The Potential of Modulating the Reducing Sugar Released (and the Potential Glycemic Response) of Muffins Using a Combination of a Stevia Sweetener and Cocoa Powder †
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Muffin Preparation
2.3. Muffin Height
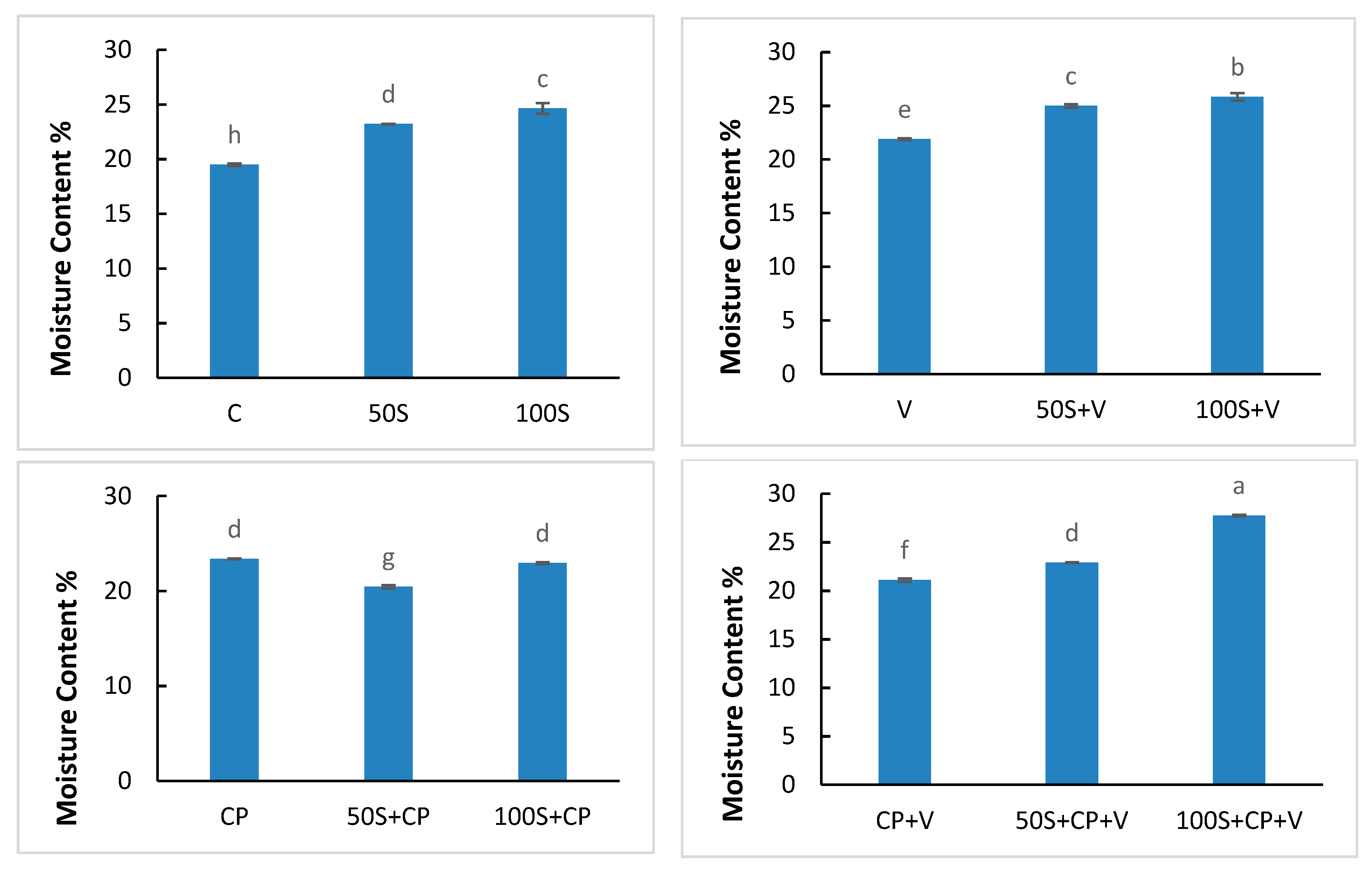
2.4. Moisture Content
2.5. Muffin Volume
2.6. Muffin Texture
2.7. Muffin Total Starch
2.8. In Vitro Predictive Glycemic Response Digestion Analysis
2.9. Statistical Analyses
3. Results and Discussion
3.1. Moisture Content
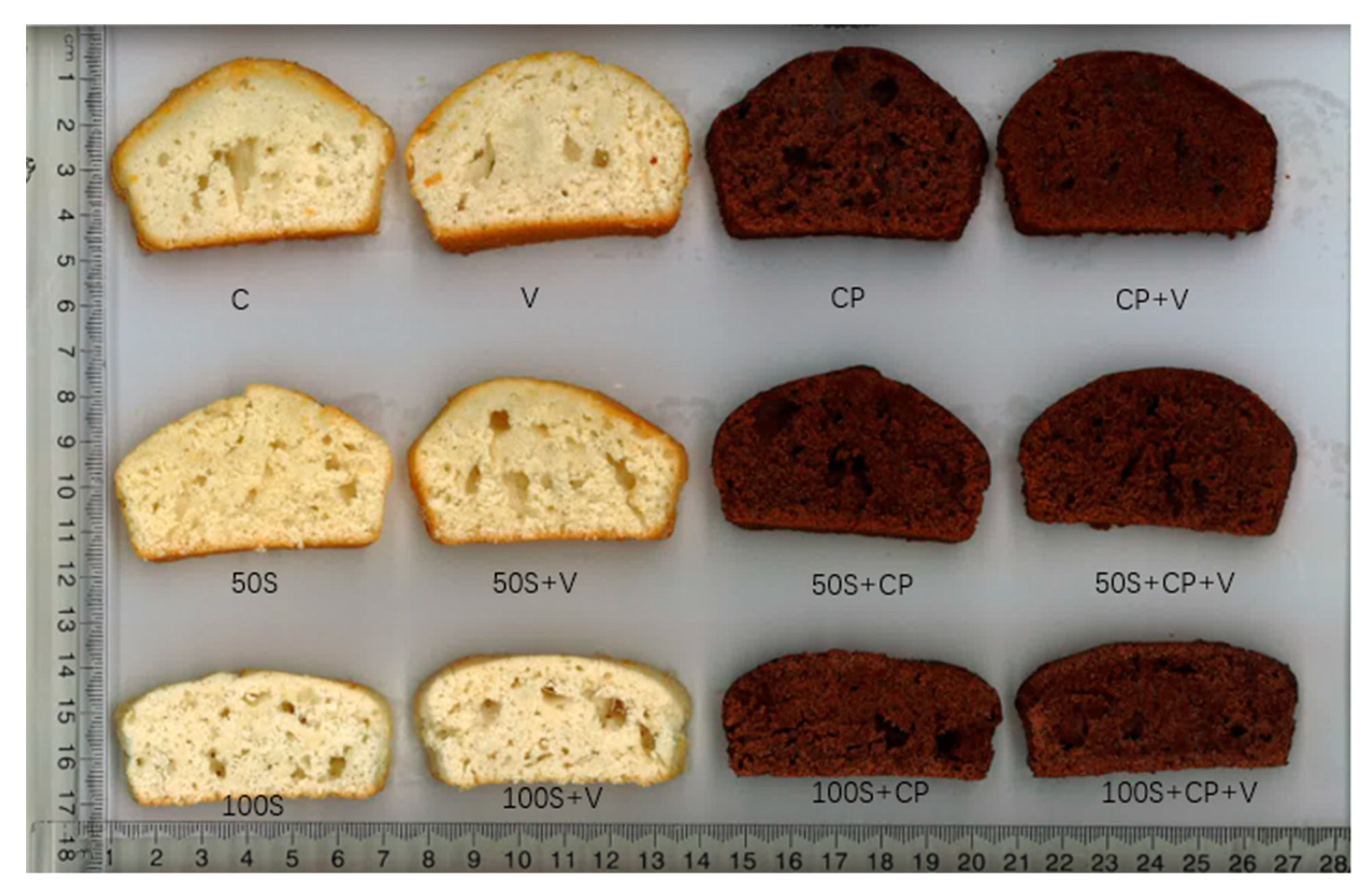
3.2. The Impact of Sugar Replacement on Product Physico-Chemical Characteristics
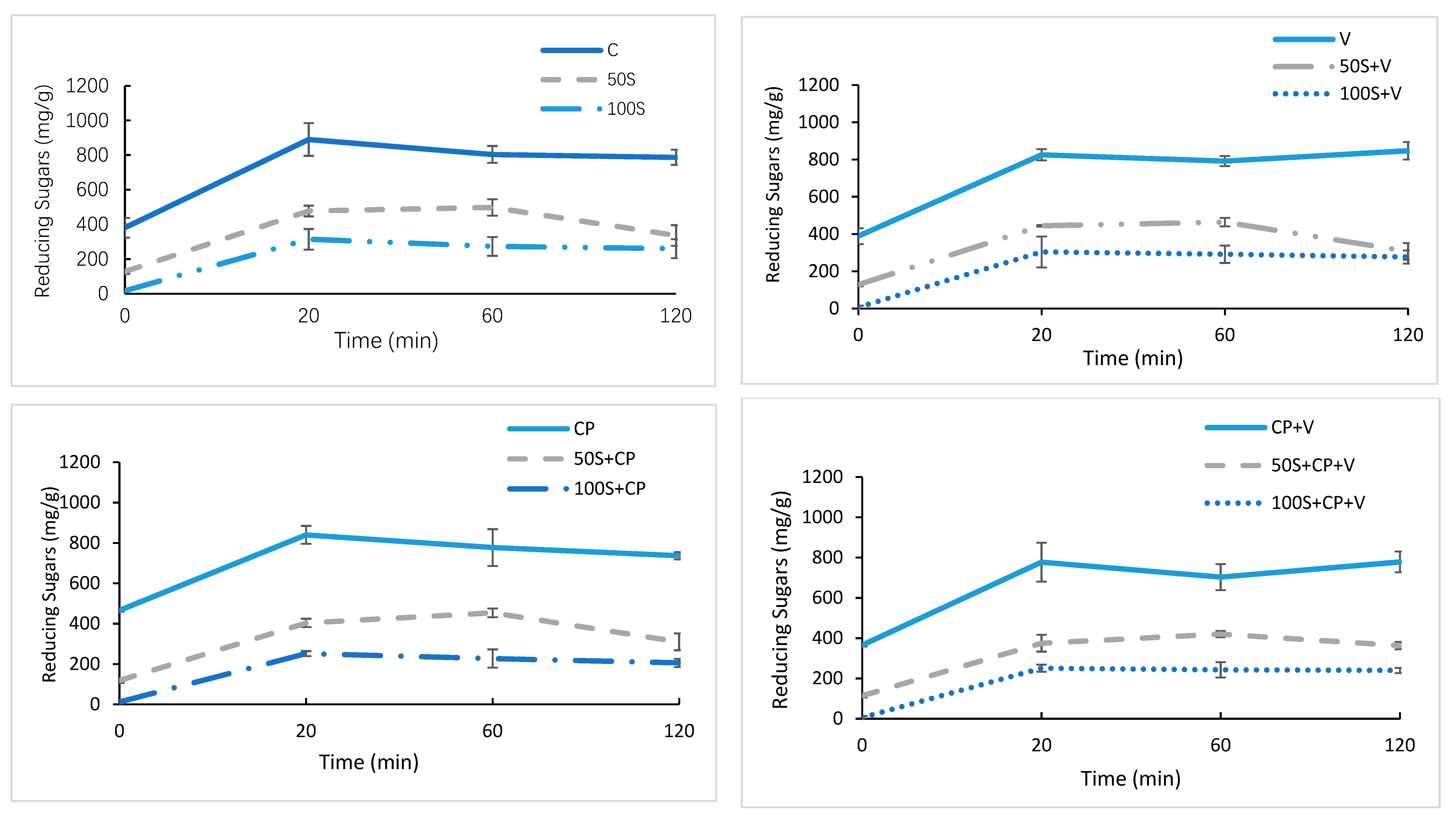
3.3. The Impact of Sugar Replacement on the In Vitro Predictive Glycemic Response
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Giuberti, G.; Gallo, A. Reducing the glycaemic index and increasing the slowly digestible starch content in gluten-free cereal-based foods: A review. Int. J. Food Sci. Technol. 2018, 53, 50–60. [Google Scholar] [CrossRef]
- Sopade, P.A. Cereal processing and glycaemic response. Int. J. Food Sci. Technol. 2017, 52, 22–37. [Google Scholar] [CrossRef]
- Connolly, A.; O’Keeffe, M.B.; Nongonierma, A.B.; Piggott, C.O.; FitzGerald, R.J. Isolation of peptides from a novel brewers spent grain protein isolate with potential to modulate glycaemic response. Int. J. Food Sci. Technol. 2017, 52, 146–153. [Google Scholar] [CrossRef]
- Brennan, C.S. Dietary fibre, glycaemic response, and diabetes. Mol. Nutr. Food Res. 2005, 49, 560–570. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Barker, H.M.; Fielden, H.; Jenkins, A.L. Effect of guar crispbread with cereal products and leguminous seeds on blood glucose concentrations of diabetics. Br. Med. J. 1980, 281, 1248–1250. [Google Scholar] [CrossRef]
- Monro, J.A. Faecal bulking efficacy of Australasian breakfast cereals. Asia Pac. J. Clin. Nutr. 2002, 11, 176–185. [Google Scholar] [CrossRef]
- Monro, J.A.; Shaw, M. Glycemic impact, glycemic glucose equivalents, glycemic index, and glycemic load: Definitions, distinctions, and implications. Am. J. Clin. Nutr. 2008, 87, 237S–243S. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Dong, Z.; Zhou, W. Structural and mechanical characteristics of bread and their impact on oral processing: A review. Int. J. Food Sci. Technol. 2018, 53, 858–872. [Google Scholar] [CrossRef]
- Klunklin, W.; Savage, G. Physicochemical, antioxidant properties and in vitro digestibility of wheat–purple rice flour mixtures. Int. J. Food Sci. Technol. 2018, 53, 1962–1971. [Google Scholar] [CrossRef]
- Barros, C.M.M.R.; Lessa, R.Q.; Grechi, M.P.; Mouço, T.L.M.; Souza, M.; das, G.C.; Wiernsperger, N.; Bouskela, E. Substitution of drinking water by fructose solution induces hyperinsulinemia and hyperglycemia in hamsters. Clinics 2007, 62, 327–334. [Google Scholar] [CrossRef]
- Bartkiene, E.; Sakiene, V.; Bartkevics, V.; Wiacek, C.; Rusko, J.; Lele, V.; Ruzauskas, M.; Juodeikiene, G.; Klupsaite, D.; Bernatoniene, J.; et al. Nutraceuticals in gummy candies form prepared from lacto-fermented lupine protein concentrates, as high-quality protein source, incorporated with Citrus paradise L. essential oil and xylitol. Int. J. Food Sci. Technol. 2018, 53, 2015–2025. [Google Scholar] [CrossRef]
- Lê, K.A.; Ith, M.; Kreis, R.; Faeh, D.; Bortolotti, M.; Tran, C.; Tappy, L. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am. J. Clin. Nutr. 2009, 89, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Burton, P.; Lightowler, H.J. Influence of bread volume on glycaemic response and satiety. Brit. J. Nutr. 2006, 96, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Bae, I.Y.; Jun, Y.; Lee, S.; Lee, H.G. Characterization of apple dietary fibers influencing the in vitro starch digestibility of wheat flour gel. LWT Food Sci. Technol. 2016, 65, 158–163. [Google Scholar] [CrossRef]
- Baeva, M.R.; Panchev, I.N.; Terzieva, V.V. Comparative study of texture of normal and energy reduced sponge cakes. Nahrung 2000, 44, 242–246. [Google Scholar] [CrossRef]
- Karp, S.; Wyrwisz, J.; Kurek, M.A.; Wierzbicka, A. Combined use of cocoa dietary fibre and steviol glycosides in low-calorie muffins production. Int. J. Food Sci. Technol. 2017, 52, 944–953. [Google Scholar] [CrossRef]
- Kulthe, A.A.; Pawar, V.D.; Kotecha, P.M.; Chavan, U.D.; Bansode, V.V. Development of high protein and low calorie cookies. Int. J. Food Sci. Technol. 2014, 51, 153–157. [Google Scholar] [CrossRef]
- Livesey, G. Health potential of polyols as sugar replacers, with emphasis on low glycaemic properties. Nutr. Res. Rev. 2003, 16, 163–191. [Google Scholar] [CrossRef]
- Oehme, A.; Wüst, M.; Wölwer-Rieck, U. Steviol glycosides are not altered during commercial extraction and purification processes. Int. J. Food Sci. Technol. 2017, 52, 2156–2162. [Google Scholar] [CrossRef]
- Azevedo, B.M.; Morais-Ferreira, J.M.; Luccas, V.; Bolini, H.M. Bittersweet chocolates containing prebiotic and sweetened with stevia (Stevia rebaudiana Bertoni) with different Rebaudioside A contents: Multiple time-intensity analysis and physicochemical characteristics. Int. J. Food Sci. Technol. 2017, 52, 1731–1738. [Google Scholar] [CrossRef]
- Wardy, W.; Jack, A.R.; Chonpracha, P.; Alonso, J.R.; King, J.M.; Prinyawiwatkul, W. Gluten-free muffins: Effects of sugar reduction and health benefit information on consumer liking, emotion, and purchase intent. Int. J. Food Sci. Technol. 2018, 53, 262–269. [Google Scholar] [CrossRef]
- Gregersen, S.; Jeppesen, P.B.; Holst, J.J.; Hermansen, K. Antihyperglycemic effects of stevioside in type 2 diabetic subjects. Metabolism 2004, 53, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.K.; Goyal, R.K. Stevia (Stevia rebaudiana) a bio-sweetener: A review. Int. J. Food Sci. Nutr. 2010, 61, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Phimolsiripol, Y.; Siripatrawan, U.; Teekachunhatean, S.; Wangtueai, S.; Seesuriyachan, P.; Surawang, S.; Laokuldilok, T.; Regenstein, J.M.; Henry, C.J. Technological properties, in vitro starch digestibility and in vivo glycaemic index of bread containing crude malva nut gum. Int. J. Food Sci. Technol. 2017, 52, 1035–1041. [Google Scholar]
- Quiles, A.; Llorca, E.; Schmidt, C.; Reißner, A.-M.; Struck, S.; Rohm, H.; Hernando, I. Use of berry pomace to replace flour, fat or sugar in cakes. Int. J. Food Sci. Technol. 2018, 53, 1579–1587. [Google Scholar] [CrossRef]
- Manisha, G.; Soumya, C.; Indrani, D. Studies on interaction between stevioside, liquid sorbitol, hydrocolloids and emulsifiers for replacement of sugar in cakes. Food Hydrocoll. 2012, 29, 363–373. [Google Scholar] [CrossRef]
- Chang, J.C.; Wu, M.C.; Liu, I.M.; Cheng, J.T. Increase of insulin sensitivity by stevioside in fructose-rich chow-fed rats. Horm. Metab. Res. 2005, 37, 610–616. [Google Scholar] [CrossRef]
- O’Donnell, K.; Kearsley, M. Sweeteners and Sugar Alternatives in Food Technology, 2nd ed.; Wiley-Blackwell: Oxford, UK, 2012; pp. 215–218. [Google Scholar]
- Wheeler, A.; Boileau, A.C.; Winkler, P.C.; Compton, J.C.; Prakash, I.; Jiang, X.; Mandarino, D.A. Pharmacokinetics of rebaudioside A and stevioside after single oral doses in healthy men. Food Chem. Toxicol. 2008, 46, S54–S60. [Google Scholar] [CrossRef]
- Gao, J.; Brennan, M.A.; Mason, S.L.; Brennan, C.S. Effect of sugar replacement with stevianna and inulin on the texture and predictive glycaemic response of muffins. Int. J. Food Sci. Technol. 2016, 51, 1979–1987. [Google Scholar] [CrossRef]
- Alencar, N.M.M.; de Morais, E.C.; Steel, C.J.; Bolini, H.M.A. Sensory characterisation of gluten-free bread with addition of quinoa, amaranth flour and sweeteners as an alternative for coeliac patients. Int. J. Food Sci. Technol. 2017, 52, 872–879. [Google Scholar] [CrossRef]
- Abdel-Salam, A.M.; Ammar, A.S.; Galal, W.K. Evaluation and properties of formulated low calories functional yoghurt cake. J. Food Agric. Environ. 2009, 7, 218–221. [Google Scholar]
- Edelstein, S.; Smith, K.; Worthington, A.; Gillis, N.; Bruen, D.; Kang, S.H.; Guiducci, G. Comparisons of Six New Artificial Sweetener Gradation Ratios with Sucrose in Conventional-Method Cupcakes Resulting in Best Percentage Substitution Ratios. J. Culin. Sci. Technol. 2007, 5, 61–74. [Google Scholar] [CrossRef]
- Struck, S.; Jaros, D.; Brennan, C.S.; Rohm, H. Sugar replacement in sweetened bakery goods. Int. J. Food Sci. Technol. 2014, 49, 1963–1976. [Google Scholar] [CrossRef]
- Röper, H.; Goossens, J. Erythritol, a new raw material for food and non-food applications. Starch 1993, 45, 400–405. [Google Scholar] [CrossRef]
- Lin, S.D.; Hwang, C.F.; Yeh, C.H. Physical and Sensory Characteristics of Chiffon Cake Prepared with Erythritol as Replacement for Sucrose. J. Food Sci. 2003, 68, 2107–2110. [Google Scholar] [CrossRef]
- Bornet, F.R. Undigestible sugars in food products. Am. J. Clin. Nutr. 1994, 59, 763S–769S. [Google Scholar] [CrossRef]
- Lin, S.D.; Lee, C.C.; Mau, J.L.; Lin, L.Y.; Chiou, S.Y. Effect of Erythritol on Quality Characteristics of Reduced-Calorie Danish Cookies. J. Food Qual. 2010, 33, 14–26. [Google Scholar] [CrossRef]
- Bornet, F.R.J.; Blayo, A.; Dauchy, F.; Slama, G. Plasma and Urine Kinetics of Erythritol after Oral Ingestion by Healthy Humans. Regul. Toxicol. Pharm. 1996, 24, S280–S285. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Li, Y.; Li, Y.; Yan, M.; Chen, K.; Xu, L. Efficient enzymatic production of rebaudioside A from stevioside. Biosci. Biotech. Bioch. 2016, 80, 67–73. [Google Scholar] [CrossRef]
- Zahn, S.; Forker, A.; Krügel, L.; Rohm, H. Combined use of rebaudioside A and fibres for partial sucrose replacement in muffins. LWT Food Sci. Technol. 2013, 50, 695–701. [Google Scholar] [CrossRef]
- AACC International. Approved Methods of Analysis, 9th ed.; AACC International: St. Paul, MN, USA, 1995; p. 76. [Google Scholar]
- Martínez-Cervera, S.; Hera, E.; de la Sanz, T.; Gómez, M.; Salvador, A. Effect of using Erythritol as a Sucrose Replacer in Making Spanish Muffins Incorporating Xanthan Gum. Food Bioprocess Tech. 2012, 5, 3203–3216. [Google Scholar] [CrossRef]
- Martínez-Cervera, S.; Sanz, T.; Salvador, A.; Fiszman, S.M. Rheological, textural and sensorial properties of low-sucrose muffins reformulated with sucralose/polydextrose. LWT Food Sci. Technol. 2012, 45, 213–220. [Google Scholar] [CrossRef]
- Ghosh, S.; Sudha, M.L. A review on polyols: New frontiers for health-based bakery products. Int. J. Food Sci. Nutr. 2012, 63, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.; Morris, G.A. The effect of inulin and fructo-oligosaccharide supplementation on the textural, rheological and sensory properties of bread and their role in weight management: A review. Food Chem. 2012, 133, 237–248. [Google Scholar] [CrossRef]
- Rößle, C.; Ktenioudaki, A.; Gallagher, E. Inulin and oligofructose as fat and sugar substitutes in quick breads (scones): A mixture design approach. Eur. Food Res. Technol. 2011, 233, 167–181. [Google Scholar] [CrossRef]
- Psimouli, V.; Oreopoulou, V. The Effect of Fat Replacers on Batter and Cake Properties. J. Food Sci. 2013, 78, C1495–C1502. [Google Scholar]
- Ronda, F.; Gómez, M.; Blanco, C.A.; Caballero, P.A. Effects of polyols and nondigestible oligosaccharides on the quality of sugar-free sponge cakes. Food Chem. 2005, 90, 549–555. [Google Scholar] [CrossRef]
- Alizadeh, M.; Azizi-Lalabadi, M.; Kheirouri, S. Impact of Using Stevia on Physicochemical, Sensory, Rheology and Glycemic Index of Soft Ice Cream. FNS 2014, 5, 390. [Google Scholar] [CrossRef]
- Ishikawa, M.; Miyashita, M.; Kawashima, Y.; Nakamura, T.; Saitou, N.; Modderman, J. Effects of Oral Administration of Erythritol on Patients with Diabetes. Regul. Toxicol. Pharm. 1996, 24, S303–S308. [Google Scholar] [CrossRef]
- Granfeldt, Y.; Eliasson, A.-C.; Björck, I. An Examination of the Possibility of Lowering the Glycemic Index of Oat and Barley Flakes by Minimal Processing. J. Nutr. 2000, 130, 2207–2214. [Google Scholar] [CrossRef]
- Anton, S.D.; Martin, C.K.; Han, H.; Coulon, S.; Cefalu, W.T.; Geiselman, P.; Williamson, D.A. Effects of stevia, aspartame, and sucrose on food intake, satiety, and postprandial glucose and insulin levels. Appetite 2010, 55, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.; Renwick, A.G. Comparative toxicokinetics and metabolism of rebaudioside A, stevioside, and steviol in rats. Food Chem. Toxicol. 2008, 46, S31–S39. [Google Scholar] [CrossRef] [PubMed]
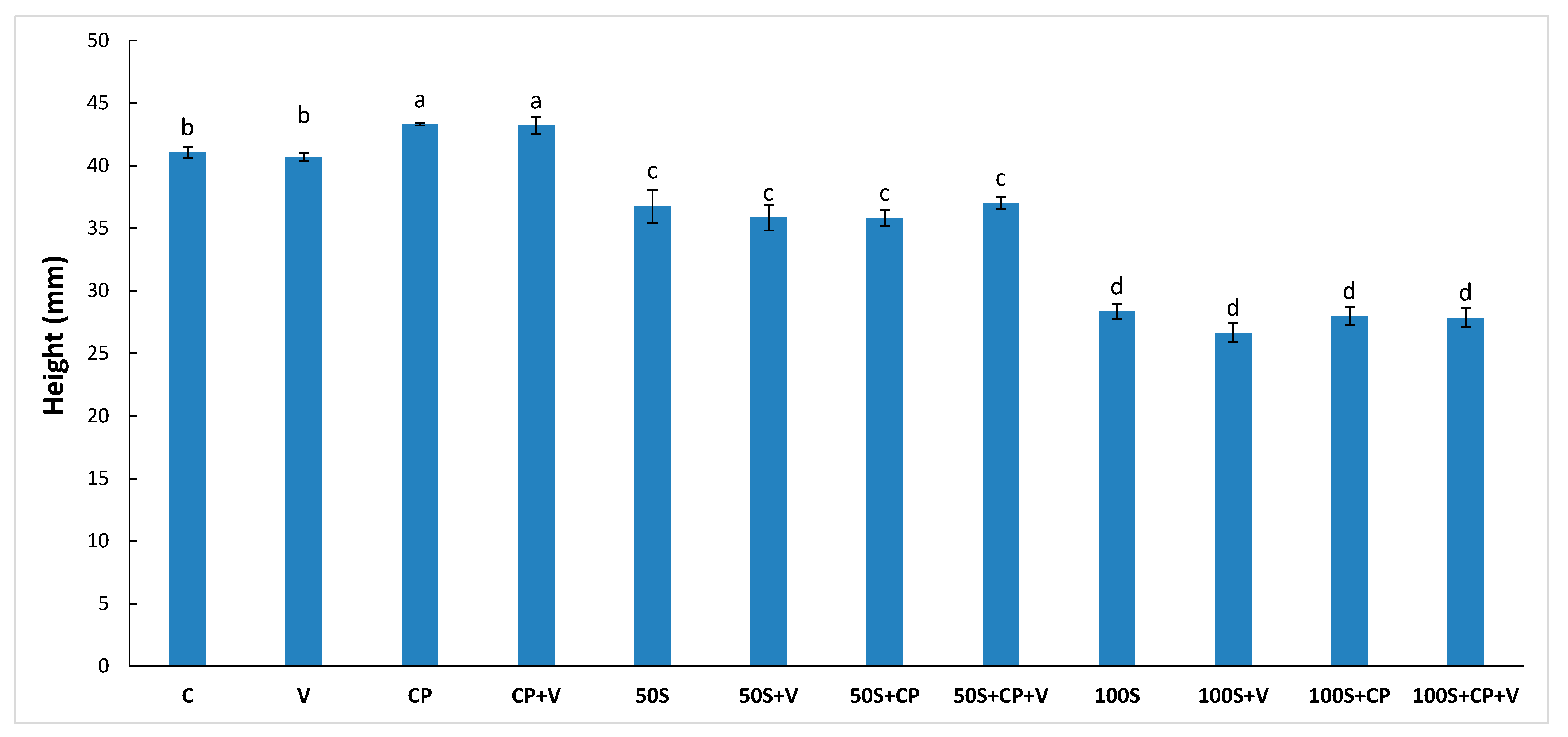
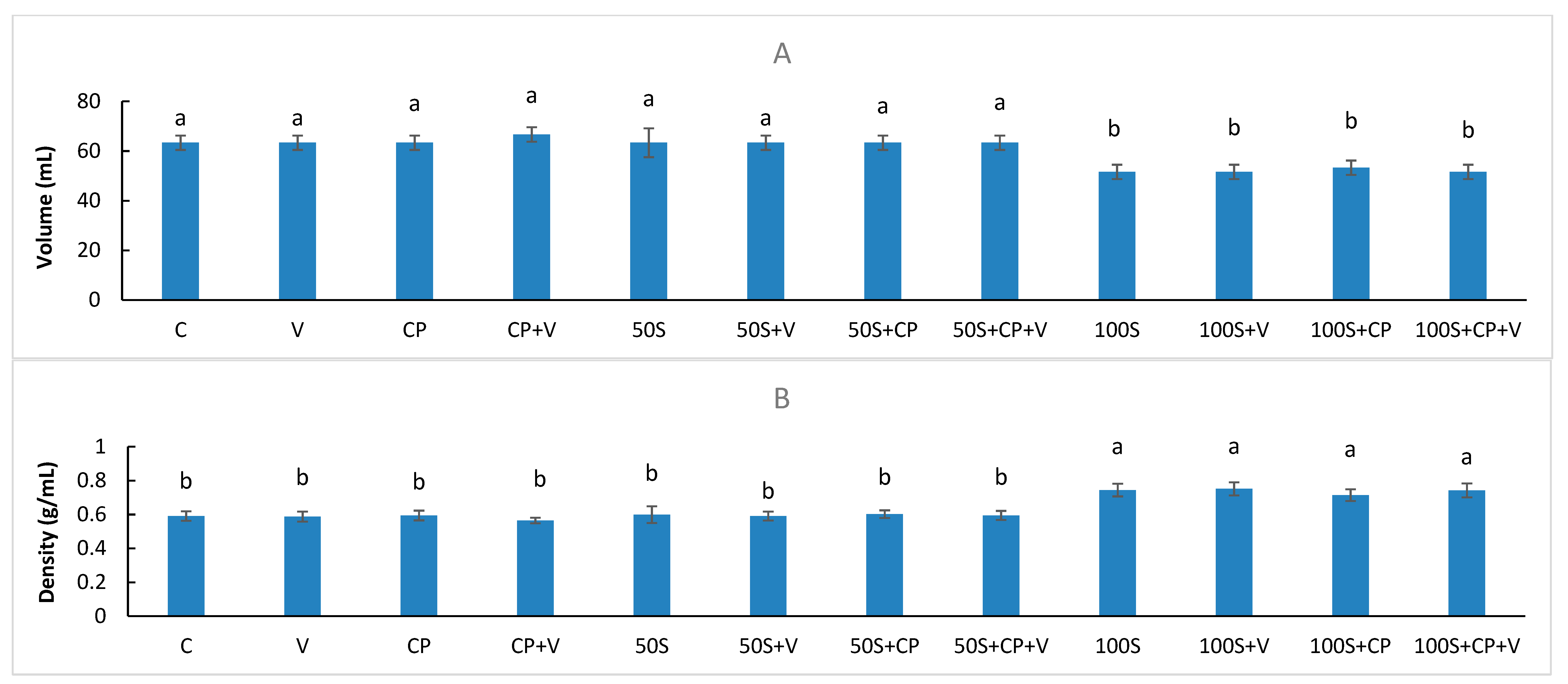

| Formulation a | C | V | CP | CP + V | 50S | 50S + V | 50S + CP | 50S + CP + V | 100S | 100S + V | 100S + CP | 100S + CP + V |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ingredients | Mass (g) | |||||||||||
| Wheat flour | 138.4 | 138.4 | 115.3 | 115.3 | 138.4 | 138.4 | 115.3 | 115.3 | 138.4 | 138.4 | 115.3 | 115.3 |
| Sugar | 92.2 | 92.2 | 92.2 | 92.2 | 46.1 | 46.1 | 46.1 | 46.1 | 0 | 0 | 0 | 0 |
| Baking powder | 6.5 | 6.5 | 6.5 | 6.5 | 6.5 | 6.5 | 6.5 | 6.5 | 6.5 | 6.5 | 6.5 | 6.5 |
| Salt | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 |
| Skim milk powder | 8.7 | 8.7 | 8.7 | 8.7 | 8.7 | 8.7 | 8.7 | 8.7 | 8.7 | 8.7 | 8.7 | 8.7 |
| Oil | 77.6 | 77.6 | 77.6 | 77.6 | 77.6 | 77.6 | 77.6 | 77.6 | 77.6 | 77.6 | 77.6 | 77.6 |
| Liquid whole egg | 34.6 | 34.6 | 34.6 | 34.6 | 34.6 | 34.6 | 34.6 | 34.6 | 34.6 | 34.6 | 34.6 | 34.6 |
| Top water | 97.6 | 97.6 | 97.6 | 97.6 | 97.6 | 97.6 | 97.6 | 97.6 | 97.6 | 97.6 | 97.6 | 97.6 |
| Cocoa powder | 0 | 0 | 23.1 | 23.1 | 0 | 0 | 23.1 | 23.1 | 0 | 0 | 23.1 | 23.1 |
| Vanilla | 0 | 3 | 0 | 3 | 0 | 3 | 0 | 3 | 0 | 3 | 0 | 3 |
| Stevia | 0 | 0 | 0 | 0 | 46.1 | 46.1 | 46.1 | 46.1 | 92.2 | 92.2 | 92.2 | 92.2 |
| Product | Firmness (g) | Springiness (%) | Total Starch (%) |
|---|---|---|---|
| C | 746.06 ± 44.10 b | 51.29 ± 0.44 ab | 26.83 ± 1.92 abc |
| V | 763.51 ± 51.48 b | 51.66 ± 0.09 a | 27.93 ± 0.42 ab |
| CP | 680.99 ± 30.33 b | 49.26 ± 0.54 ab | 26.14 ± 0.60 abcd |
| CP + V | 662.97 ± 68.46 b | 49.99 ± 0.43 ab | 24.43 ± 1.06 bcde |
| 50S | 906.07 ± 111.09 b | 51.51 ± 0.62 ab | 28.50 ±0.85 a |
| 50S + V | 1102.18 ± 102.10 b | 51.49 ± 0.78 a | 29.03 ± 0.36 a |
| 50S + CP | 987.03 ± 68.00 b | 48.67 ± 0.52 a | 22.72 ± 0.39 de |
| 50S + CP + V | 890.78 ± 76.18 b | 49.59 ± 0.54 b | 23.40 ± 0.09 cde |
| 100S | 4512.78 ± 399.65 a | 45.07 ± 0.71 c | 26.60 ± 0.94 abc |
| 100S + V | 4419.70 ± 409.69 a | 45.44 ± 0.56 c | 29.09 ± 2.56 a |
| 100S + CP | 3868.00 ± 300.87 a | 44.74 ± 1.12 c | 22.62 ± 1.42 e |
| 100S + CP + V | 3839.94 ± 522.34 a | 43.11 ± 1.36 c | 26.17 ± 1.14 abcd |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Guo, X.; Brennan, M.A.; Mason, S.L.; Zeng, X.-A.; Brennan, C.S. The Potential of Modulating the Reducing Sugar Released (and the Potential Glycemic Response) of Muffins Using a Combination of a Stevia Sweetener and Cocoa Powder. Foods 2019, 8, 644. https://doi.org/10.3390/foods8120644
Gao J, Guo X, Brennan MA, Mason SL, Zeng X-A, Brennan CS. The Potential of Modulating the Reducing Sugar Released (and the Potential Glycemic Response) of Muffins Using a Combination of a Stevia Sweetener and Cocoa Powder. Foods. 2019; 8(12):644. https://doi.org/10.3390/foods8120644
Chicago/Turabian StyleGao, Jingrong, Xinbo Guo, Margaret A. Brennan, Susan L. Mason, Xin-An Zeng, and Charles S. Brennan. 2019. "The Potential of Modulating the Reducing Sugar Released (and the Potential Glycemic Response) of Muffins Using a Combination of a Stevia Sweetener and Cocoa Powder" Foods 8, no. 12: 644. https://doi.org/10.3390/foods8120644
APA StyleGao, J., Guo, X., Brennan, M. A., Mason, S. L., Zeng, X.-A., & Brennan, C. S. (2019). The Potential of Modulating the Reducing Sugar Released (and the Potential Glycemic Response) of Muffins Using a Combination of a Stevia Sweetener and Cocoa Powder. Foods, 8(12), 644. https://doi.org/10.3390/foods8120644







