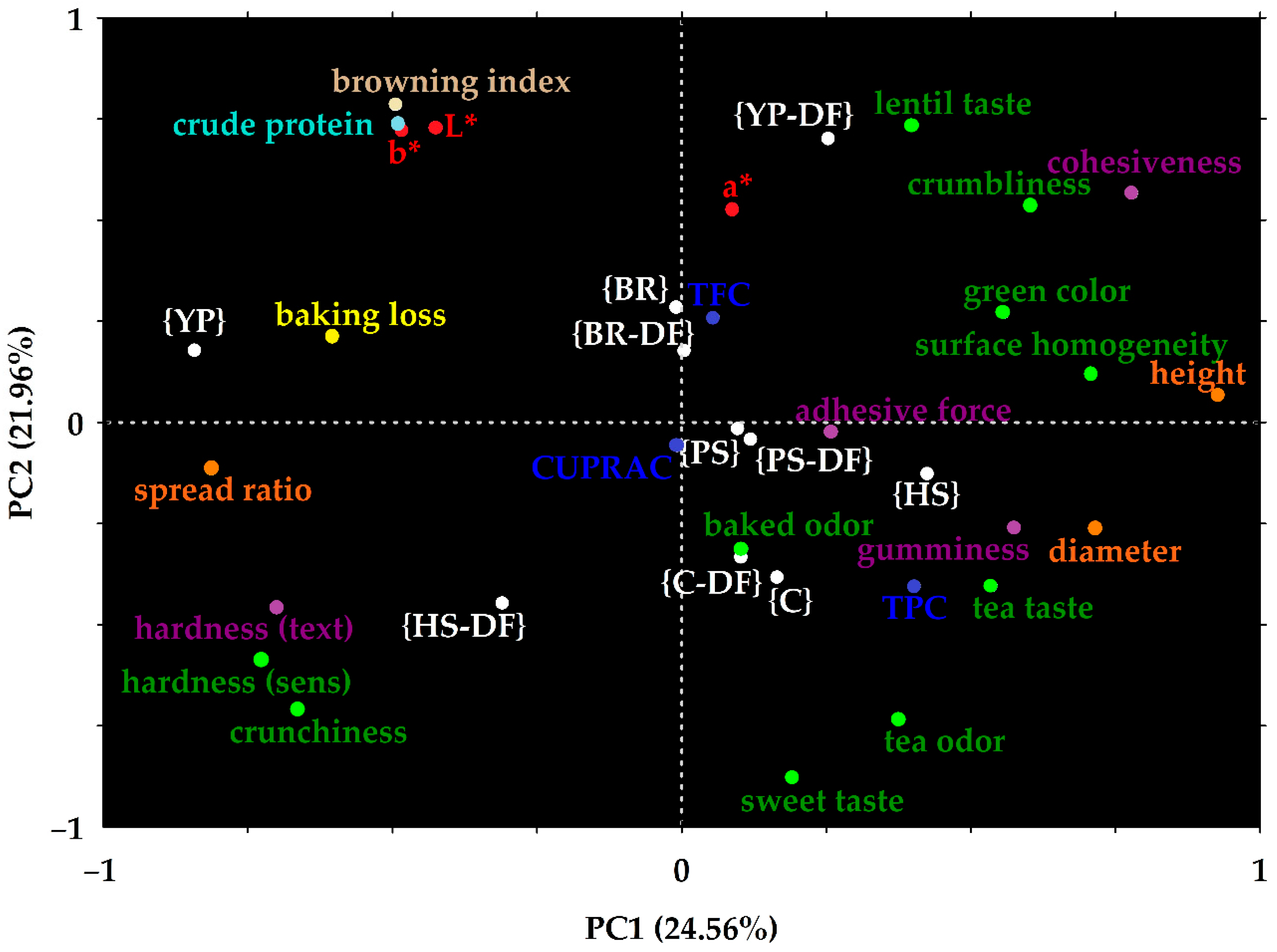
3.4. Principal Components Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flores-Hernández, M.N.; Martínez-Coria, H.; López-Valdés, H.E.; Arteaga-Silva, M.; Arrieta-Cruz, I.; Gutiérrez-Juárez, R. Efficacy of a High-Protein Diet to Lower Glycemic Levels in Type 2 Diabetes Mellitus: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 10959. [Google Scholar] [CrossRef] [PubMed]
- Tormási, J.; Benes, E.; Kónya, É.L.; Berki, M.; Abrankó, L. Evaluation of Protein Quantity and Protein Nutritional Quality of Protein Bars with Different Protein Sources. Sci. Rep. 2025, 15, 9388. [Google Scholar] [CrossRef]
- Hajas, L.; Sipos, L.; Csobod, É.C.; Bálint, M.V.; Juhász, R.; Benedek, C. Lentil (Lens Culinaris Medik.) Flour Varieties as Promising New Ingredients for Gluten-Free Cookies. Foods 2022, 11, 2028. [Google Scholar] [CrossRef]
- Nemś, A.; Miedzianka, J.; Kita, A. Quality and Nutritional Value of Cookies Enriched with Plant-based Protein Preparations. J. Sci. Food Agric. 2022, 102, 4629–4639. [Google Scholar] [CrossRef]
- Sahagún, M.; Gómez, M. Influence of Protein Source on Characteristics and Quality of Gluten-Free Cookies. J. Food Sci. Technol. 2018, 55, 4131–4138. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Núñez, Á.; Gómez, M. Enrichment of Cakes and Cookies with Pulse Flours. A Review. Food Rev. Int. 2023, 39, 2895–2913. [Google Scholar] [CrossRef]
- Park, S.; Church, D.D.; Schutzler, S.E.; Azhar, G.; Kim, I.-Y.; Ferrando, A.A.; Wolfe, R.R. Metabolic Evaluation of the Dietary Guidelines’ Ounce Equivalents of Protein Food Sources in Young Adults: A Randomized Controlled Trial. J. Nutr. 2021, 151, 1190–1196. [Google Scholar] [CrossRef]
- Zahari, I.; Östbring, K.; Purhagen, J.K.; Rayner, M. Plant-Based Meat Analogues from Alternative Protein: A Systematic Literature Review. Foods 2022, 11, 2870. [Google Scholar] [CrossRef]
- Kołodziejczak, K.; Onopiuk, A.; Szpicer, A.; Poltorak, A. Meat Analogues in the Perspective of Recent Scientific Research: A Review. Foods 2021, 11, 105. [Google Scholar] [CrossRef]
- Vignesh, K.; Yadav, D.K.; Wadikar, D.D.; Semwal, A.D. Exploring Sustenance: Cereal Legume Combinations for Vegan Meat Development. Sustain. Food Technol. 2024, 2, 32–47. [Google Scholar] [CrossRef]
- Janssen, M.; Busch, C.; Rödiger, M.; Hamm, U. Motives of Consumers Following a Vegan Diet and Their Attitudes towards Animal Agriculture. Appetite 2016, 105, 643–651. [Google Scholar] [CrossRef]
- Bohrer, B.M. An Investigation of the Formulation and Nutritional Composition of Modern Meat Analogue Products. Food Sci. Hum. Wellness 2019, 8, 320–329. [Google Scholar] [CrossRef]
- Fouillet, H.; Dussiot, A.; Perraud, E.; Wang, J.; Huneau, J.-F.; Kesse-Guyot, E.; Mariotti, F. Plant to Animal Protein Ratio in the Diet: Nutrient Adequacy, Long-Term Health and Environmental Pressure. Front. Nutr. 2023, 10, 1178121. [Google Scholar] [CrossRef] [PubMed]
- Sobiecki, J.G.; Appleby, P.N.; Bradbury, K.E.; Key, T.J. High Compliance with Dietary Recommendations in a Cohort of Meat Eaters, Fish Eaters, Vegetarians, and Vegans: Results from the European Prospective Investigation into Cancer and Nutrition–Oxford Study. Nutr. Res. 2016, 36, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, F.; Gardner, C.D. Dietary Protein and Amino Acids in Vegetarian Diets—A Review. Nutrients 2019, 11, 2661. [Google Scholar] [CrossRef]
- Bartholomae, E.; Johnston, C.S. Nitrogen Balance at the Recommended Dietary Allowance for Protein in Minimally Active Male Vegans. Nutrients 2023, 15, 3159. [Google Scholar] [CrossRef]
- Cairano, M.D.; Galgano, F.; Tolve, R.; Caruso, M.C.; Condelli, N. Focus on Gluten Free Biscuits: Ingredients and Issues. Trends Food Sci. Technol. 2018, 81, 203–212. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F. Gluten-Free Diet: Gaps and Needs for a Healthier Diet. Nutrients 2019, 11, 170. [Google Scholar] [CrossRef]
- Jnawali, P.; Kumar, V.; Tanwar, B. Celiac Disease: Overview and Considerations for Development of Gluten-Free Foods. Food Sci. Hum. Wellness 2016, 5, 169–176. [Google Scholar] [CrossRef]
- Rojas Conzuelo, Z.; Bez, N.S.; Theobald, S.; Kopf-Bolanz, K.A. Protein Quality Changes of Vegan Day Menus with Different Plant Protein Source Compositions. Nutrients 2022, 14, 1088. [Google Scholar] [CrossRef]
- Aaslyng, M.D.; Dam, A.B.; Petersen, I.L.; Christoffersen, T. Protein Content and Amino Acid Composition in the Diet of Danish Vegans: A Cross-Sectional Study. BMC Nutr. 2023, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P. Functional Foods-Based Diet as a Novel Dietary Approach for Management of Type 2 Diabetes and Its Complications: A Review. World J. Diabetes 2014, 5, 267. [Google Scholar] [CrossRef]
- Liberal, Â.; Almeida, D.; Fernandes, Â.; Pereira, C.; Ferreira, I.C.; Vivar-Quintana, A.M.; Barros, L. Nutritional, Chemical, and Antioxidant Screening of Selected Varieties of Lentils (Lens Culinaris spp.) from Organic and Conventional Agriculture. J. Sci. Food Agric. 2024, 104, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Keskin, S.O.; Sumnu, G. Versatile Functions of Raw and Modified Lentils/Lentil Components in Food Applications: A Review. Legum. Sci. 2024, 6. [Google Scholar] [CrossRef]
- Alrosan, M.; Tan, T.-C.; Easa, A.M.; Gammoh, S.; Alu’datt, M.H.; Kubow, S.; Almajwal, A.M.; Razzak Mahmood, A.A.; Al-Qaisi, A.; Bawadi, H. Enhancing the Quality of Lentil Proteins via Combination with Whey Proteins Based on a Dual Process: A Novel Strategy through the Incorporation of Complexation and Fermentation. Food Sci. Biotechnol. 2025, 34, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Alrosan, M.; Tan, T.-C.; Mat Easa, A.; Gammoh, S.; Alu’datt, M.H. Recent Updates on Lentil and Quinoa Protein-Based Dairy Protein Alternatives: Nutrition, Technologies, and Challenges. Food Chem. 2022, 383, 132386. [Google Scholar] [CrossRef]
- Cauvain, S.P.; Clark, R.H. Baking Technology and Nutrition: Towards a Healthier World; Wiley: Hoboken, NJ, USA, 2019; ISBN 978-1-119-38715-2. [Google Scholar]
- AACC (American Association of Cereal Chemists). Approved Methods of the American Association of Cereal Chemists. In Method 10-50D, 10th ed.; American Association of Cereal Chemists: St. Paul, MN, USA, 2000. [Google Scholar]
- Hajas, L.; Benedek, C.; Csajbókné Csobod, É.; Juhász, R. Development of Protein- and Fiber-Enriched, Sugar-Free Lentil Cookies: Impact of Whey Protein, Inulin, and Xylitol on Physical, Textural, and Sensory Characteristics. Foods 2022, 11, 3819. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods. Off. J. Eur. Union 2006, L404, 9–25.
- Buera, M.P.; Lozano, R.D.; Petriella, C. Definition of Colour in the Non Enzymatic Browning Proces. Die Farbe 1985, 32/33, 318–326. [Google Scholar]
- Feumba Dibanda, R.; Panyoo Akdowa, E.; Rani, P.A.; Metsatedem Tongwa, Q.; Mbofung, F.C.M. Effect of Microwave Blanching on Antioxidant Activity, Phenolic Compounds and Browning Behaviour of Some Fruit Peelings. Food Chem. 2020, 302, 125308. [Google Scholar] [CrossRef]
- CIE (Commission Internationale de l’Éclairage). Technical Report: Colorimetry, 3rd ed.; Commission Internationale de l’Éclairage: Washington, DC, USA, 2004; ISBN 390-190-633-9. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of Total Flavonoid Content in Propolis by Two Complementary Colometric Methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Demirata, B.; Özyürek, M.; Çelik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Özyurt, D. Comparative Evaluation of Various Total Antioxidant Capacity Assays Applied to Phenolic Compounds with the CUPRAC Assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef] [PubMed]
- Bender, D.A. A Dictionary of Food and Nutrition, 4th ed.; Oxford University Press: Oxford, UK, 2014; ISBN 978-019-175-239-1. [Google Scholar]
- Park, H.-R.; Kim, G.-H.; Na, Y.; Oh, J.-E.; Cho, M.-S. Physicochemical and Sensory Properties of Protein-Fortified Cookies According to the Ratio of Isolated Soy Protein to Whey Protein. Food Sci. Biotechnol. 2021, 30, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Adeola, A.A.; Ohizua, E.R. Physical, Chemical, and Sensory Properties of Biscuits Prepared from Flour Blends of Unripe Cooking Banana, Pigeon Pea, and Sweet Potato. Food Sci. Nutr. 2018, 6, 532–540. [Google Scholar] [CrossRef]
- Pereira, D.; Correia, P.M.R.; Guiné, R.P.F. Analysis of the Physical-Chemical and Sensorial Properties of Maria Type Cookies. Acta Chim. Slovaca 2013, 6, 269–280. [Google Scholar] [CrossRef]
- Liu, S.; Sun, H.; Ma, G.; Zhang, T.; Wang, L.; Pei, H.; Li, X.; Gao, L. Insights into Flavor and Key Influencing Factors of Maillard Reaction Products: A Recent Update. Front. Nutr. 2022, 9, 973677. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, X.; Wang, Y.; Ye, W.; Lin, Y.; Zhang, Y.; Zhang, K.; Zhao, K.; Guo, H. The Non-Covalent and Covalent Interactions of Whey Proteins and Saccharides: Influencing Factor and Utilization in Food. Crit. Rev. Food Sci. Nutr. 2024, 1–15. [Google Scholar] [CrossRef]
- Kutzli, I.; Weiss, J.; Gibis, M. Glycation of Plant Proteins Via Maillard Reaction: Reaction Chemistry, Technofunctional Properties, and Potential Food Application. Foods 2021, 10, 376. [Google Scholar] [CrossRef]
- Kathuria, D.; Hamid; Gautam, S.; Thakur, A. Maillard Reaction in Different Food Products: Effect on Product Quality, Human Health and Mitigation Strategies. Food Control 2023, 153, 109911. [Google Scholar] [CrossRef]
- Bandyopadhyay, P.; Ghosh, A.K.; Ghosh, C. Recent Developments on Polyphenol–Protein Interactions: Effects on Tea and Coffee Taste, Antioxidant Properties and the Digestive System. Food Funct. 2012, 3, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K.; Sjaarda, C.; Maldonado-Alvarez, L. A New Look on Protein-Polyphenol Complexation during Honey Storage: Is This a Random or Organized Event with the Help of Dirigent-Like Proteins? PLoS ONE 2013, 8, e72897. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein Content and Amino Acid Composition of Commercially Available Plant-Based Protein Isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef]
- Sá, A.G.A.; Pacheco, M.T.B.; Moreno, Y.M.F.; Carciofi, B.A.M. Processing Effects on the Protein Quality and Functional Properties of Cold-Pressed Pumpkin Seed Meal. Food Res. Int. 2023, 169, 112876. [Google Scholar] [CrossRef] [PubMed]
- Lanzoni, D.; Skřivanová, E.; Rebucci, R.; Crotti, A.; Baldi, A.; Marchetti, L.; Giromini, C. Total Phenolic Content and Antioxidant Activity of In Vitro Digested Hemp-Based Products. Foods 2023, 12, 601. [Google Scholar] [CrossRef] [PubMed]
- Bala, A.; Gul, K.; Riar, C.S. Functional and Sensory Properties of Cookies Prepared from Wheat Flour Supplemented with Cassava and Water Chestnut Flours. Cogent Food Agric. 2015, 1, 1019815. [Google Scholar] [CrossRef]
- Dogruer, I.; Baser, F.; Gulec, S.; Tokatli, F.; Ozen, B. Formulation of Gluten-Free Cookies Utilizing Chickpea, Carob, and Hazelnut Flours through Mixture Design. Foods 2023, 12, 3689. [Google Scholar] [CrossRef]
- Tang, Y.R.; Stone, A.K.; Wang, Y.; Zhou, L.; Kimmel, J.; House, J.D.; Nickerson, M.T. Effect of a PH Shift Treatment on the Functional Properties of Individual and Blended Commercial Plant Protein Ingredients. Eur. Food Res. Technol. 2023, 249, 1969–1977. [Google Scholar] [CrossRef]
- Zhu, X.; Tan, B.; Li, K.; Liu, S.; Gu, Y.; Xia, T.; Bai, Y.; Wang, P.; Wang, R. The Impacts of Different Pea Protein Isolate Levels on Functional, Instrumental and Textural Quality Parameters of Duck Meat Batters. Foods 2022, 11, 1620. [Google Scholar] [CrossRef]
- Peters, J.P.C.M.; Vergeldt, F.J.; Boom, R.M.; van der Goot, A.J. Water-Binding Capacity of Protein-Rich Particles and Their Pellets. Food Hydrocoll. 2017, 65, 144–156. [Google Scholar] [CrossRef]
- Drakos, A.; Tsakiroglou, E.; Evageliou, V.; Mandala, I. The Effect of Inulin on the Physical and Textural Properties of Biscuits Containing Jet Milled Barley Flour. Polysaccharides 2021, 2, 39–46. [Google Scholar] [CrossRef]
- Struck, S.; Gundel, L.; Zahn, S.; Rohm, H. Fiber Enriched Reduced Sugar Muffins Made from Iso-Viscous Batters. LWT 2016, 65, 32–38. [Google Scholar] [CrossRef]
- Hayat, I.; Ahmad, A.; Rafique, N.; Rafiq, S.; Bashir, S.; Ijaz, R.; Qayyum, S. Quality Attributes of Cookies Enriched with Functional Protein Isolate from Red Kidney Beans. Czech J. Food Sci. 2022, 40, 367–374. [Google Scholar] [CrossRef]
- De Angelis, D.; Vurro, F.; Santamaria, M.; Garzon, R.; Rosell, C.M.; Summo, C.; Pasqualone, A. Effect of Dry-Fractionated Pea Protein on the Physicochemical Properties and the Nutritional Features of Gluten-Free Focaccia Flat Bread. LWT 2023, 182, 114873. [Google Scholar] [CrossRef]
- Ke, C.; Li, L. Influence Mechanism of Polysaccharides Induced Maillard Reaction on Plant Proteins Structure and Functional Properties: A Review. Carbohydr. Polym. 2023, 302, 120430. [Google Scholar] [CrossRef]
- Sim, S.Y.J.; SRV, A.; Chiang, J.H.; Henry, C.J. Plant Proteins for Future Foods: A Roadmap. Foods 2021, 10, 1967. [Google Scholar] [CrossRef]
- Xue, H.; Feng, J.; Tang, Y.; Wang, X.; Tang, J.; Cai, X.; Zhong, H. Research Progress on the Interaction of the Polyphenol–Protein–Polysaccharide Ternary Systems. Chem. Biol. Technol. Agric. 2024, 11, 95. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Cookie Texture, Spread Ratio and Sensory Acceptability of Cookies as a Function of Soluble Dietary Fiber, Baking Time and Different Water Levels. LWT 2017, 80, 537–542. [Google Scholar] [CrossRef]
- Handa, C.; Goomer, S.; Siddhu, A. Physicochemical Properties and Sensory Evaluation of Fructoligosaccharide Enriched Cookies. J. Food Sci. Technol. 2012, 49, 192–199. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Zhong, M.; Xie, F.; Wang, H.; Li, L.; Qi, B.; Zhang, S. Digestibility, Textural and Sensory Characteristics of Cookies Made from Residues of Enzyme-Assisted Aqueous Extraction of Soybeans. Sci. Rep. 2020, 10, 4222. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture. Agricultural Research Service Beltsville Human Nutrition Research Center FoodData Central. French Green Lentils. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/453943/nutrients (accessed on 23 November 2024).
- Polat, H.; Dursun Capar, T.; Inanir, C.; Ekici, L.; Yalcin, H. Formulation of Functional Crackers Enriched with Germinated Lentil Extract: A Response Surface Methodology Box-Behnken Design. LWT 2020, 123, 109065. [Google Scholar] [CrossRef]
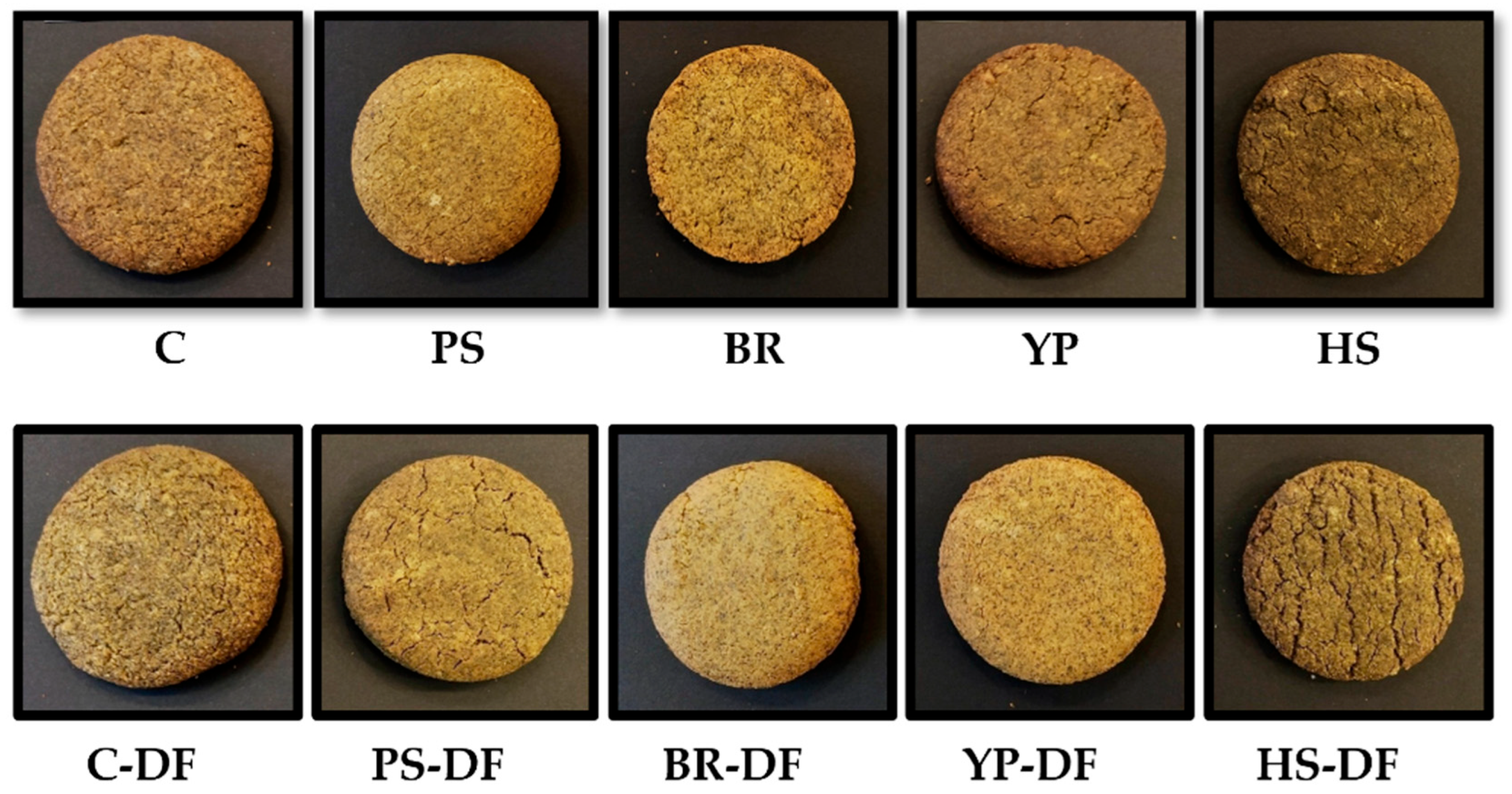
| Ingredient | Control | With Added Protein | With Added Fiber and Protein |
|---|---|---|---|
| Green lentil flour | 100.0 g | 75.0 g | 62.4 g |
| Powdered sugar | 57.8 g | 57.8 g | 57.8 g |
| Margarine | 28.4 g | 28.4 g | 28.4 g |
| Salt | 0.93 g | 0.93 g | 0.93 g |
| Sodium bicarbonate | 1.11 g | 1.11 g | 1.11 g |
| Distilled water | 7.11 g | 7.11 g | 7.11 g |
| Glucose solution (5 g/ 100 mL) | 14.6 g | 14.6 g | 14.6 g |
| Protein powder | -- | 25.0 g | 25.0 g |
| Inulin | -- | -- | 12.6 g |
| Matcha tea | 4.2 g | 4.2 g | 4.2 g |
| Protein Type | Without Dietary Fiber | With Dietary Fiber |
|---|---|---|
| No added protein | C | C-DF |
| Pumpkin seed protein | PS | PS-DF |
| Brown rice protein | BR | BR-DF |
| Yellow split pea protein | YP | YP-DF |
| Hemp seed protein | HS | HS-DF |
| Sample 1 | L* | a* | b* | BI 2 | ΔE* 3 | |
|---|---|---|---|---|---|---|
| C | 36.45 ± 0.84 C,bc | 2.22 ± 0.18 B,ab | 13.98 ± 0.38 B,bc | 51.11 ± 0.54 B,b | -- | -- |
| PS | 34.52 ± 0.48 B,ab | 2.48 ± 0.43 B,bc | 13.11 ± 0.33 B,b | 51.39 ± 1.37 B,b | 2.13 | -- |
| BR | 42.32 ± 0.17 D,d | 1.19 ± 0.11 A,a | 20.11 ± 0.09 C,d | 63.45 ± 0.55 C,cd | 8.55 | -- |
| YP | 42.71 ± 0.71 D,d | 2.74 ± 0.36 B,bc | 20.30 ± 0.31 C,d | 66.36 ± 1.04 C,d | 8.92 | -- |
| HS | 32.29 ± 0.72 A,a | 2.47 ± 0.46 B,bc | 10.25 ± 0.55 A,a | 42.63 ± 2.15 A,a | 5.59 | -- |
| C-DF | 36.72 ± 0.81 A,bc | 1.03 ± −0.74 A,a | 14.29 ± 0.09 A,c | 49.49 ± 2.67 A,b | -- | 1.26 |
| PS-DF | 36.94 ± 0.62 A,c | 1.77 ± 0.40 A,ab | 14.57 ± 0.66 A,c | 51.80 ± 0.88 A,b | 0.84 | 2.92 |
| BR-DF | 42.49 ± 0.23 B,d | 1.05 ± 0.07 A,a | 19.42 ± 0.15 B,d | 60.13 ± 0.55 B,c | 5.80 | 0.72 |
| YP-DF | 42.22 ± 1.56 B,d | 3.64 ± 0.75 B,c | 19.44 ± 0.45 B,d | 65.58 ± 2.99 C,d | 7.97 | 1.34 |
| HS-DF | 35.00 ± 0.96 A,bc | 1.04 ± 0.16 A,a | 13.72 ± 0.39 A,bc | 50.03 ± 0.55 A,b | 1.82 | 4.62 |
| Sample 1 | TPC mg GAE/100 g | TFC mg CE/100 g | CUPRAC mmol TE/100 g | CP 2 g/100 g |
|---|---|---|---|---|
| C | 552 ± 13 D,h | 133 ± 9 AB,cd | 3.13 ± 0.07 D,f | 12.4 ± 0.8 A,a |
| PS | 410 ± 12 B,ef | 128 ± 11 A,c | 2.14 ± 0.05 A,c | 17.1 ± 0.7 C,d |
| BR | 391 ± 9 B,de | 186 ± 10 C,e | 2.15 ± 0.06 A,c | 19.0 ± 0.2 D,ef |
| YP | 356 ± 21 A,c | 152 ± 11 B,d | 2.69 ± 0.05 C,e | 20.4 ± 0.2 E,f |
| HS | 463 ± 16 C,g | 208 ± 5 C,e | 2.39 ± 0.08 B,d | 15.1 ± 0.3 B,bc |
| C-DF | 425 ± 11 D,f | 80.1 ± 6.3 B,b | 2.17 ± 0.06 C,c | 12.5 ± 0.1 A,a |
| PS-DF | 368 ± 13 C,cd | 36.2 ± 6.6 A,a | 1.92 ± 0.05 B,b | 15.9 ± 1.0 BC,cd |
| BR-DF | 261 ± 6 A,a | 125 ± 2 C,c | 1.47 ± 0.09 A,a | 16.5 ± 1.2 C,cd |
| YP-DF | 349 ± 12 C,c | 122 ± 3 C,c | 2.27 ± 0.17 C,cd | 17.2 ± 0.4 C,de |
| HS-DF | 311 ± 6 B,b | 89.1 ± 16.9 B,b | 1.58 ± 0.01 A,a | 14.0 ± 0.1 AB,ab |
| Sample 1 | Baking Loss, % | Diameter, mm | Height, mm | Spread Ratio (Diameter/Height) |
|---|---|---|---|---|
| C | 12.6 ± 4.5 A,a | 56.8 ± 1.2 D,f | 8.5 ± 0.3 B,cd | 6.69 ± 0.30 C,f |
| PS | 13.8 ± 0.9 A,abc | 53.0 ± 1.0 C,d | 8.3 ± 0.3 B,bc | 6.39 ± 0.24 B,de |
| BR | 12.3 ± 1.1 A,a | 50.8 ± 0.6 B,b | 8.9 ± 0.1 C,e | 5.71 ± 0.09 A,ab |
| YP | 17.9 ± 2.2 B,d | 47.8 ± 0.5 A,a | 5.7 ± 0.1 A,a | 8.38 ± 0.19 D,g |
| HS | 13.3 ± 0.9 A,ab | 52.4 ± 1.1 C,cd | 9.3 ± 0.1 D,f | 5.63 ± 0.11 A,a |
| C-DF | 16.1 ± 1.9 C,cd | 56.8 ± 0.6 D,f | 8.6 ± 0.2 B,d | 6.60 ± 0.16 C,ef |
| PS-DF | 12.4 ± 1.3 A,a | 53.3 ± 0.8 B,d | 8.6 ± 0.2 B,d | 6.20 ± 0.20 B,cd |
| BR-DF | 14.4 ± 1.1 B,abc | 52.1 ± 0.8 A,bcd | 8.9 ± 0.1 C,e | 5.85 ± 0.11 A,ab |
| YP-DF | 15.7 ± 0.8 BC,bcd | 54.8 ± 1.2 C,e | 9.2 ± 0.2 D,f | 5.96 ± 0.23 A,bc |
| HS-DF | 14.5 ± 0.8 B,abc | 51.2 ± 0.9 A,bc | 8.1 ± 0.1 A,b | 6.32 ± 0.16 B,d |
| Sample 1 | Hardness, g | Adhesive Force, g | Cohesiveness, - | Gumminess, g |
|---|---|---|---|---|
| C | 817 ± 186 B,d | 106 ± 30.1 AB,ab | 0.092 ± 0.012 B,b | 74.6 ± 23.2 B,cd |
| PS | 926 ± 114 B,d | 110 ± 84.5 AB,ab | 0.098 ± 0.012 B,bc | 91.2 ± 17.6 BC,de |
| BR | 629 ± 88 A,c | 170 ± 3.6 B,b | 0.126 ± 0.008 C,d | 81.8 ± 3.6 BC,cde |
| YP | 1354 ± 216 C,f | 72.7 ± 62.9 A,a | 0.030 ± 0.018 A,a | 39.0 ± 18.3 A,a |
| HS | 820 ± 94 B,d | 124 ± 76.9 AB,ab | 0.118 ± 0.014 C,d | 98.1 ± 20.5 C,e |
| C-DF | 557 ± 104 C,bc | 81.8 ± 31.8 A,a | 0.115 ± 0.012 B,cd | 63.7 ± 12.0 C,bc |
| PS-DF | 426 ± 47 B,b | 94.8 ± 38.5 A,a | 0.127 ± 0.012 B,d | 55.1 ± 8.4 BC,ab |
| BR-DF | 473 ± 64 BC,bc | 105 ± 20.2 A,ab | 0.120 ± 0.009 B,d | 55.2 ± 9.6 BC,ab |
| YP-DF | 213 ± 22 A,a | 84.9 ± 18.6 A,a | 0.184 ± 0.018 C,e | 40.2 ± 5.7 A,a |
| HS-DF | 1190 ± 123 D,e | 118 ± 97.4 A,ab | 0.036 ± 0.008 A,a | 44.9 ± 12.0 AB,ab |
| Sample 1 | Surface Homogeneity | Green Color | Tea Odor | Baked Odor | Tea Taste |
|---|---|---|---|---|---|
| C | 7.0 A | 4.0 A | 8.0 A | 3.0 A | 7.0 A |
| PS | 6.2 ± 1.6 B,b | 4.6 ± 2.0 A,ab | 5.1 ± 3.4 B,a | 4.9 ± 2.2 B,ab | 4.7 ± 2.5 B,a |
| BR | 5.1 ± 1.9 B,b | 3.3 ± 2.3 A,a | 4.5 ± 3.1 B,a | 4.7 ± 2.5 B,ab | 3.6 ± 2.8 B,a |
| YP | 2.9 ± 1.2 B,a | 3.6 ± 2.4 A,a | 3.4 ± 2.8 B,a | 2.9 ± 2.4 A,a | 4.6 ± 2.9 B,a |
| HS | 5.2 ± 1.8 B,b | 6.4 ± 2.3 B,b | 5.2 ± 3.8 B,a | 5.4 ± 2.5 B,b | 7.0 ± 2.1 A,b |
| C-DF | 7.0 A | 4.0 A | 8.0 A | 3.0 A | 7.0 A |
| PS-DF | 6.0 ± 2.2 B,b | 5.0 ± 2.2 B,a | 5.0 ± 2.6 B,a | 4.9 ± 2.5 B,b | 6.0 ± 2.1 B,a |
| BR-DF | 7.1 ± 2.4 A,b | 6.0 ± 2.8 B,a | 4.2 ± 2.7 B,a | 4.7 ± 2.5 B,b | 5.4 ± 2.4 B,a |
| YP-DF | 6.3 ± 2.5 A,b | 6.1 ± 3.1 B,a | 3.8 ± 2.8 B,a | 2.8 ± 2.7 A,a | 6.3 ± 2.9 A,a |
| HS-DF | 2.1 ± 1.7 B,a | 4.6 ± 3.1 A,a | 5.2 ± 3.1 B,a | 6.2 ± 2.4 B,b | 5.6 ± 2.4 B,a |
| Sample 1 | Sweet Taste | Lentil Taste | Hardness | Crunchiness | Crumbliness |
|---|---|---|---|---|---|
| C | 6.0 A | 4.0 A | 7.0 A | 8.0 A | 3.0 A |
| PS | 5.1 ± 2.2 A,a | 4.2 ± 2.1 A,a | 5.8 ± 2.4 A,a | 6.9 ± 2.2 A,ab | 5.7 ± 3.0 B,b |
| BR | 5.1 ± 2.4 A,a | 5.0 ± 2.7 A,a | 6.1 ± 2.5 A,a | 6.4 ± 2.4 B,a | 4.4 ± 2.7 B,ab |
| YP | 4.4 ± 2.5 B,a | 3.8 ± 3.0 A,a | 8.8 ± 2.3 B,b | 8.6 ± 1.9 A,b | 2.9 ± 3.0 A,a |
| HS | 5.9 ± 2.3 A,a | 4.6 ± 2.9 A,a | 5.1 ± 1.8 B,a | 5.7 ± 2.1 B,a | 5.4 ± 2.6 B,b |
| C-DF | 6.0 A | 4.0 A | 7.0 A | 8.0 A | 3.0 A |
| PS-DF | 5.3 ± 2.5 A,ab | 3.9 ± 2.0 A,a | 7.2 ± 2.5 A,b | 7.0 ± 2.8 A,b | 5.2 ± 3.0 B,b |
| BR-DF | 5.7 ± 2.0 A,b | 4.1 ± 2.3 A,a | 7.5 ± 2.1 A,b | 7.0 ± 2.3 A,b | 4.6 ± 2.5 B,ab |
| YP-DF | 4.0 ± 2.0 B,a | 6.6 ± 3.0 B,b | 3.5 ± 2.2 B,a | 4.7 ± 1.8 B,a | 5.4 ± 2.6 B,b |
| HS-DF | 6.6 ± 2.4 A,b | 4.1 ± 2.2 A,a | 9.9 ± 0.3 B,c | 9.8 ± 0.7 B,c | 2.8 ± 3.6 A,a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juhász, R.; Hajas, L.; Csajbókné Csobod, É.; Pálinkás, Z.; Szilágyi-Utczás, M.; Benedek, C. Impact of Pumpkin Seed, Brown Rice, Yellow Pea, and Hemp Seed Proteins on the Physicochemical, Technological, and Sensory Properties of Green Lentil Cookies. Foods 2025, 14, 1518. https://doi.org/10.3390/foods14091518
Juhász R, Hajas L, Csajbókné Csobod É, Pálinkás Z, Szilágyi-Utczás M, Benedek C. Impact of Pumpkin Seed, Brown Rice, Yellow Pea, and Hemp Seed Proteins on the Physicochemical, Technological, and Sensory Properties of Green Lentil Cookies. Foods. 2025; 14(9):1518. https://doi.org/10.3390/foods14091518
Chicago/Turabian StyleJuhász, Réka, Lívia Hajas, Éva Csajbókné Csobod, Zoltán Pálinkás, Margita Szilágyi-Utczás, and Csilla Benedek. 2025. "Impact of Pumpkin Seed, Brown Rice, Yellow Pea, and Hemp Seed Proteins on the Physicochemical, Technological, and Sensory Properties of Green Lentil Cookies" Foods 14, no. 9: 1518. https://doi.org/10.3390/foods14091518
APA StyleJuhász, R., Hajas, L., Csajbókné Csobod, É., Pálinkás, Z., Szilágyi-Utczás, M., & Benedek, C. (2025). Impact of Pumpkin Seed, Brown Rice, Yellow Pea, and Hemp Seed Proteins on the Physicochemical, Technological, and Sensory Properties of Green Lentil Cookies. Foods, 14(9), 1518. https://doi.org/10.3390/foods14091518










