Sustainable Absorbent Pads from Polybutylene Adipate Terephthalate/Thermoplastic Starch Films Combined with Hairy Basil (Ocimum basilicum) Powder to Enhance Meat Shelf Life
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biocomposite Film Preparation and Characterization
2.2.1. Biocomposite Film Preparation
2.2.2. PBAT/TPS Biocomposite Film Characterization
2.3. Hairy Basil Seed Powder (HBP) Preparation
2.4. Fabrication and Characterization of the Biodegradable Pad
2.4.1. Fabrication of the Absorbent Pad
2.4.2. Determination of Water Absorbing Capacity (WAC)
2.5. Application of the Pad to Chilled Pork Packaging
2.5.1. Chilled Pork Packaging Preparation
2.5.2. Measurement of Drip Loss
2.5.3. Color Change
2.5.4. TVB-N
2.5.5. Microbial Analysis
2.5.6. pH Measurement
2.6. Statistical Analysis
3. Results and Discussion
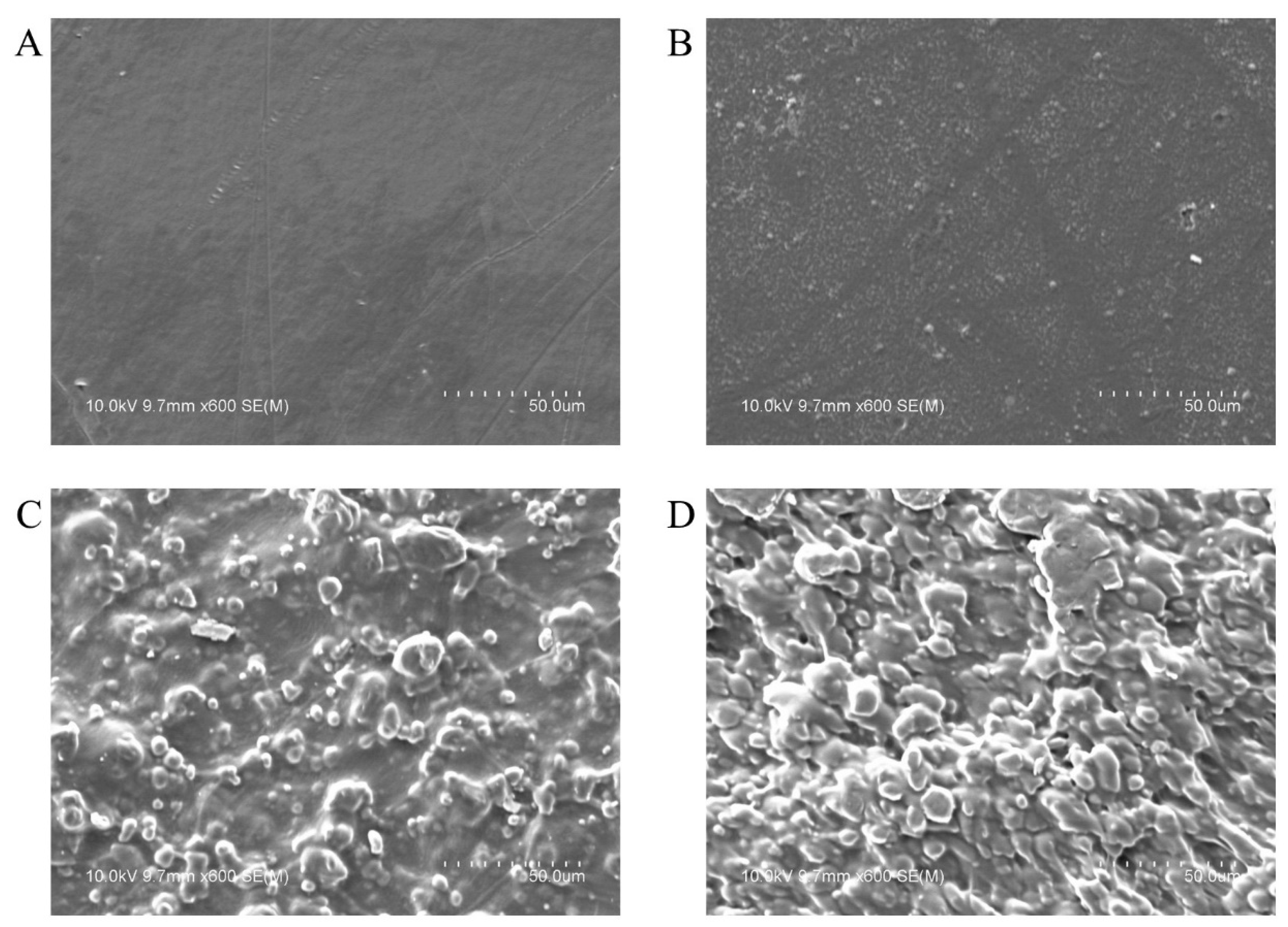
3.1. Morphology
3.2. Mechanical and Barrier Properties
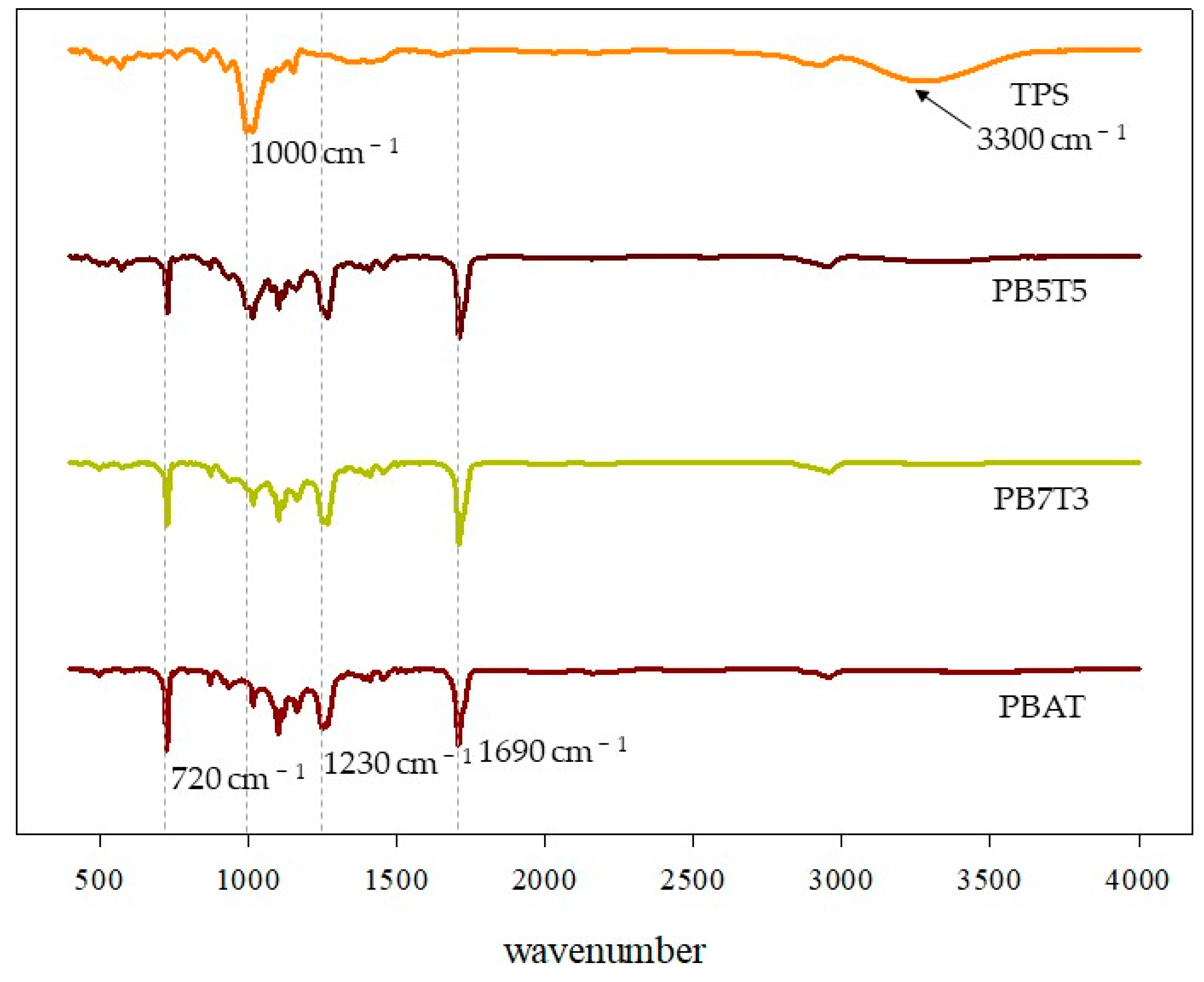
3.3. ATR-FTIR Analysis
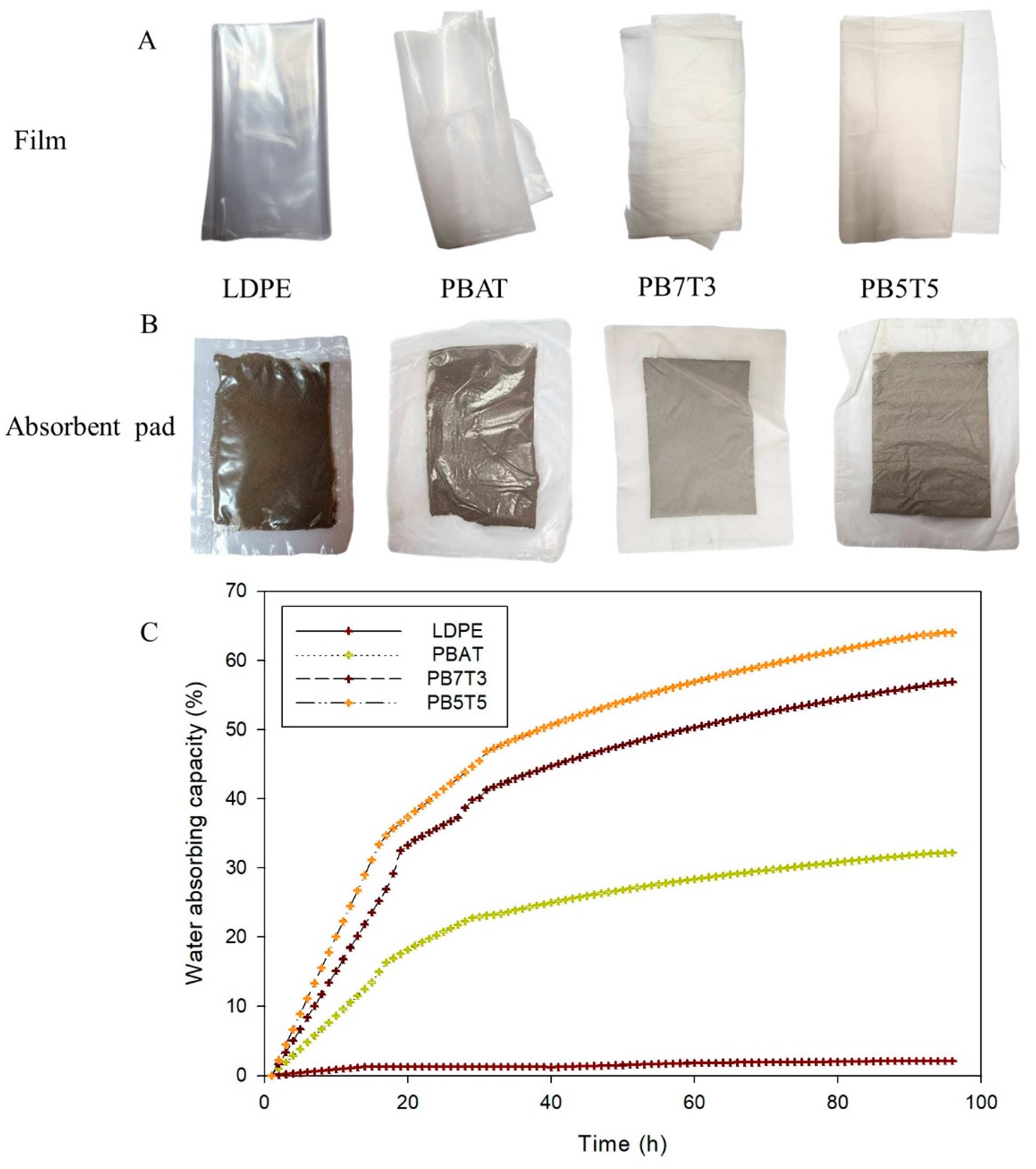
3.4. Characterization of the Absorbent Pads
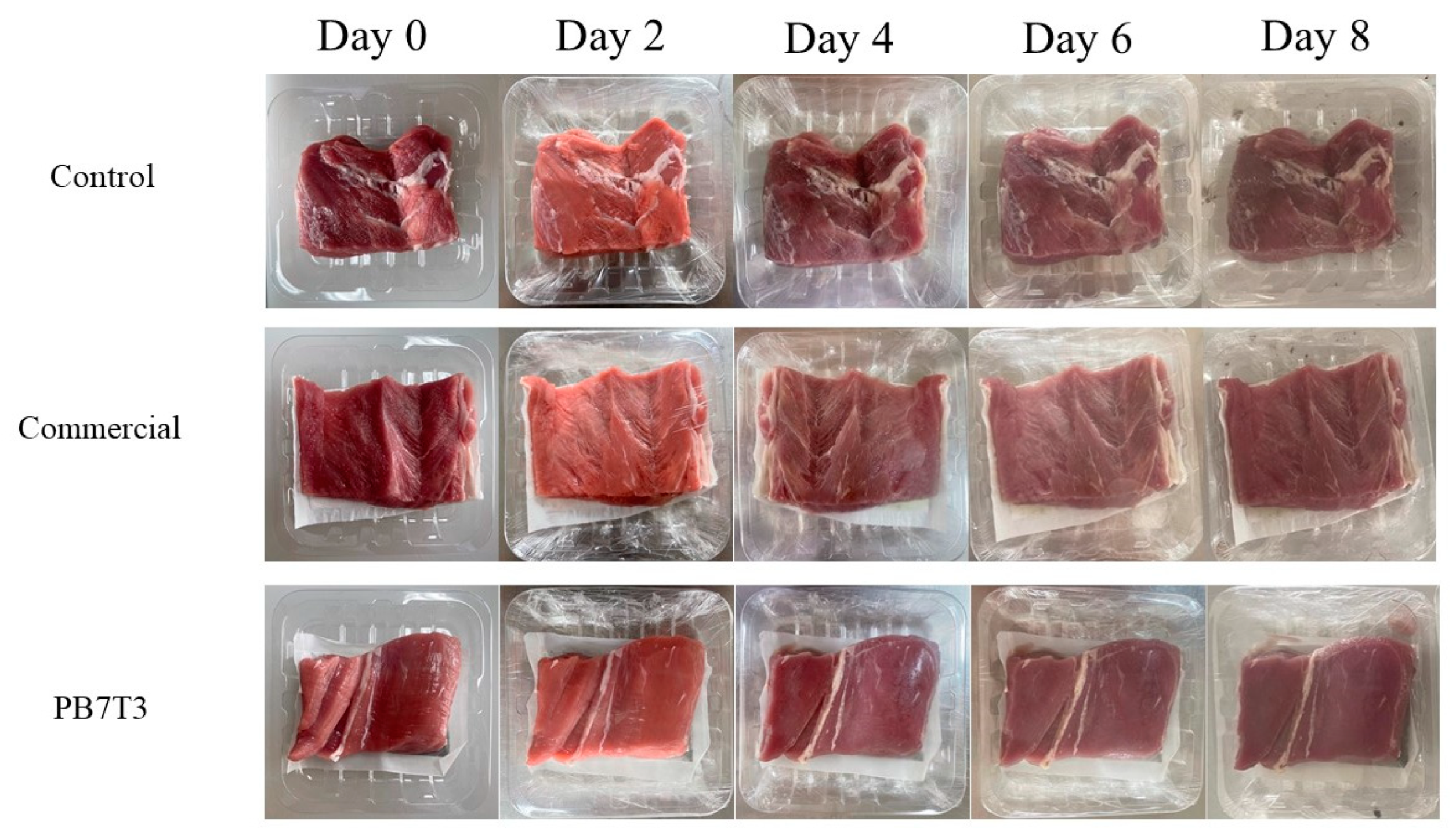
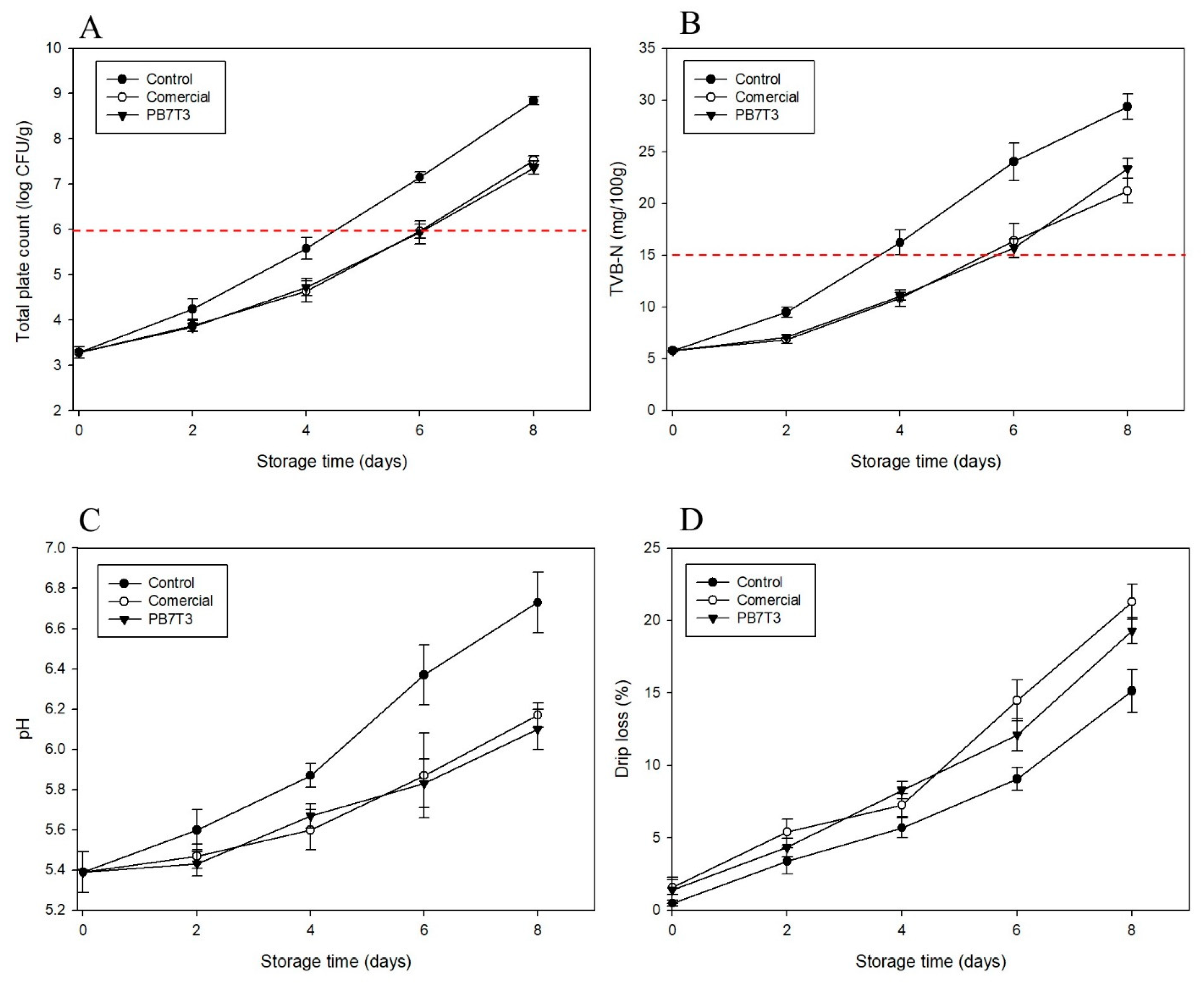
3.5. Application of PBAT/TPS Biocomposites in Fresh Pork Packaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, C.; Yu, G.; Wang, P.; Yao, Z.; Mu, L.; Li, Q.; Shi, Y. Preparation of delignified wood fibers based absorbent pad for tray package and its application in preservation of cold and fresh pork. Ind. Crops Prod. 2024, 219, 119097. [Google Scholar] [CrossRef]
- Shao, L.; Chen, S.; Wang, H.; Zhang, J.; Xu, X.; Wang, H. Advances in understanding the predominance, phenotypes, and mechanisms of bacteria related to meat spoilage. Trends Food Sci. Technol. 2021, 118, 822–832. [Google Scholar] [CrossRef]
- Ren, T.; Hayden, M.; Qiao, M.; Huang, T.S.; Ren, X.; Weese, J. Absorbent Pads Containing N-Halamine Compound for Potential Antimicrobial Use for Chicken Breast and Ground Chicken. J. Agric. Food Chem. 2018, 66, 1941–1948. [Google Scholar] [CrossRef]
- Anto, T.; Rejeesh, C.R. Synthesis and characterization of recycled HDPE polymer composite reinforced with nano-alumina particles. Mater. Today Proc. 2023, 72, 3177–3182. [Google Scholar] [CrossRef]
- Ha, P.; The, S.; Khoi, N.; Pham, T.; Duc, N.; Anh, P.; Thanh Tung, N. Poly(butylene adipate-co-terephthalate)/thermoplastic canna starch (Canna edulis ker.) (PBAT/TPS) blend film—A novel biodegradable material. J. Indian Chem. Soc. 2024, 101, 101245. [Google Scholar] [CrossRef]
- Lestido-Cardama, A.; Barbosa-Pereira, L.; Sendón, R.; Bustos, J.; Paseiro Losada, P.; Rodríguez Bernaldo de Quirós, A. Chemical safety and risk assessment of bio-based and/or biodegradable polymers for food contact: A review. Food Res. Int. 2025, 202, 115737. [Google Scholar] [CrossRef] [PubMed]
- Bumbudsanpharoke, N.; Nurhadi, R.P.; Chongcharoenyanon, B.; Kwon, S.; Harnkarnsujarit, N.; Ko, S. Effect of migration on the functionality of zinc oxide nanoparticle in polybutylene adipate terephthalate/thermoplastic starch films: A food simulant study. Int. J. Biol. Macromol. 2024, 263, 130232. [Google Scholar] [CrossRef]
- Colussi, R.; Pinto, V.Z.; El Halal, S.L.M.; Biduski, B.; Prietto, L.; Castilhos, D.D.; Zavareze, E.d.R.; Dias, A.R.G. Acetylated rice starches films with different levels of amylose: Mechanical, water vapor barrier, thermal, and biodegradability properties. Food Chem. 2017, 221, 1614–1620. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Hernández, A.; Aparicio-Saguilán, A.; Reynoso-Meza, G.; Carrillo-Ahumada, J. Multi-objective optimization of process conditions in the manufacturing of banana (Musa paradisiaca L.) starch/natural rubber films. Carbohydr. Polym. 2017, 157, 1125–1133. [Google Scholar] [CrossRef]
- Sahraee, S.; Milani, J.M.; Ghanbarzadeh, B.; Hamishehkar, H. Effect of corn oil on physical, thermal, and antifungal properties of gelatin-based nanocomposite films containing nano chitin. LWT-Food Sci. Technol. 2017, 76, 33–39. [Google Scholar] [CrossRef]
- Behrooznia, Z.; Nourmohammadi, J. Polysaccharide-based materials as an eco-friendly alternative in biomedical, environmental, and food packaging. Giant 2024, 19, 100301. [Google Scholar] [CrossRef]
- Hosseini-Parvar, S.H.; Matia-Merino, L.; Golding, M. Effect of basil seed gum (BSG) on textural, rheological and microstructural properties of model processed cheese. Food Hydrocoll. 2015, 43, 557–567. [Google Scholar] [CrossRef]
- Naji-Tabasi, S.; Razavi, S.M.A. Functional properties and applications of basil seed gum: An overview. Food Hydrocoll. 2017, 73, 313–325. [Google Scholar] [CrossRef]
- Nakhonchai, N.; Prompila, N.; Ponhong, K.; Siriangkhawut, W.; Vichapong, J.; Supharoek, S.-a. Green hairy basil seed mucilage biosorbent for dispersive solid phase extraction enrichment of tetracyclines in bovine milk samples followed by HPLC analysis. Talanta 2024, 271, 125645. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Lee, J.; Kim, M. Inhibition of Salmonella growth in exudates drained from poultry meat by bacteriophage cocktail-containing absorbent food pad. LWT 2024, 197, 115908. [Google Scholar] [CrossRef]
- Wang, X.; Yan, X.; Xu, Y.; Liu, J.; Chen, D. Changes in the quality and microbial compositions of ground beef packaged on food absorbent pads incorporated with levulinic acid and sodium dodecyl sulfate. Int. J. Food Microbiol. 2022, 376, 109771. [Google Scholar] [CrossRef]
- Yimenu, S.M.; Koo, J.; Kim, B.S.; Kim, J.H.; Kim, J.Y. Freshness-based real-time shelf-life estimation of packaged chicken meat under dynamic storage conditions. Poult. Sci. 2019, 98, 6921–6930. [Google Scholar] [CrossRef]
- Miao, J.; Peng, W.; Liu, G.; Chen, Y.; Chen, F.; Cao, Y. Biopreservative effect of the natural antimicrobial substance from Lactobacillus paracasei subsp. tolerans FX-6 on fresh pork during chilled storage. Food Control 2015, 56, 53–56. [Google Scholar] [CrossRef]
- Zhai, X.; Han, J.; Chang, L.; Zhao, F.; Zhang, R.; Wang, W.; Hou, H. Effects of starch filling on physicochemical properties, functional activities, and release characteristics of PBAT-based biodegradable active films loaded with tea polyphenols. Int. J. Biol. Macromol. 2024, 277, 134505. [Google Scholar] [CrossRef]
- Zhai, X.; Zhang, R.; Wang, W.; Hou, H. Relationship between phase morphologies and mechanical properties of thermoplastic starch/poly(butylene adipate-co-terephthalate) composite films prepared by extrusion blowing. Int. J. Biol. Macromol. 2023, 224, 1356–1360. [Google Scholar] [CrossRef]
- Gui, H.; Ma, W.; Cao, Y.; Chao, H.; Fan, M.; Dong, Q.; Li, L. Sustained release, antimicrobial, and antioxidant properties of modified porous starch-based biodegradable polylactic acid/polybutylene adipate-co-terephthalate/thermoplastic starch active packaging film. Int. J. Biol. Macromol. 2024, 267, 131657. [Google Scholar] [CrossRef]
- Phothisarattana, D.; Wongphan, P.; Promhuad, K.; Promsorn, J.; Harnkarnsujarit, N. Blown film extrusion of PBAT/TPS/ZnO nanocomposites for shelf-life extension of meat packaging. Colloids Surf. B Biointerfaces 2022, 214, 112472. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, X.; Wang, Q.; Lin, G.; Yang, H.; Yu, D.; Cui, S.; Xia, W. Antimicrobial and antioxidant films formed by bacterial cellulose, chitosan and tea polyphenol—Shelf life extension of grass carp. Food Packag. Shelf Life 2022, 33, 100866. [Google Scholar] [CrossRef]
- Abdullah, A.H.D.; Chalimah, S.; Primadona, I.; Hanantyo, M.H.G. Physical and chemical properties of corn, cassava, and potato starchs. IOP Conf. Ser. Earth Environ. Sci. 2018, 160, 012003. [Google Scholar] [CrossRef]
- Wang, F.; Xu, Z.; Chen, L.; Qiao, Z.; Hu, Y.; Fan, X.; Liu, Y.; Kang, Z.; Huang, F.; Han, M.; et al. Super absorbent resilience antibacterial aerogel with curcumin for fresh pork preservation. Food Control 2024, 159, 110289. [Google Scholar] [CrossRef]
- Zhou, X.; Zong, X.; Zhang, M.; Ge, Q.; Qi, J.; Liang, J.; Xu, X.; Xiong, G. Effect of konjac glucomannan/carrageenan-based edible emulsion coatings with camellia oil on quality and shelf-life of chicken meat. Int. J. Biol. Macromol. 2021, 183, 331–339. [Google Scholar] [CrossRef]
- Dai, H.; Lv, T.; Liu, S.; Luo, Y.; Wang, Y.; Wang, H.; Ma, L.; Wu, J.; Zhang, Y. Preparation of nanocellulose light porous material adsorbed with tannic acid and its application in fresh-keeping pad. Food Chem. 2024, 444, 138676. [Google Scholar] [CrossRef]
- Sun, P.; Zhu, Y.; Yin, L.; Kong, B.; Xia, X.; Liu, Q.; Wang, H. Development of active hydrogel absorbent pads based on sodium alginate/potassium-doped carbon dots for chilled pork preservation. Food Hydrocoll. 2024, 155, 110229. [Google Scholar] [CrossRef]
- Wang, C.; Yang, J.; Zhu, X.; Lu, Y.; Xue, Y.; Lu, Z. Effects of Salmonella bacteriophage, nisin and potassium sorbate and their combination on safety and shelf life of fresh chilled pork. Food Control 2017, 73, 869–877. [Google Scholar] [CrossRef]
| Sample | Properties | |||
|---|---|---|---|---|
| Thickness (μm) | Tensile (N) | Elongation (%) | WVP (g·mm/m2·day·KPa) | |
| LDPE | 28.00 ± 0.90 c | 11.71 ± 1.13 b | 366.69 ± 42.88 c | 0.76 ± 0.04 d |
| PBAT | 28.40 ± 0.94 c | 29.02 ± 1.26 a | 595.11 ± 34.42 a | 2.76 ± 0.14 c |
| PB7T3 | 29.80 ± 0.70 b | 6.07 ± 0.96 c | 399.77 ± 19.45 b | 4.89 ± 0.43 b |
| PB5T5 | 32.20 ± 0.80 a | 2.15 ± 1.58 d | 60.18 ± 17.58 d | 5.51 ± 0.24 a |
| Treatment | Storage Time (Days) | |||||
|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | ||
| L* | Control | 42.67 ± 2.08 NS | 44.00 ± 1.53 NS | 41.67 ± 1.53 NS | 37.00 ± 1.68 b | 36.30 ± 1.55 b |
| Commercial Pad | 42.67 ± 2.08 NS | 44.83 ± 0.29 NS | 41.33 ± 0.58 NS | 41.00 ± 1.69 a | 39.47 ± 1.65 a | |
| PB7T3 Pad | 42.67 ± 2.08 NS | 44.00 ± 1.73 NS | 42.00 ± 1.25 NS | 41.33 ± 1.53 a | 39.07 ± 1.10 a | |
| a* | Control | 10.73 ± 0.64 NS | 9.50 ± 0.70 NS | 8.13 ± 0.32 NS | 7.40 ± 0.20 NS | 5.97 ± 0.44 b |
| Commercial Pad | 10.73 ± 0.64 NS | 10.53 ± 0.76 NS | 9.40 ± 0.87 NS | 8.20 ± 0.53 NS | 7.19 ± 0.23 a | |
| PB7T3 Pad | 10.73 ± 0.64 NS | 11.23 ± 1.16 NS | 9.17 ± 0.58 NS | 8.37 ± 0.72 NS | 7.73 ± 0.71 a | |
| b* | Control | 9.00 ± 0.20 NS | 11.60 ± 0.69 NS | 13.83 ± 0.67 a | 16.27 ± 0.9 a | 19.47 ± 1.62 NS |
| Commercial Pad | 9.00 ± 0.20 NS | 10.78 ± 0.30 NS | 12.13 ± 0.40 b | 14.33 ± 1.2 ab | 17.40 ± 1.25 NS | |
| PB7T3 Pad | 9.00 ± 0.20 NS | 11.03 ± 0.72 NS | 12.53 ± 0.95 ab | 14.07 ± 0.8 b | 16.70 ± 1.39 NS | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khunta, F.; Charoensri, K.; Nurhadi, R.P.; Bumbudsanpharoke, N.; Itkor, P.; Lee, Y.-S.; Boonsiriwit, A. Sustainable Absorbent Pads from Polybutylene Adipate Terephthalate/Thermoplastic Starch Films Combined with Hairy Basil (Ocimum basilicum) Powder to Enhance Meat Shelf Life. Foods 2025, 14, 1525. https://doi.org/10.3390/foods14091525
Khunta F, Charoensri K, Nurhadi RP, Bumbudsanpharoke N, Itkor P, Lee Y-S, Boonsiriwit A. Sustainable Absorbent Pads from Polybutylene Adipate Terephthalate/Thermoplastic Starch Films Combined with Hairy Basil (Ocimum basilicum) Powder to Enhance Meat Shelf Life. Foods. 2025; 14(9):1525. https://doi.org/10.3390/foods14091525
Chicago/Turabian StyleKhunta, Fuengnapha, Korakot Charoensri, Rineta Pertiwi Nurhadi, Nattinee Bumbudsanpharoke, Pontree Itkor, Youn-Suk Lee, and Athip Boonsiriwit. 2025. "Sustainable Absorbent Pads from Polybutylene Adipate Terephthalate/Thermoplastic Starch Films Combined with Hairy Basil (Ocimum basilicum) Powder to Enhance Meat Shelf Life" Foods 14, no. 9: 1525. https://doi.org/10.3390/foods14091525
APA StyleKhunta, F., Charoensri, K., Nurhadi, R. P., Bumbudsanpharoke, N., Itkor, P., Lee, Y.-S., & Boonsiriwit, A. (2025). Sustainable Absorbent Pads from Polybutylene Adipate Terephthalate/Thermoplastic Starch Films Combined with Hairy Basil (Ocimum basilicum) Powder to Enhance Meat Shelf Life. Foods, 14(9), 1525. https://doi.org/10.3390/foods14091525






