Influence of Ripening Stage and Cultivar on Physicochemical Properties and Antioxidant Compositions of Aronia Grown in South Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemical Reagents
2.3. Extraction for Measurement of Antioxidant Compounds and Activities
2.4. Color Analysis
2.5. Soluble Solid Content, Firmness, Titratable Acidity, pH and Moisture Content
2.6. Organic Acid Compositions
2.7. Sugar Composition
2.8. Total Anthocyanin Analysis
2.9. Total Flavonoid Analysis
2.10. Total Phenolic Analysis
2.11. Polyphenol Analysis
2.12. DPPH Radical Scavenging Activity Analysis
2.13. ABTS Radical Scavenging Activity Analysis
2.14. Statistical Analysis
3. Results and Discussion
3.1. Color
3.2. Soluble Solid Content, Firmness, Titratable Acidity, pH, and Moisture Content
3.3. Quantification of Individual Organic Acids
3.4. Quantification of Individual Sugars
3.5. Total Anthocyanin Content
3.6. Total Flavonoid Content
3.7. Total Phenolics Content
3.8. Polyphenol Content
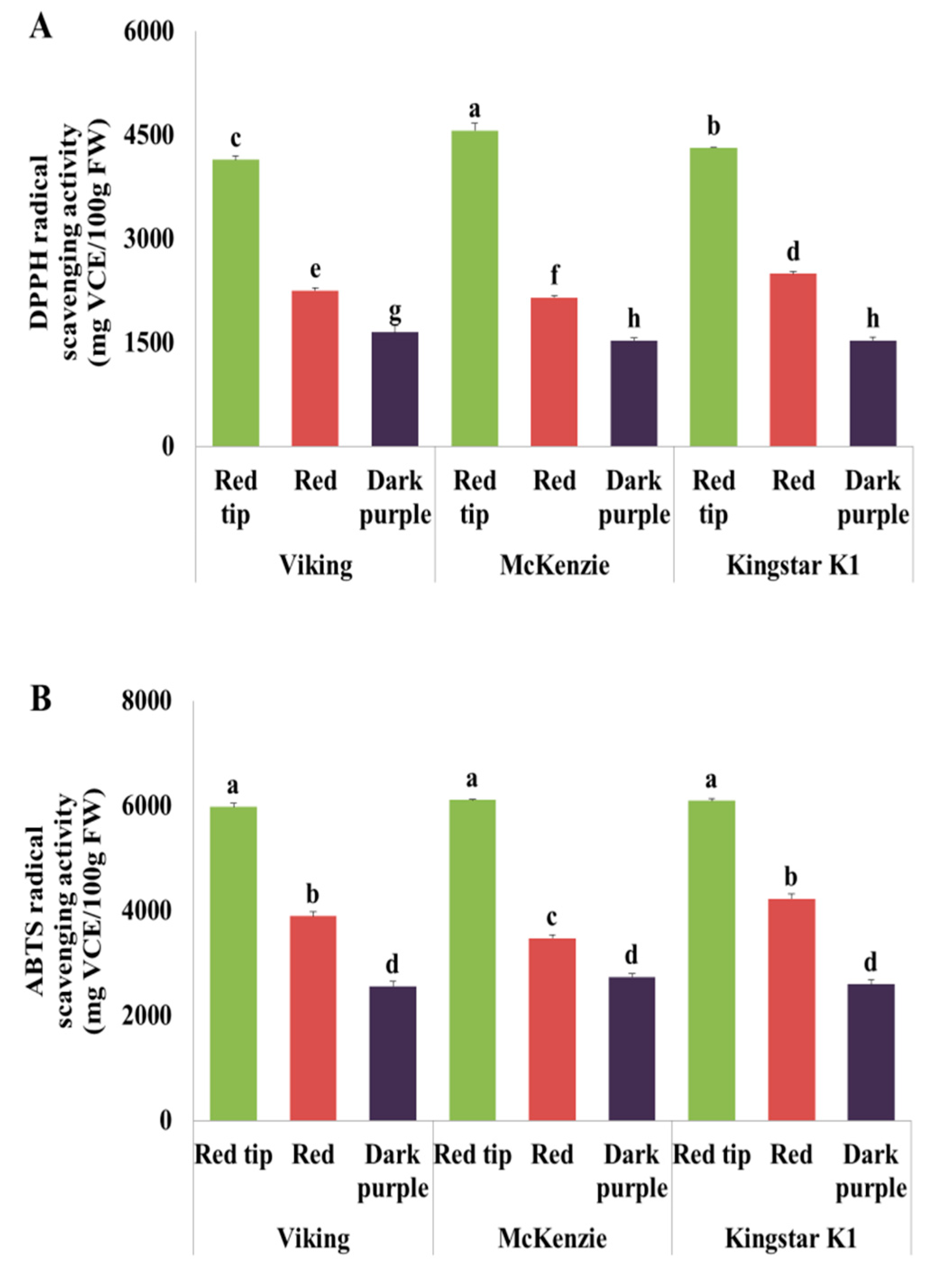
3.9. DPPH Radical Scavenging Activity
3.10. ABTS Radical Scavenging Activity Analysis
3.11. Correlation between Physicochemical Quality, Antioxidant Substances and Antioxidant Activity in Aronia Fruit
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rissanen, T.H.; Voutilainen, S.; Virtanen, J.K.; Venho, B.; Vanharanta, M.; Mursu, J.; Salonen, J.T. Low intake of fruits, and vegetables is associated with excess mortality in men: The Kuopio Ischaemic Heart Disease Risk Factor (KIHD) Study. J. Nutr. 2003, 133, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T. Dietary anthocyanin-rich plants: Biochemical basis and recent progress in health benefits studies. Mol. Nutr. Food Res. 2012, 56, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Wangensteen, H.; Bräunlich, M.; Nikolic, V.; Malterud, K.E.; Slimestad, R.; Barsett, H. Anthocyanins, proanthocyanidins and total phenolics in four cultivars of aronia: Antioxidant and enzyme inhibitory effects. J. Funct. Foods 2014, 7, 746–752. [Google Scholar] [CrossRef]
- Kokotkiewicz, A.; Jaremicz, Z.; Luczkiewicz, M. Aronia plants: A review of traditional use, biological activities, and perspectives for modern medicine. J. Med. Food 2010, 13, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Snebergrova, J.; Cizkova, H.; Neradova, E.; Kapci, B.; Rajchl, A.; Voldrich, M. Variability of Characteristic Components of Aronia. Czech J. Food Sci. 2014, 32, 25–30. [Google Scholar] [CrossRef]
- Denev, P.N.; Kratchanov, C.G.; Ciz, M.; Lojek, A.; Kratchanova, M.G. Bioavailability and antioxidant activity of black chokeberry (Aronia melanocarpa) polyphenols: In vitro and in vivo evidences and possible mechanisms of action: A review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 471–489. [Google Scholar] [CrossRef]
- Kim, Y.J.; Shin, Y. Antioxidant profile, antioxidant activity, and physicochemical characteristics of strawberries from different cultivars and harvest locations. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 587–595. [Google Scholar] [CrossRef]
- Meyers, K.J.; Watkins, C.B.; Pritts, M.P.; Liu, R.H. Antioxidant and antiproliferative activities of strawberries. J. Agric. Food Chem. 2003, 51, 6887–6892. [Google Scholar] [CrossRef]
- Shin, Y. Correlation between antioxidant concentrations and activities of Yuja (Citrus junos Sieb ex Tanaka) and other citrus fruits. J. Agric. Food Chem. 2012, 21, 1477–1482. [Google Scholar] [CrossRef]
- Chen, H.; Zuo, Y.; Deng, Y. Separation and determination of flavonoids and other phenolic compounds in cranberry juice by high-performance liquid chromatography. J. Chromatogr. A 2001, 913, 387–395. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Kaack, K.; Kühn, B.F. Black chokeberry (Aronia melanocarpa) for manufacture of a food colorant. Tidsskr. Planteavl. 1992, 96, 183–196. [Google Scholar]
- Taheri, R. Polyphenol Composition of Underutilized Aronia Fruits and Changes in Aronia Berry Polyphenol Content through Ripening. Master’s Thesis, University of Connecticut, Storrs, CT, USA, 2013. [Google Scholar]
- Jeppsson, N.; Johansson, R. Changes in fruits quality in black chokeberry (Aronia melanocarpa) during maturation. J. Hortic. Sci. Biotechnol. 2015, 75, 340–345. [Google Scholar] [CrossRef]
- Tosun, I.; Ustun, N.S.; Tekguler, B. Physical and chemical changes during ripening of blackberry fruits. Sci. Agric. 2008, 65, 87–90. [Google Scholar] [CrossRef]
- Tolić, M.T.; Krbavčić, I.P.; Vujević, P.; Milinović, B.; Jurčević, I.L.; Vahčić, N. Effects of weather conditions on phenolic content and antioxidant capacity in juice of chokeberries (Aronia melanocarpa L.). Pol. J. Food Nutr. Sci. 2017, 67, 67–74. [Google Scholar]
- Cordenunsi, B.R.; Oliveira do Nascimento, J.R.; Genovese, M.I.; Lajolo, F.M. Influence of cultivar on quality parameters and chemical composition of strawberry fruits grown in Brazil. J. Agric. Food Chem. 2002, 50, 2581–2586. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of sugars, organic acids, and total phenolics in 25 wild or cultivated berry Species. J. Food Sci. 2012, 77, C1064–C1070. [Google Scholar] [CrossRef]
- Siriwoharn, T.; Wrolstad, R.E.; Finn, C.E.; Pereira, C.B. Influence of cultivar, maturity, and sampling on blackberry (Rubus L. Hybrids) anthocyanins, polyphenolics, and antioxidant properties. J. Agric. Food Chem. 2004, 52, 8021–8030. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lin, H.S. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef]
- Benvenuti, S.; Pellati, F.; Melegari, M.; Bertelli, D. Polyphenols, anthocyanins, ascorbic acid, and radical scavenging activity of Rubus, Ribes, and Aronia. J. Food Sci. 2004, 69, 164–169. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, C.T.; Wang, C.Y. The influence of light and maturity on fruits quality and flavonoid content of red raspberries. Food Chem. 2009, 112, 676–684. [Google Scholar] [CrossRef]
- Acosta-Montoya, Ó.; Vaillant, F.; Cozzano, S.; Mertz, C.; Pérez, A.M.; Castro, M.V. Phenolic content and antioxidant capacity of tropical highland blackberry (Rubus adenotrichus Schltdl.) during three edible maturity stages. Food Chem. 2010, 119, 1497–1501. [Google Scholar] [CrossRef]
- Jakobek, L.; Drenjančević, M.; Jukić, V.; Šeruga, M. Phenolic acids, flavonols, anthocyanins and antiradical activity of “Nero”, “Viking”, “Galicianka” and wild chokefruits. Sci. Hort. 2012, 147, 56–63. [Google Scholar] [CrossRef]
- Sidor, A.; Gramza-Michałowska, A. Black Chokeberry Aronia Melanocarpa L.—A qualitative composition, phenolic profile and antioxidant potential. Molecules 2019, 24, 3710. [Google Scholar] [CrossRef]
- Määttä-Riihinen, K.R.; Kamal-Eldin, A.; Mattila, P.H.; González-Paramás, A.M.; Törrönen, A.R. Distribution and contents of phenolic compounds in eighteen Scandinavian berry species. J. Agric. Food Chem. 2004, 52, 4477–4486. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef]
- Amil-Ruiz, F.; Blanco-Portales, R.; Munoz-Blanco, J.; Caballero, J.L. The strawberry plant defense mechanism: A molecular review. Plant. Cell Physiol. 2011, 52, 1873–1903. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A.; Sroka, P.; Satora, P.; Michalik, J. Transformations of phenolic compounds in an in vitro model simulating the human alimentary tract. Food Technol. Biotechnol. 2009, 47, 456–463. [Google Scholar]
- Castrejón, A.D.R.; Eichholz, I.; Rohn, S.; Kroh, L.W.; Huyskens-Keil, S. Phenolic profile and antioxidant activity of highbush blueberry (Vaccinium corymbosum L.) during fruits maturation and ripening. Food Chem. 2008, 109, 564–572. [Google Scholar]


| Cultivar | Maturity Stage | L* | a* | b* |
|---|---|---|---|---|
| Viking | Red tip | 43.53 ± 1.99 b | −7.74 ± 1.36 e | 12.17 ± 2.14 a |
| Viking | Red | 27.23 ± 1.35 e | 9.86 ± 0.31 a | 2.33 ± 1.20 d |
| Viking | Dark purple | 23.81 ± 1.03 g | 1.02 ± 0.31 c | −1.84 ± 0.15 e |
| McKenzie | Red tip | 44.63 ± 2.66 a | −6.34 ± 1.46 d | 9.83 ± 2.46 b |
| McKenzie | Red | 28.75 ± 1.57 d | 10.18 ± 1.85 a | 3.54 ± 1.34 c |
| McKenzie | Dark purple | 25.28 ± 0.74 f | 1.00 ± 0.36 c | −2.42 ± 0.36 e |
| Kingstar K1 | Red tip | 42.65 ± 1.17 c | −6.77 ± 0.84 d | 9.90 ± 1.14 b |
| Kingstar K1 | Red | 28.22 ± 1.56 d | 9.14 ± 1.51 b | 2.72 ± 1.44 d |
| Kingstar K1 | Dark purple | 22.26 ± 0.72 h | 1.19 ± 0.44 c | −1.78 ± 0.15 e |
| Cultivar | Maturity Stage | SSC (°Brix) | Firmness (N/12 mmØ) | Titratable Acidity (%) | Malic Acid (mg/100 g) | pH | SSC/TA Ratio | Moisture Content (%) |
|---|---|---|---|---|---|---|---|---|
| Viking | Red tip | 16.00 ± 1.00 b | 3.95 ± 0.34 b | 1.26 ± 0.04 b | 448.62 ± 6.50 e | 3.52 ± 0.06 de | 12.72 ± 0.87 b | 36.92 ± 1.03 d |
| Viking | Red | 12.33 ± 0.58 d | 2.05 ± 0.21 d | 1.30 ± 0.01 a | 658.29 ± 31.30 c | 3.47 ± 0.03 e | 9.48 ± 0.50 d | 41.08 ± 0.82 c |
| Viking | Dark purple | 13.66 ± 1.53 cd | 0.99 ± 0.23 f | 1.29 ± 0.01 a | 827.78 ± 42.63 a | 3.68 ± 0.06 c | 10.57 ± 1.23 cd | 54.87 ± 1.14 a |
| McKenzie | Red tip | 20.33 ± 1.53 a | 4.04 ± 0.18 ab | 1.28 ± 0.01 ab | 434.15 ± 16.78 e | 3.79 ± 0.02 b | 15.94 ± 1.29 a | 38.58 ± 1.28 cd |
| McKenzie | Red | 13.00 ± 1.00 cd | 2.26 ± 0.25 c | 1.29 ± 0.01 a | 669.78 ± 22.02 c | 3.58 ± 0.09 d | 10.09 ± 0.78 cd | 39.21 ± 0.87 cd |
| McKenzie | Dark purple | 14.67 ± 0.58 bc | 1.15 ± 0.20 e | 1.28 ± 0.01 ab | 815.00 ± 21.42 a | 4.01 ± 0.02 a | 11.50 ± 0.49 bc | 46.64 ± 1.70 b |
| Kingstar K1 | Red tip | 20.67 ± 1.53 a | 4.09 ± 0.26 a | 1.29 ± 0.01 ab | 448.73 ± 16.77 e | 3.79 ± 0.02 b | 16.04 ± 1.20 a | 40.06 ± 0.69 c |
| Kingstar K1 | Red | 13.00 ± 1.00 cd | 2.34 ± 0.27 c | 1.29 ± 0.01 a | 578.43 ± 29.32 d | 3.55 ± 0.03 de | 10.06 ± 0.88 cd | 38.44 ± 2.17 cd |
| Kingstar K1 | Dark purple | 14.00 ± 1.00 bcd | 0.88 ± 0.15 f | 1.28 ± 0.01 ab | 758.33 ± 36.45 b | 3.81 ± 0.06 b | 10.90 ± 0.78 cd | 45.92 ± 2.69 b |
| Cultivar | Maturity Stage | Fructose (mg/100 g FW) | Glucose (mg/100 g FW) | Sorbitol (mg/100 g FW) | Total Sum (mg/100 g FW) |
|---|---|---|---|---|---|
| Viking | Red tip | 113.51 ± 33.48 f | 272.83 ± 16.64 f | 1343.97 ± 45.38 e | 1730.31 |
| Viking | Red | 841.90 ± 85.80 e | 972.32 ± 76.02 e | 1962.84 ± 66.08 d | 3777.05 |
| Viking | Dark purple | 2103.11 ± 194.48 b | 2366.75 ± 250.94 b | 3882.51 ± 536.95 b | 8352.37 |
| McKenzie | Red tip | 17.63 ± 11.53 f | 191.35 ± 8.13 f | 1333.54 ± 37.89 e | 1542.51 |
| McKenzie | Red | 1065.64 ± 108.32 d | 1145.51 ± 105.51 e | 2518.72 ± 323.36 c | 4729.86 |
| McKenzie | Dark purple | 2477.12 ± 74.65 a | 2677.70 ± 119.95 a | 4831.56 ± 129.08 a | 9986.37 |
| Kingstar K1 | Red tip | 10.51 ± 14.92 f | 232.09 ± 21.57 f | 1432.02 ± 18.61 e | 1674.62 |
| Kingstar K1 | Red | 1252.83 ± 108.20 c | 1405.74 ± 95.03 d | 2747.56 ± 210.53 c | 5406.13 |
| Kingstar K1 | Dark purple | 1961.00 ± 110.10 b | 2080.48 ± 121.71 c | 3593.35 ± 173.51 b | 7634.84 |
| Cultivar | Maturity Stage | Catechol (mg/100 g FW) | Chlorogenic Acid (mg/100 g FW) | Total Sum (mg/100 g FW) |
|---|---|---|---|---|
| Viking | Red tip | 256.11 ± 19.27 c | 120.82 ± 7.91 b | 376.92 |
| Viking | Red | 134.18 ± 6.48 d | 72.31 ± 4.81 c | 206.49 |
| Viking | Dark purple | 95.05 ± 6.93 ef | 64.69 ± 3.09 cd | 159.74 |
| McKenzie | Red tip | 399.01 ± 22.22 a | 176.62 ± 15.57 a | 575.63 |
| McKenzie | Red | 118.02 ± 1.87 de | 68.75 ± 1.59 cd | 186.78 |
| McKenzie | Dark purple | 81.80 ± 3.50 fg | 56.72 ± 3.83 de | 138.53 |
| Kingstar K1 | Red tip | 289.03 ± 10.88 b | 132.58 ± 5.39 b | 421.61 |
| Kingstar K1 | Red | 118.33 ± 1.59 de | 72.87 ± 2.81 c | 191.20 |
| Kingstar K1 | Dark purple | 70.69 ± 0.27 g | 49.76 ± 0.95 e | 120.45 |
| a* | b* | SSC | Firmness | Titratable Acidity | Malic Acid | pH | Moisture Content | Fructose | Glucose | Sorbitol | Total Anthocyanin | Total Flavonoids | Total Phenolics | DPPH | ABTS | Catechol | Chlorogenic Acid | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | −0.739 ** | 0.959 ** | 0.788 ** | 0.963 ** | −0.305 | −0.916 ** | −0.071 | −0.614 ** | −0.885 ** | −0.868 ** | −0.798 ** | −0.723 ** | 0.986 ** | 0.986 ** | 0.981 ** | 0.967 ** | 0.935 ** | 0.925 ** |
| a* | −0.592 ** | −0.792 ** | −0.578 ** | 0.456 * | 0.508 ** | −0.384 * | 0.068 | 0.441 * | 0.406 * | 0.335 | 0.124 | −0.726 ** | −0.757 ** | −0.694 ** | −0.622 ** | −0.708 ** | −0.706 ** | |
| b* | 0.660 ** | 0.962 ** | −0.191 | −0.950 ** | −0.267 | −0.723 ** | −0.936 ** | −0.926 ** | −0.884 ** | −0.835 ** | 0.953 ** | 0.927 ** | 0.958 ** | 0.961 ** | 0.897 ** | 0.855 ** | ||
| SSC | 0.715 ** | −0.461 * | −0.659 ** | 0.370 | −0.292 | −0.606 ** | −0.576 ** | −0.487 * | −0.384 * | 0.805 ** | 0.837 ** | 0.790 ** | 0.731 ** | 0.830 ** | 0.820 ** | |||
| Firmness | −0.192 | −0.961 ** | −0.212 | −0.731 ** | −0.954 ** | −0.943 ** | −0.889 ** | −0.857 ** | 0.971 ** | 0.960 ** | 0.980 ** | 0.987 ** | 0.906 ** | 0.892 ** | ||||
| Titratable acidity | 0.225 | −0.429 * | 0.080 | 0.037 | 0.028 | −0.093 | −0.038 | −0.287 | −0.286 | −0.243 | −0.179 | −0.282 | −0.272 | |||||
| Malic acid | 0.276 | 0.791 ** | 0.941 ** | 0.933 ** | 0.891 ** | 0.893 ** | −0.938 ** | −0.909 ** | −0.949 ** | −0.971 ** | −0.869 ** | −0.853 ** | ||||||
| pH | 0.362 | 0.383 * | 0.399 * | 0.488 ** | 0.543 ** | −0.087 | −0.013 | −0.126 | −0.200 | 0.000 | 0.005 | |||||||
| Moisture content | 0.748 ** | 0.768 ** | 0.733 ** | 0.885 ** | −0.629 ** | −0.580 ** | −0.642 ** | −0.711 ** | −0.535 ** | −0.515 ** | ||||||||
| Fructose | 0.998 ** | 0.981 ** | 0.911 ** | −0.912 ** | −0.883 ** | −0.926 ** | −0.942 ** | −0.857 ** | −0.835 ** | |||||||||
| Glucose | 0.985 ** | 0.920 ** | −0.895 ** | −0.862 ** | −0.909 ** | −0.929 ** | −0.837 ** | −0.813 ** | ||||||||||
| Sorbitol | 0.907 ** | −0.835 ** | −0.794 ** | −0.854 ** | −0.877 ** | −0.781 ** | −0.758 ** | |||||||||||
| Total anthocyanin | −0.753 **z | −0.705 ** | −0.780 ** | −0.839 ** | −0.674 ** | −0.655 ** | ||||||||||||
| Total flavonoids | 0.991 ** | 0.997 ** | 0.979 ** | 0.961 ** | 0.949 ** | |||||||||||||
| Total phenolics | 0.988 ** | 0.970 ** | 0.953 ** | 0.940 ** | ||||||||||||||
| DPPH | 0.987 ** | 0.954 ** | 0.943 ** | |||||||||||||||
| ABTS | 0.917 ** | 0.903 ** | ||||||||||||||||
| Catechol | 0.995 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Kim, Y.-J.; Shin, Y. Influence of Ripening Stage and Cultivar on Physicochemical Properties and Antioxidant Compositions of Aronia Grown in South Korea. Foods 2019, 8, 598. https://doi.org/10.3390/foods8120598
Yang H, Kim Y-J, Shin Y. Influence of Ripening Stage and Cultivar on Physicochemical Properties and Antioxidant Compositions of Aronia Grown in South Korea. Foods. 2019; 8(12):598. https://doi.org/10.3390/foods8120598
Chicago/Turabian StyleYang, Haejo, Young-Jun Kim, and Youngjae Shin. 2019. "Influence of Ripening Stage and Cultivar on Physicochemical Properties and Antioxidant Compositions of Aronia Grown in South Korea" Foods 8, no. 12: 598. https://doi.org/10.3390/foods8120598
APA StyleYang, H., Kim, Y.-J., & Shin, Y. (2019). Influence of Ripening Stage and Cultivar on Physicochemical Properties and Antioxidant Compositions of Aronia Grown in South Korea. Foods, 8(12), 598. https://doi.org/10.3390/foods8120598





