Protective Effect of Whey Protein and Polysaccharide Complexes on Lactobacillus paracasei F50: Comparative Analysis of Powder Characteristics and Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of L. paracasei F50 Suspension
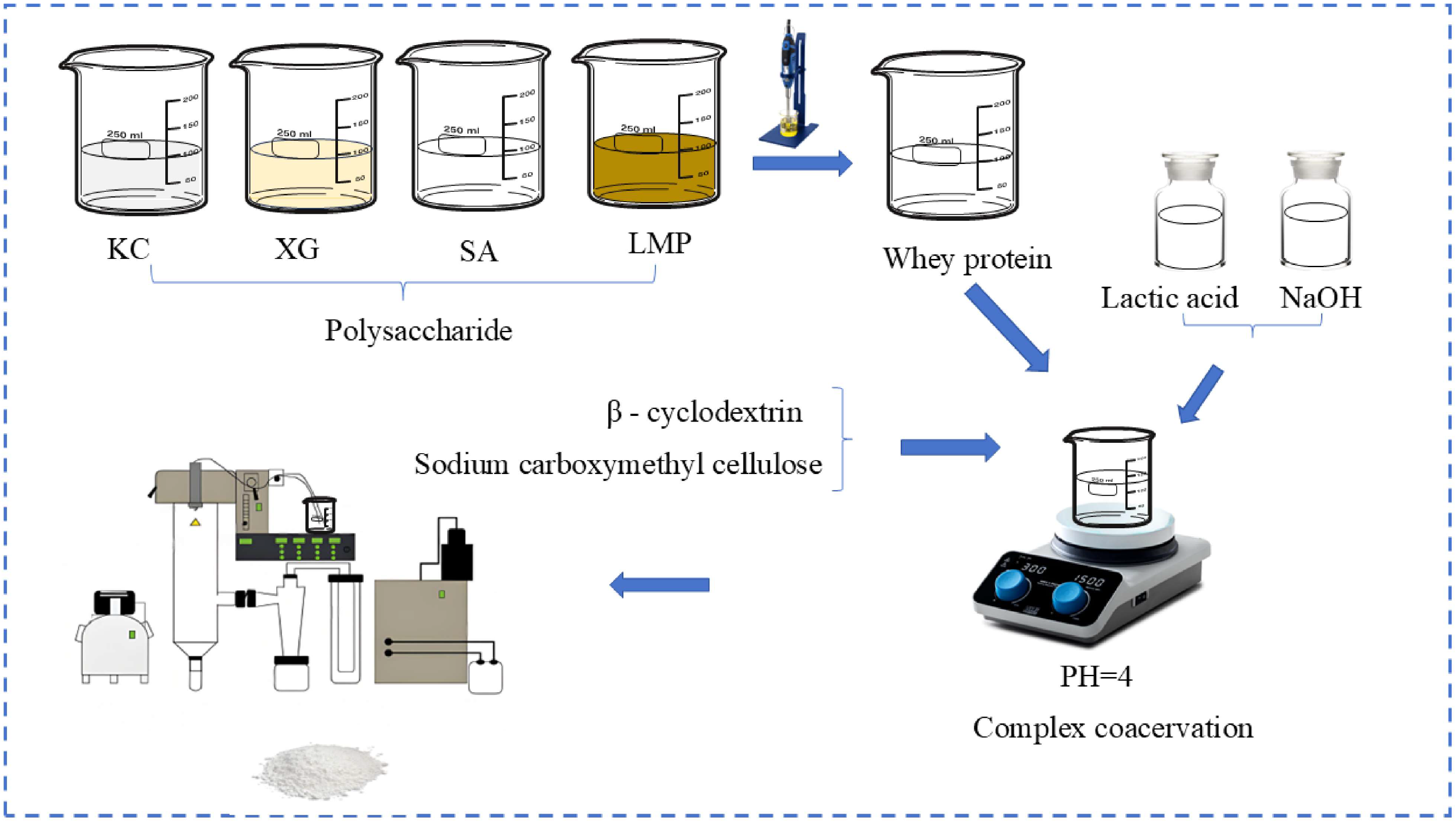
2.3. Preparation of Microcapsule Wall Material
2.4. Preparation of Microcapsules
2.5. Characterization of Microcapsules
2.5.1. Cell Survival
2.5.2. Physical Properties
2.5.3. Morphological Analysis
2.5.4. Particle Size and Zeta Potential Analysis
2.5.5. Differential Scanning Calorimetric Analysis of Thermal Stability
2.5.6. Infrared Spectroscopy Analysis
2.5.7. Crystal Structure Analysis
2.6. Determination of WP-KC Microcapsule Properties
2.6.1. The Storage Stability of the Microcapsules
2.6.2. Growth Curve and Acid Production Capacity
2.7. Effects of Food Processing Conditions on Microcapsules
2.7.1. Thermal Stability
2.7.2. Ultraviolet Radiation
2.8. Statistical Analysis
3. Results and Discussion
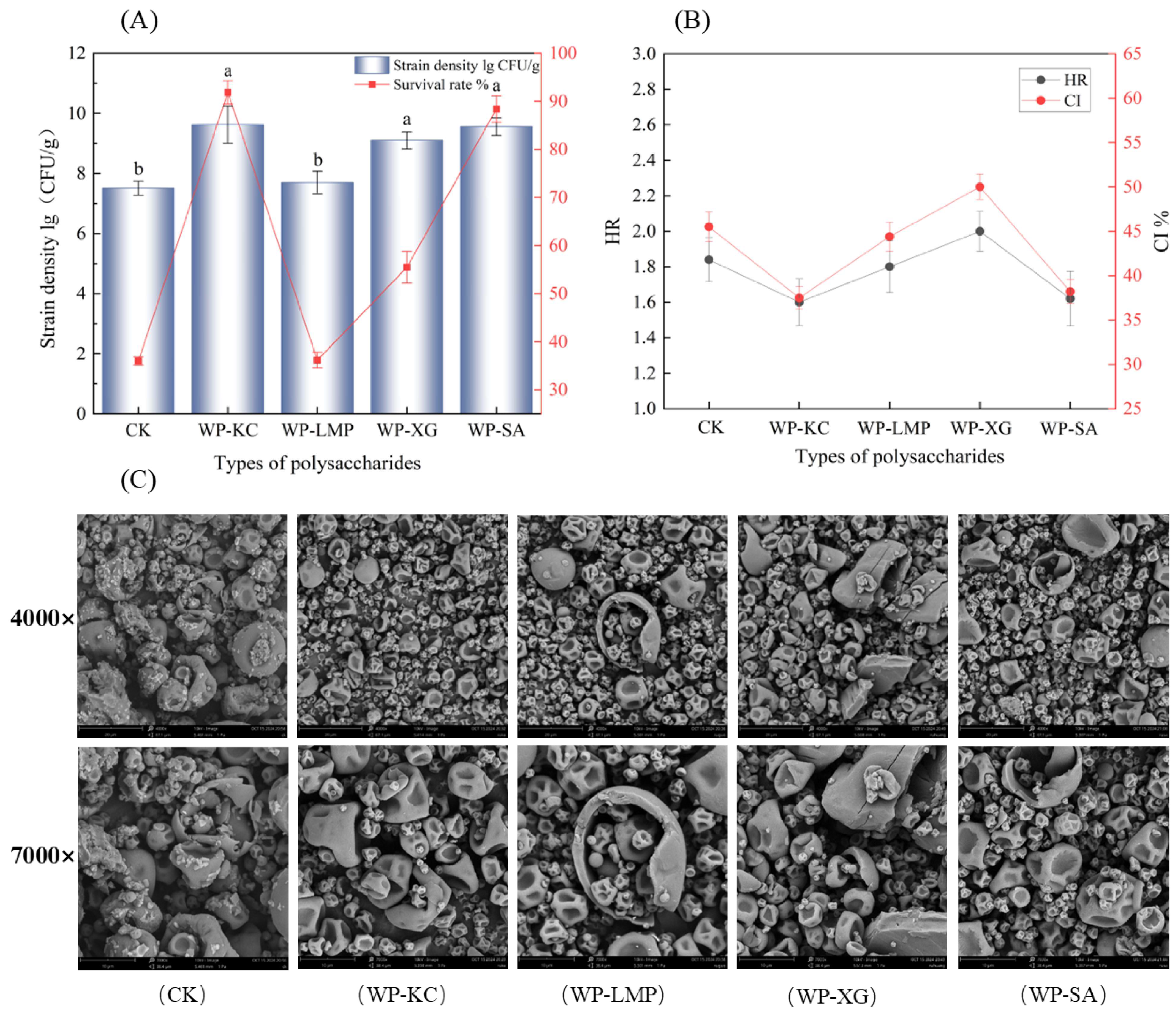
3.1. Strain Density and Survival Rate of Microcapsules
3.2. Moisture Content, Solubility, Hygroscopicity, Hausner Ratio (HR), and Carr Index (CI)
3.3. Scanning Electron Microscopy of the Microcapsules
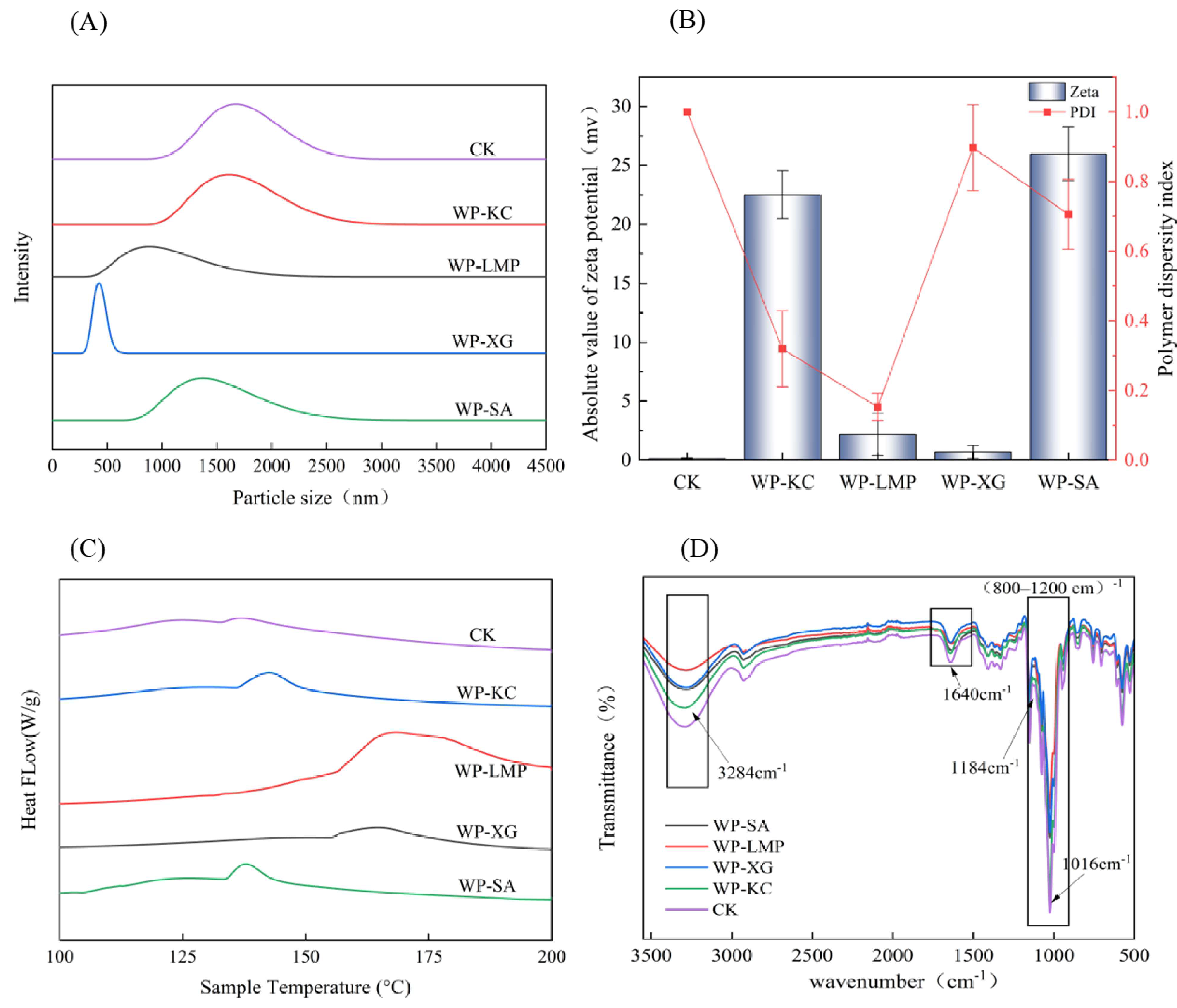
3.4. Particle Size and Zeta Potential of Microcapsules
3.5. The Differential Scanning Calorimetry of the Microcapsules
3.6. IR of Microcapsules
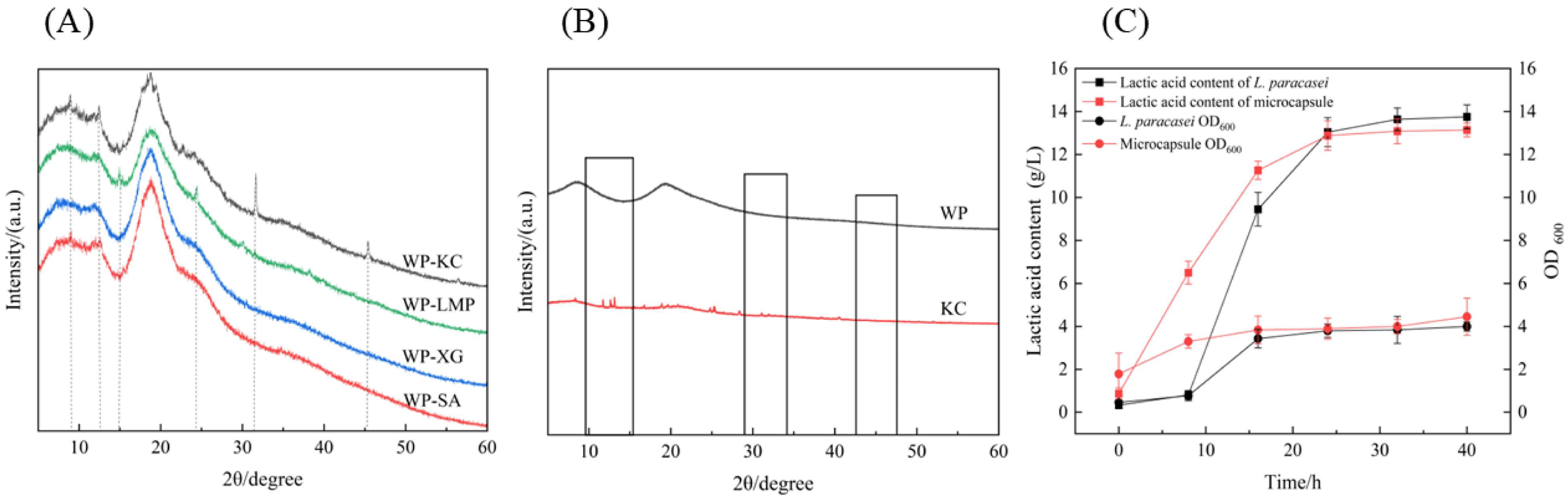
3.7. XRD of Microcapsules
3.8. WP-KC Microcapsule Properties
3.8.1. Storage Stability
3.8.2. Growth and Acid-Producing Ability
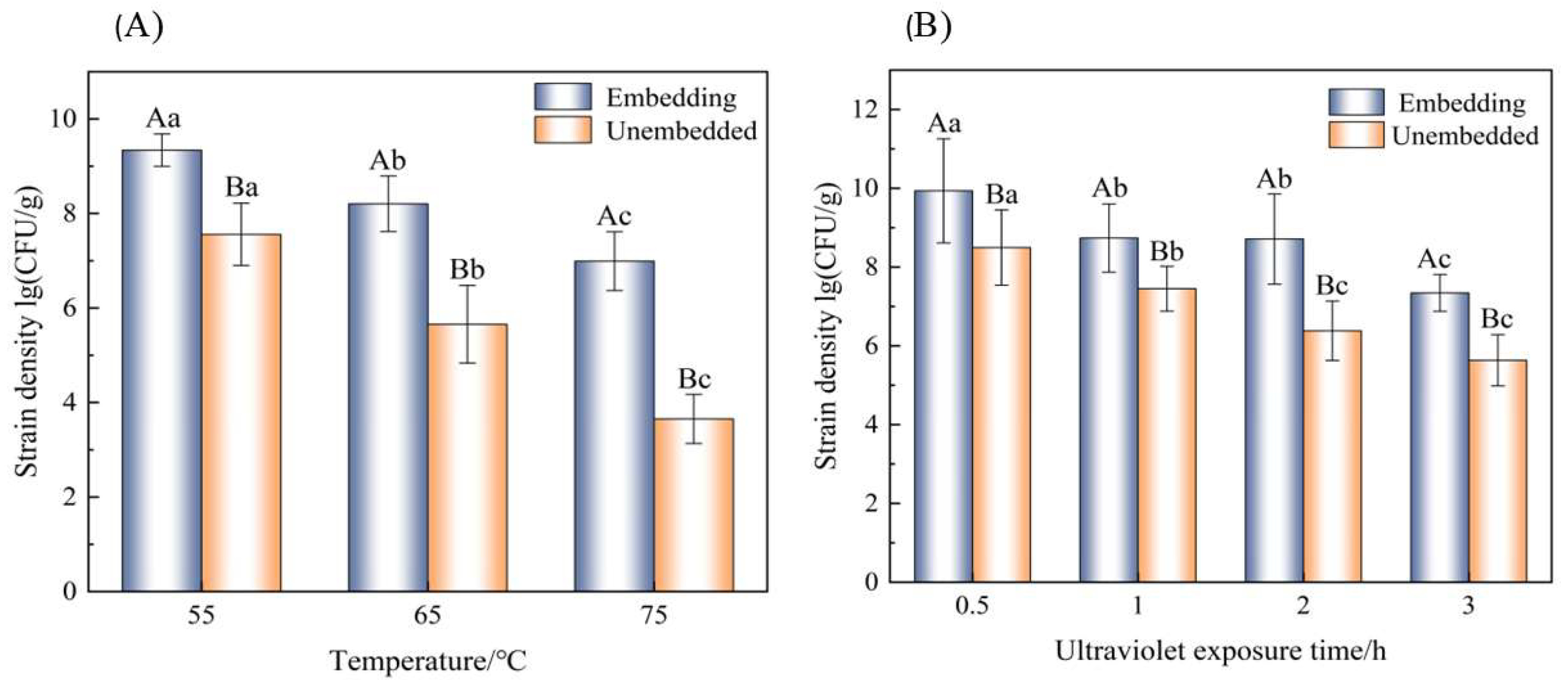
3.9. Stability of Microcapsules in Processing
3.9.1. Thermal Processing
3.9.2. Ultraviolet Irradiation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, S.; Na, G.H.; Yim, D.J.; Liu, C.-F.; Lin, T.-H.; Shih, T.-W.; Pan, T.-M.; Lee, C.-L.; Koo, Y.K. Lactobacillus paracasei Subsp. Paracasei NTU 101 Prevents Obesity by Regulating AMPK Pathways and Gut Microbiota in Obese Rat. Biochem. Biophys. Res. Commun. 2024, 731, 150279. [Google Scholar] [CrossRef]
- Yang, R.; Yang, Y.; Teng, L. 175P Lactobacillus Paracasei ZJUZ2-3 Inhibits the Gastric Tumorigenesis by Inhibit NF-κB Pathway via 3-IAA. Ann. Oncol. 2024, 35, S1472. [Google Scholar] [CrossRef]
- Lei, G.; Khan, A.; Budryn, G.; Grzelczyk, J. Probiotic Products from Laboratory to Commercialization. Trends Food Sci. Technol. 2025, 155, 104807. [Google Scholar] [CrossRef]
- Bu, W.; McClements, D.J.; Zhang, Z.; Zhang, R.; Jin, Z.; Chen, L. Encapsulation Method of Probiotic Embedded Delivery System and Its Application in Food. Food Hydrocoll. 2025, 159, 110625. [Google Scholar] [CrossRef]
- Ananta, E.; Volkert, M.; Knorr, D. Cellular Injuries and Storage Stability of Spray-Dried Lactobacillus rhamnosus GG. Int. Dairy J. 2005, 15, 399–409. [Google Scholar] [CrossRef]
- Paula, D.d.A.; Martins, E.M.F.; Costa, N.d.A.; de Oliveira, P.M.; de Oliveira, E.B.; Ramos, A.M. Use of Gelatin and Gum Arabic for Microencapsulation of Probiotic Cells from Lactobacillus plantarum by a Dual Process Combining Double Emulsification Followed by Complex Coacervation. Int. J. Biol. Macromol. 2019, 133, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Babu, A.; Shams, R.; Dash, K.K.; Shaikh, A.M.; Kovács, B. Protein-Polysaccharide Complexes and Conjugates: Structural Modifications and Interactions under Diverse Treatments. J. Agric. Food Res. 2024, 18, 101510. [Google Scholar] [CrossRef]
- Yan, Y.; Tang, R.; Li, F.; Huang, L.; Chen, Y.; He, R. Spectral Analysis of the Impact of Various Polysaccharides on the Entrapment of Curcumin by Whey Protein Isolate. Food Chem. 2025, 468, 142441. [Google Scholar] [CrossRef]
- Qiao, D.; Zhang, Y.; Sun, F.; Yoo, M.; Zhao, G.; Zhang, B. Enhancement Mechanism of ι-Carrageenan on the Network Structure and Gel-Related Properties of Soy Protein Isolate/λ-Carrageenan System. Food Chem. 2025, 468, 142476. [Google Scholar] [CrossRef]
- Ngamekaue, N.; Dumrongchai, T.; Rodklongtan, A.; Chitprasert, P. Improving Probiotic Survival through Encapsulation in Coconut Oil in Whey Protein Isolate Emulsions during Spray Drying and Gastrointestinal Digestion. LWT 2024, 198, 116061. [Google Scholar] [CrossRef]
- Liang, Z.; Chu, H.; Hou, Z.; Wang, C.; Zhang, G.; Liu, L.; Ma, X.; Li, C.; He, J. W/O/W emulsions stabilized with whey protein concentrate and pectin: Effects on storage, pasteurization, and gastrointestinal viability of lacticaseibacillus rhamnosus. Int. J. Biol. Macromol. 2023, 232, 123477. [Google Scholar] [CrossRef] [PubMed]
- Rajam, R.; Karthik, P.; Parthasarathi, S.; Joseph, G.S.; Anandharamakrishnan, C. Effect of whey protein—Alginate wall systems on survival of microencapsulated lactobacillus plantarum in simulated gastrointestinal conditions. J. Funct. Foods 2012, 4, 891–898. [Google Scholar] [CrossRef]
- da Silva Menegazzi, G.; Ribeiro, E.S.; de Farias, B.S.; da Luz, G.D.Q.; Oliveira, G.M.; Cadaval Junior, T.R.S.; Pinto, L.A.d.A.; Diaz, P.S. Microencapsulation of lacticaseibacillus casei CSL3 using cheese whey, fructo-oligosaccharide and xanthan gum by spray drying. Food Biosci. 2023, 56, 103348. [Google Scholar]
- de la Fuente, M.A.; Hemar, Y.; Singh, H. Influence of κ-carrageenan on the aggregation behaviour of proteins in heated whey protein isolate solutions. Food Chem. 2004, 86, 1–9. [Google Scholar] [CrossRef]
- Rivera Patiño, J.D.; Torrejón-Cabello, A. Survival of Lactic Acid Bacteria and Yeasts to Encapsulation Process Comparing Spray Drying to Electrostatic Spray Drying. Appl. Food Res. 2025, 5, 100756. [Google Scholar] [CrossRef]
- Sharifi, S.; Rezazad-Bari, M.; Alizadeh, M.; Almasi, H.; Amiri, S. Use of Whey Protein Isolate and Gum Arabic for the Co-Encapsulation of Probiotic Lactobacillus plantarum and Phytosterols by Complex Coacervation: Enhanced Viability of Probiotic in Iranian White Cheese. Food Hydrocoll. 2021, 113, 106496. [Google Scholar] [CrossRef]
- Zacarías, M.F.; Cuatrin, A.; Lavari, L.; Taverna, M.; Páez, R.; Vinderola, G. Biomass Production in Whey Permeate and Spray Drying of a Potential Probiotic Strain from Human Breast Milk, Bifidobacterium animalis Subsp. Lactis INL1. Int. Dairy. J. 2025, 163, 106181. [Google Scholar] [CrossRef]
- Luo, J.; Jia, M.; Yang, X.; Chai, Y.; Bao, Y. Interaction between Lactic Acid Bacteria and Polygonatum sibiricum Saponins and Its Application to Microencapsulated Co-Delivery. Food Chem. 2024, 448, 138959. [Google Scholar] [CrossRef] [PubMed]
- Nasir, N.N.; Othman, S.A. The Effect of Diammonium Phosphate and Potassium Nitrate as Filler on Moisture Content and Rate of Water Absorption of Corn-Based Film. Mater. Today Proc. 2024, 109, 22–26. [Google Scholar] [CrossRef]
- Kalman, H. Effect of Moisture Content on Flowability: Angle of Repose, Tilting Angle, and Hausner Ratio. Powder Technol. 2021, 393, 582–596. [Google Scholar] [CrossRef]
- Kamble, M.; Singh, A.; Singh, S.V.; Upadhyay, A.; Kondepudi, K.K.; Chinchkar, A.V. Effect of Gastrointestinal Resistant Encapsulate Matrix on Spray Dried Microencapsulated Lacticaseibacillus rhamnosus GG Powder and Its Characterization. Food Res. Int. 2024, 192, 114804. [Google Scholar] [CrossRef]
- Poddar, D.; Das, S.; Jones, G.; Palmer, J.; Jameson, G.B.; Haverkamp, R.G.; Singh, H. Stability of Probiotic Lactobacillus paracasei during Storage as Affected by the Drying Method. Int. Dairy. J. 2014, 39, 1–7. [Google Scholar] [CrossRef]
- Srivastava, S.; Mishra, H.N. Development of Microencapsulated Vegetable Oil Powder Based Cookies and Study of Its Physicochemical Properties and Storage Stability. LWT 2021, 152, 112364. [Google Scholar] [CrossRef]
- Sogut, E.; Filiz, B.E.; Seydim, A.C. Whey Protein Isolate- and Carrageenan-Based Edible Films as Carriers of Different Probiotic Bacteria. J. Dairy. Sci. 2022, 105, 4829–4842. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rodríguez, L.; Lobato-Calleros, C.; Pimentel-González, D.J.; Vernon-Carter, E.J. Lactobacillus plantarum Protection by Entrapment in Whey Protein Isolate: κ-Carrageenan Complex Coacervates. Food Hydrocoll. 2014, 36, 181–188. [Google Scholar] [CrossRef]
- Zhang, C.; van de Weert, M.; Bjerregaard, S.; Rantanen, J.; Yang, M. Leucine as a Moisture-Protective Excipient in Spray-Dried Protein/Trehalose Formulation. J. Pharm. Sci. 2024, 113, 2764–2774. [Google Scholar] [CrossRef] [PubMed]
- Resende, I.F.; Martins, P.M.M.; de Souza Melo, D.; Magnani, M.; Dias, D.R.; Schwan, R.F. Development and Characterization of Microencapsulated Pichia kluyveri CCMA 0615 with Probiotic Properties and Its Application in Fermented Beverages. Int. J. Food Microbiol. 2025, 427, 110967. [Google Scholar] [CrossRef]
- Alfaro-Galarza, O.; López-Villegas, E.O.; Rivero-Perez, N.; Tapia- Maruri, D.; Jiménez-Aparicio, A.R.; Palma-Rodríguez, H.M.; Vargas-Torres, A. Protective Effects of the Use of Taro and Rice Starch as Wall Material on the Viability of Encapsulated Lactobacillus paracasei Subsp. Paracasei. LWT 2020, 117, 108686. [Google Scholar] [CrossRef]
- Karaogul, E.; Ugurtay, A. Unveiling Modeling and SEM/XRD Insights into Enhanced Antibacterial, Antioxidant, and Bioactive Potentials of Micro-Encapsulated Pistacia vera Hull Extract. Food Chem. 2025, 477, 143510. [Google Scholar] [CrossRef]
- Hundre, S.Y.; Karthik, P.; Anandharamakrishnan, C. Effect of Whey Protein Isolate and β-Cyclodextrin Wall Systems on Stability of Microencapsulated Vanillin by Spray–Freeze Drying Method. Food Chem. 2015, 174, 16–24. [Google Scholar] [CrossRef]
- Guo, Q.; Li, S.; Tang, J.; Chang, S.; Qiang, L.; Du, G.; Yue, T.; Yuan, Y. Microencapsulation of Lactobacillus plantarum by Spray Drying: Protective Effects during Simulated Food Processing, Gastrointestinal Conditions, and in Kefir. Int. J. Biol. Macromol. 2022, 194, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Bommasamudram, J.; Muthu, A.; Devappa, S. Effect of Sub-Lethal Heat Stress on Viability of Lacticaseibacillus casei N. in Spray-Dried Powders. LWT 2022, 155, 112904. [Google Scholar] [CrossRef]
- Liang, B.; Feng, S.; Zhang, X.; Ye, Y.; Sun, C.; Ji, C.; Li, X. Physicochemical Properties and in Vitro Digestion Behavior of Emulsion Micro-Gels Stabilized by κ-Carrageenan and Whey Protein: Effects of Sodium Alginate Addition. Int. J. Biol. Macromol. 2024, 271, 132512. [Google Scholar] [CrossRef] [PubMed]
- Kriegseis, S.; Vogl, A.Y.; Aretz, L.; Tonnesen, T.; Telle, R. Zeta Potential and Long-Term Stability Correlation of Carbon-Based Suspensions for Material Jetting. Open Ceram. 2020, 4, 100037. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, S.; Liu, D.; Wang, Q.; Feng, X.; Chu, L. Coenzyme Q10-Loaded Microcapsules Stabilized by Glyceryl Monostearate and Soy Protein Isolates-Flaxseed Gum: Characterization, in Vitro Release and Digestive Behavior. Int. J. Biol. Macromol. 2024, 278, 134680. [Google Scholar] [CrossRef]
- Gu, C.-Y.; Shao, J.-Q.; Liu, X.-L.; Wei, J.-T.; Huang, G.-Q.; Xiao, J.-X. Spray Drying the Pickering Emulsions Stabilized by Chitosan/Ovalbumin Polyelectrolyte Complexes for the Production of Oxidation Stable Tuna Oil Microcapsules. Int. J. Biol. Macromol. 2024, 273, 133139. [Google Scholar] [CrossRef] [PubMed]
- Uko, L.; Noby, H.; Zkria, A.; Elkady, M. Novel Eco-Friendly Nanodiamonds@chitosan Hybrid Microcapsules via Facile Electrospraying for Self-Healing and Anticorrosion Coating. Diamond Relat. Mater. 2024, 148, 111436. [Google Scholar] [CrossRef]
- Ma, J.; Tan, Z.; Wei, X.; Tian, Z.; Wang, V.Y.; Wang, E.; Xu, D. Electrospray Whey Protein-Polysaccharide Microcapsules Co-Encapsulating Lactiplantibacillus plantarum and EGCG: Enhanced Probiotic Viability and Antioxidant Activity. Int. J. Biol. Macromol. 2025, 290, 138734. [Google Scholar] [CrossRef]
- Liu, S.; Sheng, W.; Zheng, H.; Pan, S.; Wang, Y.; Bayizi, M. Preparation and Study of N-Tridecane@silica Low-Temperature Nano-Encapsulated Phase Change Microcapsules. Energy Build. 2025, 334, 115490. [Google Scholar] [CrossRef]
- Barreto Pinilla, C.M.; Galland, F.; Bertoldo Pacheco, M.T.; Bócoli, P.J.; Fidelis Borges, D.; Dutra Alvim, I.; Spadoti, L.M.; Torres Silva e Alves, A. Unraveling the Real Potential of Liquid Whey as Media Culture and Microencapsulation Material for Lactic Acid Bacteria. Innov. Food Sci. Emerg. Technol. 2025, 100, 103885. [Google Scholar] [CrossRef]
- Gedik, O.; Karahan, A.G. Properties and Stability of Lactiplantibacillus plantarum AB6-25 and Saccharomyces boulardii T8-3C Single and Double-Layered Microcapsules Containing Na-Alginate and/or Demineralized Whey Powder with Lactobionic Acid. Int. J. Biol. Macromol. 2024, 271, 132406. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Chen, Y.; Li, X.; Zhou, X.; Ding, Y. Enhanced Probiotic Viability in Innovative Double-Network Emulsion Gels: Synergistic Effects of the Whey Protein Concentrate-Xanthan Gum Complex and κ-Carrageenan. Int. J. Biol. Macromol. 2024, 270, 131758. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Qiu, X.; Liu, H.; Han, Y. Microencapsulated Phase Change Materials (MicroPCMs) with TiO2-Modified Natural Polymer Shell and Macrocapsules Containing MicroPCMs for Thermal Energy Storage and UV-Shielding. Sol. Energy Mater. Sol. Cells 2024, 271, 112860. [Google Scholar] [CrossRef]
| Sample | Moisture Content (%) | Solubility (s) | Hygroscopicity (g/100 g) |
|---|---|---|---|
| CK | 2.12 ± 0.01 b | 900 ± 20.53 a | 2.08 ± 0.24 a |
| WP-KC | 2.12 ± 0.01 b | 780 ± 13.42 b | 1.02 ± 0.35 b |
| WP-LMP | 2.35 ± 0.02 a | 540 ± 8.34 d | 1.03 ± 0.03 b |
| WP-XG | 2.12 ± 0.01 b | 660 ± 12.84 c | 1.02 ± 0.52 b |
| WP-SA | 2.12 ± 0.02 b | 300 ± 15.36 e | 1.02 ± 0.33 b |
| Sample | Peak Temperature (°C) | Initial Temperature (°C) | Termination Temperature (°C) | Enthalpy Change (J/g) |
|---|---|---|---|---|
| CK | 136.96 ± 0.85 | 132.64 ± 0.72 | 142.36 ± 1.10 | 30.21 ± 0.43 |
| WP-KC | 142.63 ± 1.23 | 135.90 ± 0.95 | 149.38 ± 1.65 | 9.73 ± 0.28 |
| WP-LMP | 168.37 ± 2.15 | 157.23 ± 1.80 | 196.52 ± 3.40 | 2.06 ± 0.15 |
| WP-XG | 164.62 ± 1.90 | 155.22 ± 1.45 | 175.15 ± 2.20 | 9.86 ± 0.31 |
| WP-SA | 137.94 ± 0.78 | 133.89 ± 0.68 | 143.11± 1.05 | 6.82 ± 0.22 |
| Time (Day) | −20 °C (lg CFU/g) | 4 °C (lg CFU/g) | 25 °C (lg CFU/g) |
|---|---|---|---|
| 0 | 12.41 ± 0.14 | 12.41 ± 0.14 | 12.41 ± 0.14 |
| 15 | 12.35 ± 0.06 | 12.22 ± 0.06 | 11.59 ± 0.24 |
| 30 | 10.26 ± 0.27 | 11.94 ± 0.14 | 10.26 ± 0.05 |
| 45 | 10.88 ± 0.20 | 10.55 ± 0.34 | 9.94 ± 0.04 |
| 60 | 9.88 ± 0.03 | 9.91 ± 0.08 | 8.81 ± 0.14 |
| 75 | 8.34 ± 0.14 | 9.98 ± 0.11 | 8.43 ± 0.23 |
| 90 | 7.32 ± 0.22 | 9.65 ± 0.11 | 7.46 ± 0.16 |
| 105 | 6.32 ± 0.35 | 8.72 ± 0.03 | 5.46 ± 0.43 |
| 120 | 6.34 ± 0.13 | 8.68 ± 0.03 | 4.27 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Peng, X.; Chen, H.; Li, A.; Yang, G.; Kan, J. Protective Effect of Whey Protein and Polysaccharide Complexes on Lactobacillus paracasei F50: Comparative Analysis of Powder Characteristics and Stability. Foods 2025, 14, 1555. https://doi.org/10.3390/foods14091555
Zhang X, Peng X, Chen H, Li A, Yang G, Kan J. Protective Effect of Whey Protein and Polysaccharide Complexes on Lactobacillus paracasei F50: Comparative Analysis of Powder Characteristics and Stability. Foods. 2025; 14(9):1555. https://doi.org/10.3390/foods14091555
Chicago/Turabian StyleZhang, Xinrui, Xiaowei Peng, Huijing Chen, Aijun Li, Gang Yang, and Jianquan Kan. 2025. "Protective Effect of Whey Protein and Polysaccharide Complexes on Lactobacillus paracasei F50: Comparative Analysis of Powder Characteristics and Stability" Foods 14, no. 9: 1555. https://doi.org/10.3390/foods14091555
APA StyleZhang, X., Peng, X., Chen, H., Li, A., Yang, G., & Kan, J. (2025). Protective Effect of Whey Protein and Polysaccharide Complexes on Lactobacillus paracasei F50: Comparative Analysis of Powder Characteristics and Stability. Foods, 14(9), 1555. https://doi.org/10.3390/foods14091555





